Abstract
The development of a subunit vaccine for smallpox represents a potential strategy to avoid the safety concerns associated with replication-competent vaccinia virus. Preclinical studies to date with subunit smallpox vaccine candidates, however, have been limited by incomplete information regarding protective antigens and the requirement for multiple boost immunizations to afford protective immunity. Here we explore the protective efficacy of replication-incompetent, recombinant adenovirus serotype 35 (rAd35) vectors expressing the vaccinia virus intracellular mature virion (IMV) antigens A27L and L1R and extracellular enveloped virion (EEV) antigens A33R and B5R in a murine vaccinia virus challenge model. A single immunization with the rAd35-L1R vector effectively protected mice against a lethal systemic vaccinia virus challenge. The rAd35-L1R vector also proved more efficacious than the combination of four rAd35 vectors expressing A27L, L1R, A33R, and B5R. Moreover, serum containing L1R-specific neutralizing antibodies afforded postexposure prophylaxis after systemic vaccinia virus infection. In contrast, the combination of rAd35-L1R and rAd35-B5R vectors was required to protect mice against a lethal intranasal vaccinia virus challenge, suggesting that both IMV- and EEV-specific immune responses are important following intranasal infection. Taken together, these data demonstrate that different protective antigens are required based on the route of vaccinia virus challenge. These studies also suggest that rAd vectors warrant further assessment as candidate subunit smallpox vaccines.
The current smallpox vaccine (Dryvax) is a replication-competent vaccinia virus that is highly efficacious but associated with rare but serious adverse reactions (6, 25). Therefore, the development of novel smallpox vaccines with improved safety profiles would be highly desirable. Attenuated viruses, such as modified vaccinia virus Ankara, represent one promising strategy (9). An alternative strategy involves subunit vaccines, such as plasmid DNA vaccines and recombinant proteins (5, 11, 14-17). Subunit vaccines, however, have been limited by the need for multiple antigens and the requirement for several boost immunizations to afford protection in preclinical studies. The identification of critical protective antigens and the development of vaccination strategies that can generate protective immunity after a single immunization are therefore important, particularly for a vaccine that needs to generate rapid protective immunity in a potential outbreak setting.
Vaccinia virus virions exist in two major forms with distinct surface proteins. Intracellular mature virions (IMV) have single envelopes and are released by cellular lysis, and they are believed to be critical for person-to-person transmission. In contrast, extracellular enveloped virions (EEV) have double membranes and are formed by the extrusion of virions through the cell surface membrane, and they are thought to be important for virus propagation within the host (21, 24, 26). Vaccinia virus (Dryvax) vaccination has been shown to induce neutralizing antibodies (NAbs) against membrane glycoproteins of both variants, including IMV antigens L1R and A27L and EEV antigens B5R and A33R (12, 19, 23).
Preclinical studies with plasmid DNA and purified protein subunit smallpox vaccine candidates have required multiple immunizations with combinations of IMV and EEV antigens to afford protection in vaccinia virus and monkeypox challenge models (5, 11, 14-17). Unlike plasmid DNA and purified protein vaccines, recombinant adenovirus (rAd) vectors have been shown to generate protective immunity to Ebola virus after a single immunization (27). Given the importance of rapidly inducing protective immunity in a potential outbreak setting, we explored the utility of single-shot immunizations with recombinant, replication-incompetent rAd vectors as a novel candidate subunit smallpox vaccine.
In this study, we explored the immunogenicity and protective efficacy of rAd vectors expressing A27L, A33R, B5R, and L1R antigens against lethal systemic and intranasal (i.n.) vaccinia virus challenges in mice. We utilized the rare serotype rAd35 vector (30) rather than the common rAd5 vector, given the high frequency of preexisting anti-Ad5 immunity that is present in human populations and that likely would suppress vaccine immunogenicity (3, 7, 29). We observed that a single intramuscular (i.m.) immunization with the rAd35-L1R vector was sufficient to protect mice against systemic vaccinia virus challenges but that a combination of rAd35-L1R and rAd35-B5R vectors was required to protect mice against i.n. vaccinia virus challenges. Sera from vaccinated mice also proved partially effective in postexposure prophylaxis studies. These data suggest that rare serotype rAd vectors are useful in the development of subunit smallpox vaccines and highlight the importance of the route of infection in defining protective vaccine antigens.
MATERIALS AND METHODS
Vector production.
Recombinant, replication-incompetent, E1/E3-deleted rAd35 vectors expressing vaccinia virus Western Reserve A27L, A33R, B5R, and L1R proteins under the control of a cytomegalovirus promoter and a polyadenylation signal were produced by homologous recombination of the pAdApt35 adaptor plasmid expressing the antigens with the structural cosmid pWE.Ad35.pIX-rITR.dE3.5orf6 in adherent PER.C6 packaging cells as previously described (30). The plasmids were linearized prior to transfection of PER.C6 cells using Lipofectamine in T25 flasks. Cells were passaged into T75 flasks after 48 h and maintained until virus cytopathic effect was observed. The vectors were plaque purified, analyzed for transgene expression, amplified in 24 triple-layer T175 flasks, purified by double CsCl gradient ultracentrifugation, and dialyzed into phosphate-buffered saline (PBS) containing 5% sucrose. Purified rAd vectors were stored at −80°C. Virus particle (vp) titers were determined by spectrophotometry. Specific infectivity was assessed by PFU assays.
Animals, immunizations, and vaccinia virus challenges.
Six- to 8-week-old BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) or Taconic (Hudson, NY). Mice were injected once i.m. with 1010 or 109 vp of rAd35 vectors expressing no transgene (sham vaccination) or one or more vaccinia virus antigens in both quadriceps muscles. At week 4 following immunization, mice were infected i.p. with 2 × 108 PFU or i.n. with 2 × 107 PFU vaccinia virus Western Reserve (10 50% lethal doses [LD50]; Therion Biologics, Cambridge, MA). These doses were selected based on preliminary in vivo titration experiments that demonstrated 100% mortality in unvaccinated mice. For postexposure prophylaxis studies, 200 μl serum from vaccinated mice was administered i.m. 12 h following vaccinia virus infection. Infected mice were monitored daily for clinical status and body weights, and animals were sacrificed if they lost >20% body weight, as required by our institutional guidelines. All animal studies were approved by our institutional animal care and use committee.
ELISA.
Maxisorp enzyme-linked immunosorbent assay (ELISA) plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl carbonate coating buffer containing 1 μg/ml recombinant baculovirus-expressed A27L, A33R, B5R, or L1R protein (BEI Resources, Manassas, VA). All subsequent steps were performed at room temperature. Plates were washed three times with PBS-0.05% Tween 20 wash buffer and blocked for 2 h with a blocking buffer containing 5% nonfat dry milk, 5% fetal bovine serum (FBS), and 0.05% Tween 20 in PBS. Serum samples were serially diluted in 100 μl blocking buffer and added to the ELISA plate in duplicate. The plates were incubated for 2 h and washed five times, and a secondary peroxidase-conjugated affinity-purified rabbit anti-mouse secondary antibody (Jackson Laboratories, Bar Harbor, ME) diluted 1:2,500 in blocking buffer was added. Plates were incubated for 1 h, washed five times, and developed with tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) for 15 min. The reaction was stopped by the addition of 1% HCl stop solution, and the plates were analyzed at 450 nm with a Multiskan EX ELISA reader (Thermo Electron, Vantaa, Finland). The endpoint titer for each serum sample represented the greatest dilution with an optical density greater than twice the background values.
Virus neutralization assay.
NAb responses against vaccinia virus were measured using a luciferase-based assay in HeLa cells essentially as previously described for a β-galactosidase neutralization assay (20). We measured the reduction in luciferase reporter gene expression in target cells after a single round of virus infection. The recombinant vaccinia virus Western Reserve, expressing the luciferase reporter gene (VV:Luc), was a generous gift from David Bartlett, University of Pittsburgh. Briefly, threefold serial dilutions of mouse serum samples in 10% Dulbecco's modified Eagle's medium (DMEM) growth medium were assayed in triplicate in 100-μl volumes in a 96-well flat-bottom plate. VV:Luc (1 × 105 PFU) in a volume of 50 μl was added to each well, and the plates were incubated for 1 h at 37°C. HeLa cells (1 × 105) in 50 μl 10% DMEM growth medium then were added to achieve a multiplicity of infection of 1:1. Cytosine arabinofuranoside (Sigma, St. Louis, MO) was added at a final concentration of 20 μg/ml to prevent secondary rounds of infection. Assay controls included replicate wells of target cells alone (cell control) and target cells with virus (virus control). Following a 16-h incubation at 37°C, 100 μl was removed from each well, and 100 μl of Bright-Glo luciferase reagent (Promega, Madison, WI) was added. The cells were allowed to lyse for 2 min, 150 μl of the cell lysate was transferred to a 96-well black solid plate, and luminescence was measured using a Victor 3 luminometer (Perkin Elmer, Waltham, MA). The 50% inhibitory dose titer was calculated as the serum dilution that caused a 50% reduction in relative luminescence units compared to the level of virus in control wells after the subtraction of cell control relative luminescence units. Vaccinia virus immune globulin (VIG; Centers for Disease Control and Protection, Atlanta, GA) was utilized as a positive control reagent for all neutralization assays performed.
Comet reduction assay.
Confluent monolayers of CV-1 cells in 6-well plates (Costar, Gaithersburg, MD) were infected with VV:IHD-J (a generous gift from Bernard Moss, NIAID, NIH) diluted in 10% DMEM growth medium to achieve approximately 25 plaques per well. Following 2 h of rocking at 37°C, the virus-containing inoculum was aspirated and replaced with 2 ml fresh 10% DMEM. Mouse serum samples then were added to achieve a 1:50 final dilution. The plates were incubated for 2 days at 37°C and then stained with crystal violet. The degree of comet inhibition in wells containing mouse serum was compared to that of wells containing virus alone and graded semiquantitatively as follows: 0 (no inhibition), + (some inhibition), ++ (moderate inhibition), and +++ (complete inhibition).
ELISPOT assay.
Vaccinia virus A27L-, A33R-, B5R-, and L1R-specific cellular immune responses in vaccinated mice were assessed by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays essentially as described previously (3). Overlapping 15-amino-acid peptides spanning these specific vaccinia virus proteins were utilized. Ninety-six-well multiscreen plates (Millipore, Bedford, MA) were coated overnight with 100 μl/well of 10 μg/ml anti-mouse IFN-γ (BD Pharmingen, San Diego, CA) in endotoxin-free Dulbecco's PBS (D-PBS). The plates then were washed three times with D-PBS containing 0.25% Tween 20 (D-PBS-Tween), blocked for 2 h with D-PBS containing 5% FBS at 37°C, washed three times with D-PBS-Tween, rinsed with RPMI 1640 containing 10% FBS to remove the Tween 20, and incubated with 2 μg/ml each peptide and 5 × 105 murine splenocytes in triplicate in 100-μl reaction volumes. Following an 18-h incubation at 37°C, the plates were washed nine times with D-PBS-Tween and once with distilled water. The plates then were incubated with 2 μg/ml biotinylated anti-mouse or anti-human IFN-γ (BD Pharmingen, San Diego, CA) for 2 h at room temperature, washed six times with D-PBS-Tween, and incubated for 2 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL). Following five washes with D-PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, Rockford, IL), the reactions were stopped by washing the plates with tap water, and the plates were air dried and read using an ELISPOT reader (Cellular Technology Ltd., Cleveland, OH). The numbers of spot-forming cells (SFC) per 106 cells were calculated. Medium background values were consistently <15 SFC per 106 cells.
Statistical analyses.
Immunogenicity and challenge studies show representative data from experiments that were repeated at least three times. Immune responses among groups of mice are presented as means with standard errors. Mortality in these studies was defined as animals that were found dead or were humanely euthanized per institutional guidelines. Comparisons of survival and weight loss among groups of mice following challenge were performed using two-sided Fisher's exact tests. In all cases, P values of less than 0.05 were considered significant.
RESULTS
Humoral and cellular immune responses elicited by rAd35 vectors expressing vaccinia virus antigens.
We produced replication-incompetent rAd35 vectors expressing four membrane proteins from the IMV or EEV forms of vaccinia virus. Two IMV antigens (A27L and L1R) and two EEV antigens (A33R and B5R) were selected for detailed study based on previous preclinical vaccine studies (14, 16, 17). Antigen expression from these vectors was verified by Western blot analyses (data not shown). To assess the immunogenicity of these vaccine vectors, groups of BALB/c mice (n = 4/group) were injected once i.m. with 1010 vp of rAd35-Empty (sham vaccine), rAd35-A27L, rAd35-A33R, rAd35-B5R, rAd35-L1R, or a mixture of all four rAd35 vaccine vectors (each at 2.5 × 109 vp). Vaccinia virus-specific humoral and cellular immune responses were assessed at week 4 following immunization.
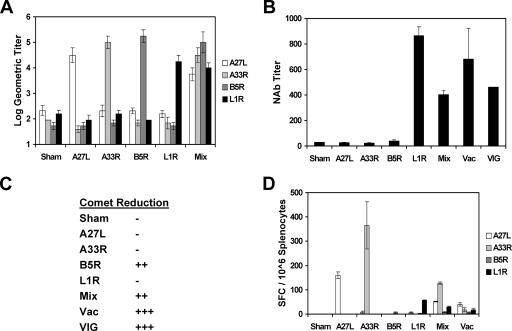
As depicted in Fig. 1A, each vector induced high-titer antibody responses by ELISA to the cognate antigen after a single i.m. immunization, as expected. Moreover, the mixture of all four vectors elicited comparable antibody titers to all four antigens. Subtyping studies demonstrated that these antibodies were predominantly IgG2a and IgG1 (data not shown). We next assessed vaccinia virus IMV-specific NAb titers by using a luciferase-based virus neutralization assay (20). As shown in Fig. 1B, sera from mice that received rAd35-L1R contained high titers of vaccinia virus-specific NAbs that were comparable to those observed in mice following a sublethal infection with 2 × 105 PFU of vaccinia virus and that exceeded titers found in VIG. Sera from mice immunized with the mixture of all four vectors, however, exhibited lower NAb titers than those of sera from mice immunized only with rAd35-L1R, presumably reflecting the fourfold lower dose of the rAd35-L1R vector in the mixture. Interestingly, sera from mice that received rAd35-A27L exhibited no detectable neutralizing activity despite the presence of high titers of A27L antibodies by ELISA, indicating that the vaccine-elicited A27L antibodies were largely nonneutralizing. As expected, sera from mice that received rAd35 vectors expressing the EEV antigens A33R and B5R did not contain IMV-specific NAbs.
FIG. 1.
Construction and immunogenicity of rAd35 vaccine vectors expressing vaccinia virus antigens. Four rAd35 vectors expressing vaccinia virus A27L, A33R, B5R, and L1R under the control of a cytomegalovirus promoter and polyadenylation signal were produced. A27L and L1R represent IMV antigens; A33R and B5R represent EEV antigens. BALB/c mice (n = 4/group) were injected once i.m. with 1010 vp of rAd35-Empty (sham vaccine), rAd35-A27L, rAd35-A33R, rAd35-B5R, rAd35-L1R, or a mixture of the four rAd35 vaccine vectors (Mix; each at 2.5 × 109 vp). At week 4 following immunization, antigen-specific antibody titers were assessed by ELISA (A), IMV-specific NAb titers were determined by luciferase-based virus neutralization assays (B), EEV-specific NAbs were assessed semiquantitatively by comet reduction assays (C), and antigen-specific cellular immune responses were assessed by pooled peptide IFN-γ ELISPOT assays (D). Positive controls included VIG and splenocytes and serum from vaccinia virus-infected mice (Vac).
We assessed EEV-specific NAbs by using a comet reduction assay that measures the inhibition of the cell-to-cell spread of vaccinia virus (18). As shown in Fig. 1C, sera from mice that received rAd35-B5R or the mixture of all four vectors exhibited significant EEV-specific NAbs. No comet inhibition, however, was observed by using sera from mice that received rAd35-A33R, suggesting that the vaccine-induced A33R antibodies were largely nonneutralizing. As expected, sera from mice that received the vectors expressing the IMV antigens A27L and L1R did not exhibit neutralizing activity in this assay. These data demonstrate that the rAd35-L1R and rAd35-B5R vectors induced IMV- and EEV-specific NAbs, respectively, after a single immunization in mice.
Vaccinia virus-specific T-lymphocyte responses were assessed by IFN-γ ELISPOT assays using pools of 15-amino-acid peptides that overlapped by 11 amino acids and covered the entire A27L, A33R, B5R, and L1R proteins. As shown in Fig. 1D, rAd35-A27L, A33R, and L1R vectors induced detectable cellular immune responses against their cognate antigens, although rAd35-B5R induced only minimal ELISPOT responses. Immunization of mice with the mixture of all four vectors induced ELISPOT responses to multiple antigens, although the response against each antigen was diminished in magnitude by approximately twofold. The ELISPOT responses to these four antigens that were induced by vaccination were higher than those induced by a sublethal infection with 2 × 105 PFU vaccinia virus. We did not measure total vaccinia virus-specific T-cell responses in this study (13).
A single immunization with rAd35-L1R protects mice against systemic vaccinia virus challenge.
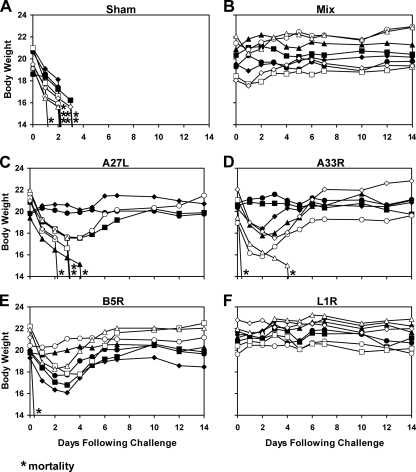
We next explored the capacity of the rAd35 vaccine vectors to protect against lethal systemic vaccinia virus challenge. Groups of BALB/c mice (n = 8/group) were immunized once i.m. with 1010 vp of rAd35-Empty (sham vaccine), each individual rAd35 vaccine vector, or a mixture of all four rAd35 vaccine vectors (each at 2.5 × 109 vp) as described above. At week 4 following immunization, mice were challenged systemically by the intraperitoneal (i.p.) route with a lethal dose (2 × 108 PFU; 10 LD50) of vaccinia virus. This dose was selected based on preliminary in vivo titration experiments that demonstrated that this dose, delivered i.p., reliably resulted in 100% mortality in unvaccinated mice (data not shown). Infected mice were monitored daily for clinical status and body weights, and animals were sacrificed if they lost >20% body weight as required by our institutional guidelines.
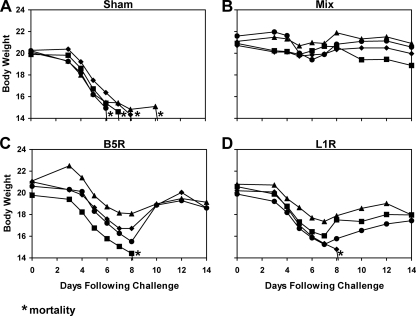
As depicted in Fig. 2A, all sham-immunized control mice rapidly lost weight and either were found dead or were sacrificed by day 3 following infection. In contrast, as shown in Fig. 2B, the mixture of all four rAd35 vaccine vectors effectively protected mice against both mortality and weight loss (P = 0.0002 by a Fisher's exact test that compared results for vaccinated mice to those for sham-immunized control mice). Mice that received a sublethal immunization with vaccinia virus were similarly protected (data not shown). Interestingly, as depicted in Fig. 2C to F, the single rAd35-L1R vector also afforded robust protection against both mortality and weight loss (P = 0.0002). The other individual vectors, however, provided only partial protection. In particular, four of eight mice vaccinated with rAd35-A27L (P = 0.08; not significant [NS]), six of eight mice vaccinated with rAd35-A33R (P = 0.007), and seven of eight mice vaccinated with rAd35-B5R (P = 0.001) survived. However, only two of eight mice in each of these groups exhibited stable body weights following challenge (P = 0.5; NS). These data indicate that rAd35-L1R was the optimal individual vector for protection against i.p. vaccinia virus challenge, demonstrating the importance of L1R as a protective antigen in this model. Comparable results were obtained after intravenous vaccinia virus challenge (data not shown).
FIG. 2.
Vaccine protection against i.p. vaccinia virus challenge. BALB/c mice (n = 8/group) were immunized once i.m. with 1010 vp of rAd35-Empty (sham vaccine) (A), the mixture of all four rAd35 vaccine vectors (each at 2.5 × 109 vp) (B), rAd35-A27L (C), rAd35-A33R (D), rAd35-B5R (E), or rAd35-L1R (F). At week 4 following immunization, mice were challenged systemically by the i.p. route with a lethal dose (2 × 108 PFU) of vaccinia virus. Body weights were determined daily, and mice were euthanized if they lost >20% of their body weight based on institutional animal guidelines. Asterisks indicate mortality.
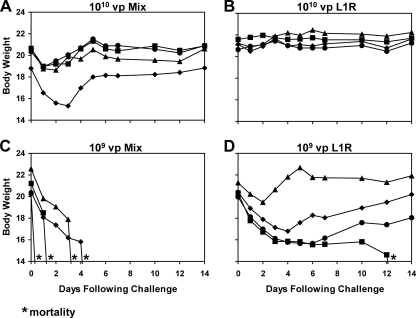
To explore further whether L1R was in fact the critical protective antigen in the mixture of the four vectors, we performed a dose reduction study. As shown in Fig. 3, groups of BALB/c mice (n = 4/group) were vaccinated with 1010 or 109 vp of the single vector rAd35-L1R or the mixture of all four vectors (each at one quarter of the total dose). Mice then were challenged i.p. with vaccinia virus at week 4 following immunization as described above. Interestingly, all mice vaccinated with the lower 109-vp dose of the mixture of all four antigens exhibited no detectable vaccinia virus-specific NAb titers (data not shown) and rapidly lost weight and either were found dead or were sacrificed by day 4 following challenge (Fig. 3C), similarly to sham-vaccinated control mice (Fig. 2A). In contrast, three of four mice vaccinated with 109 vp rAd35-L1R survived, although they all experienced at least transient weight loss (Fig. 3D). Thus, the single rAd35-L1R vector appeared superior to the mixture of all four vectors in protecting against the i.p. vaccinia virus challenge. These data suggest that the potential advantage of including A27L, A33R, and B5R antigens in the vaccine was outweighed by the fourfold-reduced dose of the critical L1R antigen in the mixture of all four vectors.
FIG. 3.
Low-dose vaccine protection against i.p. vaccinia virus challenge. BALB/c mice (n = 4/group) were immunized once i.m. with 1010 vp (A and B) or 109 vp (C and D) of the mixture of all four rAd35 vaccine vectors (each at one quarter of the total dose) (A and C) or the single rAd35-L1R vector (B and D). At week 4 following immunization, mice were challenged systemically by the i.p. route with a lethal dose (2 × 108 PFU) of vaccinia virus. Body weights were determined daily. Asterisks indicate mortality.
Postexposure prophylaxis with serum containing L1R-specific antibodies partially protects mice against systemic vaccinia virus challenge.
To explore the protective capacity of L1R-specific NAbs in the absence of T-lymphocyte responses, we performed postexposure prophylaxis studies following lethal systemic vaccinia virus infection in mice. Postexposure prophylaxis is clinically important in the management of adverse reactions to Dryvax vaccination and also likely would be critical in the event of an outbreak of variola virus or other orthopoxviruses. Groups of BALB/c mice (n = 8/group) were infected systemically by the i.p. route with a lethal dose (2 × 108 PFU) of vaccinia virus as described above. Twelve hours after infection, mice received an i.m. injection of 200 μl serum derived from mice previously vaccinated with rAd35-Empty (sham vaccine), each individual rAd35 vaccine vector, or the mixture of all four rAd35 vaccine vectors as described for Fig. 1 essentially as described previously (28).
As shown in Fig. 4, postexposure prophylaxis with hyperimmune serum in general afforded less robust protection than did the active vaccination depicted in Fig. 2. Sera from sham-immunized mice failed to rescue mice from the lethal vaccinia virus challenge as expected, but sera from mice that received all four vectors rescued only two of eight mice (P = 0.5; NS). Sera containing A27L, A33R, or B5R antibody also failed to have any detectable effect in this setting (P = 1.0; NS), but sera containing L1R antibodies rescued six of eight mice from mortality (P = 0.007). The apparent increased utility of sera containing only L1R antibodies compared to that of sera containing antibodies against all four antigens may reflect the higher L1R-specific NAb titers in sera from mice that received only rAd35-L1R (Fig. 1B). These data suggest that the L1R-specific NAb titer represents an important correlate of protective immunity in this model.
FIG. 4.
Postexposure prophylaxis against i.p. vaccinia virus challenge. BALB/c mice (n = 8/group) were infected systemically by the i.p. route with a lethal dose (2 × 108 PFU) of vaccinia virus. Twelve hours after infection, mice received an i.m. injection of 200 μl serum derived from mice previously vaccinated with 1010 vp of rAd35-Empty (sham vaccine) (A), the mixture of all four rAd35 vaccine vectors (each at 2.5 × 109 vp) (B), rAd35-A27L (C), rAd35-A33R (D), rAd35-B5R (E), or rAd35-L1R (F) as described in the legend to Fig. 1. Body weights were determined daily. Asterisks indicate mortality.
A single immunization with rAd35-B5R and rAd35-L1R protects mice against i.n. vaccinia virus challenge.
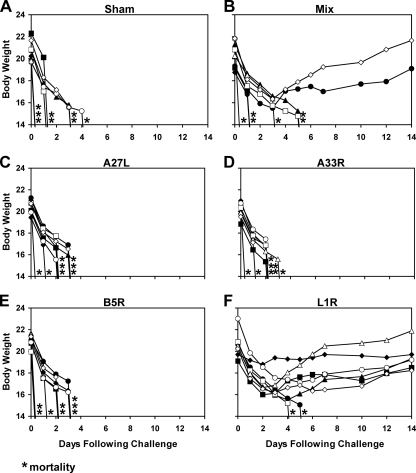
We hypothesized that EEV-specific immunity is more important following i.n. challenge than systemic challenge as a result of extensive EEV replication in local tissues following mucosal infection. To explore this hypothesis, we assessed the protective efficacy of rAd35-L1R and rAd35-B5R, both individually and in combination, against mucosal i.n. vaccinia virus challenge. As shown previously (Fig. 1B and C), rAd35-L1R and rAd35-B5R induced potent IMV- and EEV-specific NAbs, respectively, after a single immunization. Groups of BALB/c mice (n = 4/group) were immunized once i.m. with 1010 vp of rAd35-Empty (sham vaccine), rAd35-B5R, rAd35-L1R, or the mixture of these two rAd35 vaccine vectors (each at 5 × 109 vp). Mice then were challenged i.n. at week 4 following immunization with 2 × 107 PFU (10 LD50) vaccinia virus, and weight loss and clinical status were monitored. This dose was selected based on preliminary in vivo titration experiments that demonstrated that this dose, delivered i.n., reliably resulted in 100% mortality in unvaccinated mice (data not shown).
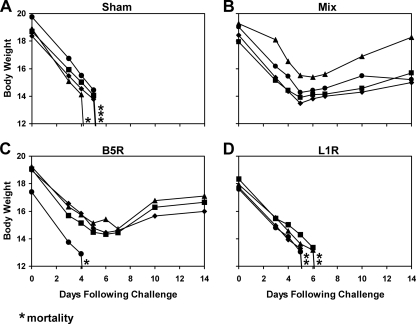
As shown in Fig. 5, sham-vaccinated mice lost weight and were sacrificed between days 6 and 12 following infection. We suspect that the slower clinical disease progression following i.n. infection (Fig. 5A) compared to that of i.p. infection (Fig. 2A) reflects the time required for the virus to replicate in local mucosal tissues prior to systemic spread. Mice vaccinated with both rAd35-B5R and rAd35-L1R vectors were completely protected against both weight loss and mortality (P = 0.02), but rAd35-B5R or rAd35-L1R alone afforded only partial protection (P = 0.1; NS). The mixture of all four rAd35 vectors as well as a sublethal immunization with vaccinia virus also afforded complete protection in this model (data not shown). Importantly, these results contrast with the results of the i.p. vaccinia virus challenge studies in which the single rAd35-L1R vector was sufficient to provide complete protection (Fig. 2F).
FIG. 5.
Vaccine protection against i.n. vaccinia virus challenge. BALB/c mice (n = 4/group) were immunized once i.m. with 1010 vp of rAd35-Empty (sham vaccine) (A), the mixture of rAd35-B5R and rAd35-L1R (each at 5 × 109 vp) (B), rAd35-B5R (C), and rAd35-L1R (D). At week 4 following immunization, mice were challenged mucosally by the i.n. route with a lethal dose (2 × 107 PFU) of vaccinia virus. Body weights were determined daily. Asterisks indicate mortality.
Postexposure prophylaxis with serum containing B5R- and L1R-specific antibodies partially protects mice against i.n. vaccinia virus challenge.
We next performed a postexposure prophylaxis study analogous to the one described in Fig. 4 but that utilized a mucosal i.n. vaccinia virus challenge. Groups of BALB/c mice (n = 4/group) were infected i.n. with 2 × 107 PFU vaccinia virus. Twelve hours after infection, mice were injected i.m. with 200 μl serum derived from mice previously vaccinated with rAd35-Empty (sham vaccine), both rAd35-B5R and rAd35-L1R, rAd35-B5R alone, or rAd35-L1R alone. As shown in Fig. 6, sera containing B5R-specific antibodies (P = 0.1; NS) or B5R- and L1R-specific antibodies (P = 0.02) proved partially protective in this setting. These data suggest that EEV B5R-specific antibodies were important in controlling the i.n. vaccinia virus infection. These results contrast with the results of the postexposure prophylaxis studies that used i.p. vaccinia virus challenges in which IMV L1R-specific antibodies appeared to be primarily responsible for protection (Fig. 4F).
FIG. 6.
Postexposure prophylaxis against i.n. vaccinia virus challenge. BALB/c mice (n = 4/group) were infected mucosally by the i.n. route with a lethal dose (2 × 107 PFU) of vaccinia virus. Twelve hours after infection, mice received an i.m. injection of 200 μl serum derived from mice previously vaccinated with 1010 vp of rAd35-Empty (sham vaccine) (A), the mixture of rAd35-B5R and rAd35-L1R (each at 5 × 109 vp) (B), rAd35-B5R (C), and rAd35-L1R (D). Body weights were determined daily. Asterisks indicate mortality.
DISCUSSION
Serious adverse events following vaccinia virus (Dryvax) vaccination (6, 25) have highlighted the need for the development of safer smallpox vaccines. We observed that a single immunization with replication-incompetent rAd35 vectors expressing key vaccinia virus antigens was sufficient to afford robust protective immunity against lethal systemic and i.n. vaccinia virus challenges in mice. The single rAd35-L1R vector efficiently protected against systemic i.p. vaccinia virus challenges, but the combination of rAd35-B5R and rAd35-L1R vectors was required to protect against mucosal i.n. vaccinia virus challenges. These data demonstrate that different protective antigens are required based on the route of virus challenge. Both EEV- and IMV-specific immunities likely are needed to protect against i.n. vaccinia virus infection, since EEV represents the predominant form of replicating virus in mucosal tissues (22).
Several prior studies have evaluated plasmid DNA vaccines and recombinant protein subunit vaccines to protect against poxvirus infections in murine and nonhuman primate challenge models (5, 11, 14-17). In these studies, multiple immunizations and the inclusion of four vaccine antigens were required to afford protective immunity. Here we extend these reports by showing that a single immunization with only one or two rAd35 vectors generated potent vaccinia virus-specific immunity that provided robust protection against lethal vaccinia virus challenges in mice. The capacity of a subunit vaccine to generate protective immunity after a single immunization is critical for a vaccine that might need to be deployed rapidly in a potential outbreak setting.
The partial efficacy of hyperimmune sera in the postexposure prophylaxis studies highlights the importance of humoral immunity in protecting against vaccinia virus in mice. This observation is consistent with previous reports that demonstrated that humoral immunity was a critical component of long-term protection against poxviruses (2) and that monoclonal and polyclonal antibodies against envelope proteins mediated protection in a postexposure prophylaxis setting (8, 19). Our data also are consistent with lymphocyte depletion studies of nonhuman primates that demonstrated that protection against monkeypox challenge was mediated primarily by antibodies (10). Our results demonstrate that L1R-specific NAbs are important for protecting against systemic vaccinia virus challenges in mice, whereas both B5R- and L1R-specific NAbs appear to be important for protecting against i.n. vaccinia virus challenges. Interestingly, B5R but not L1R was identified as a key target of the humoral immune response following the Dryvax vaccination of humans (12, 23). The importance of L1R as a protective antigen in the present study suggests that the optimal antigens for inclusion in a prophylactic vaccine may not always precisely mirror the dominant antigens targeted by the immune system following infection.
Cellular immunity also has been shown to contribute to immune protection against orthopoxviruses (4, 31). We observed that active vaccination (Fig. 2 and 5) afforded more robust protection than did the adoptive transfer of hyperimmune serum (Fig. 4 and 6). It is unclear, however, whether these differences represent the contribution of cellular immunity in the mice that received the rAd35 vaccines or simply the dilution of the serum containing antibodies in the recipient mice following adoptive transfer. It also is certainly possible that important T-lymphocyte responses are directed against antigens other than those included in this study (13).
Taken together, our data demonstrate the potential utility of rAd35 vectors expressing vaccinia virus B5R and L1R antigens as a novel subunit smallpox vaccine that requires only minimal numbers of vaccine components and that affords robust protection after a single immunization against lethal vaccinia virus challenges in mice. These data extend previous studies that demonstrated the potential utility of rAd35 and other rare serotype rAd vectors as candidate vaccines for other pathogens (1, 3, 30). Further studies will be required to assess whether rAd35-B5R and rAd35-L1R vaccines will similarly afford robust protection in nonhuman primate challenge studies and whether B5R- and L1R-specific monoclonal antibodies will prove useful for postexposure prophylaxis.
Acknowledgments
We thank Jinyan Liu, Diana Lynch, Nate Simmons, Raphael Dolin, Lindsey Baden, Jonathan Duke-Cohen, and Ellis Reinherz for generous advice, assistance, and reagents.
We acknowledge support from NIH grants AI066305, AI066924, and AI058727 (D.H.B.).
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 814654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211320-337. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 1726290-6297. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 1009458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berhanu, A., R. L. Wilson, D. L. Kirkwood-Watts, D. S. King, T. K. Warren, S. A. Lund, L. L. Brown, A. K. Krupkin, E. Vandermay, W. Weimers, K. M. Honeychurch, D. W. Grosenbach, K. F. Jones, and D. E. Hruby. 2008. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 823517-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey, C. G., J. K. Iskander, M. H. Roper, E. E. Mast, X. J. Wen, T. J. Torok, L. E. Chapman, D. L. Swerdlow, J. Morgan, J. D. Heffelfinger, C. Vitek, S. E. Reef, L. M. Hasbrouck, I. Damon, L. Neff, C. Vellozzi, M. McCauley, R. A. Strikas, and G. Mootrey. 2005. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA 2942734-2743. [DOI] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., A. J. Bett, T. M. Fu, M. E. Davies, A. Tang, K. A. Wilson, M. Chen, R. Long, T. McKelvey, M. Chastain, S. Gurunathan, J. Tartaglia, E. A. Emini, and J. Shiver. 2004. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J. Virol. 7811434-11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 1031882-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428182-185. [DOI] [PubMed] [Google Scholar]
- 10.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11740-747. [DOI] [PubMed] [Google Scholar]
- 11.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 7810230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 25471-80. [DOI] [PubMed] [Google Scholar]
- 13.Harrington, L. E., R. van der Most, J. L. Whitton, and R. Ahmed. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 763329-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heraud, J. M., Y. Edghill-Smith, V. Ayala, I. Kalisz, J. Parrino, V. S. Kalyanaraman, J. Manischewitz, L. R. King, A. Hryniewicz, C. J. Trindade, M. Hassett, W. P. Tsai, D. Venzon, A. Nalca, M. Vaccari, P. Silvera, M. Bray, B. S. Graham, H. Golding, J. W. Hooper, and G. Franchini. 2006. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 1772552-2564. [DOI] [PubMed] [Google Scholar]
- 15.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266329-339. [DOI] [PubMed] [Google Scholar]
- 16.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 784433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83209-222. [DOI] [PubMed] [Google Scholar]
- 19.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 7913454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manischewitz, J., L. R. King, N. A. Bleckwenn, J. Shiloach, R. Taffs, M. Merchlinsky, N. Eller, M. G. Mikolajczyk, D. J. Clanton, T. Monath, R. A. Weltzin, D. E. Scott, and H. Golding. 2003. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 188440-448. [DOI] [PubMed] [Google Scholar]
- 21.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 5089-100. [DOI] [PubMed] [Google Scholar]
- 22.Payne, L. G., and K. Kristensson. 1985. Extracellular release of enveloped vaccinia virus from mouse nasal epithelial cells in vivo. J. Gen. Virol. 66643-646. [DOI] [PubMed] [Google Scholar]
- 23.Pütz, M. M., C. M. Midgley, M. Law, and G. L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 121310-1315. [DOI] [PubMed] [Google Scholar]
- 24.Roos, N., M. Cyrklaff, S. Cudmore, R. Blasco, J. Krijnse-Locker, and G. Griffiths. 1996. A novel immunogold cryoelectron microscopic approach to investigate the structure of the intracellular and extracellular forms of vaccinia virus. EMBO J. 152343-2355. [PMC free article] [PubMed] [Google Scholar]
- 25.Sejvar, J. J., R. J. Labutta, L. E. Chapman, J. D. Grabenstein, J. Iskander, and J. M. Lane. 2005. Neurologic adverse events associated with smallpox vaccination in the United States, 2002-2004. JAMA 2942744-2750. [DOI] [PubMed] [Google Scholar]
- 26.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 832915-2931. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, D. J. Shedlock, L. Xu, L. Lamoreaux, J. H. Custers, P. M. Popernack, Z. Y. Yang, M. G. Pau, M. Roederer, R. A. Koup, J. Goudsmit, P. B. Jahrling, and G. J. Nabel. 2006. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 3e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumida, S. M., D. M. Truitt, M. G. Kishko, J. C. Arthur, S. S. Jackson, D. A. Gorgone, M. A. Lifton, W. Koudstaal, M. G. Pau, S. Kostense, M. J. Havenga, J. Goudsmit, N. L. Letvin, and D. H. Barouch. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 782666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorner, A. R., R. Vogels, J. Kaspers, G. J. Weverling, L. Holterman, A. A. Lemckert, A. Dilraj, L. M. McNally, P. M. Jeena, S. Jepsen, P. Abbink, A. Nanda, P. E. Swanson, A. T. Bates, K. L. O'Brien, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2006. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J. Clin. Microbiol. 443781-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 778263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, R., A. J. Johnson, D. Liggitt, and M. J. Bevan. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J. Immunol. 1726265-6271. [DOI] [PubMed] [Google Scholar]