Abstract
Spores of Bacillus subtilis spoVF strains that cannot synthesize dipicolinic acid (DPA) but take it up during sporulation were prepared in medium with various DPA concentrations, and the germination and viability of these spores as well as the DPA content in individual spores were measured. Levels of some other small molecules in DPA-less spores were also measured. These studies have allowed the following conclusions. (i) Spores with no DPA or low DPA levels that lack either the cortex-lytic enzyme (CLE) SleB or the receptors that respond to nutrient germinants could be isolated but were unstable and spontaneously initiated early steps in spore germination. (ii) Spores that lacked SleB and nutrient germinant receptors and also had low DPA levels were more stable. (iii) Spontaneous germination of spores with no DPA or low DPA levels was at least in part via activation of SleB. (iv) The other redundant CLE, CwlJ, was activated only by the release of high levels of DPA from spores. (v) Low levels of DPA were sufficient for the viability of spores that lacked most α/β-type small, acid-soluble spore proteins. (vi) DPA levels accumulated in spores prepared in low-DPA-containing media varied greatly between individual spores, in contrast to the presence of more homogeneous DPA levels in individual spores made in media with high DPA concentrations. (vii) At least the great majority of spores of several spoVF strains that contained no DPA also lacked other major spore small molecules and had gone through some of the early reactions in spore germination.
Spores of various Bacillus species are metabolically dormant and extremely resistant to a variety of environmental stress factors, including heat, UV and gamma radiation, desiccation, high pressure, and toxic chemicals (31). While many factors are responsible for spore resistance, among the most notable is the environment of the spore's central region or core (31), which is the location of the spore's DNA, RNA, and most enzymes. Among the novel protective features of the spore core are (i) the saturation of spore DNA with α/β-type small, acid-soluble spore proteins (SASP) that protect the DNA from many types of damage; (ii) the low water content, ranging from 25 to 50% of wet weight, that likely protects spore proteins from heat inactivation; and (iii) the high levels (∼25% of core dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) plus its associated divalent cations, predominantly as a 1:1 chelate with Ca2+ (Ca-DPA) (5, 9, 31). The high core Ca-DPA level helps to reduce core water content, an important element in spore resistance to wet heat, and Ca-DPA also plays a more direct role in protecting spore DNA against several types of damage (9, 21, 26, 31). Spores that lack the majority of their α/β-type SASP are fully viable, as are spores lacking all of their Ca-DPA if they also carry appropriate stabilizing mutations (see below), although both of these types of spores exhibit some decreases in spore resistance properties (21, 31). However, spores that lack both Ca-DPA and α/β-type SASP die rapidly during spore formation, most likely due to DNA damage (26).
DPA is made only during sporulation in the mother cell compartment of the sporulating cell and is then taken up into the developing spore (6). The final step in DPA synthesis is catalyzed by DPA synthase, comprised of the SpoVFA and -B proteins (6). The mechanism of DPA uptake into the forespore is not known, but it may involve proteins encoded by the sporulation-specific spoVA operon, which is expressed in the developing forespore just prior to DPA uptake (6, 32, 34). While the Ca-DPA pool in the dormant spore is stable over extremely long periods, DPA and its associated cations are released rapidly when spores initiate germination (31). This DPA release can take as little as 1 to 2 min for individual spores (3) and again may involve one or more of the SpoVA proteins (31, 34, 35). Ca-DPA release is an important event in the signal transduction process in spore germination, since at least with B. subtilis spores, DPA release in what is termed stage I of germination triggers subsequent stage II events, most importantly the hydrolysis of the spore's peptidoglycan (PG) cortex by either of two redundant cortex-lytic enzymes (CLEs), CwlJ or SleB (27, 30).
Much of our knowledge of specific roles of DPA in spore properties has come from analysis of spores that lack DPA due to specific mutations. DPA-less B. subtilis spores, for example those of spoVF or spoVA strains, are normally very unstable and spontaneously initiate hydrolysis of the spore cortex and thus complete the germination process (22, 32). Mutants producing stable DPA-less spores of several Bacillus species were isolated more than 40 years ago (1, 10, 36), but these strains have not been characterized and may have had multiple mutations. B. cereus spores containing no DPA or low DPA levels have also been generated by addition of phenylalanine to or omission of particular nutrients or Ca2+ from sporulation media (4, 8, 15). Notably, a significant percentage of the latter DPA-deficient B. cereus spores germinated spontaneously in the absence of nutrient germinants. More recently, somewhat stable DPA-less B. subtilis spores were isolated by combining a spoVF mutation with mutations that inactivate operons encoding the spore's receptors that recognize and respond to nutrients that trigger spore germination (the ger3 phenotype) (22). While the ger3 spoVF spores lack DPA and are sufficiently stable to allow their isolation and study, they are much less stable than wild-type spores and germinate slowly even when incubated at 8°C in the absence of germinants (22). However, the reason(s) for the instability of these or other DPA-less spores is not clear. Apparently more stable B. subtilis DPA-less spores were generated by combining a spoVF mutation with a mutation in sleB, a gene that encodes one of the spore's two redundant CLEs (20). The other CLE, CwlJ, exhibits a strong, possibly absolute requirement for Ca-DPA, either released from the spore core or supplied exogenously, to promote its action on cortex PG (20). The DPA-less sleB spoVF spores are extremely stable and can be stored for weeks with no apparent germination as indicated by the lack of both cortex PG hydrolysis and full hydration of the spore core. While these spores do contain CwlJ, this CLE cannot normally be activated, since the enzyme's essential activator, Ca-DPA, is absent. However, DPA-less sleB spoVF spores can be germinated efficiently by high concentrations of exogenous Ca-DPA (20).
It should also be possible to prepare spores with intermediate DPA levels by sporulation of spoVF strains with low DPA concentrations in the medium, since developing spoVF sporulating cells and thus the developing forespores take up exogenous DPA (6, 21, 32). It was thus of interest to examine in detail the effects of varying DPA levels on B. subtilis spore germination and viability and to correlate these properties with the levels of DPA in individual spores in populations. In addition, we have examined levels of several additional small molecules in DPA-less spores of various strains. The results of these studies have provided new insight into the dormancy and germination of spores of Bacillus species and the role of DPA in these processes.
MATERIALS AND METHODS
Strains used and spore preparation and purification.
The B. subtilis strains used in this work are described in Table 1; the wild-type strain was PS533, and all strains are derived from strain PS832, a laboratory strain of B. subtilis 168. Strain PS4138 was constructed by sequential transformation (22) of strain PS3610; this was first transformed to Spr with chromosomal DNA from strain FB122 and the resulting Cmr Emr Kmr Spr transformant (strain PS4137) was transformed to Tcr with chromosomal DNA from strain FB122 again, giving strain PS4138.
TABLE 1.
B. subtilis strains used
| Strain | Genotype and phenotypea | Source or reference |
|---|---|---|
| PS533 | pUB110 Kmr | 28 |
| PS832 | Wild type | Laboratory stock |
| PS3406 | ΔsleB ΔspoVA Spr Tcr | 32 |
| PS3413 | Pxyl::spoVA Spr | 11 |
| PS3610 | ΔgerA ΔgerB ΔgerK Cmr Emr Kmr | 12 |
| PS3664 | ΔsleB ΔspoVF ΔsspA ΔsspB Spr Tcr | 26 |
| PS4137 | ΔgerA ΔgerB ΔgerK ΔsleB Cmr Emr Kmr Spr | FB122→PS3610b |
| PS4138 | ΔgerA ΔgerB ΔgerK ΔsleB ΔspoVF Cmr Emr Kmr Spr Tcr | FB122→PS4137 |
| FB72 | ΔgerA ΔgerB ΔgerK Cmr Emr Spr | 22 |
| FB108 | ΔgerA ΔgerB ΔgerK ΔspoVF Cmr Emr Spr Tcr | 21 |
| FB122 | ΔsleB ΔspoVF Spr Tcr | 20 |
Cmr, resistant to chloramphenicol (5 μg/ml); Emr, resistant to erythromycin (3 μg/ml); Kmr, resistant to kanamycin (10 μg/ml); Spr, resistant to spectinomycin (100 μg/ml); Tcr, resistant to tetracycline (10 μg/ml).
Transformation of the strain to the right of the arrow was with chromosomal DNA from the strain to the left of the arrow.
Spores of all strains were routinely prepared in liquid 2× SG medium for 1.5 to 2 days (19, 22) without antibiotics and with various amounts of DPA in the medium. Spores were harvested and cleaned by sonication, centrifugation, and washing with water as described previously (19) over periods of 1 to 3 days and were kept on ice during this time. Spores cleaned in this manner [0.1 ml at an optical density at 600 nm (OD600) of 100 to 400 in either water or 20% 5-(N-2,3-dihydroxypropylacetamido)-2,4,6-triiodo-N,N′-bis(2,3-dihydroxypropyl)-isophthalamide (Nycodenz)] were further purified by layering on 2 ml of a solution of 50% Nycodenz in water, and the tube was centrifuged at 20°C for 45 min at 14,000 rpm in a TLS55 rotor in a Beckman TL-100 ultracentrifuge as described previously (5, 23, 24). Under these conditions, DPA-replete and DPA-less spoVF spores in which the spore cortex has not been hydrolyzed pellet because of their low core water content and high core wet density, while fully germinated spores in which the core water content is similar to that in growing cells float, as do sporulating cells and cell debris. All spore preparations were routinely stored on ice protected from light and were free (>95%) of fully germinated spores (dark in the phase-contrast microscope) and sporulating cells.
Spore germination.
Several different methods were used to assess the degree of germination of spore populations. In one, spores at an OD600 of ∼1 (∼108 spores/ml) were first heated at 60 or 65°C for 30 min to inactivate growing or sporulating cells. These time-temperature combinations do not inactivate DPA-less spores (21). After cooling on ice, aliquots of appropriate dilutions were spotted on LB medium (22) plates with one or two appropriate antibiotics, the plates were incubated for ∼24 h at 37°C, and colonies were counted. Previous studies have shown that the mutations in the strains used in this work affect only spore germination or viability, not cell growth rate. Thus, the efficiency of colony formation from these spores is one measure of spore germination proficiency, although also of spore viability. In a second method to measure spore germination, spores at an OD600 of 10 were heat shocked as described above, diluted 1/10 in 60 mM DPA-60 mM CaCl2 adjusted to pH 8 with Tris base, incubated for 60 to 90 min at 23°C, and then diluted and plated as described above (20). This assay also measures spore germination and viability, but the Ca-DPA will trigger the germination of any viable spores that contain CwlJ, whether these spores do or do not contain Ca-DPA (20, 30).
Two other assays for spore germination were direct ones in which spores at an OD600 of 1 were incubated in 10 mM KPO4 buffer (pH 7.4) at 37°C. At various times, 1-ml aliquots either were examined in a phase-contrast microscope or were stained with the dye Syto 16 (Molecular Probes, Eugene, OR), and the degree of spore germination was determined by flow cytometry as described previously (29). These direct methods score spores as germinated only if the spore cortex has been hydrolyzed and both inner membrane permeability and core water content have become similar to that in growing cells (2, 9, 30).
Analytical methods.
Small molecules were extracted from spores (5 to 10 mg [dry weight]) by boiling for 30 min in 1 ml water, the suspension was centrifuged, and the supernatant fluid was saved. The pellet fraction was washed twice with 1 ml water, and supernatant fluids were pooled and run through a 1.5-ml Chelex column to remove divalent cations, in particular the Mn2+ that interferes with subsequent nuclear magnetic resonance (NMR) spectroscopy. The run-through fraction was lyophilized, and the dry residue was dissolved in D2O (22). Proton NMR spectroscopy on samples dissolved in D2O was as described previously (17, 33, 35). Analysis of the level of DPA in individual spores was by laser-tweezers Raman spectroscopy (LTRS) as described previously (3, 5, 11), with 100 to 200 spores in each sample analyzed individually for their DPA content.
RESULTS
Germination of spoVF spores lacking nutrient germinant receptors and with various amounts of DPA.
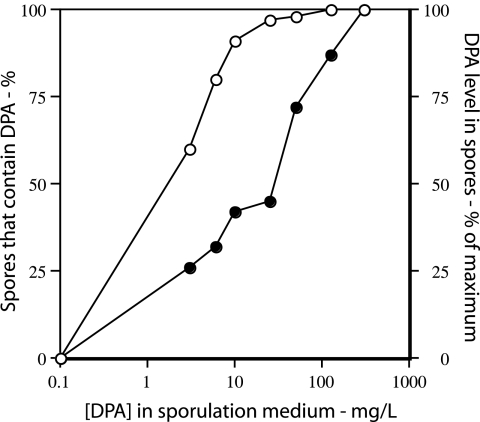
As noted previously, DPA-less spoVF spores are extremely unstable and germinate spontaneously during sporulation. However, DPA-less spores that also lack the spores’ functional nutrient germinant receptors (the ger3 genotype) (22) can be isolated and maintained with only slow completion of germination if they are kept on ice. The ger3 spoVF strain can take up exogenous DPA into the forespore during sporulation, which raises the possibility that spores with various amounts of DPA could be generated depending on the DPA concentration in the sporulation medium. Indeed, sporulation of strain FB108 (ger3 spoVF) in medium with 0 to 300 μg/ml DPA always gave at least some dormant spores (see below), although a significant percentage of spores prepared with lower DPA concentrations contained no DPA (Fig. 1; Table 2). The purified spores made even with no DPA appeared to be dormant, as they were bright in the phase-contrast microscope. However, the spores made with low DPA levels or no DPA were noticeably less bright than wild-type spore (data not shown). It was also notable that individual spores from preparations with low DPA levels exhibited the largest heterogeneity in their DPA levels, as the standard deviations of DPA levels relative to the actual DPA levels in individual spores were greater for preparations that overall had lower DPA levels (Table 2). Thus, spores from preparations made at low DPA concentrations exhibited the largest heterogeneity in their DPA levels. This was also observed for spores containing only 45% of wild-type DPA levels that were generated by allowing only low levels of expression of the spoVA operon during spore formation (11).
FIG. 1.
DPA levels in ger3 spoVF spores prepared with different DPA concentrations. Strain FB108 (ger3 spoVF) was sporulated for 2 days with different DPA concentrations, the spores were purified, and the DPA level in the purified spores was determined by examination of ∼200 spores by LTRS. ○, percentage of spores that contain DPA; •, levels of DPA in spores that do contain DPA. The DPA level in spores prepared with 300 μg/ml DPA was set at 100%. Note that for spores made with no added DPA, the data have been plotted as if the DPA concentration in the medium was 0.1 μg/ml in order to readily display all the data.
TABLE 2.
Levels of DPA in DPA-containing spores made with different DPA concentrationsa
| DPA concn (μg/ml) in sporulation medium | % of maximum DPA level (mean ± SD)b in strain:
|
||
|---|---|---|---|
| FB108 (ger3 spoVF) | FB122 (sleB spoVF) | PS4138 (ger3 sleB spoVF) | |
| 3 | 26 ± 15 | 6 ± 11 | 7 ± 8 |
| 6 | 32 ± 15 | 15 ± 20 | 28 ± 25 |
| 10 | 40 ± 20 | 23 ± 16 | 73 ± 33 |
| 25 | 45 ± 20 | 58 ± 22 | 92 ± 23 |
| 50 | 72 ± 25 | ||
| 75 | 95 ± 18 | 91 ± 21 | |
| 125 | 87 ± 24 | ||
| 300 | 100 ± 23 | 100 ± 19 | 100 ± 18 |
Spores of various strains sporulated for 1.5 to 3 days with different DPA concentrations in the sporulation medium were purified, and the DPA level in 200 individual spores was determined as described in Materials and Methods.
Values are only for those individual spores in populations that actually contained DPA and are expressed as the percentage relative to the DPA level in spores made with 300 μg/ml DPA, with the latter value set at 100%. The absolute values for the DPA levels in the spores of the different strains made with 300 μg/ml DPA were all within 12% of each other and were ∼85% of that in wild-type (PS533) spores.
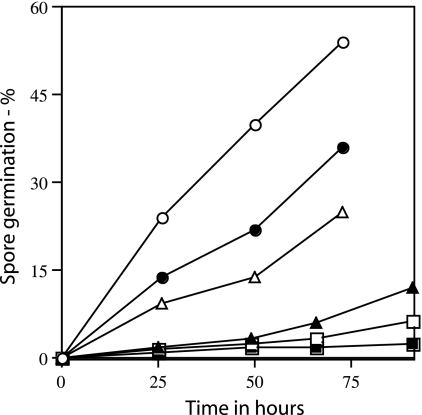
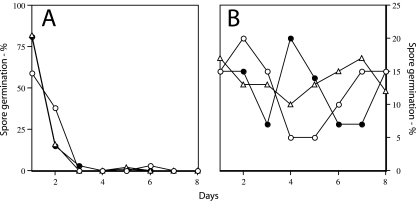
As expected from previous work (17), DPA-replete spores of a strain (FB72) that lacked only the nutrient germinant receptors exhibited little if any germination when incubated with buffer alone (Fig. 2). However, when the ger3 spoVF spores were incubated at 37°C in buffer alone and the completion of spore germination was measured by flow cytometry (Fig. 2) or phase-contrast microscopy (data not shown), spore preparations with lower DPA levels germinated fastest, while spore preparations with the highest DPA levels germinated poorly if at all. In addition, when spores of strain PS3413, in which the spoVA operon is under the control of a xylose-inducible promoter, were made with xylose concentrations of ≤0.5%, the DPA level in individual spores averaged ≤45% of that in wild-type spores, and these low-DPA-containing spores also exhibited significant spontaneous germination (reference 11 and data not shown). Thus, as noted previously for spores that lack DPA (22, 32), spore preparations with low DPA levels also exhibited more rapid spontaneous germination. However, since a significant percentage of the ger3 spoVF spore preparations made at low DPA concentrations actually had no DPA (Fig. 1), it is possible that it is primarily the DPA-free spores that are germinating fastest in this experiment, although this seems most unlikely to be the case for the spoVA spores with low DPA levels. Since the ger3 spoVF spore preparations with no DPA or low DPA levels completed germination fastest, it seems a reasonable hypothesis that it is activation of the CLE SleB in spores with low DPA levels that triggers these spores’ spontaneous germination, since the other CLE, CwlJ, requires Ca-DPA for its activation and indeed requires quite high exogenous Ca-DPA levels for this activation (20; see below).
FIG. 2.
Spontaneous germination of ger3 spoVF spores prepared with different DPA concentrations. Spores of strain FB108 (ger3 spoVF) prepared with different DPA levels as described in the legend to Fig. 1 were incubated at an OD600 of 1 at 37°C in 10 mM KPO4 buffer (pH 7.5). At various times 1-ml samples were taken and stained with the dye Syto 16, and the degree of spore germination was determined by flow cytometry as described in Materials and Methods. Spores were prepared with different DPA concentrations as follows: ○, no DPA; •, 3 μg/ml DPA; ▵, 6 μg/ml DPA, ▴, 50 μg/ml DPA; and □, 300 μg/ml DPA. ▪, germination of spores of strain FB72 (ger3) analyzed similarly.
Germination of sleB spoVF spores containing various amounts of DPA.
While the ger3 spoVF spore preparations with no DPA or low DPA levels germinated spontaneously rather rapidly, previous work has indicated that the combination of a sleB mutation with a spoVF mutation gives much more stable DPA-less spores, presumably because the remaining CLE, CwlJ, requires Ca-DPA to trigger its activity (20). Consequently, DPA-less sleB spores do not receive an endogenous signal to activate cortex hydrolysis, although CwlJ in such spores can be activated by exogenous Ca-DPA (20, 26, 32). When sleB spoVF (strain FB122) or ger3 sleB spoVF (strain PS4138) spores prepared with various DPA concentrations were purified and applied directly to nutrient plates, the apparent spore viability increased 102- to 103-fold from spores made with no DPA to spores that were made with the highest DPA concentration (Fig. 3A and 4A). While the apparent viability of the DPA-replete FB122 spores was essentially identical to (within 25% of) that of wild-type spores (data not shown), the apparent viability of the DPA-replete PS4138 spores was <1% that of DPA-replete FB122 spores (Fig. 3A and 4A and data not shown). This was not unexpected, because the absence of nutrient germinant receptors in PS4138 spores abolishes the response of these spores to nutrient germinants, and these spores will presumably only give colonies on nutrient plates due to spontaneous germination (22). However, pretreatment of these spores, including those that completely lacked DPA, with Ca-DPA prior to plating restored their apparent viability to that of wild-type spores (Fig. 3A and 4A and data not shown).
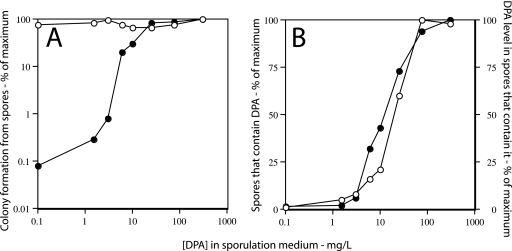
FIG. 3.
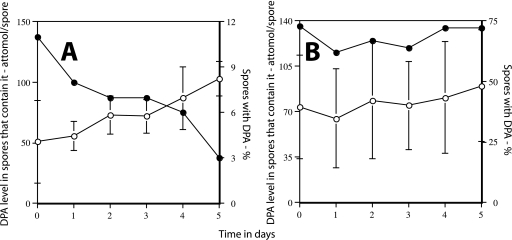
Colony formation from (A) and DPA levels in (B) sleB spoVF spores prepared with different DPA concentrations. (A) Strain FB122 (sleB spoVF) was sporulated for 2 days with different DPA concentrations, spores were purified, aliquots were applied to LB medium agar plates without (•) or after (○) pretreatment with Ca-DPA, and colonies were counted after incubation for ∼24 h at 37°C. Spore viability is expressed relative to that of spores made with 300 μg/ml DPA plated without Ca-DPA treatment. (B) The level of DPA in ∼200 FB122 spores made at different DPA concentrations was determined by LTRS as described in Materials and Methods. ○, percentage of spores that contain DPA; •, level of DPA in spores that contain DPA. All values for DPA levels are expressed relative to the level in spores made with 300 μg/ml DPA, which was set at 100%. Note that for spores made with no added DPA, the data have been plotted as if the DPA concentration in the medium was 0.1 μg/ml in order to readily display all the data.
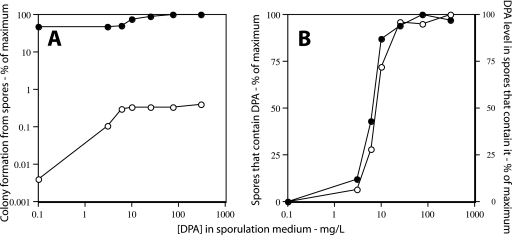
FIG. 4.
Colony formation from (A) and DPA level in (B) ger3 sleB spoVF spores prepared with different DPA concentrations. (A) Strain PS4138 (ger3 sleB spoVF) was sporulated for 2 days with different DPA concentrations, spores were purified, aliquots were applied to LB medium agar plates without (○) or after (•) pretreatment with Ca-DPA, and colonies were counted after incubation for ∼24 h at 37°C. Spore viability is expressed relative to that of spores made with 300 μg/ml DPA plated without Ca-DPA treatment, which was set at 100%. (B) The level of DPA in ∼200 PS4138 spores made at different DPA concentrations was determined by LTRS as described in Materials and Methods. ○, percentage of spores that contain DPA; •, level of DPA in spores that contain DPA. All values for DPA levels are expressed relative to the level in spores made with 300 μg/ml DPA, which was set at 100%. Note that for spores made with no added DPA, the data have been plotted as if the DPA concentration in the medium was 0.1 μg/ml in order to readily display all the data.
Analysis of the levels of DPA in individual spores in sleB spoVF and ger3 sleB spoVF spore preparations indicated that (i) of the spores made with 1.5 to 3 μg/ml DPA, only a small percentage actually contained DPA and then at only a low level; (ii) of the spores made with 6 to 10 μg/ml DPA, a large fraction again contained no DPA, although this fraction was smaller than that in spore preparations made with 1.5 to 3 μg/ml DPA; and (iii) of the spores that did contain DPA, the spore DPA level increased as the DPA concentration in the sporulation medium increased (Fig. 3B and 4B; Table 2). It was also notable that the variability in DPA levels in those individual spores that contained DPA was again highest in spore preparations that had the lowest DPA levels (Table 2). However, the standard deviations of the DPA levels in individual spores made with 300 μg/ml DPA were only slightly higher than the ±15% seen previously with wild-type spores (11).
One significant difference between the DPA levels in individual spores of the ger3 spoVF strain and the two sleB spoVF strains was that a higher percentage of the ger3 spoVF spores made with lower DPA concentrations (3 to 6 μg/ml) actually contained DPA (compare Fig. 1 with Fig. 3B and 4B). While the reason for this difference is not known with certainty, it may be because ger3 spoVF spore preparations with low DPA levels or no DPA readily germinate completely during sporulation and cleaning (Fig. 2), since these spores contain both CwlJ and SleB. These fully germinated spores will then be removed in the final step in our spore purification regimen. Consequently, ger3 spoVF spore preparations made with low DPA concentrations may be preferentially enriched in spores with significant DPA levels, and presumably this enrichment does not take place or is minimal with sleB spoVF spore preparations. Indeed, during sporulation of strain FB108 (ger3 spoVF) in the absence of DPA or with 1.5 to 6 μg/ml DPA either in liquid or on plates, phase-contrast microscopy showed that the released spores were a mixture of phase-dark spores (fully germinated), lysed spore “ghosts” composed of undigested spore coats (16), and phase-bright (still dormant) spores, and after 3 days of sporulation >90% of the released spores prepared without DPA had germinated (data not shown). In contrast, ≤10% of the released FB122 (sleB spoVF) or PS4138 (ger3 sleB spoVF) spores prepared in a DPA-free medium had fully germinated after 5 days of sporulation as determined by examination in the phase-contrast microscope (data not shown).
Germination of spores of sleB spoVF strains with various DPA levels.
Although sleB spoVF spores prepared without DPA exhibited no obvious germination throughout sporulation, analysis of the kinetics of colony formation (and thus spore germination) from FB122 (sleB spoVF) spores with different DPA levels on rich nutrient plates revealed that whether these spores had no DPA, were DPA replete, or had intermediate DPA levels, >95% of these spores triggered germination within ∼48 h (Fig. 5A). In contrast, with PS4138 (ger3 sleB spoVF) spores, which lack all functional nutrient germinant receptors in addition to SleB, colony formation (and thus spore germination) from spores with DPA levels from none to normal took place at a relatively constant rate over at least 7 days (Fig. 5B), as seen previously for DPA-replete spores that lack only the nutrient germinant receptors (22). Note, however, that the percentage of the FB122 and PS4318 spores that germinated in the entire 7-day period varied greatly, as shown by the large differences in the apparent viability of these spores over a 24-h period (Fig. 3A and 4A). These results with the PS4138 spores in particular are in sharp contrast to the relatively rapid spontaneous completion of germination of ger3 spoVF spores with low DPA levels (Fig. 2), and this further supports the hypothesis that it is largely by activation of sleB that low DPA levels result in spontaneous completion of spore germination.
FIG. 5.
Kinetics of colony formation from sleB spoVF spores and ger3 sleB spoVF spores. Spores of strains FB122 (sleB spoVF) (A) and PS4138 (ger3 sleB spoVF) (B) were prepared as described in the legends to Fig. 3 and 4, purified, and applied to LB medium plates; the plates were incubated at 37°C, and colonies that appeared over a 7-day period were counted every 24 h. Since colony formation from spores requires spore germination, the colony formation was expressed as percent spore germination, with the total colonies formed in 7 days defined as 100%. The DPA concentrations in the medium in which the spores were prepared were as follows: ○, no DPA; ▵, 10 μg/ml DPA; and •, 300 μg/ml DPA.
The sleB spoVF spores with low DPA levels exhibited no significant spontaneous germination upon extended incubation in sporulation medium, as noted above, or when purified spores were incubated for 5 days at 37°C in buffer alone and germination was assessed by phase-contrast microscopy (data not shown). The latter result was not too surprising since only a rather low concentration of DPA was present in those spores that contained DPA (Fig. 6A), and cortex PG hydrolysis through activation of CwlJ by at least exogenous Ca-DPA requires a Ca-DPA concentration of ≥25 mM (20). However, it was more notable that when spore germination was assessed as the percentage of spores that released DPA, sleB spoVF spores did exhibit some germination in 5 days (Fig. 6A). Since the mean DPA level in those sleB spoVF spores that did not germinate in 5 days increased significantly over this time (Fig. 6A), it seems likely that spores with the least DPA had been more likely to germinate. In contrast to these results, ger3 sleB spoVF spores exhibited neither a significant decrease in the percentage of DPA-containing spores nor an increase in the mean DPA content during 5 days of incubation at 37°C in buffer alone (Fig. 6B). Thus, these spores appeared to be extremely stable even when spore DPA levels were low.
FIG. 6.
Germination of sleB spoVF and ger3 sleB spoVF spores prepared with a low DPA concentration. Spores of strains FB122 (sleB spoVF) (A) and PS4138 (ger3 sleB spoVF) (B) were sporulated for 2 days with 8 μg/ml DPA and purified as described in Materials and Methods, and the purified spores were incubated at 37°C and an OD600 of 1 in 10 mM KPO4 buffer (pH 7.5). Every 24 h, aliquots were frozen for subsequent analysis of individual spores by LTRS to determine the percentage of spores that retained DPA (•), and the level of DPA in spores that contained it (○) as well as the standard deviations of this value. Phase-contrast microscopy over the 5 days of incubation indicated that <5% of the spores became phase dark (data not shown).
Viability of spores lacking α/β-type SASP and with different DPA levels.
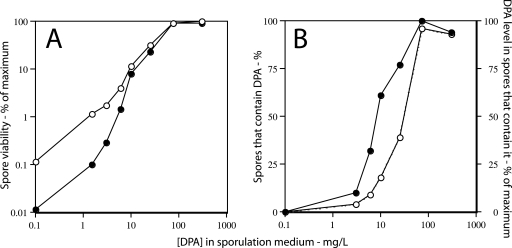
The ability to generate relatively stable spore preparations with various levels of DPA by mutations in sleB and spoVF has also provided a means of asking whether subnormal DPA levels in spores are effective in restoring spore viability. Probably the most dramatic effect of DPA on spore properties is seen with spores of strain PS3664, which in addition to SleB and SpoVF also lacks the majority of the DNA-protective α/β-type SASP (26). When spores of this strain were sporulated with DPA concentrations of from 0 to 300 μg/ml, the apparent viability of these spores upon direct application to nutrient plates increased dramatically, with the spores prepared with the two highest DPA concentrations having essentially the same apparent viability as wild-type spores (Fig. 7A and data not shown). Since spores of strain PS3664 that have no DPA will germinate poorly if at all due to the lack of both DPA and SleB, spore viability was also determined after pretreatment of the spores with Ca-DPA in order to fully germinate spores via activation of CwlJ (20). The viability of the spores made with DPA concentrations of ≤3 μg/ml was increased slightly by this treatment, but these spores’ viability remained 2 to 3 log units below that of DPA-replete spores (Fig. 7A). Analysis of the DPA levels in individual PS3664 spores prepared with various DPA concentrations indicated that only a small percentage of the spores made with 0 to 10 μg/ml DPA actually contained DPA (as seen with sleB spoVF spores) and that spores prepared in medium with less than 25 μg/ml DPA contained DPA levels that were <50% of those in DPA-replete spores (Fig. 7B). However, comparison of the data on spore viability and DPA levels (Fig. 7A and B) indicated that even submaximum DPA levels were sufficient to restore significant viability to spores that lacked α/β-type SASP (see Discussion).
FIG. 7.
Apparent spore viability of (A) and DPA level in (B) sleB spoVF sspA sspB spores made with different DPA concentrations. (A) Spores of strain PS3664 (sleB spoVF sspA sspB) were sporulated for 1.5 days with different DPA concentrations, the spores were purified and applied to LB medium plates without (•) or after (○) prior treatment with Ca-DPA, and colonies were counted after incubation for ∼24 h at 37°C as described in Materials and Methods. Spore viability was expressed relative to that of spores prepared with 300 μg/ml DPA and applied to plates after Ca-DPA treatment, and this value was set as 100%. The actual viability of the latter spores was essentially identical to that for wild-type spores (±25%). (B) The DPA levels in individual spores prepared with different DPA concentrations were determined by LTRS as described in Materials and Methods. The DPA level in spores prepared with 300 μg/ml DPA in the sporulation medium was set at 100%, and values for all other spore preparations were expressed relative to this value. The symbols used are: ○, percentage of spores that contain DPA; •, level of DPA in those spores that have DPA. Note that for spores made with no added DPA, the data have been plotted as if the DPA concentration in the medium was 0.1 μg/ml in order to readily display all the data.
Levels of other small molecules in spores that lack DPA.
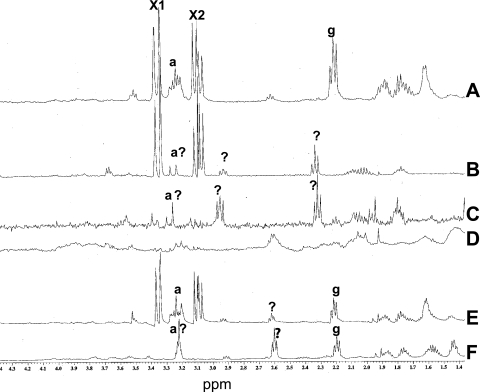
As noted above, ger3 spoVF spore preparations that lack or have low levels of DPA completed spore germination spontaneously at a relatively high rate, and this was greatly slowed by a mutation inactivating the CLE SleB. This observation suggested that DPA-less spores, in particular those spores in which cortex lysis is blocked by a sleB mutation, might not be equivalent to dormant spores but have actually gone through some reactions associated with germination, specifically those in stage I (27, 30). One of the major events in stage I of spore germination is the rapid release of DPA and its associated cations (27). However, B. subtilis spores contain a number of other small molecules, albeit at levels well below those of DPA, including arginine, glutamate, and one or more unidentified compounds (33, 35). Recent work has shown that these additional small molecules are also released essentially in parallel with DPA during spore germination triggered by both nutrients and nonnutrient agents such as high pressure or a cationic surfactant (29, 33, 35). Given the latter results, we used proton NMR spectroscopy to assess the levels of small molecules in spores of a number of strains that did or did not contain DPA (Fig. 8 and data not shown). As found previously, spores of strain PS533 (wild type) made without added DPA and of strains FB108 (ger3 spoVF), FB122 (sleB spoVF), and PS4138 (ger3 sleB spoVF) made with 300 μg/ml DPA had similar levels of DPA, although DPA levels in the spoVF spores were generally only ∼85% of those in wild-type spores (Table 2 and data not shown). Levels of glutamate and unidentified small molecules (Fig. 8, peaks g, X1, and X2) differed by at most twofold in PS533, FB122, and PS4138 spores that contained DPA, although levels of glutamate and perhaps arginine appeared to be much lower in FB108 spores made with DPA (Fig. 8A, B, and E and data not shown). The apparent absence of glutamate from FB108 spores made with DPA was seen with two independent spore preparations, as was the appearance of several new peaks due to small molecules in the spectrum from extracts of these spores (Fig. 8B, peaks labeled ?) Spores of strain FB108 (ger3 spoVF) made without DPA lacked DPA as expected (Fig. 1) but also lacked most of the other abundant small molecules found in wild-type spores, with the exception of two groups of peaks in the NMR spectrum due to an unidentified small molecule(s) and perhaps arginine (Fig. 8C, peaks labeled ? and a?, respectively). FB122 (sleB spoVF) spores made without DPA also lacked this compound (Fig. 3B), and these spores also lacked all the abundant unidentified small molecules found in wild-type spores (Fig. 8D). PS4138 (ger3 sleB spoVF) spores made without DPA also lacked this molecule (Fig. 4B), as expected. In addition, the NMR spectrum of small molecules from the latter spores lacked the peaks due to the major unidentified small molecules (Fig. 8E, peaks labeled X1 and X2 in Fig. 8A), although significant levels of glutamate and perhaps arginine as well as several peaks due to unidentified compounds remained (Fig. 8F, peaks labeled g, a?, and ?). DPA, arginine, glutamate, and the unknown small molecules were also almost completely absent from purified dormant sleB spoVA spores (strain PS3406) (data not shown) that synthesize DPA in the mother cell but do not take up this molecule into the forespore (6, 32). These DPA-less dormant spores were also extremely stable (data not shown).
FIG. 8.
NMR spectra of small molecules extracted from wild-type and spoVF spores. Spores of strains PS533 (wild-type) (A), FB108 (ger3 spoVF) (B and C), FB122 (sleB spoVF) (D), and PS4138 (ger3 sleB spoVF) (E and F) were prepared with 300 μg/ml DPA (B and E) or without DPA (A, C, D, and F); spores were purified; small molecules were extracted from 10 (A), 6 (B and C), 8 (D), or 5 (E and F) mg (dry weight) of spores; extracts were processed, and NMR spectra were determined as described in Materials and Methods. The peaks due to known compounds (a, arginine; g, glutamate) are labeled, as are peaks due to unknown compounds (the doublet X1 and quadruple peak X2 in spectra A, B, and E and peaks labeled ? in spectra B, C, E, and F). The peaks labeled a? in spectra B, C, and F may be from arginine, but this assignment is not yet definitive. The magnification of spectra B to F has been adjusted slightly to account somewhat for the different amounts of spores extracted (spectra B and C expanded 1.2-fold, spectra D expanded 2-fold, and spectra E and F expanded 1.4-fold.
DISCUSSION
The work presented in this paper allows us to make a number of new conclusions about the role of DPA in B. subtilis spore germination, spore stability, and viability. First, as indicated by previous work, DPA-less spores are quite unstable. Even if the stability of these spores was improved by addition of the ger3 mutations, a sleB mutation, or both types of mutations, the spores that lacked DPA spontaneously and relatively rapidly appeared to go through either all germination events (ger3 spoVF spores) or at least some early germination reactions (sleB spoVF and ger3 sleB spoVF spores). If cortex hydrolysis was blocked in DPA-less spoVF spores by the absence of SleB alone, these spores still appeared to have gone through some early germination events, since in addition to lacking DPA they also lacked other small molecules normally released in the first minutes of spore germination (29). It is formally possible that the latter additional small molecules are just not taken up into the forespore of spoVF strains sporulated without DPA. However, this seems much less likely than that these molecules are indeed accumulated and then released subsequently during sporulation, much as spores of a strain lacking SpoVF proteins alone form during sporulation without DPA but then spontaneously germinate and lyse. Thus, it appears more likely that it is the absence of DPA alone that renders B. subtilis spores unstable, although why the DPA-less spores are so unstable is not clear. Consequently, it appears that even when cortex hydrolysis is blocked, DPA-less spores are still not true dormant spores but rather are only blocked at some point in stage I of germination. It is, however, notable that sleB spoVF spores that contain low DPA levels continued to germinate slowly as measured by DPA release when incubated for 5 days in buffer alone, while comparable ger3 sleB spoVF spores did not. This suggests that the slow but consistent DPA release during extended incubation of sleB spoVF spores is due to slow activation of one or more of the spore's nutrient germinant receptors in these spores, either by nutrient germinants released from the spores that germinate or present as low-level contaminants in spore preparations. However, this does not take place in ger3 sleB spoVF spores, which lack the nutrient germinant receptors. Since DPA-less ger3 sleB spoVF spores retained at least much of the glutamate and perhaps the arginine that is released from sleB spoVF spores, this further suggests that the nutrient germinant receptors may be directly involved in the release of amino acids in some situations. While the mechanism whereby the nutrient germinant receptors might be involved in amino acid release from spores is not clear, one of the protein components of the nutrient germinant receptors exhibits weak but significant sequence homology to a superfamily of proteins, some of which are involved in amino acid transport (14).
A second conclusion is that it appears most likely that low or minimal DPA levels trigger completion of spore germination by activating the CLE SleB somehow, either directly or indirectly via some stage I event triggered by low DPA as noted above. It has been suggested that SleB may work only on cortical PG that is under strain (7, 18), and perhaps such strain increases as core DPA levels fall and the core water content rises, although the evidence for this is minimal at present. It is, however, notable that completion of germination of spores of ger3 spoVF strains with no DPA took several days, as measured by completion of cortex PG hydrolysis (Fig. 2), especially since the initial DPA-less spore preparations appeared to have undergone at least one early step in spore germination, i.e., the release of additional small molecules. Thus, the activation of cortex hydrolysis by SleB in these DPA-less spores does not appear to be immediate but takes place over several days. This suggests that these DPA-less spores are in some sort of metastable state such that while poised to complete germination, they only very slowly complete this process. Interestingly, there is evidence for such a metastable state in the germination of B. subtilis spores by high pressures of 500 to 600 MPa (37), While these pressures cause the release of DPA as well as other small molecules from spores, these spores appear to remain in stage I of germination for long periods and complete germination only very slowly (33, 37).
It was also notable that hydrolysis of cortex PG by the other CLE, CwlJ, was triggered only by the release of high Ca-DPA levels from spores. Indeed, a third new conclusion is that release of a low level of Ca-DPA from a spore is not sufficient to trigger CwlJ action in the same spore. This was seen most notably with sleB spoVF spores prepared in medium with 1.5 and 3 μg/ml DPA, as the colony-forming ability of these spores on nutrient plates compared to that of these spores made with 300 μg/ml DPA was 10- to 15-fold less than predicted based on the percentage of these spores that contained DPA (Fig. 3A and B). While the levels of DPA in spores made with 1.5 to 3 μg/ml DPA averaged between 4 to 9% of that in spores made with 300 μg/ml DPA, the former DPA levels were too low to activate CwlJ upon DPA release triggered by nutrients. In contrast, the spores of this strain prepared with 6 and 10 μg/ml DPA had colony-forming abilities that were 19 and 28%, respectively, of that of DPA-replete spores, similar to the percentage of the spores made with 6 and 10 μg/ml DPA that actually contained DPA, and the DPA levels in the latter spores were 30 to 40% of that in DPA-replete spores. Thus, while low DPA levels triggered spore germination through activation of SleB, release of relatively high Ca-DPA levels was needed to activate the other CLE, CwlJ, and release of low Ca-DPA levels was ineffective. The latter conclusion agrees with previous work showing that there is a threshold of Ca-DPA concentration needed for CwlJ activation by exogenous Ca-DPA, as concentrations below 25 mM are ineffective, while 50 mM is optimal (20). Presumably the high threshold for Ca-DPA activation of CwlJ also ensures that this CLE is not activated during spore formation when Ca-DPA is taken up from the mother cell, where presumably its concentration always remains well below 25 mM. Ca-DPA activation of the autoprocessing of the zymogen of the protease GPR, which initiates SASP degradation in the spore core during spore germination, also exhibits a very similar threshold of dependence on Ca-DPA concentration (13). This suggests that spores with low Ca-DPA levels may not contain significant levels of the active form of GPR and thus might degrade SASP only very slowly during spore germination. However, even gpr B. subtilis spores give colonies on rich nutrient plates, as spore outgrowth is delayed only somewhat (25).
A final new conclusion is that the DPA level needed to restore viability to spores that lack α/β-type SASP is relatively low. Of sspA sspB sleB spoVF spores made with no DPA, at most 0.1% were viable, while of those made with 3 μg/ml, 1% were viable and only 2% had DPA at a level ∼15% that of wild-type spores (Fig. 4A and B). For these spores made with 6 μg/ml DPA, 5% were viable and 5% contained DPA at an average level of 50% that of spores made with 300 μg/ml DPA (Fig. 4A and B). Thus, the endogenous DPA requirement for spore viability when most DNA-protective α/β-SASP are absent is significantly lower than the DPA requirement for activation of CwlJ.
In addition to the conclusions noted above, there are also a number of observations made in this work that we cannot yet explain. First, it was surprising that when spoVF spores were made with low exogenous DPA concentrations, so many of the purified spores contained no DPA, and this percentage was higher with lower DPA concentrations in the sporulation medium. However, for spores made with very high DPA concentrations, >95% of the spores routinely contained DPA. It was also notable that of the spores made at low DPA concentrations that actually contained DPA, there was a large variation in their DPA content, with this variation decreasing markedly as average spore DPA content increased. One partial explanation for this phenomenon is that spores with the lowest DPA contents more rapidly released the DPA that they had taken up in germination-like reactions. However, it is also possible that there is significant heterogeneity in DPA uptake at low DPA concentrations. Unfortunately, it is not known how exogenous DPA is taken up by sporulating cells to give DPA in the mother cell that can then be taken up into the forespore.
A final point of uncertainty concerns the germination of DPA-less ger3 spoVF and sleB spoVF spores when incubated in the absence of nutrients. Is this spontaneous germination, in which spores with normal DPA levels but without all nutrient germinant receptors exhibit slow but steady germination of 0.01 to 0.1% of total spores when incubated at 37°C? Is this due to occasional activation of SleB either spontaneously or accelerated by low spore DPA levels, or is there some low-level stimulation of nutrient germinant receptors by endogenous or exogenous compounds? Clearly there are questions about the role of DPA in the stability and germination of bacterial spores that remain to be answered.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM19698) and the Army Research Office to P.S.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Balassa, G., P. Milhaud, E. Raulet, M. T. Silva, and J. C. F. Sousa. 1979. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J. Gen. Microbiol. 110365-379. [DOI] [PubMed] [Google Scholar]
- 2.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 715879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, D., S. S. Huang, and Y. Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 786936-6941. [DOI] [PubMed] [Google Scholar]
- 4.Church, B. D., and H. Halvorson. 1959. Dependence of the heat resistance of bacterial spores on their dipicolinic acid content. Nature 183124-125. [DOI] [PubMed] [Google Scholar]
- 5.Coleman, W. H., D. Chen, Y.-Q. Li, A. E. Cowan, and P. Setlow. 2007. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 1898458-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 571-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster, S. J., and K. Johnstone. 1987. Purification and properties of a germination-specific cortex-lytic enzyme from spores of Bacillus megaterium. Biochem. J. 242573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank, H. A., and K. I. Tonaki. 1971. Pseudogermination in dipicolinic acid-less spores of a Bacillus cereus T mutant. J. Bacteriol. 106292-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-63. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC.
- 10.Hanson, R. S., M. V. Curry, J. V. Garner, and H. O. Halvorson. 1972. Mutants of Bacillus cereus strain T that produce thermoresistant spores lacking dipicolinic acid have low levels of calcium. Can. J. Microbiol. 181139-1143. [DOI] [PubMed] [Google Scholar]
- 11.Huang, S. S., D. Chen, P. L. Pelczar, V. R. Vepachedu, P. Setlow, and Y.-Q. Li. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 1894681-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi, T., B. Setlow, M. Paidhungat, and P. Setlow. 2004. Analysis of the effects of a gerF (lgt) mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 1862984-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illades-Aguiar, B., and P. Setlow. 1994. Autoprocessing of the protease that degrades small, acid-soluble proteins of spores of Bacillus species is triggered by low pH, dehydration, and dipicolinic acid. J. Bacteriol. 1767032-7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack, D. L., I. T. Paulsen, and M. H. Saier, Jr. 2000. The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology 1461797-1814. [DOI] [PubMed] [Google Scholar]
- 15.Keynan, A., W. G. Murrell, and H. O. Halvorson. 1962. Germination properties of spores with low dipicolinic acid content. J. Bacteriol. 83395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klobutcher, L. A., K. Ragkousi, and P. Setlow. 2006. The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by the protozoan Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 103165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loshon, C. A., P. G. Wahome, M. W. Maciejewski, and P. Setlow. 2006. Levels of glycine betaine in growing cells and spores of Bacillus species and lack of effect of glycine betaine on spore properties. J. Bacteriol. 1883153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino, S., N. Ito, S. Miyata, and R. Moriyama. 1994. A spore-lytic enzyme released from Bacillus cereus spores during germination. Microbiology 1401403-1410. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England.
- 20.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 1834886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 1825505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paidhungat, M., and P. Setlow. 2000. Role of Ger− proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 1822513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 9315405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickwood, D., T. Ford, and J. Graham. 1982. Nycodenz: a new nonionic iodinated gradient medium. Anal. Biochem. 12323-31. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Salas, J. L., M. L. Santiago-Lara, B. Setlow, M. D. Sussman, and P. Setlow. 1992. Properties of Bacillus megaterium and Bacillus subtilis mutants which lack the protease that degrades small, acid-soluble proteins during spore germination. J. Bacteriol. 174807-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setlow, B., S. Atluri, R. Kitchel, K. Koziol-Dube, and P. Setlow. 2006. Role of dipicolinic acid in the resistance and stability of spores of Bacillus subtilis. J. Bacteriol. 1883740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 1834894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 1783486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setlow, B., P. G. Wahome, and P. Setlow. 2008. Release of small molecules during the germination of spores of Bacillus species. J. Bacteriol. 1904759-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 31.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101514-525. [DOI] [PubMed] [Google Scholar]
- 32.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vepachedu, V. R., K. Hirneisen, D. G. Hoover, and P. Setlow. 2007. Studies of the release of small molecules during pressure germination of spores of Bacillus subtilis. Lett. Appl. Microbiol. 45342-348. [DOI] [PubMed] [Google Scholar]
- 34.Vepachedu, V. R., and P. Setlow. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 23971-77. [DOI] [PubMed] [Google Scholar]
- 35.Vepachedu, V. R., and P. Setlow. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 1891565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise, J., A. Swanson, and H. O. Halvorson. 1967. Dipicolinic acid-less mutants of Bacillus cereus. J. Bacteriol. 942075-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wuytack, E. Y., S. Boven, and C. W. Michiels. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressure. Appl. Environ. Microbiol. 643220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]