Abstract
l-(−)-Azetidine-2-carboxylate (AC) is a toxic, natural product analog of l-proline. This study revealed the genes and biochemical strategy employed by Pseudomonas sp. strain A2C to detoxify and assimilate AC as its sole nitrogen source. The gene region from Pseudomonas sp. strain A2C required for detoxification was cloned into Escherichia coli and sequenced. The 7.0-kb region contained eight identifiable genes. Four encoded putative transporters or permeases for γ-amino acids or drugs. Another gene encoded a homolog of 2-haloacid dehalogenase (HAD). The encoded protein, denoted l-azetidine-2-carboxylate hydrolase (AC hydrolase), was highly overexpressed by subcloning. The AC hydrolase was shown to catalyze azetidine ring opening with the production of 2-hydroxy-4-aminobutyrate. AC hydrolase was further demonstrated to be a new hydrolytic member of the HAD superfamily by showing loss of activity upon changing aspartate-12, the conserved active site nucleophile in this family, to an alanine residue. The presence of a gene encoding a potential export chaperone protein, CsaA, adjacent to the AC hydrolase gene suggested that AC hydrolase might be found inside the periplasm in the native Pseudomonas strain. Periplasmic and cytoplasmic cell fractions from Pseudomonas sp. strain A2C were prepared. A higher specific activity for AC hydrolysis was found in the periplasmic fraction. Protein mass spectrometry further identified AC hydrolase and known periplasmic marker proteins in the periplasmic fraction. A model was proposed in which AC is hydrolyzed in the periplasm and the product of that reaction is transported into and further metabolized in the cytoplasm.
Plants produce many toxins that offer protection against grazing by insects and mammals (11). Some of those toxins act by mimicking essential amino acids. One such natural product is S-(−)-azetidine-2-carboxylate (AC), which can comprise up to 7% of the leaf mass of Lily of the Valley plants (6, 7) and has more recently been found to be present in sugar beets (26). AC mimics the amino acid l-proline. Upon ingestion by a susceptible animal, AC becomes incorporated into proteins in place of l-proline. The different bond angles in AC compared to l-proline causes change in the protein backbone, which can result in disruption of function (8). AC is also produced by Streptomyces spp. and is considered to be an antibiotic, but it is too toxic to humans to be useful clinically.
AC is toxic to microorganisms if it is transported into the cell and incorporated into proteins (8). However, Saccharomyces cerevisiae is known to detoxify AC via acetylation of the carboxylic acid group (32). Additionally, bacteria are known that not only tolerate AC but use it as the sole source of nitrogen (5, 35, 36). Bacteria growing on AC are reported to open the azetidine ring and susbsequently capture the nitrogen atom via a transamination reaction. The data are consistent with a mechanism involving an enzyme-catalyzed hydrolytic opening of the azetidine ring. However, until the present study, the gene(s) and enzyme(s) responsible for this activity had not been identified. It has also been unclear how cells that metabolize AC might manage to protect themselves against toxic effects of the compound. The present study has addressed those unresolved issues.
MATERIALS AND METHODS
Bacterial strains.
Agrobacterium radiobacter DSM 5172, Enterobacter cloacae DSM 30054, and Enterobacter amnigenus DSM 4486 were obtained from the DSMZ Culture Collection. Sinorhizobium meliloti RM1021 and Escherichia coli K-12 were kindly provided by Michael Sadowsky, University of Minnesota. Ralstonia eutrophus JMP134 was kindly provided by Robert Hausinger, Michigan State University. Pseudomonas sp. strain A2C was obtained by enrichment culturing as described below.
Bacteria capable of growing on AC were isolated by enrichment culture with an inoculum from soil surrounding roots of Lily of the Valley plants. The minimal medium (30) contained 0.4% (wt/vol) glucose as the sole carbon source and 2.5 mM AC as the sole nitrogen source. Liquid cultures were transferred several times and then plated to obtain isolated colonies.
Chemicals and chemical identification.
(S)-(−)-Azetidine-2-carboxylate (99%) was obtained from Toronto Research Chemicals, Inc. (North York, Ontario, Canada). o-Phthalaldehyde and (S)-2-hydroxy-4-aminobutyrate were obtained from Sigma-Aldrich (St. Louis, MO). All other commercial chemicals were the highest grade of purity available. Identification of the chemical product of the AC hydrolase reaction and standard compounds was carried out on a Varian VXR 300 MHz nuclear magnetic resonance (NMR) spectrometer. 1H-NMR spectra were recorded with the compound in D2O containing the chemical shift standard 3-(trimethylsilyl)-propionic acid, d4 sodium salt.
Molecular methods.
The Pseudomonas genomic library was created by partial digestion of DNA with EcoRI and ligation into pUC119 using standard methods (24). E. coli strain DH5α cells were transformed with the ligation mixture. Then, the cells were allowed to recover for 45 min, concentrated by centrifugation, resuspended in minimal medium, and plated onto minimal agar containing AC (27). The few colonies observed were picked, inoculated into liquid culture, and then tested for AC hydrolase activity as described below. The putative clones tested positive, and the DNA sequence of the insert was subsequently determined. Inverse PCR was used to extend the flanking DNA sequence beyond the initial 3.4-kb clone obtained in the initial screen, to 7.0 kb (Fig. 1). DNA encoding the putative AC hydrolase was isolated from Pseudomonas genomic DNA by PCR using PfuUltraHS (Stratagene, La Jolla, CA) via the following primers: 5′ AZC PCR, GTGGTGGTTCATATGCAACTGACCGACTTCAAAGCG; 3′ AZC PCR, GTTGTTGTTCTCGAGTTAGCCCTTCAGCGCTTGCTT ATG. The PCR product was cloned as an NdeI/XhoI fragment into pET29 (Novagen, Madison, WI) and transformed into E. coli strain BLR(DE3) (Novagen) for overexpression of the AC hydrolase. Plates used for selection of clones expressing the AC hydrolase gene were composed of morpholinepropanesulfonic acid minimal medium (19), 1.5% agar, 0.3 M NaCl, 5 mM AC, 1 mM each thiamine, proline, and arginine, and ampicillin.
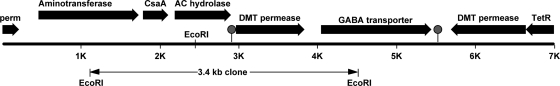
FIG. 1.
Map of the 7.0-kb region containing the AC resistance and accessory genes. The gene regions represent the following annotated or determined protein assignments (left to right): a GABA permease (perm), GABA aminotransferase, a CsaA export chaperone, the demonstrated AC hydrolase (this study), DMT permease, GABA transporter, DMT permease, and a TetR transcriptional regulatory protein. The region of the 3.4-kb clone is shown at the bottom. The circles represent predicted transcription termination sites (http://www.softberry.com/berry.phtml?topic=fgenesb&group=help&subgroup=gfindb).
Site-directed mutagenesis by the QuikChange method (Stratagene) was used to create the D12A mutant of AC hydrolase. Primers used were oligo A, CGCTGACCTTCGCCTGCTACGGCACC, and oligo B, GGTGCCGTAGCAGGCGAAGGTCAGCG.
Sequencing and sequence analysis.
The 16S rRNA gene from Pseudomonas sp. strain AC and the cloned gene region were sequenced by the University of Minnesota BioMedical Genomics Center using an Applied Biosystems 3100 Genetic Analyzer. Sequence analysis was performed using the program FGENESB (http://www.softberry.com/berry.phtml?topic=fgenesb&group=help&subgroup =gfindb) and the BLAST algorithm to search against the GenBank database (1). For each translated gene product in the AC hydrolase locus, the BLAST hit with the lowest e-value was selected for comparison as presented in the Results section, below.
Separation of cytoplasmic and periplasmic fractions and identification of proteins.
Periplasmic and cytoplasmic preparations were prepared by the procedure of Franklin (9). Proteins in each fraction were analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and, subsequently, mass spectrometry (MS). Protein bands were eluted from the gel and trypsin treated, and peptides were analyzed by capillary high-performance liquid chromatography and tandem mass spectrometry (MS/MS) as previously described (20). Tandem mass spectra were acquired using a QSTAR Pulsari Q-TOF MS apparatus (ABI, Foster City, CA). Data were analyzed using Mascot v 2.0 (Matrix Science Ltd., Boston, MA) and ProID 1.1/BioAnalyst 1.1/Analyst QS 1.1 (ABI, Foster City, CA). The sequences were run against the NCBI-RefSeq bacterial database from 20 March 2006 with the addition of the AC sequence obtained in this study. The total number of proteins in the final database consisted of 941,110 sequences.
Enzyme assays.
AC hydrolase was assayed using a Molecular Devices Gemini XPS spectrofluorimeter (Sunnyvale, CA) to detect primary amines. The procedures were similar to those described for proline reductase (29) with the following exceptions: AC was used in place of l-proline, the reaction mixture was reduced to 0.25 ml and incubated at 35°C, 25 μl of the reaction mixture was added to 1 ml of the o-phthalaldehyde mixture, and 200 μl of each assay mixture was analyzed for fluorescence emission. A standard curve using authentic 2-hydroxy-4-aminobutyrate was linear from 10 to 1,000 nmol.
RESULTS
Bacterial prevalence of AC growth activity.
Previously, AC metabolism was reported to occur in cultures of bacteria representing the genera Agrobacterium, Rhizobium, and Enterobacter. In this context, the following bacteria were screened for the ability to grow on AC as the sole nitrogen source: Escherichia coli K-12, Agrobacterium radiobacter DSM 5172, Sinorhizobium meliloti, Ralstonia eutropha JMP134, Enterobacter cloacae DSM 30054, and Enterobacter amnigenus DSM 4486. Several of these strains have been subjected to complete genomic sequencing, and different strains of the latter two species had previously been reported to express AC hydrolase activity. However, in the present study, all test strains were negative, suggesting that growth on AC is not a common phenotype in microbes.
Subsequently, bacteria capable of growth on AC as a sole nitrogen source were obtained by culturing bacteria from soil found beneath a well-established bed of Lily of the Valley plants. Luxuriant growth was observed. One bacterium was observed to grow with AC as the sole source of carbon and nitrogen. It was chosen for further study. Morphology, Gram staining, and 16S rRNA sequencing (accession number EU599183) indicated that the bacterium was a Pseudomonas sp., but it did not match any known species closely enough to be given a species name. Hence, it was denoted as Pseudomonas sp. strain A2C.
Identification and cloning of the AC resistance gene region.
AC was found to be toxic to E. coli DH5α strains, and the toxicity was accentuated by the addition of NaCl to the growth medium, as had been observed previously (10, 24, 31). This served as the basis for the selective screening of an E. coli library prepared from total genomic DNA obtained from Pseudomonas sp. strain A2C. It was reasoned that the AC-metabolizing genes, if expressed in E. coli, would protect the cell against growth inhibition by AC. This strategy proved successful.
Putative clones grown on AC-containing agar plates, and thus resistant to AC, were checked for inserts. Several resistant clones were found to contain an identical 3.4-kb insert. Deletion mutagenesis by restriction with EcoR1 resulted in the loss of AC resistance.
Identification of initial product from AC metabolism.
A cell-free enzyme preparation from recombinant E. coli containing the intact 3.4-kb insert clone metabolized AC to a water-soluble metabolite. The metabolite reacted with o-phthalaldehyde to produce a fluorescent product. Secondary amines do not produce this product, indicating that the compound was a primary amine. Wild-type E. coli strains did not transform AC to any detectable product.
A primary amine product of AC would only be generated by the cleavage of one of the two different carbon-nitrogen bonds in the azetidine ring. One ring opening would produce 2-hydroxy-4-aminobutyrate (HAB), and the other would yield 4-hydroxy-2-aminobutyrate (homoserine). In this study, NMR spectroscopy unambiguously identified the first detectable metabolite of AC metabolism as HAB [δ 1.9-2.16 (m, 2H, 3-H), 3.0-3.2 (m, 2H, 4-H), 4.15 (dd, 1H, 2-H, J = 7.5, 4.2)]. The NMR spectrum of the biological material was identical to that of commercially available (S)-HAB. Different HAB enantiomers would be indistinguishable by conventional NMR spectroscopy. The NMR spectrum of authentic homoserine (23) was very different from that of the biological product obtained here. Moreover, (l)- and (d,l)-homoserine were tested in growth experiments and shown not to serve as a sole nitrogen source for Pseudomonas sp. strain A2C.
DNA sequencing and annotation of the AC resistance gene region.
The 3.4-kb inserted DNA was sequenced, and then further sequence was obtained from each end to generate a total sequence of 7,001 bp (accession number 1076384) (Fig. 1). Potential open reading frames were found in the 7-kb fragment using BlastX on the NCBI server and the Softberry program FGENESB. Open reading frames were located and annotated as follows: (i) a 4-aminobutyrate (GABA) permease (bp 1 to 219), (ii) an aminotransferase (bp 454 to 1737), (iii) the export chaperone CsaA (bp 1784 to 2113), (iv) a hydrolase of the HAD superfamily shown here to hydrolyze AC (bp 2182 to 2904), (v) a drug/metabolite transporter (DMT) permease family member (bp 2966 to 3835), (vi) a GABA transporter (bp 4041 to 5450), (vii) another DMT permease family member (bp 5687 to 6643), and (viii) part of a TetR family transcriptional regulator (bp 6640 to 7001).
All of the proteins inferred from the 7.0-kb DNA region showed matches to known proteins with a percent sequence identity above 50%, except for one of the putative DMT permeases (Table 1). The two end genes were fragments, but all other translated proteins matched known proteins over the entire sequence length. The highest identities, for the aminotransferase at 66% and the GABA transporter at 76%, were to proteins from other Pseudomonas strains.
TABLE 1.
Sequence analysis of translated putative gene regions in the 7-kb clonea
| Protein | Size (aa)b | % IDc | % Similarity | Organismd | Accession no.e |
|---|---|---|---|---|---|
| Permease | 72 | 57 | 75 | P. putida F1 | ABQ78912 |
| Aminotransferase | 427 | 66 | 78 | P. syringae R728aj | AAY35219 |
| CsaA | 109 | 59 | 76 | T. thermophilus HB27 | AAS580482 |
| AC hydrolase | 240 | 57 | 74 | Mesorhizobium loti MAFF303099 | BAB51201 |
| DMT permease | 275 | 39 | 60 | R. eutropha JMP134 | AAZ65013 |
| GABA transporter | 469 | 76 | 85 | P. putida KT2440 | AAN68152 |
| DMT permease | 318 | 60 | 76 | Yersinia intermedia ATCC 29909 | ZP_00835500 |
| TetR | 119 | 55 | 71 | Ochrobacterium anthropi ATCC 49188 | YP_001371895 |
The proteins correspond to those demarcated in Fig. 1 from left to right. The percent identity (ID), percent similarity, and gaps were determined by using the BLAST algorithm using default conditions and by comparison with the protein match in GenBank yielding the lowest expect value (e-value).
Designates the number of amino acids in the protein inferred by translation of the identified gene in the AC resistance locus.
Percent amino acid sequence identity.
Organism containing the gene encoding the protein that was the top match in GenBank as determined with the BLAST algorithm.
GenBank accession number for the protein in the indicated organism.
Confirmation of and analysis of the AC ring-opening gene.
The HAD family gene product identified from a gene found near the middle of the sequenced DNA region was deemed most likely to be the AC hydrolase that produced HAB, and this was further confirmed in several experiments described below. First, the 3.4-kb clone contained only three complete genes, encoding a putative CsaA protein, an HAD homolog protein, and a presumptive DMT permease protein (Fig. 1). This three-gene region conferred onto the recombinant E. coli strain the ability to produce HAB, as described above.
Subsequently, the AC hydrolase DNA coding sequence was cloned behind the T7 promoter in E. coli to yield a strain that, when induced, expressed high levels of a 27,000 molecular weight (MW) protein that was ostensibly absent in the wild-type E. coli or in an uninduced recombinant (Fig. 2). The observed major protein migrated in the gel to a position that is close to the predicted molecular weight for the HAD homolog (MW, 27,111). Moreover, higher expression of the 27,000 MW protein led to a dramatic increase in the measured AC hydrolase activity in cell extracts. Whereas the original clone showed a specific activity of 94 nmol per min per mg, the overexpressing clone gave a specific activity of 2,054 nmol per min per mg. The latter protein extract did not show any detectable 2-haloacid dehalogenase activity with either (R)-(+)- or (S)-(−)-2-chloropropionate as the substrate. Moreover, no activity could be detected with l-proline as the substrate.
FIG. 2.
Proteins produced by E. coli strain BLR(DE3) containing a 3.4-kb insert conferring on the bacterium resistance to AC. Protein expression is under control of the T7 promoter. Lanes: (A) uninduced (no isopropyl-β-d-thiogalactopyranoside [IPTG]) cells grown to an absorbance at 600 nm of 0.6; (B) cells induced with IPTG, added initially at an absorbance of 0.6, for 1 hour and reaching an absorbance at 600 nm of 1.3; (C) cells induced with IPTG for 4 h and reaching an absorbance at 600 nm of 2.4. The protein extracts were obtained by boiling whole-cell pellets in SDS and β-mercaptoethanol. The position of the major recombinant protein is shown by the arrow.
Site-directed mutagenesis confirming the HAD homolog as the A2C hydrolase activity.
The HAD superfamily proteins are characterized by the presence of a catalytically important aspartic acid residue near their N termini (12). This conserved aspartate has been demonstrated to function in nucleophilic catalysis by displacing the halogen atom from haloacid substrates (4, 16, 25). Alignments suggested the presence of a similarly conserved aspartate residue in the HAD homolog identified in this study. To confirm that the HAD homolog studied here was the AC hydrolase, and to begin to study the mechanism of this putative new superfamily member, the aspartate residue was modified by site-directed mutagenesis to an alanine residue (D12A). The resultant mutant enzyme was tested for AC hydrolase activity in whole cells and cell extracts, and none was detected. The mutant clone expressed the recombinant protein at a very visible level as demonstrated by SDS-PAGE. This result was consistent with the HAD superfamily homolog identified here representing the AC hydrolase activity, and it is further consistent with its assignment as an HAD superfamily member with a unique catalytic activity.
Identification and localization of AC-metabolizing enzymes.
The csaA gene adjacent to the identified AC hydrolase gene (Fig. 1), after translation, showed strongest sequence relatedness (Table 1) to a protein from Thermus thermophilus, for which an X-ray structure has been determined and an export-related chaperone function has been established (14, 33). Other strong sequence matches were also identified as putative export chaperones. A related observation was that another Pseudomonas strain that grew on AC accumulated high levels of HAB in the growth medium that were subsequently taken up and metabolized to support growth as a nitrogen source (21). In tandem, these observations supported the hypothesis that the AC hydrolase was transported into, and active in, the periplasm.
To test this hypothesis, cells of the wild-type Pseudomonas sp. strain A2C were disrupted and fractionated into periplasmic and cytoplasmic protein fractions using published procedures as described in Materials and Methods. The polypeptides in each fraction were then separated by denaturing SDS-PAGE (Fig. 3). The periplasmic fraction (Fig. 3A) showed different bands than the cytosolic fraction (Fig. 3B); the latter showed a larger number of protein bands, as expected. The gel regions containing proteins close in molecular weight to the A2C hydrolase (MW, approximately 21,000 to 35,000) in the periplasm and the predicted HAB aminotransferase protein (MW, approximately 42,000 to 52,000) in the cytoplasm were analyzed by elution of polypeptides, digestion with trypsin, and tandem mass spectrometry.
FIG. 3.
SDS-PAGE and tandem mass spectrometry of selected protein bands. (A) SDS-PAGE of periplasmic protein extract and mass spectrum of the peptide in the region of MW 27,000 in the periplasmic fraction and identified as AC hydrolase. (B) SDS-PAGE and mass spectrum of the peptide in the region of MW 46,000 in the cytoplasmic fraction and identified as a homolog to aminohydroxybutyrate aminotransferase. Observed fragment ions are labeled according to the nomenclature of Biemann (3).
Multiple proteins were identified by searching for the predicted A2C hydrolase and aminobutyrate aminotransferase protein sequences and searching the databases for matches to proteins from other organisms. This confirmed the fidelity of the fractionation technique, as the search for homologous proteins yielded known periplasmic proteins in the periplasmic fraction and cytosolic proteins in the cytosolic fraction. For example, the periplasmic fraction contained peptides homologous to the periplasmic ABC transporter (accession number NP_742282) and periplasmic basic amino acid transporter (accession number NP_746597), both from Pseudomonas putida KT2440. Moreover, the proteins in each respective fraction were unique with the exception of the AC hydrolase protein, which was found in both periplasmic and cytosolic fractions. By contrast, peptides corresponding to the HAB aminotransferase were found only in the cytoplasmic fractions. The A2C hydrolase protein was rigorously identified. The identified fragments covered over 40% of the protein, and multiple fragments matched the predicted masses with a resolution of 0.1 atomic mass unit or less. Similarly, the aminotransferase showed three peptides which exactly matched the predicted HAB aminotransferase sequence with a high confidence level. Representative peptide matches are shown in Fig. 3.
Initial attempts to determine AC hydrolase activity in the periplasmic fraction using the standard fluorimetric assay were confounded by the presence of EDTA in the periplasmic fraction. Removal of the EDTA by dialysis allowed activity to be measured. There was activity in both fractions; however, the specific activity was 40% higher in the periplasmic protein fraction.
DISCUSSION
Prior to this study, bacteria had been reported to metabolize AC to HAB (5, 36). The ring-opened product had been shown to undergo transamination, allowing bacteria to grow on AC as a nitrogen source. The present study significantly extended previous work. First, the product of the ring-opening reaction was rigorously identified as HAB. Second, genes encoding AC detoxification were cloned into an E. coli strain. One of the gene products was identified as a member of the HAD superfamily of proteins. The HAD homolog was not observed to show haloacid dehalogenase activity with the substrates tested; however, it did have substantial AC hydrolase activity. The HAD superfamily is represented by well over 1,000 sequences and several metabolic activities: dehalogenation, phosphate ester hydrolysis, and dephosphonylation (4). All of the proteins for which an X-ray structure has been determined show an alpha/beta-hydrolase fold and contain an active site aspartic acid nucleophile. Our sequence alignment of AC hydrolase with members of the HAD superfamily revealed a highly conserved region in the vicinity of the known active site aspartate. Site-directed mutagenesis of the single aspartic acid abolished all AC hydrolase activity, consistent with the assignment of AC hydrolase in the HAD superfamily and suggesting mechanistic similarities. Evidence was also presented to indicate that the AC hydrolase is largely expressed in the periplasmic cell cavity.
In light of these findings, a putative role for the other gene products is immediately suggested. The product of A2C hydrolytic ring opening, 2-hydroxy-4-aminobutyrate, is proposed to be transported into the cytoplasm by the GABA transporter. The GABA transporter homolog identified in this study showed 76% sequence identity to a similar protein in P. putida KT2440. Transport of HAB by this transporter would allow cytoplasmic metabolism of this intermediate. The present study showed that the aminotransferase was found in the cytoplasm of Pseudomonas sp. strain A2C. The HAB aminotransferase described here showed 66% amino acid sequence identity with the annotated 4-aminobutryate aminotransferase from Pseudomonas syringae (Table 1). The protein identified here also showed 46% amino acid sequence identity to the E. coli 4-aminobutyrate aminotransferase for which an X-ray structure is known (17).
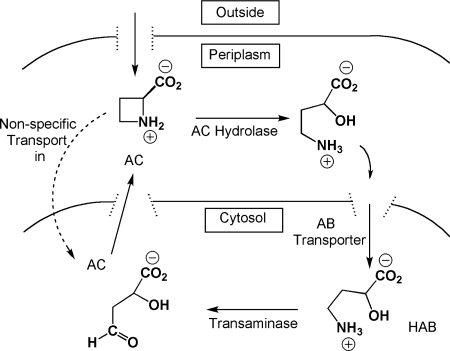
Taken in total, the data suggest the evolution of a multiply tiered strategy for resistance to, and assimilation of, AC. First, if any AC adventitiously enters the cell, a DMT (13) may transport AC outside the cell (Fig. 4). AC in the periplasm is acted upon by AC hydrolase that has been exported outside the cytoplasmic membrane with the possible assistance of the csaA gene product. AC ring opening requires AC hydrolase, and water as a cosubstrate, to produce 2-hydroxy-4-aminobutyrate as the product. 2-Hydroxy-4-aminobutyrate could be transported into the cytoplasm by the action of a GABA transporter homolog and then undergo transamination in the cytoplasm. This not only detoxifies AC but also provides nitrogen to support growth.
FIG. 4.
Proposed model for AC detoxification and assimilation in Pseudomonas sp. strain A2C. The AB transporter is the 4-aminobutyrate transporter; AC is putatively transported out of the cell by the DMT.
The present study highlights a strategy of combining detoxification and catabolism with A2C. Moreover, the system is designed to minimize risk by having the initial detoxification reaction, azetidine ring opening, catalyzed in the periplasm. There are other detoxification reactions known to be mediated by periplasmic enzymes (34). For example, four classes of β-lactamases are widely found in the periplasm of diverse gram-negative bacteria, where they hydrolytically open the four-membered β-lactam ring of penicillins, cephalosporins, carbapenams, monobactams, and penams (22). The β-lactamases derive from a different protein fold than AC hydrolase. The detoxification of reactive oxygen species is also known to be mediated by periplasmic enzymes (18, 28). This class includes superoxide dismutase, catalase, and peroxidases. In other examples, periplasmic enzymes are involved in catabolism for cell protein cycling and nutrient acquisition. For example, E. coli is reported to express 20 periplasmic proteases (15). A number are involved in d-alanine-d-alanine peptide hydrolysis as part of cell wall formation, but some are likely involved in general polypeptide hydrolysis and amino acid acquisition.
Periplasmic AC hydrolase, mediating both detoxification and catabolism, is relatively unique. Generally, enzymes that catabolize potential growth substrates are not simultaneously protecting against toxins. The most analogous system that we are aware of is a periplasmic catabolic trehalase; however, that protects cells against alginate secreted by the same cell and it is thought to prevent reentry of toxic alginate (2, 9).
Acknowledgments
We thank the Institute for Renewable Energy and the Environment, University of Minnesota, for support.
We acknowledge Jack Richman for skillful assistance in chemical analyses and for helpful discussions.
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-441. [DOI] [PubMed] [Google Scholar]
- 2.Bakkevig, K., H. Sletta, M. Gimmestad, R. Aune, H. Ertesvag, K. Degnes, B. E. Christensen, T. E. Ellingsen, and S. Valla. 2005. Role of the Pseudomonas fluorescens extracellular environment. J. Bacteriol. 1878375-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biemann, K. 1988. Contributions of mass spectrometry to peptide and protein structure. Biomed. Environ. Mass Spectrom. 1699-111. [DOI] [PubMed] [Google Scholar]
- 4.Burroughs, A. M., K. N. Allen, D. Dunaway-Mariano, and L. Aravind. 2006. Evolutionary genomics of the HAD superfamily: understanding the structural adaptions and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J. Mol. Biol. 3611003-1034. [DOI] [PubMed] [Google Scholar]
- 5.Dunnill, P. M., and L. Fowden. 1965. Azetidine-2-carboxylic acid breakdown by soil microorganism. Phytochemistry 4445-451. [Google Scholar]
- 6.Fowden, L. 1955. Azetidine-2-carboxylic acid: a new constituent of plants. Nature 176347-348. [Google Scholar]
- 7.Fowden, L. 1956. Azetidine-2-carboxylic acid: a new cyclic imino acid occuring in plants. Biochem. J. 64323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowden, L., and M. H. Richmond. 1963. Replacement of proline by azetidine-2-carblxylic acid during biosynthesis of protein. Biochim. Biophys. Acta 71459-461. [Google Scholar]
- 9.Franklin, M. J., and D. E. Ohman. 2002. Mutant analysis and cellular localization of the AlgI, AlgJ, and AlgF proteins required for O-acetylation of alginate in Pseudomonas aeruginosa, J. Bacteriol. 1843000-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant, M., and A. S. Brown. 1975. Effect of L-azetidine 2-carboxylic acid on growth and proline metabolism in Escherichia coli. Biochim. Biophys. Acta 404180-187. [DOI] [PubMed] [Google Scholar]
- 11.Harbourne, J. B. 1988. Ecological biochemistry, 3rd ed.. Academic Press, London, England.
- 12.Hisano. T., Y. Hata, T. Fujii, J. Q. Liu, T. Kurihara, N. Esaki, and K. Soda. 1996. Crystal structure of L-2-haloacid dehalogenase from Pseudomonas sp. YL. An alpha/beta hydrolase structure that is different from the the alpha/beta hydrolase fold. J. Biol. Chem. 27120322-20330. [DOI] [PubMed] [Google Scholar]
- 13.Jack, D. L., N. M. Yang, and M. H. Saier, Jr. 2001. The drug/metabolite transporter superfamily. Eur. J. Biochem. 2683620-3639. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi, S., J. Muller, D. Linde, S. Kuramitsu, T. Shibata, Y. Inoue, D. G. Vassylyev, and S. Yokoyama. 2001. The crystal structure of the ttCsaA protein: an export chaperone from Thermus thermophilus. EMBO J. 20562-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuez, N., M. Meltzer, and M. Ehrmann. 2007. Periplasmic proteases and protease inhibitors, p. 150-170. In M. Ehrmann (ed.), The periplasm. ASM Press, Washington, DC.
- 16.Li, Y., F. Y. Hata, T. Fujii, T. Hisano, M. Nishihara, T. Kurihara, and N. Esaki. 1998. Crystal structure of reaction intermediates of L-2-haloacid dehalogenase and implications for the reaction mechanism. J. Biol. Chem. 27315035-15044. [DOI] [PubMed] [Google Scholar]
- 17.Liu, W., P. E. Peterson., J. A. Langston, X. Jin, X. Zhou, A. J. Fisher, and M. D. Toney. 2005. Kinetic and crystallographic analysis of active site mutants of Escherichia coli gamma-aminobutyrate aminotransferase. Biochemistry 442982-2992. [DOI] [PubMed] [Google Scholar]
- 18.Lumppio, H. L., N. V. Shenvi, A. O. Summers, G. Voordouw, and D. M. Kurtz, Jr. 2001. Rubrerythrin and rubredoxin oxidoreductase in Desulfovibrio vulgaris: a novel oxidative stress protection system. J. Bacteriol. 183101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelsestuen, G. L., Y. Zhang, M. B. Martinez, N. S. Key, B. Jilma, M Verneris, A. Sinaiko, and R. S. Kasthuri. 2005. Plasma protein profiling: unique and stable features of individuals. Proteomics 54012-4024. [DOI] [PubMed] [Google Scholar]
- 21.Ottinger, P. 2001. Enrichment of soil bacteria on novel compounds and characterization of azetidine-2-carboxylate metabolism. M.S. thesis. University of Applied Sciences, Waedenswil, Switzerland.
- 22.Poole, K. 2007. Antimicrobial and stress resistance, p. 304-324. In M. Ehrmann (ed.), The periplasm. ASM Press, Washington, DC.
- 23.Pouchert, C. J., and J. Behnke. 1993. The Aldrich library of 13C and 1H FT-NMR spectra, 1st ed. Aldrich Chemical Company, Inc., St. Louis, MO.
- 24.Reese, L. M., K. O. Cutler, and C. E. Deutch. 1996. Sensitivity of Eschericia coli to proline analogues during osmotic stresss and anaerobiosis. Lett. Appl. Microbiol. 22202-205. [DOI] [PubMed] [Google Scholar]
- 25.Ridder. I. S., H. J. Rozeboom, K. H. Kalk, D. B. Janssen, and B. W. Dijkstra. 1999. Three-dimensional structure of L-2-haloacid dehalogenase from Xanthobacter autotrophicus GJ10 complexed with the substrate-analogue formate. J. Biol. Chem. 27233015-33022. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein, E., H. Zhou, K. M. Krasinska, A. Chien, and C. H. Becker. 2006. Azetidine-2-carboxylic acid in garden beets (Beta vulgaris), Phytochemistry 67898-903. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. Russell. 2001. Molecular cloning. CSHL Press, Cold Spring Harbor, NY.
- 28.San Mateo, L. R., M. M. Hobbs, and T. H. Kawula. 1998. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol. Microbiol. 27391-404. [DOI] [PubMed] [Google Scholar]
- 29.Seto, B. 1979. Proline reductase: a sensitive fluorometric assay with o-phthalaldehyde. Anal. Biochem. 9544-47. [DOI] [PubMed] [Google Scholar]
- 30.Stanier, R. Y., N. J. Palleroni, and M. Duodoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43159-271. [DOI] [PubMed] [Google Scholar]
- 31.Sugiura, M., and M. Kisumi. 1985. Proline-hyperproducing strains of Serratia marcesens: enhancement of proline analog-mediated growth inhibition by increasing osmotic stress. Appl. Environ. Microbiol. 49782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takagi, H., M. Shichiri, M. Takemura, M. Mohri, and S. Nakamori. 2000. Saccharomyces cerevisiae E1278b has novel genes of the N-acetyltransferase gene superfamily required for l-proline analog resistance. J. Bacteriol. 1824249-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkmer-Engert, L. D., S. Schreiber, and J. P. Muller. 2003. Interaction of the Bacillus subtilis chaperone CsaA with the secretory protein YvaY. FEMS Microbiol. Lett. 22693-100. [DOI] [PubMed] [Google Scholar]
- 34.Walker, K. W., and H. F. Gilbert. 1994. Effect of redox environment on the in vitro and in vivo folding of RTEM-1 β-lactamase and Escherichia coli alkaline phosphatase. J. Biol. Chem. 26928487-28493. [PubMed] [Google Scholar]
- 35.Yeung, K. F. 1987. Degradation of L-azetidine-2-carboxylic acid by Enterobacter aggiomerans and E. aminigenus. Ph.D. thesis, University of Michigan, Ann Arbor.
- 36.Yeung, K. F., K. M. Lee, and R. W. Woodward. 1998. Isolation and identification of two L-azetidine-2-carboxylic acid-degrading soil organisms, Enterobacter ammlomerans and Enterobacter amnigenus. J. Nat. Prod. 61207-211. [DOI] [PubMed] [Google Scholar]