Abstract
Cyanobacteria produce phycobilisomes, which are macromolecular light-harvesting complexes mostly assembled from phycobiliproteins. Phycobiliprotein beta subunits contain a highly conserved γ-N-methylasparagine residue, which results from the posttranslational modification of Asn71/72. Through comparative genomic analyses, we identified a gene, denoted cpcM, that (i) encodes a protein with sequence similarity to other S-adenosylmethionine-dependent methyltransferases, (ii) is found in all sequenced cyanobacterial genomes, and (iii) often occurs near genes encoding phycobiliproteins in cyanobacterial genomes. The cpcM genes of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 were insertionally inactivated. Mass spectrometric analyses of phycobiliproteins isolated from the mutants confirmed that the CpcB, ApcB, and ApcF were 14 Da lighter than their wild-type counterparts. Trypsin digestion and mass analyses of phycobiliproteins isolated from the mutants showed that tryptic peptides from phycocyanin that included Asn72 were also 14 Da lighter than the equivalent peptides from wild-type strains. Thus, CpcM is the methyltransferase that modifies the amide nitrogen of Asn71/72 of CpcB, ApcB, and ApcF. When cells were grown at low light intensity, the cpcM mutants were phenotypically similar to the wild-type strains. However, the mutants were sensitive to high-light stress, and the cpcM mutant of Synechocystis sp. strain PCC 6803 was unable to grow at moderately high light intensities. Fluorescence emission measurements showed that the ability to perform state transitions was impaired in the cpcM mutants and suggested that energy transfer from phycobiliproteins to the photosystems was also less efficient. The possible functions of asparagine N methylation of phycobiliproteins are discussed.
In order to power the electron transfer reactions of photosystems (PS) I and II, cyanobacteria and red algae use pigment-protein supercomplexes, called phycobilisomes (PBS), to harvest light energy efficiently (5, 7, 20, 22, 55). In many cyanobacteria, including Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803, the orange- and red-light-absorbing proteins, phycocyanin (PC) and allophycocyanin (AP), respectively, are the major phycobiliproteins (PBP). All PBP are assembled as heterodimeric protomers (αβ) that can further assemble into toroid-shaped trimers [(αβ)3] and/or hexamers [(αβ)6] (5, 55). PBP subunits carry at least one and up to three linear tetrapyrrole chromophores, called phycobilins, which are covalently attached through cysteinyl thioether linkages to the polypeptides (20, 55). The most common PBS structures in cyanobacteria are hemidiscoidal PBS, which have a core assembled from AP cylinders. Six peripheral rods composed of PC hexamers typically radiate from two sides of the core substructure (5, 7, 20, 55). In some species or under specific growth conditions, additional hexamers of phycoerythrin (PE) or phycoerythrocyanin (PEC) may be attached at distal ends of the peripheral rods to enhance light absorption at between 500 and 600 nm (20, 55). The positions of the various PBP within the PBS are determined by protein-protein interactions between the various PBP as well as by so-called linker proteins, which provide the scaffolding information necessary to direct the correct assembly of PBS (5, 20, 55).
We have characterized the assembly of PBS of the unicellular marine cyanobacterium Synechococcus sp. strain PCC 7002 in considerable detail (5, 6, 11, 12, 18, 19, 23, 38, 48). More recently, we have been studying the posttranslational processes that lead to maturation of holo-PBP in this cyanobacterium. A heterodimeric CpcE-CpcF lyase attaches a phycocyanobilin (PCB) chromophore to Cys84 of apo-CpcA (α-PC) (14, 15, 59, 68). The CpcT lyase ligates the PCB chromophore to Cys153 of β-PC (53), and a heterodimeric CpcS-I-CpcU lyase attaches PCB to Cys82 of β-PC (CpcB), to Cys81 of α-AP (ApcA), and to β-AP (ApcB) (46, 54). In some other cyanobacteria, a lyase comprised of a single polypeptide related to CpcS-I is responsible for PCB attachment at Cys81/82 of all PBP subunits except CpcA and possibly ApcE (47, 66, 67). Not only does PCB attachment to apo-PBP confer their ability to act as light-harvesting proteins, but these posttranslational modifications stabilize PBP, allowing PBS to assemble (46, 53, 54).
Posttranslational modification is an important mechanism to modulate the structural and functional properties of proteins. In PBP biogenesis, a second type of posttranslational modification distinct from PCB attachment is known to play a role. Minami et al. (39) reported that ApcB of Anabaena cylindrica contained a modified aspartate residue at position 71, and shortly thereafter, Klotz et al. (31) showed that this modified amino acid residue was γ-N-methylasparagine (γ-N-methyl-Asn). Klotz and Glazer subsequently showed that this modification is almost universally present in the β subunits of PBP (30). X-ray crystallography has confirmed that Asn methylation occurs in β-PC (56) and β-AP (4), and the structure of a PC variant that lacks this methylation has also been determined (1). Swanson and Glazer demonstrated that methylation of this Asn residue was catalyzed by a specific methyltransferase, which they partially purified; they also characterized two mutant strains lacking this methyltransferase (58). Although these authors did not identify the gene encoding this enzyme, they concluded that N methylation of Asn72 is important for efficient energy transfer in PBS.
The goal of this study was to identify the gene that encodes the cyanobacterial PBP Asn-methyltransferase and to study the role(s) of PBP methylation in PBS assembly and energy transfer. Since the studies of Swanson and Glazer (58), many cyanobacterial genomes have been sequenced, including those of Synechocystis sp. strain PCC 6803 (29), Nostoc sp. strain PCC 7120 (28), and Synechococcus sp. strain PCC 7002 (GenBank accession no. NC_010475 to NC_010480). By using a comparative bioinformatics approach and gene neighborhood analysis, we identified highly conserved open reading frames, sll0487 in Synechocystis sp. strain PCC 6803 and SYNPCC7002_A2010 in Synechococcus sp. strain PCC 7002, which encode a previously uncharacterized methyltransferase family that occurs only in the genomes of organisms that synthesize PBP. Using a reverse genetics approach, we show that this gene, which we have named cpcM, encodes the enzyme that methylates the amide nitrogen of Asn71/72 in CpcB, ApcB, and ApcF. The cpcM mutants are much more sensitive to high light intensities than the corresponding wild-type strains.
MATERIALS AND METHODS
Strains of cyanobacteria and culture conditions.
Synechocystis sp. strain PCC 6803 was grown in B-HEPES medium at 30°C at a light intensity of 100 μmol photons m−2 s−1 and was bubbled with 1% (vol/vol) CO2 in air (standard conditions) as previously described (51). For the cpcM mutant, 50 μg kanamycin ml−1 was added to the solid or liquid growth medium. The wild-type and mutant strains of Synechococcus sp. strain PCC 7002 were grown in medium A supplemented with 1 mg NaNO3 ml−1 (medium A+) at 38°C at a light intensity of 250 μmol photons m−2 s−1 and were bubbled with 1% (vol/vol) CO2 in air (standard conditions) (57). For the cpcM mutant strain, 50 μg gentamicin ml−1 was added to medium A+. The photoautotrophic growth rates of wild-type and mutant strains were measured by monitoring the optical density at 730 nm (OD730) using a Genesys 10 spectrophotometer (ThermoSpectronic, Rochester, NY). Chromosomal DNA was isolated from wild-type and mutant cells of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 as described previously (52). Routine DNA manipulations were performed in Escherichia coli strain DH5α using standard laboratory procedures.
Construction of the cpcM mutants.
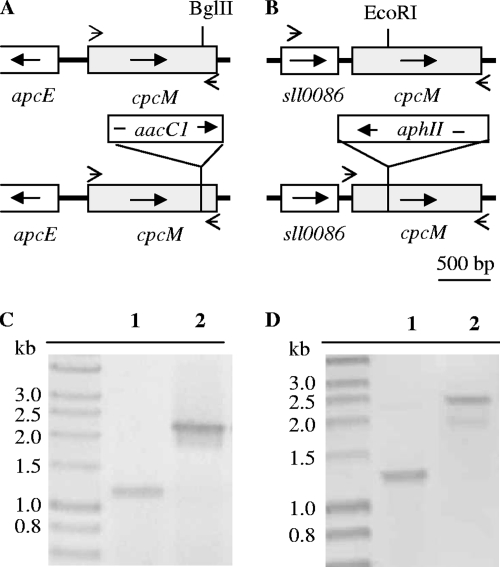
To generate a Synechococcus sp. strain PCC 7002 cpcM mutant, a 2,080-bp DNA fragment containing the ∼1,200-bp cpcM gene and flanking sequences including the 5′ end sequence of apcE gene were amplified by PCR using DNA of wild-type Synechococcus sp. strain PCC 7002 as the template and primers 17002MF (5′ CGAGGTAAGCAAGGGGGGATCCACCAC 3′) and 27002MR (5′ TAGGTGTCAGCATAATCAATTGTCTGG 3′). The resulting amplicon was cloned into pUC19 and sequenced. For insertional inactivation of the cpcM gene, a 1-kb DNA fragment, which encodes the aacCI gene and confers resistance to gentamicin, was inserted into the unique BglII site within the cpcM coding sequence (Fig. 1A). This cpcM::aacCI inactivation construct was linearized and used to transform cells of Synechococcus sp. strain PCC 7002 as described previously (16). Transformants were selected on medium A+ plates containing 50 μg gentamicin ml−1 and were streaked several times on selective medium.
FIG. 1.
Cloning and mutagenesis of the cpcM genes of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803. (A and B) The physical maps show the DNA fragments containing the cpcM gene from wild-type and cpcM mutants of Synechococcus sp. strain PCC 7002 (A) and Synechocystis sp. strain PCC 6803 (B). The arrows in the boxes indicate the direction of transcription of each gene. Small arrows outside the boxes show the approximate positions of primers used for PCR amplification. (C and D) PCR analysis was used to verify segregation of wild-type and mutant alleles of the cpcM loci in Synechococcus sp. strain PCC 7002 (C) and Synechocystis sp. strain PCC 6803 (D). For lanes 1, the DNA template was prepared from wild-type cells; for lanes 2, the DNA template was prepared from a representative cpcM mutant.
For mutagenesis of the cpcM gene in Synechocystis sp. strain PCC 6803, a 1.3-kb DNA fragment carrying the open reading frame sll0487 was amplified by PCR using primers sll04875.2 (5′ TTCGGATCCATGTTGTCCAACTCCGAC 3′) and sll0487.3.2 (5′ AATTCCCGGGCATCGAGAAGTCG 3′). A 1.3-kb DNA fragment carrying the aphII gene and conferring resistance to kanamycin was inserted at the unique EcoRI site in the cpcM gene to produce an inactivation construct, cpcM::aphII (Fig. 1B). This construct was linearized and used to transform cells of Synechocystis sp. strain PCC 6803 (16, 51). Transformants were screened on B-HEPES plates supplemented with 50 μg kanamycin ml−1.
Isolation of PBS.
PBS were isolated from wild-type and mutant cells of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 as described previously (7, 53). Liquid cultures were grown to late exponential growth phase (OD730 of 1.5 to 2.0 for Synechocystis sp. strain PCC 6803 and 3.0 to 3.5 for Synechococcus sp. strain PCC 7002). Cells were harvested by centrifugation and lysed by two passages through a chilled French pressure cell at 138 MPa. PBS were isolated on discontinuous sucrose gradients by ultracentrifugation (53).
Protein assays, electrophoresis, and pigment analyses.
Protein concentrations were determined using a protein assay kit (Pierce Biotechnology, Rockford, IL). The polypeptide compositions of isolated PBS were resolved by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate, and polypeptides were visualized by staining with Coomassie blue as described previously (53, 54). PCB-linked proteins were visualized by UV-induced fluorescence after soaking gels in 100 mM ZnSO4 for 2 min (42, 53). Chlorophyll (Chl) a concentrations were determined for cells grown to late exponential growth phase (OD730 of 0.8 to 1.2) as described previously (34, 35). The relative PBP contents of cells were estimated by heat-induced bleaching at 65°C for 5 min as described previously (44, 64). The absorption difference was measured at 635 nm for Synechococcus sp. strain PCC 7002 cells and at 630 nm for Synechocystis sp. strain PCC 6803 cells.
Absorption and fluorescence spectroscopy.
Absorption spectra of whole cells, purified PBS, and PBP were recorded with a Genesys 10 spectrophotometer (ThermoSpectronic, Rochester, NY). Data were analyzed using the Igor-Pro (Wavemetrics, Inc., Lake Oswego, OR). Fluorescence excitation and emission spectra were recorded by using an SLM 8000C spectrofluorometer (52). For state transition measurements, cells (1.0 OD730 unit per ml) were resuspended in liquid A+ medium for Synechococcus sp. strain PCC 7002 cells and in B-HEPES medium for Synechocystis strain 6803 cells. Cells were illuminated for 2 min with blue light provided by a 460-nm short-pass filter (Corion, Holliston, MA) to produce state 1. Cells were incubated in the dark for at least 5 min to produce state 2 (65).
HPLC-MS and trypsin digestion.
The Proteomics and Mass Spectrometry Core Facility (Pennsylvania State University) performed high-pressure liquid chromatography-mass spectrometry (HPLC-MS) analyses of PBS and tryptic digests of PBP. For identification of methylated peptides, total proteins from the PBS were digested with trypsin. The PBS proteins (∼150 to 200 μg) were resolved by HPLC, and fractions were collected and dried by evaporation under reduced pressure. The resulting protein pellets were dissolved in 200 μl of 100 mM ammonium bicarbonate, reduced with tributylphosphine (5 μl), and incubated for 30 min. Proteins were alkylated with iodoacetamide (6 μl), and after a 60-min incubation, the reaction was quenched with tributylphosphine (5 μl). Samples were then digested with trypsin at a ratio of 1:50 (wt/wt) for 18 h at 37°C.
Sequence analysis and bioinformatics.
DNA and protein sequence analyses were performed with MacVector software (MacVector, Inc., Cary, NC). Homolog sequences were retrieved from the JGI database (http://img.jgi.doe.gov/cgi-bin/pub/main.cgi) by using the CpcM sequences of Synechococcus sp. strain PCC 7002 (SYNPCC7002_A2010) and Synechocystis sp. strain PCC 6803 (sll0487) as query sequences. Sequence alignments were generated using the ClustalW module in MacVector version 9.5.2. Neighbor-joining phylogenetic trees were produced from the ClustalW alignment using the PAUP Phylogenetic Analysis program (Sinauer Associates, Sunderland, MA).
RESULTS
Identification of cpcM genes in cyanobacteria.
Three criteria were applied in our primary screening for candidate genes that could encode the Asn72 methyltransferase for PBP β subunits. First, the gene product should display sequence similarity to the methyltransferase protein superfamily (33, 36). Second, orthologs of this gene should occur only in chlorophototrophs that synthesize PBP. Third, we further screened all candidate genes encoding methyltransferases for those that occur in the vicinity of genes encoding other PBP- or PBS-related proteins. A gene matching all of these criteria had first attracted our attention several years ago, when a DNA fragment carrying the apcE gene of Synechococcus sp. strain PCC 7002 was cloned and sequenced (GenBank accession no. AF059340.1). A putative methyltransferase gene was observed upstream from and divergently transcribed from apcE, which encodes the largest linker polypeptide of PBS in Synechococcus sp. strain PCC 7002. ApcE organizes the AP of the core and attaches the PBS to the thylakoid membrane (2, 3, 5, 8, 9, 19). Many cyanobacterial genomes have subsequently been sequenced, and this same gene organization is found in at least 17 cyanobacterial genomes, including, among others, those of Synechococcus sp. strains WH8102, PCC 7942, and PCC 6301; Lyngbya sp. strain PCC 8106; and Anabaena variabilis strain ATCC 29413. In many of these organisms, apcE is found upstream from, and is probably cotranscribed with, the apcABC operon, which encodes the AP alpha and beta subunits as well as the 8-kDa LC core linker protein of PBS core subassemblies. The putative methyltransferase gene, designated open reading frame SYNPCC7002_A2010 in Synechococcus sp. strain PCC 7002 and open reading frame sll0487 in Synechocystis sp. strain PCC 6803, occurs as a single-copy gene in all sequenced cyanobacterial genomes. Based upon its distribution, the linkage information, and the results obtained from the characterization of null mutants of two different cyanobacterial strains (see below), we suggest that genes orthologous to SYNPCC7002_A2010 and sll0487 be renamed cpcM.
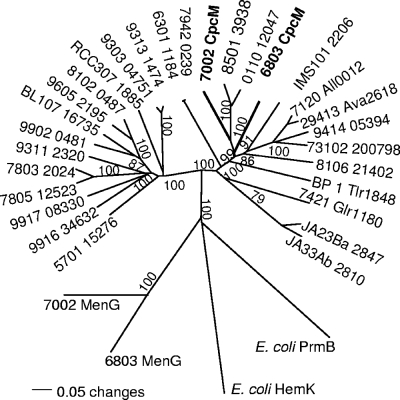
Cyanobacterial CpcM proteins form two distinctive clades (Fig. 2). Clade 1, including the CpcM proteins of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803, contains CpcM proteins of strains that assemble PBS containing AP, PC, and sometimes PE or PEC. However, clade 2 mostly contains the CpcM proteins of marine Synechococcus sp. strains, which can assemble PBS, and PE-containing Prochlorococcus sp. strains, which do not produce PBS but synthesize PBP. Interestingly and as noted above, the cpcM gene is found upstream from the apcEABC gene cluster in most marine Synechococcus spp. As illustrated by the relatively short branch lengths within these two clades, cyanobacterial CpcM proteins share significant amino acid sequence similarity, which suggests that these proteins are structurally and functionally related. For comparison, two much more distantly related MenG methyltransferases from the phylloquinone biosynthetic pathway in cyanobacteria (45) were included as outgroup sequences in the phylogenetic analysis. The E. coli proteins HemK/PrmC and PrmB, both of which methylate glutamine residues in proteins (see Discussion), were also included as outgroup sequences. Although exhibiting some distant similarity to CpcM, especially in the N-terminal methyltransferase domain (amino acids 39 to 262 of Synechococcus sp. strain PCC 7002 CpcM), the MenG, HemK/PrmC, and PrmB proteins are highly divergent from all CpcM proteins (Fig. 2).
FIG. 2.
Phylogenetic analysis of CpcM proteins from cyanobacteria. Amino acid sequences of CpcM proteins from Synechococcus sp. strain PCC 7002 (7002), Synechocystis sp. strain PCC 6803 (6803), Nostoc sp. strain PCC 7120 (7120), Nostoc punctiforme strain PCC 73102 (73102), Anabaena variabilis strain ATCC 29413 (29413), Thermosynechococcus elongatus strain BP-1 (BP 1), Cyanothece sp. strain CCY 0110 (0110), Crocosphaera watsonii strain WH8501 (8501), Trichodesmium erthraeum strain IMS101 (IMS101), Gloeobacter violaceus strain PCC 7421 (7421), Synechococcus elongatus strain PCC 7942 (7942), Synechococcus sp. strain PCC 6301 (6301), Nodularia spumigena strain CCY9414 (9414), Lyngbya sp. strain PCC 8106 (8106), Synechococcus sp. strain JA-3-3Ab (JA33Ab), Synechococcus sp. strain JA-2-3Ba (JA23Ba), Synechococcus sp. strain RCC307 (RCC307), Synechococcus sp. strain WH5701 (5701), Synechococcus sp. strain CC9902 (9902), Synechococcus sp. strain BL107 (BL107), Synechococcus sp. strain WH8102 (8102), Synechococcus sp. strain CC9605 (9605), Synechococcus sp. strain CC9311 (9311), Synechococcus sp. strain RS9916 (9916), Synechococcus sp. strain RS9917 (9917), Synechococcus sp. strain WH7803 (7803), Synechococcus sp. strain WH7805 (7805), Prochlorococcus marinus strain MIT9303 (9303), Prochlorococcus marinus strain MIT9313 (9313), Prochlorococcus sp. strain CC9605 (9605), and Prochlorococcus sp. strain CC9902 (CC9902) were compared. The sequence alignment was generated using the ClustalW module within the MacVector program, version 9.5 (MacVector, Inc., Cary, NC). The phylogenetic tree was generated using the phylogenetic analysis program PAUP (Sinauer Associates, Sunderland, MA). The MenG methyltransferases, which perform the last step in phylloquinone synthesis, from Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 7002 and HemK/PrmC and PrmB from E. coli were chosen as outgroup members for this analysis. Bootstrap values are based upon 1,000 replicates and are indicated for most branches.
Verification of the cpcM mutants.
As illustrated in Fig. 1A, the cpcM gene of Synechococcus sp. strain PCC 7002 was inactivated by inserting the aacC1 gene into the coding sequence of the gene at a unique BglII site. As shown in Fig. 1C, PCR amplification of the cpcM locus in wild-type cells produced a 1.2-kb amplicon, while PCR amplification of the cpcM locus in a Gmr transformant produced a 2.3-kb amplicon. These results showed that the cpcM locus was insertionally inactivated and that the cpcM and cpcM::aacC1 alleles had fully segregated. The cpcM gene of Synechocystis sp. strain PCC 6803 was similarly inactivated by inserting the aphII gene into the coding sequence of the gene at a unique EcoRI site (Fig. 1B). PCR amplification of the cpcM locus in wild-type cells produced a 1.3-kb amplicon, while PCR amplification of the cpcM locus in a Kmr transformant produced a 2.5-kb amplicon (Fig. 1D). These results showed that the cpcM locus had been inactivated and that the cpcM and cpcM::aphII alleles had fully segregated.
HPLC-MS of the isolated PBS.
To determine whether the loss of CpcM had any effect on the posttranslational modification of PBP, PBS were isolated from wild-type and mutant cells of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803, which had been grown under standard growth conditions. The sucrose gradients employed for the PBS isolation from wild-type and cpcM mutant cells of both cyanobacterial strains were similar in appearance, and the majority of the PBP were recovered from the PBS fraction in the 1.0 M sucrose zone (data not shown). The polypeptide composition of the PBS was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the composition and the relative amounts of the polypeptide components of the PBS of the cpcM mutant strains were similar to those of the respective wild-type strains (data not shown). These results indicated that the loss of CpcM had no major effect on the assembly of PBP and PBS complexes in either strain under standard growth conditions.
For further analyses of the PBP, PBS samples isolated from the wild-type and cpcM mutant strains were analyzed by HPLC-MS. The AP and PC subunits could be observed among polypeptides derived from PBS isolated from wild-type Synechococcus sp. strain PCC 7002 cells (Table 1; see Fig. S1 in the supplemental material). The methylated β-AP (ApcB), β-PC (CpcB), and β18 (ApcF) polypeptides were identified by their observed masses of 17,823 Da, 19,525 Da, and 19,326 Da, respectively (Table 1). However, polypeptides with these masses were not observed in the PBS isolated from the cpcM mutant of Synechococcus sp. strain PCC 7002. Instead, polypeptides with molecular masses of 17,809 Da and 19,511 Da were observed for β-AP (ApcB) and β-PC (CpcB), respectively (Table 1) (the β18 subunit [ApcF] was not detected). These masses, which are 14 Da lower than those of the respective polypeptides from the wild-type strain, correspond to the calculated masses of unmethylated ApcB (17,810 Da) and CpcB (19,512 Da), which carry one and two PCB chromophores (mass, 588.3 Da), respectively (Table 1).
TABLE 1.
Observed and predicted masses for PBP from wild-type and cpcM mutants of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803
| Strain and PBP | Mass (Da)a
|
|||
|---|---|---|---|---|
| WT
|
CpcM−
|
|||
| Observed | Calculated | Observed | Calculated | |
| PCC 7002 | ||||
| ApcA | 17,742 | 17,742 | 17,742 | 17,742 |
| ApcB | 17,823 | 17,824 | 17,809 | 17,810 |
| CpcA | 18,210 | 18,210 | 18,209 | 18,210 |
| CpcB | 19,525 | 19,526 | 19,511 | 19,512 |
| ApcF | 19,326 | 19,325 | NDb | 19,311 |
| PCC 6803 | ||||
| ApcA | 17,868 | 17,868 | 17,867 | 17,868 |
| ApcB | 17,817 | 17,817 | 17,803 | 17,803 |
| CpcA | 18,174 | 18,174 | 18,173 | 18,174 |
| CpcB | 19,315 | 19,316 | 19,301 | 19,302 |
| ApcF | 19,356 | 19,362 | 19,342 | 19,348 |
Observed and calculated masses for proteins from wild-type (WT) and CpcM-less (CpcM−) strains. ApcA, ApcB, CpcA, and ApcF each carry one PCB chromophore (588.3 Da), while CpcB carries two PCB chromophores (1,176.6 Da). ApcB, Cpc,B, and ApcF additionally are assumed to carry one methyl group.
ND, not detected.
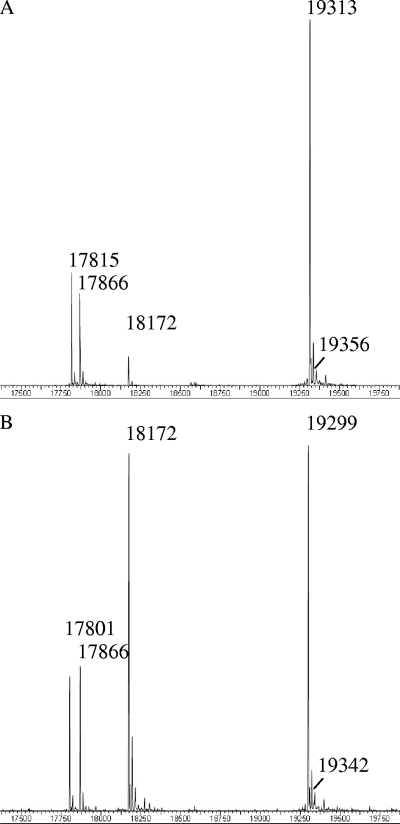
The results of HPLC-MS analyses of PBS isolated from wild-type Synechocystis sp. strain PCC 6803 and the cpcM mutant of this strain are shown in Fig. 3. For the PBS isolated from the wild type (Fig. 3A and Table 1), polypeptides with masses of 17,817 Da, 19,315 Da, and 19,356 Da were observed, which correspond closely to the calculated masses expected for the methylated ApcB, CpcB, and ApcF polypeptides, respectively. For PBS isolated from the cpcM mutant of Synechocystis sp. strain PCC 6803, polypeptides with masses of 17,803, 19,301, and 19,342 Da were observed, which correspond closely to the predicted masses of unmethylated ApcB (17,803 Da), CpcB (19,302 Da), and ApcF (19,348 Da) carrying one (ApcB and ApcF) or two (CpcB) PCB chromophores (Fig. 3B and Table 1). It should be noted that inactivation of the cpcM gene had no effect on the observed masses of the α subunits (ApcA and CpcA) of AP and PC in either strain (Table 1). These results suggested that CpcM is uniquely responsible for posttranslational methylation of three PBP β subunits: ApcB, ApcF, and CpcB.
FIG. 3.
HPLC-MS analysis of the PBP from isolated PBS of wild-type Synechocystis sp. strain PCC 6803 (A) and its cpcM mutant (B). The masses of some PBP are indicated in Da (Table 1).
Trypsin digestion and HPLC-MS analyses.
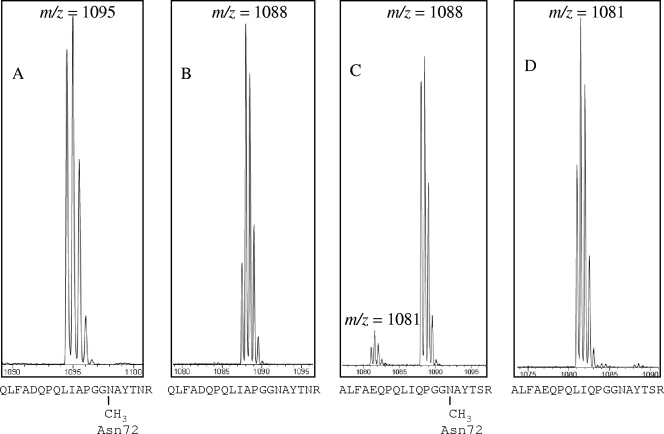
Based on amino acid sequencing (30, 31, 39, 43) and the X-ray structures of methylated and unmethylated PC (1, 56), the amide nitrogen of Asn72 is the methylation site for β-PC. To verify further that CpcM is the Asn72 methyltransferase, the PBP derived from PBS of the two wild-type and two cpcM mutant strains were digested with trypsin. A comparison of the tryptic peptides of PBP from the wild-type and the corresponding cpcM mutant strains provided additional information about the site at which methylation occurs in each strain. Figure 4 shows the results of HPLC-MS analyses of tryptic peptides derived from PBS. As shown in Fig. 4A, a doubly protonated peptide with a mass of 2,190 Da (m/z = 1,095) was observed by HPLC-MS analysis of a tryptic digest of PBP isolated from wild-type Synechococcus sp. strain PCC 7002; however, the same tryptic peptide from PBS purified from the cpcM mutant had a mass 14 Da lighter, i.e., 2,176 Da (Fig. 4B) (m/z = 1,088 Da). The mass of this peptide corresponds exactly to the calculated mass of the same tryptic peptide, which includes Asn72 but which lacked a methyl group. Similarly, a doubly protonated 2,176-Da peptide (Fig. 4C) (m/z = 1,088 Da) containing Asn72 was observed by HPLC-MS analysis of the tryptic digest of PBP from wild-type Synechocystis strain 6803. No peptide with this mass was observed in the tryptic digest of PBP of the Synechocystis strain 6803 cpcM mutant; instead, the corresponding peptide had a mass that was 14 Da lighter (Fig. 4D) (m/z = 1,081 Da). These results confirmed that CpcM is responsible for methylation of Asn72 of β-PC. As indicated by a sequence comparison of the CpcB, ApcB, and ApcF proteins from Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 (see Fig. S2 in the supplemental material), the Asn72 residue occurs 10 residues N-terminal to the absolutely conserved Cys82/81 residue to which PCB is ligated in all three PBP β subunits.
FIG. 4.
HPLC-MS analysis of peptides from PBP digested with trypsin. PBP, which had been isolated from the wild-type (A and C) and cpcM mutants of (B and D) Synechococcus sp. strain PCC 7002 (A and B) and Synechocystis strain 6803 (C and D), were recovered from PBS, digested with trypsin, and subjected to HPLC-MS analysis. The calculated masses of the doubly protonated CpcB peptides from the wild-type and the cpcM mutant of Synechococcus sp. strain PCC 7002 are 2,190 Da and 2,176 Da, respectively. The calculated masses of the doubly protonated CpcB peptides from the wild-type and the cpcM mutant of Synechocystis sp. strain PCC 6803 are 2,176 Da and 2,162 Da, respectively. Since the peptides shown are doubly protonated, the masses are obtained by multiplying the m/z values by two. Note that a very small amount of the unmethylated form of the CpcB tryptic peptide (2,160 Da) is observed in wild-type Synechocystis sp. strain PCC 6803.
Light sensitivity of the cpcM mutants.
The cpcM mutants of both Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 could grow photoautotrophically. As shown in Table 2, when cells were grown at a low light intensity (50 μmol photons m−2 s−1), no significant difference could be seen between the doubling times of the wild type (8.5 h) and the cpcM mutant (8.7 h) of Synechococcus sp. strain PCC 7002. Likewise, the doubling times of wild-type (12.3 h) and the cpcM mutant of (12.8 h) Synechocystis sp. strain PCC 6803 were similar. The growth rates of these four strains were also measured at light intensities of up to 390 μmol photons m−2 s−1. For Synechococcus sp. strain PCC 7002, wild-type cells grew faster as the light intensity increased, and the fastest doubling time was observed at the highest light intensity. Although the cpcM mutant also grew faster as the light intensity increased, the mutant consistently grew more slowly than the wild-type strain. These results suggested that the cpcM mutant is more sensitive to photoinhibition than the wild type.
TABLE 2.
Doubling times of wild-type and cpcM mutants of Synechococcus sp. strain PCC 7002 and of Synechocystis sp. strain PCC 6803 under different light conditions
| Light intensity (μmol photons m−2 s−1) | Mean doubling time (h) ± SD for strain:
|
|||
|---|---|---|---|---|
| 7002
|
6803
|
|||
| Wild type | cpcM mutant | Wild type | cpcM mutant | |
| 50 | 8.5 ± 0.3 | 8.7 ± 0.4 | 12.3 ± 0.3 | 12.8 ± 0.4 |
| 140 | 5.8 ± 0.2 | 6.1 ± 0.3 | 8.2 ± 0.3 | 11.4 ± 0.3 |
| 260 | 4.3 ± 0.2 | 4.8 ± 0.3 | 6.7 ± 0.3 | 48.4 ± 2.2 |
| 390 | 3.6 ± 0.1 | 4.4 ± 0.3 | 7.4 ± 0.3 | NMa |
NM, not measurable. Cells did not grow under this condition.
Synechocystis sp. strain PCC 6803 characteristically grows more slowly and is more sensitive to high light than Synechococcus sp. strain PCC 7002 (Table 2). The growth rate of the wild type increased with increasing light intensity up to ∼260 μmol photons m−2 s−1, but a further increase in light intensity caused photoinhibition and a decreased growth rate for Synechocystis sp. strain PCC 6803 (Table 2). This photoinhibitory effect was much more dramatic in the case of the cpcM mutant of Synechocystis sp. strain PCC 6803. Although the cpcM mutant grew at approximately the same rate as the wild type at the lowest light intensity tested (50 μmol photons m−2 s−1), the doubling time for the cpcM mutant was nearly sevenfold higher than that for the wild type at 260 μmol photons m−2 s−1, and the cpcM mutant did not grow at 390 μmol photons m−2 s−1 (Table 2). Thus, PBP methylation is clearly not essential for PBS assembly or for efficient light harvesting at low light intensities. However, the mutant strains with unmethylated PBP were much more sensitive to photoinhibition than the corresponding wild-type strains, and this effect was most pronounced for Synechocystis sp. strain PCC 6803.
The light sensitivity of the cpcM mutant strains was easily observed from the color of the liquid cultures grown at increasing light intensities (see Fig. S3 in the supplemental material). The Chl and PBP contents were determined for cells grown under different light conditions (Table 3). In general, for the wild-type Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803, an increase in the growth light intensity led to a reduction in the Chl and PBP contents of the cells, and the reduction was much greater for the PBP than for Chl. For Synechococcus sp. strain PCC 7002, a very high light intensity (1,200 μmol photons m−2 s−1) was required to produce a dramatic change in the pigmentation (Table 3; see Fig. S3 in the supplemental material) Compared to the respective wild-type strains, however, the cpcM mutants of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 exhibited obvious differences in pigmentation when cells were grown at high light intensities. This was most evident for the cpcM mutant of Synechocystis sp. strain PCC 6803. Compared to wild-type cells grown at 260 μmol photons m−2 s−1, cells of the cpcM mutant exhibited a 33% reduction in Chl content and a 50% reduction in PBP content.
TABLE 3.
Pigment contents of cells from liquid cultures of wild-type and cpcM mutants of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 grown at different light intensities
| Strain | Mean Chl a content (μg Chl OD unit−1 ml−1) ± SD at the following light intensity (μmol photons m−2 s−1):
|
Relative PBP content (%)a at the following light intensity (μmol photons m−2 s−1):
|
||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 140 | 260 | 390 | 50 | 140 | 260 | 390 | |
| PCC 7002 | ||||||||
| Wild type | 5.5 ± 0.3 | 5.2 ± 0.2 | 4.8 ± 0.1 | 4.7 ± 0.2 | 100 | 95 | 92 | 86 |
| cpcM mutant | 5.4 ± 0.2 | 5.3 ± 0.3 | 4.6 ± 0.2 | 4.3 ± 0.2 | 97 | 89 | 84 | 76 |
| PCC 6803 | ||||||||
| Wild type | 6.1 ± 0.3 | 5.7 ± 0.3 | 5.4 ± 0.1 | 5.1 ± 0.2 | 100 | 97 | 92 | 74 |
| cpcM mutant | 5.6 ± 0.3 | 4.8 ± 0.2 | 3.6 ± 0.3 | NMb | 92 | 76 | 50 | NM |
PBP content is reported as the percentage relative to the content of the respective wild-type cells grown at 50 μmol m−2 s−1. Standard errors were about ±3 to 5%.
NM, not measurable. Cells did not grow under this condition.
Energy transfer and state transitions.
The absolute conservation of Asn methylation of PBP β subunits implies that this posttranslational modification must play an important functional role(s). When cyanobacterial cells are incubated in blue light or darkness, the cells exhibit characteristic changes in the transfer of absorbed light energy from PBS to PS I and PS II, a change known as the state transition (17, 65). In dark-adapted cells (state 2), more light energy is transferred from PBS to PS I; however, when cells are preilluminated with blue light, light absorbed by PBS is preferentially transferred to PS II (state 1). This pattern is clearly observed for the wild-type Synechococcus sp. strain PCC 7002 (see Fig. S4A in the supplemental material) and Synechocystis sp. strain PCC 6803 (see Fig. S4C in the supplemental material). At 77 K, fluorescence emission from PC, AP, and the terminal emitters of the PBS occurs at about 650 nm, 660 nm, and 685 nm, respectively. Fluorescence emission from the Chls of PS II is observed at 685 nm and 695 nm and that from PS I at ∼717 nm for Synechococcus strain 7002 and ∼725 nm for Synechocystis sp. strain PCC 6803 (65). For both wild-type strains, increased fluorescence from PS II is observed after cells are pretreated with blue light, as expected. The cpcM mutants are still able to redirect light energy between PS I and PS II by the state transition mechanism (see Fig. S4B and S4C in the supplemental material). However, the fluorescence emission from PBP was significantly higher for both mutant strains, and this was especially true for Synechocystis sp. strain PCC 6803 cells in state 1 (see Fig. S4D in the supplemental material). For the cpcM mutant of Synechococcus sp. strain PCC 7002, energy transfer to PS II, as reflected by relatively low fluorescence emission at 695 nm, was impaired in both states 1 and 2. Since the PBP contents of the cells are lower in the cpcM mutants (Table 3), the fluorescence emission spectra indicated that the cpcM mutants have a much lower efficiency of light energy transfer from PBS to both PS I and PS II.
DISCUSSION
Distribution of CpcM and its relationship to other methyltransferases.
Although γ-N-methylasparagine was recently found in polytheonamides A and B, which are cytotoxic linear peptides from a marine sponge (24), to our knowledge the only proteins that have been shown to contain γ-methylasparagine are the β subunits of PBP. Archaeal proteins (13) and components of the transcription and translation apparatus in eukaryotes (41) do not appear to have this posttranslational modification. However, several proteins, including protein L3 of ribosomes and release factors (RF) 1 and 2 of E. coli contain δ-N-methylglutamine (10, 27, 32, 40, 50). PrmB methylates ribosomal protein L3 on a Gln residue within the sequence GSIGQNQTPGKVF (10, 27), and this sequence has no similarity to the sequence methylated by CpcM (LIAPGGNAYTNRR in CpcB of Synechococcus sp. strain PCC 7002; see Fig. S2 in the supplemental material). Interestingly, HemK (also known as PrmC) methylates the Gln residue in the sequence SGAGGQHVN, which occurs in both RF1 and RF2 and is similar to the motif surrounding Asn71/72 of PBP β subunits (27, 40, 50).
Cyanobacterial CpcM methyltransferases, which methylate the amide nitrogen of Asn71/72 of PBP beta subunits, contain a typical methyltransferase domain of the Rossman fold type (33, 37). Similar structurally conserved domains occur in many other S-adenosylmethionine-dependent methyltransferases, including those of the UbiE/COQ5/MenG, SmtA, and HemK/PrmC families (33, 36, 45, 49, 50). Sequence conservation suggests that CpcM probably shares mechanistic similarities with these other methyltransferases. However, when cyanobacterial CpcM proteins are compared with MenG and PrmC/HemK, only a very low level of sequence similarity is found for these three methyltransferases (Fig. 2). Although residues 32 to 350 of Synechococcus sp. strain PCC 7002 CpcM can be aligned with PrmC/HemK, these two proteins are only ∼14% identical and ∼27% similar in the region in which their amino acid sequences overlap. Compared to PrmC/HemK, CpcM has N-terminal and C-terminal extensions of 31 and 49 residues, respectively. The precise roles of these extensions are unknown, but the X-ray structure of PrmC/HemK shows that the N and C termini extend away from the protein core (50). Thus, it is likely that the CpcM and PrmC/HemK core structures are similar, and these enzymes may have similar catalytic mechanisms. Interestingly, when Asn72 of CpcB was replaced by Gln in Synechococcus sp. strain PCC 7002, some methylation of the Gln72 residue occurred (62). This observation suggests that CpcM is specific for the residue at position 72 but that its active site can accommodate the larger Gln side chain.
Sequenced cyanobacterial genomes carried only single copies of cpcM-like genes. Based upon the characterization of cpcM mutants from two unicellular cyanobacterial strains, Synechococcus sp. strain PCC 7002 (marine) and Synechocystis sp. strain PCC 6803 (freshwater), CpcM is the only enzyme that posttranslationally methylates Asn71/72 residues of CpcB, ApcB, and ApcF. Previous studies have shown that Asn72 is also methylated in the β subunits of PE and PEC (30). Since several of the organisms included in phylogenetic analyses shown in Fig. 2 synthesize PE or PEC but have only a single CpcM-type methyltransferase, it can be surmised that CpcM methylates Asn72 in the β subunits of PE (CpeB) and PEC (PecB) in those organisms. Thus, we conclude that CpcM can recognize and methylate the amide nitrogen of Asn71/72 in all PBP β-type subunits in any given cyanobacterium.
Functional role of Asn methylation of PBP.
Swanson and Glazer (58) previously characterized two nitrosoguanidine-induced mutants of Synechococcus sp. strain PCC 7942 that lacked detectable Asn methylation of PBP. They showed that unmethylated PBP had absorption spectra that were similar to those of the methylated proteins, were no more sensitive to thermal denaturation than methylated PBP, and, as observed here, were able to assemble PBS normally. However, similar to the results for whole cells of the cpcM null mutants described here (see Fig. S4 in the supplemental material), isolated PBS exhibited increased fluorescence emission from AP and PC. A decrease in the quantum yield of fluorescence emission in the methylation-defective mutant strains indicated that excitation energy losses were occurring through decay pathways without fluorescence emission in the mutants lacking methyltransferase activity. Swanson and Glazer (58) concluded that Asn methylation of PBP contributes significantly to the overall efficiency of directional energy transfer from PBS to PS I and PS II. It should be noted that those authors did not measure the growth rates of their mutants; moreover, those authors did not specify the light intensity under which their cells were grown. Thomas et al. (61) subsequently used these same mutants to demonstrate lower rates of electron transfer through PS II in vivo. Thomas et al. (62) later studied two site-specific PC mutants with Asp or Gln in place of the Asn located at β-72 in the cyanobacterium Synechococcus sp. strain PCC 7002. Spectroscopic measurements demonstrated that these substitutions affected both the ground-to-excited-state transition and the excited-state characteristics of the β-84 chromophore, and they concluded that γ-N-methylasparagine plays an important role in establishing an environment that minimizes nonradiative energy loss from the PCB chromophore bound at β-Cys84 (62).
In the cpcM null mutant strains described here, only a very slight increase in the doubling time was observed at low light intensity for Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803. This result, together with the observation that most of the PBP in the mutant strains were assembled into PBS, indicates that Asn71/72 methylation of PBP β subunits is not essential for PBS assembly in the two cyanobacteria studied here and that energy transfer losses, while measurable, did not impair growth at low light intensity. The results obtained for the two cpcM mutants differed somewhat at higher light intensities (Table 2). For Synechococcus sp. strain PCC 7002, the growth rate of the cpcM mutant was only slightly lower than that of the wild type as the light intensity increased. Although the Chl contents of the wild type and cpcM mutant were similar at different light intensities, the PBP content of the cpcM mutant was consistently 5 to 10% lower than that of the wild type. The lower PBP content in the mutant cells suggests that the absence of Asn72 methylation of PBP beta subunits decreases the stability of the PBP and leads to lower PBP levels in cells. However, the PBP contents of cells clearly decreased as the growth light intensity increased. This is obvious when one compares the cpcM mutant and wild-type cells after growth at very high light intensity (1,200 μmol photons m−2 s−1) (see Fig. S3A and S3B in the supplemental material).
The situation for Synechocystis sp. strain PCC 6803 was somewhat different. At low light intensities, the doubling time of the cpcM mutant was already longer than that of the wild type, and at higher light intensities, the cpcM mutant grew very slowly or did not grow at all (Table 2; see Fig. S3C and S3D in the supplemental material). The PBP content of the cpcM mutant was significantly lower than that of wild-type cells at all light intensities (Table 3). Thus, it appears that the lack of Asn methylation had a much greater effect on PBP levels in Synechocystis sp. strain PCC 6803, but again the degree of PBP destabilization increased dramatically as the light intensity increased. The molecular mechanism by which light might destabilize unmethylated PC or AP is not known at this time, but the effect presumably arises from structural changes that occur in the vicinity of the chromophores bound at Cys81 of ApcB and at Cys82 of CpcB. As shown in the X-ray structures of PBP (4, 56) (see Fig. S5 in the supplemental material), the γ-N-methyl-Asn72 residue lies immediately adjacent to the PCB chromophore that is attached at Cys82 of CpcB and Cys81 of ApcB. The β-81/82 PCB chromophores are more exposed in PC and AP of the cpcM mutant strains because of the absence of the γ-N-methyl group on Asn72 (1). It has been reported that PBP can sensitize the formation of reactive oxygen species (25, 26, 63) and that PBP are sensitive to bleaching by reactive oxygen species (21, 60, 63). In future studies we will determine whether PBP lacking methylation at Asn71/72 produce more reactive oxygen species, whether the PBP themselves are more sensitive to reactive oxygen species, or both. Because low growth light intensities were probably employed in previous studies, it should be noted that the photoinhibition and lower PBP contents of cpcM mutants were not previously reported. These effects are distinct from the inefficient energy transfer properties that were previously reported (58, 61, 62) and confirmed here.
In summary, using bioinformatics and reverse genetics approaches, we have identified the cpcM gene, encoding the methyltransferase that posttranslationally methylates Asn71/72 in CpcB (β-PC), ApcB (β-AP), and ApcF (β18) in two cyanobacteria. As demonstrated through biochemical characterization of cpcM mutants here and in vitro biochemical studies to be reported elsewhere (38a), CpcM methylates all apo-PBP β subunits. Characterization of cpcM mutants of Synechococcus sp. strain PCC 7002 and Synechocystis sp. strain PCC 6803 revealed that the inability to methylate Asn71/72 of PBP causes reduced energy transfer from PBS to PS I and PS II as well as sensitivity to high light intensity. Surprisingly, the methylation of Asn71/72 in PBP β subunits is a life-or-death issue for Synechocystis sp. strain PCC 6803 cells growing at even moderately high light intensity. The severe photoinhibition observed for cpcM mutants may explain why this posttranslational modification occurs almost universally in PBP β subunits.
Supplementary Material
Acknowledgments
This research was supported by National Science Foundation grants to W.M.S. (MCB-0133441) and to D.A.B. (MCB-0077586 and MCB-0519743). H.S.L. was supported by a grant from the Louisiana Board of Regents to W.M.S. (LEQSF(1999-2002)-RD-A-5). The W. M. Keck Foundation provided support for equipment utilized for this study and located in the Keck Conservation and Molecular Genetics lab at the University of New Orleans.
We gratefully acknowledge Eric Snyder and James R. Miller (The Pennsylvania State University) for assistance with the MS.
Footnotes
Published ahead of print on 9 May 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Adir, N., and N. Lerner. 2003. The crystal structure of a novel unmethylated form of C-phycocyanin, a possible connector between cores and rods in phycobilisomes. J. Biol. Chem. 27825926-25932. [DOI] [PubMed] [Google Scholar]
- 2.Ajlani, G., and C. Vernotte. 1998. Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol. Biol. 37577-580. [DOI] [PubMed] [Google Scholar]
- 3.Bald, D., J. Kruip, and M. Rögner. 1996. Supramolecular architecture of cyanobacterial thylakoid membranes: how is the phycobilisome connected with the photosystems? Photosynth. Res. 49103-118. [DOI] [PubMed] [Google Scholar]
- 4.Brejc, K., R. Ficner, R. Huber, and S. Steinbacher. 1995. Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution. J. Mol. Biol. 249424-440. [DOI] [PubMed] [Google Scholar]
- 5.Bryant, D. A. 1991. Cyanobacterial phycobilisomes: progress toward complete structural and functional analysis via molecular genetics, p. 257-300. In L. Bogorad and I. K. Vasil, (ed.) The photosynthetic apparatus: molecular biology and operation. Academic Press, New York, NY.
- 6.Bryant, D. A., R. de Lorimier, G. Guglielmi, and S. E. Stevens, Jr. 1990. Structural and compositional analyses of the phycobilisomes of Synechococcus sp. PCC 7002. Analyses of the wild-type strain and a phycocyanin-less mutant constructed by interposon mutagenesis. Arch. Microbiol. 153550-560. [DOI] [PubMed] [Google Scholar]
- 7.Bryant, D. A., G. Gugliemi, N. Tandeau de Marsac, A. M. Castets, and G. Cohen-Bazire. 1979. The structure of cyanobacterial phycobilisomes: a model. Arch. Microbiol. 123113-127. [Google Scholar]
- 8.Capuano, V., A. S. Braux, N. Tandeau de Marsac, and J. Houmard. 1991. The “anchor polypeptide” of cyanobacterial phycobilisomes. Molecular characterization of the Synechococcus sp. PCC 6301 apcE gene. J. Biol. Chem. 2667239-7247. [PubMed] [Google Scholar]
- 9.Capuano, V., J. C. Thomas, N. Tandeau de Marsac, and J. Houmard. 1993. An in vivo approach to define the role of the LCM, the key polypeptide of cyanobacterial phycobilisomes. J. Biol. Chem. 2688277-8283. [PubMed] [Google Scholar]
- 10.Clarke, S. 2002. The methylator meets the terminator. Proc. Natl. Acad. Sci. USA 991104-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lorimier, R., D. A. Bryant, and S. E. Stevens, Jr. 1990. Genetic analysis of a 9 kDa phycocyanin-associated linker polypeptide. Biochim. Biophys. Acta 101929-41. [DOI] [PubMed] [Google Scholar]
- 12.de Lorimier, R., G. Guglielmi, D. A. Bryant, and S. E. Stevens, Jr. 1990. Structure and mutation of a gene encoding a Mr 33,000 phycocyanin-associated linker polypeptide. Arch. Microbiol. 153541-549. [DOI] [PubMed] [Google Scholar]
- 13.Eichler, J., and M. W. W. Adams. 2005. Posttranslational protein modification in Archaea. Microbiol. Mol. Biol. Rev. 69393-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairchild, C. D., and A. N. Glazer. 1994. Oligomeric structure, enzyme kinetics, and substrate specificity of the phycocyanin alpha subunit phycocyanobilin lyase. J. Biol. Chem. 2698686-8694. [PubMed] [Google Scholar]
- 15.Fairchild, C. D., J. Zhao, J. Zhou, S. E. Colson, D. A. Bryant, and A. N. Glazer. 1992. Phycocyanin alpha-subunit phycocyanobilin lyase. Proc. Natl. Acad. Sci. USA 897017-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frigaard, N.-U., Y. Sakuragi, and D. A. Bryant. 2004. Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. Methods Mol. Biol. 274325-340. [DOI] [PubMed] [Google Scholar]
- 17.Fujita, Y., A. Murakami, and K. Aizawa. 1994. Short-term and long-term adaptation of the photosynthetic apparatus: homeostatic properties of thylakoids, p. 677-692. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Acadmic Publishers, Dordrecht, The Netherlands.
- 18.Gindt, Y. M., J. Zhou, D. A. Bryant, and K. Sauer. 1992. Core mutations of Synechococcus sp. PCC 7002 phycobilisomes: a spectroscopic study. J. Photochem. Photobiol. 1575-89. [DOI] [PubMed] [Google Scholar]
- 19.Gindt, Y. M., J. Zhou, D. A. Bryant, and K. Sauer. 1994. Spectroscopic studies of phycobilisome subcore preparations lacking key core chromophores: assignment of excited state energies to the Lcm, beta 18 and alpha AP-B chromophores. Biochim. Biophys. Acta 1186153-162. [DOI] [PubMed] [Google Scholar]
- 20.Glazer, A. N. 1989. Light guides. Directional energy transfer in a photosynthetic antenna. J. Biol. Chem. 2641-4. [PubMed] [Google Scholar]
- 21.Glazer, A. N. 1994. Phycobiliproteins: a family of valuable, widely used fluorophores. J. Appl. Phycol. 6105-112. [Google Scholar]
- 22.Glazer, A. N., D. L. Lundell, G. Yamanka, and R. C. Williams. 1983. The structure of a ‘simple’ phycobilisome. Ann. Microbiol. 134B159-180. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Lojero, C., B. Perez-Gomez, G. Shen, W. M. Schluchter, and D. A. Bryant. 2003. Interaction of ferredoxin:NADP+ oxidoreductase with phycobilisomes and phycobilisome substructures of Synechococcus sp. strain PCC 7002. Biochemistry 4213800-13811. [DOI] [PubMed] [Google Scholar]
- 24.Hamada, T., S. Matsunaga, G. Yano, and N. Fusetani. 2005. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J. Am. Chem. Soc. 127110-118. [DOI] [PubMed] [Google Scholar]
- 25.He, J.-A., Y.-Z. Hu, and L.-J. Jiang. 1996. Photochemistry of phycobiliproteins: first observation of reactive oxygen species generated from phycobiliproteins on photosensitization. J. Am. Chem. Soc. 1188957-8958. [Google Scholar]
- 26.He, J.-A., Y.-Z. Hu, and L.-J. Jiang. 1997. Photodynamic action of phycobiliproteins: in situ generation of reactive oxygen species. Biochim. Biophys. Acta 1320165-174. [Google Scholar]
- 27.Heurgué-Hamard, V., S. Champ, A. Engström, M. Ehrenberg, and R. H. Buckingham. 2002. The hemK gene in Escherichia coli encodes the N(5)-glutamine methyltransferase that modifies peptide release factors. EMBO J. 21769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko, T., Nakamura, Y. C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8205-213. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. PCC6803. II. Sequence determination of the entire genome and assignment of potential protein coding regions. DNA Res. 3109-136. [DOI] [PubMed] [Google Scholar]
- 30.Klotz, A. V., and A. N. Glazer. 1987. Gamma-N-methylasparagine in phycobiliproteins. Occurrence, location and biosynthesis. J. Biol. Chem. 26217350-17355. [PubMed] [Google Scholar]
- 31.Klotz, A. V., J. A. Leary, and A. N. Glazer. 1986. Post-translational methylation of asparaginyl residues. Identification of β-71 γ-N-methylasparagine in allophycocyanin. J. Biol. Chem. 26115891-15894. [PubMed] [Google Scholar]
- 32.Klotz, A. V., B. A. Thomas, A. N. Glazer, and R. W. Blacher. 1990. Detection of methylated asparagines and glutamine residues in polypeptides. Anal. Biochem. 1895-100. [DOI] [PubMed] [Google Scholar]
- 33.Kozbial, P. Z., and A. R. Mushegian. 2005. Natural history of S-adenosylmethionine-binding proteins. BMC Struct. Biol. 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtenthaler, H. K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148350-382. [Google Scholar]
- 35.MacKinney, G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140315-322. [Google Scholar]
- 36.Marchler-Bauer, A., J. B. Anderson, M. K. Derbyshire, C. DeWeese-Scott, N. R. Gonzales, M. Gwadz, L. Hao, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, D. Krylov, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, S. Lu, G. H. Marchler, M. Mullokandov, J. S. Song, N. Thanki, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2007. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35D237-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, J. L., and F. M. McMillan. 2002. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr. Opin. Struct. Biol. 12783-793. [DOI] [PubMed] [Google Scholar]
- 38.Maxson, P., K. Sauer, J. Zhou, D. A. Bryant, and A. N. Glazer. 1989. Spectroscopic studies of cyanobacterial phycobilisomes lacking core polypeptides. Biochim. Biophys. Acta 97740-51. [DOI] [PubMed] [Google Scholar]
- 38a.Miller, C. A., H. S. Leonard, I. G. Pinsky, B. M. Turner, S. R. Williams, L. Harrison, Jr., A. F. Fletcher, G. Shen, D. A. Bryant, and W. M. Schlucter. 15 May 2008, posting date. Biogenesis of phycobiliproteins. III. CpcM is the asparagine methyltransferase for phycobiliprotein β subunits in cyanobacteria. J. Biol. Chem. doi: 10.1074/jbc.M802734200. [DOI] [PubMed]
- 39.Minami, Y., F. Yamada, T. Hase, H. Matsubara, A. Murakami, Y. Fujita, T. Takao, and Y. Shimonishi. 1985. Amino acid sequences of allophycocyanin α- and β-subunits isolated from Anabaena cylindrica: presence of an unknown derivative of aspartic acid in the β-subunit. FEBS Lett. 191216-220. [Google Scholar]
- 40.Nakahigashi, K., H. Kubo, S. Narita, T. Shimaoka, S. Goto, T. Oshima, H. Mori, M. Maeda, C. Wada, and H. Inokuchi. 2002. HemK, a class of protein methyl transferase with similarity to DNA methyl transferases, methylates polypeptide chain release factors, and hemK knockout induces defects in translational termination. Proc. Natl. Acad. Sci. USA 991473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polevoda, B., and F. Sherman. 2007. Methylation of proteins involved in translation. Mol. Microbiol. 65590-606. [DOI] [PubMed] [Google Scholar]
- 42.Raps, S. 1990. Differentiation between phycobiliprotein and colorless linker polypeptides by fluorescence in the presence of ZnSO4. Plant Physiol. 92358-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rümbeli, R., F. Suter, M. Wirth, W. Sidler, and H. Zuber. 1987. Isolation and localization of N4-methylasparagine in phycobiliproteins from the cyanobacterium Mastigocladus laminosus. Biol. Chem. Hoppe-Seyler 3681401-1406. [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto, T., and D. A. Bryant. 1998. Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. PCC 7002. Arch. Microbiol. 16910-19. [DOI] [PubMed] [Google Scholar]
- 45.Sakuragi, Y., B. Zybailov, G. Shen, A. D. Jones, P. R. Chitnis, A. van der Est, R. Bittl, S. Zech, D. Stehlik, J. H. Golbeck, and D. A. Bryant. 2002. Insertional inactivation of the menG gene, encoding 2-phytyl-1,4-naphthoquinone methyltransferase of Synechocystis sp. PCC 6803, results in the incorporation of 2-phytyl-1,4-naphthoquinone into the A1 site and alteration of the equilibrium constant between A1 and FX in photosystem I. Biochemistry 41394-405. [DOI] [PubMed] [Google Scholar]
- 46.Sauneé, N. A., S. R. Williams, D. A. Bryant, and W. M. Schluchter. 2008. Biogenesis of phycobiliproteins. II. CpcS-I and CpcU comprise the heterodimeric bilin lyase that attaches phycocyanobilin to Cys-82 of beta-phycocyanin and Cys-81 of allophycocyanin subunits in Synechococcus sp. PCC 7002. J. Biol. Chem. 2837513-7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheer, H., and K. H. Zhao. 2008. Biliprotein maturation: the chromophore attachment. Mol. Microbiol. 68263-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schluchter, W. M., and D. A. Bryant. 1992. Molecular characterization of ferredoxin-NADP+ reductase in cyanobacteria: cloning and sequence of the petH gene of Synechococcus sp. PCC 7002 and studies on the gene product. Biochemistry 313092-3102. [DOI] [PubMed] [Google Scholar]
- 49.Schubert, H. L., R. M. Blumenthal, and X. Cheng. 2003. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem. Sci. 28329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert, H. L., J. D. Phillips, and C. P. Hill. 2003. Structures along the catalytic pathway of PrmC/HemK, an N5-glutamine AdoMet-dependent methyltransferase. Biochemistry 425592-5599. [DOI] [PubMed] [Google Scholar]
- 51.Shen, G., S. Boussiba, and W. F. J. Vermaas. 1993. Synechocystis sp. PCC 6803 strains lacking photosystem I and phycobilisome function. Plant Cell 51853-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen, G., and D. A. Bryant. 1995. Characterization of a Synechococcus sp. strain PCC 7002 mutant lacking photosystem I. Protein assembly and energy distribution in the absence of the photosystem I reaction center core complex. Photosynth. Res. 4141-53. [DOI] [PubMed] [Google Scholar]
- 53.Shen, G., N. A. Saunée, S. R. Williams, E. F. Gallo, W. M. Schluchter, and D. A. Bryant. 2006. Identification and characterization of a new class of bilin lyase: the cpcT gene encodes a bilin lyase responsible for attachment of phycocyanobilin to Cys-153 on the β-subunit of phycocyanin in Synechococcus sp. PCC 7002. J. Biol. Chem. 28117768-17778. [DOI] [PubMed] [Google Scholar]
- 54.Shen, G., W. M. Schluchter, and D. A. Bryant. 2008. Biogenesis of phycobiliproteins. I. cpcS-I and cpcU mutants of the cyanobacterium Synechococcus sp. PCC 7002 define a heterodimeric phycocyanobilin lyase specific for beta-phycocyanin and allophycocyanin subunits. J. Biol. Chem. 2837503-7512. [DOI] [PubMed] [Google Scholar]
- 55.Sidler, W. A. 1994. Phycobilisome and phycobiliprotein structure, p. 139-216. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 56.Stec, B., R. F. Troxler, and M. M. Teeter. 1999. Crystal structure of C-phycocyanin from Cyanidium caldarium provides a new perspective on phycobilisome assembly. Biophys. J. 762912-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens, S. E., Jr, and R. D. Porter. 1980. Transformation in Agmenellum quadruplicatum. Proc. Natl. Acad. Sci. USA 776052-6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanson, R. V., and A. N. Glazer. 1990. Phycobiliprotein methylation: effect of the γ-N-methylasparagine residue on energy transfer in phycocyanin and the phycobilisome. J. Mol. Biol. 214787-796. [DOI] [PubMed] [Google Scholar]
- 59.Swanson, R. V., J. Zhou, J. A. Leary, T. Williams, R. de Lorimier, D. A. Bryant, and A. N. Glazer. 1992. Characterization of phycocyanin produced by cpcE and cpcF mutants and identification of an intergenic suppressor of the defect in bilin attachment. J. Biol. Chem. 26716146-16154. [PubMed] [Google Scholar]
- 60.Tapia, G., A. Galetovic, E. Lemp, E. Pino, and E. Lissi. 1999. Singlet oxygen-mediated photobleaching of the prosthetic group in hemoglobins and C-phycocyanin. Photochem. Photobiol. 70499-504. [PubMed] [Google Scholar]
- 61.Thomas, B. A., T. M. Bricker, and A. V. Klotz. 1993. Postranslational methylation of phycobilisomes and oxygen evolution efficiency in cyanobacteria. Biochim. Biophys. Acta 1143104-108. [Google Scholar]
- 62.Thomas, B. A., L. McMahon, and A. V. Klotz. 1995. N5-methylasparagine and energy-transfer efficiency in C-phycocyanin. Biochemistry 343758-3770. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, S. P., J. Q. Zhao, and L. J. Jiang. 2000. Photosensitized formation of singlet oxygen by phycobiliproteins in neutral aqueous solutions. Free Rad. Res. 33489-498. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, J., and J. J. Brand. 1989. Specific bleaching of phycobiliproteins from cyanobacteria and red algae at high temperature in vivo. Arch. Microbiol. 152447-452. [Google Scholar]
- 65.Zhao, J., G. Shen, and D. A. Bryant. 2001. Photosystem stoichiometry and state transitions in a mutant of the cyanobacterium Synechococcus sp. PCC 7002 lacking phycocyanin. Biochim. Biophys. Acta 1505248-257. [DOI] [PubMed] [Google Scholar]
- 66.Zhao, K. H., P. Su, J. M. Tu, X. Wang, H. Liu, M. Plöscher, L. Eichacker, C. Bubenzer, H. Scheer, X. Wang, and M. Zhou. 2007. Lyase activities of CpcS and CpcT-like proteins from Nostoc PCC7120 and sequential reconstitution of binding sites of phycoerythrocyanin and phycocyanin beta-subunits. J. Biol. Chem. 28234093-34103. [DOI] [PubMed] [Google Scholar]
- 67.Zhao, K. H., P. Su, J. M. Tu, X. Wang, H. Liu, M. Plöscher, L. Eichacker, B. Yang, M. Zhou, and H. Scheer. 2007. Phycobilin:cysteine-84 biliprotein lyase, a near-universal lyase for cysteine-84-binding sites in cyanobacterial phycobiliproteins. Proc. Natl. Acad. Sci. USA 10414300-14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou, J., G. E. Gasparich, V. L. Stirewalt, R. de Lorimier, and D. A. Bryant. 1992. The cpcE and cpcF genes of Synechococcus sp. PCC 7002. Construction and phenotypic characterization of interposon mutants. J. Biol. Chem. 26716138-16145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.