Abstract
We have functionally produced the outer membrane cytochrome OmcA from Shewanella oneidensis in Escherichia coli. Substrate accessibility experiments indicate that OmcA is surface exposed in an E. coli B strain but not in a K-12 strain. We show that a functional type II secretion system is required for surface localization.
Under anaerobic growth conditions, Shewanella oneidensis MR-1 can use iron(III) as a terminal electron acceptor (16, 20, 27). Iron respiration in this organism requires electron transfer components spanning the cell envelope from the external surface of the outer membrane to the quinone pool in the cytoplasmic membrane. The mtrD-mtrE-mtrF-omcA-mtrC-mtrA-mtrB gene cluster encodes components necessary for dissimilatory metal reduction, and it is likely that at least some of these have overlapping functions (26, 27). One route to dissecting the pathway of electron flow is to establish the pathway in a heterologous host (22). To this end, we have expressed the omcA gene from S. oneidensis MR-1 in Escherichia coli.
OmcA is a probable decaheme c-type cytochrome which is anchored at the exterior face of the Shewanella outer membrane via an N-terminal phospholipid modification (19). The type II protein export machinery has been implicated in the export of OmcA and the related outer membrane c-type cytochome, MtrC, across the outer membrane (5, 10, 12). To express omcA in E. coli, we cloned the gene into pUNI-PROM (14), a medium-copy-number vector in which the expression of omcA is driven by the constitutive tat promoter and tatA ribosome binding site (13). To maximize the likelihood of heme insertion into heterologously produced OmcA, we coexpressed the E. coli cytochrome c maturation genes from plasmid pEC86 (1).
As shown in Fig. 1A, Western blot analysis clearly detected the presence of OmcA in whole cells of E. coli laboratory strains MC4100 (a K-12 derivative [3]) and BL21 (an E. coli B strain) when the strains were transformed with pOmcA and pEC86. Analysis of a number of Western blots did not reveal any reproducible differences in the amounts of OmcA produced in the two strains. Subcellular fractionation revealed that OmcA was present in the inner and outer membrane fractions of both strains, with no antigen detectable in the soluble protein fraction. Since a significant amount of OmcA was clearly detected in the membranes of both strains, we next examined the absorption spectra of whole cells of MC4100 and BL21 expressing omcA.
FIG. 1.
Localization of OmcA in E. coli. (A) OmcA is found in the inner and outer membranes after heterologous production in E. coli. Four-hundred-milliliter cultures of strains MC4100 (F−ΔlacU169 araD139 rpsL150 relA1 ptsF rbs flbB5301), BL21 [F− ompT hsdSB(rB− mB−) dcm gal λ(DE3)], and JDW1 (as BL21, ΔgspD::Apra), transformed with pEC86 and pOmcA grown aerobically in LB medium supplemented with 20 μg/ml chloramphenicol and 125 μg/ml ampicillin, were harvested and fractionated to give soluble and membrane fractions, and the inner and outer membranes were separated by sucrose density gradient centrifugation (21). Whole cells (WC) (equivalent to an optical density at 600 nm of 0.05 of cell suspension), soluble fractions (Sol) (250 μg protein), inner membranes (IM) (10 μg protein), and outer membranes (OM) (2 μg protein) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (15), electroblotted (28), and probed with anti-OmcA antiserum (1:5,000 dilution). (B) OmcA produced in E. coli is released into outer membrane vesicles (OMVs). Cells of MC4100 (MC), BL21 (BL), and JWD1 producing the OmcA and Ccm proteins were washed with a high-salt buffer (100 mM Tris-HCl, pH 7.4, 500 mM KCl, 40% sucrose, supplemented with Roche complete EDTA-free protease inhibitor). The wash fraction was ultracentrifuged and the pellet resuspended, separated by SDS-PAGE, and blotted with antiserum to OmcA (left), β-lactamase (Bla; Sigma) (left), or TatA (right) (25). (C) Production of OmcA in E. coli leads to enhanced outer membrane vesicle release. Twenty-five-milliliter cultures of MC4100 or BL21 harboring either pEC86 plus pUNI-PROM or pEC86 plus pOmcA were centrifuged twice and filtered through a 0.45-μm filter to remove all cells, after which vesicular material was pelleted by ultracentrifugation, washed in phosphate-buffered saline (24), and repelleted as described previously (18). (Left) The vesicular material was resuspended in 50 μl of the same buffer, and the optical density of the suspension at 280 nm was determined. Error bars represent standard errors of the means (n = 3). (Right) Sixteen microliters of each of these samples was subjected to SDS-PAGE and blotted with antiserum to OmpA.
For visible spectroscopy analysis, cell suspensions of each strain coexpressing the omcA and ccm genes were incubated in sealed 1-ml cuvettes and the headspace was sparged with nitrogen. From spectra collected from the turbid cell samples, it was possible to resolve clearly the Soret absorption band of cytochromes, with a maximum wavelength (λmax) of ∼410 nm, for both MC4100 (Fig. 2A and B) and BL21 (Fig. 3A and B). As shown in Fig. 2 and 3, for each strain, reduction of the cytochrome pool by the addition of sodium dithionite (10 μl of a 0.2% solution) resulted in a red spectral shift of this band (λmax of ∼420 nm). An increase in absorbance at ∼552 nm was also observed upon the addition of dithionite. This peak is characteristic of the reduced α-band of c-type cytochromes, and its appearance confirms that these cytochromes dominate the dithionite-reducible cytochrome pool of E. coli MC4100(pOmcA) and E. coli BL21(pOmcA).
FIG. 2.
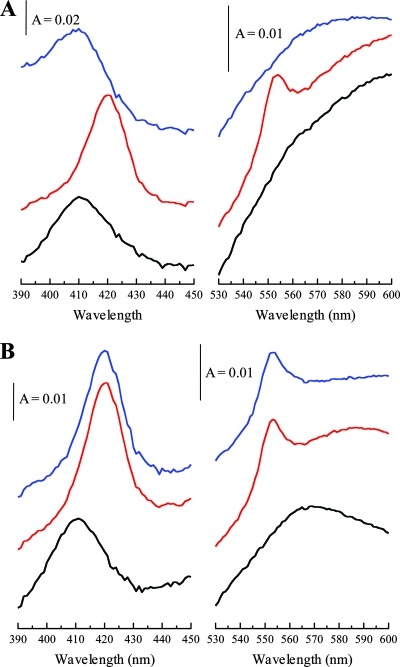
Whole-cell spectroscopy of E. coli MC4100 producing OmcA. (A) Treatment with Fe(III)-NTA. (B) Treatment with insoluble Fe(III) oxide. Black spectra, air oxidized; red spectra, dithionite reduced; blue spectra, 5 min after treatment with Fe(III)-NTA (A) or Fe(III) oxide (B). The Soret (γ) region is shown between 390 and 450 nm, and the α-band region is shown between 530 and 600 nm. The sloping baselines are a result of the turbid whole-cell suspensions used. The cell concentration was ∼0.15 mg ml−1. A, absorbance.
FIG. 3.
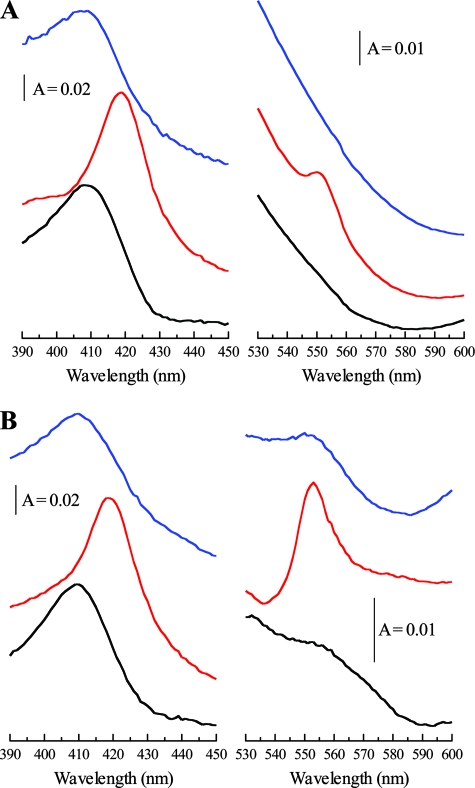
Whole-cell spectroscopy of E. coli BL21 producing OmcA. (A) Treatment with Fe(III)-NTA. (B) Treatment with insoluble Fe(III) oxide. Black spectra, air oxidized; red spectra, dithionite reduced; blue spectra, 5 min after treatment with Fe(III)-NTA (A) or Fe(III) oxide (B). The Soret (γ) region is shown between 390 and 450 nm, and the α-band region is shown between 530 and 600 nm. The sloping baselines are a result of the turbid whole-cell suspensions used. The cell concentration was ∼0.15 mg ml−1. A, absorbance.
OmcA is implicated in the reduction of Fe(III), and the purified protein has been shown to reduce soluble Fe(III) in the form of Fe(III)-nitrilotriacetic acid (NTA) (23, 26). The addition of the soluble Fe(III)-NTA complex to cell suspensions of either MC4100(pOmcA) (Fig. 2A) or BL21(pOmcA) (Fig. 3A) resulted in the reoxidation of this c-type cytochrome pool, as judged by the disappearance of the 552-nm band and the shift of the λmax of the Soret band from 420 nm back to 410 nm. This demonstrated that, in both strains, the OmcA hemes were accessible to soluble Fe(III) and indicates that OmcA is functional for substrate reduction when it is heterologously produced in E. coli.
Interestingly, however, the reoxidation states of the c-type cytochrome pool following the addition of insoluble amorphous iron (hydr)oxide to the reduced cell suspensions were quite different in the two strains. In the case of E. coli MC4100(pOmcA), no reoxidation of the c-type cytochrome pool was observed, suggesting that the hemes were not accessible to the insoluble oxidant (Fig. 2B). By contrast, in the case of E. coli BL21(pOmcA), the addition of insoluble amorphous iron (hydr)oxide fully reoxidized the c-type cytochrome pool over a timescale similar to that observed with soluble Fe(III)-NTA (Fig. 3B). These results strongly suggest that for E. coli BL21(pOmcA), the OmcA hemes are accessible to the insoluble Fe(III) oxidant and therefore must be located on the outside of the cell. By contrast, for E. coli MC4100(pOmcA), the observations are consistent with the likelihood that the OmcA hemes are located on the inside of the cell, i.e., on the inner leaflet of the outer membrane. This latter observation would be entirely in keeping with previous studies which have shown that although E. coli K-12 strains carry genes for a type II secretion system, these genes are cryptic in the MC4100 strain and are not expressed under any laboratory growth conditions studied thus far (8, 9). An alternative explanation might be that the surface properties of the outer membrane differ between the two strains, differentially preventing access to the insoluble oxidant for the MC4100 strain only.
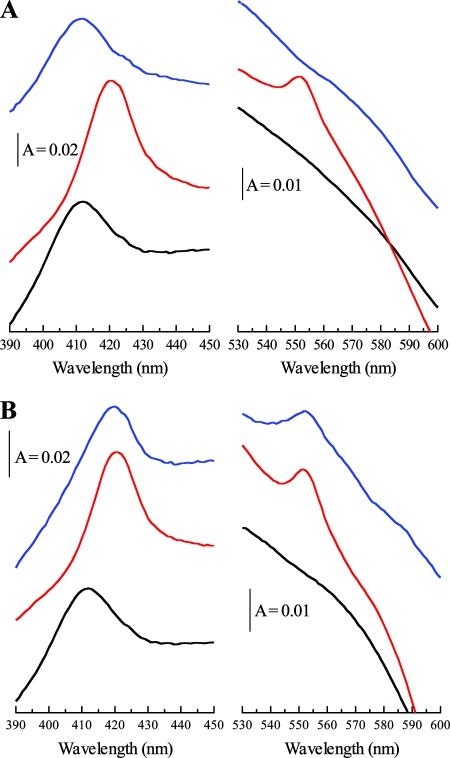
In order to test whether the type II secretion machinery was required for the surface exposure of the OmcA hemes in BL21, we constructed an in-frame, polar deletion in the gspD gene by insertion of the apramycin resistance cassette from plasmid pIJ773 (11) according to the method of Datsenko and Wanner (4). We cotransformed the resultant strain, JWD1, with pOmcA and pEC86. Western blot analysis of the gspD strain (Fig. 1A) showed clearly detectable OmcA that was found in both the inner and outer membrane fractions, as was the case for the gspD+ strain. Whole-cell visible spectroscopy of the BL21 gspD strain, shown in Fig. 4A, indicated that, as with the parent strain carrying omcA, the c-type cytochrome pool was oxidizable by soluble Fe(III)-NTA in intact cells. However, the inactivation of gspD resulted in an inability of the c-type cytochrome pool to be reoxidized by insoluble Fe(III) (hydr)oxide (Fig. 4B), indicating that the hemes of OmcA were no longer surface exposed. These data strongly suggest that the type II secretion system present in E. coli BL21 is able to recognize and transport the OmcA heme domain to the surface of the cell. This observation is quite unexpected since type II-dependent secretion of an exoprotein expressed in a heterologous host is rarely observed (2, 7). It is likely that the OmcA we detect in the inner membrane in each of our strains (Fig. 1A) reflects a transit stage in the export of OmcA to the outer membrane. It should be noted that although inner membrane localization of outer membrane cytochromes is not normally observed in Shewanella, it can be detected upon inactivation of the type II secretion system, consistent with the idea that it represents a transit form of the protein (5).
FIG. 4.
Whole-cell spectroscopy of a gspD mutant derivative of E. coli BL21 producing OmcA. (A) Treatment with Fe(III)-NTA. (B) Treatment with insoluble Fe(III) oxide. Black spectra, air oxidized; red spectra, dithionite reduced; blue spectra, 5 min after treatment with Fe(III)-NTA (A) or Fe(III) oxide (B). The Soret (γ) region is shown between 390 and 450 nm, and the α-band region is shown between 530 and 600 nm. The sloping baselines are a result of the turbid whole-cell suspensions used. The cell concentration was ∼0.15 mg ml−1. A, absorbance.
It has previously been reported that incubation of intact cells of Shewanella in high-salt buffer resulted in the release of OmcA into the wash buffer, which was taken as an indication that the protein was attached to the exterior face of the outer membrane (6). When we carried out similar experiments with heterologously produced OmcA, we also observed release of the protein from intact cells of E. coli expressing omcA into the wash fraction. However, OmcA release occurred regardless of the strain used for the experiments, and since the accessibility experiments described above have already demonstrated that the catalytic domain of the protein is not surface exposed in either MC4100 or JWD1, we reasoned that the OmcA that apparently washed off from the cell surface could not result from the release of surface-exposed protein in this case. Instead, as shown in Fig. 1B, ultracentrifugation of the cell washes from each strain resulted in pelleting of the OmcA material, suggesting that the protein was associated with a particulate fraction. Western blotting analysis of the particulate material showed that the periplasmic protein β-lactamase was also present (Fig. 1B), as was the outer membrane protein OmpA (Fig. 1C), but that the inner membrane protein TatA was not (Fig. 1B). Thus, we conclude that the washing step releases sealed outer membrane vesicles containing OmcA from the surface of E. coli cells.
The release of outer membrane vesicles in gram-negative bacteria is a general stress response that correlates with the accumulation of protein in the cell envelope (18). To determine whether heterologous production of OmcA was giving rise to the production of outer membrane vesicles in E. coli strains, we compared the quantities of outer membrane vesicles (determined by measuring the absorbance at 280 nm of the resuspended outer membrane vesicle fraction according to the methods described in references 17 and 18) shed from strains MC4100 and BL21 harboring pEC86 and either pOmcA or pUNI-PROM as our negative control. As shown in Fig. 1C, heterologous production of OmcA in either E. coli strain clearly resulted in a notable increase in the shedding of outer membrane vesicles, possibly as a stress response induced by the increased amount of lipoprotein anchored in the outer membrane.
In summary, in this report we have demonstrated the functional production and correct localization of the S. oneidensis decaheme cytochrome OmcA in E. coli. Our results represent a key step toward reconstituting the type II secretion-dependent pathway for dissimilatory metal reduction in a heterologous host.
Acknowledgments
We thank Matt Marshall, Liang Shi, and Alex Balieav, Pacific North National Laboratories (PNNL), Richland, WA, for the gift of anti-OmcA antiserum and for helpful discussions and Roland Freudl for the gift of OmpA antiserum. Tony Pugsley and Olivera Francetic are thanked for helpful advice, and Brian Jepson is thanked for his help in collecting absorbance spectra. D.J.R. thanks Jim Fredrickson and John Zachara (PNNL) for coordinating this activity and for stimulating discussions.
This work is supported by an MRC Senior Non-Clinical Fellowship to T.P. The BBSRC is acknowledged for providing a studentship to J.W.D. D.J.R. also thanks the U.S. DOE for financial support through the Biogeochemistry Grand Challenge.
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Arslan, E., H. Schulz, R. Zufferey, P. Kunzler, and L. Thöny-Meyer. 1998. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251744-747. [DOI] [PubMed] [Google Scholar]
- 2.Bouley, J., G. Condemine, and V. E. Shevchik. 2001. The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II Out pathway of Erwinia chrysanthemi. J. Mol. Biol. 308205-219. [DOI] [PubMed] [Google Scholar]
- 3.Casadaban, M. J., and S. N. Cohen. 1979. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc. Natl. Acad. Sci. USA 764530-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiChristina, T. J., C. M. Moore, and C. A. Haller. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J. Bacteriol. 184142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field, S. J., P. S. Dobbin, M. R. Cheesman, N. J. Watmough, A. J. Thomson, and D. J. Richardson. 2000. Purification and magneto-optical spectroscopic characterization of cytoplasmic membrane and outer membrane multiheme c-type cytochromes from Shewanella frigidimarina NCIMB400. J. Biol. Chem. 2758515-8522. [DOI] [PubMed] [Google Scholar]
- 7.Filloux, A. 2004. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694163-719. [DOI] [PubMed] [Google Scholar]
- 8.Francetic, O., D. Belin, C. Badaut, and A. P. Pugsley. 2000. Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J. 196697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francetic, O., and A. P. Pugsley. 1996. The cryptic general secretory pathway (gsp) operon of Escherichia coli K-12 encodes functional proteins. J. Bacteriol. 1783544-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorby, Y. A., S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 10311358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 1001541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartshorne, R. S., B. N. Jepson, T. Clarke, S. J. Field, J. Fredrickson, J. Zachara, L. Shi, J. N. Butt, and D. J. Richardson. 2007. Characterization of Shewanella oneidensis MtrC: a cell surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J. Inorg. Biol. Chem. 121083-1094. [DOI] [PubMed] [Google Scholar]
- 13.Jack, R. L., F. Sargent, B. C. Berks, G. Sawers, and T. Palmer. 2001. Constitutive expression of Escherichia coli tat genes indicates an important role for the twin-arginine translocase during aerobic and anaerobic growth. J. Bacteriol. 1831801-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack, R. L., G. Buchanan, A. Dubini, K. Hatzixanthis, T. Palmer, and F. Sargent. 2004. Coordinating assembly and export of complex bacterial proteins. EMBO J. 233962-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49219-286. [DOI] [PubMed] [Google Scholar]
- 17.Mashburn, L. M., and M. Whiteley. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437422-425. [DOI] [PubMed] [Google Scholar]
- 18.McBroom, A. J., and M. J. Kuehn. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63545-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers, C. R., and J. M. Myers. 2004. The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett. Appl. Microbiol. 39466-470. [DOI] [PubMed] [Google Scholar]
- 20.Nealson, K. H., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48311-433. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido, H. 1994. Isolation of outer membranes. Methods Enzymol. 235225-234. [DOI] [PubMed] [Google Scholar]
- 22.Pitts, K. E., P. S. Dobbin, F. Reyes-Ramirez, A. J. Thomson, D. J. Richardson, and H. E. Seward. 2003. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA: expression in Escherichia coli confers the ability to reduce soluble Fe(III) chelates. J. Biol. Chem. 27827758-27765. [DOI] [PubMed] [Google Scholar]
- 23.Ross, D. E., S. S. Ruebush, S. L. Brantley, R. S. Hartshorne, T. A. Clarke, D. J. Richardson, and M. Tien. 2007. Characterization of protein-protein interactions involved in iron reduction by Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 735797-5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell, D. W., and J. Sambrook. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Sargent, F., U. Gohlke, E. de Leeuw, N. R. Stanley, T. Palmer, H. R. Saibil, and B. C. Berks. 2001. Purified components of the Escherichia coli Tat protein transport system form a double-layered ring structure. Eur. J. Biochem. 2683361-3367. [DOI] [PubMed] [Google Scholar]
- 26.Shi, L., B. Chen, Z. Wang, D. A. Elias, M. U. Mayer, Y. A. Gorby, S. Ni, B. H. Lower, D. W. Kennedy, D. S. Wunschel, H. Z. Mottaz, M. J. Marshall, E. A. Hill, A. S. Beliaev, J. M. Zachara, J. K. Fredrickson, and T. C. Squier. 2006. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J. Bacteriol. 1884705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi, L., T. C. Squier, J. M. Zachara, and J. K. Fredrickson. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol. Microbiol. 6512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]