Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) genomes are tethered to the host chromosomes and partitioned faithfully into daughter cells with the host chromosomes. The latency-associated nuclear antigen (LANA) is important for segregation of the newly synthesized viral genomes to the daughter nuclei. Here, we report that the nuclear mitotic apparatus protein (NuMA) and LANA can associate in KSHV-infected cells. In synchronized cells, NuMA and LANA are colocalized in interphase cells and separate during mitosis at the beginning of prophase, reassociating again at the end of telophase and cytokinesis. Silencing of NuMA expression by small interfering RNA and expression of LGN and a dominant-negative of dynactin (P150-CC1), which disrupts the association of NuMA with microtubules, resulted in the loss of KSHV terminal-repeat plasmids containing the major latent origin. Thus, NuMA is required for persistence of the KSHV episomes in daughter cells. This interaction between NuMA and LANA is critical for segregation and maintenance of the KSHV episomes through a temporally controlled mechanism of binding and release during specific phases of mitosis.
Kaposi's sarcoma-associated herpesvirus (KSHV) is a gammaherpesvirus that is associated with Kaposi's sarcoma, body cavity-based lymphomas (BCBL), and multicentric Castleman's disease (48). During latent infection, in which no progeny virus is produced, KSHV genomes are maintained as multiple episomes in the host cells. In a lifelong latent state, KSHV genomic DNA exists as a closed circular episome, similar to the host chromosomal DNA, packaged onto the nucleosomes with cellular histones (11, 49, 54). KSHV genomes are replicated once per cell cycle and are partitioned faithfully into daughter cells, along with host chromosomes, during mitosis (29, 62, 63). The association of KSHV DNA with host chromosomes was shown to be mediated by the latency-associated nuclear antigen (LANA), which acts as a linker to tether viral DNA onto to the host chromosomes (4, 11, 64).
LANA, encoded by open reading frame 73 (ORF73), is consistently expressed in Kaposi's sarcoma lesions and is crucial for episome maintenance in proliferating cells (4, 36). LANA, a large nuclear protein (222 to 234 kDa) with a multifunctional role, is critical for episome maintenance and oncogenesis mediated by KSHV (64). LANA has three distinct domains: a proline-rich N-terminal region, which is important for its binding with host chromosomes; a long glutamic acid-rich internal repeat domain; and a carboxy-terminal domain (64). LANA has been shown to modulate cellular transcription by altering various transcription factors, such as transcription factor 4/cyclic AMP response element binding protein 2, mSin3A, CBP, RING3, and GSK-3b (37, 39, 64); can repress the transcription activity of p53 (2, 19); and induces chromosomal instability (55). In addition, LANA was found to interact with pRb and concurrently stimulates transcription from the cyclin E promoter (51). Furthermore, it is also important for maintenance of latency by repressing the transcriptional activity of Rta, a KSHV gene that activates lytic replication (38). In addition to modulating the transcription of viral and cellular genes, LANA recruits a number of molecules to regulate replication of the viral episome and the segregation of the newly synthesized genome copies to daughter nuclei by tethering to the host chromosomes (21, 40, 52, 54, 61). A simplified model suggests that LANA can mediate tethering of the KSHV genome to specific components of the chromatin structure through binding of its carboxy terminus with the terminal repeat (TR) and associating with components of the human chromatin at its amino terminus, which includes histones and MeCP2 (6, 11, 36).
Nuclear mitotic apparatus (NuMA) protein, a 238-kDa human protein, is a component of the nuclear mitotic apparatus and is distributed throughout the nucleus, excluding nucleoli, in interphase cells (10, 26). In dividing cells, NuMA redistributes to the spindle poles at metaphase and anaphase to organize microtubule movement and to stabilize the mitotic spindle (14, 15, 34). NuMA is vital for mitosis, since microinjection of NuMA antibodies and mutations of NuMA result in a block in mitosis and formation of micronuclei (20, 68). It has an intrinsic ability to self-assemble into fibrous structures (25, 26) and to interact with a number of essential mitotic components, including microtubules (17, 28), dynein/dynactin (47), and the mammalian Pins homolog, LGN (16). The structure of NuMA resembles the intermediate filaments, because of the coiled-coil and globular-head domains, and it may also form ordered structures in the interphase nucleus (1, 26). NuMA has also been shown to bind with the DNA matrix attachment regions (44), and the dissociation precedes DNA degradation during apoptosis (67). Recently, it has been shown that NuMA can influence the organization and maintenance of higher-order chromatin structure associated with the differentiation of mammary epithelium (1). These studies suggest that NuMA has multiple functions in cell cycle progression.
Although it has been shown that the KSHV episomes are associated with host chromosomes and maintain constant episomal copies after successive passages, the underlying mechanism of the maintenance and segregation of the KSHV genome during cell division is largely unknown. Here, we show that NuMA and LANA can interact with each other during interphase and that this interaction is temporally lost as the cells enter mitosis. This interaction also involves the molecular motor complex dynein/dynactin and microtubules, as inhibition of NuMA function by blocking its association with dynein/dynactin and microtubules resulted in loss of episomes in the daughter cells. Thus, the interaction of NuMA with LANA contributes to KSHV genome maintenance and segregation.
MATERIALS AND METHODS
Plasmids, antibodies, and cell lines.
pA3M-LANA, the pA3M-LANA deletion constructs carrying the c-myc-tagged ORF73 amino-terminal domain (amino acids [aa] 1 to 435) and carboxy-terminal domain (aa 752 to 1162), and red fluorescent protein (RFP)-LANA were used as expression vectors and were described previously (24). The pA3M-LANA deletion constructs carrying Myc-tagged ORF73 aa 840 to 963 and aa 840 to 1067 were constructed by PCR amplification from pA3M LANA carboxy-terminal constructs. pBSpuro-EGFP-3TR was constructed by subcloning the puromycin resistance expression cassette from pBABEpuro into the SalI and ClaI sites of pBS-EFGP (Stratagene Inc., La Jolla, CA) containing multiple cloning sites. The complete TR unit of KSHV (801 bp) was excised from a cosmid clone, Z6, with restriction endonuclease NotI and ligated into pBSpuro at the NotI site to obtain pBSpuro-EGFP-3TR, which contains three copies of the TR. GFP-NuMA plasmid expressing GFP-tagged NuMA protein was obtained from Andreas Merdes (University of Edinburgh, Edinburgh, United Kingdom) (46). RFP-p150-CC1 expressing aa 217 to 548 of the p150Glued subunit of dynactin was obtained from Trina A. Schroer (Johns Hopkins University, Baltimore, MD) (50). Myc-LGN plasmid expressing Myc-tagged LGN protein was obtained from Q. Du (Medical College of Georgia, Augusta) (17). Myc-tagged proteins were detected using mouse hybridoma 9E10 obtained from the University of Michigan Hybridoma Core Facility. NuMA protein was detected with goat anti-NuMA antibody obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
The KSHV-negative cell lines BJAB and DG75 and the KSHV-positive cell lines BC-3 and BCBL1 were cultured in RPMI 1640 medium supplemented with 7% fetal bovine serum, 2 mM l-glutamine, and penicillin-streptomycin (5 U/ml and 5 μg/ml, respectively). The human embryonic kidney 293 (HEK293) cell line and human osteosarcoma cell line U2OS were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum, 2 mM l-glutamine, and penicillin-streptomycin (5 U/ml and 5 μg/ml, respectively). All cell lines were grown at 37°C in a humidified environment supplemented with 5% CO2.
Western blot analysis.
Electrophoresed proteins were blotted onto 0.45-μm nitrocellulose membranes (Osmonics, Inc., Minnetonka, MN) at 100 V for 1 to 2 h. The blots were blocked with 5% milk in phosphate-buffered saline (PBS) and washed three times with TBST buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) before overnight incubation with the mouse 9E10 hybridoma or goat anti-NuMA (Santa Cruz Inc., Santa Cruz, CA) at 4°C. The blots were washed three times with TBST and incubated with appropriate AlexaFluor 680 or IRDye 800 secondary antibody (1:10,000; Molecular Probes, Carlsbad, CA). The membranes were scanned with an Odyssey Infrared scanner (Li-Cor Biosciences, Lincoln, NE). Densitometric analysis was performed with the Odyssey scanning software.
Cell cycle arrest and synchronization.
U2OS cells transfected with green fluorescent protein (GFP)-NuMA and RFP-LANA were serum starved to synchronize the cells in G0/G1 phase and subjected to double thymidine treatment to synchronize the cells in S phase and nocodazole to synchronize them in mitosis. In brief, for G0 phase synchronization, the cells were washed twice with serum-free DMEM and incubated for 48 h in the absence of serum. The cells were released from starvation by the addition of fetal bovine serum to 20%. To synchronize the cells in S phase, the cells were treated with 2 mM thymidine for 22 h, grown in DMEM with 5% serum for 10 h, and treated with 2 mM thymidine again for 14 h. The cells were treated with 1.5 μg/ml nocodazole for 5 h for mitotic synchronization. The cells were released from the treatment by washing them twice in 1× PBS and analyzed by live-cell microscopy. The fixed cells were washed again twice with PBS and stained with DAPI (4′,6′-diamidino-2-phenylindole). The slides were examined with an Olympus Fluoview FV300 confocal microscope, and images were analyzed with FLUOVIEW software (Olympus Inc., Melville, NY).
Immunofluorescence assays.
BC3 and BJAB cells were washed with PBS and spread evenly on a slide. The cells were fixed with 1:1 methanol/acetone for 10 min at −20°C, dried, and rehydrated with PBS. For blocking, cells were incubated with PBS containing 3% bovine serum albumin and 1% glycine for 30 min. The cells were then cross-reacted with appropriate antibodies. Slides were detected with 1:1,000 chicken anti-goat and then goat anti-mouse or -rabbit immunoglobulin-fluorescein isothiocyanate-conjugated secondary antibodies. The slides were examined with a Fluoview FV300 (Olympus Inc., Melville, NY) confocal microscope, and the images were analyzed with FLUOVIEW software (Olympus Inc., Melville, NY). An average of 20 scans per cell were obtained for Z-stack analysis.
Live-cell imaging.
To examine the accurate time point for association of NuMA and LANA, U2OS cells transfected with GFP-NuMA and RFP-LANA and synchronized in S phase or mitosis were harvested by washing them twice in 1× PBS. Coverslips with cells were mounted in Rose chambers in L15 medium supplemented with 10% fetal bovine serum. The temperature was maintained at 37°C with a custom-made microscope stage heater. U2OS cells were filmed on a Leica inverted microscope equipped with a 63× 1.4-numerical-aperture (NA) PlanApo objective lens. Images were recorded at 5-minute intervals.
Immunoprecipitation.
For immunoprecipitation, 2 × 107 transfected HEK293 cells or 8 × 107 BJAB and BC-3 cells were lysed on ice with 1 ml of RIPA buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.1% NP-40, 1 mM EDTA [pH 8.0]) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg of pepstatin/ml, 10 μg of leupeptin/ml, and 10 μg of aprotinin/ml). The lysates were centrifuged at high speed to remove the cell debris. A control serum was used to preclear the lysate before it was incubated with specific antibodies. The precleared lysates were then incubated with anti-NuMA or anti-LANA antibody overnight at 4°C with rotation and further incubated with protein A and G Sepharose beads at 4°C for 1 h with rotation. The resulting immunoprecipitates were collected by centrifugation at 2,000 × g for 3 min at 4°C, and the pellets were washed four times with 1 ml of ice-cold RIPA butter. The immunoprecipitated pellets were resuspended in 30 μl of 2× sodium dodecyl sulfate (SDS) protein sample buffer (62.5 mM Tris [pH 6.8], 40 mM dithiothreitol, 2% SDS, 0.025% bromophenol blue, and 10% glycerol) and then resolved on SDS-polyacrylamide gel electrophoresis (PAGE) with an 8% polyacrylamide gel. The separated proteins were transferred to a nitrocellulose membrane. Western blot analysis was performed for the detection of LANA protein by the use of an anti-rabbit polyclonal antibody. Similarly, reverse immunoprecipitation with an anti-LANA polyclonal serum was performed for BJAB and BC-3 cells, which were probed for the detection of NuMA coimmunoprecipitation (co-IP) with LANA.
Short-term replication assay.
pBSpuro-EGFP containing three copies of the TR was cotransfected into HEK293 and DG75 cells in four sets: (i) without LANA expression, (ii) with LANA expression, (iii) with LANA and LGN expression, and (iv) with LANA and RFP-N1-p150-CC1 expression vector. Twenty-four hours posttransfection, 1× puromycin (2 μg/ml) was added to the cells for selection. Relative numbers of GFP-expressing cells, which was the measure of the presence of TR plasmids and protein expression, were measured at 1, 5, and 9 days posttransfection. To measure survival, the cells at each time point were trypsinized and counted using trypan blue exclusion. To isolate DNA template for real-time tests, plasmid DNA was extracted using a modified Hirt's procedure. Briefly, medium from the six-well plates was removed and the cells were washed with 1× PBS, followed by lysing the cells in plates with a 1:2 mixture of solutions I and II (solution I, Tris, glucose, and EDTA; solution II, SDS and NaOH). The lysed cells were transferred to a tube, and 1.5 volumes of solution III (3 M potassium acetate) were added, followed by incubation on ice for 10 min and centrifugation at 8,000 rpm for 10 min. The supernatant was further extracted with phenol using phenol-chloroform-isoamyl alcohol, and DNA was precipitated using 0.6 volume of 2-propanol. The pellet was dried, dissolved in Tris-EDTA with RNase, and incubated at 37°C for 30 min, followed by proteinase K treatment. Proteins were removed by a second round of phenol extraction, followed by precipitation of DNA. The extracted DNA was dissolved in Tris-EDTA buffer.
Real-time PCR analysis of viral and cellular transcription.
Quantitative real-time PCR was performed in a total volume of 25 μl, including 12.5 μl of Sybr green PCR Master Mix (New England Biolabs, Beverly, MA). The GAPDH (glyceraldehyde-3-phosphate dehydrogenase) content in each sample was first amplified as an internal control to normalize DNA input for TR amplification. DNA samples were then amplified with primers (forward, 5′ GGGGGACCCCGGGCAGCGAG 3′, and reverse, 5′ GGCTCCCCCAAACAGGCTCA 3′) flanking TR nucleotides 677 to 766.
RNA interference (RNAi).
Small interfering RNAs (siRNAs) complementary to the GGCGUGGCAGGAGAAGUUC fragment of NuMA (8) were cloned into the pSIREN vector according to the instructions of the manufacturer (Clontech, Palo Alto, CA) to generate the si-NuMA construct. pSIREN vector with luciferase target sequence was used as a control. Ten million HEK293 and BJAB cells were transfected by electroporation. NuMA knockdown stable cells were selected and maintained in 4 μg/ml puromycin.
RESULTS
NuMA interacts with the carboxy-terminal domain of KSHV-encoded LANA.
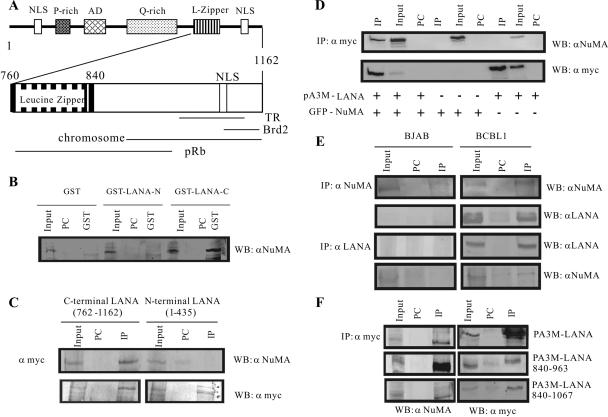
LANA is consistently expressed in KSHV-infected cells and contains three distinct domains (64). The carboxy terminus is important for interaction with the TRs, as well as other cellular proteins (Fig. 1A). A yeast two-hybrid screen, using the carboxy terminus of LANA as bait with a cDNA library from lymphoblastoid cell lines, identified NuMA as a candidate LANA-interacting molecule. Additionally, recent data from a proteomic study using the carboxy terminus of LANA also detected NuMA, suggesting strong association of NuMA with LANA (31). To further corroborate the interaction, we performed GST-binding assays using vector, amino terminus, and the carboxy terminus of LANA fused to GST (Fig. 1B) with the nuclear extracts from KSHV-positive BCBL1 cells. Western blot analysis detecting NuMA indicated a specific association of NuMA with the carboxy terminus of LANA (Fig. 1B).
FIG. 1.
The carboxy terminus of LANA binds with NuMA. (A) Schematic showing the domains of the LANA protein. LANA contains two nuclear localization sequences (NLS), a proline-rich domain (P-rich) and a glutamine-rich repeat region (Q-rich), an acidic domain (AD), and a putative leucine zipper (L-Zipper). C-terminal LANA mediates TR DNA binding, Brd2, MeCP2, pRb binding, and chromosome association. Brd2, bromodomain containing 2. (B) GST, GST-LANA-N, and GST-LANA-C fusion proteins were expressed in Escherichia coli and purified with glutathione-Sepharose beads. Nuclear extracts from BCBL-1 cells were incubated with either GST control or GST-LANA truncations normalized by Coomassie staining. The precipitated proteins were resolved by SDS-PAGE and detected with goat anti-NuMA antibody. In each case, 5% of the cell lysates were used as input for comparison. PC, precleared fraction. (C) Twenty million HEK293T cells were transfected with 20 μg of GFP-NuMA and 20 μg of either pA3M-LANA-C or pA3M-LANA-N expression plasmid. The cells were harvested at 36 h posttransfection, and the lysates were immunoprecipitated (IP) with 1 μg of anti-Myc antibody. Samples were resolved on SDS-6% PAGE and probed with anti-NuMA antibody. LANA truncations were detected with 9E10 Myc hybridoma supernatant. (D) Twenty million HEK293T cells were transfected with 20 μg of GFP-NuMA and 20 μg of pA3M-LANA expression plasmids as indicated. The cells were harvested at 36 h and were immunoprecipitated with 1 μg of anti-Myc antibody. Samples were resolved on SDS-6% PAGE and immunoblotted with anti-NuMA antibody to detect NuMA and 9E10 Myc hybridoma for LANA. (E) Fifty million KSHV-positive and negative BCBL1 and BJAB cells, respectively, were harvested and immunoprecipitated with 3 μl of polyclonal anti-NuMA antibody. Reverse immunoprecipitations were performed with 3 μl of rabbit anti-LANA antibody. LANA and NuMA were detected using the respective antibodies. (F) Twenty million HEK293T cells were transfected with 20 μg of GFP-NuMA and 20 μg of either pA3M-LANA aa 840 to 963 or pA3M-LANA aa 840 to 1067 expression plasmid. The cells were harvested at 36 h and immunoprecipitated with 1 μg of anti-Myc antibody.
The interaction of LANA with NuMA was also confirmed by co-IP analysis. co-IP was performed using cell lysates from HEK293T cells expressing NuMA and Myc-tagged amino-terminal LANA (aa 1 to 435) or carboxy-terminal LANA (aa 762 to 1162). The cell lysates were subjected to immunoprecipitation with anti-Myc antibody, and the coimmunoprecipitated NuMA was detected by anti-NuMA antibody. The results showed that the NuMA protein was detected only with the carboxy terminus of LANA (Fig. 1C). Therefore, GST binding and co-IP assays demonstrated that NuMA is a binding partner of LANA and specifically associates at the carboxy terminus of LANA.
LANA can associate with NuMA as a complex in human cells.
To determine if this association could also be seen for full-length LANA, lysates from HEK293T cells expressing Myc-tagged LANA and NuMA were subjected to co-IP analysis. Cell lysates were subjected to anti-Myc antibody immunoprecipitation and the coimmunoprecipitated protein, NuMA, was detected by immunobloting using anti-NuMA antibodies. Immunoprecipitation with anti-Myc antibody and subsequent detection with anti-NuMA antibody showed that anti-Myc antibody precipitated NuMA protein only when LANA was present (Fig. 1D, lane 1). As expected, LANA was detected in cells transfected with pA3M-LANA and immunoprecipitated with anti-Myc antibody (Fig. 1D). These results demonstrated that NuMA coimmunoprecipitated specifically with LANA and could form a complex in the cells.
To determine the interaction of NuMA and LANA in KSHV-positive cells, a co-IP assay was performed using the KSHV-positive cell line BCBL1 and a negative cell line, BJAB. Immunoprecipitation with anti-NuMA antibody and subsequent detection with anti-LANA antibody showed that NuMA precipitated LANA from the KSHV-positive cells (Fig. 1E). Reverse co-IP analysis with anti-LANA antibody from BCBL1 and BJAB cells showed that NuMA was coimmunoprecipitated only from the KSHV-positive cells (Fig. 1E). These results indicate that NuMA is capable of forming a complex with LANA in KSHV-infected cells, as well as in cells expressing exogenous LANA.
The residues located between aa 840 and 963 of the carboxy terminus of LANA are responsible for association with NuMA.
The carboxy-terminal domain of LANA has been shown to be involved in various functions, including binding to the TRs and transcriptional modulation (65). To determine the minimal residues of LANA responsible for NuMA interaction, we expressed two truncations of the carboxy terminus of LANA tagged with the Myc epitope, along with NuMA, in HEK293T cells. Immunoprecipitation analysis with anti-Myc antibody and subsequent detection with anti-NuMA antibody showed that NuMA was precipitated with LANA carboxy-terminal polypeptide located between amino acid residues 840 and 1067. Further truncation of the carboxy terminus to a domain located within amino acid residues 840 to 963 also showed binding (Fig. 1F). Therefore, the residues located between aa 840 and 963 of the carboxy terminus of LANA are responsible for association with NuMA.
NuMA and LANA associate in a cell cycle-dependent manner.
To further confirm the association of LANA and NuMA, we cotransfected RFP-LANA and GFP-NuMA expression constructs into U2OS cells and analyzed the distribution of fluorescence at different stages of the cell cycle by confocal microscopy (see Materials and Methods).
To examine the distribution of LANA relative to the centrosome and NuMA, the above-mentioned transfected cells were synchronized at the different stages of the cell cycle, assessed by the state of chromosome condensation as seen by DAPI staining (Fig. 2). On average, 30 cells were viewed at each stage described below. In interphase cells, NuMA was present throughout the nucleus, except for the nucleolus (Fig. 2A). At this stage, LANA was seen throughout the nuclei and shared compartments similar to those of NuMA (Fig. 2A). On extending mitosis, the redistribution of the NuMA protein commenced early in prophase and appeared to coincide with chromosome condensation. However, NuMA was not a component of the mitotic chromosome. In cells with condensed DNA, LANA was distinctly separated from NuMA signals but was still distributed on the condensed chromosomes (Fig. 2B). In the mid-prometaphase, while the NuMA proteins began to form spindles, LANA was still condensed on the chromosomes (Fig. 2C). However, in metaphase, LANA signals on the chromosomes were aligned with the spindle equator, whereas NuMA was detected at the spindle poles (Fig. 2D). In anaphase, the spindle poles as seen by NuMA staining moved further apart, and the sister chromatids, as well as LANA, separated and moved toward the respective spindle poles (Fig. 2E). The two groups of sister chromosomes then became two well-separated sister nuclei, followed by division of the cytoplasm (cytokinesis) in telophase (9). During this process, NuMA reassociates early with the reforming nuclei, once again colocalizing with LANA, which is associated with the decondensed chromosomes (Fig. 2F). Thus, LANA and NuMA can occupy the same nuclear compartment in a cell-cycle-dependent manner during interphase. In interphase, both LANA and NuMA were seen diffusely associated throughout the nuclei and stayed in the same compartments. However, the proteins efficiently separated once the cells entered mitosis. NuMA was concentrated and formed new spindle poles, whereas LANA remained on the chromosomes. After the segregation of daughter cells and reformation of the nuclei, the association was once again reestablished, and NuMA again redistributed to the nucleus of each daughter cell (Fig. 2).
FIG. 2.
NuMA and LANA colocalize in a cell cycle-dependent manner. U2OS cells cotransfected with GFP-NuMA and RFP-LANA were synchronized at specific cell cycle stages as indicated. GFP and RFP signals were visualized using confocal microscopy as described in Materials and Methods. The nuclei were stained with DAPI.
LANA can colocalize with NuMA during interphase but is lost as the cells enter mitosis.
As seen above, NuMA and LANA resided in the same compartment during certain phases of the cell cycle. Therefore Z-stack confocal microscopic analysis was performed to verify colocalization of the two proteins. To determine the colocalization of LANA and NuMA, fluorescent images of the z axis were collected, together with the x and y dimensions, from U2OS cells with coexpression of RFP-LANA and GFP-NuMA. During interphase and cytokinesis, on overlay pictures of all three dimensions, yellow signals were observed, indicating colocalization of LANA and NuMA in all three dimensions (Fig. 3A and B). Notably, in addition to the yellow signals in the overlay pictures, scattered green and red spots were also seen. This was likely due to partial colocalization, which suggests that these two proteins may also be involved in additional activities independent of the link to KSHV replication and genome segregation.
FIG. 3.
Colocalization of NuMA and LANA in U2OS cells. U2OS cells expressing GFP-NuMA and RFP-LANA were synchronized at interphase (A), cytokinesis (B), and metaphase (C). GFP-NuMA and RFP-LANA proteins were visualized by confocal microscopy. The nuclei were counterstained with DAPI. The images were analyzed with FLUOVIEW software (Olympus), and the colocalization of the two proteins is shown in both x and y sections.
As cells entered mitosis, LANA remained bound to the chromosomes, and NuMA was seen condensed at both spindles poles (Fig. 3C). No colocalization was observed in any of the three dimensions. The results of these three-dimensional analyses suggest that LANA and NuMA do not colocalize and thus do not interact once the cell enters mitosis.
Dynamic association of LANA and NuMA in actively dividing living cells.
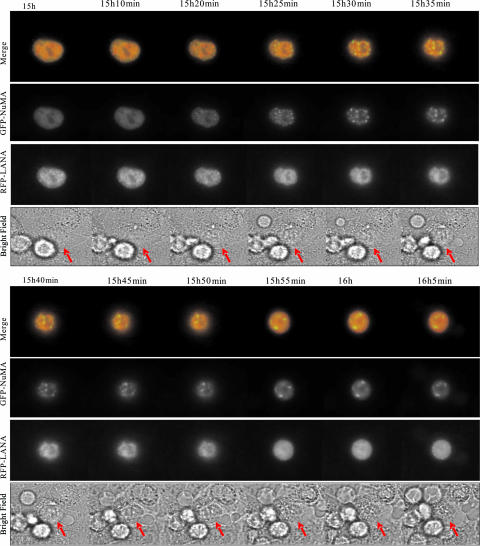
To confirm the biological significance of the observed colocalization of LANA and NuMA in fixed cells, we investigated their intracellular movements in living U2OS cells. Moreover, in vivo imaging experiments provided us with additional insights into the dynamics of NuMA and LANA association. RFP-LANA and GFP-NuMA were coexpressed in U2OS cells, and the cells coexpressing both proteins were synchronized. The movements of NuMA and LANA proteins were monitored using time-lapse fluorescence microscopy by taking images of the cotransfected cells from the S phase to the mitotic phase at 5-min intervals.
The colocalization and association of fluorescently labeled NuMA with LANA was documented in the nuclei of all 15 cells observed. The cells were synchronized by traditional double thymidine treatment to enrich for cells in S phase. As a representative example, fluorescent images of two cells taken at 12 time points are shown in Fig. 4 (see video S1 in the supplemental material). In the nuclei of both cells, NuMA (green) and LANA (red) were detected to colocalize as the cells progressed through S phase. As the DNA became condensed during the progression of the cell cycle, signals for both NuMA and LANA were also condensed in the nuclei (Fig. 4, 15 h to 15 h 20 min). However, the green signal became more condensed at 15 h 25 min after release from thymidine treatment. Thereafter, the two proteins were seen to separate as NuMA became more condensed and started to form spindles (Fig. 4, 15 h 20 min to 16 h 5 min). This dynamic activity is consistent with our previous observation in fixed cells, which showed that LANA and NuMA are separated as the cells enter mitosis. NuMA molecules began to assemble at the poles in early prophase, while LANA signals were predominantly seen on the condensed chromosomes.
FIG. 4.
NuMA and LANA separate during prophase. U2OS cells transfected with GFP-NuMA and RFP-LANA were synchronized at S phase. Cells released from treatment continued to grow in a chamber in L15 medium supplemented with 10% fetal bovine serum. U2OS cells were filmed on a Leica inverted microscope equipped with a 63× 1.4-NA PlanApo objective lens. Images were recorded at 5-minute intervals and analyzed with Image J software. Selected frames from the two-color time-lapse recording are shown. Arrows in Phase panels indicate the cells analyzed for GFP-NuMA and RFP-LANA.
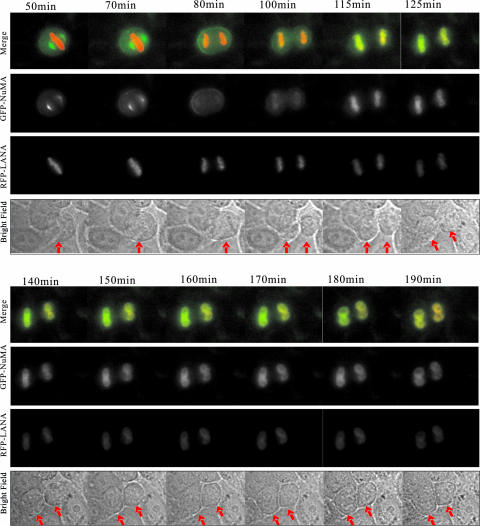
We also monitored GFP-NuMA- and RFP-LANA-coexpressing cells after synchronization in mitosis. A representative cell undergoing mitosis with GFP-NuMA and RFP-LANA expression is shown in Fig. 5 (see video S2 in the supplemental material). Based on monitoring cells over a period of 3 h, as seen by the position of LANA on the chromosome, this cell proceeded from prophase to telophase and eventually formed two daughter cells. At 50 min after release from the treatment, highly condensed chromosomes and spindle poles were formed, and the chromosomes began to align at the central plane of the cell (Fig. 5). A clear metaphase plate was seen at 70 min, and the cell proceeded to anaphase at 100 min. The sister chromatids were pulled apart toward the spindle poles, and the cell membrane was clearly narrowed at the midpoint as the two daughter cells were generated. In this series of studies, the daughter cells were formed at 115 min. During the entire mitosis process, both LANA and NuMA signals were in separate compartments and were not colocalized. However, these proteins colocalized (as seen by the yellow color) when the chromosomes began to decondense in the daughter cells at 125 min (Fig. 5). More intense signals demonstrating colocalization were seen at 150 min, as the yellow overlay was clearly dominant in the nuclei (Fig. 5). Taken together, these dynamic studies clearly showed that NuMA and LANA colocalized during interphase, separated at the beginning of mitosis, and reassociated in the daughter cells when the cells exited mitosis.
FIG. 5.
NuMA and LANA reunite in daughter nuclei after cytokinesis. U2OS cells were transfected with GFP-NuMA and RFP-LANA and synchronized at mitosis. Cells released from treatment continued to grow in a chamber in L15 medium supplemented with 10% fetal bovine serum. U2OS cells were filmed on a Leica inverted microscope equipped with a 63× 1.4-NA PlanApo objective lens. Images were recorded at 5-minute intervals and analyzed with Image J software. Selected frames from the two-color time-lapse recording are shown. Bright field images are shown under the merged images. Arrows in Phase panels indicate the cells focused for the localization of GFP-NuMA and RFP-LANA.
NuMA is required for efficient maintenance of KSHV-TR.
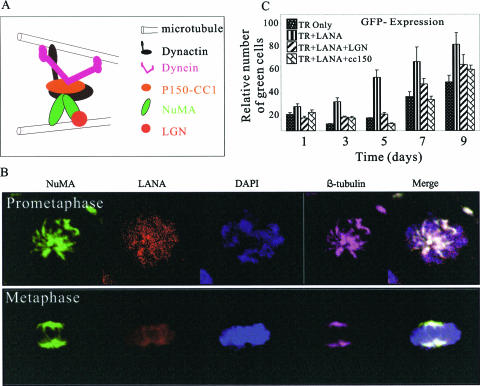
In mammalian cells, dynein, dynactin, and microtubules assemble to form a complex required for spindle assembly and maintenance (50). NuMA binds directly to stationary microtubules and anchors other microtubules at the spindle pole through interaction with dynactin and the attached dynein motor (Fig. 6A) (46). In somatic cells, this interaction is restricted to mitosis, because NuMA is segregated to the nucleus during interphase and spatially separated from cytoplasmic dynein and dynactin by the nuclear membrane. Additionally, previous proteomic studies from our laboratory have shown that dynein can bind to LANA (31, 56). Thus, we wanted to see if these proteins were functionally involved in KSHV genome persistence. In an initial examination of expression pattern, NuMA, LANA, and β-tubulin were investigated in KSHV-positive (BC-3) cells. NuMA and tubulin were colocalized in cells undergoing prometaphase and metaphase (Fig. 6B). LANA was distributed throughout the chromosome and did not show colocalization with either NuMA or β-tubulin (Fig. 6B).
FIG. 6.
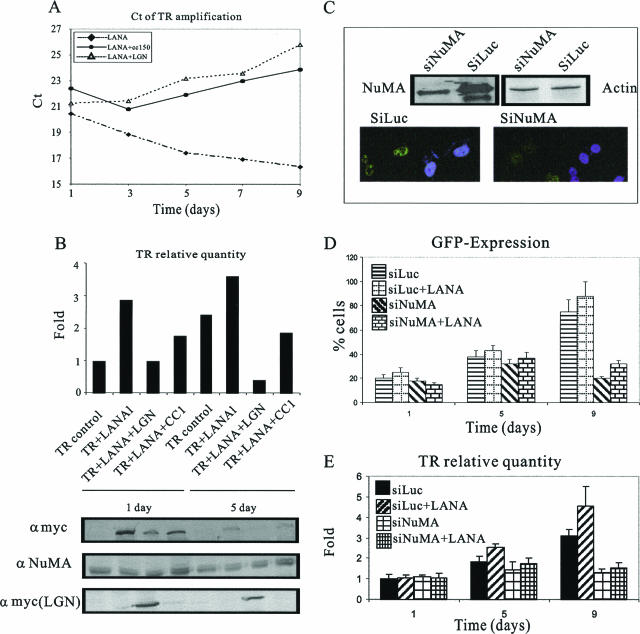
The NuMA-dynein/dynactin complex is important in KSHV-TR maintenance. (A) Model of NuMA, dynein/dynactin, and microtubule complex. (B) Colocalization of NuMA (green) and microtubules in mitotic cells. BC-3 cells were synchronized at prometaphase (top) and metaphase (bottom). The cells were fixed and probed with anti-NuMA goat polyclonal antibody, anti-LANA rabbit polyclonal antibody, and anti-β-tubulin mouse monoclonal antibody. Staining was visualized by confocal microscopy with donkey anti-goat (green), goat anti-rabbit (red), and donkey anti-mouse antibodies. The nuclei were counterstained with DAPI. NuMA, green; tubulin, pink; LANA, red; DNA, blue. (C) HEK293 cells were transfected with pBSpuro-EGFP-3TR and expression constructs as indicated. Twenty hours posttransfection, the cells were subjected to selection with 1 μg/ml puromycin. Expression of GFP was measured and imaged at days 1, 3, 5, 7, and 9 posttransfection. The error bars indicate standard deviations.
To elucidate the functional role of NuMA in maintenance of the KSHV genome, we used a dominant-negative molecule of dynactin, p150-CC1, to block the NuMA function (33, 50). p150-CC1 is a truncated polypeptide consisting of aa 217 to 548 of the p150Glued subunit of dynactin and has been shown to bind dynein in vitro and also to inhibit NuMA function (33, 50). NuMA binds directly to microtubules through its microtubule binding domain at its carboxy terminus (13). Additionally, we used LGN, a mammalian Pins homologue shown to be associated with the spindle poles during mitosis. Microtubules and LGN share the same domain for binding to NuMA, and so, overexpression of LGN disrupts interaction between NuMA and microtubules and further disrupts the function of NuMA (13, 14).
To determine if NuMA is important for maintenance of the KSHV genome, a short-term replication assay with the KSHV TR was conducted. Four sets of constructs were introduced into both HEK293 and DG75 cells: pBSpuro-EGFP with three copies of the TR, (i) without LANA expression, (ii) with LANA expression, (iii) with LANA and LGN expression, and (iv) with LANA and RFP-N1-p150-CC1 expression constructs. Under puromycin selection, the relative proportions of cells expressing EGFP were calculated at 1, 3, 5, 7, and 9 days posttransfection. The ratio of cells with EGFP expression increased throughout the selection because the nontransfected cells were sensitive to the puromycin and thus died. In contrast to this, LANA-expressing cells showed a much higher proportion of enhanced-GFP-positive cells than the other groups (Fig. 6C; see Fig. S1 in the supplemental material). Cells expressing LGN and p150-CC1, along with LANA, showed significantly reduced numbers of GFP-expressing cells, most likely due to the inefficient passage of the pBSpuro-EGFP-TR plasmid in the daughter cells. These data show that destabilization of NuMA function leads to the loss of TR-containing plasmids, suggesting its role in KSHV genome segregation.
The same trend in the number of TR-containing plasmids, from a similar number of cells in each set, was seen throughout the experiments in a semiquantitative PCR assay (reflected by the threshold cycle [CT] value) (Fig. 7A). A lower number of plasmid copies yields a higher CT value, and vice versa. The number of TR-containing plasmids decreased (increased CT value) in cells in which NuMA function was disrupted (Fig. 7A). After normalizing the cells, we calculated the number of pBSpuro-EGFP-TR plasmids per cell in each experiment. A dramatic difference was seen in the groups with disrupted NuMA function, specifically in cells where a p150-CC1 dominant-negative of dynactin was expressed (Fig. 7B). These data again demonstrated that the normal function of NuMA is important for maintenance of the KSHV genome. In the absence of an intact NuMA/microtubule complex, the maintenance of the KSHV genome exhibited a dramatic decrease in copy numbers, especially when direct interaction between NuMA and microtubules was inhibited by expression of LGN, which disrupts NuMA interaction with the microtubules (Fig. 7A and B).
FIG. 7.
Disruption of normal NuMA function inhibits KSHV maintenance. (A) Hirt's DNA was extracted on days 1, 3, 5, 7, and 9 posttransfection from cells with pBSpuro-EGFP-3TR with LANA, LANA plus p150-CC1, and LANA plus LGN. DNA from equal numbers of cells was subjected to real-time quantitation of the TR plasmids in these three sets. Representative CT values of the amplifications from these three samples at different time points are plotted. (B) Equal amounts of Hirt's DNA were amplified with TR-specific primers and normalized with GAPDH copies to get the relative number of copies of TR plasmid. Relative copy numbers of the TR plasmid in a representative experiment in these samples on day 1 and day 5 are plotted. Protein expression on days 1 and 5 was detected by immunoblotting. (C) siRNAs for NuMA cloned in pSIREN vector were introduced into 10 million HEK293 cells to generate si-NuMA stable cells. pSIREN vector with firefly luciferase siRNA was used as a control. NuMA knockdown stable cells were selected and maintained in 4 μg/ml puromycin. Expression of NuMA was examined by immunoblotting and immunofluorescence. (D) si-NuMA and si-Luc stable cells were transfected with pBSpuro-EGFP-3TR and subjected to selection. Expression of GFP was measured and imaged at days 1, 5, and 9 posttransfection.The error bars indicate standard deviations. (E) Hirt's DNA from si-Luc and si-NuMA with and without LANA-expressing cells containing pBSpuro-EGFP-3TR was extracted at days 1, 5, and 9 posttransfection. Equal amounts of Hirt's DNA were subjected to TR amplification, and the numbers of copies of TR plasmids in these cells relative to GAPDH are plotted.
Knockdown of NuMA expression by RNAi deregulates segregation and TR plasmid maintenance.
The results described above suggested that NuMA can play a critical role in segregation and maintenance of the KSHV genome. To test this hypothesis more directly, we sought to disrupt the expression of endogenous NuMA using an RNAi strategy. A 19-nucleotide RNA sequence was reported to suppress the expression of endogenous NuMA in several different mammalian cell lines (8). Therefore, we transfected HEK293 cells with an siRNA duplex that corresponded to specific sequences of the human NuMA. Two days after transfection, the level of endogenous NuMA was significantly reduced compared to the controls as detected by immunoblotting and in situ immunostaining experiments (Fig. 7C). In siRNA-treated cells, the percentage of GFP-TR-expressing cells and the relative number of TR plasmid copies increased in controls, including si-Luc and si-Luc with LANA (Fig. 7C and D). However, the GFP-TR signals and the relative numbers of TR plasmids in experimental groups (si-NuMA expression only or expressed with LANA) showed negligible increase by day 5 and began to decrease on day 9 (Fig. 7D and E, respectively). Notably, both the percentage of GFP-TR signals and the relative number of TR plasmids on day 9 had dramatically decreased by about 60% compared to cells with intact NuMA and exogenous LANA expression. Therefore, these results strongly support the hypothesis that NuMA is important for segregation and maintenance of the KSHV genome.
DISCUSSION
The identification of the cellular binding partners of KSHV-encoded LANA in this study allowed us to demonstrate for the first time that NuMA can play an important role in viral-genome maintenance and segregation. In human cells, NuMA associated with LANA in interphase and disassociated as the cells entered mitosis. NuMA is one of the nuclear matrix proteins and can self-assemble into a fibrous network (26). In vitro, NuMA has the potential to form dimers, which can further assemble into multiarm oligomers by associating with its globular-head domains. NuMA forms a hexagonal lattice by associating with multiple molecules in the cells (26). The functional role of NuMA in the interphase is not entirely clear compared to that during mitosis and needs to be determined. NuMA has been shown to associate with small nuclear ribonucleoproteins and splicing factors involved in recycling and phosphorylation of RNA-processing factors and thus has been implicated in the regulation of DNA replication and transcription (22, 57, 70). As a nuclear matrix protein, NuMA is thought to support the nuclear shape in differentiating cells (45). In addition, a role for NuMA in DNA anchoring has also been proposed based on its interaction with matrix attachment region sequences, which anchor DNA on the nuclear matrix (44). A link between NuMA and chromatin structure is implied by the observation that expression of NuMA constructs lacking C-terminal residues in HeLa cells is accompanied by redistribution of nuclear components, such as DNA and histone H1, to the nuclear rim (25). NuMA has also been shown to colocalize with the multifunctional, DNA-binding, high-mobility group proteins I/Y (58) and binds to the putative transcription factor GAS41 (27). Recently, NuMA was reported to be present in chromatin compartments and can influence the organization and maintenance of higher-order chromatin structure associated with the differentiation of mammary epithelium (1). In this study, a strong association between LANA and NuMA was seen in interphase; thus, NuMA may also function as a matrix to support specific biological processes of KSHV, including genome maintenance and segregation into new daughter cells. This is the first report showing that a viral protein can interact with NuMA and that it helps in segregation of the viral genome into the dividing daughter cells. A role for KSHV LANA protein in attaching the viral genome to the nuclear matrix was shown before by our group, in that CENP-F binds to LANA and helps in tethering the viral genome to the nuclear matrix (31). A human papillomavirus 16-encoded protein, E7, has also been shown to colocalize with the nuclear matrix during infection (23).
The spindle apparatus is formed to move and separate sister chromatids into two daughter cells during cell divisions. After releasing from the nucleus, NuMA associates with cytoplasmic dynein/dynactin to form a complex and translocates to the spindle poles, where it organizes and tethers microtubules to the spindle poles (46). After the onset of anaphase, the association of NuMA with dynein/dynactin is lost, and it releases from the spindle poles to allow spindle disassembly and the reformation of interphase daughter nuclei (26, 28, 46, 47). A functional role of NuMA in KSHV cell division has been suggested based on our observation that the maintenance of TR plasmids was inhibited on disruption of either dynein/dynactin association or microtubule association in the presence of NuMA. Dynein is a minus-end-directed microtubule motor that consists of multiple polypeptide chains arranged in a large multisubunit complex. Dyneins are large, multisubunit ATPases that interact with microtubules and dynactin to generate force. Dyneins are involved in the transport of particles and organelles along microtubules and in the transport of condensed chromosomes during mitosis (18). In our previous proteomic studies, heavy-chain dyneins have been found to bind both the KSHV TR and LANA (31, 56). Since dynein/dynactin and microtubule association are important in transportation of both NuMA and mitotic chromosomes to the spindle poles, we speculate that NuMA may also help in efficient segregation of the replicated KSHV genome into daughter cells.
NuMA has a role in the maintenance of KSHV DNA in a cell cycle-dependent manner. In interphase, it can act as a nuclear matrix to tether the KSHV genome, which is important for persistence. However, in mitosis, it forms complexes with dynein/dynactin and microtubules, which is critical for the segregation of replicated viral DNA. The amino and carboxy termini of LANA have different functions and can cooperate to tether the KSHV genome on human chromosomes (65). The amino-terminal LANA binds chromosomes diffusely through histones and is essential for LANA-mediated DNA replication and episome persistence (6, 11, 42). The carboxy terminus of LANA contains a TR DNA-binding region between aa 996 and 1139 that is important for mediating DNA replication and episome persistence (5, 21, 35, 41, 54). This region also mediates chromosome association and is important for LANA self-association (32, 36). The carboxy terminus of LANA contains a DNA-binding region and can associate with the mitotic chromosomes by interacting with cellular proteins, including DEK and Brd4 (32, 36, 66, 69). Interestingly, the carboxy-terminal chromatin-binding domain of LANA is thought to be important for interaction with Brd2/RING3 (66) and can bind to Brd4 through interaction with the extraterminal domain of Brd4 (69). In this study, the carboxy terminus of LANA has been shown to bind to the nuclear mitotic apparatus. The association between LANA and NuMA, we now show, involves a complex of dynein/dynactin and microtubules. The involvement of this motor complex indicates that NuMA is important for segregation of the KSHV genome. We further determined that NuMA binds to LANA between aa 840 and 963, which is adjacent to the TR-binding region but does not overlap with it. It is likely that other cellular proteins involved in regulation of the mitotic apparatus, as well as CENP-E and CENP-F, the members of the tethering complex, make LANA important for KSHV genome segregation.
Several lines of evidences have suggested that LANA is closely linked to the protection of infected host cells from apoptosis. First, Pim-1 is highly expressed in LANA-expressing cells, and LANA can enhance the transcription activity of the Pim-1 promoter (3). Additionally, Pim-1 can bind to the carboxy terminus of LANA (3) and inhibit NuMA-associated apoptosis (7). Second, LANA is also closely associated with apoptosis protection in KSHV-infected cells (19, 30). LANA-expressing cells can proliferate faster and are better protected from apoptosis (3, 19, 30, 55). Third, LANA was shown to be a good target molecule for KSHV therapy through induction of apoptosis (12). Finally, reduced levels of LANA lead to p53 activation and result in G1 cell cycle arrest, DNA fragmentation, and oxidative-stress-mediated apoptosis (12). Moreover, disruption of the p53-MDM2-LANA complex by the MDM2 inhibitor Nutlin-3a selectively induced massive apoptosis in PEL cells (53). However, the mechanism involved in apoptotic protection by LANA is largely unknown. Several observations suggest that the apoptotic degradation of NuMA may be related to chromatin condensation and micronucleation (43). Four apoptotic cleavage sites were identified at a junction between the globular tail and the central coiled-coil domains of NuMA. A NuMA deletion mutant missing the entire cleavage region resisted degradation and protected cells from nuclear disruption upon apoptotic attack (43). NuMA is specifically targeted by human rhinovirus type 1B or measles virus during virus-induced programmed cell death (59). A virus-specific mechanism of NuMA cleavage was shown in human rhinovirus type 1B- and measles virus-infected cells and suggests that these viruses activate different sets of proteases (59). In addition, NuMA may influence the kinetics of nuclear degradation during apoptosis as a caspase substrate (60). All these observations suggest that NuMA is a target for virus-induced cell death and that it may also function as a downstream effecter of KSHV-mediated protection from apoptosis, as well as regulation of genome segregation and persistence through interaction with LANA.
Supplementary Material
Acknowledgments
We thank Andreas Merdes, Trina A. Schroer, and Quansheng Du for generously providing reagents. We are grateful to Thomai Gocstooulos and Evangelia Athanasoula for their technical support.
This work was supported by grants from the Leukemia and Lymphoma Society of America and by public health service grants NCI CA072510, CA108461, and CA091792; NIDCR DEO017338 and DE01436; and NIAID AI067037 (to E.S.R.). E.S.R. is a scholar of the Leukemia and Lymphoma Society of America. S.C.V is supported by an NIH pathway to independence award (K99CA126182).
Footnotes
Published ahead of print on 16 April 2008.
Supplemental material for this article can be found at http://jvi.asm.org/.
REFERENCES
- 1.Abad, P. C., J. Lewis, I. S. Mian, D. W. Knowles, J. Sturgis, S. Badve, J. Xie, and S. A. Lelievre. 2007. NuMA influences higher order chromatin organization in human mammary epithelium. Mol. Biol. Cell 18348-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2004. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from P16INK4A-induced cell cycle arrest. J. Biol. Chem. 2803862-3874. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj, B. G., S. C. Verma, K. Lan, M. A. Cotter, Z. L. Woodman, and E. S. Robertson. 2006. KSHV encoded LANA upregulates Pim-1 and is a substrate for its kinase activity. Virology 35118-28. [DOI] [PubMed] [Google Scholar]
- 4.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284641-644. [DOI] [PubMed] [Google Scholar]
- 5.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 753250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbera, A. J., M. E. Ballestas, and K. M. Kaye. 2004. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 78294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya, N., Z. Wang, C. Davitt, I. F. McKenzie, P. X. Xing, and N. S. Magnuson. 2002. Pim-1 associates with protein complexes necessary for mitosis. Chromosoma 11180-95. [DOI] [PubMed] [Google Scholar]
- 8.Chang, P., M. Coughlin, and T. J. Mitchison. 2005. Tankyrase-1 polymerization of poly(ADP-ribose) is required for spindle structure and function. Nat. Cell Biol. 71133-1139. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland, D. W. 1995. NuMA: a protein involved in nuclear structure, spindle assembly, and nuclear re-formation. Trends Cell Biol. 560-64. [DOI] [PubMed] [Google Scholar]
- 10.Compton, D. A., I. Szilak, and D. W. Cleveland. 1992. Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J. Cell Biol. 1161395-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264254-264. [DOI] [PubMed] [Google Scholar]
- 12.Curreli, F., A. E. Friedman-Kien, and O. Flore. 2005. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J. Clin. Investig. 115642-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du, M. Q., T. C. Diss, H. Liu, H. Ye, R. A. Hamoudi, J. Cabecadas, H. Y. Dong, N. L. Harris, J. K. Chan, J. W. Rees, A. Dogan, and P. G. Isaacson. 2002. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood 1003415-3418. [DOI] [PubMed] [Google Scholar]
- 14.Du, M. Q., H. Liu, T. C. Diss, H. Ye, R. A. Hamoudi, N. Dupin, V. Meignin, E. Oksenhendler, C. Boshoff, and P. G. Isaacson. 2001. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood 972130-2136. [DOI] [PubMed] [Google Scholar]
- 15.Du, Q., and I. G. Macara. 2004. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119503-516. [DOI] [PubMed] [Google Scholar]
- 16.Du, Q., P. T. Stukenberg, and I. G. Macara. 2001. A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat. Cell Biol. 31069-1075. [DOI] [PubMed] [Google Scholar]
- 17.Du, Q., L. Taylor, D. A. Compton, and I. G. Macara. 2002. LGN blocks the ability of NuMA to bind and stabilize microtubules. A mechanism for mitotic spindle assembly regulation. Curr. Biol. 121928-1933. [DOI] [PubMed] [Google Scholar]
- 18.Fant, X., A. Merdes, and L. Haren. 2004. Cell and molecular biology of spindle poles and NuMA. Int. Rev. Cytol. 2381-57. [DOI] [PubMed] [Google Scholar]
- 19.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402889-894. [DOI] [PubMed] [Google Scholar]
- 20.Gaglio, T., A. Saredi, and D. A. Compton. 1995. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J. Cell Biol. 131693-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 757882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobert, G. N., C. N. Hueser, E. M. Curran, Q. Y. Sun, V. V. Glinsky, W. V. Welshons, A. Eisenstark, and H. Schatten. 2001. Immunolocalization of NuMA and phosphorylated proteins during the cell cycle in human breast and prostate cancer cells as analyzed by immunofluorescence and postembedding immunoelectron microscopy. Histochem. Cell Biol. 115381-395. [DOI] [PubMed] [Google Scholar]
- 23.Greenfield, I., J. Nickerson, S. Penman, and M. Stanley. 1991. Human papillomavirus 16 E7 protein is associated with the nuclear matrix. Proc. Natl. Acad. Sci. USA 8811217-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groves, A. K., M. A. Cotter, C. Subramanian, and E. S. Robertson. 2001. The latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus activates two major essential Epstein-Barr virus latent promoters. J. Virol. 759446-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gueth-Hallonet, C., J. Wang, J. Harborth, K. Weber, and M. Osborn. 1998. Induction of a regular nuclear lattice by overexpression of NuMA. Exp. Cell Res. 243434-452. [DOI] [PubMed] [Google Scholar]
- 26.Harborth, J., J. Wang, C. Gueth-Hallonet, K. Weber, and M. Osborn. 1999. Self assembly of NuMA: multiarm oligomers as structural units of a nuclear lattice. EMBO J. 181689-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harborth, J., K. Weber, and M. Osborn. 2000. GAS41, a highly conserved protein in eukaryotic nuclei, binds to NuMA. J. Biol. Chem. 27531979-31985. [DOI] [PubMed] [Google Scholar]
- 28.Haren, L., and A. Merdes. 2002. Direct binding of NuMA to tubulin is mediated by a novel sequence motif in the tail domain that bundles and stabilizes microtubules. J. Cell Sci. 1151815-1824. [DOI] [PubMed] [Google Scholar]
- 29.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 7611677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katano, H., Y. Sato, and T. Sata. 2001. Expression of p53 and human herpesvirus-8 (HHV-8)-encoded latency-associated nuclear antigen with inhibition of apoptosis in HHV-8-associated malignancies. Cancer 923076-3084. [DOI] [PubMed] [Google Scholar]
- 31.Kaul, R., S. C. Verma, and E. S. Robertson. 2007. Protein complexes associated with the Kaposi's sarcoma-associated herpesvirus-encoded LANA. Virology 364317-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley-Clarke, B., M. E. Ballestas, V. Srinivasan, A. J. Barbera, T. Komatsu, T. A. Harris, M. Kazanjian, and K. M. Kaye. 2007. Determination of Kaposi's sarcoma-associated herpesvirus C-terminal latency-associated nuclear antigen residues mediating chromosome association and DNA binding. J. Virol. 814348-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King, S. J., C. L. Brown, K. C. Maier, N. J. Quintyne, and T. A. Schroer. 2003. Analysis of the dynein-dynactin interaction in vitro and in vivo. Mol. Biol. Cell 145089-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kisurina-Evgenieva, O., G. Mack, Q. Du, I. Macara, A. Khodjakov, and D. A. Compton. 2004. Multiple mechanisms regulate NuMA dynamics at spindle poles. J. Cell Sci. 1176391-6400. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu, T., M. E. Ballestas, A. J. Barbera, B. Kelley-Clarke, and K. M. Kaye. 2004. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology 319225-236. [DOI] [PubMed] [Google Scholar]
- 36.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 7611596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 749637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan, K., D. A. Kuppers, S. C. Verma, and E. S. Robertson. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J. Virol. 786585-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 27631016-31022. [DOI] [PubMed] [Google Scholar]
- 40.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 812645-2652. [DOI] [PubMed] [Google Scholar]
- 41.Lim, C., H. Sohn, D. Lee, Y. Gwack, and J. Choe. 2002. Functional dissection of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J. Virol. 7610320-10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim, L. L., J. Druce, and A. C. Street. 2004. Human herpesvirus type 8-associated episodic multisystem illness in an HIV-infected patient in the absence of hemophagocytic lymphohistiocytosis. Clin. Infect. Dis. 381640-1641. [DOI] [PubMed] [Google Scholar]
- 43.Lin, H. H., H. L. Hsu, and N. H. Yeh. 2007. Apoptotic cleavage of NuMA at the C-terminal end is related to nuclear disruption and death amplification. J. Biomed. Sci. 14681-694. [DOI] [PubMed] [Google Scholar]
- 44.Luderus, M. E., J. L. den Blaauwen, O. J. de Smit, D. A. Compton, and R. van Driel. 1994. Binding of matrix attachment regions to lamin polymers involves single-stranded regions and the minor groove. Mol. Cell. Biol. 146297-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merdes, A., and D. W. Cleveland. 1998. The role of NuMA in the interphase nucleus. J. Cell Sci. 11171-79. [DOI] [PubMed] [Google Scholar]
- 46.Merdes, A., R. Heald, K. Samejima, W. C. Earnshaw, and D. W. Cleveland. 2000. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 149851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merdes, A., K. Ramyar, J. D. Vechio, and D. W. Cleveland. 1996. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87447-458. [DOI] [PubMed] [Google Scholar]
- 48.Moore, P. S., and Y. Chang. 2003. Kaposi's sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu. Rev. Microbiol. 57609-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore, P. S., S. J. Gao, G. Dominguez, E. Cesarman, O. Lungu, D. M. Knowles, R. Garber, P. E. Pellett, D. J. McGeoch, and Y. Chang. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quintyne, N. J., S. R. Gill, D. M. Eckley, C. L. Crego, D. A. Compton, and T. A. Schroer. 1999. Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 147321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 61121-1127. [DOI] [PubMed] [Google Scholar]
- 52.Sakakibara, S., K. Ueda, K. Nishimura, E. Do, E. Ohsaki, T. Okuno, and K. Yamanishi. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 787299-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarek, G., S. Kurki, J. Enback, G. Iotzova, J. Haas, P. Laakkonen, M. Laiho, and P. M. Ojala. 2007. Reactivation of the p53 pathway as a treatment modality for KSHV-induced lymphomas. J. Clin. Investig. 1171019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 748532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Si, H., and E. S. Robertson. 2006. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J. Virol. 80697-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Si, H., S. C. Verma, and E. S. Robertson. 2006. Proteomic analysis of the Kaposi's sarcoma-associated herpesvirus terminal repeat element binding proteins. J. Virol. 809017-9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sparks, C. A., E. G. Fey, C. A. Vidair, and S. J. Doxsey. 1995. Phosphorylation of NUMA occurs during nuclear breakdown and not mitotic spindle assembly. J. Cell Sci. 1083389-3396. [DOI] [PubMed] [Google Scholar]
- 58.Tabellini, G., M. Riccio, G. Baldini, R. Bareggi, A. M. Billi, V. Grill, P. Narducci, and A. M. Martelli. 2001. Further considerations on the intranuclear distribution of HMGI/Y proteins. Ital. J. Anat. Embryol. 106251-260. [PubMed] [Google Scholar]
- 59.Taimen, P., H. Berghall, R. Vainionpaa, and M. Kallajoki. 2004. NuMA and nuclear lamins are cleaved during viral infection—inhibition of caspase activity prevents cleavage and rescues HeLa cells from measles virus-induced but not from rhinovirus 1B-induced cell death. Virology 32085-98. [DOI] [PubMed] [Google Scholar]
- 60.Taimen, P., and M. Kallajoki. 2003. NuMA and nuclear lamins behave differently in Fas-mediated apoptosis. J. Cell Sci. 116571-583. [DOI] [PubMed] [Google Scholar]
- 61.Verma, S. C., T. Choudhuri, R. Kaul, and E. S. Robertson. 2006. Latency-associated nuclear antigen (LANA) of Kaposi's sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 802243-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma, S. C., T. Choudhuri, and E. S. Robertson. 2007. The minimal replicator element of the Kaposi's sarcoma-associated herpesvirus terminal repeat supports replication in a semiconservative and cell-cycle-dependent manner. J. Virol. 813402-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verma, S. C., K. Lan, T. Choudhuri, M. A. Cotter, and E. S. Robertson. 2007. An autonomous replicating element within the KSHV genome. Cell Host Microbe 2106-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma, S. C., K. Lan, and E. Robertson. 2007. Structure and function of latency-associated nuclear antigen. Curr. Top. Microbiol. Immunol. 312101-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma, S. C., and E. S. Robertson. 2003. Molecular biology and pathogenesis of Kaposi sarcoma-associated herpesvirus. FEMS Microbiol. Lett. 222155-163. [DOI] [PubMed] [Google Scholar]
- 66.Viejo-Borbolla, A., M. Ottinger, E. Bruning, A. Burger, R. Konig, E. Kati, J. A. Sheldon, and T. F. Schulz. 2005. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 7913618-13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver, V. M., C. E. Carson, P. R. Walker, N. Chaly, B. Lach, Y. Raymond, D. L. Brown, and M. Sikorska. 1996. Degradation of nuclear matrix and DNA cleavage in apoptotic thymocytes. J. Cell Sci. 10945-56. [DOI] [PubMed] [Google Scholar]
- 68.Yang, C. H., and M. Snyder. 1992. The nuclear-mitotic apparatus protein is important in the establishment and maintenance of the bipolar mitotic spindle apparatus. Mol. Biol. Cell 31259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You, J., V. Srinivasan, G. V. Denis, W. J. Harrington, Jr., M. E. Ballestas, K. M. Kaye, and P. M. Howley. 2006. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 808909-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng, C., D. He, S. M. Berget, and B. R. Brinkley. 1994. Nuclear-mitotic apparatus protein: a structural protein interface between the nucleoskeleton and RNA splicing. Proc. Natl. Acad. Sci. USA 911505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.