Abstract
Mammalian cells express several factors that inhibit lentiviral infection and that have been under strong selective pressure. One of these factors, TRIM5, targets the capsid protein of incoming retrovirus particles and inhibits subsequent steps of the replication cycle. By substituting human immunodeficiency virus type 1 capsid, we were able to show that a set of divergent primate lentivirus capsids was generally not susceptible to restriction by TRIM5 proteins from higher primates. TRIM5α proteins from other primates exhibited distinct restriction specificities for primate lentivirus capsids. Finally, we identified novel primate lentiviral capsids that are targeted by TRIMCyp proteins.
Primates have been colonized by retroviruses at various times during their evolution, leading to the selection of species-specific variants of genes encoding restriction factors that defend host cells from infection. Reciprocally, in order to colonize a particular species, retroviruses have evolved resistance to these species-specific barriers by changing their protein sequences to avoid interactions with restriction factors or by expressing small proteins that specifically neutralize them. However, the specialization involved in overcoming restriction factors present in one host species can come at the expense of acquiring susceptibility to those of another. This could, in principle, limit cross-species transmission. For example, endogenous levels of the capsid (CA)-targeting restriction factor TRIM5α do not inhibit human immunodeficiency virus type 1 (HIV-1) replication in humans, yet rhesus TRIM5α is a major barrier to HIV-1 replication in rhesus macaque cells (10, 12, 14, 20, 24, 32). To explore whether TRIM5α is a general barrier to cross-species primate lentivirus transmission, we determined the abilities of TRIM5 proteins from various primate species to restrict divergent primate lentiviruses.
A limiting factor in undertaking studies of diverse primate lentiviruses is that the complete genome sequence and infectious molecular clones are not yet available for a number of these lentiviruses. Furthermore, the generation of virus isolates and infectious molecular clones often involves passage in human cells, which might lead to the selection of mutations that alter sensitivity to human restriction factors such as TRIM5α. However, TRIM5 proteins target the viral CA pro-tein (6, 9-11, 25), and the replacement of human immunodeficiency virus type 1 (HIV-1) CA by that of simian immunodeficiency virus strain MAC239 (SIVMAC) generates a recombinant virus that displays the TRIM5α sensitivity of SIVMAC rather than HIV-1 (12). Therefore, we employed the same strategy and introduced CA-coding sequences from various primate lentiviruses (Fig. 1) into an HIV-1 GagPol expression plasmid (9). Where possible, we used Gag sequences obtained without in vitro cultivation in human cells. A second advantage of this approach is that the GagPol chimeras package HIV-1 vector genomes expressing reporter genes, and therefore, chimeric virion infectivity can be easily measured in the absence of variables that might arise due to other viral proteins. This approach has thus far been successful for the following chimeras (Fig. 1): HIV-Gb1 expressing CA from SIVcpzGab1 derived from a human peripheral blood mononuclear cell-cultured isolate (13), HIV-Gb2 expressing CA from SIVcpzGab2 derived from uncultured lymphocyte DNA from a naturally infected Pan troglodytes troglodytes ape (3), HIV-TN1B expressing CA from the TAN1.910 clone constructed based on the consensus of viral sequences from fecal RNA of a naturally infected Pan troglodytes schweinfurthii ape (27), HIV-MAC expressing CA from SIVMAC (12), HIV-SAB expressing CA from SIVagmSAB (9), HIV-GSN expressing CA from SIVgsn71 amplified from uncultured lymphocyte DNA of a wild-caught greater spot-nosed monkey (Cercopithecus nictitans) (5), and HIV-DEB expressing CA sequences from SIVdebCNE40 amplified from uncultured lymphocytes of a naturally infected De Brazza's monkey (Cercopithecus neglectus) (2).
FIG. 1.
Generation of lentiviral CA chimeras. Shown is an amino acid sequence alignment of CA proteins from the indicated lentiviruses. Sequence identities are shaded. Three regions defined as loops 1, 2, and 3 are indicated. The double arrow indicates the boundary of the N- and C-terminal domains. The second arrow indicates the C-terminal junction between HIV-1 and the other lentiviral CAs used in the generation of the chimeric GagPol constructs.
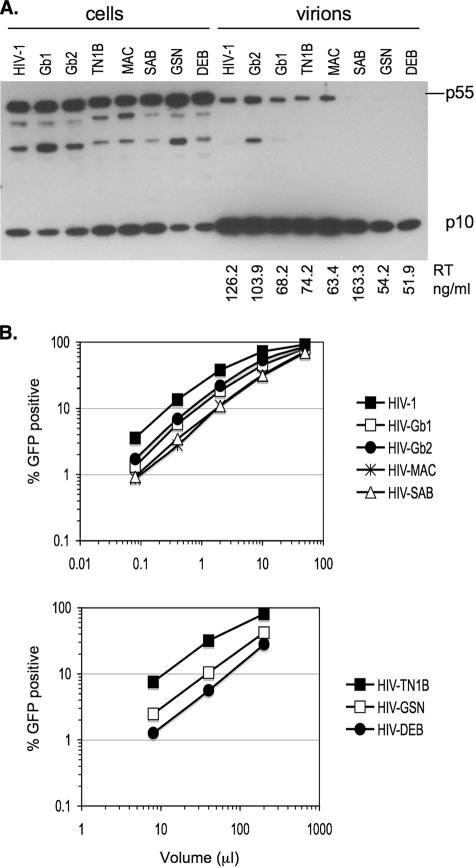
In each case, Gag expression, processing, and particle release, determined by Western blot analysis with an anti-HIV-1-matrix antibody, and particle reverse transcriptase (RT) activity were comparable to those of wild-type HIV-1 GagPol (Fig. 2A). HIV-GSN and HIV-DEB produced somewhat lower levels of Gag and particles than those of the other chimeras and wild-type HIV-1 GagPol (Fig. 2A). All chimeras produced infectious particles when cotransfected with an HIV-1 green fluorescent (GFP) protein vector (CSGW) and a vesicular stomatitis virus G envelope glycoprotein expression plasmid (28), although infectious titers varied (Fig. 2B). As expected, HIV-GSN and HIV-DEB titers were lower than those obtained with other chimeras (Fig. 2B), probably due to the fact that particle production was less efficient (Fig. 2A). Surprisingly, HIV-TN1B titers were also lower than those of wild-type HIV-1, even though protein expression and particle release levels were comparable (Fig. 2).
FIG. 2.
Characterization of lentiviral CA chimeras. (A) 293T cells (10-cm dishes) were transfected with wild-type or chimeric HIV-1 GagPol (5 μg), HIV-1 GFP vector (5 μg), and vesicular stomatitis virus G (1 μg) expression plasmids and harvested 2 days posttransfection. Virions were purified by ultracentrifugation through a 20% sucrose cushion. Cell and virion lysates were analyzed by immunoblotting with an antibody against HIV-1 matrix (p17). The RT activity of viral supernatants, measured using a commercial RT kit (Cavidi), is indicated (representative of two independent experiments). (B) Infectivity of viruses generated in A was measured using hamster CHO cells. Infected cells were enumerated by fluorescence-activated cell sorter (FACS) analysis. A representative of three independent experiments is shown.
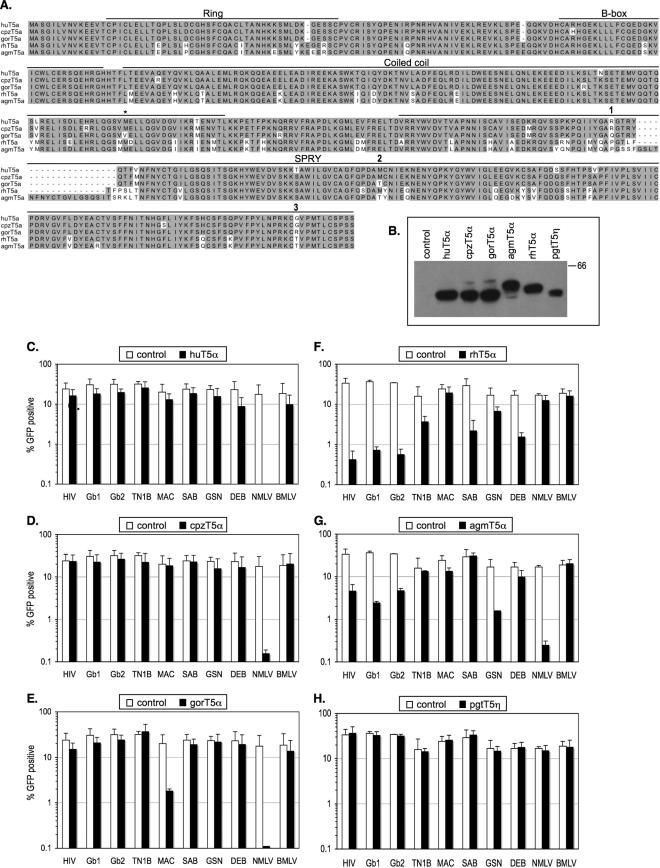
In addition to the previously described human, rhesus macaque, pigtailed macaque, African green monkey, and owl monkey TRIM5α, TRIM5η, and TRIMCyp proteins, we isolated chimpanzee TRIM5α (cpzTRIM5α) and gorilla TRIM5α (gorTRIM5α) cDNAs from Pan troglodytes verus peripheral blood mononuclear cells and Gorilla gorilla fibroblasts, respectively. The cpzTRIM5α amino acid sequence was identical to previously published sequences, whereas gorTRIM5α differed in a single amino acid position in the coiled coil (Fig. 3A) (18, 22, 23). Both proteins were closely related to each other and to human TRIM5α (huTRIM5α), from which they differed only at 9 to 10 amino acid positions (Fig. 3A). We generated single-cell clones of CHO cells expressing C-terminally hemagglutinin (HA) epitope-tagged versions of these proteins as previously described (11). Protein expression levels were comparable for all TRIM5α proteins, and only pigtailed macaque TRIM5η (pgtTRIM5η) was expressed at somewhat lower levels (Fig. 3B).
FIG. 3.
Restriction specificities of primate TRIM5 proteins. (A) Amino acid alignment of primate TRIM5α proteins. Functional domains are indicated. The asterisk indicates the position of the amino acid difference with the published variants of gorTRIM5α. Numbers indicate the three residues that differ in the gorTRIM5α SPRY domain compared to huTRIM5α and cpzTRIM5α. (B) Expression of primate TRIM5 HA-tagged proteins in stable CHO-derived cell lines as determined by Western blot analysis using an anti-HA antibody (Covance). (C to H) Infection of CHO cells that were unmodified (white bars) or that stably expressed the indicated primate TRIM5 proteins (black bars) by wild-type and chimeric HIV-1 or NMLV or B-tropic murine leukemia virus (BMLV). Infected cells were enumerated by FACS analysis. The dose of each virus was selected to infect 15% to 30% of unmodified CHO cells.
Notably, neither huTRIM5α nor cpzTRIM5α inhibited the infectivity of any of the chimeric lentiviruses viruses tested, but both inhibited N-tropic murine leukemia virus (NMLV) infection by 100-fold (Fig. 3C and D). gorTRIM5α was also inactive against most chimeric viruses but did inhibit HIV-MAC infectivity by about 10-fold (Fig. 3E), in agreement with a previous study showing that chimeric huTRIM5α expressing the gorTRIM5α SPRY domain inhibits SIVMAC infection (18). Additionally, like huTRIM5α and cpzTRIM5α, gorTRIM5α strongly inhibited NMLV infection (Fig. 3E). Rhesus macaque TRIM5α (rhTRIM5α) and African green monkey (agmTRIM5α) inhibited HIV-Gb1 and HIV-Gb2 infection as strongly, about 50- and 10-fold, respectively, as they inhibited wild-type HIV-1 (Fig. 2F and G). This was somewhat expected, given the extensive amino acid sequence homology between the HIV-1, Gab1, and Gab2 CAs (Fig. 1A). Surprisingly, however, the inhibition of HIV-TN1B by the Old World monkey TRIM5 proteins was significantly less potent, fivefold by rhTRIM5α and negligible by agmTRIM5α (Fig. 2F and G). SIVagmSab has been shown to be susceptible to rhTRIM5α but resistant to agmTRIM5α (9), and HIV-SAB recapitulated this phenotype (Fig. 3F and G). rhTRIM5α and agmTRIM5α exhibited opposing specificities in terms of the restriction of HIV-GSN and HIV-DEB. Specifically, rhTRIM5α reduced HIV-GSN infectivity moderately (threefold) and reduced HIV-DEB infectivity considerably (10-fold) (Fig. 3F). In contrast, agmTRIM5α strongly inhibited HIV-GSN infection (10-fold) but had only marginal effects (less than twofold) on the infectivity of HIV-DEB (Fig. 3G). Finally, pgtTRIM5η did not significantly inhibit any of the chimeric viruses tested (Fig. 3H). Of note, the addition of cyclosporine A (CsA) during infection had either no or marginal (twofold) effects on lentivirus restriction by the various TRIM5 proteins shown in Fig. 3 (data not shown).
Recently, we and others have shown that a TRIMCyp fusion protein has arisen independently in pigtailed macaques and owl monkeys (4, 16, 29, 30), and remarkably, unlike owl monkey TRIMCyp (omkTRIMCyp), pgtTRIMCyp is completely inactive against HIV-1 even though it can restrict other lentiviruses, including feline immunodeficiency virus (FIV) (29). Furthermore, we identified a single amino acid change (R69H) in pgtTRIMCyp, compared to pgtCypA or omkTRIMCyp, that results in a loss of the interaction with the HIV-1 CA but a “gain” of interaction with the FIV CA, insofar as it became more difficult to abolish FIV restriction with CsA (29). These observations suggest that some ancient retroviral infection led to the selection of H69 in pgtTRIMCyp, because H69 enhances the restriction of some as-yet-unknown (perhaps FIV-like) virus.
Like the HIV-1 CA, the SIVcpz (SIV from chimpanzees) CAs were strongly inhibited by omkTRIMCyp (100-fold) but not by pgtTRIMCyp (Fig. 4B and C). However, both omkTRIMCyp and pgtTRIMCyp inhibited HIV-GSN. Notably, a single amino acid change in pgtTRIMCyp, H69R, which reverses a change in CypA that was acquired after its LINE-mediated retrotransposition into pgtTRIM5, was able to confer the ability to restrict SIVcpz as well as HIV-1 CAs (Fig. 4D). The reversal of a second postretrotransposition change, N66D, had no significant effects (data not shown).
FIG. 4.
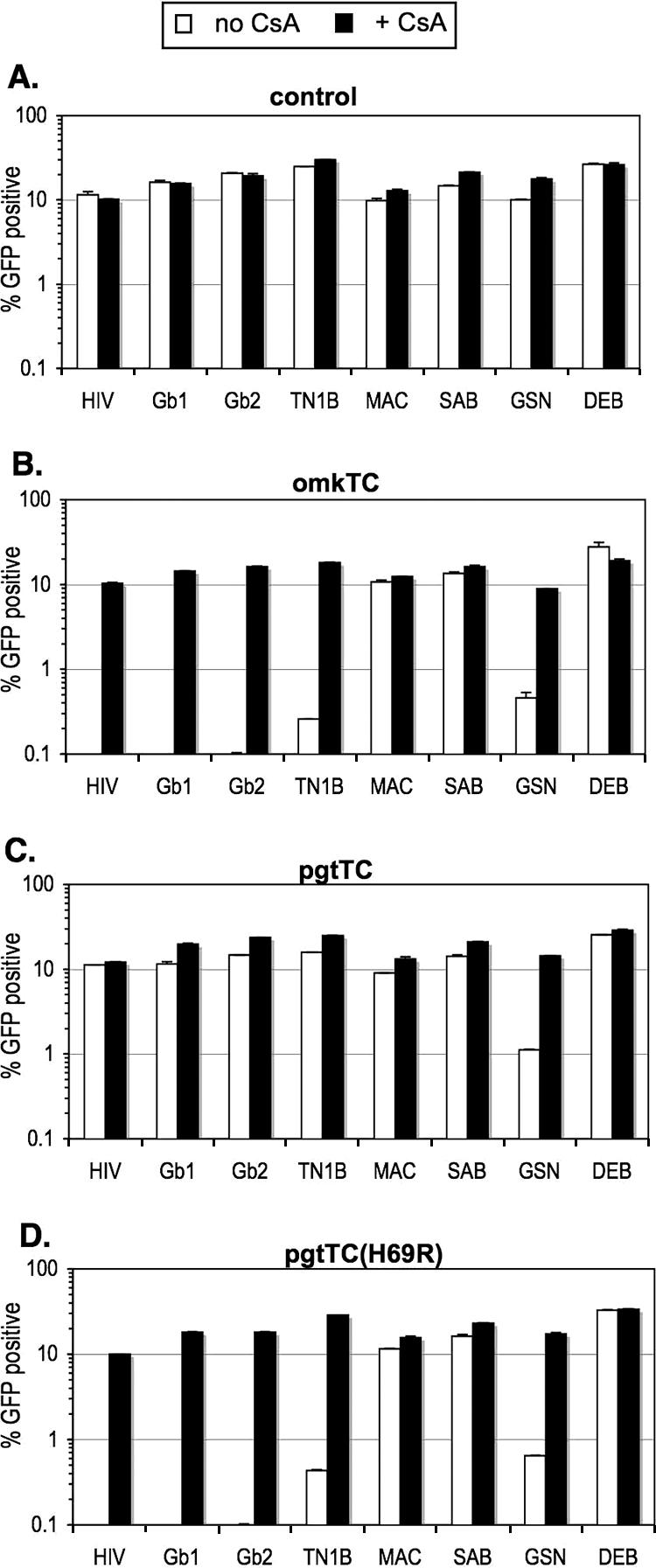
Restriction specificity of wild-type and mutant TRIMCyp proteins. Unmodified hamster CHO cells (A) or CHO cells stably expressing the TRIMCyp protein indicated (B to D) were infected with wild-type or chimeric HIV-1 in the absence (white bars) or presence (black bars) of 5 μM CsA. Infected cells were enumerated by FACS analysis. The expression of each TRIMCyp protein in these stable cell lines was previously described (29).
Overall, these studies describe methods by which the “repertoire” of modern primate lentiviruses that can be inhibited by modern TRIM5 proteins can be determined. Two conclusions are particularly noteworthy; first, the ability of lentiviral CAs to interact with CypA is significantly more widespread than originally thought (7, 17, 34), and since SIVgsn CA was sensitive to both omkTRIMCyp and pgtTRIMCyp, SIVgsn represents another example of a lentivirus CA that is capable of binding CypA. CA-CypA interactions likely provided the impetus for the selection of ancestral owl and macaque monkey individuals that harbored CypA sequences inserted into the TRIM5 locus, and the striking, convergent TRIM5 evolution at the single-amino-acid level following CypA retrotransposition in these species (21, 29) suggests that the selection pressure imposed by retroviruses in each species was very powerful. Second, it was surprising that higher-primate TRIM5α proteins did not appear to restrict the majority of lentiviruses tested. Indeed, gorTRIM5α was the only higher primate protein capable of restricting a primate lentivirus (SIVMAC). The C-terminal TRIM5α SPRY domain, which determines restriction specificity (18, 19, 26, 33) differs at only three positions in gorTRIM5α compared to huTRIM5α and cpzTRIM5α that are presumably responsible for this phenotype (Fig. 2A).
SIVcpz, the immediate ancestor of HIV-1, has been transmitted to humans from chimpanzees of the P. t. troglodytes subspecies on three occasions, yet there is no evidence for SIVcpz transmission from P. t. schweinfurthii to humans (15, 31). Interestingly, the CA sequences of these viruses are rather different (Fig. 1), particularly in the regions predicted to lie on the exposed surface of the three-dimensional CA structure, and affect its ability to be recognized by various TRIM5 proteins (10). These differences probably account for the ability of HIV-TN1B to resist rhTRIM5α while maintaining its ability to bind CypA. However, because both SIVcpz P. t. troglodytes- and SIVcpz P. t. schweinfurthii-derived CAs were resistant to huTRIM5α, it is unlikely that huTRIM5α is responsible for the absence of SIVcpz P. t. schweinfurthii strains in humans. Indeed, huTRIM5α did not inhibit any of the CAs tested, suggesting that it does not impose a major barrier to colonization of humans by nonhuman primate lentiviruses in general. Similarly, it was previously shown that huAPOBEC3 activity does not completely account for the inability of certain simian immunodeficiency viruses to infect humans (8), suggesting that these intrinsic antiviral factors are not the sole determinants of primate lentivirus cross-species transmissibility.
SIVcpz is itself a recombinant virus that derives its 5′ half, including CA, from SIV from red-capped mangabeys and its 3′ half from SIVgsn (1). Since SIVgsn CA was not sensitive to cpzTRIM5α, it is unlikely that TRIM5α drove the selection against SIVgsn CA sequences during the genesis of SIVcpz. Rather, the inability of higher-primate TRIM5α proteins to inhibit infection by lower-primate lentiviruses would, predictably, facilitate the coinfection of chimpanzees by multiple lentiviruses. Such permissiveness likely facilitated the birth of a recombinant lentivirus (SIVcpz/HIV-1) that has become well adapted to a close relative of humans, ultimately leading to the initiation of the current pandemic.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Bailes, E., F. Gao, F. Bibollet-Ruche, V. Courgnaud, M. Peeters, P. A. Marx, B. H. Hahn, and P. M. Sharp. 2003. Hybrid origin of SIV in chimpanzees. Science 3001713. [DOI] [PubMed] [Google Scholar]
- 2.Bibollet-Ruche, F., E. Bailes, F. Gao, X. Pourrut, K. L. Barlow, J. P. Clewley, J. M. Mwenda, D. K. Langat, G. K. Chege, H. M. McClure, E. Mpoudi-Ngole, E. Delaporte, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2004. New simian immunodeficiency virus infecting De Brazza's monkeys (Cercopithecus neglectus): evidence for a cercopithecus monkey virus clade. J. Virol. 787748-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibollet-Ruche, F., F. Gao, E. Bailes, S. Saragosti, E. Delaporte, M. Peeters, G. M. Shaw, B. H. Hahn, and P. M. Sharp. 2004. Complete genome analysis of one of the earliest SIVcpzPtt strains from Gabon (SIVcpzGAB2). AIDS Res. Hum. Retrovir. 201377-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan, G., Y. Kozyrev, and S.-L. Hu. 2008. TRIMCyp expression in Old World primates Macaca nemestrina and Macaca fascicularis. Proc. Natl. Acad. Sci. USA. [Epub ahead of print.] doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed]
- 5.Courgnaud, V., M. Salemi, X. Pourrut, E. Mpoudi-Ngole, B. Abela, P. Auzel, F. Bibollet-Ruche, B. Hahn, A. M. Vandamme, E. Delaporte, and M. Peeters. 2002. Characterization of a novel simian immunodeficiency virus with a vpu gene from greater spot-nosed monkeys (Cercopithecus nictitans) provides new insights into simian/human immunodeficiency virus phylogeny. J. Virol. 768298-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 9911914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Griffero, F., N. Vandegraaff, Y. Li, K. McGee-Estrada, M. Stremlau, S. Welikala, Z. Si, A. Engelman, and J. Sodroski. 2006. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology 351404-419. [DOI] [PubMed] [Google Scholar]
- 8.Gaddis, N. C., A. M. Sheehy, K. M. Ahmad, C. M. Swanson, K. N. Bishop, B. E. Beer, P. A. Marx, F. Gao, F. Bibollet-Ruche, B. H. Hahn, and M. H. Malim. 2004. Further investigation of simian immunodeficiency virus Vif function in human cells. J. Virol. 7812041-12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatziioannou, T., S. Cowan, U. K. Von Schwedler, W. I. Sundquist, and P. D. Bieniasz. 2004. Species-specific tropism determinants in the human immunodeficiency virus type 1 capsid. J. Virol. 786005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc. Natl. Acad. Sci. USA 10110774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatziioannou, T., M. Princiotta, M. Piatak, Jr., F. Yuan, F. Zhang, J. D. Lifson, and P. D. Bieniasz. 2006. Generation of simian-tropic HIV-1 by restriction factor evasion. Science 31495. [DOI] [PubMed] [Google Scholar]
- 13.Huet, T., R. Cheynier, A. Meyerhans, G. Roelants, and S. Wain-Hobson. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345356-359. [DOI] [PubMed] [Google Scholar]
- 14.Keckesova, Z., L. M. Ylinen, and G. J. Towers. 2004. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc. Natl. Acad. Sci. USA 10110780-10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keele, B. F., F. Van Heuverswyn, Y. Li, E. Bailes, J. Takehisa, M. L. Santiago, F. Bibollet-Ruche, Y. Chen, L. V. Wain, F. Liegeois, S. Loul, E. M. Ngole, Y. Bienvenue, E. Delaporte, J. F. Brookfield, P. M. Sharp, G. M. Shaw, M. Peeters, and B. H. Hahn. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao, C.-H., Y.-Q. Kuang, H.-L. Liu, Y.-T. Zheng, and B. Su. 1997. A novel fusion gene, TRIM5-cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS. 21S19-S26. [DOI] [PubMed] [Google Scholar]
- 17.Lin, T. Y., and M. Emerman. 2006. Cyclophilin A interacts with diverse lentiviral capsids. Retrovirology 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkura, S., M. W. Yap, T. Sheldon, and J. P. Stoye. 2006. All three variable regions of the TRIM5α B30.2 domain can contribute to the specificity of retrovirus restriction. J. Virol. 808554-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Caballero, D., T. Hatziioannou, A. Yang, S. Cowan, and P. D. Bieniasz. 2005. Human tripartite motif 5α domains responsible for retrovirus restriction activity and specificity. J. Virol. 798969-8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perron, M. J., M. Stremlau, B. Song, W. Ulm, R. C. Mulligan, and J. Sodroski. 2004. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc. Natl. Acad. Sci. USA 10111827-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribeiro, I. P., A. N. Menezes, M. A. Moreira, C. R. Bonvicino, H. N. Seuánez, and M. A. Soares. 2005. Evolution of cyclophilin A and TRIMCyp retrotransposition in New World primates. J. Virol. 7914998-15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawyer, S. L., L. I. Wu, M. Emerman, and H. S. Malik. 2005. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. USA 1022832-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song, B., B. Gold, C. O'Huigin, H. Javanbakht, X. Li, M. Stremlau, C. Winkler, M. Dean, and J. Sodroski. 2005. The B30.2(SPRY) domain of the retroviral restriction factor TRIM5α exhibits lineage-specific length and sequence variation in primates. J. Virol. 796111-6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stremlau, M., C. M. Owens, M. J. Perron, M. Kiessling, P. Autissier, and J. Sodroski. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427848-853. [DOI] [PubMed] [Google Scholar]
- 25.Stremlau, M., M. Perron, M. Lee, Y. Li, B. Song, H. Javanbakht, F. Diaz-Griffero, D. J. Anderson, W. I. Sundquist, and J. Sodroski. 2006. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA 1035514-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stremlau, M., M. Perron, S. Welikala, and J. Sodroski. 2005. Species-specific variation in the B30.2(SPRY) domain of TRIM5α determines the potency of human immunodeficiency virus restriction. J. Virol. 793139-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takehisa, J., M. H. Kraus, J. M. Decker, Y. Li, B. F. Keele, F. Bibollet-Ruche, K. P. Zammit, Z. Weng, M. L. Santiago, S. Kamenya, M. L. Wilson, A. E. Pusey, E. Bailes, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2007. Generation of infectious molecular clones of simian immunodeficiency virus from fecal consensus sequences of wild chimpanzees. J. Virol. 817463-7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virgen, C. A., and T. Hatziioannou. 2007. Antiretroviral activity and Vif sensitivity of rhesus macaque APOBEC3 proteins. J. Virol. 8113932-13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virgen, C. V., Z. Kratovac, P. D. Bieniasz, and T. Hatziioannou. 2008. Independent genesis of chimeric TRIM-cyclophilin proteins in two primate species. Proc. Natl. Acad. Sci. USA. [Epub ahead of print.] doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed]
- 30.Wilson, S. J., B. L. J. Webb, L. M. J. Ylinen, E. Verschoor, J. L. Heeney, and G. J. Towers. 2008. Independent evolution of an antiviral TRIMCyp in rhesus macaques. Proc. Natl. Acad. Sci. USA. [Epub ahead of print.] doi: 10.1073/pnas.0709258105. [DOI] [PMC free article] [PubMed]
- 31.Worobey, M., M. L. Santiago, B. F. Keele, J. B. Ndjango, J. B. Joy, B. L. Labama, A. B. Dhed, A. Rambaut, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2004. Origin of AIDS: contaminated polio vaccine theory refuted. Nature 428820. [DOI] [PubMed] [Google Scholar]
- 32.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. USA 10110786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap, M. W., S. Nisole, and J. P. Stoye. 2005. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr. Biol. 1573-78. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, F., T. Hatziioannou, D. Perez-Caballero, D. Derse, and P. D. Bieniasz. 2006. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology 353396-409. [DOI] [PubMed] [Google Scholar]