Abstract
The herpes simplex virus (HSV) virion host shutoff (Vhs) protein is an endoribonuclease that accelerates decay of many host and viral mRNAs. Purified Vhs does not distinguish mRNAs from nonmessenger RNAs and cuts target RNAs at many sites, yet within infected cells it is targeted to mRNAs and cleaves those mRNAs at preferred sites including, for some, regions of translation initiation. This targeting may result in part from Vhs binding to the translation initiation factor eIF4H; in particular, several mutations in Vhs that abrogate its binding to eIF4H also abolish its mRNA-degradative activity, even though the mutant proteins retain endonuclease activity. To further investigate the role of eIF4H in Vhs activity, HeLa cells were depleted of eIF4H or other proteins by transfection with small interfering RNAs (siRNAs) 48 h prior to infection or mock infection in the presence of actinomycin D. Cellular mRNA levels were then assayed 5 h after infection. In cells transfected with an siRNA for the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase, wild-type HSV infection reduced β-actin mRNA levels to between 20 and 30% of those in mock-infected cells, indicative of a normal Vhs activity. In contrast, in cells transfected with any of three eIF4H siRNAs, β-actin mRNA levels were indistinguishable in infected and mock-infected cells, suggesting that eIF4H depletion impeded Vhs-mediated degradation. Depletion of the related factor eIF4B did not affect Vhs activity. The data suggest that eIF4H binding is required for Vhs-induced degradation of many mRNAs, perhaps by targeting Vhs to mRNAs and to preferred sites within mRNAs.
The herpes simplex virus (HSV) virion host shutoff (Vhs) protein (UL41) is an endoribonuclease (9, 14, 83, 84, 87) that is a minor structural component of virions (57, 58, 67) and affects the half-lives of many host and viral mRNAs (19, 23, 24, 30, 38, 39, 47-49, 55-57, 66, 68, 77). At early times, copies of Vhs from infecting virions degrade many host mRNAs in the cytoplasm. This can be seen most dramatically following infection with UV light-inactivated virus or with infectious virus in the presence of drugs, such as actinomycin D, that block viral and cellular transcription (19-23, 56, 57, 66, 77). Under these conditions, Vhs significantly reduces the levels of a majority of cellular mRNAs, indicating that it degrades many different mRNAs and does not recognize a specific sequence within target molecules.
This downturn in host mRNA levels is not as pronounced when viral and new cellular transcription is allowed. While many mRNAs still decline in abundance, a number of host mRNAs actually accumulate to higher levels (11-13, 34, 54, 80-82). The reason, no doubt, is complex. Some mRNAs may be refractory to Vhs-mediated degradation, either because some feature of their structure renders them resistant to the nuclease or because Vhs is not targeted to those mRNAs. Other mRNAs may still be degraded by the Vhs protein, but their transcription rates are stimulated by infection to such an extent as to more than compensate for the rate of Vhs-induced decay. Vhs may actually stabilize some mRNAs, either by reducing the levels of cellular factors involved in their normal decay or by binding to and inhibiting a key component of the normal turnover apparatus for that mRNA. In addition, some host mRNAs appear to be stabilized by viral gene products, other than Vhs, synthesized after infection (6, 29). Although the UL41 gene product was given the moniker Vhs based on the first phenotype associated with it (39, 56), it is not exclusively a host shutoff factor. Rather, it alters the balance between the synthesis and decay rates of host mRNAs, which may have many downstream effects on gene expression, both negative and positive. In addition to its effect on host mRNAs, following the onset of viral transcription Vhs accelerates the turnover of viral mRNAs belonging to every kinetic class (38, 47, 48, 55, 56, 77). In this role, it helps determine viral mRNA levels and facilitates the sequential expression of different classes of viral genes (38, 47, 48, 55).
While mutations that inactivate Vhs have only a modest effect upon virus production during single-step growth experiments (56, 57), wild-type virus rapidly outgrows Vhs mutants over several replication cycles during mixed infections in cell culture (38). Much more striking is the dramatic effect of Vhs mutations upon HSV virulence in animals (2, 8, 25, 32, 33, 40, 46, 69, 70, 73-76). The mechanism by which Vhs affects virulence is unknown but may involve inhibitory effects on key components of the adaptive and innate immune responses (68). Vhs impedes antigen presentation by both major histocompatibility complex class I (85) and class II (86) molecules and inhibits the secretion of cytokines that would otherwise recruit lymphocytes and neutrophils to the site of infection (65, 78). Recently, Vhs has been shown to inhibit the replenishment of the short-lived receptor for tumor necrosis factor alpha (41) and has been reported to assist infection by helping to temporarily block infected cells from entering apoptosis (1). In addition, Vhs mutants of HSV-2 are severely attenuated in normal mice (46, 70) but are almost as virulent as wild-type virus in alpha/beta interferon receptor-knockout animals (46), suggesting that an important role of Vhs is to inhibit the host alpha/beta interferon-mediated antiviral response (5, 8, 46).
Purified or partially purified Vhs lacks the specificity that it displays in vivo (14, 83, 84, 87). Thus, in the absence of cellular factors Vhs does not distinguish mRNAs from nonmessenger RNAs and cuts target RNAs at many sites (14, 83, 84, 87). Recently, a purified glutathione S-transferase (GST)-Vhs fusion protein was shown to have a substrate specificity similar to that of RNase A (83, 84). In contrast, within infected cells or unfractionated cytoplasmic extracts, Vhs is targeted to mRNAs (37, 47, 48, 66, 72, 77, 87), as opposed to nonmessenger RNAs, and cleaves mRNAs at preferred sites (9-11, 31, 44, 50, 82), including, for some mRNAs, regions of translation initiation (9, 10, 31, 44, 50). This specificity may result, in part, from the ability of Vhs to bind host translation initiation factors, including eIF4H (14, 17, 18) and/or eIF4B (7). eIF4H and eIF4B share a region of sequence homology, and both stimulate the ATP-dependent RNA helicase activity of eIF4AI and eIF4AII (28, 59-64). eIF4AI and eIF4AII, in turn, are components of the cap-binding complex eIF4F, which also includes the cap-binding protein eIF4E and the scaffolding protein eIF4G (26, 28, 61, 71, 79). The RNA helicase activity of eIF4AI/II is thought to be important for unwinding secondary structure at the 5′ end of the mRNA to allow binding of the 40S ribosome subunit and the initiation of cap-dependent scanning (52).
Binding of Vhs to eIF4H has been demonstrated in Saccharomyces cerevisiae two-hybrid and GST pull-down assays, and the two proteins can be coimmunoprecipitated from mammalian cells (14, 17, 18). In addition, a complex of Vhs and a GST-eIF4H fusion protein has been purified from lysates of bacteria in which the two proteins were coexpressed, indicating that the interaction is direct and not mediated by another eukaryotic protein (14). The complex is an active endonuclease but remains untargeted, since it cuts target RNAs at many sites (14). The data for Vhs interacting with eIF4B are not as extensive. The domain of eIF4H required for Vhs binding overlaps the region of sequence homology shared by eIF4H and eIF4B (17, 18), and an interaction between Vhs and eIF4B has been demonstrated by far-Western blotting (7). In addition, eIF4B and eIF4H both stimulate the basal nuclease activity of Vhs that is expressed in yeast (7). However neither factor alone, or in combination, is sufficient to fully reconstitute the targeting of yeast-expressed Vhs in vitro, suggesting that additional mammalian factors are required (7).
To date, every Vhs mutation that abrogates eIF4H binding also abolishes its ability to degrade mRNAs that are translated by cap-dependent scanning (14, 17, 18). Conversely, so far, every Vhs polypeptide that degrades scanned mRNAs retains the ability to bind eIF4H (14, 17, 18). While these data strongly suggest that binding to eIF4H is required for in vivo Vhs activity, one cannot exclude the possibility that the mutant Vhs proteins fail to degrade mRNAs, not because they do not bind eIF4H but because they are defective in some other unknown function. To address this potential problem, HeLa cells were depleted of eIF4H or other cellular proteins by transfection with small interfering RNAs (siRNAs) 48 h prior to infection or mock infection in the presence of actinomycin D. The levels of cellular mRNAs were then assayed 5 h after infection or mock infection to determine the extent of Vhs-mediated mRNA degradation following depletion of various cellular proteins. In cells transfected with no siRNA, or transfected with an siRNA for the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), wild-type HSV infection reduced the levels of β-actin mRNA to between 20 and 30% of those in mock-infected cells, indicative of a normal Vhs activity. In contrast, in cells transfected with any of three eIF4H siRNAs, β-actin mRNA levels were indistinguishable in infected and mock-infected cells, indicating that eIF4H depletion impeded Vhs-mediated degradation. Surprisingly, siRNA-mediated depletion of eIF4B did not affect Vhs activity. In accord with this, evaluation of a collection of Vhs mutants showed that the abilities of Vhs to bind GST-eIF4H and GST-eIF4B are distinguishable genetically. Mutant polypeptides were identified which bind eIF4B and retain endonuclease activity but which do not degrade scanned mRNAs in vivo, suggesting that the binding of an active Vhs endonuclease to eIF4B is not sufficient to induce mRNA decay. Taken together, the data suggest that binding to eIF4H, but probably not eIF4B, is required for Vhs-induced degradation of many mRNAs, perhaps by targeting Vhs to mRNAs and to preferred sites within mRNAs.
MATERIALS AND METHODS
Cells and virus.
HeLa S3 cells and Vero cells were purchased from the American Type Culture Collection and maintained in Eagle's minimum essential medium (MEM) (GIBCO) supplemented with 10% (vol/vol) calf serum and antibiotics as described previously (15, 16, 48, 57). Wild-type HSV-1, strain KOS, was grown, and its titers were determined, on Vero cells (36, 37, 47, 48), while HeLa cells were used for all siRNA transfections and assays of Vhs-mediated mRNA degradation.
Antibodies.
A polyclonal rabbit antiserum raised against a Vhs-LacZ fusion protein has been described elsewhere (57). Monoclonal antibodies against human eIF4H (WBSCR1) and eIF4B were purchased from ProteinTech Group, Inc., and Cell Signaling Technology, Inc., respectively.
Plasmids.
pKOSamp contains the Vhs open reading frame from HSV-1(KOS) cloned into pcDNA1.1amp (Invitrogen) downstream from a promoter for T7 RNA polymerase and the cytomegalovirus immediate-early promoter (15, 16). It is suitable for expressing Vhs by in vitro transcription and translation or following transfection of mammalian cells. The mutant Vhs alleles D34N, D82N, E192Q, D194N, D195N, T211S, T211A, D213N, D215N, D261N, R435H, K(89-489), K(1-453), K(1-382), and ΔSma have been described previously (14-16, 18, 49, 57). T214I contains a point mutation that changes threonine 214 to isoleucine and is the allele carried by the mutant virus Vhs 1 (15, 16, 49, 56, 57). All mutant Vhs alleles were cloned into pcDNA1.1amp.
pGST-4H contains the eIF4H open reading frame cloned into the vector pGEX-5-3 (Amersham Pharmacia) (17). It encodes a GST-eIF4H fusion protein that can be expressed in bacteria. To construct pGST-4B, the human eIF4B open reading frame was PCR amplified and cloned between the BamHI and SalI sites of pGEX-5-3. It encodes a fusion protein of GST and eIF4B. pcDNA-4AII (18) and pcDNA-4B contain the eIF4AII and eIF4B open reading frames, respectively, cloned into pcDNA1.1amp downstream from a promoter recognized by T7 RNA polymerase. They were used to produce eIF4AII and eIF4B, respectively, by in vitro transcription and translation.
siRNA transfections.
siRNAs against human GAPDH, eIF4H, and eIF4B were purchased from Ambion. The structures of the siRNAs and their locations within the eIF4H and eIF4B mRNAs are shown in Fig. 1. siRNA transfections of HeLa cells in six-well trays were performed using the siPORT NeoFX transfection reagent (Ambion) as recommended by the manufacturer. In each experiment, each transfection was performed in triplicate. Briefly, 2 μM solutions of the siRNAs were prepared in nuclease-free water. For each transfection, 5 μl of siPORT NeoFX transfection reagent was mixed with 295 μl of Opti-MEM I medium (Ambion) and incubated at room temperature for 10 min. This was then mixed with a mixture containing 45 μl of a 2 μM stock of siRNA and 255 μl of Opti-MEM I, and incubation continued for 10 min. During this interval, HeLa cells were harvested and resuspended at a concentration of 1 × 105 cells/ml in MEM containing 10% calf serum. For each transfection, 600 μl of the mixture of siRNA, siPORT NeoFX, and Opti-MEM I was added to an empty well of a six-well plate, after which each well received 2.4 ml of the HeLa cell suspension. Cultures were incubated at 37°C for 24 h, at which point the medium was replaced with fresh MEM plus 10% (vol/vol) calf serum, and incubation continued for another 24 h. At 48 h after transfection, the cells were infected with 20 PFU/cell of wild-type HSV-1(KOS) or mock infected, both in the presence of 5 μg/ml of actinomycin D. Total cytoplasmic RNAs were isolated 5 h after infection or mock infection (47, 48) and analyzed by real-time quantitative reverse transcription-PCR to determine the levels of β-actin mRNA, using 18S rRNA as an internal control. β-Actin mRNA levels were compared in infected and mock-infected cells to determine the extent of Vhs-mediated mRNA degradation.
FIG. 1.
Structures of eIF4H and eIF4B siRNAs. The structures of eIF4B and eIF4H are depicted at the top of the figure, with the regions of sequence homology shared by eIF4B (amino acids 90 to 172) and eIF4H (amino acids 36 to 117) represented by the black rectangles. The region of eIF4H shown by deletion analysis to be required for Vhs binding (amino acids 90 to 136) (17) is depicted by the solid line below the rectangle for eIF4H. Alteration of any of three amino acids (E97, D102, or D114) to alanine significantly reduces the binding of eIF4H to Vhs (18). The locations of these amino acids are depicted by the arrows below the rectangle for eIF4H. The sequences of the three eIF4H siRNAs and one eIF4B siRNA used in these experiments are shown at the bottom of the figure, along with their positions within eIF4H or eIF4B mRNAs. The identification number assigned by the manufacturer (Ambion) to each siRNA is shown at the left. UTR, untranslated region.
Western blotting.
To determine the effect of transfected siRNAs upon the expression of cellular translation factors, cells were harvested 48 h after transfection and lysed by boiling in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris [pH 7.0], 2% [wt/vol] SDS, 10% [vol/vol] glycerol, 5% [vol/vol] beta-mercaptoethanol). Proteins in whole-cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting (57), using antibodies against eIF4H, eIF4B, or GAPDH.
Real-time quantitative reverse transcription-PCR.
The relative amounts of β-actin mRNA in mock-infected and infected cells were quantified by real-time quantitative reverse transcription-PCR using an Applied Biosystems Model 7500 Real Time PCR system. Each sample of cytoplasmic RNA was reverse transcribed using a High Capacity cDNA Reverse Transcription kit from Applied Biosystems. Quadruplicate aliquots of each cDNA reaction mixture were amplified using TaqMan primers and probes for human β-actin mRNA, and another four aliquots were amplified with primers and probes for 18S rRNA. Primers and probes were purchased from Applied Biosystems. β-Actin mRNA levels were determined using 18S rRNA as an internal standard, since Vhs does not affect rRNAs (37, 47, 48, 66, 77). For each type of transfection the relative amounts of β-actin mRNA in mock-infected and infected cultures were determined using the threshold cycle method described in the Model 7500 manual (4). Propagation of standard deviations from multiple experiments was performed according to established procedures (3).
In vitro transcription and translation.
[35S]methionine-labeled eIF4AII, eIF4B, and wild-type or mutant Vhs were produced by in vitro transcription and translation from pcDNA-4AII, pcDNA-4B, pKOSamp, or plasmids containing mutant Vhs alleles, using the TnT T7 Coupled Transcription/Translation system from Promega (17, 18).
GST pull-down assays.
For in vitro binding assays, GST, GST-eIF4H, or GST-eIF4B was expressed in Escherichia coli strain BL21 and isolated by binding to glutathione-Sepharose 4B as previously described (17, 18). Beads with bound GST, GST-eIF4H, or GST-eIF4B were resuspended in 0.4-ml portions of rabbit reticulocyte lysate containing [35S]methionine-labeled eIF4B, eIF4AII, or wild-type or mutant Vhs. After 30 min of incubation with constant end-over-end agitation, the beads were pelleted and washed, and complexes containing GST, GST-eIF4H, or GST-eIF4B and any bound proteins eluted as described previously (17, 18). Bound proteins were resolved by SDS-PAGE, and the relative amounts of [35S]methionine-labeled Vhs, eIF4B, or eIF4AII were quantified using a Storm Model 840 Phosphoimager (Molecular Dynamics, Inc.). Binding of any of the three proteins to GST was virtually undetectable, indicating that the binding to GST-eIF4B or GST-eIF4H was specific.
To compare the relative binding of wild-type and mutant Vhs polypeptides to GST-eIF4B, binding reactions were performed with mixtures containing a [35S]methionine-labeled Vhs protein and [35S]methionine-labeled eIF4B. The binding of [35S]methionine-labeled eIF4B to GST-eIF4B reflected the fact that eIF4B exists as a dimer in vivo (13, 17, 24, 25) and provided an internal control for the amount of protein loaded onto the gel. The ratio (bound Vhs/bound eIF4B) for any Vhs allele was divided by the ratio (input Vhs/input eIF4B) determined for an aliquot of the input material that had not been exposed to the GST-eIF4B Sepharose 4B beads. This ratio (bound Vhs/bound eIF4B)/(input Vhs/input eIF4B) was normalized to 1.0 for wild-type Vhs, and the ratio (bound Vhs/bound eIF4B)/(input Vhs/input eIF4B) was calculated for each mutant Vhs polypeptide relative to the wild-type ratio. While this did not provide a measurement of the relative binding affinities of different Vhs polypeptides, it did provide a measure of the relative amounts of wild-type Vhs and mutant Vhs polypeptides that bound GST-eIF4B under these experimental conditions.
RESULTS
One of the strongest indications that binding to eIF4H is important for Vhs-induced decay is the observation that, to date, every Vhs mutation that abrogates its binding to eIF4H greatly reduces its ability to degrade mRNAs that are translated by cap-dependent scanning, even if the mutant protein retains endonuclease activity (14, 17, 18). Conversely, every Vhs polypeptide that degrades scanned mRNAs binds eIF4H. While these data are very suggestive, one cannot exclude the possibility that the mutant Vhs polypeptides fail to degrade mRNAs not because they do not bind eIF4H but because they are defective in some other unknown function. To address this potential problem, we tested whether siRNA-mediated knockdown of the level of eIF4H prior to infection would affect the ability of wild-type Vhs to induce mRNA decay.
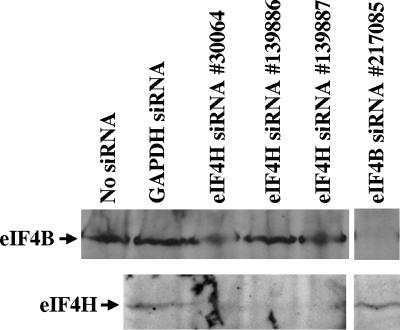
The first step was to check whether transfection of cells with various eIF4H siRNAs reduces the level of eIF4H protein. HeLa cells were transfected with three different eIF4H siRNAs, one directed against a sequence within the open reading frame and two against sequences in the 3′ untranslated region (Fig. 1). Parallel cultures were transfected with siRNAs against eIF4B or the housekeeping enzyme GAPDH or mock transfected. Cell lysates were prepared 48 h after transfection and examined by SDS-PAGE and Western blotting to determine the levels of eIF4H and eIF4B protein (Fig. 2). None of the siRNAs had an apparent effect upon cell number or morphology, as determined by light microscopy 48 h after transfection, nor did they affect the amount of 18S rRNA that could be recovered from the cultures (data not shown), further indicating that none of the siRNAs, or the transfection procedure itself, had a significant cytotoxic effect upon the cells over the first 48 h after transfection. Most importantly, each eIF4H siRNA significantly reduced the level of eIF4H relative to that in cells transfected with a GAPDH siRNA (Fig. 2). Two of the siRNAs (139886 and 139887) did not appreciably affect the level of the related factor eIF4B. On this gel the lane for the third eIF4H siRNA (30064) was compressed, making it unclear whether transfection with this siRNA had induced a small decrease in the level of eIF4B. Examination of other gels from this or other transfections indicates that this was a gel artifact and that siRNA 30064 caused little, if any, decrease in the level of eIF4B (data not shown). Conversely, an eIF4B siRNA (217085) reduced the level of eIF4B but not that of eIF4H.
FIG. 2.
Transfection with siRNAs depletes the levels of eIF4H or eIF4B. HeLa cells were transfected with no siRNA, with siRNAs for GAPDH or eIF4B, or with three different siRNAs for eIF4H as indicated above the gel lanes. Cell lysates were prepared 48 h after transfection and assayed by Western blotting for eIF4B or eIF4H as indicated to the left of the gel. Each eIF4H-specific siRNA significantly reduced the level of eIF4H, but not eIF4B. The eIF4B siRNA depleted the level of eIF4B, but not eIF4H.
To examine the effect of eIF4H depletion upon Vhs activity, HeLa cells were infected 48 h after siRNA transfection with 20 PFU/cell of wild-type HSV-1 (KOS) or mock infected, both in the presence of 5 μg/ml of actinomycin D. Cytoplasmic RNAs were prepared 5 h later, and the levels of β-actin mRNA were determined by real-time quantitative reverse transcription-PCR using 18S rRNA as an internal control. Comparison of mRNA levels following infection or mock infection in the presence of actinomycin D is a standard assay for Vhs activity (20-23, 39, 47, 55, 57, 66, 77). In cells transfected with no siRNA or an siRNA for GAPDH, wild-type HSV infection reduced the level of β-actin mRNA to between 20 and 30% of that in mock-infected cells (Fig. 3), indicative of a normal Vhs activity. In contrast, in cells transfected with two of the three eIF4H siRNAs (139887 and 30064), β-actin mRNA levels were indistinguishable in infected and mock-infected cells, indicating that transfection with these siRNAs prevented detectable Vhs-mediated mRNA degradation. In cells transfected with the third siRNA (139886), the level of β-actin mRNA was 70% of that in mock-infected cells, indicating that, while Vhs-mediated degradation was not completely abolished, it was significantly impeded.
FIG. 3.
siRNA-mediated depletion of eIF4H impedes Vhs-induced degradation of β-actin mRNA. HeLa cells were transfected with no siRNA, with an siRNA for GAPDH, or with three different siRNAs for eIF4H as indicated. The cells were infected 48 h after transfection with 20 PFU/cell of wild-type (WT) HSV-1 or mock infected, both in the presence of actinomycin D. Total cytoplasmic RNAs were prepared 5 h after infection or mock infection and analyzed by real-time reverse transcription-PCR for the relative amounts of the housekeeping β-actin mRNA, using 18S rRNA as an endogenous control. For each type of transfection, the amount of β-actin mRNA in infected cells (gray bar) was normalized to the amount in mock-infected cells (black bar). Error bars represent the standard deviations of replicate samples and multiple experiments.
Because of the functional similarities between eIF4H and eIF4B (28, 59-64), it was important to examine whether eIF4B also plays an important role in Vhs-mediated mRNA decay. The region of eIF4H shown by deletion mapping to be necessary and sufficient for binding Vhs overlaps the region of sequence homology shared by eIF4H and eIF4B (17), and several point mutations in eIF4H which reduce binding to Vhs fall within this region of shared homology (Fig. 1) (18). In addition, purified eIF4H and eIF4B both stimulate the basal Vhs endonuclease activity that is observed in whole extracts of yeast that have been engineered to express Vhs, although neither fully reconstitutes the targeted cleavage of mRNAs that is observed for in vitro-translated Vhs in rabbit reticulocyte lysates (7, 43). The potential role of eIF4B in Vhs-mediated decay was examined in two ways. The first involved comparing the binding of mutant and wild-type Vhs polypeptides to eIF4H and eIF4B.
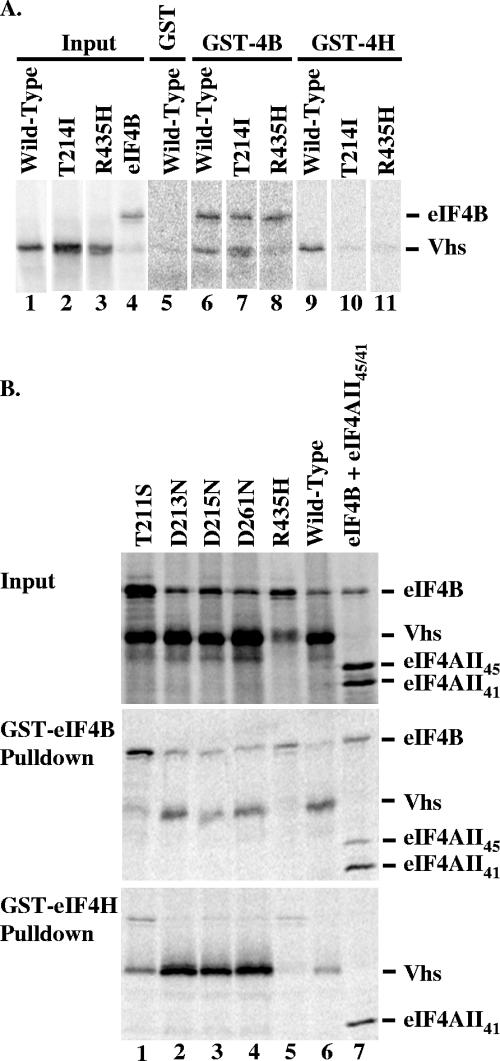
To date, binding of Vhs to eIF4B has been demonstrated only using a far-Western blotting assay in which soluble in vitro-translated Vhs bound to denatured eIF4B on a nitrocellulose membrane (7). In the present study we examined the binding of Vhs to GST-eIF4B or GST-eIF4H in solution, focusing on wild-type Vhs and a collection of mutant Vhs polypeptides, each of which lacks in vivo mRNA-degradative activity and has been characterized previously with respect to binding eIF4H (see Fig. 6) (14, 17, 18). In this experiment, each binding reaction mixture contained an excess of GST-eIF4B or GST-eIF4H and a mixture of [35S]methionine-labeled, in vitro-translated Vhs and eIF4B (Fig. 4 and 5).Control reactions showed that GST-eIF4B readily binds in vitro-translated eIF4B, as well as two forms of eIF4AII: the full-length 407-amino-acid polypeptide (labeled eIF4AII45) and a truncated form (labeled eIF4AII41) containing amino acids 38 through 407 of the full-length protein (Fig. 4B, lane 7, middle panel). Previous studies showed that eIF4AII41 is produced during in vitro translation by initiation at an AUG codon that encodes methionine 38 of the full-length protein (18). Binding of GST-eIF4B to in vitro-translated eIF4B reflected the fact that eIF4B exists as a dimer in vivo (28, 45, 61, 63) and provided an internal control for the amount of protein loaded onto the gel. GST-eIF4H differed from GST-eIF4B in that it did not interact appreciably with eIF4B (Fig. 4B, lane 7, bottom panel). This was unsurprising because the region of homology shared by eIF4H and eIF4B does not contain the dimerization domain of eIF4B (18, 28). GST-eIF4H also differed from GST-eIF4B in that it bound the truncated form of eIF4AII (eIF4AII41) more robustly than the full-length protein (eIF4AII45) (Fig. 4B, lane 7, bottom panel). In accord with previous results (18), binding to eIF4AII45 was observed (data not shown), but only upon longer exposure of the gel.
FIG. 6.
Summary of the structures and in vivo mRNA-degradative activities of wild-type and mutant Vhs polypeptides and their abilities to bind eIF4H and eIF4B. The Vhs polypeptide encoded by wild-type HSV-1(KOS) is represented by the solid rectangle in line 1, and the structures of deletion and point mutants of the HSV-1(KOS) polypeptide are in lines 2 through 17. For deletion mutants, the Vhs residues included in the mutant proteins are indicated. For each point mutant, the location of the altered residue is indicated by the vertical line above the bar representing the protein. The in vivo mRNA-degradative activity of each Vhs protein is shown in the third column from the right and summarizes, in a qualitative fashion, quantitative measurements that were reported previously (14-16, 49, 57). Activity was assayed for all Vhs alleles using a cotransfection assay of Vhs activity and during virus infections for those alleles marked by an asterisk. The double plus sign indicates activity similar to that of wild-type HSV-1(KOS), and the minus sign indicates no detectable mRNA-degradative activity. The second column from the right indicates whether a Vhs protein binds (++) or does not bind (−) eIF4H and summarizes data that have been reported previously (14, 17, 18). The binding of various mutant Vhs polypeptides to GST-eIF4B was expressed relative to the binding of wild-type Vhs to GST-eIF4B, as explained in Materials and Methods, and is shown in the rightmost column.
FIG. 4.
Binding of wild-type and mutant Vhs polypeptides to GST-eIF4B and GST-eIF4H. (A) [35S]methionine-labeled wild-type polypeptide and the mutant Vhs polypeptides T214I and R435H were produced by in vitro transcription and translation, as was [35S]methionine-labeled eIF4B. Aliquots of the in vitro-translated material are shown in lanes 1 through 4. Each of the Vhs polypeptides was mixed with an approximately equal amount (as judged by [35S]methionine incorporation) of eIF4B and analyzed for the ability to bind GST-eIF4B and GST-eIF4H as described previously (14, 17, 18). Equal aliquots of the same mixture of wild-type Vhs and eIF4B were assayed for binding GST (lane 5), GST-eIF4B (lane 6), and GST-eIF4H (lane 9) (14, 17, 18). Similarly, equal aliquots of the same mixture of the T214I polypeptide and eIF4B were assayed for binding GST-eIF4B (lane 7) and GST-eIF4H (lane 10), while equal aliquots of the same mixture of the R435H polypeptide and eIF4B were assayed for binding GST-eIF4B (lane 8) and GST-eIF4H (lane 11). Bound proteins were eluted from the beads and analyzed by SDS-PAGE and autoradiography. (B) [35S]methionine-labeled HSV-1(KOS) and mutant Vhs polypeptides were produced by in vitro transcription and translation and mixed with [35S]methionine-labeled, in vitro-translated eIF4B (lanes 1 to 6). The Vhs polypeptides and eIF4B were analyzed for the ability to bind GST-eIF4B and GST-eIF4H as described previously (14, 17, 18). Proteins that bound to GST-eIF4B (middle panel) and GST-eIF4H (bottom panel) were analyzed by SDS-PAGE and autoradiography. Aliquots of the input in vitro-translated material are shown in the top panel. The wild-type HSV-1(KOS) and mutant Vhs polypeptides are indicated at the top of each lane. Their structures are diagrammed in Fig. 6. In a parallel reaction, [35S]methionine-labeled eIF4B was mixed with full-length in vitro-translated eIF4AII (eIF4AII45) and a truncated form of eIF4AII containing amino acids 38 through 407 of the full-length protein (lane 7) and analyzed for binding to GST-eIF4B (middle panel) and GST-eIF4H (bottom panel).
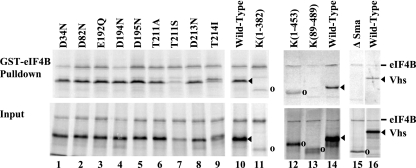
FIG. 5.
Binding of wild-type HSV-1(KOS) and mutant Vhs polypeptides to GST-eIF4B. [35S]methionine-labeled HSV-1(KOS) and mutant Vhs polypeptides were produced by in vitro transcription and translation, mixed with [35S]methionine-labeled in vitro-translated eIF4B, and analyzed for the ability to bind GST-eIF4B (14, 17, 18). Proteins that bound to GST-eIF4B were analyzed by SDS-PAGE and autoradiography in the upper panel (GST-eIF4B Pulldown), while aliquots of the input in vitro-translated material are shown in the lower panel (Input). The wild-type HSV-1(KOS) and mutant Vhs polypeptides are indicated at the top of each lane. Their structures are diagrammed in Fig. 6. All binding reactions were performed in the same experiment, but lanes 1 through 9 were run on one gel, lanes 10 and 11 on another, lanes 12 through 14 on a third gel, and lanes 15 and 16 on a fourth. The gels were not run for precisely the same length of time, which is why the electrophoretic mobility of wild-type Vhs, relative to eIF4B, was not the same for the different gels. The location of the wild-type Vhs protein is indicated by filled arrowheads to the right of lanes 10, 14, and 16. The locations of mutant Vhs polypeptides that are not full length are shown by open circles to the right of lanes 11 to 13 and 15.
Analysis of the Vhs mutations revealed a number, such as D34N or D194N, that had no appreciable effect upon the amount of Vhs that bound to either GST-eIF4H or GST-eIF4B (Fig. 4, 5, and 6) (17). Many of these were point mutations that altered key conserved residues in the nuclease motif of Vhs (14). Several other mutations—typified by R435H, K(89-489), and ΔSma—greatly reduced binding to both GST-eIF4H and GST-eIF4B (Fig. 4, 5, and 6) (17). However, a number of mutations had different effects upon Vhs binding to GST-eIF4H and GST-eIF4B (Fig. 6). The alleles K(1-382) and K(1-453) contain nonsense mutations that result in truncated Vhs polypeptides containing the first 382 and 453 amino acids of the wild-type protein, respectively (Fig. 6) (17, 49). These mutations abolished detectable binding to GST-eIF4H (Fig. 6) (17) but either had little [K(1-382)] or much less [K(1-453)] of an effect on binding to GST-eIF4B (Fig. 5 and 6). Similarly, the point mutations T211A and T214I greatly reduced Vhs binding to eIF4H (17) but did not affect binding to eIF4B (Fig. 5 and 6). T214I was particularly informative. This mutant polypeptide lacks detectable in vivo mRNA-degradative activity for mRNAs that are translated by cap-dependent scanning (48, 56) but still cuts downstream of an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) in vitro (44), indicating that it retains endonuclease activity. It fails to bind eIF4H (17, 18) but still binds eIF4B (Fig. 5 and 6), indicating not only that binding to eIF4B and eIF4H can be distinguished genetically but that binding of an active Vhs endonuclease to eIF4B is not sufficient for in vivo degradation of scanned mRNAs.
The second, and complementary, approach that was used to analyze the involvement of eIF4B in Vhs activity was to determine whether siRNA-mediated knockdown of the level of eIF4B inhibits Vhs-mediated degradation of β-actin mRNA. As with the experiment in Fig. 3, HeLa cells were transfected with no siRNA or with siRNAs for GAPDH or eIF4B (Fig. 7). Transfection with this eIF4B siRNA (217085) significantly reduces the level of eIF4B while not affecting the level of eIF4H (Fig. 2). At 48 h after transfection, the cells were infected with 20 PFU/cell of wild-type HSV-1(KOS) or mock infected, both in the presence of 5 μg/ml of actinomycin D. Cytoplasmic RNAs were analyzed 5 h later, and the relative levels of β-actin mRNA in infected and mock-infected cultures were taken as a measure of the extent of Vhs-mediated mRNA degradation (Fig. 7). In cells transfected with the eIF4B siRNA, wild-type HSV infection reduced the level of β-actin mRNA to between 10 and 20% of that in mock-infected cells (Fig. 7), an effect similar to that seen in cells transfected with no siRNA or a GAPDH siRNA and indicative of a normal Vhs activity. Thus, in contrast to the situation for eIF4H, significant siRNA-mediated depletion of the level of eIF4B did not impede Vhs-induced degradation of β-actin mRNA.
FIG. 7.
siRNA-mediated depletion of eIF4B does not affect Vhs-induced degradation of β-actin mRNA. HeLa cells were transfected with no siRNA or with siRNAs for GAPDH or eIF4B as indicated. The cells were infected 48 h after transfection with 20 PFU/cell of wild-type (WT) HSV-1 or mock infected, both in the presence of actinomycin D. Total cytoplasmic RNAs were prepared 5 h after infection or mock infection and analyzed by real-time reverse transcription-PCR for the relative amounts of β-actin mRNA, using 18S rRNA as an endogenous control. For each type of transfection, the amount of β-actin mRNA in infected cells (gray bar) was normalized to the amount in mock-infected cells (black bar). Error bars represent the standard deviations of replicate samples and multiple experiments.
DISCUSSION
These studies demonstrate that transfection of cells with any of three different siRNAs, which deplete the level of eIF4H, inhibits Vhs-mediated degradation of β-actin mRNA, while depletion of the related factor eIF4B has no detectable effect on Vhs activity. These results are significant because they bolster, through a complementary approach, the results of genetic and protein-protein interaction experiments (14, 17, 18) suggesting that the binding of Vhs to eIF4H, but not to eIF4B, is required for Vhs-mediated degradation of many cellular and viral mRNAs for which translation is initiated by cap-dependent ribosome scanning.
The most straightforward interpretation of these results is that siRNA-mediated depletion of eIF4H is the direct cause of the failure of Vhs to degrade β-actin mRNA, presumably because if eIF4H is not present, Vhs cannot bind to it. One cannot rule out, however, the possibility that the failure of Vhs results, not from the depletion of eIF4H, but from an as-yet-unidentified off-target effect on expression of some other cellular gene(s). The fact that Vhs is inhibited by three different eIF4H siRNAs suggests that this is not the case. Although all three siRNAs knock down expression of eIF4H, they are unrelated in sequence. One, therefore, would not expect the same off-target effect to be caused by all three siRNAs.
Another possibility is that the inhibition of Vhs is caused by eIF4H depletion, but not because binding to eIF4H is required for Vhs activity. Instead, Vhs inhibition might be an indirect effect of eIF4H knockdown on the expression of another cellular protein that is directly required for Vhs activity. The strongest argument against this is our previous data showing that, to date, every Vhs protein that degrades scanned mRNAs binds eIF4H, while T214I, and every other mutation that abrogates eIF4H binding, impedes the Vhs degradation of many viral and constitutively expressed cellular mRNAs (14, 17, 18). siRNA-mediated knockdowns and experiments with mutant Vhs proteins must both be interpreted with caveats, but not the same caveats. The fact that these different, yet complementary, approaches both point to the same conclusion strongly supports the notion that binding to eIF4H is required for Vhs-mediated degradation of β-actin mRNA. In addition, the fact that Vhs mutations, which abrogate eIF4H binding, affect expression of many cellular genes indicates that the Vhs-eIF4H interaction is required for degradation not just of β-actin mRNA but of many cellular and viral mRNAs.
A similar combination of siRNA-mediated knockdowns and the analysis of Vhs mutants suggests that binding to eIF4B does not play a critical role in Vhs activity. Transfection with an eIF4B siRNA caused significant depletion of eIF4B levels but did not affect Vhs-mediated mRNA degradation. Although suggestive, these experiments do not absolutely prove that eIF4B is not required for Vhs activity, since it is unlikely that the cells were depleted of all eIF4B, and whatever eIF4B remained may have been enough for Vhs activity. However, the Vhs mutant T214I fails to bind eIF4H (17, 18) and lacks detectable mRNA-degradative activity against mRNAs that are translated by cap-dependent scanning (47-49, 56), even though the mutant polypeptide retains endonuclease activity (44) and still binds eIF4B (Fig. 5 and 6). Thus, not only is Vhs activity unaffected by significant siRNA-mediated reduction of eIF4B levels, but the ability of an active Vhs endonuclease to bind eIF4B is not sufficient for it to induce degradation of scanned mRNAs.
Although eIF4H appears to be required for Vhs degradation of many mRNAs, it cannot be the only factor required for appropriate targeting of the endonuclease within infected cells, since a purified complex of recombinant Vhs and GST-eIF4H remains a sequence-nonspecific endonuclease that does not distinguish mRNAs from nonmessenger RNAs and cleaves target RNAs at many sites (18). An attractive candidate as an additional factor that helps target Vhs is eIF4AI/II, the RNA helicase whose activity is stimulated by eIF4H and eIF4B and which is a component of the cap-binding complex eIF4F. Vhs has been shown to bind eIF4AII (18) and can be isolated as a component of cap-binding complexes prepared by chromatography of cytoplasmic extracts on 7-methyl GTP Sepharose 4B (H. Page and G. S. Read, unpublished data), an observation that may explain why, for some mRNAs, the preferred sites of Vhs cleavage are near regions of translation initiation. To date, attempts to test whether siRNA-mediated depletion of eIF4AI and II affects Vhs activity have not yielded easily interpretable results (data not shown), perhaps because eIF4AI/II depletion has multiple downstream effects. Clearly, more needs to be done to test the role of eIF4AI/II in Vhs activity.
While the current studies indicate the importance of eIF4H in the Vhs-mediated decay of many mRNAs, it is important to identify any mRNAs that are susceptible to Vhs but whose degradation does not require the binding of Vhs to eIF4H, as well as any mRNAs that are not degraded by Vhs. An apparent example of the first possibility is provided by mRNAs that contain an EMCV IRES. Vhs cuts downstream from an EMCV IRES in rabbit reticulocyte lysates (10), and this activity is retained by the mutant T214I polypeptide (44), even though it does not bind eIF4H (14, 17, 18) or degrade mRNAs translated by cap-dependent scanning (37, 47, 48, 56). In a reconstituted in vitro translation system, initiation at an EMCV IRES does not require eIF4H but does involve binding of a complex of eIF4G and eIF4A directly to the IRES (27, 35, 42, 51, 53). Targeting of the T214I protein to the IRES may result from the fact that it still binds eIF4AII. The data raise the intriguing possibility that Vhs degradation of mRNAs containing an EMCV IRES requires a different set of cellular factors than those required for Vhs degradation of mRNAs translated by cap-dependent ribosome scanning. An example of the second possibility is IEX-1 mRNA, a cellular mRNA whose expression is stimulated following HSV infection (82) and which is apparently refractory to Vhs-mediated degradation. IEX-1 mRNA is stabilized early after infection by the HSV immediate-early protein ICP27, through a process involving activation of the p38 mitogen-activated protein kinase pathway (6, 29), although the details of how this impedes Vhs-mediated degradation remain to be determined.
Without knowledge of the structure of Vhs bound to eIF4H and of the interactions of the complex with mRNA and other factors, one cannot propose a detailed model for how eIF4H participates in Vhs-mediated mRNA turnover. Nevertheless, current data suggest that its role may involve more than simply tethering Vhs in the vicinity of the 5′ cap. Vhs can be found associated with the cap-binding complex eIF4F, although the association apparently is not dependent upon its ability to bind eIF4H, since the mutant T214I polypeptide, which does not bind eIF4H, still associates with eIF4F. Although only a limited number of Vhs mutants have been examined so far, the ability of Vhs to associate with eIF4F appears to correlate with its ability to bind eIF4AII (H. Page and G. S. Read, unpublished data), which is itself a component of the cap-binding complex. The data for T214I also suggest that the simple association of an active Vhs endonuclease with the cap-binding complex is not sufficient to induce degradation of scanned mRNAs. While it remains possible that eIF4H is also required to tether or orient Vhs in the appropriate conformation, it is possible that successful Vhs cleavage of mRNAs is dependent upon some other activity of eIF4H, such as its ability to stimulate the ATP-dependent RNA helicase activity of eIF4AI and -II. Finally, given the functional similarities between eIF4H and eIF4B, it is a puzzle that eIF4H apparently plays an important role in Vhs activity, while eIF4B does not. Efforts are under way to further elucidate the differences between eIF4H and eIF4B and to determine what features and activities of eIF4H are required for its role in Vhs-mediated mRNA degradation.
Acknowledgments
We thank Heidi Page, June Deng, and our other colleagues at the University of Missouri—Kansas City for many helpful discussions. We thank Steve Appier and the other science teachers at Shawnee Mission East High School, Prairie Village, KS, for help and guidance to N.S., who is a student at the school.
This work was supported by Public Health Service grant RO1 AI-21501 to G.S.R. from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Barzilai, A., I. Zivony-Elbom, R. Sarid, E. Noah, and N. Frenkel. 2006. The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery. J. Virol. 80505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, Y., E. Tavor, Y. Asher, C. Berkowiltz, and M. Moyal. 1993. Effect of herpes simplex virus type-1 UL41 gene on the stability of mRNA from the cellular genes: beta-actin, fibronectin, glucose transporter-1, and docking protein, and on virus intraperitoneal pathogenicity of newborn mice. Virus Genes 7133-143. [DOI] [PubMed] [Google Scholar]
- 3.Bevington, P. R. 1969. Data reduction and error analysis for the physical sciences. McGraw-Hill Book Company, New York, NY.
- 4.Bookout, A. L., C. L. Cummins, D. J. Mangelsdorf, J. M. Pesola, and M. F. Kramer. 2006. High-throughput real-time quantitative reverse transcription PCR, p. 15.8.1-15.8.28. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed]
- 5.Chee, A. V., and B. Roizman. 2004. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 784185-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcoran, J. A., W. L. Hsu, and J. R. Smiley. 2006. Herpes simplex virus ICP27 is required for virus-induced stabilization of the ARE-containing IEX-1 mRNA encoded by the human IER3 gene. J. Virol. 809720-9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doepker, R. C., W. L. Hsu, H. A. Saffran, and J. R. Smiley. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 784684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duerst, R. J., and L. A. Morrison. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 322158-167. [DOI] [PubMed] [Google Scholar]
- 9.Elgadi, M. M., C. E. Hayes, and J. R. Smiley. 1999. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J. Virol. 737153-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elgadi, M. M., and J. R. Smiley. 1999. Picornavirus internal ribosome entry site elements target RNA cleavage events induced by the herpes simplex virus virion host shutoff protein. J. Virol. 739222-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esclatine, A., B. Taddeo, L. Evans, and B. Roizman. 2004. The herpes simplex virus 1 UL41 gene-dependent destabilization of cellular RNAs is selective and may be sequence-specific. Proc. Natl. Acad. Sci. USA 1013603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esclatine, A., B. Taddeo, and B. Roizman. 2004. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 788582-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esclatine, A., B. Taddeo, and B. Roizman. 2004. The UL41 protein of herpes simplex virus mediates selective stabilization or degradation of cellular mRNAs. Proc. Natl. Acad. Sci. USA 10118165-18170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 768560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everly, D. N., Jr., and G. S. Read. 1997. Mutational analysis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): characterization of HSV type 1 (HSV-1)/HSV-2 chimeras. J. Virol. 717157-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everly, D. N., Jr., and G. S. Read. 1999. Site-directed mutagenesis of the virion host shutoff gene (UL41) of herpes simplex virus (HSV): analysis of functional differences between HSV type 1 (HSV-1) and HSV-2 alleles. J. Virol. 739117-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, P., D. N. Everly, Jr., and G. S. Read. 2001. mRNA decay during herpesvirus infections: interaction between a putative viral nuclease and a cellular translation factor. J. Virol. 7510272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, P., D. N. Everly, Jr., and G. S. Read. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 799651-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenwick, M. L. 1984. The effects of herpesviruses on cellular macromolecular synthesis, p. 359-390. In H. Fraenkel-Conrat and R. R. Wagner (ed.), Comprehensive virology, vol. 19. Plenum Publishing Corp., New York, NY. [Google Scholar]
- 20.Fenwick, M. L., and J. Clark. 1982. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J. Gen. Virol. 61121-125. [DOI] [PubMed] [Google Scholar]
- 21.Fenwick, M. L., and R. D. Everett. 1990. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J. Gen. Virol. 712961-2967. [DOI] [PubMed] [Google Scholar]
- 22.Fenwick, M. L., and R. D. Everett. 1990. Transfer of UL41, the gene controlling virion-associated host cell shutoff, between different strains of herpes simplex virus. J. Gen. Virol. 71411-418. [DOI] [PubMed] [Google Scholar]
- 23.Fenwick, M. L., and M. M. McMenamin. 1984. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J. Gen. Virol. 651225-1228. [DOI] [PubMed] [Google Scholar]
- 24.Fenwick, M. L., and S. A. Owen. 1988. On the control of immediate early (alpha) mRNA survival in cells infected with herpes simplex virus. J. Gen. Virol. 692869-2877. [DOI] [PubMed] [Google Scholar]
- 25.Geiss, B. J., T. J. Smith, D. A. Leib, and L. A. Morrison. 2000. Disruption of virion host shutoff activity improves the immunogenicity and protective capacity of a replication-incompetent herpes simplex virus type 1 vaccine strain. J. Virol. 7411137-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68913-963. [DOI] [PubMed] [Google Scholar]
- 27.Hellen, C. U., and P. Sarnow. 2001. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 151593-1612. [DOI] [PubMed] [Google Scholar]
- 28.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-89. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Hsu, W. L., H. A. Saffran, and J. R. Smiley. 2005. Herpes simplex virus infection stabilizes cellular IEX-1 mRNA. J. Virol. 794090-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, F. E., C. A. Smibert, and J. R. Smiley. 1995. Mutational analysis of the herpes simplex virus virion host shutoff protein: evidence that vhs functions in the absence of other viral proteins. J. Virol. 694863-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karr, B. M., and G. S. Read. 1999. The virion host shutoff function of herpes simplex virus degrades the 5′ end of a target mRNA before the 3′ end. Virology 264195-204. [DOI] [PubMed] [Google Scholar]
- 32.Keadle, T. L., K. A. Laycock, J. L. Morris, D. A. Leib, L. A. Morrison, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic vaccination with vhs(-) herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J. Gen. Virol. 832361-2365. [DOI] [PubMed] [Google Scholar]
- 33.Keadle, T. L., L. A. Morrison, J. L. Morris, J. S. Pepose, and P. M. Stuart. 2002. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J. Virol. 763615-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khodarev, N. N., S. J. Advani, N. Gupta, B. Roizman, and R. R. Weichselbaum. 1999. Accumulation of specific RNAs encoding transcriptional factors and stress response proteins against a background of severe depletion of cellular RNAs in cells infected with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 9612062-12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolupaeva, V. G., T. V. Pestova, C. U. Hellen, and I. N. Shatsky. 1998. Translation eukaryotic initiation factor 4G recognizes a specific structural element within the internal ribosome entry site of encephalomyocarditis virus RNA. J. Biol. Chem. 27318599-18604. [DOI] [PubMed] [Google Scholar]
- 36.Krikorian, C. R., and G. S. Read. 1989. Proteins associated with mRNA in cells infected with herpes simplex virus. Biochem. Biophys. Res. Commun. 164355-361. [DOI] [PubMed] [Google Scholar]
- 37.Krikorian, C. R., and G. S. Read. 1991. In vitro mRNA degradation system to study the virion host shutoff function of herpes simplex virus. J. Virol. 65112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong, A. D., and N. Frenkel. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. USA 841926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwong, A. D., J. A. Kruper, and N. Frenkel. 1988. Herpes simplex virus virion host shutoff function. J. Virol. 62912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang, L., and B. Roizman. 2006. Herpes simplex virus 1 precludes replenishment of the short-lived receptor of tumor necrosis factor alpha by virion host shutoff-dependent degradation of its mRNA. J. Virol. 807756-7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomakin, I. B., C. U. Hellen, and T. V. Pestova. 2000. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol. Cell. Biol. 206019-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu, P., F. E. Jones, H. A. Saffran, and J. R. Smiley. 2001. Herpes simplex virus virion host shutoff protein requires a mammalian factor for efficient in vitro endoribonuclease activity. J. Virol. 751172-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, P., H. A. Saffran, and J. R. Smiley. 2001. The vhs1 mutant form of herpes simplex virus virion host shutoff protein retains significant internal ribosome entry site-directed RNA cleavage activity. J. Virol. 751072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Methot, N., M. S. Song, and N. Sonenberg. 1996. A region rich in aspartic acid, arginine, tyrosine, and glycine (DRYG) mediates eukaryotic initiation factor 4B (eIF4B) self-association and interaction with eIF3. Mol. Cell. Biol. 165328-5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 779337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oroskar, A. A., and G. S. Read. 1987. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J. Virol. 61604-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oroskar, A. A., and G. S. Read. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 631897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pak, A. S., D. N. Everly, K. Knight, and G. S. Read. 1995. The virion host shutoff protein of herpes simplex virus inhibits reporter gene expression in the absence of other viral gene products. Virology 211491-506. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Parada, J., H. A. Saffran, and J. R. Smiley. 2004. RNA degradation induced by the herpes simplex virus vhs protein proceeds 5′ to 3′ in vitro. J. Virol. 7813391-13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pestova, T. V., C. U. Hellen, and I. N. Shatsky. 1996. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 166859-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pestova, T. V., J. R. Lorsch, and C. U. T. Hellen. 2007. The mechanism of translation initiation in eukaryotes, p. 87-128. In M. B. Mathews, N. Sonenberg, and J. W. B. Hershey (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 53.Pestova, T. V., I. N. Shatsky, and C. U. Hellen. 1996. Functional dissection of eukaryotic initiation factor 4F: the 4A subunit and the central domain of the 4G subunit are sufficient to mediate internal entry of 43S preinitiation complexes. Mol. Cell. Biol. 166870-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray, N., and L. W. Enquist. 2004. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J. Virol. 783489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Read, G. S. 1997. Control of mRNA stability during herpes simplex virus infections, p. 311-321. In J. B. Harford and D. R. Morris (ed.), mRNA metabolism and post-transcriptional gene regulation. Wiley-Liss, Inc., New York, NY.
- 56.Read, G. S., and N. Frenkel. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate-early) viral polypeptides. J. Virol. 46498-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 677149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Read, G. S., and M. Patterson. 2007. Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions. J. Virol. 811148-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richter, N. J., G. W. Rogers, Jr., J. O. Hensold, and W. C. Merrick. 1999. Further biochemical and kinetic characterization of human eukaryotic initiation factor 4H. J. Biol. Chem. 27435415-35424. [DOI] [PubMed] [Google Scholar]
- 60.Richter-Cook, N. J., T. E. Dever, J. O. Hensold, and W. C. Merrick. 1998. Purification and characterization of a new eukaryotic protein translation factor. Eukaryotic initiation factor 4H. J. Biol. Chem. 2737579-7587. [DOI] [PubMed] [Google Scholar]
- 61.Rogers, G. W., Jr., A. A. Komar, and W. C. Merrick. 2002. eIF4A: the godfather of the DEAD box helicases. Prog. Nucleic Acid Res. Mol. Biol. 72307-331. [DOI] [PubMed] [Google Scholar]
- 62.Rogers, G. W., Jr., W. F. Lima, and W. C. Merrick. 2001. Further characterization of the helicase activity of eIF4A. Substrate specificity. J. Biol. Chem. 27612598-12608. [DOI] [PubMed] [Google Scholar]
- 63.Rogers, G. W., Jr., N. J. Richter, W. F. Lima, and W. C. Merrick. 2001. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J. Biol. Chem. 27630914-30922. [DOI] [PubMed] [Google Scholar]
- 64.Rogers, G. W., Jr., N. J. Richter, and W. C. Merrick. 1999. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J. Biol. Chem. 27412236-12244. [DOI] [PubMed] [Google Scholar]
- 65.Samady, L., E. Costigliola, L. MacCormac, Y. McGrath, S. Cleverley, C. E. Lilley, J. Smith, D. S. Latchman, B. Chain, and R. S. Coffin. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 773768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schek, N., and S. L. Bachenheimer. 1985. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J. Virol. 55601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smibert, C. A., D. C. Johnson, and J. R. Smiley. 1992. Identification and characterization of the virion-induced host shutoff product of herpes simplex virus gene UL41. J. Gen. Virol. 73467-470. [DOI] [PubMed] [Google Scholar]
- 68.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 781063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith, T. J., C. E. Ackland-Berglund, and D. A. Leib. 2000. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J. Virol. 743598-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith, T. J., L. A. Morrison, and D. A. Leib. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 762054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sonenberg, N., and T. E. Dever. 2003. Eukaryotic translation initiation factors and regulators. Curr. Opin. Struct. Biol. 1356-63. [DOI] [PubMed] [Google Scholar]
- 72.Sorenson, C. M., P. A. Hart, and J. Ross. 1991. Analysis of herpes simplex virus-induced mRNA destabilizing activity using an in vitro mRNA decay system. Nucleic Acids Res. 194459-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strand, S. S., and D. A. Leib. 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J. Virol. 7813562-13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strelow, L., T. Smith, and D. Leib. 1997. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology 23128-34. [DOI] [PubMed] [Google Scholar]
- 75.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 696779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strelow, L. I., and D. A. Leib. 1996. Analysis of conserved domains of UL41 of herpes simplex virus type 1 in virion host shutoff and pathogenesis. J. Virol. 705665-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strom, T., and N. Frenkel. 1987. Effects of herpes simplex virus on mRNA stability. J. Virol. 612198-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzutani, T., M. Nagamine, T. Shibaki, M. Ogasawara, I. Yoshida, T. Daikoku, Y. Nishiyama, and M. Azuma. 2000. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 811763-1771. [DOI] [PubMed] [Google Scholar]
- 79.Svitkin, Y. V., A. Pause, A. Haghighat, S. Pyronnet, G. Witherell, G. J. Belsham, and N. Sonenberg. 2001. The requirement for eukaryotic initiation factor 4A (eIF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA 7382-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taddeo, B., A. Esclatine, and B. Roizman. 2002. The patterns of accumulation of cellular RNAs in cells infected with a wild-type and a mutant herpes simplex virus 1 lacking the virion host shutoff gene. Proc. Natl. Acad. Sci. USA 9917031-17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taddeo, B., A. Esclatine, and B. Roizman. 2004. Post-transcriptional processing of cellular RNAs in herpes simplex virus-infected cells. Biochem. Soc. Trans. 32697-701. [DOI] [PubMed] [Google Scholar]
- 82.Taddeo, B., A. Esclatine, W. Zhang, and B. Roizman. 2003. The stress-inducible immediate-early responsive gene IEX-1 is activated in cells infected with herpes simplex virus 1, but several viral mechanisms, including 3′ degradation of its RNA, preclude expression of the gene. J. Virol. 776178-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taddeo, B., and B. Roizman. 2006. The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 809341-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taddeo, B., W. Zhang, and B. Roizman. 2006. The U(L)41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 1032827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J. Immunol. 1563901-3910. [PubMed] [Google Scholar]
- 86.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 766974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zelus, B. D., R. S. Stewart, and J. Ross. 1996. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J. Virol. 702411-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]