Abstract
Human noroviruses cause more than 90% of epidemic nonbacterial gastroenteritis. However, the role of B cells and antibody in the immune response to noroviruses is unclear. Previous studies have demonstrated that human norovirus specific antibody levels increase upon infection, but they may not be protective against infection. In this report, we used murine norovirus (MNV), an enteric norovirus, as a model to determine the importance of norovirus specific B cells and immune antibody in clearance of norovirus infection. We show here that mice genetically deficient in B cells failed to clear primary MNV infection as effectively as wild-type mice. In addition, adoptively transferred immune splenocytes derived from B-cell-deficient mice or antibody production-deficient mice were unable to efficiently clear persistent MNV infection in RAG1−/− mice. Further, adoptive transfer of either polyclonal anti-MNV serum or neutralizing anti-MNV monoclonal antibodies was sufficient to reduce the level of MNV infection both systemically and in the intestine. Together, these data demonstrate that antibody plays an important role in the clearance of MNV and that immunoglobulin G anti-norovirus antibody can play an important role in clearing mucosal infection.
Extensive studies have demonstrated that humoral immune responses are generated by challenge with various norovirus strains in humans, pigs, cattle, and mice (5, 10, 13, 22, 23, 27, 37, 39, 44-46). Studies of natural norovirus infections in human populations show that the lowest rates of seroconversion are in the 0- to 5-year-old age group and, by adulthood, seroconversion rates range from 80 to 100% in most countries (reviewed in reference 32). Among children <5 years old, a higher baseline titer of norovirus antibody appears to correlate with protection from infection; however, in adults, a preexisting titer does not appear to be protective (37). This suggests that in children, antibody may be protective, whereas in adults, seropositivity may merely be a sign of previous infection. However, a Norwalk virus challenge study that examined the timing of virus-specific immunoglobulin A (IgA) production demonstrated that an elevation in salivary IgA occurred more than 5 days after infection in susceptible individuals, whereas in individuals resistant to infection, IgA levels were elevated earlier, 1 to 5 days postchallenge (28). This suggests a correlation between the timing of an increase in norovirus specific mucosal IgA production and whether virus established a productive infection in the host.
In humans, several studies have focused on antibody production in response to inoculation with norovirus viruslike particles (VLPs) assembled in the absence of viral replication by expression of viral capsid proteins (1, 2, 15, 41, 44-47). These VLPs share epitopes with virions but do not carry viral genome (14, 21). High doses of VLPs administered intranasally or perorally (p.o.), with or without adjuvants such as cholera toxin or Escherichia coli labile toxin, induced mucosal IgA and serum IgG in human volunteers, calves, pigs, and mice (1, 2, 15, 16, 19, 41, 44-47). The antibody responses that are elicited following infection with noroviruses are cross-reactive between strains within the same genogroup, but much less cross-reactive between strains from different genogroups (20, 21, 27). Importantly, antisera from infected human volunteers and experimentally vaccinated mice are able to block binding of Norwalk and Lordsdale VLPs to ABH histo-blood group antigens (16, 19, 29). The presence of antibodies that block norovirus receptor binding suggests that such antibodies could exert a protective effect against infection or promote resolution of symptoms. In addition, inoculating mice with vaccine cocktails comprised of multiple norovirus VLPs enhances the production of blocking antibodies, as well as heterotypic antibodies against strains not included in the cocktail (29). However, no formal assessment of the physiologic importance of either induced polyclonal or specific antibody isotypes has been undertaken to date.
Murine norovirus (MNV) is an enteric virus that, like its human counterparts, is spread by the fecal-oral route (58). MNV-infected mice make a significant antibody response (17, 18, 23, 40, 53), which can be neutralizing (48). The availability of a culture system and plaque assay has allowed the isolation of neutralizing monoclonal antibodies (MAbs) (57). One such MAb, A6.2, recognizes a structurally constrained epitope that is present in the surface exposed hypervariable P2 domain of VP1, the major capsid protein (24, 30, 57). These data suggest that the antibody response may be important for the control of MNV infection, but this has not been shown. The question of whether systemic IgG can play a role in control of mucosal infection remains controversial.
Here, we report on the role of B cells, polyclonal immune antibody, and neutralizing IgG MAbs in the clearance of MNV infection. Mice that are genetically deficient in B cells (μMT mice) failed to clear primary MNV infection as effectively as did wild-type mice. Adoptively transferred immune splenocytes from mice either lacking B cells (μMT mice) or mice lacking B cells capable of making virus-specific antibody (HELMET mice) were unable to clear MNV infection from the intestine of persistently infected RAG1−/− mice. Further, adoptive transfer of polyclonal anti-MNV serum and IgG anti-MNV MAbs were sufficient to reduce the level of MNV infection both systemically and in the intestine. Together, these data demonstrate that antibody, including IgG, plays an important role in the clearance of MNV.
MATERIALS AND METHODS
Virus, viral stocks, and plaque assays.
MNV strain MNV1.CW3 was used in all virus infections (57). To generate a concentrated virus stock, RAW 264.7 cells were infected in VP-SFM media (Gibco, Carlsbad, CA) for 2 days at a multiplicity of infection of 0.05. Supernatants were clarified by low-speed centrifugation for 20 min at 3,000 rpm. Virus was concentrated by centrifugation at 4°C for 3 h at 27,000 rpm (90,000 g) in an SW32 rotor. Viral pellets were resuspended in phosphate-buffered saline, and titers were determined on RAW 264.7 cells as previously described (57). Plaque assays were performed as previously described (57) with the following modifications. Tissues were harvested into sterile, screw-top 2-ml tubes containing 500 μl of 1-mm zirconia/silica beads (BioSpec Products, Bartlesville, OK) and stored at −80°C. To obtain virus titers in these tissues, 1 ml of complete Dulbecco modified Eagle medium was added to each sample on ice, followed by homogenization using a MagNA Lyser (Roche Applied Science, Indianapolis, IN) prior to plaque assay. The limit of detection was 20 PFU/ml.
Cell culture and antibodies.
RAW 264.7 cells (ATCC, Manassas, VA) were maintained as described previously (57). MAb 9BG5, specific to the reovirus type 3 hemagglutinin (4, 7), and anti-MNV MAbs A6.1, A6.2, and H6.1 were produced in INTEGRA Celline CL1000 flasks (Integra Biosciences, Ijamsville, MD) as previously described (33). Anti-MNV MAb A6.2 has been previously reported (24, 57). We used the same methods to isolate additional neutralizing MAbs A6.1 and H6.1 specific for the MNV capsid (data not shown). The titer of anti-MNV antibody in serum was determined by using enzyme-linked immunosorbent assay (ELISA) (23).
Mice and infections.
All mice were bred and housed at Washington University School of Medicine in accordance with all federal and university policies. Wild-type C57BL/6/J (B6; Jackson Laboratory, catalog no. 000664), B6RAG1−/− (RAG1−/−, Jackson Laboratory, catalog no. 002216), and μMT (25) mice backcrossed onto a C57BL/6 background (μMT; Jackson Laboratory, catalog no. 002288) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). HELMET mice, which express an IgM/IgD transgenic B-cell receptor specific for hen egg lysozyme, were generated and bred as previously described (34). Wild-type mice were tested by ELISA for the presence of MNV antibody prior to experiments (23). All mice used in these studies were seronegative. RAG1−/−, and all splenocyte donor mice were infected with 3 × 106 PFU of virus p.o. in 25 μl of Dulbecco modified Eagle medium supplemented with 10% fecal bovine serum (HyClone, Logan, UT). All other mice were infected with 3 × 107 PFU p.o. In RAG1−/− mice, two segments of the small intestine were harvested: a 1-in. section of the small intestine immediately distal to the pylorus of the stomach (designated the duodenum/jejunum) and a 1-in. section of the small intestine immediately proximal to the cecum (designated the distal ileum). In all other mice the distal ileum and three mesenteric lymph nodes (MLN) were harvested. The duodenum/jejunum was not harvested from wild-type mice since virus titers could not be detected at this site.
Adoptive and passive transfer studies.
Spleens were harvested from mice, and single-cell suspensions were generated. Cells were counted and diluted in RPMI 1640 media (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (SH30071.03; HyClone), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 10 mM HEPES, 1 mM sodium pyruvate, 2-mercaptoethanol, and 2 mM l-glutamine (cRPMI). A total of 107 cells were injected into persistently infected RAG1−/− mice by intraperitoneal (i.p.) injection in 0.5 ml of cRPMI. Immune and control antiserum was obtained from either immunized or control mice, filtered (0.2-μm pore size), heat fixed for 30 min at 55°C, and then stored for use. Sera and MAbs were passively transferred into recipient mice i.p.
Statistical methods.
All data were analyzed by using Prism software (GraphPad Software, San Diego, CA). Virus titers were analyzed with the nonparametric Mann-Whitney test. All differences not specifically stated to be significant were insignificant (P > 0.05).
RESULTS
B cells are required to control early mucosal MNV replication and long-term clearance in MLN but not intestine.
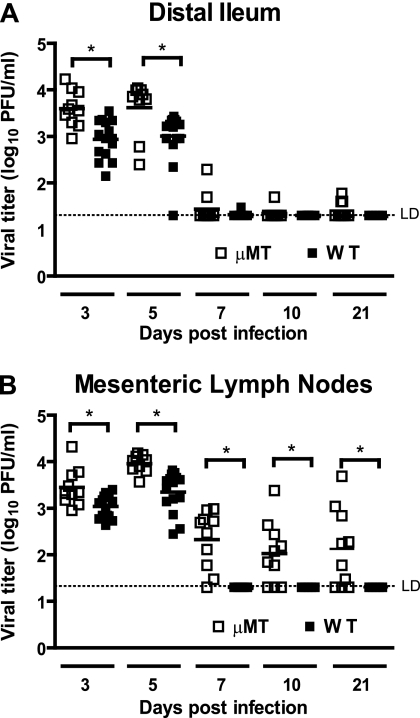
We have previously demonstrated that RAG1−/− mice, which are deficient in both T cells and B cells, develop a chronic persistent MNV infection (23), whereas wild-type mice efficiently clear infection. This indicates that adaptive immunity is important for MNV clearance. To evaluate the role of B cells in the control of primary MNV infection, we compared MNV titers in μMT (B-cell-deficient) mice and wild-type B6 mice in the distal ileum and the MLN after p.o. infection. Previous work has shown that MNV replicates in both the distal ileum and the MLN (17, 18, 35, 48). At 3 and 5 days postinfection, μMT mice had significantly higher virus titers compared than did wild-type controls in the distal ileum (P = 0.002 and P = 0.014, Fig. 1A) and also in the MLN (P = 0.009 and P = 0.0004, Fig. 1B). The majority of both wild-type B6 and μMT mice cleared ileal infection by day 7 postinfection, with only 2 of 10 μMT mice having any measurable ileal titer at this time point (Fig. 1A). In the MLN, wild-type mice cleared infection by day 7, whereas in μMT mice titers on average decreased ∼100-fold from their peak at 5 days postinfection. However, MLN titers in μMT mice remained detectable at days 7, 10, and 21 postinfection. The difference between wild-type B6 and μMT mice was statistically significant at these time points (day 7, P = 0.0004; day 10, P = 0.045; and day 21, P = 0.045 [Fig. 1B]). These data showed that B cells were important in the control of MNV infection at days 3 and 5 in both the distal ileum and the MLN. However, while B cells were dispensable for clearance of MNV in the distal ileum, they were required for the clearance of infection from MLN.
FIG. 1.
B cells limit MNV replication and are required for MNV clearance in the distal ileum and MLN. Virus titers in the distal ilea (A) and MLN (B) of B6 and μMT mice. These data are pooled from at least two independent experiments with five mice per group in each experiment, and each symbol indicates a sample from an individual mouse. *, P < 0.05. LD, limit of detection.
B cells are required to efficiently clear ileal MNV infection after adoptive transfer of immune cells.
The experimental results depicted in Fig. 1 showed a critical role for B cells in the control of ileal replication early after infection and for elimination of MNV infection from the mesenteric lymphatics. B cells can function to control viral infection via either antibody production or via antibody-independent effects such as priming an effective T-cell response (34). To address the mechanism of B-cell action, we turned to passive and adoptive-transfer experiments.
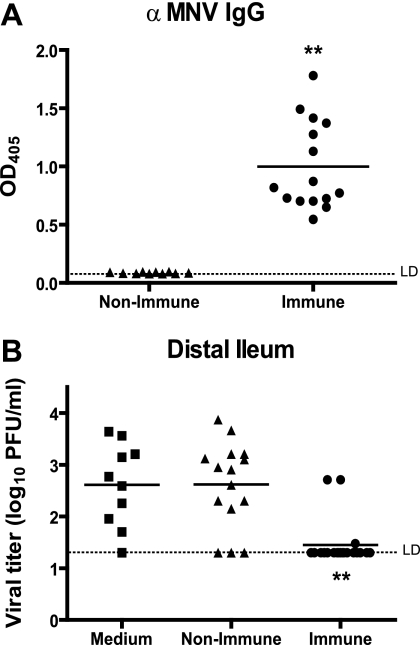
We first determined whether mice that have made a serologic response to MNV contain immune cells capable of clearing ileal MNV infection. We mock and MNV infected wild-type mice, and at 35 to 42 days postinfection we harvested sera and splenocytes from these mice. Compared to mock-infected mice, MNV-infected B6 mice developed a substantial IgG response to MNV virions by 35 to 42 days postinfection (P < 0.0001, Fig. 2A). This is consistent with our previous reports and the work of others (17, 18, 23, 40, 48, 53).
FIG. 2.
Antibody responses and capacity of adoptively transferred immune splenocytes to clear enteric MNV infection. (A) Levels of serum anti-MNV IgG antibody determined by ELISA (1:100 serum dilution) in mock- and MNV-immunized wild-type B6 mice 35 to 42 days postinfection. (B) Virus titers in the distal ileum of persistently infected RAG1−/− mice 6 days after transfer of medium alone, nonimmune splenocytes, or immune splenocytes. These data are pooled from three independent experiments with three to five mice per group in each experiment. **, P < 0.0001; *, P < 0.05. LD, limit of detection.
Since MNV-infected RAG1−/− mice develop a persistent ileal infection, these mice provide an excellent model for defining immune mechanisms of clearance of mucosal MNV infection. We chose to further explore the clearance of MNV infection because we were interested in determining the immune factors that are required for the abrogation of an already-established viral infection. Persistently infected RAG1−/− mice provide a broad time frame in which an established infection is not cleared and, in the absence of an adaptive immune response, we could test individual aspects of adaptive immunity and determine their role in the clearance of MNV infection. Further, the lack of an adaptive immune response allows establishment of MNV infection in the duodenum/jejunum, a site where MNV replication cannot be detected in wild-type B6 mice (data not shown). This shows that either T or B cells or both lymphocyte subsets are important for resistance to MNV infection in this tissue. The duodenum/jejunum provides a second tissue for analysis of the role of B cells and antibody in control of MNV infection.
Compared to nonimmune splenocytes, splenocytes harvested from immune wild-type mice were able to effectively clear MNV infection from the distal ileum 6 days after adoptive transfer into persistently infected RAG1−/− mice (Fig. 2B, P < 0.0001). This system allowed us to (i) test whether B cells were necessary for the clearance of persistent MNV infection and (ii) test whether the capacity of B cells to make antiviral antibodies was important.
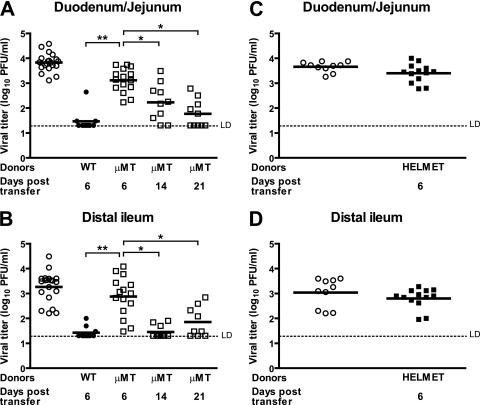
To determine whether B cells were necessary for the clearance of MNV, splenocytes were obtained from μMT mice at 35 to 42 days postinfection. At this time there was no detectable virus titer in the spleens of these mice (data not shown). These μMT splenocytes were adoptively transferred into persistently infected RAG1−/− recipients. In contrast to immune splenocytes from wild-type mice, the splenocytes from μMT mice were unable to clear MNV infection in either the duodenum/jejunum (Fig. 3A) or the distal ileum (Fig. 3B) of RAG1−/− recipients at 6 days after transfer.
FIG. 3.
B cells and antiviral antibody production are essential for efficient clearance of MNV infection from intestine of persistently infected RAG1−/− mice. Virus titers in the duodena/jejuna (A and C) and distal ilea (B and D) of persistently infected RAG1−/− recipients after adoptive transfer of medium (mock), immune wild type, μMT splenocytes (A and B) or HELMET splenocytes (C and D). These data are pooled from three independent experiments with three to five mice per group in each experiment. **, P < 0.0001; *, P < 0.05. LD, limit of detection.
We wanted to determine whether, in the absence of B cells and antibody, the cells that remained in the spleen (including T cells) were capable of clearing MNV infection or whether B cells were essential for clearance at later times after the transfer of immune splenocytes into RAG1−/− recipients. We therefore measured virus titers at 14 and 21 days after transfer of μMT cells into recipient mice. At 14 and 21 days after transfer, there was a significant reduction in the virus titers in the duodenum and jejunum (P = 0.005 and P = 0.0001, respectively; Fig. 3A), as well as in the distal ileum (P = 0.0002 and P = 0.004, respectively; Fig. 3B). This showed that immune cells other than B cells can decrease mucosal infection with MNV. However, MNV was not cleared completely from mucosal sites in all RAG1−/− recipients even 21 days after transfer. We concluded two things from these experiments. First, B cells or antibody were critical for efficient clearance of MNV infection from mucosal sites. Second, in the absence of B cells, the remaining immune cells, presumably T cells, were able to reduce virus titers but not completely clear infection.
The requirement for B cells is antibody dependent: antiviral antibody is involved in the clearance of mucosal MNV infection.
We next wanted to determine whether the requirement for B cells was due to the production of antiviral antibody. We therefore studied HELMET mice, which are HEL-specific IgM/IgD B-cell receptor mice bearing the IgMa allotype bred onto the B cell−/− background (34). These mice have B cells that produce only HEL-specific antibody and do not mount an antiviral antibody response (34). However, these B cells can support normal levels of T-cell responses (34). We obtained splenocytes from MNV-immunized HELMET mice at 35 to 42 days postinfection, at which time no virus titers could be detected in the spleen (data not shown). Splenocytes from HELMET mice were adoptively transferred into persistently infected RAG1−/− mice to determine whether B cells have a role in MNV clearance that is independent of antibody production. This was a particularly relevant question since T cells had some capacity to control mucosal MNV infection (Fig. 3 and data not shown) and B cells can support the development of antiviral T cells (34). We measured virus titers in the intestine of recipient RAG1−/− mice 6 days after adoptive transfer. No significant difference was noted between control mice that did not receive any donor splenocytes and mice that received splenocytes from HELMET donors in either the duodenum/jejunum or the distal ileum (Fig. 3C and D). In addition, no significant difference was noted between recipients of splenocytes from μMT donors and recipients of splenocytes from HELMET donors (compare Fig. 3A and B with Fig. 3C and D). Thus, we concluded that the requirement for B cells in the efficient control of MNV infection was due to the production of virus-specific antibody.
MNV specific polyclonal sera and IgG are sufficient to limit MNV replication in the intestine and spleen.
Since the transfer of immune HELMET splenocytes linked the importance of B cells in the efficient clearance of MNV infection to a role for MNV-specific antibody, we directly tested the ability of passively transferred anti-MNV antibody to limit viral replication.
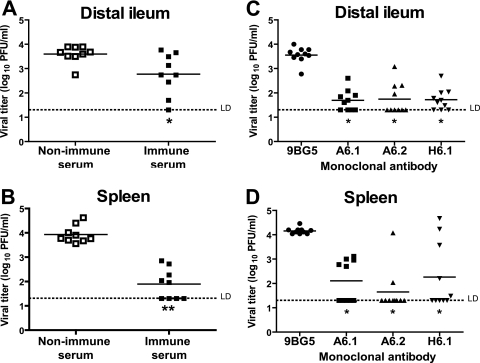
To obtain MNV specific polyclonal antibody, we mock immunized or MNV immunized wild-type mice and 35 to 42 days postinfection obtained nonimmune and immune serum. We transferred 500 μl of serum via i.p. injection into persistently infected RAG1−/− mice and measured virus titers in the distal ileum and spleen 6 days after transfer. In the distal ileum, transfer of immune serum led to a modest, but significant decrease in the levels of MNV titer compared to nonimmune serum transfers (P = 0.013, Fig. 4A). Similar results were obtained in the duodenum/jejunum (data not shown). In the spleen, the transfer of immune serum had a much more significant effect of reducing the levels of MNV titer to below the levels of detection in half of the mice examined (P < 0.0001). This demonstrated that MNV immune polyclonal serum is sufficient to reduce MNV titers in the intestine and spleen.
FIG. 4.
Immune serum and neutralizing IgG MAbs reduce MNV infection from intestines and spleens of RAG1−/− mice. Virus titers in distal ilea (A and C) and spleens (B and D) of persistently infected RAG1−/− recipients 6 days after passive transfer of either immune serum (A and B) or neutralizing MAbs specific for capsid protein (C and D). These data are pooled from three independent experiments with three mice per group in each experiment. **, P < 0.0001; *, P < 0.05. LD, limit of detection.
Even though IgA is classically thought of as the main antibody isotype present at the mucosal surface (reviewed in reference 36), there are reports of IgG antibody controlling mucosal viral infection (26, 38, 55). We therefore wanted to determine whether IgG antibodies against MNV capsid alone could reduce MNV titers. We obtained three anti-MNV capsid MAbs—A6.1, A6.2, and H6.1, all of the IgG2a isotype—that had previously been shown to neutralize MNV in vitro (24, 57; unpublished data). We administered 500 μg of each antibody via i.p. injection into persistently infected RAG1−/− mice. In the distal ileum, each of the MAbs was able to significantly reduce the levels of MNV titers compared to mice injected with the 9BG5 control antibody (P = 0.0002) in each case (Fig. 4C). Furthermore, in the spleen, all three MAbs (A6.1, A6.2, and H6.1) were also able to significantly reduce the levels of MNV titers (P = 0.0002, P = 0.0004, and P = 0.0207, respectively; Fig. 4D). This demonstrated that MAbs of the IgG isotype directed against MNV capsid were able to control MNV infection in the distal ileum and spleen.
DISCUSSION
Noroviruses are the cause of more than 95% of epidemic nonbacterial gastroenteritis worldwide and are also a common cause of intestinal infectious disease in the community (8, 11, 49, 56). The immune mechanisms involved in clearance of norovirus infection are not completely understood. Human volunteer studies show short-lived immunity to homologous viral challenge, but the role of antibody in this protection is not known (9, 39, 59). The availability of a culturable, enteric MNV allows the use of immunodeficient mice to identify the immune mechanisms required to control and eliminate mucosal, lymphatic, and systemic norovirus infection (23, 24, 35, 54, 57). It is important to note that the relationship between the pathogenesis of MNV infection and human norovirus infection is not clear (58). However, MNV is an efficient enteric virus, infecting a broad array of mice in research colonies (18, 48, 53) and sharing fundamental mechanisms of replication (43) and structure (24) with human noroviruses. We therefore believe that mechanistic studies of MNV immunity in well-defined mouse systems may provide valuable insights into conserved aspects of norovirus immunity.
In the present study we focus on the role of B cells and antibody, deriving four primary conclusions. First, B cells are important for control of ileal and lymphatic MNV infection early (3 to 5 days after) after oral infection. Second, the role of B cells later in infection is tissue specific; the ileum can be cleared of infection without B cells, but B cells are important for the clearance of infection from the enteric lymphatic system even 3 weeks after infection. Third, the role of B cells is in large part due to the production of antiviral antibody. Fourth, IgG antibodies can play a role in clearance of mucosal MNV infection. Together, these findings provide the first comprehensive view of the role of the serologic response in control of mucosal and lymphatic norovirus infection.
Role of B cells early after oral infection.
B-cell-deficient μMT mice were defective in the control of MNV replication in the distal ileum and MLN at 3 and 5 days postinfection. At such early times after infection no virus-specific IgG can be detected. However, the strain of μMT mice used in these experiments (μMT mice) can produce small amounts of virus- specific IgA (31). Thus, increased titers of MNV early after infection might be due to a relative defect in IgA production by μMT mice compared to wild-type B6 mice. However, 3 days is a very short time to mount a physiologically significant virus-specific antibody response. We therefore believe it more likely that the increased replication of MNV in μMT mice compared to wild-type mice early after infection may reflect a role for natural antibody in MNV infection. Validating this hypothesis will require further experiments. Natural antibodies produced by B1 B cells can play a role in the control of some mucosal pathogens, including viruses. However, other explanations are possible, and these are the subject of ongoing investigations.
Tissue-specific role of B cells during MNV infection.
Although the distal ileum of B-cell-deficient mice was cleared of MNV infection by 7 days after infection, the MLN was not. Thus, the clearance of MNV infection from intestinal lymphatic tissue is more dependent on B cells than the clearance of ileal infection. The reason for this is not clear. It may be that the cellular tropism of MNV is different in these different sites and that B cells are more important for elimination of MNV from some tissues than others. Of note, however, passive transfer of polyclonal immune serum and MAbs into persistently infected RAG1−/− mice was capable of limiting ileal MNV replication. Thus, IgG does have access to relevant sites of MNV replication in the ileum and can be effective in this tissue. An alternative explanation is that other effector mechanisms, such as T cells, are more effective in the ileum than in the lymphatic system. Arguing against this, however, is the fact that adoptively transferred immune cells lacking B cells were not completely able to clear MNV from the ileum even late after infection. However, since RAG1−/− mice do not have normal MLN, these adoptive- and passive-transfer experiments into persistently infected RAG1−/− mice shed no light on the role of IgG per se in lymphatic infection.
Role of antiviral IgG in B-cell-dependent control of mucosal MNV infection.
The role of B cells in the control of MNV infection appears to be primarily due to the production of antiviral antibody. The data supporting this conclusion include the lack of effectiveness of immune cells from both B-cell-deficient μMT mice and HELMET mice in controlling mucosal MNV infection in adoptive-transfer experiments. This is consistent with a role for antiviral antibody since HELMET mice have B cells and produce antibody to HEL but cannot mount an antiviral antibody response (33, 34). Furthermore, passive transfer of polyclonal antiserum decreased mucosal MNV infection. This latter result was obtained via passive transfer of 0.5 ml of serum, which represents less than the volume of serum in a naturally immune mouse. Thus, by definition, the effect of immune serum was observed at physiologically relevant antibody concentrations. We further found that IgG MAbs can significantly decrease MNV infection at mucosal sites. Together, these data argue that systemic IgG can have a significant effect on mucosal norovirus infection. Although our experiments with IgG MAbs provide proof of concept that IgG can have significant mucosal effects, the amount of specific IgG isotypes, or the specific epitope targets of such IgGs, that would have to be elicited by a vaccine to alter mucosal infection remains an open question. Our data show that the capsid protein is a target for neutralizing IgG. Additional viral proteins may be targets of protective antibodies or may induce protective immunity by other mechanisms.
Comparison of our results to those obtained for other enteric viruses.
The role of B cells and antibody has been extensively documented in both reovirus and rotavirus infection. Using B-cell-deficient mice, it has been determined that B cells are required for long-term robust protection against rotaviruses (12). In mice, both IgG and IgA are important in control of rotavirus (60). However, while intestinal IgA is an efficient mechanism of rotavirus clearance, it is clear that virus specific IgG or IgM, if present in the intestine or present in large quantities in serum, can also mediate protection in the intestine (26, 38, 55). In both rotavirus and reovirus, virus specific antibody can control systemic infection and spread (6, 42, 50-52) as well as primary intestinal infection (3). In this aspect, murine norovirus infections is similar to both rotavirus and reovirus infection, indicating a conserved role for systemic antibody at mucosal sites. Targeting robust systemic IgG responses may therefore be a valid goal for establishing effective mucosal protection against norovirus and other enteric virus infections.
Acknowledgments
This study was supported by National Institutes of Health grants RO1AI054483 and RO1AI065982 to H.W.V. K.A.C. was supported by Ruth L. Kirschstein National Research Service Awards 5F31AI56665 and 5T32GM007200 and a UNCF.Merck Graduate Science Dissertation Fellowship.
We thank Lindsay Droit for technical assistance in screening mice for MNV status prior to the initiation of experiments at Washington University and Darren Kreamalmayer for his outstanding expertise in the breeding of animals.
Washington University holds U.S. patent 7,041,444 B2 (murine calicivirus; 9 May 2006) and has pending patent applications related to the field. Washington University, H.W.V., and C.E.W. receive income based on licenses for MNV technology.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Ball, J. M., D. Y. Graham, A. R. Opekun, M. A. Gilger, R. A. Guerrero, and M. K. Estes. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 11740-48. [DOI] [PubMed] [Google Scholar]
- 2.Ball, J. M., M. E. Hardy, R. L. Atmar, M. E. Conner, and M. K. Estes. 1998. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J. Virol. 721345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkon, M. L., B. L. Haller, and H. W. Virgin. 1996. Circulating IgG can play a critical role in clearance of intestinal reovirus infection. J. Virol. 701109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstin, S. J., D. R. Spriggs, and B. N. Fields. 1982. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virol. 117146-155. [DOI] [PubMed] [Google Scholar]
- 5.Cheetham, S., M. Souza, T. Meulia, S. Grimes, M. G. Han, and L. J. Saif. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 8010372-10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuff, C. F., E. Lavi, C. K. Cebra, J. J. Cebra, and D. H. Rubin. 1990. Passive immunity to fatal reovirus serotype 3-induced meningoencephalitis mediated by both secretory and transplacental factors in neonatal mice. J. Virol. 641256-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dermody, T. S., M. L. Nibert, R. Bassel Duby, and B. N. Fields. 1990. A sigma 1 region important for hemagglutination by serotype 3 reovirus strains. J. Virol. 645173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wit, M. A., M. P. Koopmans, L. M. Kortbeek, W. J. Wannet, J. Vinje, F. van Leusden, A. I. Bartelds, and Y. T. van Duynhoven. 2001. Sensor, a population-based cohort study on gastroenteritis in The Netherlands: incidence and etiology. Am. J. Epidemiol. 154666-674. [DOI] [PubMed] [Google Scholar]
- 9.Dolin, R., N. R. Blacklow, H. DuPont, R. F. Buscho, R. G. Wyatt, J. A. Kasel, R. Hornick, and R. M. Chanock. 1972. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc. Soc. Exp. Biol. Med. 140578-583. [DOI] [PubMed] [Google Scholar]
- 10.Dolin, R., A. G. Levy, R. G. Wyatt, T. S. Thornhill, and J. D. Gardner. 1975. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am. J. Med. 59761-768. [DOI] [PubMed] [Google Scholar]
- 11.Fankhauser, R. L., J. S. Noel, S. S. Monroe, T. Ando, and R. I. Glass. 1998. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1781571-1578. [DOI] [PubMed] [Google Scholar]
- 12.Franco, M. A., and H. B. Greenberg. 1999. Immunity to rotavirus infection in mice. J. Infect. Dis. 179(Suppl. 3)S466-S469. [DOI] [PubMed] [Google Scholar]
- 13.Graham, D. Y., X. Jiang, T. Tanaka, A. R. Opekun, H. P. Madore, and M. K. Estes. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 17034-43. [DOI] [PubMed] [Google Scholar]
- 14.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 312185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero, R. A., J. M. Ball, S. S. Krater, S. E. Pacheco, J. D. Clements, and M. K. Estes. 2001. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 759713-9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington, P. R., L. Lindesmith, B. Yount, C. L. Moe, and R. S. Baric. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J. Virol. 7612335-12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu, C. C., L. K. Riley, H. M. Wills, and R. S. Livingston. 2006. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp. Med. 56247-251. [PubMed] [Google Scholar]
- 18.Hsu, C. C., C. E. Wobus, E. K. Steffen, L. K. Riley, and R. S. Livingston. 2005. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 121145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutson, A. M., R. L. Atmar, D. M. Marcus, and M. K. Estes. 2003. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J. Virol. 77405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, X., D. O. Matson, G. M. Ruizpalacios, J. Hu, J. Treanor, and L. K. Pickering. 1995. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J. Clin. Microbiol. 331452-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 666527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, P. C., J. J. Mathewson, H. L. DuPont, and H. B. Greenberg. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J. Infect. Dis. 16118-21. [DOI] [PubMed] [Google Scholar]
- 23.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 2991575-1578. [DOI] [PubMed] [Google Scholar]
- 24.Katpally, U., C. E. Wobus, K. Dryden, H. W. Virgin, and T. J. Smith. 2008. Structure of antibody neutralized murine norovirus and unexpected differences to viruslike particles. J. Virol. 822079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350423-426. [DOI] [PubMed] [Google Scholar]
- 26.Kuklin, N. A., L. Rott, N. Feng, M. E. Conner, N. Wagner, W. Muller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B-cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J. Immunol. 1661894-1902. [DOI] [PubMed] [Google Scholar]
- 27.Lindesmith, L., C. Moe, J. LePendu, J. A. Frelinger, J. Treanor, and R. S. Baric. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J. Virol. 792900-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9548-553. [DOI] [PubMed] [Google Scholar]
- 29.LoBue, A. D., L. Lindesmith, B. Yount, P. R. Harrington, J. M. Thompson, R. E. Johnston, C. L. Moe, and R. S. Baric. 2006. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 245220-5234. [DOI] [PubMed] [Google Scholar]
- 30.Lochridge, V. P., and M. E. Hardy. 2007. A single amino acid substitution in the P2 domain of VP1 of murine norovirus is sufficient for escape from antibody neutralization. J. Virol. [DOI] [PMC free article] [PubMed]
- 31.Macpherson, A. J., A. Lamarre, K. McCoy, G. R. Harriman, B. Odermatt, G. Dougan, H. Hengartner, and R. M. Zinkernagel. 2001. IgA production without mu or delta chain expression in developing B cells. Nat. Immunol. 2625-631. [DOI] [PubMed] [Google Scholar]
- 32.Matsui, S. M., and H. B. Greenberg. 2000. Immunity to calicivirus infection. J. Infect. Dis. 181(Suppl. 2)S331-S335. [DOI] [PubMed] [Google Scholar]
- 33.McClellan, J. S., S. A. Tibbetts, S. Gangappa, K. A. Brett, and H. W. Virgin. 2004. Critical role of CD4 T cells in an antibody-independent mechanism of vaccination against gamma-herpesvirus latency. J. Virol. 786836-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClellan, K. B., S. Gangappa, S. H. Speck, and H. W. Virgin. 2006. Antibody-independent control of gamma-herpesvirus latency via B-cell induction of antiviral T-cell responses. PLoS Pathog. 2e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mumphrey, S. M., H. Changotra, T. N. Moore, E. R. Heimann-Nichols, C. E. Wobus, M. J. Reilly, M. Moghadamfalahi, D. Shukla, and S. M. Karst. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 813251-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagler-Anderson, C., C. Terhoust, A. K. Bhan, and D. K. Podolsky. 2001. Mucosal antigen presentation and the control of tolerance and immunity. Trends Immunol. 22120-122. [DOI] [PubMed] [Google Scholar]
- 37.Okhuysen, P. C., X. Jiang, L. Ye, P. C. Johnson, and M. K. Estes. 1995. Viral shedding and fecal IgA response after Norwalk virus infection. J. Infect. Dis. 171566-569. [DOI] [PubMed] [Google Scholar]
- 38.O'Neal, C. M., G. R. Harriman, and M. E. Conner. 2000. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J. Virol. 744102-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrino, T. A., D. S. Schreiber, J. S. Trier, A. Z. Kapikian, and N. R. Blacklow. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N. Engl. J. Med. 29786-89. [DOI] [PubMed] [Google Scholar]
- 40.Perdue, K. A., K. Y. Green, M. Copeland, E. Barron, M. Mandel, L. J. Faucette, E. M. Williams, S. V. Sosnovtsev, W. R. Elkins, and J. M. Ward. 2007. Naturally occurring murine norovirus infection in a large research institution. J. Am. Assoc. Lab. Anim. Sci. 4638-44. [PubMed] [Google Scholar]
- 41.Periwal, S. B., K. R. Kourie, N. Ramachandaran, S. J. Blakeney, S. DeBruin, D. Zhu, T. J. Zamb, L. Smith, S. Udem, J. H. Eldridge, K. E. Shroff, and P. A. Reilly. 2003. A modified cholera holotoxin CT-E29H enhances systemic and mucosal immune responses to recombinant Norwalk virus-virus like particle vaccine. Vaccine 21376-385. [DOI] [PubMed] [Google Scholar]
- 42.Sherry, B., X.-Y. Li, K. L. Tyler, J. M. Cullen, and H. W. Virgin. 1993. Lymphocytes protect against and are not required for reovirus induced myocarditis. J. Virol. 676119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sosnovtsev, S. V., G. Belliot, K. O. Chang, V. G. Prikhodko, L. B. Thackray, C. E. Wobus, S. M. Karst, H. W. Virgin, and K. Y. Green. 2006. Cleavage map and proteolytic processing of the murine norovirus nonstructural polyprotein in infected cells. J. Virol. 807816-7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souza, M., M. S. Azevedo, K. Jung, S. Cheetham, and L. J. Saif. 2008. Pathogenesis and immune responses in gnotobiotic calves after infection with human norovirus (HuNoV) genogroup II.4-HS66 strain. J. Virol. 821777-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Souza, M., S. M. Cheetham, M. S. Azevedo, V. Costantini, and L. J. Saif. 2007. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II.4 (HS66 strain). J. Virol. 819183-9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souza, M., V. Costantini, M. S. Azevedo, and L. J. Saif. 2007. A human norovirus-like particle vaccine adjuvanted with ISCOM or mLT induces cytokine and antibody responses and protection to the homologous GII. 4 human norovirus in a gnotobiotic pig disease model. Vaccine 258448-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tacket, C. O., M. B. Sztein, G. A. Losonsky, S. S. Wasserman, and M. K. Estes. 2003. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin. Immunol. 108241-247. [DOI] [PubMed] [Google Scholar]
- 48.Thackray, L. B., C. E. Wobus, K. A. Chachu, B. Liu, E. R. Alegre, K. S. Henderson, S. T. Kelley, and H. W. Virgin. 2007. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J. Virol. 8110460-10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tompkins, D. S., M. J. Hudson, H. R. Smith, R. P. Eglin, J. G. Wheeler, M. M. Brett, R. J. Owen, J. S. Brazier, P. Cumberland, V. King, and P. E. Cook. 1999. A study of infectious intestinal disease in England: microbiological findings in cases and controls. Commun. Dis. Public Health 2108-113. [PubMed] [Google Scholar]
- 50.Tyler, K. L., M. A. Mann, B. N. Fields, and H. W. Virgin. 1993. Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J. Virol. 673446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyler, K. L., H. W. Virgin, R. Bassel Duby, and B. N. Fields. 1989. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J. Exp. Med. 170887-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virgin, H. W., R. Bassel-Duby, B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 624594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward, J. M., C. E. Wobus, L. B. Thackray, C. R. Erexson, L. J. Faucette, G. Belliot, E. L. Barron, S. V. Sosnovtsev, and K. Y. Green. 2006. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol. Pathol. 34708-715. [DOI] [PubMed] [Google Scholar]
- 54.Ward, V. K., C. J. McCormick, I. N. Clarke, O. Salim, C. E. Wobus, L. B. Thackray, H. W. Virgin, and P. R. Lambden. 2007. Recovery of infectious murine norovirus using pol II-driven expression of full-length cDNA. Proc. Natl. Acad. Sci. USA 10411050-11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westerman, L. E., H. M. McClure, B. Jiang, J. W. Almond, and R. I. Glass. 2005. Serum IgG mediates mucosal immunity against rotavirus infection. Proc. Natl. Acad. Sci. USA 1027268-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler, J. G., D. Sethi, J. M. Cowden, P. G. Wall, L. C. Rodrigues, D. S. Tompkins, M. J. Hudson, and P. J. Roderick. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 3181046-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wobus, C. E., S. M. Karst, L. B. Thackray, K. O. Chang, S. V. Sosnovtsev, G. Belliot, A. Krug, J. M. Mackenzie, K. Y. Green, and H. W. Virgin. 2004. Replication of a Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLOS Biol. 2e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wobus, C. E., L. B. Thackray, and H. W. Virgin. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 805104-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wyatt, R. G., R. Dolin, N. R. Blacklow, H. L. DuPont, R. F. Buscho, T. S. Thornhill, A. Z. Kapikian, and R. M. Chanock. 1974. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J. Infect. Dis. 129709-714. [DOI] [PubMed] [Google Scholar]
- 60.Youngman, K. R., M. A. Franco, N. A. Kuklin, L. S. Rott, E. C. Butcher, and H. B. Greenberg. 2002. Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J. Immunol. 1682173-2181. [DOI] [PubMed] [Google Scholar]