Abstract
TH-17 cells have been shown to play a role in bacterial defense, acute inflammation, and autoimmunity. We examined the role of interleukin 17 (IL-17) production in human immunodeficiency virus type 1 (HIV-1) infection. Both HIV-1- and cytomegalovirus (CMV)-specific IL-17-producing CD4+ T cells were detectable in early HIV-1 infection but were reduced to nondetectable levels in chronic and nonprogressive HIV-1 infection. IL-17-producing CMV-specific cells were not detected in blood from HIV-1-uninfected normal volunteers. Virus-specific TH-17 cells could coexpress other cytokines and could express CCR4 or CXCR3. Although the etiology of these cells has yet to be established, we propose that microbial translocation may induce them.
T-helper cells that produce interleukin 17 (IL-17) (TH-17) have recently been found in mice as a distinct subset of CD4+ T cells that differentiate from naive CD4+ T cells in response to combined IL-6 and transforming growth factor beta stimulation, acquire the transcription factor RORγt, and are expanded and maintained in the presence of IL-23(9, 14, 19, 20). Surprisingly, in humans, induction of TH-17 cells was shown to require IL-6 and IL-1β but not transforming growth factor beta (1). Murine and human TH-17 cells have been shown to produce IL-17A and IL-17F, which then act on local tissues to release proinflammatory cytokines, including IL-6, tumor necrosis factor alpha, and IL-1β, chemokines, including CXCL-6, CXCL-7, CXCL-8, and MCP-1, and metalloproteinases. Altogether, this results in the recruitment, mobilization, and activation of neutrophils characteristic of acute inflammatory responses leading to abscess formation and tissue destruction (3, 10, 18). The emerging data suggest that TH-17 cells play a role in autoimmunity and defense against bacterial and fungal pathogens. The presence of TH-17 rather than TH-1 cells has been strongly associated with the destructive effects of autoimmunity for the murine extrinsic allergic encephalitis model (6, 11, 17). TH-17 cells have also been identified in human autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis (8, 12, 15, 21). IL-17 has been shown to play a role in mobilizing neutrophils to infections caused by bacteria, such as Klebsiella pneumoniae, and intra-abdominal abscesses due to Bacteroides fragilis. Acosta-Rodriguez et al. (1) recently showed that in humans, Candida albicans can induce TH-17 cells that produce only IL-17 and not gamma interferon (IFN-γ) via the effects of C. albicans inducing IL-23 but not IL-12 on antigen-presenting cells, whereas Mycobacterium tuberculosis mainly elicited IFN-γ-producing cells. It was subsequently demonstrated that C. albicans-induced TH-17 cells expressed the chemokine receptor CCR4, whereas M. tuberculosis could induce T cells that produce IL-17 but only in concert with IFN-γ and these cells expressed the TH1 marker, CXCR3, but not CCR4 (2). It was thus suggested that CCR4 could identify “true” IL-17-only-producing cells whereas CXCR3 identified TH1 cells which also could produce IL-17. Little is known about TH-17 cells in other types of infections. Human immunodeficiency virus type 1 (HIV-1) infection is characterized by chronic immune activation and is associated with increases in proinflammatory cytokines (7). In order to better understand the nature of TH-17 cells in humans and to further understand the immmunopathogenesis of HIV-1 infection, we characterized IL-17-producing CD4+ T cells in HIV-1-infected individuals.
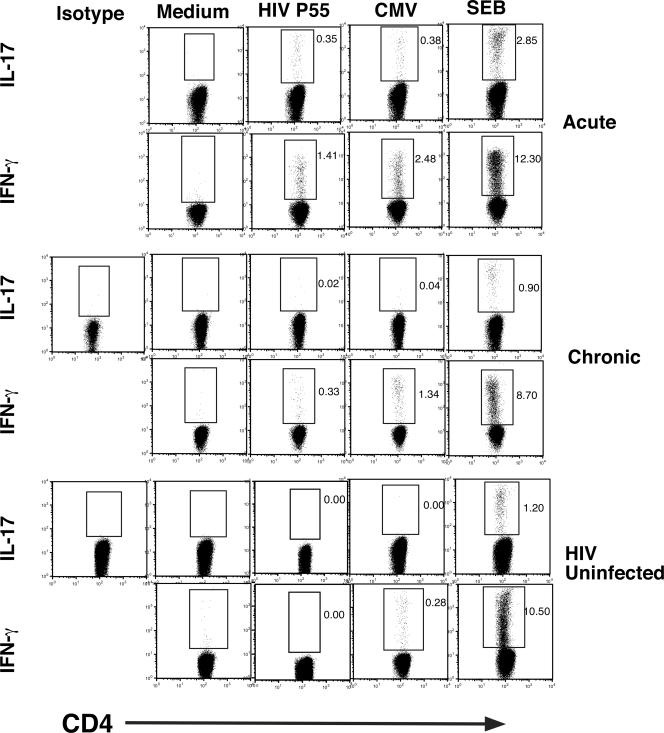
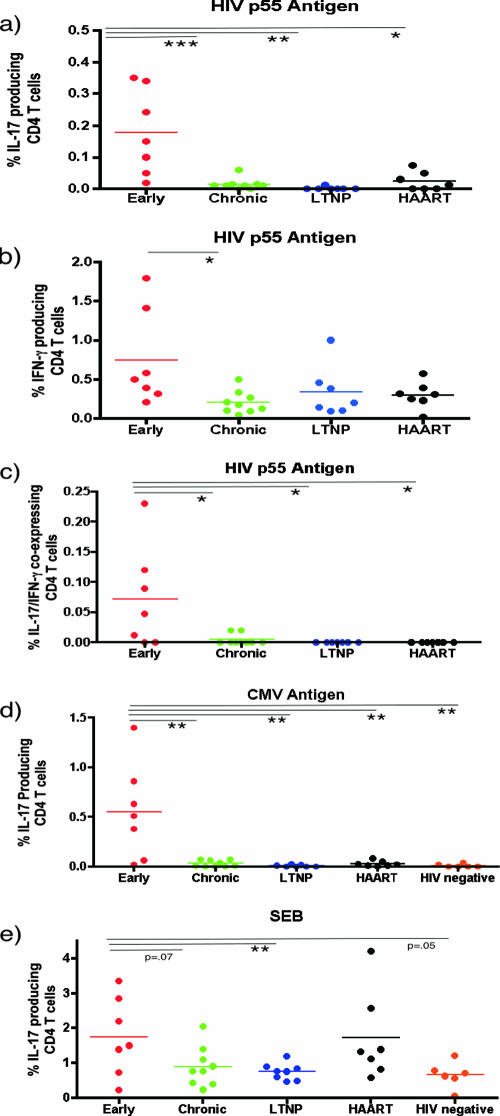
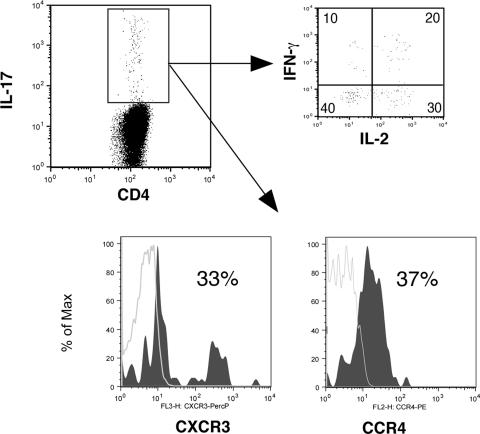
In order to determine the role of IL-17-producing CD4+ T cells in HIV-1 infection, we studied 30 HIV-1-infected individuals in various clinical stages of disease (Table 1), which included the following: (i) early infection, defined as infected for less than 1 year (five out of seven individuals had evolving Western blots); (ii) chronic HIV-1 infection, defined as HIV-1 infected for greater than 1 year; (iii) long-term nonprogression, defined as infected with HIV-1 for greater than 10 years with no evidence of decline in the number of CD4+ T cells below 500/mm3; and (iv) individuals who had progressed and were treated with highly active antiretroviral therapy (HAART) for at least 1 year. In addition, six HIV-1-uninfected normal volunteers were studied as controls. Informed consent was obtained from participants in accordance with the guidelines for conduct of clinical research at the University of Toronto and St. Michael's Hospital, Toronto. Ex vivo peripheral blood mononuclear cells (PBMC) from Ficoll-treated blood samples were incubated with the following antigens for 6 h: (i) medium and control antigens, (ii) 5 μg/ml of HIV-1 p55 (Austral Biologicals, San Ramon, CA), (iii) cytomegalovirus (CMV) antigen (CMV lysate, 1/40 dilution; Virion, Switzerland), or (iv) 3 μg/ml staphylococcal enterotoxin B (SEB) (Toxin Technologies, Sarasota, FL) as a mitogen control, in the presence of 1 μg/ml of antibodies to CD49d and CD28 for costimulation (BD Biosciences) and 1 μg/ml brefeldin A. Cells were then harvested, fixed, and stained using antibodies to CD4-PerCP, IFN-γ-phycoerythrin, IL-2-fluorescein isothiocyanate, and IL-17A-allophycocyanin (BD BioSciences and eBioscience, San Diego, CA). In some experiments (three samples), antibodies to CCR4 and CXCR3 were also used (BD BioSciences). Data were acquired by using Cell Quest software (BD Biosciences, San Diego, CA) and analyzed using the FloJo software program (Treestar Inc., San Carlos, CA). From 100,000 to 200,000 events in the lymphocyte gate were acquired per sample. Using four-color intracellular flow cytometry, we identified antigen-specific memory CD4+ T cells by gating on CD4+ T cells within the lymphocyte gate, which express cytokine after brief exposure to the specific antigen in question (HIV-1 and CMV for HIV-1-infected individuals and CMV for HIV-1-uninfected individuals) as previously described (22). Antigen-specific cells were quantitated by calculating the frequency of cytokine-producing cells under antigen-stimulated conditions and subtracting the number of cytokine-producing cells in control antigen-stimulated conditions. Representative experiments with individuals with acute and chronic HIV-1 infection and an uninfected individual are shown in Fig. 1, and summary data are depicted in Fig. 2. Statistical comparisons between the early-infection group and other groups used the unpaired Student t test. As expected, we were able to detect HIV-1-specific IFN-γ-producing CD4+ T cells at all stages of infection (Fig. 1 and 2a). We were able to detect the presence of HIV-1- and CMV-specific CD4+ T cells that produce IL-17A predominantly in early HIV-1 infection (individuals infected for less than 1 year), which were significantly greater in frequency than for individuals with chronic HIV-1 infection or for HIV-1-uninfected individuals for CMV-specific cells. However, lower levels of IL-17-producing, HIV-1-specific CD4+ T cells were also detected for some HAART-treated individuals (Fig. 2). The frequency of IL-17-producing cells was much lower than that of IFN-γ-producing cells for a given antigen (Fig. 2), e.g., in the early-infection group we observed a fourfold greater average frequency of p55 IFN-γ-producing cells than IL-17-producing cells (0.8% versus 0.2% of CD4+ T cells, respectively). CMV-specific IL-17-producing CD4+ T cells were observed only in early infection and were not seen in normal volunteers, although all clinical stages and normal volunteers had detectable CMV-specific IFN-γ-producing cells (Fig. 1; also data not shown). We observed significantly greater IL-17 production from CD4+ T cells in response to SEB for early-infected individuals than for long-term nonprogressors but only a trend to increased production compared to results for chronically infected or uninfected controls (Fig. 2e). These findings support in part the recent observations of an increased capacity for CD4+ T cells from HIV-1-infected individuals to produce IL-17 in response to phorbol myristate acetate-ionomycin compared to results for uninfected individuals (13). IL-17-producing virus-specific cells in our individuals were heterogenous in their phenotype in that there were cells that expressed only IL-17 and others that coexpressed other cytokines, including IFN-γ and IL-2. A representative sample is depicted in Fig. 3, and summary data for IFN-γ-positive, IL-17-coexpressing, HIV-1-specific cells are shown in Fig. 2c. In addition, subsets of virus-specific IL-17-producing cells could express the TH1-associated marker CXCR3 or the TH17-associated marker CCR4 (Fig. 3).
TABLE 1.
Clinical characteristics of subjects
| Clinical stage (na) | Mean no. of CD4+ T cells/mm3 | Range (CD4+ T cells/mm3) | Mean no. of viral load copies/ml bDNA | Range (viral load copies/ml bDNA) |
|---|---|---|---|---|
| Early HIV infection (7) | 503 | 320-720 | 27,295 | 139-89,000 |
| Chronic infection (9) | 415 | 161-770 | 71,564 | 2,955-300,000 |
| Long-term nonprogressor (7) | 852 | 560-1,200 | 5,106b | <50-29,652 |
| Chronic progressor on HAARTb (7) | 450 | 148-1,050 | 69b | <50-180 |
n, no. of subjects.
Undetectable viral loads (<50 copies/ml bDNA) were scored as “50 copies/ml” for calculations of mean viral load. Two of seven long-term nonprogressors had undectectable viral loads, viral loads in six of seven HAART-treated progressors were undetectable, and one had a viral load of 180 copies/ml.
FIG. 1.
Virus-specific IL-17-producing cells are detectable in early HIV-1 infection. Representative data from an HIV-1-infected acute seroconverter, a chronically infected individual, and an HIV-1-uninfected individual are shown. The frequencies of antigen-specific cells are indicated as percentages of total CD4+ T cells.
FIG. 2.
Summary data on cytokine-producing cells in response to HIV-1, CMV, and SEB. The x axis represents the clinical groups. The y axis represents the percentages of CD4+ T cells producing cytokines. ***, P < 0.005; **, P < 0.01; *, P < 0.05.
FIG. 3.
Representative cytokine coexpression of virus-specific IL-17-producing cells. Representative data for p55 HIV-1 antigen-stimulated PBMC from an acute seroconverter are shown. PBMC in all individuals were stimulated for 6 h with p55 antigen and then stained for CD4/IL-17/IL-2/IFN-γ. In a subset of individuals (three), cells were also costained for surface expression of CXCR3 and CCR4. IL-17-producing CD4+ T cells in response to p55 antigen are shown, which were then gated to show coexpression of IL-2 or IFN-γ or to show surface expression of the chemokine receptors CXCR3 and CCR4. Number in quadrants represent percentages of IL-17-expressing CD4+ T cells also staining for IL-2 and/or IFN-γ. In the histograms, shaded regions represent chemokine receptor expression and the control (fluorescence minus one), shown as gray lines.
Thus, we have shown that both HIV-1- and CMV-specific TH-17 cells are produced in early HIV-1 infection. The fact that we did not detect significant numbers of CMV-specific TH-17 cells in normal volunteers with detectable CMV-specific TH-1 cells suggests this to be a phenomenon specific to early HIV-1 infection. Recently, Acosta-Rodriguez et al. (2) described how human TH-17 cells that produced only IL-17 could be induced by C. albicans. These cells could be identified by CCR4 expression. These authors also described IL-17/IFN-γ-coexpressing cells that were induced by mycobacteria and which were CCR4 negative but expressed CXCR3 and thus were postulated to be a subset of TH1 cells. The virus-specific TH-17 cells we describe here for early HIV-1 infection produce IL-17 but can also coexpress other cytokines, including IFN-γ. The cytokine expression pattern correlated with our observed expression of CCR4 and CXCR3, in which only a subset of cells stained for CCR4 or CXCR3. The CCR4-expressing cells may thus reflect “IL-17-only”-producing cells, whereas CXCR3-expressing cells may reflect IL-17/IFN-γ-coexpressing cells. These findings thus suggest that virus-specific IL-17 cells in our cohort are a heterogeneous population containing both TH1 and “true” TH17 cells. Our data do not explain why virus-specific cells should induce IL-17 and why they are not seen during chronic progressive or nonprogressive infection or in normal uninfected individuals. Recent studies have shown that monocytes stimulated with peptidoglycan and lipopolysaccharide (TLR2 and four agonists, respectively) produce IL-1β and a little IL-12, and this cytokine milieu will differentiate naive CD4+ T cells into IL-17-expressing or IL-17/IFN-γ-coexpressing cells (1). In early HIV-1 infection, there is dramatic replication of virus in gut CD4+ T cells, resulting in their loss and the eventual translocation of bacterial products into the blood (4, 5, 16). This scenario may provide the necessary signals to alter the phenotype of some virus-specific cells that are also migrating to the gut or being primed in gut-associated lymphoid tissue to a TH-17 phenotype. Alternatively, translocated lipopolysaccharide in the blood may alter the phenotype of virus-specific CD4+ T cells circulating in the blood, thus potentially explaining the lack of detection of IL-17-producing CMV-specific cells in peripheral blood of healthy volunteers. Thus, IL-17 expression may be an aberrant phenotype of virus-specific cells which are primed in a milieu of microbial translocation. Since gut-derived CD4+ T cells are almost completely depleted in chronic HIV-1 infection, our not finding IL-17-producing cells in chronic infection may also reflect this depletion. Since TH-17 cells promote the recruitment of neutrophils and inflammation, such cells may help fuel ongoing activation to allow further HIV-1 replication. We did not observe a correlation between plasma viral load levels in early infection and the frequency of HIV-1-specific IL-17-producing cells; however, viral replication in the gut was not examined in this study. Thus, further studies will be needed to determine the role of these cells in contributing to the control or enhancement of viral replication in early HIV-1 infection. If they do enhance viral replication, they may represent a potential therapeutic target for dampening inflammatory responses to reduce viral replication.
Acknowledgments
We extend special thanks to all the participants of this study for contributing to our work.
This study was carried out with funding from Canadian Institutes of Health Research (CIHR). M.A.O. receives salary support from the OHTN (Ontario HIV Treatment Network).
Footnotes
Published ahead of print on 23 April 2008.
REFERENCES
- 1.Acosta-Rodriguez, E. V., G. Napolitani, A. Lanzavecchia, and F. Sallusto. 2007. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8942-949. [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Rodriguez, E. V., L. Rivino, J. Geginat, D. Jarrossay, M. Gattorno, A. Lanzavecchia, F. Sallusto, and G. Napolitani. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8639-646. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli, E., M. Oukka, and V. K. Kuchroo. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8345-350. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 121365-1371. [DOI] [PubMed] [Google Scholar]
- 5.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y., C. L. Langrish, B. McKenzie, B. Joyce-Shaikh, J. S. Stumhofer, T. McClanahan, W. Blumenschein, T. Churakovsa, J. Low, L. Presta, C. A. Hunter, R. A. Kastelein, and D. J. Cua. 2006. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Investig. 1161317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauci, A. S., G. Pantaleo, S. Stanley, and D. Weissman. 1996. Immunopathogenic mechanisms of HIV infection. Ann. Intern. Med. 124654-663. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto, T., K. Akiyama, N. Kobayashi, and A. Mori. 2005. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch. Allergy Immunol. 137(Suppl. 1)51-54. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov, I. I., B. S. McKenzie, L. Zhou, C. E. Tadokoro, A. Lepelley, J. J. Lafaille, D. J. Cua, and D. R. Littman. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 1261121-1133. [DOI] [PubMed] [Google Scholar]
- 10.Kolls, J. K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity 21467-476. [DOI] [PubMed] [Google Scholar]
- 11.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linden, A., H. Hoshino, and M. Laan. 2000. Airway neutrophils and interleukin-17. Eur. Respir. J. 15973-977. [DOI] [PubMed] [Google Scholar]
- 13.Maek-A-Nantawat, W., S. Buranapraditkun, J. Klaewsongkram, and K. Ruxrungtham. 2007. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2066-75. [DOI] [PubMed] [Google Scholar]
- 14.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441231-234. [DOI] [PubMed] [Google Scholar]
- 15.Matusevicius, D., P. Kivisakk, B. He, N. Kostulas, V. Ozenci, S. Fredrikson, and H. Link. 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 5101-104. [DOI] [PubMed] [Google Scholar]
- 16.Mehandru, S., K. Tenner-Racz, P. Racz, and M. Markowitz. 2005. The gastrointestinal tract is critical to the pathogenesis of acute HIV-1 infection. J. Allergy Clin. Immunol. 116419-422. [DOI] [PubMed] [Google Scholar]
- 17.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y. H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 61133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman, L. 2007. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13139-145. [DOI] [PubMed] [Google Scholar]
- 19.Stockinger, B., and M. Veldhoen. 2007. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 19281-286. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24179-189. [DOI] [PubMed] [Google Scholar]
- 21.Wong, C. K., C. Y. Ho, E. K. Li, and C. W. Lam. 2000. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9589-593. [DOI] [PubMed] [Google Scholar]
- 22.Yue, F. Y., C. M. Kovacs, R. C. Dimayuga, P. Parks, and M. A. Ostrowski. 2004. HIV-1-specific memory CD4(+) T cells are phenotypically less mature than cytomegalovirus-specific memory CD4(+) T cells. J. Immunol. 1722476-2486. [DOI] [PubMed] [Google Scholar]