Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is a member of the gammaherpesvirus family. KSHV is the etiologic agent of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. The first open reading frame of the KSHV genome encodes a type 1 transmembrane glycoprotein named K1. K1 is structurally similar to the B-cell receptor (BCR), and its cytoplasmic tail contains an immunoreceptor tyrosine-based activation motif that can activate Syk kinase and the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Recent evidence suggests that receptor signaling occurs not only at the cell membrane, but from intracellular compartments as well. We have found that K1 is internalized in a clathrin-dependent manner, and efficient internalization is coupled to its signaling function. Once internalized, K1 traffics from the early endosome to the recycling endosome. Interestingly, blocking K1's activation of Syk and PI3K prevents K1 from internalizing. We have also found that blocking clathrin-mediated endocytosis prevents downstream signaling by K1. These results strongly suggest that internalization of K1 is intimately associated with normal signaling. When K1 internalization was examined in B lymphocytes, we found that K1 cointernalized with the BCR. Altogether, these results suggest that K1's signaling function is tightly coupled to its internalization.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) (also called human herpesvirus 8) is a gammaherpesvirus that was first identified in KS biopsies (5). KSHV has since been found in all epidemiological forms of KS (18). Viral DNA has been consistently isolated in AIDS-associated KS and almost all European/Mediterranean KS (9, 13, 30). KSHV has also been associated with lymphoproliferative diseases, such as primary effusion lymphoma and multicentric Castleman's disease (44), both of which are of B-cell origin. The exact mechanism by which KSHV induces transformation has not yet been completely dissected.
The far-left end of the KSHV genome encodes a 46-kDa transmembrane glycoprotein called K1. This position is equivalent to that of the saimiri transformation protein of herpesvirus saimiri (32) and the R1 oncogene of rhesus monkey rhadinovirus (12). K1 is expressed in KS lesions, primary effusion lymphoma cells, and multicentric Castleman's disease (1, 19, 24, 39). K1 is structurally similar to the B-cell receptor (BCR). The cytoplasmic tail contains an immunoreceptor tyrosine-based activation motif (ITAM), which has been shown to be capable of activating a signal profile (21, 26) similar to that activated by the BCR in B lymphocytes (38). The ITAM is essentially comprised of two SH2 binding motifs. Unlike the BCR, K1 is constitutively active, possibly due to oligomerization via conserved extracellular cysteine residues (21). K1 has been shown to interact with multiple cellular proteins containing SH2 domains, including Lyn, Syk, p85, PLCγ2, RasGAP, Vav, and Grb2. This interaction is thought to occur through the phosphorylated SH2 binding motifs that constitute the ITAM in the C terminus of K1 (25). Furthermore, K1 expression has also been shown to promote the production and secretion of vascular endothelial growth factor in both epithelial and endothelial cells and to increase matrix metalloproteinase 9 expression in endothelial cells, all of which is dependent on the SH2 binding motifs in the K1 cytoplasmic tail (50). Transgenic K1 mice develop tumors with features similar to those of spindle-cell sarcomatoid and malignant plasmablastic lymphoma. Moreover, lymphocytes isolated from these transgenic mice showed constitutive activation of NF-κB and Oct-2 and enhanced Lyn activity (35, 36). Additionally, our laboratory has previously shown that K1 activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in both B cells and endothelial cells, protecting cells from apoptosis (45, 49).
Activation of cell surface receptors by specific ligands often results in internalization via clathrin-dependent and -independent pathways, and internalization of receptors is considered an important mechanism by which cells control the intensity and duration of signal transduction. Recent findings indicate that internalization of receptors can allow signal propagation and amplification due to the high order of regulation of the endosome, using the compartmentalized organization of the endocytic pathway, going beyond the conventional role of receptor/cargo degradation. Some receptors, such as epidermal growth factor (EGF) or fibroblast growth factor, can maintain their signaling activities from within intracellular compartments (3, 41).
In this study, we show that K1 is internalized via clathrin-mediated endocytosis and that K1's ability to signal is linked to its internalization. We further demonstrate that blocking internalization prevents K1 activation of the PI3K/Akt pathway.
MATERIALS AND METHODS
Reagents and antibodies.
LY294002 and amantadine were purchased from Sigma Chemicals (St. Louis, MO). Piceatannol was purchased from Calbiochem (La Jolla, CA). Anti-FLAG M1 and M2-Cy3 antibodies were purchased from Sigma Chemicals. Anti-clathrin-HC antibody was purchased from Santa Cruz Biotechnology. Anti-TfR-Alexa 647, anti-immunoglobulin M (IgM)-Alexa 647 and anti-rabbit Alexa 647 were purchased from Molecular Probes, Invitrogen (Carlsbad, CA). Goat anti-mouse IgG, goat anti-mouse horseradish peroxidase (HRP), and 1-step ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] were purchased from Pierce (Rockford, IL). Anti-Akt (S473), anti-Akt (total), and anti-rabbit HRP were purchased from Cell Signaling (Danvers, MA). Anti-EEA1-fluorescein isothiocyanate (FITC) was purchased from BD Pharmingen (Franklin Lakes, NJ).
cDNAs, cell lines, and transfections.
pEF-K1WT and pEF-K1ITAM− have been previously described (45). pEF-K1ΔC was constructed by deleting the C terminus of K1 (Fig. 1). A cDNA encoding the clathrin hub fragment containing an amino-terminal T7 epitope (MASMTGGQQMG) was provided by J. Trejo (University of North Carolina). Rab11-green fluorescent protein (GFP) was a kind gift from Stephen S. G. Ferguson (University of Western Ontario).
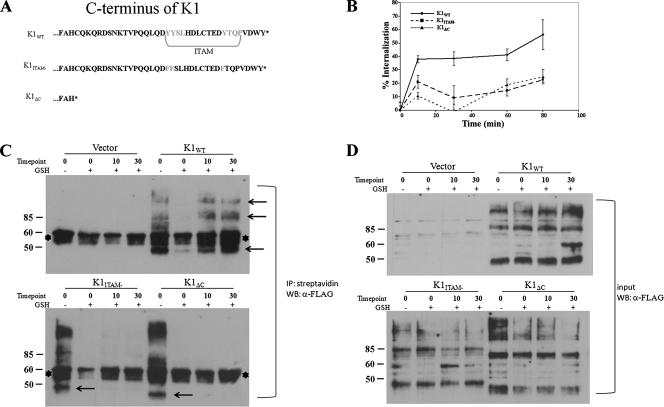
FIG. 1.
Internalization of K1 in HeLa cells. (A) Diagram of the amino acids in the C termini of K1 and mutants. The ITAM is highlighted. (B) Transfected cells were surface labeled with M1 anti-FLAG antibody at 4°C for 1 h, washed, and incubated for the times indicated at 37°C. Antibody remaining on the surface was stripped off, and the cells were lysed. Internalized antibody was quantified by ELISA. The data are expressed as a percentage of the initial amount of antibody bound to the cell surface at 0 min at 4°C. The error bars indicate standard deviations. (C) Endocytosis of K1 in a biotin endocytosis assay. HeLa cells were transfected with K1 and mutant expression plasmids as indicated above each gel. The samples were processed for the biotinylation endocytosis assay as described in Materials and Methods. The biotinylated proteins were internalized by transferring the cells to 37°C for 10 and 30 min. The + and − symbols indicate presence and absence of glutathione (GSH) treatment. The cells were then lysed, and all biotinylated proteins were immunoprecipitated (IP) using streptavidin beads. FLAG-K1 was detected in cell surface and intracellular pools by immunoblotting. The arrows indicate K1-specific bands. The asterisks denote nonspecific bands. (D) The same samples as in panel C, representing 1% of the input sample before immunoprecipitation.
HeLa cells stably expressing the tetracycline-regulated chimeric transcription factor (tetR-VP16) (generously provided by J. Trejo, University of North Carolina) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 4.5 mg/ml glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml G418. The cells were plated at 1.5 × 105 or 2.5 × 105 cells per well of 12-, or 6-well plates, respectively, and grown overnight. The cells were transiently transfected with a total of 0.8 or 2.0 μg per well of 12- or 6-well plates, respectively, of plasmids encoding FLAG-tagged wild-type (WT) K1 (K1WT) or mutants and either pcDNA vector or GFP fusion constructs, using Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions. The cells were incubated in complete medium for 48 h prior to being assayed as indicated below.
DG-75 cells, a human B-cell line that is KSHV negative and Epstein-Barr virus negative (23), were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. Forty micrograms of pEF-K1WT, mutants, or empty vector was electroporated in serum-free medium into 8 × 106 DG-75 cells at 300 V and 950 μF.
Internalization assay.
To assess the internalization of receptors, HeLa cells transiently expressing similar amounts of FLAG-K1 (WT or mutant proteins) were incubated with the calcium-dependent M1 anti-FLAG antibody for 1 h at 4°C. Under these conditions, only receptors present on the cell surface bind antibody. The cells were washed and incubated for various times at 37°C, allowing the M1 anti-FLAG-bound K1 protein to internalize. The remaining surface-bound antibody was then removed by three washes with phosphate-buffered saline (PBS) (Ca2+ and Mg2+ free) containing 0.04% EDTA for 5 min at 4°C. The cells were lysed in Triton lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 3% bovine serum albumin [BSA], and 1% Triton X-100). The amount of internalized anti-FLAG antibody in each cell lysate was then determined by sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates (Greiner Bio-one; catalog no. 655061) were coated with 1 μg/well goat anti-mouse antibody (Pierce) in 0.1 ml of PBS overnight at 4°C. Each well was incubated with 0.2 ml of 3% BSA in PBS for 3 h to block nonspecific binding. Cellular lysates were applied to the plates, and the samples were incubated for 3 h at room temperature, followed by three washes with PBS. Next, 0.5 μg of HRP-coupled goat anti-mouse antibody in 0.1 ml of PBS containing 3% BSA was added to each well. After five washes in PBS, 0.2 ml of HRP substrate (One Step ABTS solution; Pierce) was added to each well. HRP activity was determined by measuring the absorbance at 405 nm using a Fluostar-Optima microplate spectrophotometer (BMG Laboratories). To determine the amount of antibody initially bound to the surface, lysates were prepared from cells washed in PBS without EDTA.
Cell surface biotinylation and endocytosis assay.
HeLa cells were transfected with WT or mutant K1 expression plasmids as described above. Forty-eight hours posttransfection, the cells were incubated with 1.5 mg/ml of sulfosuccinimidyl 2-(biotinamido) ethyl-dithioproprionate (sulfo-NHS-SS-biotin) (Pierce Chemical Company) at 4°C for 1 h and washed with 15 mM glycine in PBS to quench any free sulfo-NHS-SS-biotin. This was followed by several washes with PBS. To measure cell surface biotinylated proteins, the cells were then lysed in 0.5 ml of RIPA buffer (20 mm Tris-HCl, pH 7.4, with 150 mm NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1% deoxycholate, 5 mM EDTA) containing protease inhibitors. To measure internalized biotinylated proteins, PBS was replaced with complete DMEM at 37°C for 10 and 30 min. The cells were washed twice for 30 min each time in glutathione stripping solution (60 mM glutathione and 0.83 mM NaCl, with 0.83 mM NaOH and 1% BSA added before use) at 4°C to remove all cell surface biotin groups. The remaining biotinylated proteins that were endocytosed were sequestered inside the cell and were therefore protected from glutathione stripping. The cell extracts were centrifuged to remove cell debris. The supernatant was incubated with streptavidin beads (Sigma Chemical Company) to collect bound, biotinylated proteins. These samples were then analyzed by SDS-polyacrylamide gel electrophoresis (PAGE), and Western blots were performed to identify K1-FLAG fusion proteins.
Immunofluorescence confocal microscopy.
HeLa cells plated on collagen-coated glass bottom dishes (MatTek, Ashland, MA) or DG-75 cells in suspension were transfected with FLAG-tagged WT or mutant K1 plasmids, as described above. Forty-eight hours posttransfection, the cells were preincubated with rabbit polyclonal anti-FLAG-Cy3 antibody for 1 h at 4°C to label K1 proteins expressed on the cell surface. The cells were washed and incubated for various times at 37°C. The cells were fixed and processed for confocal microscopy. Briefly, the cells were fixed in 2% paraformaldehyde and permeabilized with 100% methanol. The cells were blocked with 5% normal goat serum. Colocalization of K1 with subcellular structures was assessed by incubating permeabilized cells with antibody for 1 h at 25°C, followed by species-specific fluorophore-conjugated secondary antibodies and imaging using an Olympus FV500 confocal laser scanning microscope.
Amantadine and hypertonic treatments.
Clathrin-mediated endocytosis was inhibited using amantadine (2 mM amantadine in DMEM with 1% BSA) or hypertonic medium (20% sucrose in DMEM with 1% BSA) for 30 min as described previously (16, 34) and assayed as indicated below.
Western blot assays.
To detect Akt phosphorylation, transfected HeLa cells were serum starved for 24 h; the cells were harvested and lysed in RIPA buffer containing protease and phosphatase inhibitors. One hundred micrograms of each protein sample was subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and probed with anti-Akt-Ser473 (Cell Signal). After detection, the membranes were stripped and reprobed using Akt (total) antibody (Cell Signal).
RESULTS
Rate of K1 internalization.
In order to determine how surface expression of K1 is regulated, we first compared the internalization rates of K1WT, a K1ITAM− mutant in which both tyrosines present in the two SH2 binding motifs had been mutated to phenlyalanines (45), and a mutant of K1 in which the entire carboxyl terminus had been deleted, K1ΔC (Fig. 1A). HeLa cells were chosen for endocytosis assays, since they have a larger cytoplasmic compartment than B cells and are easy to transfect. The cells were transiently transfected with either FLAG-tagged K1WT, FLAG-tagged K1ITAM−, or FLAG-tagged K1ΔC for 48 h and then labeled with the calcium-dependent M1-FLAG antibody for 1 h at 4°C. The cells were washed to remove unbound antibody and incubated at 37°C for various times. After incubation, the remaining surface-bound calcium-sensitive M1-FLAG antibody was stripped using an EDTA/PBS solution. The cells were lysed, and the amount of internalized M1-FLAG was quantified by a FLAG ELISA. For K1WT, after 10 min at 37°C, ∼40% of the receptor had been internalized and appeared to reach a steady-state level of internalization. There was a slight increase in internalized K1 to ∼60% after 80 min at 37°C (Fig. 1B). For the K1ITAM− mutant, ∼20% of receptor had been internalized by 10 min, and this appeared to be a steady-state level. After 10 min, approximately 10% of K1ΔC surface receptor had been internalized, reaching ∼20% internalization after 80 min at 37°C (Fig. 1B.) These results suggest that elements in the C terminus of K1 are involved in internalization, allowing K1WT to internalize most efficiently.
To confirm that K1 internalization is not dependent on antibody cross-linking, cell surface biotinylation and internalization of K1 and the mutants were analyzed (Fig. 1C). We used a biotin endocytosis assay that employed the disulfide-linked NHS-SS-biotin, which permits cleavage of any biotin from the cell surface with glutathione after the 37°C endocytosis step (2, 4, 33, 51). Internalization of biotinylated glycoprotein protects the biotin from cleavage. The biotinylated proteins were then immunoprecipitated with streptavidin-conjugated beads and subjected to SDS-PAGE analysis. Membranes were then probed with antibody specific to the N-terminal FLAG epitopes on each construct. The zero-minute lane showed total cell surface biotinylation of K1 proteins before the glutathione cleavage step. The zero-minute-minus lane indicated that the cells were not returned to 37°C and were instead immediately treated with glutathione; the absence of K1-specific bands demonstrated that the cleavage step removed all remaining cell surface biotin to an undetectable level. For the 10-min-plus and 30-min-plus lanes, cells were incubated at 37°C for the indicated times before the cleavage step. Thus, if K1were internalized, a band would be detectable in these lanes. As can be seen in Fig. 1C, K1WT internalization was detectable by 10 min after incubation at 37°C and continued after 30 min at 37°C. However, for the two mutants, K1ITAM− and K1ΔC, no specific bands appeared at the times tested, indicating that neither of the two mutants was protected from cleavage by glutathione, since they were not endocytosed. The banding pattern seen for the K1 constructs is typical, showing a major band at 46 kDa and larger bands running at 100, 150, and 200 kDa, most likely due to oligomerization through extracellular disulfide bonds (20, 21, 27). Input levels of biotinylated proteins are also shown (Fig. 1D). Before immunoprecipitations were performed, a fraction of the lysate from each sample was removed and subjected to SDS-PAGE analysis. Membranes were probed with the same antibody as in Fig. 1C.
Confocal microscopy analysis of HeLa cells transiently transfected with the WT and mutant K1 constructs supported our ELISA data. Cells transfected with FLAG-tagged K1WT, FLAG-tagged K1ITAM−, or FLAG-tagged K1ΔC were labeled with an anti-FLAG-CY3 antibody, washed, and incubated for various times at 37°C. After incubation, the cells were fixed and analyzed by confocal microscopy. After 20 min of incubation, K1WT localized predominantly to endocytic-like structures (Fig. 2). In contrast, K1ITAM− and K1ΔC mutants were maintained predominantly at the surface at both 20 min and 60 min, suggesting that their internalization was severely compromised.
FIG. 2.
Confocal analysis of K1 internalization. WT and mutant K1-FLAG expression plasmids were transfected into HeLa cells. Forty-eight hours posttransfection, the transfected cells were surface labeled with anti-Flag-Cy3 antibody at 4°C for 1 h and then transferred to 37°C for 20 or 60 min. The cells were fixed and analyzed by confocal microscopy.
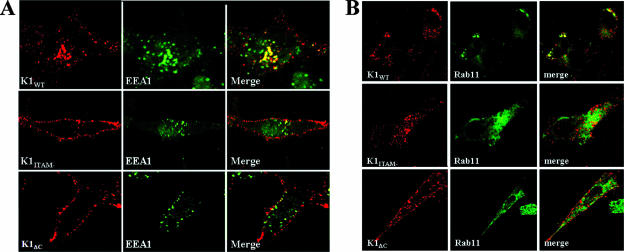
We next determined whether K1 was recycled after being endocytosed. EEA1 served as a marker of early endosomes, and Rab11 was used as a marker for recycling endosomes. HeLa cells were labeled for K1 surface expression as described above and incubated for 20 min at 37°C. Permeabilized cells were labeled with anti-EEA1-FITC. EEA1 is a Rab5 effector protein associated with the cytoplasmic side of early endosomes (6). As can be seen in Fig. 3A, K1WT colocalized with EEA1, which indicated that K1 trafficked to the early endosome. The majority of K1ITAM− and K1ΔC remained on the surfaces of the cells.
FIG. 3.
Subcellular trafficking of K1. (A) K1-expressing HeLa cells were incubated with anti-FLAG-CY3 for 1 h at 4°C. Bound receptors were allowed to internalize for 20 min at 37°C and subsequently labeled with FITC-labeled anti-EEA1 antibody. (B) HeLa cells were cotransfected with K1 expression vectors and a Rab11-GFP expression vector. The cells were surface labeled with anti-FLAG-Cy3 for 1 h at 4°C. The cells were incubated at 37°C for 90 min. The cells were fixed and imaged by confocal microscopy.
We also coexpressed K1 with Rab11-GFP. Rab11 has been shown to coordinate traffic through the recycling endosome (47). Our confocal analysis revealed that K1WT appeared to traffic to the recycling endosome, as indicated by colocalization with Rab11-GFP after incubation at 37°C for 90 min. Conversely, K1ITAM− and K1ΔC did not appear to colocalize with Rab11 (Fig. 3B).
K1 is internalized in a clathrin-dependent manner.
We next investigated whether K1 is internalized through a clathrin-dependent pathway. We first analyzed the colocalization of K1WT and endogenous clathrin. HeLa cells were transfected with WT FLAG-K1 expression plasmids and were incubated with anti-FLAG-Cy3 at 4°C to label K1 on the cell surface. The cells were placed at 37°C for 5 min, fixed, permeabilized, and labeled with anti-clathrin-HC-FITC antibody, which stains the clathrin heavy chain. The cells were examined by confocal microscopy. The staining was performed 5 min after incubation of the cells at 37°C. Although the majority of K1 remained on the cell surface, there was internalization of K1 that appeared to colocalize with clathrin (Fig. 4A). We next looked at the effect of a dominant-negative clathrin hub. The hub is the C-terminal third of the clathrin heavy chain (29) and has been shown to act as a dominant-negative clathrin inhibitor by competing for light-chain binding (28). Cells were transiently transfected as described above with the addition of an expression vector encoding the clathrin hub fragment with an amino-terminal T7 epitope. The cells were labeled with anti-FLAG-Cy3 at 4°C and moved to 37°C for 20 min. The cells were then fixed and permeabilized. Cells expressing the clathrin hub mutant were identified by costaining them for the T7 epitope. Antibody to the T7 epitope labeled the entire cytoplasm, as previously described (46). We found that cells that expressed the clathrin hub showed decreased internalization of K1, with K1 primarily localized to the cytoplasmic membrane after 20 min at 37°C (Fig. 4B), further supporting the hypothesis that K1 is internalized in a clathrin-dependent manner.
FIG. 4.
K1 is internalized in a clathrin-dependent manner. (A) Colocalization of K1WT with endogenous clathrin-HC. K1-expressing cells were surface labeled with anti-FLAG-Cy3 for 1 h at 4°C. After incubation at 37°C for 5 min, the cells were fixed and immunostained for clathrin-HC. Bound antibodies were visualized with the use of secondary antibody conjugated to FITC. The boxed region indicates the magnified area (Zoom). (B) HeLa cells were cotransfected with K1 and T7-Hub. The cells were labeled with an anti-FLAG-Cy3 antibody at 4°C, incubated at 37°C for 20 min, and then processed as described in the text. Cells expressing the clathrin hub mutant were identified by costaining them for the T7 epitope. A phase-contrast image of the cell is on the right.
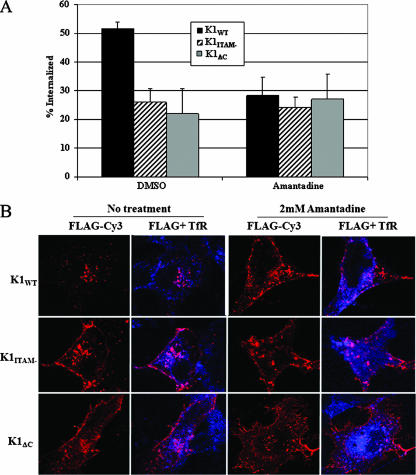
We examined the effects of clathrin inhibitors on K1 internalization. Amantadine is a drug that inhibits clathrin-coated-pit invagination at the plasma membrane (34). Cells transiently transfected with either FLAG-tagged K1WT, FLAG-tagged K1ITAM−, or FLAG-tagged K1ΔC were stained as described above and incubated in the presence or absence of amantadine for 20 min at 37°C. The cells were then assayed for internalized K1 protein via ELISA. In K1WT cells, internalization was substantially inhibited by amantadine (Fig. 5A), while both mutants were less affected by clathrin inhibition. Conversely, K1 internalization was not inhibited by the caveolin inhibitor filipin (data not shown). Shown in Fig. 5B are confocal data supporting inhibition of K1 internalization by amantadine. K1-expressing cells were stained as described above. As a positive control, we also labeled the transferrin receptor (TfR) using anti-TfR-Alexa 647 antibody (Molecular Probes). The TfR has been well studied and constitutively cycles between the plasma membrane, early endosomes, and recycling endosomes, and it has served as a classic system for the study of clathrin-mediated endocytosis (8). Amantadine prevented the majority of K1 protein from internalizing, as well as preventing TfR internalization (Fig. 5B).
FIG. 5.
Amantadine inhibits K1 internalization. (A) Transfected HeLa cells were surface labeled with M1 anti-FLAG, and surface receptors were allowed to internalize for 20 min in the presence or absence of drug. The amount of internalized antibody was quantified by ELISA. The data are expressed as a ratio of the initial amount of antibody bound to the cell surface at zero minutes at 4°C to the amount of antibody bound in the presence of drug. The error bars indicate standard deviations. (B) K1-expressing cells were surface labeled with anti-FLAG-Cy3 and anti-TfR-Alexa 647 for 1 h at 4°C. The surface receptors were allowed to internalize at 37°C for 20 min in the presence of the drug. The cells were fixed and analyzed by confocal microscopy.
K1 endocytosis and signaling are coupled.
Recent work has shown regulation of receptor signaling as a function of receptor internalization (3, 40-42). Subcellular localization can determine which effector molecules couple to activated receptors and the relative strengths of different signaling pathways. To that end, we wanted to determine if known signaling molecules activated by K1 are cointernalized and/or colocalized intracellularly. Conner and Schmid had previously shown that K1 can activate the PI3K signaling pathway in B cells and endothelial cells (8). Here, we examined whether K1 colocalized with the p85 subunit of PI3K. HeLa cells were transfected with a WT K1 FLAG expression plasmid. Forty-eight hours posttransfection, the cells were surface labeled with anti-FLAG-Cy3 to detect K1. The cells were either left on ice (Fig. 6A; t = 0) or moved to 37°C for 5 min (Fig. 6A; t = 5). Next, the cells were placed on ice, rinsed with cold PBS, and fixed. After permeabilization, the cells were incubated with anti-p85 primary antibody, followed by a FITC-conjugated secondary antibody to detect the p85 subunit of PI3K, and examined by confocal microscopy. K1WT and the p85 subunit of PI3K appeared to colocalize very well at t = 0, and there was also colocalization of K1 and p85 at t = 5 (Fig. 6A). We also analyzed K1 colocalization with p85 without prelabeling K1 surface molecules to get a better sense of their intracellular interaction. HeLa cells were transfected with a K1WT-FLAG expression plasmid. Forty-eight hours posttransfection, the cells were permeabilized and stained for FLAG-K1 and p85. As can be seen in Fig. 6B, K1 also appeared to colocalize with p85 intracellularly.
FIG. 6.
Intracellular distribution of K1 and PI3K. (A) HeLa cells were transfected with a K1WT expression plasmid. Forty-eight hours posttransfection, the cells were surface labeled with anti-FLAG-Cy3 to detect K1. After fixation and permeabilization, the cells were stained using an anti-p85 primary antibody, followed by a FITC-conjugated secondary antibody to detect the p85 subunit of PI3K. (B) Forty-eight hours posttransfection, K1WT-expressing HeLa cells were fixed, permeabilized, and stained with anti-FLAG-Cy3 and anti-p85 primary antibody, followed by a FITC-conjugated secondary antibody. The cells were analyzed by confocal microscopy.
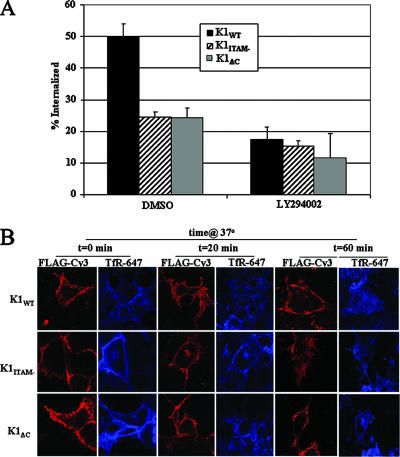
To further delineate the relationship between signaling and internalization, we utilized specific chemical inhibitors of kinases activated by K1, such as PI3K and Syk (8), and determined their effects on K1 internalization by both ELISA and confocal microscopy. LY294002 is an inhibitor of PI3K, and piceatannol is a Syk kinase inhibitor. HeLa cells were transfected with K1 and K1 mutant expression vectors and processed as described above. Additionally, cell surface-expressed TfR was labeled using anti-TfR-Alexa 647 antibody for confocal studies. LY294002 was added during subsequent 37°C incubations. As determined by ELISA, LY294002 inhibited K1WT internalization by over twofold after 20 min but had little or no effect on the K1ITAM− or K1ΔC protein (Fig. 7A). This was further corroborated by confocal data showing little or no K1WT internalization when cells were treated with LY294002 (Fig. 7B). In contrast, the TfR was internalized in an efficient manner.
FIG. 7.
Inhibition of K1 internalization by LY294002. (A) K1 internalization was measured in the presence or absence of LY294002. Transfected HeLa cells were surface labeled with M1 anti-FLAG, and surface receptors were allowed to internalize for 20 min in the presence of drug or vehicle control (dimethyl sulfoxide). The amount of internalized antibody was quantified by ELISA as described in Materials and Methods. The data are expressed as a ratio of the initial amount of antibody bound to the cell surface at 0 min at 4°C to the amount of antibody bound in the presence of drug after the cells were incubated at 37°C for 20 min. This is depicted as percent internalization on the y axis. The error bars indicate standard deviations. (B) K1 internalization in the presence or absence of LY294002 as analyzed by confocal microscopy. K1-expressing HeLa cells were surface labeled with anti-FLAG-Cy3 and anti-TfR-Alexa 647 for 1 h at 4°C. The surface receptors were allowed to internalize at 37°C for 0, 20, or 60 min in the presence of the drug. The cells were fixed and analyzed by confocal microscopy.
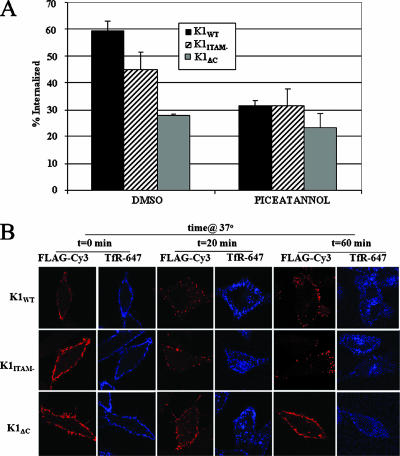
K1 has also been shown to activate Syk kinase (25). We next tested whether piceatannol, an inhibitor of Syk kinase, affected K1 endocytosis. We found that piceatannol also inhibited K1 internalization, although not to the same extent as seen with LY294002 (Fig. 8A), resulting in approximately 50% decrease after the cells were incubated for 20 min at 37°C in the presence of the drug. Confocal data also supported this, showing that the majority of K1 remained on the surface in the presence of piceatannol (Fig. 8B). The two K1 mutants, K1ITAM− and K1ΔC, were retained on the surface regardless of drug treatment.
FIG. 8.
Treatment of cells with piceatannol inhibits K1 internalization. (A) HeLa cells were transfected with WT or mutant K1 expression plasmids for 48 hours. Transfected HeLa cells were then incubated in the presence of 15 μg/ml piceatannol. The cells were surface labeled for K1. The amount of internalized receptor was quantified by ELISA as described in Materials and Methods. The data are expressed as a fraction of the initial amount of antibody bound to the cell surface at 0 min at 4°C and are depicted as percent internalization on the y axis. The error bars indicate standard deviations. (B) Confocal microscopy analysis of K1 internalization in the presence of piceatannol. K1-expressing HeLa cells were surface labeled with anti-FLAG-Cy3 and anti-TfR-Alexa 647 for 1 h at 4°C. The surface receptors were allowed to internalize at 37°C for 0, 20, or 60 min in the presence of the drug. The cells were fixed and analyzed by confocal microscopy.
Inhibition of endocytosis dampens signaling by K1.
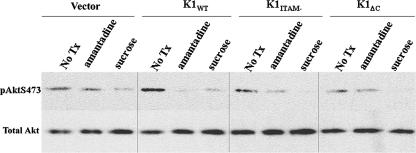
To examine whether K1 endocytosis and trafficking are important for controlling the signaling pathways and cellular responses to K1, we inhibited endocytosis by treating cells with amantadine or hypertonic medium. Hypertonic medium has been shown to inhibit clathrin-mediated endocytosis (16). HeLa cells were transiently transfected as described above. Twenty-four hours posttransfection, the cells were serum starved for an additional 24 h. The cells were pretreated for 30 min in fresh medium containing 2 mM amantadine or 20% sucrose. The cells were harvested, and the lysates were separated by SDS-PAGE, blotted onto membranes, and probed with anti-phospho-Akt (S473). As shown in Fig. 9, expression of K1WT increased Akt phosphorylation in these cells, while K1ITAM− and K1ΔC did not induce Akt phosphorylation. Treatment with the endocytosis inhibitors, amantadine and sucrose, dramatically decreased Akt phosphorylation in K1WT-expressing cells (Fig. 9).
FIG. 9.
Inhibition of clathrin-mediated endocytosis prevents K1 signaling. K1-expressing HeLa cells were treated with either amantadine or hypertonic sucrose medium to inhibit clathrin or were left untreated for 30 min prior to harvesting of the cells. The lysates were subjected to Western blot analysis. Equal amounts of proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with phosphospecific antibody to Akt S473 (top), stripped, and reprobed with antibody that recognized total Akt levels (bottom). No Tx, untreated cells.
K1 internalization in B cells.
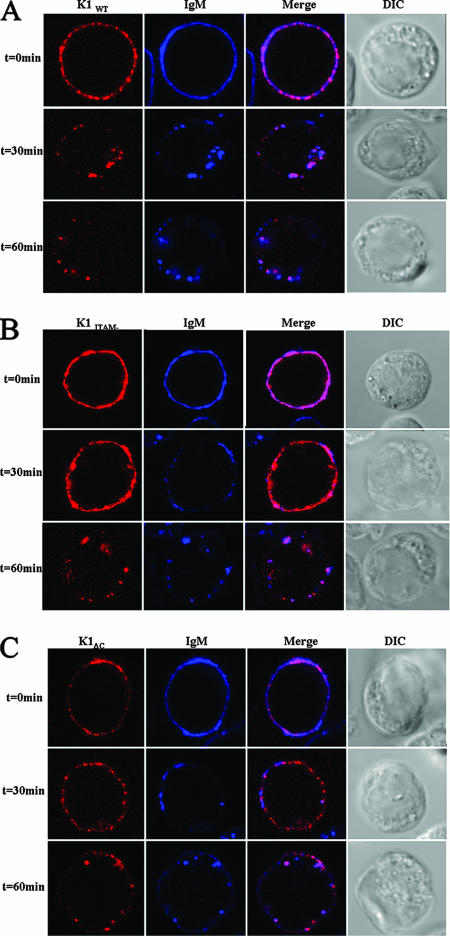
We next analyzed K1 internalization in DG75 B cells, which are KSHV negative. Since KSHV infects B lymphocytes, we wanted to determine if K1 cointernalized with the BCR. Cells were electroporated with K1 and mutant expression vectors as described in Materials and Methods. Twenty-four hours posttransfection, the cells were surface labeled for K1 using anti-FLAG-CY3 antibody and for IgM using anti-IgM-Alexa 647 antibody. After unbound antibody was rinsed away, the cells were incubated at 37°C for the indicated times, fixed, and analyzed by confocal microscopy. As can be seen in Fig. 10A, K1 and IgM colocalized and internalized together. Interestingly, after 30 min at 37°C, the K1ITAM− and K1ΔC mutants (Fig. 10B and C) remained bound together with BCR on the surface of the cells and did not internalize, in contrast to K1WT and IgM, which had been completely internalized at 30 min. By 60 min, the majority of K1ΔC and IgM remained on the surface, but the majority of K1ITAM− and IgM were internalized. These results show that K1WT cointernalizes with the BCR.
FIG. 10.
K1 cointernalizes with IgM. DG-75 cells were transfected with K1WT (A), K1ITAM− (B), or K1ΔC (C) expression vector. Twenty-four hours posttransfection, the cells were surface labeled with anti-FLAG-Cy3 (red) and anti-IgM-Alexa 647 (blue) antibodies at 4°C for 30 min. The cells were transferred to 37°C for the times indicated, fixed, and viewed by confocal microscopy. Colocalization is indicated by magenta color. DIC, differential interference contrast.
DISCUSSION
It is well established that K1 is expressed on the cell surface and activates signaling pathways, including the PI3K/Akt pathway, through its C-terminal SH2 binding motifs that encode the ITAM (21, 26, 45). In order to further elucidate the mechanism of K1 signaling, we have examined how its signaling function at the plasma membrane is regulated. In this study, we demonstrated that endocytosis and signaling of K1 are linked. The two mutants, K1ITAM− and K1ΔC, show decreased internalization compared to K1WT and are partially internalized at very late time points. At later time points, it is possible that the K1 mutants are being internalized nonspecifically. Thus, elements in the C terminus of K1 are required for efficient internalization, specifically, the ITAM motif. K1 has been shown to activate Syk and PI3K signaling pathways, all of which are dependent upon the ITAM of K1 (21, 25, 26, 45). Inhibiting K1 activation of PI3K and Syk kinase using LY294002 and piceatannol, respectively, resulted in decreased endocytosis of K1WT, with little effect on the two mutants tested, further supporting the role of signaling in the control of K1 surface expression. We have also observed the reciprocal result; inhibiting internalization hinders efficient downstream signaling by K1.
In order to determine the mechanisms controlling K1 internalization, we analyzed the trafficking pattern of surface-labeled K1WT and mutants. Perturbing clathrin-dependent endocytosis markedly decreased the rate at which K1 was internalized. We could inhibit K1 internalization by using a dominant-negative clathrin hub, further supporting our data that K1 is internalized via clathrin. Following internalization, K1 was present in both early endosomes, since it colocalized with EEA1, and in recycling endosomes, since it colocalized with Rab11. Although the question of whether K1 is truly recycled needs further investigation, it is an intriguing possibility. Rab11 overexpression has been shown to facilitate the transition of some proteins from early endosomes to late and recycling endosomal compartments, but it does not drive membrane proteins to the recycling endosome (11). Additionally, it has been shown that the overexpression of WT Rab11 does not cause a significant impairment in the capacity of the cells to recycle transferrin molecules (37). Other viral glycoproteins, such as the Us28 glycoprotein of cytomegalovirus (14), show ligand-independent constitutive internalization and recycling. Constitutive recycling may provide a convenient method for the cell to regulate surface receptor numbers. If a rapid decrease in surface receptors is needed, the internalization rate could be increased and/or recycling decreased, and vice versa.
In addition to receptor down-regulation, internalization of receptor subunits also serves to modulate receptor signaling pathways. Our laboratory has previously shown that K1 can activate Akt via a PI3K-dependent pathway (45). Here, we demonstrated that the ability of K1 to activate Akt kinase was suppressed in cells in which endocytosis had been inhibited (Fig. 9). The suppression of downstream signaling has been shown for cellular receptors, as well. Using an internalization-defective cell line, it was shown that EGF receptor (EGFR)-mediated tyrosine phosphorylation and mitogen-activated protein kinase activation were both attenuated in internalization-defective cells (48). Further, correct trafficking of EGFR is required to establish specific signaling pathways. Membrane trafficking regulates the signal transduction events of EGFR. There is also evidence that receptor tyrosine kinases signal from endosomes. It has been demonstrated that the internalized EGFR can still signal (7). EGFR remains bound to EGF within the endosomal compartment; EGF-stimulated EGFRs preserve their dimerization and their kinase activities within endosomes (43, 52). Receptor signaling from endosomes might allow spatial and temporal regulation of signal transduction. Signal transduction could be compartmentalized at the plasma membrane and on endosomes. Compartmentalization would provide high flexibility to an otherwise limited number of signaling cascades to transduce specific signals from multiple signaling cues. There is, in fact, accumulating evidence that signaling from endosomes could be used as a means to compartmentalize signal transduction (40, 42).
The colocalization of K1 and the p85 subunit of PI3K is consistent with models of PI3K function in which PI3K, a principally cytoplasmic protein, is recruited to membranes quantitatively in response to receptor activation. In cells in which endocytosis is inhibited, the p85 subunit of PI3K is hypophosphorylated (48). The endosomes are highly enriched for PI3K activity.
Mutation of the K1 ITAM has previously been shown to prevent phosphorylation, recruitment, and binding of Syk to K1 (21, 25, 26). Specifically, K1's ability to activate downstream signaling was significantly reduced in Syk-deficient B cells (21). Additionally, K1 signaling was shown to induce the phosphorylation and activation of Syk kinase in B cells (21, 25, 26). Furthermore, Syk kinase was also shown to bind to the doubly phosphorylated K1 ITAM (21). Syk kinase can regulate Akt activation through activation of PI3K (10, 15, 17, 31). Further, it has been suggested that the activation of PI3K by Syk is directly involved in endocytosis (22). Lee et al. (25) have shown that K1 interacts with different signaling proteins through its C-terminal tyrosine residues with different affinities. It is easy to imagine that this differential phosphorylation of the tyrosine residues in different locations in the cell, i.e., plasma membrane versus endocytic vesicles, allows K1 to interact with various cellular proteins. Indeed, several receptors can transmit signals from endocytic compartments, and these signals can be qualitatively different from those initiated at the plasma membrane. For example, TrkA promotes nerve growth factor (NGF)-mediated cell survival at the cell surface, whereas it induces differentiation when internalized (53). Internalization could couple activation with down-regulation, which allows precise temporal control of signaling by limiting the lifetime of the activated signaling components.
The fact that K1 cointernalizes with the BCR in B cells suggests a physiologically important role for K1 internalization. It is plausible that K1 may function to scavenge the BCR from the surfaces of newly infected B cells.
In summary, the data presented in this report demonstrate that K1 internalization occurs through a clathrin-mediated endocytic pathway. K1 protein expressed at the surface is constitutively active and recruits PI3K to the membrane. Activation of PI3K results in internalization of K1 via clathrin. Once internalized, K1 continues to transduce signals, inducing the activation of Akt and downstream signals. Inhibiting either clathrin-dependent internalization or activation of PI3K prevents efficient endocytosis and efficient signaling by K1. The signaling of K1 at the plasma membrane is regulated by both surface expression modulation and compartmentalized signaling. This is yet an additional means for KSHV to control cellular signaling pathways.
Acknowledgments
We thank JoAnn Trejo and May Paing for kindly providing us with reagents and protocols for the endocytosis ELISAs and for critical reading of the manuscript. Rab11-GFP was a kind gift from Stephen S. G. Ferguson, University of Western Ontario. We also thank members of the Damania and Dittmer laboratories for helpful discussions.
This work was supported by NIH grants CA096500 and HL083469 and AHA grant 0640041N to B.D. C.C.T was supported in part by NCI training grant T32-CA71341. B.D. is a Leukemia and Lymphoma Society Scholar and a Burroughs Welcome Fund Investigator in Infectious Disease.
Footnotes
Published ahead of print on 23 April 2008.
REFERENCES
- 1.Bowser, B. S., S. M. DeWire, and B. Damania. 2002. Transcriptional regulation of the K1 gene product of Kaposi's sarcoma-associated herpesvirus. J. Virol. 7612574-12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretscher, M. S., and R. Lutter. 1988. A new method for detecting endocytosed proteins. EMBO J. 74087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant, D. M., F. G. Wylie, and J. L. Stow. 2005. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol. Biol. Cell 1614-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busch, G., D. Hoder, W. Reutter, and R. Tauber. 1989. Selective isolation of individual cell surface proteins from tissue culture cells by a cleavable biotin label. Eur. J. Cell Biol. 50257-262. [PubMed] [Google Scholar]
- 5.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 6.Christoforidis, S., H. M. McBride, R. D. Burgoyne, and M. Zerial. 1999. The Rab5 effector EEA1 is a core component of endosome docking. Nature 397621-625. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, S., and R. A. Fava. 1985. Internalization of functional epidermal growth factor:receptor/kinase complexes in A-431 cells. J. Biol. Chem. 26012351-12358. [PubMed] [Google Scholar]
- 8.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 42237-44. [DOI] [PubMed] [Google Scholar]
- 9.Cornali, E., C. Zietz, R. Benelli, W. Weninger, L. Masiello, G. Breier, E. Tschachler, A. Albini, and M. Sturzl. 1996. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi's sarcoma. Am. J. Pathol. 1491851-1869. [PMC free article] [PubMed] [Google Scholar]
- 10.Cornall, R. J., A. M. Cheng, T. Pawson, and C. C. Goodnow. 2000. Role of Syk in B-cell development and antigen-receptor signaling. Proc. Natl. Acad. Sci. USA 971713-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale, L. B., J. L. Seachrist, A. V. Babwah, and S. S. Ferguson. 2004. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J. Biol. Chem. 27913110-13118. [DOI] [PubMed] [Google Scholar]
- 12.Damania, B., M. Li, J. K. Choi, L. Alexander, J. U. Jung, and R. C. Desrosiers. 1999. Identification of the R1 oncogene and its protein product from the rhadinovirus of rhesus monkeys. J. Virol. 735123-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupin, N., M. Grandadam, V. Calvez, I. Gorin, J. T. Aubin, S. Havard, F. Lamy, M. Leibowitch, J. M. Huraux, J. P. Escande, and H. Agut. 1995. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi's sarcoma. Lancet 345761-762. [DOI] [PubMed] [Google Scholar]
- 14.Fraile-Ramos, A., T. N. Kledal, A. Pelchen-Matthews, K. Bowers, T. W. Schwartz, and M. Marsh. 2001. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 121737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold, M. R., M. P. Scheid, L. Santos, M. Dang-Lawson, R. A. Roth, L. Matsuuchi, V. Duronio, and D. L. Krebs. 1999. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J. Immunol. 1631894-1905. [PubMed] [Google Scholar]
- 16.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong, J. J., T. M. Yankee, M. L. Harrison, and R. L. Geahlen. 2002. Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. A mechanism for negative signaling by the Lyn tyrosine kinase. J. Biol. Chem. 27731703-31714. [DOI] [PubMed] [Google Scholar]
- 18.Huang, Y. Q., J. J. Li, M. H. Kaplan, B. Poiesz, E. Katabira, W. C. Zhang, D. Feiner, and A. E. Friedman-Kien. 1995. Human herpesvirus-like nucleic acid in various forms of Kaposi's sarcoma. Lancet 345759-761. [DOI] [PubMed] [Google Scholar]
- 19.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236147-154. [DOI] [PubMed] [Google Scholar]
- 20.Lagunoff, M., D. M. Lukac, and D. Ganem. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 755891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 965704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau, C., X. Wang, L. Song, M. North, S. Wiehler, D. Proud, and C. W. Chow. 2008. Syk associates with clathrin and mediates phosphatidylinositol 3-kinase activation during human rhinovirus internalization. J. Immunol. 180870-880. [DOI] [PubMed] [Google Scholar]
- 23.Lazar, A., S. Reuveny, M. Minai, A. Traub, and A. Mizrahi. 1981. Interferon production by a human lymphoblastoid cell line (DG-75) free of the Epstein-Barr genome. Antimicrob. Agents Chemother. 20151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, B. S., M. Connole, Z. Tang, N. L. Harris, and J. U. Jung. 2003. Structural analysis of the Kaposi's sarcoma-associated herpesvirus K1 protein. J. Virol. 778072-8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, B. S., S. H. Lee, P. Feng, H. Chang, N. H. Cho, and J. U. Jung. 2005. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. J. Virol. 7912173-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, H., J. Guo, M. Li, J. K. Choi, M. DeMaria, M. Rosenzweig, and J. U. Jung. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 185219-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma- associated herpesvirus. Nat. Med. 4435-440. [DOI] [PubMed] [Google Scholar]
- 28.Liu, S. H., M. S. Marks, and F. M. Brodsky. 1998. A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J. Cell Biol. 1401023-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, S. H., M. L. Wong, C. S. Craik, and F. M. Brodsky. 1995. Regulation of clathrin assembly and trimerization defined using recombinant triskelion hubs. Cell 83257-267. [DOI] [PubMed] [Google Scholar]
- 30.Marchioli, C. C., J. L. Love, L. Z. Abbott, Y. Q. Huang, S. C. Remick, N. Surtento-Reodica, R. E. Hutchison, D. Mildvan, A. E. Friedman-Kien, and B. J. Poiesz. 1996. Prevalence of human herpesvirus 8 DNA sequences in several patient populations. J. Clin. Microbiol. 342635-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon, K. D., C. B. Post, D. L. Durden, Q. Zhou, P. De, M. L. Harrison, and R. L. Geahlen. 2005. Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J. Biol. Chem. 2801543-1551. [DOI] [PubMed] [Google Scholar]
- 32.Murthy, S. C., J. J. Trimble, and R. C. Desrosiers. 1989. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J. Virol. 633307-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 774191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phonphok, Y., and K. S. Rosenthal. 1991. Stabilization of clathrin coated vesicles by amantadine, tromantadine and other hydrophobic amines. FEBS Lett. 281188-190. [DOI] [PubMed] [Google Scholar]
- 35.Prakash, O., Z. Y. Tang, X. Peng, R. Coleman, J. Gill, G. Farr, and F. Samaniego. 2002. Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J. Natl. Cancer Inst. 94926-935. [DOI] [PubMed] [Google Scholar]
- 36.Prakash, O., O. R. Swamy, X. Peng, Z. Y. Tang, L. Li, J. E. Larson, J. C. Cohen, J. Gill, G. Farr, S. Wang, and F. Samaniego. 2005. Activation of Src kinase Lyn by the Kaposi sarcoma-associated herpesvirus K1 protein: implications for lymphomagenesis. Blood 1053987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren, M., G. Xu, J. Zeng, C. De Lemos-Chiarandini, M. Adesnik, and D. D. Sabatini. 1998. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA 956187-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reth, M., and J. Wienands. 1997. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 15453-479. [DOI] [PubMed] [Google Scholar]
- 39.Samaniego, F., S. Pati, J. Karp, O. Prakash, and D. Bose. 2001. Human herpesvirus 8 k1-associated nuclear factor-kappa b-dependent promoter activity: role in Kaposi's sarcoma inflammation? J. Natl. Cancer Inst. Monogr. 2815-23. [DOI] [PubMed] [Google Scholar]
- 40.Seto, E. S., H. J. Bellen, and T. E. Lloyd. 2002. When cell biology meets development: endocytic regulation of signaling pathways. Genes Dev. 161314-1336. [DOI] [PubMed] [Google Scholar]
- 41.Sigismund, S., T. Woelk, C. Puri, E. Maspero, C. Tacchetti, P. Transidico, P. P. Di Fiore, and S. Polo. 2005. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 1022760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorkin, A., and M. Von Zastrow. 2002. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 3600-614. [DOI] [PubMed] [Google Scholar]
- 43.Sorkin, A. D., L. V. Teslenko, and N. N. Nikolsky. 1988. The endocytosis of epidermal growth factor in A431 cells: a pH of microenvironment and the dynamics of receptor complex dissociation. Exp. Cell Res. 175192-205. [DOI] [PubMed] [Google Scholar]
- 44.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 861276-1280. [PubMed] [Google Scholar]
- 45.Tomlinson, C. C., and B. Damania. 2004. The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway. J. Virol. 781918-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trejo, J., Y. Altschuler, H. W. Fu, K. E. Mostov, and S. R. Coughlin. 2000. Protease-activated receptor-1 down-regulation: a mutant HeLa cell line suggests novel requirements for PAR1 phosphorylation and recruitment to clathrin-coated pits. J. Biol. Chem. 27531255-31265. [DOI] [PubMed] [Google Scholar]
- 47.Ullrich, O., S. Reinsch, S. Urbe, M. Zerial, and R. G. Parton. 1996. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vieira, A. V., C. Lamaze, and S. L. Schmid. 1996. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 2742086-2089. [DOI] [PubMed] [Google Scholar]
- 49.Wang, L., D. P. Dittmer, C. C. Tomlinson, F. D. Fakhari, and B. Damania. 2006. Immortalization of primary endothelial cells by the K1 protein of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 663658-3666. [DOI] [PubMed] [Google Scholar]
- 50.Wang, L., N. Wakisaka, C. C. Tomlinson, S. M. DeWire, S. Krall, J. S. Pagano, and B. Damania. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 642774-2781. [DOI] [PubMed] [Google Scholar]
- 51.Weixel, K., and N. A. Bradbury. 2002. Analysis of CFTR endocytosis by cell surface biotinylation. Methods Mol. Med. 70323-340. [DOI] [PubMed] [Google Scholar]
- 52.Zapf-Colby, A., and J. M. Olefsky. 1998. Nerve growth factor processing and trafficking events following TrkA-mediated endocytosis. Endocrinology 1393232-3240. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y., D. B. Moheban, B. R. Conway, A. Bhattacharyya, and R. A. Segal. 2000. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J. Neurosci. 205671-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]