Abstract
Blood-circulating monocytes migrate in tissues in response to danger stimuli and differentiate there into two major actors of the immune system: macrophages and dendritic cells. Given their migratory behavior and their pivotal role in the orchestration of immune responses, it is not surprising that cells of the monocyte lineage are the target of several viruses, including human immunodeficiency virus type 1 (HIV-1). HIV-1 replicates in monocytoid cells to an extent that is influenced by their differentiation status and modulated by exogenous stimulations. Unstimulated monocytes display a relative resistance to HIV infection mostly exerted during the early steps of the viral life cycle. Despite intensive studies, the identity of the affected step remains controversial, although it is generally assumed to take place after viral entry. We reexamine here the early steps of viral infection of unstimulated monocytes using vesicular stomatitis virus G protein-pseudotyped HIV-1 virions. Our data indicate that a first block to the early steps of infection of monocytes with these particles occurs at the level of viral entry. After entry, reverse transcription and integration proceed with extremely slow kinetics rather than being blocked. Once completed, viral DNA molecules delay entry into the nucleus and integration for up to 5 to 6 days. The inefficacy of these steps accounts for the resistance of monocytes to HIV-1 during the early steps of infection.
Blood-circulating monocytes differentiate in tissues into macrophages and dendritic cells in response to danger stimuli (25). Due to their migratory behavior and their key functions in immune system responses, it is not surprising that cells of the monocyte-macrophage lineage are the preferential targets of a number of lentiviruses. These viruses establish a complex relation with their host. In the case of Maedi-Visna virus infection (an ovine lentivirus), the virus adopts what has been named a Trojan horse spread mechanism (44). The name appropriately fits the behavior of this virus that remains silently hidden in circulating monocytes until cell differentiation in tissues reactivates its replication (34). In the case of Maedi-Visna infection, postintegration silencing of the viral long terminal repeat (LTR) promoter provides the covert that allows the virus to migrate undetected with monocytes (4, 13, 17, 35).
An identical behavior with respect to cells of the monocyte lineage has been evoked in the case of human immunodeficiency virus type 1 (HIV-1) to explain the passage of the virus from the blood to receptive, possibly uninfected tissues (28), as best exemplified by viral spread in the central nervous system (1, 11, 23, 24, 27). Although a vast literature exists on the ability of HIV-1 to replicate in either macrophages or dendritic cells (8, 22, 32, 38), blood-circulating monocytes are often considered as an example of a cell type restrictive to HIV-1 infection, much like quiescent lymphocytes (6, 16, 31, 33, 36, 37, 41, 43). Given that infectious virus can be isolated from monocytes of seropositive patients (7), it is clear that monocytes, albeit resistant, are not completely refractory to HIV infection.
The control of viral infection in monocytes is thought to take place during the early phases of infection after viral entry. Monocytes express both CD4 receptor and CCR5 and CXCR4 coreceptors (9, 10, 14, 20, 21, 29, 39, 40), and poor infection of monocytes is also observed with viral particles pseudotyped with pantropic envelopes, such as the vesicular stomatitis virus G protein (VSVg) (37, 43). The identity of this block remains, however, controversial. Several reports suggest it occurs prior to reverse transcription (6, 12, 36, 37), while others argue for a post-reverse transcription impairment prior to integration (16, 31, 33).
Given these discrepancies, we set out to carefully characterize the early steps of infection of unstimulated monocytes by HIV-1. In contrast to previous reports, our data indicate that HIV-1 particles are first blocked at the level of entry. This results in lower amounts of particles present in monocytes compared to more susceptible cell types, such as HeLaP4 cells or differentiated macrophages. At a post-viral entry step, reverse transcription and integration are not blocked but proceed with extremely long kinetics. Viral DNA molecules are completed within days after entry and integrate after a long delay following their synthesis.
MATERIALS AND METHODS
Cells.
Human primary monocytes were obtained from peripheral blood mononuclear cells of healthy donors by subsequent Ficoll and Percoll gradients, followed by negative selection with a cocktail of hapten CD3, CD7, CD19, CD45RA, and CD56 anti-immunoglobulin E antibodies coupled to MACS microbeads (Miltenyi Biotec). Cells were frozen at this time. The purity of the population was greater than 95% as judged by surface marker characterization, as previously described (15). Cells were maintained in complete RPMI 1640 medium supplemented with 10% fetal calf serum (BioWest). Differentiated macrophages were obtained after incubation of monocytes for 4 to 6 days in granulocyte-macrophage or macrophage colony-stimulating factor (GM-CSF and M-CSF, respectively; R&D Systems) at 100 ng/ml, as previously described (15, 19). HeLaP4 and HeLa P4/P5 cells (containing an integrated copy of β-galactosidase under the control of the viral LTR) or 293T cells were maintained in complete Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. HeLaP4 gave intense background problems in a Vpr-BLAM assay (3) and were thus not used in this assay. The antibodies used for cell surface staining and flow cytometry analysis were from Becton Dickinson. The phagocytic ability of monocytes and macrophages was determined by fluorescence microscopy by scoring the number of phycoerythrin (PE)-positive cells after 1 h of incubation with PE-labeled beads (Sigma), as described previously (19).
Viruses.
HIV-1 retroviral vectors were used in single-round infection assays (30). Vectors were produced upon transfection of three plasmids into 293T cells: the packaging vector 8.2 coding for Gag-Pro-Pol and nonstructural viral proteins, the Env-coding construct, and the transfer vector coding for a mini-viral genome expressing green fluorescent protein (GFP) under the control of a cytomegalovirus promoter. The R5-tropic envelope JRFL (obtained from Jeremy Luban, Geneva, Switzerland) was used together with VSVg. Due to their higher infectious titers, the vectors were routinely pseudotyped with VSVg. Viral particles were purified by ultracentrifugation through 45/25% sucrose cushions and resuspended, and their infectious titers were determined on HeLaP4 cells or 293T cells as described previously (15). Purification through a sucrose cushion was required for optimal infection of monocytes. Controls infections were routinely performed in the presence of reverse transcriptase (RT) inhibitors to exclude pseudotransduction.
Single-round infections, entry assays, immunofluorescence, and PCR analysis.
Monocytes were infected directly after thawing with vectors at a multiplicity of infection (MOI) of 10 (the MOI being the number of infectious particles per cell) for 2 h prior to virus removal, extensive cell washing, and cell seeding (at 105 cells/well of a 96-well plate, round bottom, in 200 μl). Differentiated macrophages were infected directly in plates. Higher MOIs did not result in increased rates of infection and were thus generally avoided. The percentage of GFP+ cells was determined by flow cytometry. During the time frame of the analysis, monocytes remained largely nonadherent. When indicated, cells were stained with a specific anti-CD14 antibody (Becton Dickinson) prior to flow cytometry analysis. The RT inhibitors nevirapine and dichetoacid integrase (L-731,988) were used at 20 μg/ml (obtained from AIDS Reagent and Reference Program of the National Institutes of Health [NIH] and as a gift from J. F. Mouscadet, ENS-Cachan, Cachan, France, respectively).
For intracellular p24 analysis, cells were infected for 2 h as described above, extensively washed, and treated with trypsin for 10 min to remove noninternalized virion particles. Cells were then permeabilized (Fix&Perm, GAS-004; Caltag) according to the manufacturer's instructions and stained with a specific anti-capsid (anti-CA) antibody prior to flow cytometry analysis (Kc57; Beckman Coulter).
The Vpr-BLAM assay was performed essentially as described previously (3), using a β-lactamase loading solution kit (K1085; Invitrogen). Infections were performed at an MOI of 3, which preliminary experiments indicated to be in the linear range of the assay (results not shown).
For PCR, infections were generally carried out at an MOI of 1, and semiquantitative PCR analysis was performed on serial fivefold dilutions of cellular lysates to ensure that PCR amplification occurred in the linear range, as described previously (15). Actin DNA was used to normalize samples, and control infections with the RT inhibitors zidovudine-dideoxyinosine were performed in parallel. PCR products were run on agarose gel, transferred onto nylon membranes, and hybridized with a specific 32P-labeled probe. The intensity of the signals obtained was quantified by phosphorimager analysis.
The primer sequences were as follows (5′ to 3′, nucleotides within parentheses refer to the complete HIV-1 sequence; GenBank accession no. M38432): full-length (FL) AC37, CACTCCCAACGAAGACAAG (nucleotides [nt] 9100 to 9120), and AC38, CAGCAAGCCGAGTCCTGCGT (nt 699 to 708); and episomal 2LTR forms (2LTRs) circles, AC34, TCCCAGGCTCAGATCTGGTCTAAC (nt 465 to 489); AC35, GCCTCAATAAAGCTTGCCTTG (nt 522 to 542); actinup, CGAGAAGATGACCCAGATC; and actindown, TGCCGCCAGACAGCACTGTG. The probe sequences were as follows: AC36, TAGAGATCCCTCAGACCCTT (nt 589 to 608), for HIV-1 FL, and actinprobe, GGAGAAGAGCTACGAGCTGC, for 2LTRs.
For confocal microscopy studies, infections were performed at an MOI of 10. Cells were then extensively washed and examined 4 to 6 h after viral addition. For the analysis, cells were fixed in 4% paraformaldehyde and processed according to standard procedures using a specific anti-CA antibody (obtained from the NIH repository). The DNA staining dye TOTO-3 (Invitrogen) was added according to the manufacturer's instructions just prior to mounting. Images were acquired with a confocal microscopy (Axiovert 100M, LSM510; Zeiss).
RESULTS
An appreciable fraction of monocytes can be infected with HIV-1.
VSVg-pseudotyped HIV-1 vectors coding for gfp were used in a single-round infectivity assay on monocytes and differentiated macrophages belonging to the same donor (Fig. 1A). VSVg pseudotypes were used for their higher infectious titers and because the restriction of monocytes to HIV-1 is also observed in this case (31, 37). Cells were infected at an MOI of 10 for 2 h (higher MOIs did not result in increased infections' rate [data not shown]). Monocytes were then extensively washed and seeded, whereas infection of differentiated macrophages was carried out directly in plates. The number of infected GFP+ cells was then determined every day over a period of 10 days by flow cytometry (Fig. 1B). During this time, monocytes, in contrast to differentiated macrophages, displayed marginal changes in the specific cell surface markers analyzed and remained unable to phagocytize PE-labeled beads (Table 1). This prolonged analysis was carried out because we fortuitously realized that higher percentages of GFP+ monocytes were detectable at late times after single-round infection. Under these conditions, GFP+ macrophages started to be clearly detectable by day 1 postinfection, and their proportion increased up to day 4 and remained steady thereafter. In contrast, the proportion of GFP+ monocytes was very low at early times and increased for up to 7 to 8 days, depending on the donor. At this time, the percentage of GFP+ monocytes was 6 to 8% of the total, that is, only three- to fivefold less than what we observed with differentiated macrophages. We do not believe that these differences are due to postintegration defects that result in lower or delayed accumulation of GFP signal in monocytes. Indeed, GFP was readily detected in these cells as early as 24 h after infection with an adenoviral vector containing an identical cytomegalovirus-GFP expression cassette (Ad5-GFP; obtained from Pierre Boulanger, LAENNEC, Lyon, France [data not shown]).
FIG. 1.
Infection of monocytes with HIV-1. (A) Purified primary blood monocytes and GM-CSF-differentiated macrophages derived from the same donor were infected with VSVg-pseudotyped HIV-1 vectors coding gfp. Vectors were produced by transient transfection of 293T cells and purified over a 25% sucrose cushion by ultracentrifugation. Their infectious titer was measured on target HeLaP4 or 293T cells. (B) Cells were infected at an MOI of 10 for 2 h prior to virus removal by cell washing and cell seeding. The percentage of infected GFP-positive cells was measured by flow cytometry every day over a period of 10 days postinfection. The graph presents values obtained with three different donors.
TABLE 1.
Cell surface marker analysis
| Cell type | Marker expressiona
|
Phagocytosisb | |||||
|---|---|---|---|---|---|---|---|
| CD14 | CD86 | CD40 | CD1a | CD64 | CXC3R1 | ||
| Monocytes | |||||||
| Day 0 | +++ | + | +++ | + | +++ | ++ | - |
| Day 8 | +++ | + | +++ | - | +++ | ++ | - |
| Macrophages | |||||||
| M-CSF | +++ | ++ | +++ | - | +++ | ++ | + |
| GM-CSF | + | ++ | +++ | - | +++ | - | + |
MFI = 1 to <10, +; MFI = 10 to <100, ++, MFI = 100 to 1,000, MFI = +++.
Scored by fluorescence microscopy analysis after incubation of cells with PE-labeled beads.
During these experiments, we noticed that viral purification through sucrose was crucial for successful infection; this was probably due to the loss of contaminant cellular debris that interferes with infection. These differences, together with an analysis prolonged over time, may explain why an appreciable portion of single-round infected monocytes is detected here, in contrast to the findings of previous reports (31, 37).
Overall, these results indicate that monocytes are susceptible to HIV. The long delay required to reach a plateau in the number of GFP-positive monocytes with respect to differentiated macrophages suggests that one or more steps of the early phases of infection may occur very slowly in these cells.
GFP-positive cells are bona fide monocytes.
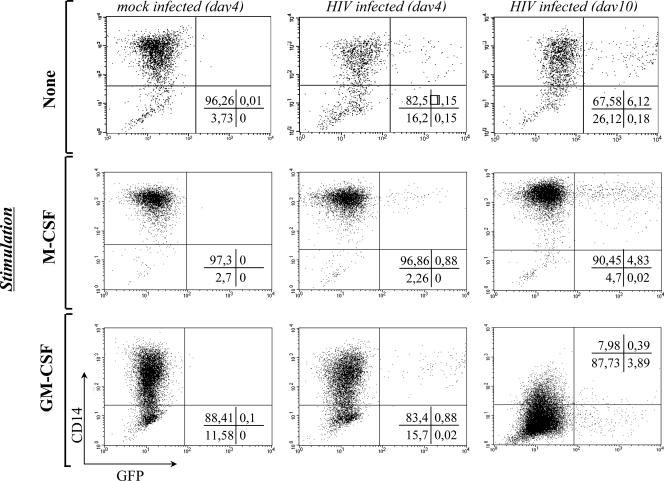
To unequivocally identify infected cells, monocytes were transduced with HIV-1, seeded in the absence or presence of either M-CSF or GM-CSF, and labeled with a monocyte-macrophage-specific marker after infection (CD14, Fig. 2). We and others have previously shown that CD14 surface levels on monocytes/macrophages are differently modulated by these cytokines: upregulated after M-CSF stimulation and downregulated after GM-CSF stimulation (2, 18, 19). Accordingly, GFP+ cells were CD14 positive in the absence of exogenous stimulation, and either upregulated or downregulated CD14 over time. As expected from the data of Fig. 1, the percentage of GFP+ cells increased over time (compare the middle to the right series of quadrants). These results indicate HIV-1 infection of true monocytes.
FIG. 2.
HIV-1 infects bona fide monocytes. To determine the identity of the small fraction of GFP+ cells, monocytes were infected with HIV-1 vectors at an MOI of 10 as described above and seeded in the absence or presence of GM-CSF or M-CSF. The expression of CD14, a specific marker of monocytes/macrophages, was examined by flow cytometry at 4 and 10 days postinfection. We and others have already shown that GM-CSF and M-CSF either decrease or increase the amount of CD14 at the cell surface (19). The results from a representative experiment out of four are shown here.
Monocytes display a first block to VSVg-pseudotyped HIV particles at the entry level.
To determine the phase(s) impaired during HIV-1 the early steps of infection of monocytes, we reexamined first the issue of viral entry. Due to the low number of infected monocytes, a number of studies, including this one, have made use of VSVg-pseudotyped particles (31, 37). The notion that viral entry is not defective in monocytes is experimentally based on a single report describing similar levels of VSVg-mediated entry between monocytes and macrophages (37) determined by using a Vpr-BLAM entry assay (3). For the rest, this notion is based on the perception that VSVg-mediated entry occurs with similar efficiency in all cell types since VSVg is a pantropic Env. It follows that if a specific cell type displays resistance to HIV-Env and VSVg-HIV-1 pseudotypes, a post-viral-entry block is most likely going to be evoked.
We reexamined the step of viral entry using two techniques. First, we decided to measure the amount of intracellular p24 (CA) 2 h after infection in a comparison between monocytes, HeLaP4 cells, and differentiated macrophages; the last of which are more permissive to viral infection than monocytes. Although this assay does not yield information on the fate of viral capsids nor on their intracellular localization (endosomes or cytoplasm) (26), it measures the amount of viral particles that entered target cells.
Cells were infected at different MOIs. At 2 h postinfection the cells were treated with trypsin to remove virus bound at the exterior of the cells, permeabilized, and labeled with an anti-CA antibody prior to flow cytometry analysis (Fig. 3A). Under these conditions, comparable numbers of CA-positive cells were obtained with increasing viral inputs after infection of HeLaP4 cells and differentiated macrophages. In contrast, the proportion of monocytes that were CA positive reached a plateau at ca. 10% and was consistently lower than that of the more permissive cells (between three- and fourfold, depending on the MOI). As a control, infections performed at a low temperature prior to trypsin treatment yielded no positive signal (not shown). When the median fluorescence intensities (MFIs; i.e., the the measure of the intensity of the fluorescence signal proportional to the amount of antigen present in the cell) of the CA-positive populations were compared, a three- to fourfold decrease was again observed in monocytes (Fig. 3B, MFI obtained after infection at an MOI of 20). The addition of proteasome inhibitors during infection did not increase the percentage of CA+ cells or their MFI (not shown). This indicates that the decrease observed in monocytes was not due to higher levels of intracellular capsids degradation, at least within the time frame of the analysis.
FIG. 3.
Monocytes display diminished intracellular CA signal after infection with VSVg-pseudotyped HIV. Monocytes were infected with increasing viral inputs for 2 h, along with HeLaP4 cells and GM-CSF-differentiated macrophages. Loosely bound and noninternalized virion particles were removed by trypsin treatment. Cells were then permeabilized and examined for their CA content by flow cytometry using a specific anti-CA antibody. The figure presents data obtained with three to four different donors showing the percentage of CA+ cells (A) and the MFIs of positive cells at an MOI of 20, normalized to the value obtained in HeLaP4 cells (B). ***, P = 0,009 (Student t test).
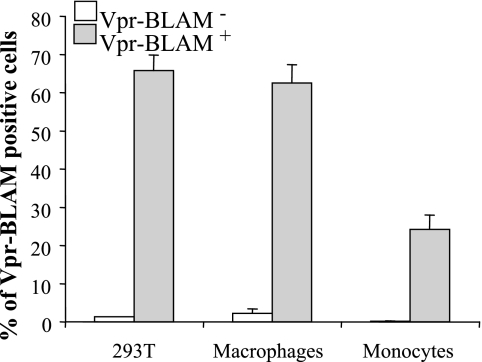
Next, viral entry was examined by using a Vpr-BLAM assay that measures the cytosolic presence of Vpr-BLAM after virus-to-cell fusion (3). HIV-1-derived vectors incorporating Vpr-BLAM were used to infect monocytes, along with 293T cells and differentiated macrophages. Cells were then loaded with CCF2 dye, and virus entry was evaluated by measuring the fluorescent shift induced by Vpr-BLAM using flow cytometry. Again, a three- to fourfold reduction in the number of Vpr-BLAM-positive monocytes was consistently observed with respect to differentiated macrophages and HeLaP4 cells (Fig. 4). Of note, the number of positive cells was always higher following the Vpr-BLAM assay than after intracellular CA staining. We believe this is due to a higher sensitivity of the former method, possibly a consequence of the enzymatic nature of the BLAM reaction. In support of this hypothesis, although 100% of the HeLaP4 cells were infected at an MOI of 20, only half of them display an amount of intracellular p24 detectable by flow cytometry (see Fig. 3).
FIG. 4.
Monocytes display an entry defect after a Vpr-BLAM entry assay. Vpr-Blam was incorporated into virion particles by transfection of 293T cells during vector production. Cells were infected with an equal number of virion particles for 2 h at an MOI of 3. After a washing step, cells were loaded with the CCF2 dye, and the Vpr-BLAM activity was revealed after 18 h as a fluorescence shift in CCF2-positive cells by flow cytometry. The graph presents the percentages of Vpr-BLAM-positive cells obtained for the different cell types examined (n = 3).
When analyzed by confocal microscopy at 4 h postinfection to visualize CA, monocytes and macrophages displayed a similar intracellular punctuate staining, which can reflect vesicular or cytoplasmic localization of viral nucleoprotein complexes (Fig. 5A and B, respectively). However, despite showing similar patterns, these results do not exclude differences in the intracellular compartment containing viral complexes after entry, an issue that merits further investigation.
FIG. 5.
Intracellular CA staining after confocal microscopy. Monocytes (A) and macrophages (B) were infected at an MOI of 10 for 2 h, washed, and analyzed by confocal microscopy. Cells were first labeled with a specific anti-CA antibody and then with the DNA dye TOTO-3 to mark their nuclei. The results of representative experiments are shown here.
Overall, these results indicate that VSVg-pseudotyped viral particles display a clear defect in viral entry in monocytes. This defect may be the founding restriction displayed by monocytes to viral infection.
Monocytes display very long reverse transcription and integration kinetics.
The three- to fourfold defect in viral entry suffices alone to explain the differences observed in the overall infections' rates between differentiated macrophages and monocytes. However, the long time frame required to reach a plateau in the number of infected monocytes suggests that one or more steps following entry may be completed very slowly.
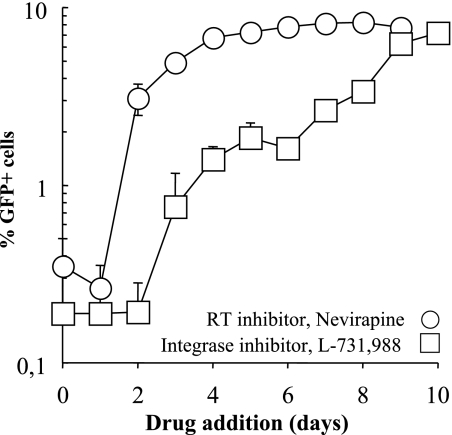
The infection of monocytes has been studied before using PCR at shortly after infection (6, 12, 16, 31, 33, 36, 37, 41). Here, we decided to measure the kinetics of reverse transcription and integration over a long time frame. Initially, the reverse transcription and integration processes were arrested at different times postinfection by the addition of specific inhibitors (nevirapine and L-731,988, respectively). The percentage of GFP+ cells obtained at each time point was then determined at day 10 after infection by flow cytometry. The inhibitors are expected to block the synthesis of elongating viral DNA molecules or the integration of formed ones but have no effect once these processes have been completed. Thus, a kinetic measurement of the completion of the reverse transcription and integration processes can be obtained (by measuring the percentage of GFP+ cells obtained at each time point with respect to untreated controls). Most importantly, this approach restricts the analysis exclusively to functional viral genomes, that is, to genomes capable of expression.
Under these conditions, synthesis of viral DNA molecules was completed very slowly in monocytes. Reverse transcription took place over days after infection of monocytes with a tRT50% value of 55 ± 8 h, where tRT50% is the time at which half of the total number of GFP+ cells and thus of the infectious viral genomes compared to untreated controls are completed (n = 8, Fig. 6). The majority of functional viral genomes were completed by day 4 to 5 postinfection, a finding similar to what has been recently described in quiescent lymphocytes (43).
FIG. 6.
The kinetics of reverse transcription and integration are extremely slow in monocytes. Monocytes were infected for 2 h with HIV-1 vectors at an MOI of 10 prior to virus removal. Reverse transcription and integration were arrested at the indicated time points by the addition of nevirapine and of the dichetoacid IN inhibitor L-731,988 (at 10 and 20 μg/ml, respectively). The percentages of GFP+ cells obtained for each time point were determined at 10 days postinfection. The graph presents data obtained from three different donors.
The kinetics of integration were also very slow in monocytes (a tIN50% of between 7 and 8 days). The comparison between the curves of RT and integrase (IN) suggest that completed viral DNA molecules require up to 5 to 6 additional days to integrate. This delay can be due to trafficking, nuclear import, or even post-nuclear-import defects specifically present in monocytes. Overall, our data suggest that the two key enzymatic processes during HIV infection occur very slowly in monocytes rather than being completely blocked.
Kinetics of overall viral DNA synthesis after HIV-1 infection of monocytes.
Next, the overall amounts of viral DNAs produced during infection were examined by PCR. Monocytes were infected for 2 h as described above, along with differentiated macrophages, and lysed at different times postinfection. The amounts of FL and 2LTRs were determined by semiquantitative PCR using primers that specifically recognize each species (Fig. 7). FL products accumulated with similar kinetics in both monocytes and differentiated macrophages, although monocytes displayed lower levels of FL DNA (three- to fourfold). This defect correlates with the diminished level of viral entry described above in monocytes (see Fig. 3 and 4). In contrast, 2LTRs accumulated faster in macrophages, largely during the first 24 h after viral addition, than in monocytes. This difference was more pronounced at early time points and diminished at later time points.
FIG. 7.
The kinetics of total viral DNA production were assessed by PCR. Monocytes were infected as described in the text, and cells were lysed at the indicated time points. Semiquantitative PCR analysis was conducted on serial fivefold dilutions of samples with primers that amplify specifically FL and 2LTR forms (i.e., FL and 2LTRs, respectively). PCR products were transferred onto a nylon membrane, hybridized with a specific 32P-labeled probe, and quantified by phosphorimager analysis. The graph presents these results after normalization of the different samples for actin DNA.
Overall, these results suggest a delay in the accumulation of nuclear forms after HIV infection of monocytes with respect to macrophages and suggest that access to the nuclear compartment is an important restrictive step in the infection of this cell type.
R5-tropic pseudotypes display a similar behavior than VSVg ones.
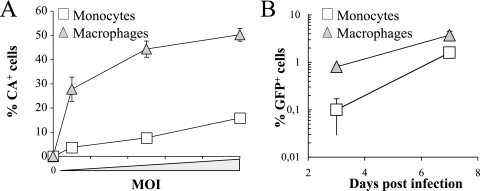
VSVg pseudotypes are widely used due to their higher infectivity, especially when target cells display low susceptibility to viral infection. However, certain aspects of infection may differ between particles entering via VSVg or via their natural Env. To determine whether this was the case, lentiviral vectors were pseudotyped with an R5-tropic Env (JR-FL) and used in two series of experiments representing the telling differences described here during monocyte infection: assessment of viral entry (by intracellular p24 assay) and determination of the percentage of infected cells over time. Monocytes and differentiated macrophages were infected with equal amounts of JR-FL-pseudotyped particles, as shown in Fig. 3, and analyzed by flow cytometry after intracellular p24 staining (Fig. 8A). Under these conditions, the percentage of monocytes displaying positive CA staining was fourfold lower than that of macrophages, as observed above for VSVg. These results indicate that monocytes display a viral entry defect most likely independent of a specific Env-receptor interaction.
FIG. 8.
Behavior of R5-tropic Env HIV-1 virion particles. Virion particles were pseudotyped with the JR-FL Env upon cotransfection of 293T cells, titers were determined on target P4P5 HeLa cells (expressing CD4 and CCR5), and the particles were evaluated on monocytes and macrophages in an intracellular p24 staining assay (A) or to determine the percentage of infected cells over time (B). For intracellular staining, cells were infected with increasing amounts of viral particles for 2 h prior to cell washing, permeabilization, and p24 staining. Cells were then assayed by flow cytometry as described in the legend to Fig. 3. To determine viral infectivity, cells were infected at an MOI of 3 for 2 h, plated, and then analyzed at 3 and 7 days postinfection. The graphs present results obtained from three independent experiments.
The susceptibility of cells to viral infection was then analyzed (Fig. 8B). At day 3, the percentage of GFP+ macrophages was ∼10-fold higher than for monocytes, but this disparity decreased when cells were analyzed at day 7. At this time, the different susceptibility of monocytes and macrophages to HIV could be entirely accounted for by the viral entry defect.
Overall, these results reveal no major differences between the behavior of HIV-1 viral particles entering cells via R5-tropic Env or VSVg.
DISCUSSION
The results presented here indicate that blood-circulating monocytes display several defects during HIV-1 infection with respect to more permissive cell types: an entry defect, a slow kinetic of reverse transcription, and delayed nuclear import and integration. However, with the exception of viral entry, these defects delay, but do not diminish, the virus' infectivity.
A major difference between our study and previous analyses is the fact that monocytes were examined here over a long period of time after infection (6, 12, 16, 31, 33, 36, 37). In this manner, we were able to show that the relative restriction of monocytes versus macrophages with respect to HIV infection (observed between days 1 and 4) lessens if the cells are analyzed at late time points (days 7 to 10). Thus, our results are not in disagreement with previous reports but do extend them by indicating that HIV-1 requires longer times to complete the infection of monocytes, much as has been recently suggested for quiescent lymphocytes (43).
When compared at late times, the numbers of infected monocytes are only three- to fourfold lower than those of macrophages. This defect correlates with the lower levels of viral particle entry observed in the former cell type. Two different assays were used to determine the extent of viral particle entry: intracellular p24 that measures the overall amount of CA accessing monocytes (irrespective of its localization) and Vpr-BLAM entry that measures the cytoplasmic delivery of the Vpr-BLAM fusion. In both cases, a decreased amount of entry was measurable in monocytes as opposed to HeLaP4 cells and macrophages. To our knowledge, this is the first report to suggest that VSVg-mediated entry may differ among cell types. It is possible that differences in receptor concentration at the cell surface (phosphatidylserine, although this has been recently questioned [5]) or in the internalization of receptor-particle complexes could be responsible for these differences. Given that these differences persist when monocytes are challenged with wild-typw Env-bearing HIV particles, it is possible that the defect in viral entry is cell type specific rather than receptor specific.
Once the entry defect of VSVg-pseudotyped particles is taken into account, macrophages and monocytes do not display different susceptibilities to HIV infection provided that cells are compared late after infection, allowing the virus to complete the early steps of its life cycle in monocytes. This is due in part to the fact that reverse transcription is completed over a period of 2 days but is mostly to the long delay elapsing between the synthesis of viral DNA forms and their integration. The kinetic curves obtained with specific inhibitors suggested an interval of 5 to 6 days (determined by comparison of the t50% values of RT and IN). This time frame can be due to trafficking, nuclear import, or even post-nuclear-import events that affect viral nucleoprotein complexes. We believe that the delay in the accumulation of nuclear viral DNA forms observed by PCR supports the hypothesis that nuclear import (and/or trafficking to the nuclear membrane) is a major constraint—albeit not a complete restriction—during HIV infection of monocytes.
A recent report suggested that viral nucleoprotein complexes is targeted and stored to microtubule organizing centers in quiescent lymphocytes prior to their rerouting to the nuclear pore (42). For the moment, it is not known whether this is a general path taken by incoming viral particles, or whether it is a phenomenon specific to cells of low metabolism. If this is true, it is possible that in monocytes that display low metabolic activity, viral nucleoprotein complexes also are directed to microtubule organizing centers and that their rerouting to the nucleus is defective.
In summary, our results indicate that monocytes support the early steps of HIV infection and that their resistance to viral infection is alleviated over time. This implies that viral nucleoprotein complexes in monocytes remain functionally stable for a long time prior to integration and expression. As such, monocytes can serve as a stable reservoir for the virus, as well as a vehicle for its dissemination. In the case of HIV-1, this “cytoplasmic delay” assures the silent cover that for other lentiviruses (such as Maedi-Visna virus, for example) is instead achieved at a postintegration step.
Acknowledgments
We thank Jeanine Bernaud for help with blood sample collection and Chantal Bella and the flow cytometry service of the IFR128. We thank the AIDS Research and Reference Reagent Program of the NIH and P. Boulanger, W. C. Greene, J. Luban, and J. F. Mouscadet for material used in this study.
This study received the support of Sidaction, ANRS, the NIH, and the TRIoH consortium of the European Community.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Banks, W. A., N. Ercal, and T. O. Price. 2006. The blood-brain barrier in neuroAIDS. Curr. HIV Res. 4259-266. [DOI] [PubMed] [Google Scholar]
- 2.Buckle, A. M., Y. Jayaram, and N. Hogg. 1990. Colony-stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin. Exp. Immunol. 81339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 201151-1154. [DOI] [PubMed] [Google Scholar]
- 4.Clements, J. E., D. H. Gabuzda, and S. L. Gdovin. 1990. Cell type specific and viral regulation of visna virus gene expression. Virus Res. 16175-183. [DOI] [PubMed] [Google Scholar]
- 5.Coil, D. A., and A. D. Miller. 2004. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 7810920-10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin, M., and S. Gordon. 1994. The kinetics of human immunodeficiency virus reverse transcription are slower in primary human macrophages than in a lymphoid cell line. Virology 200114-120. [DOI] [PubMed] [Google Scholar]
- 7.Collman, R., N. F. Hassan, R. Walker, B. Godfrey, J. Cutilli, J. C. Hastings, H. Friedman, S. D. Douglas, and N. Nathanson. 1989. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1): monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J. Exp. Med. 1701149-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collman, R. G., C. F. Perno, S. M. Crowe, M. Stevenson, and L. J. Montaner. 2003. HIV and cells of macrophage/dendritic lineage and other non-T cell reservoirs: new answers yield new questions. J. Leukoc. Biol. 74631-634. [DOI] [PubMed] [Google Scholar]
- 9.Creery, D., W. Weiss, G. Graziani-Bowering, R. Kumar, Z. Aziz, J. B. Angel, and A. Kumar. 2006. Differential regulation of CXCR4 and CCR5 expression by interleukin (IL)-4 and IL-13 is associated with inhibition of chemotaxis and human immunodeficiency virus (HIV) type 1 replication but not HIV entry into human monocytes. Viral Immunol. 19409-423. [DOI] [PubMed] [Google Scholar]
- 10.Creery, D., W. Weiss, W. T. Lim, Z. Aziz, J. B. Angel, and A. Kumar. 2004. Down-regulation of CXCR-4 and CCR-5 expression by interferon-gamma is associated with inhibition of chemotaxis and human immunodeficiency virus (HIV) replication but not HIV entry into human monocytes. Clin. Exp. Immunol. 137156-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunfee, R., E. R. Thomas, P. R. Gorry, J. Wang, P. Ancuta, and D. Gabuzda. 2006. Mechanisms of HIV-1 neurotropism. Curr. HIV Res. 4267-278. [DOI] [PubMed] [Google Scholar]
- 12.Eisert, V., M. Kreutz, K. Becker, C. Konigs, U. Alex, H. Rubsamen-Waigmann, R. Andreesen, and H. von Briesen. 2001. Analysis of cellular factors influencing the replication of human immunodeficiency virus type I in human macrophages derived from blood of different healthy donors. Virology 28631-44. [DOI] [PubMed] [Google Scholar]
- 13.Gabuzda, D. H., J. L. Hess, J. A. Small, and J. E. Clements. 1989. Regulation of the visna virus long terminal repeat in macrophages involves cellular factors that bind sequences containing AP-1 sites. Mol. Cell. Biol. 92728-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genin, P., Y. Mamane, H. Kwon, C. LePage, M. A. Wainberg, and J. Hiscott. 1999. Differential regulation of CC chemokine gene expression in human immunodeficiency virus-infected myeloid cells. Virology 261205-215. [DOI] [PubMed] [Google Scholar]
- 15.Goujon, C., L. Riviere, L. Jarrosson-Wuilleme, J. Bernaud, D. Rigal, J. L. Darlix, and A. Cimarelli. 2007. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzinger, N., L. Baca-Regen, M. Stevenson, and H. E. Gendelman. 1995. Efficient synthesis of viral nucleic acids following monocyte infection by HIV-1. Virology 206731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess, J. L., J. A. Small, and J. E. Clements. 1989. Sequences in the visna virus long terminal repeat that control transcriptional activity and respond to viral transactivation: involvement of AP-1 sites in basal activity and transactivation. J. Virol. 633001-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogasen, A. K., K. Hestdal, and T. G. Abrahamsen. 1993. Granulocyte-macrophage colony-stimulating factor, but not macrophage colony-stimulating factor, suppresses basal and lipopolysaccharide-stimulated complement factor production in human monocytes. J. Immunol. 1513215-3224. [PubMed] [Google Scholar]
- 19.Jarrosson-Wuilleme, L., C. Goujon, J. Bernaud, D. Rigal, J. L. Darlix, and A. Cimarelli. 2006. Transduction of nondividing human macrophages with gammaretrovirus-derived vectors. J. Virol. 801152-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joly, M., and J. M. Pinto. 2005. CXCR4 and CCR5 regulation and expression patterns on T- and monocyte-macrophage cell lineages: implications for susceptibility to infection by HIV-1. Math. Biosci. 19592-126. [DOI] [PubMed] [Google Scholar]
- 21.Juffermans, N. P., S. Weijer, A. Verbon, P. Speelman, and T. van der Poll. 2002. Expression of human immunodeficiency virus coreceptors CXC chemokine receptor 4 and CC chemokine receptor 5 on monocytes is down-regulated during human endotoxemia. J. Infect. Dis. 185986-989. [DOI] [PubMed] [Google Scholar]
- 22.Kedzierska, K., S. M. Crowe, S. Turville, and A. L. Cunningham. 2003. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 1339-56. [DOI] [PubMed] [Google Scholar]
- 23.Kim, W. K., S. Corey, X. Alvarez, and K. Williams. 2003. Monocyte/macrophage traffic in HIV and SIV encephalitis. J. Leukoc. Biol. 74650-656. [DOI] [PubMed] [Google Scholar]
- 24.Lane, J. H., V. G. Sasseville, M. O. Smith, P. Vogel, D. R. Pauley, M. P. Heyes, and A. A. Lackner. 1996. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J. Neurovirol. 2423-432. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani, A., A. Sica, and M. Locati. 2005. Macrophage polarization comes of age. Immunity 23344-346. [DOI] [PubMed] [Google Scholar]
- 26.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 722208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maslin, C. L., K. Kedzierska, N. L. Webster, W. A. Muller, and S. M. Crowe. 2005. Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr. HIV Res. 3303-317. [DOI] [PubMed] [Google Scholar]
- 28.Meltzer, M. S., and H. E. Gendelman. 1992. Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Curr. Top. Microbiol. Immunol. 181239-263. [DOI] [PubMed] [Google Scholar]
- 29.Naif, H. M., S. Li, M. Alali, A. Sloane, L. Wu, M. Kelly, G. Lynch, A. Lloyd, and A. L. Cunningham. 1998. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 72830-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272263-267. [DOI] [PubMed] [Google Scholar]
- 31.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 755448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 795386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien, W. A., A. Namazi, H. Kalhor, S. H. Mao, J. A. Zack, and I. S. Chen. 1994. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J. Virol. 681258-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peluso, R., A. Haase, L. Stowring, M. Edwards, and P. Ventura. 1985. A Trojan horse mechanism for the spread of visna virus in monocytes. Virology 147231-236. [DOI] [PubMed] [Google Scholar]
- 35.Small, J. A., C. Bieberich, Z. Ghotbi, J. Hess, G. A. Scangos, and J. E. Clements. 1989. The visna virus long terminal repeat directs expression of a reporter gene in activated macrophages, lymphocytes, and the central nervous systems of transgenic mice. J. Virol. 631891-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonza, S., A. Maerz, N. Deacon, J. Meanger, J. Mills, and S. Crowe. 1996. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J. Virol. 703863-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triques, K., and M. Stevenson. 2004. Characterization of restrictions to human immunodeficiency virus type 1 infection of monocytes. J. Virol. 785523-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verani, A., G. Gras, and G. Pancino. 2005. Macrophages and HIV-1: dangerous liaisons. Mol. Immunol. 42195-212. [DOI] [PubMed] [Google Scholar]
- 39.Worgall, S., R. Connor, R. J. Kaner, E. Fenamore, K. Sheridan, R. Singh, and R. G. Crystal. 1999. Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages. J. Virol. 735865-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi, Y., S. Rana, J. D. Turner, N. Gaddis, and R. G. Collman. 1998. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61213-222. [DOI] [PubMed] [Google Scholar]
- 42.Zamborlini, A., J. Lehmann-Che, E. Clave, M. L. Giron, J. Tobaly-Tapiero, P. Roingeard, S. Emiliani, A. Toubert, H. de The, and A. Saib. 2007. Centrosomal pre-integration latency of HIV-1 in quiescent cells. Retrovirology 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou, Y., H. Zhang, J. D. Siliciano, and R. F. Siliciano. 2005. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J. Virol. 792199-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zink, M. C., O. Narayan, P. G. Kennedy, and J. E. Clements. 1987. Pathogenesis of visna/maedi and caprine arthritis-encephalitis: new leads on the mechanism of restricted virus replication and persistent inflammation. Vet. Immunol. Immunopathol. 15167-180. [DOI] [PubMed] [Google Scholar]