Abstract
The rising prevalence of human immunodeficiency virus type 1 (HIV-1) infection in women, especially in resource-limited settings, accentuates the need for accessible, inexpensive, and female-controlled preexposure prophylaxis strategies to prevent mucosal transmission of the virus. While many compounds can inactivate HIV-1 in vitro, evaluation in animal models for mucosal transmission of virus may help identify which approaches will be effective in vivo. Macaques challenged intravaginally with pathogenic simian immunodeficiency virus (SIVmac251) provide a model to preclinically evaluate candidate microbicides. 2-Hydroxypropyl-β-cyclodextrin (BCD) prevents HIV-1 and SIV infection of target cells at subtoxic doses in vitro. Consistent with these findings, intravaginal challenge of macaques with SIVmac251 preincubated with BCD prevented mucosal transmission, as measured by plasma viremia and antiviral antibodies, through 10 weeks postchallenge. In an initial challenge, BCD applied topically prior to SIVmac251 prevented intravaginal transmission of virus compared to controls (P < 0.0001). However, upon a second virus challenge following BCD pretreatment, the majority of the previously protected animals became infected. The mechanism through which animals become infected at a frequency similar to that of controls after prior exposure to BCD and SIVmac251 in subsequent intravaginal virus challenges (P = 0.63), despite the potent antiviral properties of BCD, remains to be determined. These results highlight the unpredictability of antiviral compounds as topical microbicides and suggest that repeated exposures to candidate treatments should be considered for in vivo evaluation.
The prevalence of human immunodeficiency virus type 1 (HIV-1) infection is increasing at high rates in women, predominantly through heterosexual transmission, especially in resource-limited settings. Women now comprise nearly 50% of the worldwide prevalence of HIV/AIDS (41). This rise, in part, may be due to limited utilization of condoms by HIV-infected male sexual partners (7). To help stem the increasing heterosexual transmission of HIV-1 to women, a number of prophylactic approaches are being evaluated, including topically applied formulations of antiviral compounds intended to be applied by women prior to sexual activity. Such a candidate would be a topical microbicide that could prevent HIV-1 transmission by blocking virus binding and/or infection of mucosal target cells or by inactivating the virions. According to the Rockefeller Foundation, a microbicide that is 60% effective against HIV-1 transmission could avert 2.5 million infections and save $2.7 billion in health care costs and $1 billion in economic productivity costs worldwide (30).
As of May 2007, eight products were in active clinical trials as anti-HIV-1 microbicide candidates, of which Carraguard and PRO2000 were the only ones in phase III trials (1). These compounds work through a variety of mechanisms, including formation of a physical barrier; prevention of viral binding, fusion, or absorption to mucosal cells; and inhibition of viral replication. Some of these compounds act nonspecifically against a range of microbes and may also have spermicidal activity. Three of the eight compounds, tenofovir, UC781, and TMC120, act on lentiviruses by inhibiting reverse transcription of the viral genome after the virus enters the cell.
A number of microbicide candidates have been evaluated preclinically for the prevention of intravaginal transmission of lentiviruses in nonhuman primate models. Cellulose acetate phthalate (CAP) (3, 12, 22), 3HP-β-LG (47), benzalkonium chloride (37), and cyanovirin (39, 40) inhibited intravaginal transmission of simian immunodeficiency virus (SIVmac251) in macaques to various degrees by nonspecific interference with virus binding and/or attachment to cells. Estradiol implants (33) have been shown to thicken the vaginal epithelium in rhesus macaques, effectively inhibiting intravaginal transmission of SIVmac251. Specifically blocking gp120 interactions with receptor/coreceptors by single or multiple neutralizing antibodies administered topically or systemically (23, 28, 45) or by the CCR5 ligands PSC-RANTES or −2 RANTES (13, 15) was largely effective in prevention of intravaginal SIV/HIV-1 chimeric virus (SHIV) transmission. The CCR5 inhibitor CMPD 167 (43, 45, 46), the gp120 binding inhibitor BMS-378806, and the gp41 inhibitor C52L (44) can significantly prevent intravaginal infection of rhesus macaques when used topically at relatively high concentrations.
2-Hydroxypropyl-β-cyclodextrin (BCD) inhibits lentiviral infection by two different mechanisms. First, BCD has been shown to extract cholesterol and, to a lesser extent, phospholipids from mammalian cells (11). Such lipids have been shown to coalesce into lipid raft structures, which are important for HIV entry into (21) and release from target cells in vitro (24, 26). BCD appears to disrupt these cellular lipid rafts, preventing productive infection of cells (18). Second, BCD has been shown to deplete cholesterol from the envelopes of HIV and SIV virions, disrupting the viral membrane and viral contents and thereby inactivating the virus (8, 9, 19).
We hypothesized that BCD could be a microbicidal candidate to inhibit intravaginal transmission of HIV by inactivating the cell-associated and cell-free virus, as well as by alteration of the cervicovaginal target cells. BCD (3%) in phosphate-buffered saline (PBS) was shown to efficiently inhibit intravaginal transmission of cell-associated HIV-1 in a SCID-Hu mouse model of infection (10). Other attractive microbicidal characteristics of BCD were its high solubility in water, its lack of color or odor, and its low cost. In addition, BCD is used extensively as a carrier protein to increase the solubility, stability, and bioavailability of other compounds in FDA-approved drug formulations (2). In this study, BCD was assayed for the ability to prevent infection of cells in vitro, as well as to prevent infection of rhesus macaques via the intravaginal route of challenge by SIV.
MATERIALS AND METHODS
Cell lines and media.
HeLa and 293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.3 mg/ml l-glutamine (Invitrogen), called DMEM-10. Caco-2 cells were grown in DMEM containing 20% FBS, penicillin, streptomycin, and l-glutamine. GHOST-Hi5 cells, which express CCR5 and CXCR4 (4), were grown in DMEM containing 10% FBS, penicillin, streptomycin, l-glutamine, 0.5 mg/ml G418, 0.5 μg/ml puromycin, and 0.1 mg/ml hygromycin (Invitrogen). CEMx174 cells were grown in RPMI 1640 (Invitrogen) containing 10% FBS, penicillin, streptomycin, and l-glutamine. All cells were incubated at 37°C and 5% CO2.
Virus stocks.
Infectious stocks of the X4-tropic virus HIV-1LAI and the R5-tropic virus HIV-1NFNSX were made by transfecting 293T cells with pBru3Ori (16) or pNFNSX (25) plasmid using the Mammalian Transfection kit (Stratagene, La Jolla, CA). The SIVmac251 stock was described previously (42) and was used for both in vitro and in vivo experiments.
Toxicity assay.
GHOST-Hi5, Caco-2, and HeLa cells were plated at 1 × 105 to 1 × 106 cells/well in a 12-well plate and incubated at 37°C overnight. Medium containing 0, 2.5, 5, 10, and 20% (wt/vol) pharmaceutical-grade BCD (Cyclodextrin Technologies Development, High Springs, FL) was added to the wells in duplicate and incubated at 37°C for 2 h. The cells were removed from the plate with trypsin-EDTA, diluted with medium, and stained with trypan blue. Both live and dead cells were counted by light microscopy.
In vitro BCD inhibition of infection.
GHOST-Hi5 cells were plated at 2 × 104 cells/well in a 24-well plate and incubated at 37°C overnight. HIV-1LAI, HIV-1NFNSX, or SIVmac251 was added to the cells in the presence or absence of 5% BCD in duplicate and incubated at 37°C for 2 h. The cells were washed two times with PBS, fresh DMEM-10 was added to the wells, and the cells were incubated at 37°C for 48 h. The cells were removed from the plate with trypsin-EDTA, resuspended in PBS containing 2% FBS and 2% paraformaldehyde, and analyzed for green fluorescent protein expression on a FACSCalibur (Becton Dickinson, San Diego, CA).
GHOST-Hi5 cells were also incubated with HIV-1LAI or SIVmac251 at a multiplicity of infection of 0.1 in the presence or absence of 5% BCD in medium for 2 h at 37°C. The cells were then washed and incubated with medium without BCD for 2 days at 37°C. The supernatants were harvested and centrifuged to remove cells and debris and added to CEMx174 cell cultures. Inoculated CEMx174 culture supernatants were removed, filtered through a 0.45-μm filter (Pall, Ann Arbor, MI), and frozen at −80°C in aliquots every 2 to 4 days for 3 weeks. These supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) for HIV p24 antigen or SIV p27 antigen (Beckman Coulter, Miami, FL).
Animals.
Twenty-two adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center in accordance with American Association for Accreditation of Laboratory Animal Care standards. The animals were negative for serum antibodies to HIV-2, SIV, type D retrovirus, and simian T-lymphotropic virus type 1. All animal procedures were performed while the animals were sedated with 10 mg intramuscular ketamine-HCl (Parke-Davis, Morris Plains, NJ)/kg of body weight. Blood was drawn from the animals weekly for the first 8 weeks after challenge and biweekly thereafter.
In vivo BCD administration and SIV challenge.
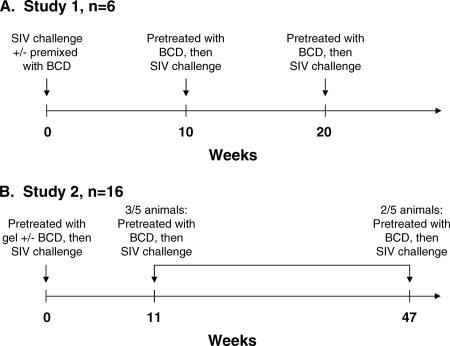
BCD stocks evaluated in cell culture were tested in vivo. In the first study (study 1) (Fig. 1 and Table 1), six macaques were randomly assigned to two experimental treatment conditions: three animals each to (i) SIV control and (ii) BCD+SIV. The three control animals were intravaginally inoculated with 1 ml (approximately 1 × 105 50% tissue culture infectious doses [TCID50]/ml) SIVmac251 and again in the same manner 4 h later (SIVmac251 is hereafter referred to as SIV in the animal infections). The three BCD+SIV macaques were inoculated with the same dose of virus that had been mixed 1:1 in a syringe with 10% BCD in KY Long-Lasting Vaginal Moisturizer gel (Johnson & Johnson, Raritan, NJ) to give a final concentration of 5% (vol/vol) BCD 5 to 15 min prior to administration. This procedure was repeated 4 h later in the same manner. At 10 weeks postchallenge, the BCD+SIV animals inoculated with BCD-inactivated virus had 10% BCD in gel administered intravaginally, followed 15 min later with 1 ml SIV. This procedure was repeated 4 h later in the same manner. At 20 weeks following the initial challenge, BCD+SIV animal 31945 was rechallenged a third time with a preadministration of 10% BCD in gel (1 ml) 15 min prior to virus challenge (1 ml; 5% final concentration of BCD). This procedure was applied again 4 h later.
FIG. 1.
Timelines for in vivo intravaginal challenges. (A) Study 1 experimental animals were challenged with SIVmac251 premixed with 5% BCD in gel at week 0. At weeks 10 and 20, the uninfected animals were inoculated with 1 ml gel containing 10% BCD, followed 5 to 15 min later with 1 ml SIVmac251. Study 1 control animals received the same dose of SIVmac251 at week 0. (B) Study 2 experimental animals were challenged with 1 ml gel containing 10% BCD, followed 5 to 15 min later with 1 ml SIVmac251. Uninfected animals were rechallenged in the same manner at week 11 or 47. Study 2 controls were challenged with the same dose of SIVmac251 alone or following 1 ml of gel without BCD. Two uninfected controls were rechallenged with SIVmac251 at week 47.
TABLE 1.
Summary of animal groups and infection outcomes
| Study | Animal | Group | Challenge 1a | Infection status after challenge 1 | Challenge 2a | Infection status after challenge 2 |
|---|---|---|---|---|---|---|
| 1 | 31930 | BCD + SIV | 0 (3/11)b | − | 10 (5/23) | + |
| 31945 | 0 (3/11)b | − | 10 (5/23) | −d | ||
| 31948 | 0 (3/11)b | − | 10 (5/23) | + | ||
| 31941 | SIV | 0 (3/11) | + | NAe | NA | |
| 31942 | 0 (3/11) | + | NA | NA | ||
| 31950 | 0 (3/11) | + | NA | NA | ||
| 2 | 24485 | BCD + SIV | 0 (5/23) | − | 11 (8/2) | + |
| 26737 | 0 (5/23) | − | 11 (8/2) | + | ||
| 27133 | 0 (5/23) | − | 11 (8/2) | + | ||
| 30042 | 0 (8/2) | + | NA | NA | ||
| 31455 | 0 (8/2) | − | 47 (6/26)c | + | ||
| 31944 | 0 (8/2) | − | 47 (6/26)c | − | ||
| 30989 | Gel + SIV | 0 (5/23) | + | NA | NA | |
| 30990 | 0 (5/23) | + | NA | NA | ||
| 31000 | 0 (5/23) | + | NA | NA | ||
| 31551 | 0 (8/2) | + | NA | NA | ||
| 28884 | 0 (8/2) | − | 47 (6/26) | + | ||
| 31920 | 0 (8/2) | + | NA | NA | ||
| 31026 | SIV | 0 (5/23) | + | NA | NA | |
| 31940 | 0 (5/23) | + | NA | NA | ||
| 29693 | 0 (8/2) | + | NA | NA | ||
| 28756 | 0 (8/2) | − | 47 (6/26) | + |
Week (month/day) of challenge.
Study 1 animals were challenged with SIV pretreated with BCD in the first challenge.
These animals were challenged the second time with SIV after 50% BCD exposure (instead of 10%).
This animal became infected upon a third challenge at week 20 (8/2).
NA, not applicable.
In the second study (study 2) (Fig. 1 and Table 1), 16 animals were randomly assigned to three treatment conditions: (i) 4 animals to SIV-alone control, (ii) 6 animals to SIV+gel control, and (iii) 6 animals to BCD+SIV. The four SIV-alone control animals were intravaginally challenged with 1 ml SIV and again 4 h later. The six SIV+gel control animals were challenged with gel alone, followed 15 min later with 1 ml virus. This procedure was repeated 4 h later. The six BCD+SIV animals had 10% BCD in gel administered intravaginally, followed 15 min later with 1 ml virus. This procedure was repeated 4 hours later. Three of the BCD+SIV treatment animals (24485, 26737, and 27133) were uninfected at week 11 and were rechallenged at week 11 in the same manner.
At week 47, two remaining uninfected BCD+SIV treatment animals (31455 and 31944) each received an atraumatic vaginal wash with 50% BCD in saline (2 ml), followed 10 min later with 50% BCD in gel (1 ml; 25% final BCD concentration), followed 15 min later with 1 ml of SIV. This procedure was repeated 4 h later in the same manner. Also, at week 47, uninfected SIV+gel control animal 28884 was given 1 ml gel alone intravaginally, followed 15 min later by 1 ml of SIV. SIV-alone control animal 28756 was challenged with 1 ml SIV twice, 4 hours apart.
Plasma viral load and anti-SIV antibody measurements.
Plasma from EDTA-treated blood was used for measurement of SIV RNA copies by real-time PCR assay as previously described (36). Plasma immunoglobulin G (IgG) binding antibody levels were measured by ELISA as previously described (20).
Statistical analyses.
Data were analyzed by descriptive and graphical means, contingency table methods, inverse regression analyses, standard repeated-measures analysis of variance, and linear mixed-effects longitudinal-regression models. To satisfy homogeneity of variance requirements, data were transformed to their common logarithms. Mixed-effects models take into account possible correlations among within-subject (i.e., within-macaque) error structures over time. In vivo infection was determined by an animal's response in two successive weeks in which viral RNA (vRNA) was detected in the plasma. Various subset analyses were performed to determine statistical equivalence in vRNA responses following successful challenge. A longitudinal repeated-measures model with autoregressive order 1 correlation structure was used for the first challenges of study 1 and study 2. Linear mixed-effects models were fitted to the log viral load data for study 2 following the second challenge. The time of the second challenge was treated as week 0 and compared to the combined controls infected at the first challenge or the second challenge. All significance tests were two sided. A probability level below 0.05 was considered statistically significant.
RESULTS
BCD in vitro toxicity.
BCD has been given intravaginally to mice at a 3% concentration in PBS (10), to rabbits at a 5% concentration in a gel formulation (5), and to women at 70% concentration in a gel formulation daily for 1 month (J. E. K. Hildreth, unpublished results) without pathological effects. To better understand the effects of short-term BCD exposure on individual cell types, we tested the toxicity of a 2-hour incubation with BCD on three cell lines: Caco-2, a human intestinal epithelial cell line; GHOST-Hi5, derived from human osteosarcoma cells; and HeLa, a human cervical epithelial cell line (Fig. 2). The 50% toxic dose, at which 50% of the cells were dead after 2 hours, as assessed by trypan blue exclusion, was approximately 8% for GHOST-Hi5 cells, 10% for Caco-2 cells, and 12% for HeLa cells.
FIG. 2.
In vitro BCD toxicities on various cell lines. GHOST-Hi5 cells, Caco-2 cells, and HeLa cells were exposed to medium containing 0, 2.5, 5, 10, or 20% BCD for 2 hours. The cells were trypsinized and counted by trypan blue exclusion. The dashed lines indicate the 50% toxic-dose concentration of BCD on each cell type. The points represent the averages of duplicate wells, and the error bars represent the standard deviations of duplicate wells. The graph is representative of two or three independent experiments.
In vitro viral inhibition with BCD.
To determine the antiviral effectiveness of transient exposure of BCD against HIV-1 and SIVmac251, inhibition assays were performed using the GHOST-Hi5 indicator cell line, which expresses CCR5 and CXCR4, incubated in the presence or absence of 5% BCD in medium (Fig. 3A). GHOST-Hi5 cells were readily infected at a high frequency with HIV-1LAI, a CXCR4-tropic virus, and HIV-1NFNSX and SIVmac251, CCR5-tropic viruses, in the absence of BCD. Inoculation in the presence of 5% BCD abolished infection of the cells by all three viruses.
FIG. 3.
(A) In vitro inhibition of HIV-1 and SIV infection by BCD. GHOST-Hi5 cells were infected with HIV-1LAI, HIV-1NFNSX, and SIVmac251 in medium containing no BCD or 5% BCD for 2 hours. The cells were washed with PBS, and the medium was replaced with new medium without BCD. The cells were incubated for 48 h, trypsinized, fixed, and analyzed for green fluorescent protein expression on a FACSCaliber. The graph is representative of three independent experiments. The error bars indicate standard deviations. (B) Replication kinetics of HIV-1 or SIV incubated in the presence or absence of BCD. Supernatants from GHOST-Hi5 cells infected with HIV-1LAI or SIVmac251 in the presence or absence of 5% BCD were added to CEMx174 cells. The cells were passaged for 3 weeks, and the supernatants were analyzed for p24 or p27 production by ELISA.
In order to determine whether any HIV-1LAI or SIVmac251 virus in the GHOST-Hi5 cell assay was not inactivated, supernatants from the cells incubated with virus in the presence or absence of BCD were added to CEMx174 cells, a cell line that supports replication of CXCR4-tropic HIV-1 and GPR15/BOB-tropic SIV isolates (Fig. 3B). The CEMx174 cells were passaged for 21 days. Cells incubated with GHOST-Hi5 cell supernatants challenged in the absence of BCD led to active production and replication of HIV-1LAI and SIVmac251. However, the cells incubated with BCD-treated GHOST-Hi5 cell supernatants did not support any virus replication, suggesting that the BCD in the GHOST-Hi5 cell cultures had efficiently inactivated the virus stocks.
Study 1: in vivo challenge with BCD-inactivated SIV.
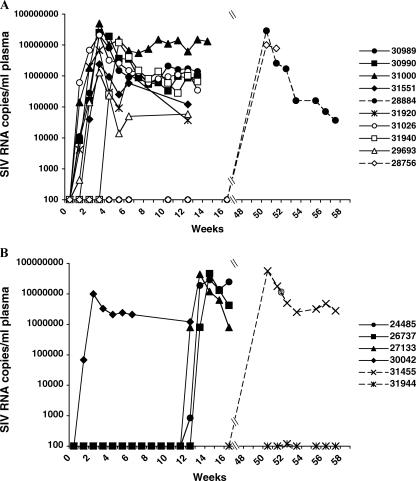
In study 1, two doses of SIV 4 hours apart were administered to the three SIV control animals; this inoculation regimen provided a robust infection challenge (42). The three BCD+SIV animals were challenged intravaginally twice in 1 day with pathogenic SIV that had been premixed with BCD in a nonprescription gel formulation at a final concentration of 5%. Plasma viral loads were measured weekly for all animals. All three SIV control animals became infected, as demonstrated by measurable plasma viremia beginning 1 to 2 weeks postchallenge (Fig. 4A and Table 1), whereas all three BCD+SIV animals remained aviremic through 10 weeks postchallenge (P < 0.0001) (Fig. 4B and Table 1). Control animal 31941 had a high viral load, along with severe weight loss and a CD4 count of 231 cells/mm3 of blood and was euthanized at 8 weeks postinfection.
FIG. 4.
Study 1 plasma vRNA levels. The plasmas of the SIVmac251-challenged animals were analyzed by quantitative reverse transcription-PCR for SIV RNA copies per milliliter. (A) SIV RNA copies in the plasma of the control animals, which were challenged with SIVmac251 at week 0. (B) The animals were challenged with BCD-inactivated SIVmac251 at week 0 and treated with BCD in gel, followed by SIVmac251 at week 10. Animal 31945 was challenged again at week 20 with BCD in gel, followed by SIVmac251. The arrows indicate when challenges took place, and the dagger indicates when animal 31941 was euthanized.
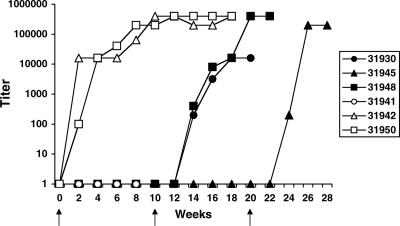
Two of the control animals (31942 and 31950) seroconverted by 2 weeks postchallenge (Fig. 5). As is typical of many monkeys with rapidly progressive SIV infection, the control animal with the highest viral load (31941) did not have measurable plasma anti-SIV antibodies by the time of necropsy. Consistent with the plasma viral load data, the BCD+SIV animals did not have detectable plasma antibodies by 10 weeks postchallenge.
FIG. 5.
Study 1 plasma antibody titers. The plasmas of the SIVmac251-challenged animals were analyzed by ELISA for anti-SIV IgG antibodies. Three animals were challenged with BCD-inactivated SIVmac251 at week 0 and BCD in gel, followed by SIVmac251 at week 10 (closed symbols). Animal 31945 was challenged again at week 20 with BCD in gel, followed by SIVmac251. Three control animals were challenged with SIVmac251 at week 0 (open symbols). The arrows indicate when challenges took place.
Study 1: in vivo intravaginal BCD pretreatment prior to SIV rechallenge.
As premixing of the virus with BCD does not reflect a clinically relevant model for application of a microbicide, we next tested preapplication of BCD gel intravaginally, followed by SIV challenge. At week 10 of study 1, all three uninfected BCD+SIV animals were pretreated with 10% BCD in gel in 1 ml, a volume sufficient for distribution and persisting coverage of the entire macaque vaginal mucosa. Fifteen minutes later, the SIV challenge inoculum was added in the same volume (final BCD concentration, 5%). This procedure was repeated 4 h later. Animals 31930 and 31948 became infected 1 week after the second challenge, as determined by their plasma viral loads (Fig. 4B); infection was subsequently confirmed by seroconversion (Fig. 5). The final uninfected BCD+SIV animal (31945) was then pretreated with BCD, followed by SIV challenge at week 20, and subsequently became infected 1 week after the third challenge (Fig. 4B and 5).
Study 2: in vivo BCD pretreatment prior to SIV challenge.
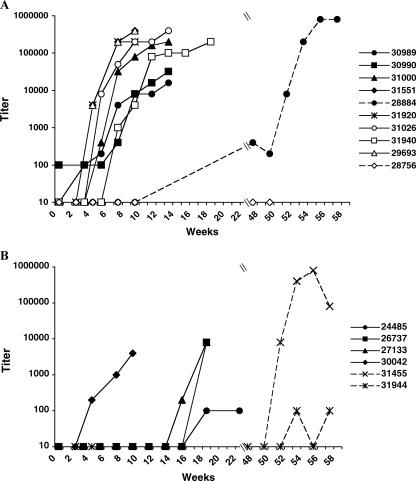
Because the study 1 animals were infected in subsequent SIV challenges despite vaginal application of BCD, we next tested macaques that had no prior exposure to BCD-inactivated virus. Study 2 animals were initially pretreated with BCD prior to SIV exposure. Four control animals were challenged with SIV only (SIV-alone control group). Although the carrier gel was not found to have antiretroviral properties in vitro (Z. Liao and J. E. K. Hildreth, unpublished results), six SIV+gel control animals were pretreated with gel alone, followed 15 min later with the same dose of virus. Of this combined control group, 8/10 animals (5/6 SIV+gel; 3/4 SIV alone) became infected (Fig. 6A and Table 1). In the BCD+SIV treatment group, six animals were pretreated with BCD in gel, followed 15 min later with the same volume of SIV. The final BCD concentration after addition of the virus was 5%. Plasma vRNA levels for the BCD+SIV animals showed that one of six (17%) became infected by 11 weeks postchallenge (Fig. 6B and Table 1), resulting in a significant difference between infection of the BCD+SIV treatment group and the combined control group (P = 0.0007, from a comparative longitudinal repeated-measures regression model with autoregressive order 1 correlation structure). Plasma anti-SIV antibodies were consistent with the viral loads; animals with measurable viremia also had detectable antiviral antibodies (Fig. 7A).
FIG. 6.
Study 2 plasma vRNA levels. (A) The plasmas of the controls were analyzed for SIV RNA copies. Control animals were challenged with SIVmac251 alone (open symbols) or following challenge with gel without drug (closed symbols). Two animals that remained uninfected were challenged a second time with SIVmac251 at week 47 (dashed lines). (B) The plasmas of the experimental animals (BCD+SIV) were analyzed by quantitative reverse transcription-PCR for SIV RNA copies per milliliter. The animals were challenged with BCD in gel, followed by SIVmac251 at week 0. Three BCD-protected animals were rechallenged in the same manner at week 11 (solid lines), and the other two BCD-protected animals were challenged a second time with SIVmac251 at week 47 in the presence of higher concentrations of BCD (dashed lines).
FIG. 7.
Study 2 plasma antibody titers. (A) The plasmas of the control animals were analyzed by ELISA for anti-SIV IgG antibodies. The animals were challenged with BCD in gel, followed by SIVmac251, at week 0. Uninfected animals were rechallenged in the same manner at week 11 (solid lines) or at week 47 (dashed lines). The animals were challenged with SIVmac251 alone (open symbols) or following challenge with gel (closed symbols). Two animals were challenged a second time with SIVmac251 at week 47 (dashed lines). (B) The plasmas of the BCD-treated animals were analyzed for anti-SIV IgG antibodies. The animals were challenged with BCD in gel, followed by SIVmac251, at week 0. Uninfected animals were rechallenged in the same manner at week 11 (solid lines) or at week 47 in the presence of increased concentrations of BCD (dashed lines).
Study 2: second in vivo BCD pretreatment prior to SIV challenge.
In order to determine if the BCD-exposed animals in study 2 would be protected from a second challenge by BCD pretreatment prior to SIV inoculation, the five remaining uninfected BCD-treated animals were rechallenged a second time (Fig. 1 and Table 1). First, three of five BCD+SIV animals (24485, 26737, and 27133) were challenged in the same manner at 11 weeks after the first challenge. The plasma viral loads indicated that all three animals became infected by 1 to 2 weeks after the second challenge (Fig. 6B). Two of these animals (27133 and 26737) also seroconverted by 7 weeks postchallenge (Fig. 7B).
We were concerned that BCD was less efficiently absorbed by the mucosa in the BCD-experienced animals upon subsequent exposures. We therefore examined whether greater concentrations of the antiviral agent could neutralize an SIV challenge in the remaining two of five animals in study 2. Thus, at week 47, the two remaining uninfected BCD+SIV animals (31455 and 31944) received a wash of 50% BCD in saline, followed by 50% BCD in gel, intravaginally prior to SIV challenge (1:1 volumes for a final BCD concentration of 25%) (Table 1). This new lot of BCD was tested in vitro and was shown to inactive HIV-1 in vitro in a manner equivalent to that of the prior lot (data not shown).
In addition, the two uninfected control animals were challenged in the same manner as in their respective first challenges: 28756 received virus alone, and 28884 received gel, followed by SIV (Table 1). One BCD+SIV animal (31455), as well as both control animals, became infected, as indicated by plasma viremia (Fig. 6). There was no difference in the infection rate of the BCD+SIV group or combined control group animals after the second challenge at week 11 or 47 (P = 0.63, from a comparative longitudinal repeated-measures regression model).
Plasma anti-SIV antibodies were detected in all study 2 animals with measurable plasma viremia, with the exception of control animal 28756, which rapidly progressed to disease following infection and had to be euthanized (Fig. 7A).
DISCUSSION
To date, a number of published reports have demonstrated that candidate topical microbicides can protect greater than 50% of treated macaques from intravaginal infection by SIVmac251 or SHIVs (3, 12, 13, 15, 22, 23, 28, 33, 37, 39, 44, 45). Compounds that nonspecifically prevent SIVmac251 binding to and/or entry into target cells, CAP and benzalkonium chloride, showed >50% protection using high doses of virus inocula (3, 12, 22, 37). Blockade of infection by antibodies or ligands used to inhibit specific binding of SHIV envelopes to receptors/coreceptors in the vaginal mucosa required relatively high concentrations (≥5 mg of antibody or ≥0.1 mM of PSC-RANTES/−2 RANTES) in order to prevent infection in the majority of treated animals (13, 15, 23, 28, 44, 45). Cyanovirin-N was able to specifically inhibit intravaginal SHIV infection of 83% of treated macaques at concentrations as low as 0.5% (39). The antibody and cyanovirin microbicide studies were performed on animals pretreated with progesterone, which decreases the thickness of the vaginal epithelium and increases susceptibility to infection, requiring much less virus to infect control macaques (34). Therefore, the candidate microbicides needed to block relatively smaller numbers of infectious particles from the smaller challenge doses. With the exception of a recent CAP study, none of the prior examinations of microbicidal compounds within the macaque model have evaluated efficacy in preventing mucosal transmission after repeated exposures to the antiviral agents (12). Indeed, the prominent but disappointing clinical trial failures of the anti-HIV-1 compounds nonoxynol 9 and cellulose sulfate have suggested that repeated exposure to the microbicide candidates may increase risk of infection (14, 29, 31, 32).
We elected to test the mucosal antiviral efficacy of BCD, as the molecule has an extensive record of safety in humans as an oral, topical, and parenteral agent. Prior investigation had demonstrated that BCD blocks HIV infection at several levels: it renders susceptible cells resistant to infection (18), it inactivates free virus (8, 19), and it prevents the release of infectious particles by infected cells (19). The mechanisms associated with these activities have been characterized. By removing cholesterol from susceptible cells, the compound disrupts chemokine receptor function, and virus-cell fusion is blocked. The depletion of cholesterol from virus particles also prevents virus-cell fusion. Finally, cholesterol removal from infected cells causes a drastic reduction in the release of virus particles and results in the assembly of virions incapable of fusing to host cells. We have also shown in preliminary studies that BCD retains its antiviral activity when incorporated into a vaginal cream (Hildreth, unpublished), a likely microbicide vehicle.
In this study, we demonstrated that BCD efficiently prevented cells from becoming infected with HIV-1 or SIV at nontoxic concentrations in cell culture. Although the amount of BCD needed to neutralize 50% of an SIV stock is close to 5 mM (or approximately 0.73%) (8), we tested the effects of 5% BCD on the actual macaque virus challenge stocks. In addition, BCD diluted as low as 5% was able to inactivate a high-titer virus infection in cell culture. BCD-treated virus did not infect GHOST cells in vitro and did not infect macaques after intravaginal challenge (study 1). In study 2, BCD was administered intravaginally to macaques prior to challenge with a high dose of SIV. A significant number of the animals did not become infected compared to the controls. Unexpectedly, when the study 2 animals were pretreated with BCD prior to a second challenge 11 or 47 weeks later, most of them became infected at a rate similar to that of the controls even when high concentrations of BCD were used. Two of the three study 1 animals also became infected when they were rechallenged with BCD pretreatment followed by SIV at week 10.
There are at least three possible explanations for the animals becoming infected by SIV following a second or third BCD pretreatment. First, BCD may cause pathology of the vaginal epithelium, allowing SIV particles that escaped inactivation to cross the epithelium to infect target cells. In our study, no gross pathology was noted for the vaginal epithelium of the BCD-treated macaques after drug administration. In addition, initial BCD exposure did not immediately cause infection in the majority of the animals, even though challenges were performed twice on the same day at a 4-h interval, but rather, they became infected upon a second exposure after extended intervals, 10 or 47 weeks later. A second possibility is that BCD-inactivated virions present in the vaginal canals of treated animals could be taken up by mucosal antigen-presenting cells to recruit SIV target cells, namely, macrophages and T lymphocytes (35), to the area. CD4+ T cells specific for recognition of HIV peptides have been shown to be preferentially infected by HIV compared to T cells specific for other antigens (6). Again, this mechanism appears unlikely, as any lymphocyte recruitment as a result of such a possible immunization of whole killed SIV virions would probably disappear after a few weeks at most and would not be present 11 to 47 weeks later. In addition, vaginal uptake of antigens has not been shown to produce robust mucosal immune responses without the use of potent adjuvants (17, 27, 38). The third and most likely explanation is that it is possible that a 5% final concentration of BCD is insufficient to inactivate as high an inoculum of SIV virions as was used in this study or that the formulation suboptimally coated the vaginal canal, allowing the epithelium to be exposed to infectious virions that escaped BCD. More animals will need to be tested with multiple challenges of SIV following BCD pretreatment to determine if this model does indeed explain the results described here.
Because 5% BCD failed to protect macaques from repeated SIV challenges, this study has important implications for the evaluation of anti-HIV-1 microbicides. It is interesting that our BCD formulation significantly prevented the infection of macaques from initial intravaginal challenge, similar to other in vivo microbicide studies. However, unlike the majority of previously published macaque studies, we conducted repeated applications of BCD, followed by SIV challenges, as presumably microbicides will be used during multiple sexual encounters. It was only upon the second or third challenges that we observed a failure of BCD to prevent intravaginal infection of macaques. Recent promising studies with CAP have used repeated applications, followed by intravaginal challenge with SIVmac251 (22) or SHIV in multiple low doses (12). Our studies strongly suggest that future testing of topical antiviral microbicides or other preexposure prophylaxis regimens within animal models should also be performed with repeated administrations in order to assess compound efficacy against multiple exposures of virus. To be more consistent with HIV-1 sexual transmission, future experiments with low-dose SIV challenges in the macaque model would likely prove more realistic and easier to extrapolate to humans. The consequence of repeated mucosal exposure to inactivated virus may also be of interest, particularly with regard to influencing future susceptibility to an infectious transmission.
Acknowledgments
We thank John Coffin, Eric Freed, and Steve Hughes for valuable discussions; Marc Busch for technical assistance; and David Graham for sharing preliminary data.
This work was supported by the National Cancer Institute's intramural Center for Cancer Research, which supports the HIV Drug Resistance Program; by Public Health Service grant U51RR00169 from the National Center for Research Resources; by fellowship number 106406-33-RFMC from the American Foundation for AIDS Research (amfAR) for Z.A.; and, in part, with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Published ahead of print on 23 April 2008.
REFERENCES
- 1.Alliance for Microbicide Development. 2007. Ongoing microbicide clinical trials (by product). Alliance for Microbicide Development, Silver Spring, MD.
- 2.Bibby, D. C., N. M. Davies, and I. G. Tucker. 2000. Mechanisms by which cyclodextrins modify drug release from polymeric drug delivery systems. Int J. Pharm. 1971-11. [DOI] [PubMed] [Google Scholar]
- 3.Boadi, T., E. Schneider, S. Chung, L. Tsai, A. Gettie, M. Ratterree, J. Blanchard, A. R. Neurath, and C. Cheng-Mayer. 2005. Cellulose acetate 1,2-benzenedicarboxylate protects against challenge with pathogenic X4 and R5 simian/human immunodeficiency virus. AIDS 191587-1594. [DOI] [PubMed] [Google Scholar]
- 4.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 726988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degim, Z., T. Degim, F. Acarturk, D. Erdogan, C. Ozogul, and M. Koksal. 2005. Rectal and vaginal administration of insulin-chitosan formulations: an experimental study in rabbits. J. Drug Target. 13563-572. [DOI] [PubMed] [Google Scholar]
- 6.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 41795-98. [DOI] [PubMed] [Google Scholar]
- 7.Fleischman, J., and K. Cravero. 8 March 2004. A focus on women and AIDS. The Boston Globe, Boston, MA.
- 8.Graham, D. R. M., E. Chertova, J. M. Hilburn, L. O. Arthur, and J. E. K. Hildreth. 2003. Cholesterol depletion of HIV-1 and SIV with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 778237-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyader, M., E. Kiyokawa, L. Abrami, P. Turelli, and D. Trono. 2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol. 7610356-10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna, K. V., K. J. Whaley, L. Zeitlin, T. R. Moench, K. Mehrazar, R. A. Cone, Z. Liao, J. E. Hildreth, T. E. Hoen, L. Shultz, and R. B. Markham. 2002. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Investig. 109205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilsdonk, E. P., P. G. Yancey, G. W. Stoudt, F. W. Bangerter, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 27017250-17256. [DOI] [PubMed] [Google Scholar]
- 12.Kim, C. N., D. R. Adams, S. Bashirian, S. Butera, T. M. Folks, and R. A. Otten. 2006. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. J. Med. Primatol. 35210-216. [DOI] [PubMed] [Google Scholar]
- 13.Kish-Catalone, T., R. Pal, J. Parrish, N. Rose, L. Hocker, L. Hudacik, M. Reitz, R. Gallo, and A. Devico. 2007. Evaluation of −2 RANTES vaginal microbicide formulations in a nonhuman primate simian/human immunodeficiency virus (SHIV) challenge model. AIDS Res. Hum. Retrovir. 2333-42. [DOI] [PubMed] [Google Scholar]
- 14.Kreiss, J., E. Ngugi, K. Holmes, J. Ndinya-Achola, P. Waiyaki, P. L. Roberts, I. Ruminjo, R. Sajabi, J. Kimata, and T. R. Fleming. 1992. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 268477-482. [PubMed] [Google Scholar]
- 15.Lederman, M. M., R. S. Veazey, R. Offord, D. E. Mosier, J. Dufour, M. Mefford, M. Piatak, Jr., J. D. Lifson, J. R. Salkowitz, B. Rodriguez, A. Blauvelt, and O. Hartley. 2004. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306485-487. [DOI] [PubMed] [Google Scholar]
- 16.Lee, P. P., and M. L. Linial. 1994. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J. Virol. 686644-6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehner, T., L. A. Bergmeier, C. Panagiotidi, L. Tao, R. Brookes, L. S. Klavinskis, P. Walker, J. Walker, R. G. Ward, L. Hussain, et al. 1992. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science 2581365-1369. [DOI] [PubMed] [Google Scholar]
- 18.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 171009-1019. [DOI] [PubMed] [Google Scholar]
- 19.Liao, Z., D. R. Graham, and J. E. Hildreth. 2003. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res. Hum Retrovir. 19675-687. [DOI] [PubMed] [Google Scholar]
- 20.Lu, X., H. Kiyono, D. Lu, S. Kawabata, J. Torten, S. Srinivasan, P. J. Dailey, J. R. McGhee, T. Lehner, and C. J. Miller. 1998. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS 121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manson, K. H., M. S. Wyand, C. Miller, and A. R. Neurath. 2000. Effect of a cellulose acetate phthalate topical cream on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 443199-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6207-210. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 743264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien, W. A., Y. Koyanagi, A. Namazie, J. Q. Zhao, A. Diagne, K. Idler, J. A. Zack, and I. S. Chen. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 34869-73. [DOI] [PubMed] [Google Scholar]
- 26.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 9813925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parr, E. L., M. B. Parr, and M. Thapar. 1988. A comparison of specific antibody responses in mouse vaginal fluid after immunization by several routes. J. Reprod. Immunol. 14165-176. [DOI] [PubMed] [Google Scholar]
- 28.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 758340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramjee, G., R. Govinden, N. S. Morar, and A. Mbewu. 2007. South Africa's experience of the closure of the cellulose sulphate microbicide trial. PLoS Med. 4e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockefeller Foundation. 2002. Mobilization for microbicides: the decisive decade. Rockefeller Foundation, New York, NY.
- 31.Roddy, R. E., L. Zekeng, K. A. Ryan, U. Tamoufe, S. S. Weir, and E. L. Wong. 1998. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 339504-510. [DOI] [PubMed] [Google Scholar]
- 32.Rustomjee, R., Q. Abdool Karim, S. S. Abdool Karim, M. Laga, and Z. Stein. 1999. Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS 131511-1515. [DOI] [PubMed] [Google Scholar]
- 33.Smith, S. M., G. B. Baskin, and P. A. Marx. 2000. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J. Infect. Dis. 182708-715. [DOI] [PubMed] [Google Scholar]
- 34.Sodora, D. L., A. Gettie, C. J. Miller, and P. A. Marx. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retrovir. 14(Suppl. 1)S119-123. [PubMed] [Google Scholar]
- 35.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14183-189. [DOI] [PubMed] [Google Scholar]
- 37.Tevi-Benissan, C., M. Makuva, A. Morelli, M. C. Georges-Courbot, M. Matta, A. Georges, and L. Belec. 2000. Protection of cynomolgus macaque against cervicovaginal transmission of SIVmac251 by the spermicide benzalkonium chloride. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 24147-153. [DOI] [PubMed] [Google Scholar]
- 38.Thapar, M. A., E. L. Parr, and M. B. Parr. 1990. The effect of adjuvants on antibody titers in mouse vaginal fluid after intravaginal immunization. J. Reprod. Immunol. 17207-216. [DOI] [PubMed] [Google Scholar]
- 39.Tsai, C. C., P. Emau, Y. Jiang, M. B. Agy, R. J. Shattock, A. Schmidt, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2004. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retrovir. 2011-18. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, C. C., P. Emau, Y. Jiang, B. Tian, W. R. Morton, K. R. Gustafson, and M. R. Boyd. 2003. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 19535-541. [DOI] [PubMed] [Google Scholar]
- 41.UNAIDS. 2006. AIDS epidemic update. Joint United Nations Programme on HIV/AIDS and World Health Organization, Geneva, Switzerland.
- 42.Van Rompay, K. K., K. A. Schmidt, J. R. Lawson, R. Singh, N. Bischofberger, and M. L. Marthas. 2002. Topical administration of low-dose tenofovir disoproxil fumarate to protect infant macaques against multiple oral exposures of low doses of simian immunodeficiency virus. J. Infect. Dis. 1861508-1513. [DOI] [PubMed] [Google Scholar]
- 43.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 1981551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veazey, R. S., P. J. Klasse, S. M. Schader, Q. Hu, T. J. Ketas, M. Lu, P. A. Marx, J. Dufour, R. J. Colonno, R. J. Shattock, M. S. Springer, and J. P. Moore. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 43899-102. [DOI] [PubMed] [Google Scholar]
- 45.Veazey, R. S., R. J. Shattock, M. Pope, J. C. Kirijan, J. Jones, Q. Hu, T. Ketas, P. A. Marx, P. J. Klasse, D. R. Burton, and J. P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9343-346. [DOI] [PubMed] [Google Scholar]
- 46.Veazey, R. S., M. S. Springer, P. A. Marx, J. Dufour, P. J. Klasse, and J. P. Moore. 2005. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat. Med. 111293-1294. [DOI] [PubMed] [Google Scholar]
- 47.Wyand, M. S., K. H. Manson, C. J. Miller, and A. R. Neurath. 1999. Effect of 3-hydroxyphthaloyl-beta-lactoglobulin on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 43978-980. [DOI] [PMC free article] [PubMed] [Google Scholar]