Abstract
Human herpesvirus 8 (HHV-8), which is associated with the endothelial tumor Kaposi's sarcoma, encodes three CC/β-chemokines. These are expressed early during productive (lytic) infection and are believed to be involved in immune evasion, in addition to viral pathogenesis via induction of angiogenic cytokines. Here we report that two of the HHV-8 chemokines, CCR8 agonists vCCL-1 and vCCL-2, have direct effects on endothelial survival and virus replication. The v-chemokines stimulated virus replication when added to infected cultures exogenously, and CCR8 knockdown absent v-chemokine supplementation inhibited virus production, indicative of autocrine effects of endogenously produced vCCLs. This was verified and proreplication functions of each chemokine were demonstrated via shRNA-mediated vCCL depletion. The v-chemokines inhibited expression of lytic cycle-induced proapoptotic protein Bim, RNA interference-mediated suppression of which mimicked v-chemokine proreplication functions. Our data show for the first time that the v-chemokines have direct effects on virus biology, independently of their postulated immune evasion functions, and suggest that in vivo the v-chemokines might play direct roles in Kaposi's sarcomagenesis via paracrine prosurvival signaling.
Human herpesvirus 8 (HHV-8) is associated etiologically with the endothelial cell neoplasm Kaposi's sarcoma (KS) and the B-cell malignancies primary effusion lymphoma (PEL) and multicentric Castleman's disease. KS, especially, has been linked with inflammatory and angiogenic cytokine dysregulation, and in this respect the proinflammatory and proangiogenic cytokine viral interleukin-6 (vIL-6), in addition to the three HHV-8-specified viral chemokines, which also induce angiogenesis, may play important pathogenic roles (35). Vascular endothelial growth factor (VEGF), induced by vIL-6 (1) and at least one of the viral chemokines (25), is believed to contribute to PEL also (2). Therefore, study of the HHV-8 cytokines is relevant to understanding the mechanisms by which HHV-8 induces neoplasia and for developing therapeutic interventions. The roles of the v-cytokines in normal virus biology have not been defined, and indeed they may represent useful antiviral targets.
HHV-8 CC/β-chemokines vCCL-1, vCCL-2, and vCCL-3 are encoded by open reading frames (ORFs) K6, K4, and K4.1, respectively. vCCL-2 binds to a broad range of CC-chemokine receptors and can also target CXCR4, CX3C, and XCR1 (6, 10, 13, 16, 23, 29, 42, 45). However, only CCR3 and CCR8 are able to support vCCL-2 signal transduction. vCCL-1 signals via CCR8, and vCCL-3 has been reported to be an agonist for CCR4 and XCR1 (13, 16, 28, 45, 47).
The functions in virus biology of the HHV-8 v-chemokines remain speculative but may include roles in immune evasion and virus dissemination via Th2 polarization and lymphocyte/monocyte recruitment mediated by their various agonist and neutral agonist activities (35). However, their potential roles in promoting productive, or lytic, virus replication via direct effects on virus-infected cells have not been investigated. In view of the previously demonstrated prosurvival activities of vCCL-1 and vCCL-2 on PEL cells (25) and vCCL-1 on murine thymic lymphoma cells (26, 46), this is a distinct possibility, potentially enabling the extended survival of cells to allow efficient virus production in the face of virally induced apoptotic signals. Such activities might also contribute to HHV-8-associated neoplasia. Primary endothelial cells have been reported to be chemotactically responsive to CCR8 agonists vCCL-1 and CCL1, and KS spindle cells express CCR8 (22), suggesting that HHV-8 v-chemokines could play a direct role in KS pathogenesis.
In this report, we present data demonstrating that the v-chemokines are able, via CCR8, to signal in and enhance the survival of endothelial cells and to promote virus productive replication. The findings suggest novel, direct functions of vCCL-1 and vCCL-2 in both virus biology and viral pathogenesis, independent of immune evasion and cytokine-inducing activities and distinct from the strictly autocrine functions of other, mechanistically distinct and intracellularly expressed viral antiapoptotic proteins.
MATERIALS AND METHODS
Recombinant proteins.
Intein-chitin-binding protein fusion proteins of CCL1, vCCL-1, vCCL-2, and CXCL1/GROα were purified from isopropyl-β-d-thiogalactopyranoside-induced pTYB4 chemokine expression vector-transformed bacteria (Rosetta; Novagen, Madison, WI) by passage of sonicated cell extracts over chitin columns (New England Biolabs, Beverly, MA) and washing with 10 bed volumes of washing buffer (20 mM HEPES [pH 8.0], 500 mM NaCl, 1 mM EDTA) four times. The chemokines were recovered by cleavage of the fusion proteins by incubation of the columns in cleavage buffer (20 mM dithiothreitol, 20 mM HEPES [pH 8.0], 500 mM NaCl, 1 mM EDTA) at 4°C overnight, followed by elution with washing buffer. Eluates were dialyzed in phosphate-buffered saline (PBS), and endotoxin was removed by using Detoxi-gel (Pierce, Rockford, IL). Recombinant human VEGF165 was purchased from Peprotech Inc. (Rocky Hill, NJ).
Antibodies.
Phospho-ERK1/2 (Thr202/Tyr 204), phospho-AKT (Ser473), phospho-FOXO3a (Ser253), phospho-Bcl-2 (Ser70), ERK1/2, FOXO3a, Bcl-2, and Bim antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Other antibodies used were to HDAC1, AKT, and Mcl-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), K8.1 and latency-associated nuclear antigen (LANA; Advanced Biotechnologies Inc., Columbia, MD), CCR8 (Epitomics, Inc., Burlingame, CA), calnexin (BD Bioscience, Franklin Lakes, NJ), β-actin (Sigma, St. Louis, MO), and DR3 (Abcam, Cambridge, MA). ORF59 antibody was kindly provided by B. Chandran.
Plasmids and oligonucleotides.
The coding sequences of CCL1, vCCL-1, vCCL-2, and GROα/CXCL-8 were cloned as NcoI and SmaI fragments into pTYB4 (New England Biolabs). The NotI and BamHI fragment containing the forkhead responsive element (FHRE) and luciferase gene sequences from pGL3/FHRE (Addgene, Cambridge, MA) was cloned into lentiviral vector pYNC320 (a derivative of pTYF [9]). The corresponding part of pGL3/basic (Promega, Madison, WI) was cloned into pYNC320. Complementary pairs of 59-nucleotide oligonucleotides encoding a 21-nucleotide short hairpin RNA (shRNA) to CCR8 (5′-CCTCCAGCGTAGACTACATTT-3′), Bim (5′-GACCGAGAAGGTAGACAATTG-3′), vCCL-1 (5′-GCGGCTGCCTGCCATAGCTTA-3′) and vCCL-2 (5′-GGTGAAGAAGCTGATGCAGCA-3′), or nonsilencing (NS) shRNA (5′-CAACAAGATGAAGAGCACCAA-3′) were designed according to the strategy of MISSION (Sigma) and annealed and cloned into the BamHI and MluI sites of the pYNC352 (pTYF derivative) or pSUPER.retro.puro (OligoEngine, Seattle, WA) vectors. Primer sequences used for quantitative PCR were the following: K8.1 forward primer, 5′-TGCCATTTTCTGCCACCACTACAACGACT-3′; K8.1 reverse primer, 5′-ACAAGTCCCAGCAATAAACCCACAGCCCA-3′; vCCL-1 forward primer, 5′-GCAGCAGCTATTAGGCGTGTA-3′; vCCL-1 reverse primer, 5′-CCACGTTTTATGCTGCGTTA-3′; vCCL-2 forward primer, 5′-GGGTACCAGCTGGACAGAAG-3′; vCCL-2 reverse primer, 5′-TGACTGCCTTGCTTTGTTTG-3′; Bim forward primer, 5′-CCTACAGACAGAGCCACAAGG-3′; Bim reverse primer, 5′-CGGGGATCTGGTAGCAAAAGG-3′; CCR8 forward primer, 5′-GGCTGCTGCTCATTGAGCTGC-3′; CCR8 reverse primer, 5′-TCAGACAGGGCCAGGTTCAAG-3′); actin forward primer, 5′-TGCCATCCTAAAAGCCACCCCACTTC-3′; actin reverse primer, 5′-AAGCAATGCTATCACCTCCCCTGTGT-3′.
Cell culture.
Dermal microvascular endothelial cells (DMVEC) and telomerase immortalized microvascular endothelial (TIME) cells were maintained in EGM-2 MV medium (Lonza, Walkersville, MD) containing 5% fetal bovine serum (FBS) and cytokine supplements. BCBL-1 cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS and gentamicin. HEK293T and Phoenix (Orbingen, San Diego, CA) packaging cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS and gentamicin.
Virus production and infection.
Infectious HHV-8 virus was obtained by inducing BCBL-1 cells with phorbol 12-myristate 13-acetate (20 ng/ml) and calcium ionophore (A23187; 500 ng/ml). After 16 to 20 h cells were pelleted and resuspended in fresh medium without phorbol 12-myristate 13-acetate and A23187. After 5 days, cells were pelleted, the supernatant filtered through 0.45-μm filters, and virions pelleted at 15,000 rpm for 2 h in an SW41 rotor. For infection of TIME cells, the viral pellet was resuspended in basal EGM-2 MV medium supplemented with 5 μg of Polybrene per ml and added to cell cultures for 2 h, prior to replacement of inoculum with fresh medium. For virus inductions, the medium was replaced (after >48 h) with fresh medium containing 20 ng/ml tetradecanoyl phorbol acetate (TPA). Lentiviruses were produced using calcium phosphate-mediated cotransfection of HEK293T cells with pYNC352- or pYNC320-based vectors together with pGag-Pol and pVSV-G. Retroviruses were produced by cotransfection of Phoenix cells with pSuper-NS, pSuper-CCR8i, or pSuper-Bimi together with pVSV-G. All virus preparations were subjected to 0.45-μm filtration.
Western blotting and immunofluorescence assay.
For immunoblotting, cells were lysed in lysis buffer (50 mM HEPES [pH 8.0], 100 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 mM Na3VO4, 50 mM NaF, and protease inhibitor cocktail). Cytoplasmic fractions were prepared by homogenizing cells with 10 strokes of a Dounce homogenizer in homogenization buffer (10 mM HEPES [pH 8.0], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and protease inhibitor cocktail) on ice and centrifuging at 3,000 × g for 5 min; the supernatant was used as the cytoplasmic fraction. Proteins were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane for immunoblotting. Immunoreactive bands were visualized in a chemiluminescence assay. For immunofluorescence assays (IFAs), cells on a 0.1% gelatin-coated coverglass were either fixed and permeabilized in chilled methanol (for detection of HHV-8 lytic antigens and Bim) or fixed in 4% paraformaldehyde without permeabilization (for detection of surface-expressed CCR8). Following incubation with blocking buffer (3% bovine serum albumin [BSA] in PBS), coverslips were incubated with primary antibody, washed with PBS, and then incubated with appropriate florescent dye-conjugated secondary antibody. Coverslips were mounted in 90% glycerol containing 10 mg/ml p-phenylenediamine. Nuclei were visualized by staining with Hoechst 33342.
Apoptosis assays.
Endothelial cells grown on a 0.1% gelatin-coated coverglass were washed with Annexin V binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, and 2.5 mM CaCl2), stained with fluorescein isothiocyanate (FITC)- or Cy-3-conjugated Annexin V (Biovision Inc., Mountain View, CA), fixed in 2% formaldehyde, mounted, and visualized by UV microscopy. For terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL), cells were fixed in chilled methanol for 5 min and preincubated in TdT reaction buffer (25 mM Tris-HCl [pH 6.6], 200 mM sodium cacodylate, 0.25 mg/ml BSA, and 1 mM CoCl2) for 10 min. TUNEL reactions were carried out at 42°C for 2 h in TdT reaction buffer containing TdT and biotin-dUTP (Roche, Indianapolis, IN) and terminated with stop solution (300 mM NaCl and 30 mM sodium citrate). LANA IFAs were performed after TUNEL assays, and biotin-dUTP and LANA were visualized by staining with FITC-avidin (Roche) or Cy-3-conjugated secondary antibody, respectively.
Quantitative PCR.
For quantitative reverse transcriptase PCR (qRT-PCR), total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized from 1 μg of total RNA using SuperScript II RT (Invitrogen) with random hexamers. For determination of encapsidated HHV-8 genome copy number, viral DNA was phenol extracted after treatment of virus suspensions with DNase I for 15 min at room temperature and then with proteinase K-sodium dodecyl sulfate at 65°C for 1 h. Excess HHV-8 bacmid DNA was treated with DNase I and processed identically to control for DNase I efficacy. All qPCRs were performed in a 96-well microplate using an ABI Prism 7500 detection system (Applied Biosystems, Foster City, CA) with the RT2Real-Time SYBR green/ROX master mix (SuperArray Bioscience Corp., Frederick, MD). Reactions were performed in a total volume of 25 μl and contained 50 ng of reverse-transcribed RNA (based on the initial RNA concentration) or an appropriate volume of viral DNA and 250 nM of each set of primers. To calculate the copy number of viral DNA, BAC-cloned HHV-8 genomic DNA (52) was used as a standard. PCR conditions included an initial incubation step of 2 min at 50°C and an enzyme heat activation step of 10 min at 95°C, followed by 45 cycles of 15 seconds at 95°C for denaturing and 1 min at 60°C for annealing and extension.
RESULTS
vCCL-1 and vCCL-2 signaling in endothelial cells.
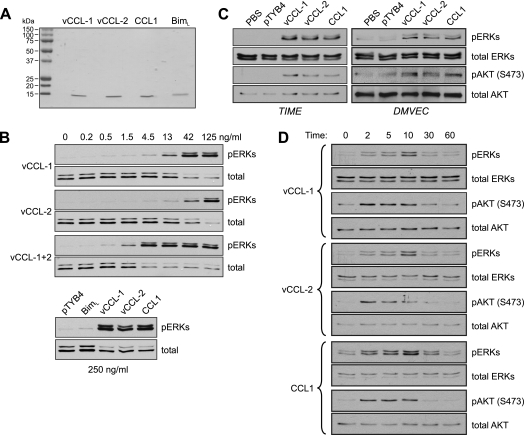
To characterize signaling by the v-chemokines, and CCL1, in endothelial cells we utilized bacterially expressed recombinant proteins (Fig. 1A) and primary and telomerase-immortalized DMVEC and TIME cells (50). Initial dose-response experiments were conducted in TIME cells, to verify the activities of the recombinant chemokines and determine their minimally and maximally effective doses (Fig. 1B). ERK activation after 10-minute stimulation was detected in cell lysates by Western analysis for the phosphorylated, activated form of the kinase. We found that vCCL-1 and vCCL-2 had somewhat different activity profiles, with detectable and maximum activities occurring at 4.5 and 42 ng/ml for vCCL-1 and 13 and at least 125 ng/ml for vCCL-2. When used in combination, the activities of vCCL-1 and vCCL-2 appeared to be synergistic at low doses, with maximum stimulation being achieved at 4.5 ng/ml of vCCL-1 and vCCL-2. This level of stimulation was maintained with up to 125 ng/ml of each v-chemokine and was equivalent to the maximum level of ERK activation obtained with individual stimulation by vCCL-1 and vCCL-2. In a parallel experiment, cells were treated with 250 ng/ml of vCCL-1, vCCL-2, or cellular CCR8 agonist CCL1, or with a pTYB4 (empty bacterial expression vector) transformant-derived negative control sample or an irrelevant recombinant protein (BimL; 250 ng/ml). The results confirmed specificity of signaling by the chemokines, with no activation of ERK in the negative controls (Fig. 1B, lower section).
FIG. 1.
Activation of AKT and ERK1/2 by viral and cellular CCR8 agonists in endothelial cells. (A) Recombinant vCCL-1, vCCL-2, and CCL1, along with Bim (negative control), were generated as described in Materials and Methods, and their integrity and purity were checked on Coomassie-stained gels. (B) Viral chemokine activities and signaling in endothelial cells were checked in ERK activation assays in TIME cells. Cells were serum and supplement deprived for 24 h and then treated with different doses of the v-chemokines, alone or combined, for 10 min prior to cell harvest for analysis of ERK activation. Phospho-specific antibody was used to identify threonine-202/tyrosine-204-phosphorylated ERKs (pERKs) by Western analysis. Antibodies recognizing ERKs independently of phosphorylation status were used to confirm equal protein loading and transfer. In a parallel experiment, cultures were treated with 250 ng of the v-chemokines, CCR1, or BimL (nonsignaling control), or with pTYB4 (empty bacterial expression vector) transformant extract processed identically and in parallel with the recombinant protein-containing samples. (C) ERK and AKT activation assays were undertaken in TIME cells and primary DMVEC treated with maximally effective doses (250 ng/ml) of recombinant vCCL-1, vCCL-2, or CCL1. Antibody recognizing serine-473-phosphorylated AKT (pAKT) was employed to identify the active form of the kinsase by immunoblotting. (D) Similar experiments were undertaken in TIME cells, which were harvested at different times poststimulation to determine the kinetics and durations of ERK and AKT activation.
Treatment of DMVEC in addition to TIME cells with the v-chemokines and CCL1 (using maximally effective, matched doses) revealed chemokine-induced ERK and AKT activation in both cell types (Fig. 1C). The kinetics of ERK and AKT activation by vCCL-1, vCCL-2, and CCL1 were examined in TIME cells. For each chemokine, maximal activation of AKT and ERK occurred at 2 and 10 min, respectively, with return to baseline or near-baseline levels by 30 min (Fig. 1D). Such transient signaling is typical of G protein-coupled receptors (GPCRs), which are negatively regulated by desensitization and internalization (38). Our data revealed distinguishable kinetics of ERK and AKT activation and equivalent signaling by vCCL-1, vCCL-2, and CCL1.
Viral chemokines signal via CCR8 in endothelial cells.
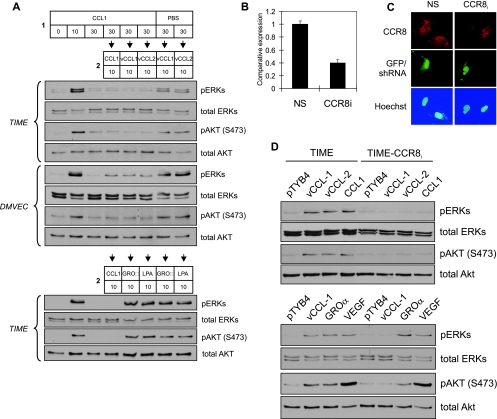
To verify utilization of CCR8 by the v-chemokines, we utilized two approaches: receptor desensitization and RNA interference-mediated CCR8 knockdown. For the first, TIME cells and DMVEC were treated with CCR8 ligand CCL1 (or PBS, the negative control) for 30 min prior to addition of vCCL-1 or vCCL-2 for 10 min. ERK and AKT were activated by the v-chemokines in PBS-treated cultures, but signaling was abrogated in the CCL1-pretreated cells (Fig. 2A, top). Non-CCR8 agonists CXCL1 (GROα) and lysophosphatidic acid were, by contrast, able to signal normally in CCL1-pretreated cultures (Fig. 2A, bottom), demonstrating that effects on v-chemokine signaling were the result of homologous (receptor-specific) rather than heterologous desensitization.
FIG. 2.
CCR8 usage by viral chemokines. (A) Receptor desensitization to investigate the utilization of CCR8 by vCCL-1 and vCCL-2 in endothelial cells. Recombinant CCL1 (250 ng/ml) was applied to TIME or DMVEC cultures for 30 min (step 1); parallel cultures were stimulated with PBS to provide a negative control. Then, optimal signaling doses (250 ng/ml) of vCCL-1 and vCCL-2, or non-CCR8 agonist controls, were applied for a further 10 min (steps 2), before cell harvest. Cell lysates were analyzed by Western blotting for detection of ERK and AKT activation (phosphorylation). Cells pretreated with CCL1, but not with PBS, were resistant to stimulation by the v-chemokines. Ten-minute stimulation with CCL1 in the pretreatment step (1) gave the expected signaling responses, which diminished to baseline by 30 min (beginning of step 2). CCL1 pretreatment did not affect signaling by CCR8-independent GROα (200 ng/ml) or lysophosphatidic acid (5 μM), demonstrating the specificity of the desensitization effect. (B to D) CCR8 knockdown to determine CCR8 utilization. TIME cells were infected to near 100% efficiency (determined by GFP fluorescence) with lentivirus (GFP+)-shRNA vectors specifying either CCR8 mRNA-directed (CCR8i) or nonsilencing (NS, control) sequences. Cultures were analyzed by qRT-PCR (B) or with a derived cell line (E2) by CCR8-specific IFA (C) for the expression of CCR8 mRNA and protein, respectively. For the latter, lentivirus-infected cells were mixed with uninfected TIME cells to provide a means of comparison; GFP-positive (lentivirus-infected) cells showed markedly decreased expression of CCR8 (red) relative to GFP/lentivirus-negative cells. (D) Signal transduction by the v-chemokines and CCL1 (250 ng/ml) in TIME-CCR8i(E2) cells was greatly diminished relative to that in TIME cells (top). Application of GROα (200 ng/ml) or VEGF (10 ng/ml) to the cultures resulted in equivalent signal transduction in TIME and TIME-CCR8i(E2) cells but reduced signaling in TIME-CCR8i(E2) cells by CCR8 agonist CCL1, demonstrating the specificity of the CCR8-shRNA effect (bottom).
To provide further evidence of v-chemokine utilization of and dependence on CCR8, signaling assays were undertaken in a TIME cell line, TIME-CCR8i(E2), stably transduced with a lentiviral vector constitutively expressing CCR8 mRNA-specific shRNA. Quantitative RT-PCR for CCR8 mRNA and IFAs to detect surface expression of CCR8 demonstrated RNA interference efficacy in TIME-CCR8i cells (Fig. 2B and C). Relative to TIME cells, TIME-CCR8i cells were greatly diminished in their ability to support vCCL-1, vCCL-2, and CCL1 signaling (Fig. 2D, top) but were unaltered in their support of signaling induced by non-CCR8 agonists CXCL1/GROα and VEGF (Fig. 2D, bottom).
Together, the data from the desensitization and CCR8 knockdown experiments demonstrated that vCCL-1 and vCCL-2, like CCL1, operate through CCR8 in endothelial cells and that other receptors are unlikely to be involved.
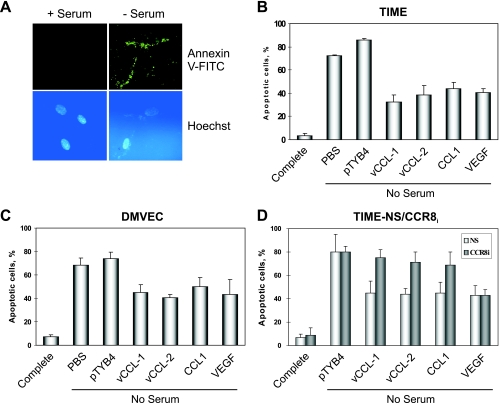
Viral chemokines protect endothelial cells from starvation-induced apoptosis.
In consideration of previously reported antiapoptotic activities of vCCL-1 and vCCL-2 on PEL and vCCL-1 on murine BW4157 thymoma cells (25, 26), the v-chemokines were tested for their abilities to protect endothelial cells also. Apoptosis in TIME and DMVEC cultures was induced by serum deprivation, and Annexin V-FITC staining (Fig. 3A) was used to detect apoptosis after 48 h in the presence and absence of chemokine supplementation. For TIME cells (Fig. 3B), the v-chemokines and CCL1 conferred protection of around 40 to 60% relative to the untreated (PBS) and pTYB4-treated controls, very similar to the level of protection obtained using VEGF, a known endothelial cell survival factor (19, 36). Significant protection of DMVEC also was mediated by each of the CCR8 agonists, and again, these levels were comparable to VEGF-mediated protection (Fig. 3C). CCR8-depleted TIME-CCR8i cells were refractory to the protective effects of vCCL-1, vCCL-2, and CCL1, but not VEGF, demonstrating that CCR8 was necessary for the prosurvival activities of the chemokines (Fig. 3D).
FIG. 3.
Viral chemokine-mediated protection of endothelial cells from starvation-induced apoptosis. (A) Examples of Annexin V-FITC staining to detect apoptosis in TIME cells deprived of serum for 48 h. (B) Quantified data from an experiment in which serum-starved cultures were supplemented with recombinant vCCL-1, vCCL-2, CCL1, or VEGF, or to which PBS or “pTYB4-extract” (negative controls) was added. (C) An analogous experiment was carried out in primary endothelial cells, DMVEC. (D) Similar analysis of TIME-CCR8i and TIME-NS (control) cells demonstrated the dependence on CCR8 for the protective effects of vCCL-1, vCCL-2, and CCL1, but not of non-CCR8 ligand VEGF. (Maximally effective chemokine and VEGF concentrations of 250 ng/ml and 10 ng/ml, respectively, were used for experiments B to D.)
Regulation of apoptotic pathways by v-chemokines.
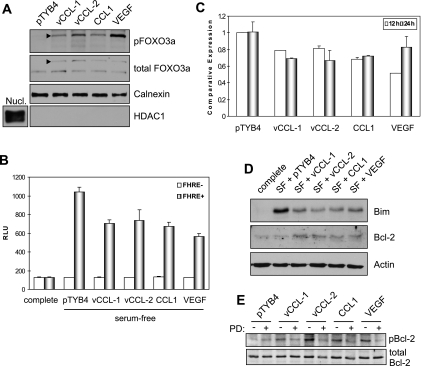
Several targets of AKT are involved in inhibiting apoptosis and promoting cell survival. These targets include the proapoptotic Bcl-2 family member Bad, caspase 9, GSK-3β, and the family of forkhead transcription factors that positively regulate the expression of proapoptotic proteins such as the BH3-only Bcl-2-family protein Bim (4, 8, 12, 14, 15). Phosphorylation of these proteins by AKT leads to their inactivation and to the promotion of cell survival. Because the forkhead transcription factor FOXO3a (FKHRL1) has been reported to be important in the regulation of endothelial cell survival (44), we investigated whether the v-chemokines could regulate FOXO3a phosphorylation in TIME cells. Serum-starved cells were treated with vCCL-1, vCCL-2, or CCL1 for 15 min, and Western analysis was used for detection of Ser-253-phosphorylated FOXO3a in cytoplasmic extracts, as the phosphorylated FOXOs localize in this compartment (4). Each of the chemokines, and VEGF, induced FOXO3a phosphorylation (Fig. 4A).
FIG. 4.
Antiapoptotic pathways induced by v-chemokines. (A) Western analysis of cytoplasmic extracts of serum-starved TIME cultures treated for 15 min with vCCL-1, vCCL-2, CCL1, VEGF, or pTYB4 (negative control) for serine-phosphorylated (inactive) FOXO3a (arrowhead). Nucl., nuclear extract. (B) Functional inactivation of FOXO transcription factors in response to the v-chemokines was confirmed by reporter assay, utilizing a lentivirus-delivered FOXO-responsive (FHRE) luciferase expression construction (or promoterless control). Cultures were serum starved for 12 h in the presence and absence of the CCR8 agonists before cell harvest. Parallel cultures were maintained in serum-supplemented medium (complete). (Data are from triplicate cultures; standard deviations from mean values are indicated.) (C) qRT-PCR determinations of Bim mRNA levels in serum-deprived TIME cultures in response to the v-chemokines, CCL1, and VEGF. Serum withdrawal and cytokine treatments were for 12 h and 24 h. (Triplicate cultures were used; standard deviations are indicated.) (D) Western analysis of Bim and Bcl-2 protein levels in serum-free (SF), cytokine-treated TIME cultures (24 h treatment). (E) Immunoblotting to investigate ERK targeting of Bcl-2 at serine-70. Phosphorylation of Bcl-2 in response to chemokine or VEGF treatment (10 min, following 12-h serum starvation) was sensitive to the ERK kinase (MEK) inhibitor PD98059 (PD) (added to cultures at 50 nM for 30 min prior to cytokine stimulation). (Chemokine and VEGF concentrations of 250 ng/ml and 10 ng/ml, respectively, were used in all experiments.)
Effects of the v-chemokines on FOXO activity were tested directly using FOXO-responsive reporter assays, utilizing a lentivirus vector for high-efficiency reporter cassette delivery. The results (Fig. 4B) confirmed inactivation of FOXO transcription factors in response to the v-chemokines, CCL1, and endothelial cell-protective VEGF, relative to cells treated with processed bacterial extract (pTYB4) lacking recombinant protein.
As Bim is regulated positively by FOXO3a, we undertook qRT-PCR experiments to determine changes in endogenous Bim mRNA expression in response to v-chemokine treatment. TIME cells were treated for 12 h or 24 h with each of the v-chemokines, CCL1, VEGF, or pTYB4-transformant sample (negative control), and cells were then harvested for mRNA preparation. Moderate reductions of Bim transcripts were identified in response to the cytokines, relative to controls (pTYB4). However, at present we cannot rule out involvement of mRNA destabilization, known to operate in response to IL-3 in Baf-3 cells (30). Western blotting revealed that each of the cytokines was able to repress Bim protein expression by at least twofold (Fig. 4D). The greater reductions in Bim protein expression relative to mRNA levels in response to cytokine treatment may indicate that posttranslational regulation, e.g., ERK-mediated Bim destabilization (24, 27), is relevant here.
Also analyzed for expression was the antiapoptotic protein Bcl-2, regulated positively by NF-κB, a target of AKT signaling that promotes NF-κB nuclear translocation and activation (31, 37). In contrast to Bim, Bcl-2 levels were increased by the CCR8 agonists and VEGF (Fig. 4D). Further investigation determined that Bcl-2 phosphorylation at Ser-70, associated with Bcl-2 activation, was induced by vCCL-1, vCCL-2, and CCL1 in a PD98059-sensitive (MEK [ERK kinase]-dependent) manner (Fig. 4E). ERK targeting of serine and threonine residues in this loop region of Bcl-2 has been reported to regulate Bcl-2 stability and activity (5, 7). Together, these data suggest that the antiapoptotic effects of the v-chemokines and CCL1, in addition to VEGF, are in part mediated via suppression of proapoptotic Bim and may also involve induction and activation of Bcl-2.
HHV-8 v-chemokines inhibit apoptosis induced by HHV-8 productive replication.
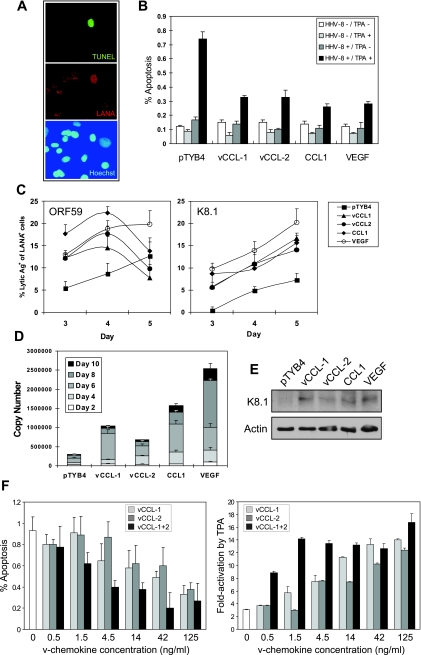
Relevant to potential roles of the v-chemokines in promoting virus replication via survival signaling, we tested their effects on apoptosis induced during lytic replication. TIME cells were infected with BCBL-1 PEL cell-derived virus, and lytic cycle replication was then induced with TPA. Apoptosis was monitored at day 6 postinduction by TUNEL staining in the absence and presence of vCCL-1, vCCL-2, and CCL1, with LANA costaining performed to identify infected cells. The results (Fig. 5A and B) revealed, firstly, that apoptosis was induced in a proportion of LANA-positive cells but not in uninfected, LANA-negative cells, and secondly, that chemokine treatment reduced significantly (around threefold) the frequency of induced apoptosis. The low frequencies of cells undergoing TPA-induced, HHV-8-dependent apoptosis are consistent with the typically very inefficient reactivation of HHV-8 from endothelial cells. TPA treatment of uninfected cells did not induce apoptosis, nor did treatment of uninfected or infected cultures with chemokine alone have any effect. These data demonstrate that v-chemokines inhibit lytic cycle-induced apoptosis, as they do apoptosis induced by serum starvation.
FIG. 5.
Chemokine modulation of lytic cycle-induced apoptosis and virus replication. (A) Example of IFA and TUNEL staining to identify infected (LANA+) and apoptotic cells in BCBL-1 virus-infected TIME cultures induced with TPA for 6 days. TUNEL staining was seen only in HHV-8-infected cells. (B) Quantified data from infected or uninfected HHV-8 cultures treated with control (pTYB4), v-chemokine, CCL1, and VEGF, uninduced or induced with TPA. (Assays were performed on triplicate cultures.) (C) Early (ORF59-specified) and late (K8.1-encoded) lytic antigen positivity among chemokine- or VEGF-treated TIME cells was determined by IFA at 3, 4, and 5 days post-TPA induction. (Data were derived from duplicate coverslips; deviations from the mean are indicated.) (D) Direct measurements by qPCR of cell-free, encapsidated (DNase I-resistant) viral DNA from TPA-treated cultures confirmed induction of virus productive replication by the chemokines and VEGF. Media were harvested for analysis every 2 days; separate and cumulative titers are presented. (Error bars represent standard deviations from mean values obtained from triplicate qPCR reactions per sample.) (E) Consistent with the qPCR data, amounts of K8.1 late antigen were higher at day-10 post-TPA treatment in the cytokine-treated cultures than in the pTYB4 control, as determined by Western analysis. (F) Dose-response experiments to determine active concentrations of the v-chemokines with respect to their individual and combined effects on virus production and cell survival. Cumulative virus production over 6 days of TPA treatment and apoptosis at day 6 were measured by qPCR and TUNEL assay, as before. (Data were derived from triplicate cultures; error bars indicate standard deviations from average values; multiple random fields were viewed to derive quantified TUNEL results.)
vCCL-1 and vCCL-2 promote HHV-8 lytic cycle.
To examine effects of the v-chemokines on virus replication, HHV-8 latently infected (LANA+) TIME cells were treated with TPA to induce lytic replication either in the absence or presence of the v-chemokines, CCL1, or VEGF, and cells were monitored by IFA for expression of virus lytic proteins encoded by ORF59 (early) and K8.1 (late). The numbers of cells expressing late lytic antigen (K8.1) were increased markedly (>2-fold) at all time points in the chemokine- and VEGF-treated cultures (Fig. 5C). Expression of early antigen (ORF59) was increased by cytokine treatment at days 3 and 4 but for the chemokine-supplemented cultures was similar to the untreated control on day 5, possibly indicative of accelerated kinetics of early lytic gene expression in response to the chemokines.
Further experiments were undertaken to investigate by qPCR the production of encapsidated (DNase I-resistant) viral genomes released into the medium over the course of 10 days post-TPA induction, allowing total virus yields to be determined. Consistent with the IFA results, we found markedly increased virus production in cells treated with the chemokines or VEGF relative to untreated controls (Fig. 5D). The cytokines had no effect on virus production in the absence of lytic induction by TPA (data not shown). In a separate experiment, Western blotting revealed higher cumulative amounts of late K8.1 virion glycoprotein produced in the cytokine-treated cultures (Fig. 5E), consistent with the qPCR data.
Lastly, experiments were undertaken to investigate the influence of v-chemokine concentration and potential cooperative effects of vCCL-1 and vCCL-2 on virus replication and lytic cycle-induced apoptosis. Different doses of vCCL-1 and vCCL-2 were added separately and in combination to TPA-treated HHV-8-infected TIME cultures, and virus production and apoptosis in infected cells were measured by qPCR and TUNEL/LANA costaining, respectively, as performed previously. The data (Fig. 5F) demonstrated clear dose-dependent effects of each of the v-chemokines on virus replication and apoptotic inhibition, with evidence of cooperativity at low concentrations. These results reflect the activity profiles of vCCL-1 and vCCL-2, individually and together, obtained in signal transduction assays (Fig. 1B).
Autocrine effects of v-chemokines.
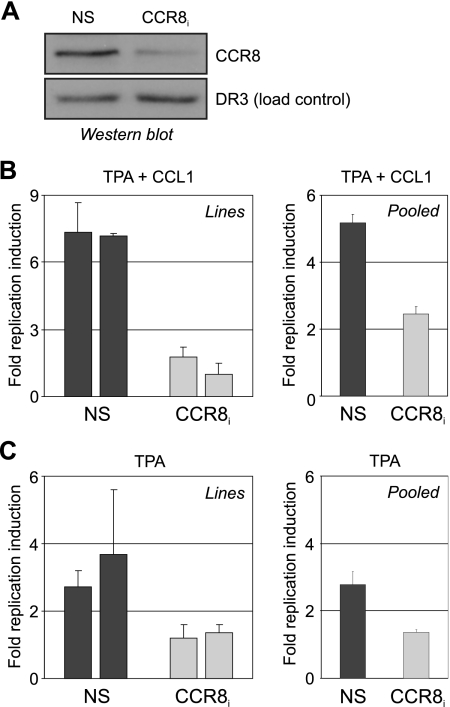
To determine whether endogenously produced HHV-8 chemokines could stimulate virus production via autocrine signaling, virus reactivation experiments were undertaken in TIME-CCR8i cell lines and “pooled” transformants (uncloned). This approach allowed assessment of both the functionality of normal, virus-produced concentrations of the v-chemokines and their effects on virus replication in the cells in which they were produced. CCR8 depletion in these experiments was functionally equivalent to a vCCL-1/vCCL-2 knockout virus. Western analysis of NS versus CCR8 shRNA-transduced cultures confirmed reduced CCR8 levels in the mixed cell population (Fig. 6A), similar to results obtained with CCR8 shRNA-expressing cell lines (Fig. 2B and C). CCR8i cells (“pooled” and lines) were resistant to chemokine-mediated promotion of virus replication upon lytic induction with TPA (for 3 days), confirming functional knockdown of CCR8 and paracrine signaling inhibition (Fig. 6B). Cultures treated with TPA alone, in the absence of exogenously added chemokines, revealed CCR8i-dependent differences, with CCR8-depleted cells showing markedly reduced virus production relative to NS-shRNA-transduced control cultures (Fig. 6C). As no induction of CCL1 was identified, by qRT-PCR, in TPA-treated (or TPA plus vCCL-1/2-treated) TIME cultures (results not shown), the data indicated that virus-produced vCCL-1 and vCCL-2 are able to promote virus replication in an autocrine manner.
FIG. 6.
CCR8 dependence and contribution of autocrine signaling to enhancement of virus productive replication by HHV-8 chemokines. (A) Western blot assay for verification of CCR8 depletion in unselected TIME cultures transduced at high efficiency with CCR8 shRNA lentivirus or the NS-shRNA lentivirus control. (B) Retrovirus-shRNA-transduced TIME-CCR8i cell lines (left) or pooled (unselected) lentivirus transformants (right) were compared with TIME-NS cultures (controls) for their abilities to respond to CCR8 agonist in replication-enhancing assays. Medium was harvested for qPCR analysis of encapsidated HHV-8 genomic DNA at 3 days post-lytic induction by TPA treatment. TIME-CCR8i cells were largely refractory to stimulation by CCL1 (250 ng/ml), demonstrating functional depletion of CCR8. (C) In the absence of exogenously added chemokine, TIME-NS cells (CCR8+), but not TIME-CCR8i cultures, were able to support significant TPA-induced HHV-8 virus production and release by 3 days posttreatment. The results indicate that endogenously produced vCCL-1 and/or vCCL-2 can enhance virus productive replication. (Data for panels A and B were derived from triplicate qPCR reactions per sample; standard deviations are shown.)
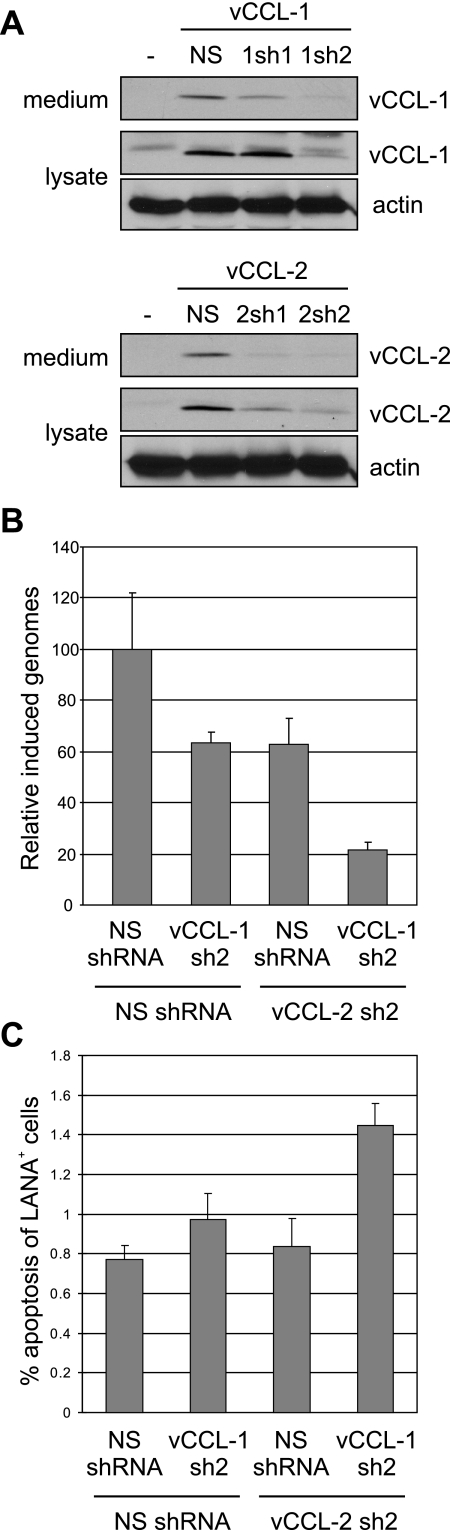
The combined and individual roles of vCCL-1 and vCCL-2 in virus replication via autocrine signaling were verified and investigated through the generation of shRNAs for vCCL-1 and vCCL-2 knockdown. Initially, candidate shRNAs were tested for efficacy in transfected HEK293T cells (Fig. 7A), and the most active for each target was subsequently utilized in functional assays in TIME cells. Single and double vCCL-depleted cultures were generated via transduction of cells with drug-selectable lentiviral vectors encoding either NS (control) or vCCL-1 shRNA followed (after drug selection) by secondary high-efficiency (>80%) transduction with GFP+ lentiviral vectors specifying NS or vCCL-2 shRNA. HHV-8 reactivation from these cultures measured by qPCR of encapsidated viral DNA identified individual and combined effects of the v-chemokines on virus yields following 6 days of TPA treatment (Fig. 6B). Single depletion of vCCL-1 and vCCL-2 led to reduced yields of virus (around 60% of control), but this effect was markedly enhanced by combined knockdown of vCCL-1 and vCCL-2, which diminished virus production by approximately 80% (Fig. 7B). TUNEL analysis for detection of apoptosis in these cultures revealed increased cell death in the double knockdown cells, although only modest effects of single vCCL depletion (Fig. 7C). We conclude that both vCCL-1 and vCCL-2 function to promote virus replication via autocrine signal transduction and that this may in part be mediated via promotion of cell survival.
FIG. 7.
Effects of viral chemokine depletion on virus productive replication. (A) Lentiviral-cloned shRNAs directed to vCCL-1 or vCCL-2 were tested for activity by cotransfection with v-chemokine expression vectors in HEK293T cells. Western analysis for detection of secreted and intracellular v-chemokines demonstrated efficacy of both of the vCCL-2 shRNAs and of vCCL-1 shRNA-2. (B) Selected shRNAs (2) were used for lentiviral-mediated single and double vCCL depletion in TIME cells (NS shRNA vectors provided negative controls) to determine the involvement of endogenously produced vCCL-1 and vCCL-2 on virus replication. Quantitative PCR of ultracentrifuge-pelleted, DNase I-resistant (encapsidated) HHV-8 DNA was undertaken to determine relative virus production from the various latently infected TIME cultures after 6 days of TPA treatment. (Data were derived from triplicate cultures; standard deviations from mean values are shown.) (C) Apoptosis in reactivated cultures, as determined by TUNEL assay. Data were derived from multiple random fields.
Bim induction in response to HHV-8 lytic replication.
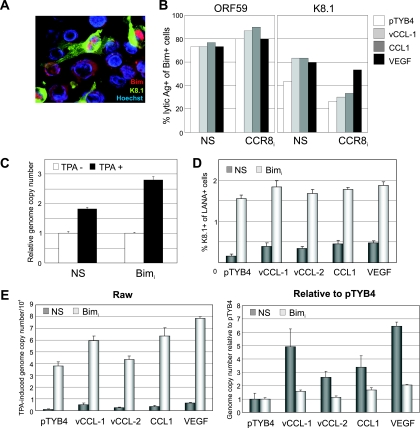
The possible involvement of Bim in virus-induced apoptosis was investigated by IFA to determine any correlation of Bim expression with virus-infected cells and, specifically, with those induced into lytic replication. Dual staining was undertaken to identify cells positive for Bim and either ORF59 (early) or K8.1 (late) antigens at 3 days post-TPA induction. An example of the IFA images obtained is shown for K8.1 plus Bim (Fig. 8A). A high proportion (73%) of Bim-positive cells also stained positively for ORF59 early antigen (Fig. 8B, left panel, NS). A lower percentage, 43%, costained for Bim and K8.1 late antigen (Fig. 8B, right panel). The data indicate that the induction of Bim expression is triggered by early events in the replicative cycle and that only a proportion of these cells progresses to full lytic replication (by day 3 post-TPA). Of significance was the finding that the proportion of Bim plus K8.1 costaining cells was increased from 43% to over 60% by vCCL-1 and CCL1 treatments of TIME-NS cells (Fig. 8B, right panel). TIME-CCR8i cultures had reduced numbers of Bim-positive cells expressing K8.1 antigen in the absence of chemokine and were, as expected, essentially unresponsive to the CCR8 agonists, although not to VEGF. Together, these IFA data demonstrate a tight correlation between Bim expression and lytic reactivation and indicate that v-chemokine signaling via CCR8 promotes lytic cycle progression in both autocrine and paracrine fashions.
FIG. 8.
Bim induction during lytic replication and influence on virus production. (A) Example of IFA staining for lytic antigen expression (late K8.1 glycoprotein) and Bim, showing costaining in a proportion of the cells. (B) Quantitation of early (ORF59) and late (K8.1) lytic antigen coexpression with Bim at 3 days post-TPA induction of the lytic cycle. There is a high correspondence (>73%) of ORF59 protein coexpression with Bim. K8.1/Bim coexpression is lower, especially in CCR8-depleted cells with or without chemokine, suggesting CCR8 signaling-dependent promotion of lytic cycle progression by both autocrine and paracrine mechanisms. (C) Measurements of HHV-8 production in retrovirus-shRNA-transduced Bim-depleted (Bimi) TIME cells and in NS-shRNA-transduced controls induced with TPA for 3 days. qPCR (performed in triplicate) was applied to ultracentrifuge-pelleted and DNase I-resistant (encapsidated) DNA. (D and E) K8.1 late lytic antigen expression (D) and virus productive replication (E) in Bim-depleted versus TIME-NS (control) cells after 6 days of TPA treatment in the absence or presence of added cytokines (250 ng/ml chemokines, 10 ng/ml VEGF). Raw (left) and adjusted (right) data are presented to show, respectively, TPA-induced genome copy numbers (background [-TPA] subtracted) and values relative to pTYB4 (negative control) for each culture type. (Data are from duplicate cultures; triplicate qPCRs [E] were performed on each culture.)
Bim suppression mimics proreplication functions of HHV-8 chemokines.
To correlate prosurvival activities of and Bim regulation by the v-chemokines with increased lytic replication induced by the CCR8 agonists, Bim-specific shRNA or NS-shRNA (negative control) was transduced by virus vector into TIME cells to generate (drug-selected) pooled cultures. These were then infected with BCBL-1-derived virus, and reactivation experiments were undertaken as before. Representative data from one such experiment are shown in Fig. 8C. Virus productive replication (at day 3) was increased in Bim-deficient cultures relative to the NS-shRNA controls, supporting the hypothesis that v-chemokine suppression of lytic cycle-induced Bim (and apoptosis) is involved centrally in v-chemokine-enhanced virus production. To provide further evidence of this mechanism of v-chemokine function, we repeated the experiment in the absence and presence of v-chemokines, CCL1, and VEGF. This time, analysis of viral antigen expression and virus production was analyzed after 6 days post-TPA, allowing a better assessment of the effects of the different conditions, most evident at around this time point (Fig. 5). IFA analysis of K8.1 expression at day 6 postinduction revealed that the percentages of HHV-8-infected (LANA+) cells expressing the late antigen were increased dramatically in the Bim-depleted cultures but, notably, the effects of the cytokines were reduced in these cells relative to TIME-NS controls (Fig. 8D). Reflecting these data, virus production was enhanced greatly but effects of the v-chemokines, CCL1, and VEGF were reduced in Bim-depleted cells relative to TIME-NS cultures (Fig. 8E). Together, the results indicate that the chemokines mediate their proreplication functions, at least in part, via Bim repression and that this enhances lytic cycle progression.
DISCUSSION
It is generally considered that functions of HHV-8 chemokines in HHV-8 productive replication include immune evasion; the chemokines are able to engage as agonists with Th2-expressed chemokine receptors and, in the case of vCCL-2, to block cellular chemokine signaling via a range of other chemokine receptors (reviewed in reference 35). The proangiogenic activities of each of the three HHV-8 chemokines (6, 47) indicate their possible contributions to virus dissemination and also, as a by-product, to virus-associated neoplasia. Under investigation here are the possible direct, autocrine activities of HHV-8 CCR8 agonists vCCL-1 and vCCL-2 to productive replication and their potential paracrine contributions to HHV-8 pathogenesis via prosurvival signaling. We have found that these viral chemokines, acting in biologically relevant endothelial cells, promote survival and enhance virus lytic replication in both autocrine and paracrine fashions.
HHV-8 encodes several proteins, both latent and lytic, that serve as survival factors for the infected cell. During latency, these are likely to be necessary for viral maintenance within the host by promoting long-term viability of infected cells. LANA and viral interferon regulatory factor 3/LANA2-inactivating interactions with p53 and the viral FLICE inhibitory protein activation of NF-κB are among latency functions that serve this role (18, 21, 39, 48). With regard to lytic replication, there are several prosurvival candidates. These include vBcl-2 (11, 40), calcium release- and caspase-inhibitory survivin-like ORF K7-encoded protein (17, 51), and p53-inhibtory viral interferon regulatory factor 1 (34, 41), all of which may serve to block lytic cycle-induced apoptosis, leading to enhanced virus production by delaying cell death long enough to allow completion of the productive replication cycle. However, similar activities of the viral chemokines are distinct because they are able to act in paracrine in addition to autocrine manners, thereby conferring protection to surrounding cells that are not undergoing lytic replication. This is of potential significance to virus-associated neoplasia, such as Kaposi's sarcoma, and provides a mechanism by which an HHV-8 lytic protein can cooperate with latency functions to promote pathogenesis or act on uninfected cells to do the same. As noted previously, native and KS endothelial cells express vCCL-1- and vCCL-2-responsive CCR8 (3, 22) and would therefore be subject to antiapoptotic activities of these lytic cell-released chemokines. During normal “nonpathogenic” infection, the v-chemokines conceivably might function to cooperate with latency functions in the maintenance of viral persistence.
Other lytic functions of HHV-8 have been speculated to play roles, via paracrine signaling, in virus-associated neoplasia, especially KS. Most notably, vGPCR induces KS-like endothelial lesions in transgenic mice but is expressed in only a small minority of cells within the tumors, and there is evidence that it cooperates with latency functions in such tumorigenesis (32). The mechanism of paracrine effects of vGPCR is via the induction of cellular secreted factors, such as VEGF, bFGF, IL-8, and IL-6, that are proangiogenic and/or proinflammatory and implicated in KS disease. However, the contribution of vGPCR to KS disease in the context of HHV-8 infection is unclear, not least because most cellular transcripts are destabilized and encoded protein expression is dramatically suppressed by the viral exonuclease (SOX) specified by ORF37 (20). The viral interleukin-6 homologue, vIL-6, also has been speculated to contribute to pathogenesis; like its cellular counterpart, it is both an angiogenic and an inflammatory cytokine. Unlike vGPCR, its contributions to pathogenesis would be independent of host shutoff functions. Thus, our work adds the viral chemokines vCCL-1 and vCCL-2 to the short list of HHV-8 lytic proteins that potentially contribute to neoplasia via paracrine signaling. Uniquely, however, we also provide evidence for enhancement of lytic replication by the v-chemokines, by a mechanism that is likely to be mediated in part via prosurvival functions.
The mechanisms of antiapoptotic signaling by vCCL-1 and vCCL-2 in response to starvation and lytic replication in endothelial cells are not fully resolved, but our data suggest that regulation of Bim is important. Firstly, Bim, a key Bcl-2-family protein in the regulation of apoptosis, is induced in both serum-starved and lytically infected endothelial cells; secondly, stress-induced Bim is repressed by the viral chemokines, to levels comparable with those seen in VEGF-treated cells; thirdly, knockdown of Bim expression both promotes virus replication and diminishes the proreplication activities of vCCL-1 and vCCL-2. In addition to Bim, Bcl-2 is targeted by v-chemokine signaling, and this may contribute to prevention of stress-induced apoptosis. We found that Bcl-2 levels were induced and ERK-targeted residue S70 phosphorylated in response to the v-chemokines. Mitogenic cytokine treatment has been shown to enhance Bcl-2 antiapoptotic activity via such phosphorylation (5). It is noteworthy that Bcl-2 is expressed at elevated levels in KS tumors, suggesting that Bcl-2 dysregulation may be significant for Kaposi's sarcomagenesis (33, 43, 49). Our data suggest a novel, paracrine mechanism by which vCCL-1 and vCCL-2 expressed from lytically infected cells might contribute to pathogenically relevant regulation of Bcl-2 via both induction and functional activation.
In summary, we have shown in this report that HHV-8 chemokines vCCL-1 and vCCL-2 inhibit stress-induced apoptosis in endothelial cells and induce virus productive replication. We provide evidence that both activities involve suppression of Bim, a proapoptotic Bcl-2 family protein that is induced by lytic replication. Thus, the v-chemokines have the potential to contribute to both virus replication and HHV-8 neoplasia, via autocrine and paracrine mechanisms.
Acknowledgments
We thank B. Chandran for providing ORF59 antibody and G. Sandford and D. Chen for their support and advice throughout the course of this project.
This work was supported by NIH grant PO1-CA113239. Y.B.C. is the recipient of a Korea Research Foundation Grant (KRF-2005-214-C00124).
Footnotes
Published ahead of print on 23 April 2008.
REFERENCES
- 1.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 934034-4043. [PubMed] [Google Scholar]
- 2.Aoki, Y., and G. Tosato. 1999. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood 944247-4254. [PubMed] [Google Scholar]
- 3.Bernardini, G., G. Spinetti, D. Ribatti, G. Camarda, L. Morbidelli, M. Ziche, A. Santoni, M. C. Capogrossi, and M. Napolitano. 2000. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood 964039-4045. [PubMed] [Google Scholar]
- 4.Biggs, W. H., III, J. Meisenhelder, T. Hunter, W. K. Cavenee, and K. C. Arden. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. USA 967421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagosklonny, M. V. 2001. Unwinding the loop of Bcl-2 phosphorylation. Leukemia 15869-874. [DOI] [PubMed] [Google Scholar]
- 6.Boshoff, C., Y. Endo, P. D. Collins, Y. Takeuchi, J. D. Reeves, V. L. Schweickart, M. A. Siani, T. Sasaki, T. J. Williams, P. W. Gray, P. S. Moore, Y. Chang, and R. A. Weiss. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278290-294. [DOI] [PubMed] [Google Scholar]
- 7.Breitschopf, K., J. Haendeler, P. Malchow, A. M. Zeiher, and S. Dimmeler. 2000. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: molecular characterization of the involved signaling pathway. Mol. Cell. Biol. 201886-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 2821318-1321. [DOI] [PubMed] [Google Scholar]
- 9.Chang, L. J., and A. K. Zaiss. 2003. Self-inactivating lentiviral vectors and a sensitive Cre-loxP reporter system. Methods Mol. Med. 76367-382. [DOI] [PubMed] [Google Scholar]
- 10.Chen, S., K. B. Bacon, L. Li, G. E. Garcia, Y. Xia, D. Lo, D. A. Thompson, M. A. Siani, T. Yamamoto, J. K. Harrison, and L. Feng. 1998. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J. Exp. Med. 188193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, E. H., J. Nicholas, D. S. Bellows, G. S. Hayward, H. G. Guo, M. S. Reitz, and J. M. Hardwick. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 94690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378785-789. [DOI] [PubMed] [Google Scholar]
- 13.Dairaghi, D. J., R. A. Fan, B. E. McMaster, M. R. Hanley, and T. J. Schall. 1999. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J. Biol. Chem. 27421569-21574. [DOI] [PubMed] [Google Scholar]
- 14.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91231-241. [DOI] [PubMed] [Google Scholar]
- 15.Dijkers, P. F., R. H. Medema, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2000. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 101201-1204. [DOI] [PubMed] [Google Scholar]
- 16.Endres, M. J., C. G. Garlisi, H. Xiao, L. Shan, and J. A. Hedrick. 1999. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 1891993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, P., J. Park, B. S. Lee, S. H. Lee, R. J. Bram, and J. U. Jung. 2002. Kaposi's sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J. Virol. 7611491-11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402889-894. [DOI] [PubMed] [Google Scholar]
- 19.Gerber, H. P., A. McMurtrey, J. Kowalski, M. Yan, B. A. Keyt, V. Dixit, and N. Ferrara. 1998. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 27330336-30343. [DOI] [PubMed] [Google Scholar]
- 20.Glaunsinger, B., and D. Ganem. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13713-723. [DOI] [PubMed] [Google Scholar]
- 21.Guasparri, I., S. A. Keller, and E. Cesarman. 2004. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 199993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Haque, N. S., J. T. Fallon, M. B. Taubman, and P. C. Harpel. 2001. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood 9739-45. [DOI] [PubMed] [Google Scholar]
- 23.Kledal, T. N., M. M. Rosenkilde, F. Coulin, G. Simmons, A. H. Johnsen, S. Alouani, C. A. Power, H. R. Luttichau, J. Gerstoft, P. R. Clapham, I. Clark-Lewis, T. N. Wells, and T. W. Schwartz. 1997. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science 2771656-1659. [DOI] [PubMed] [Google Scholar]
- 24.Ley, R., K. Balmanno, K. Hadfield, C. Weston, and S. J. Cook. 2003. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 27818811-18816. [DOI] [PubMed] [Google Scholar]
- 25.Liu, C., Y. Okruzhnov, H. Li, and J. Nicholas. 2001. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 7510933-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louahed, J., S. Struyf, J. B. Demoulin, M. Parmentier, J. Van Snick, J. Van Damme, and J. C. Renauld. 2003. CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/ CCL1 and vMIP-I. Eur. J. Immunol. 33494-501. [DOI] [PubMed] [Google Scholar]
- 27.Luciano, F., A. Jacquel, P. Colosetti, M. Herrant, S. Cagnol, G. Pages, and P. Auberger. 2003. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 226785-6793. [DOI] [PubMed] [Google Scholar]
- 28.Luttichau, H. R., A. H. Johnsen, J. Jurlander, M. M. Rosenkilde, and T. W. Schwartz. 2007. Kaposi sarcoma-associated herpes virus targets the lymphotactin receptor with both a broad spectrum antagonist vCCL2 and a highly selective and potent agonist vCCL3. J. Biol. Chem. 28217794-17805. [DOI] [PubMed] [Google Scholar]
- 29.Luttichau, H. R., I. C. Lewis, J. Gerstoft, and T. W. Schwartz. 2001. The herpesvirus 8-encoded chemokine vMIP-II, but not the poxvirus-encoded chemokine MC148, inhibits the CCR10 receptor. Eur. J. Immunol. 311217-1220. [DOI] [PubMed] [Google Scholar]
- 30.Matsui, H., H. Asou, and T. Inaba. 2007. Cytokines direct the regulation of Bim mRNA stability by heat-shock cognate protein 70. Mol. Cell 2599-112. [DOI] [PubMed] [Google Scholar]
- 31.Mayo, M. W., L. V. Madrid, S. D. Westerheide, D. R. Jones, X. J. Yuan, A. S. Baldwin, Jr., and Y. E. Whang. 2002. PTEN blocks tumor necrosis factor-induced NF-κB-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J. Biol. Chem. 27711116-11125. [DOI] [PubMed] [Google Scholar]
- 32.Montaner, S., A. Sodhi, A. Molinolo, T. H. Bugge, E. T. Sawai, Y. He, Y. Li, P. E. Ray, and J. S. Gutkind. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 323-36. [DOI] [PubMed] [Google Scholar]
- 33.Morris, C. B., R. Gendelman, A. J. Marrogi, M. Lu, J. M. Lockyer, W. Alperin-Lea, and B. Ensoli. 1996. Immunihistochemical detection of Bcl-2 in AIDS-associated and classical Kaposi's sarcoma. Am. J. Pathol. 1481055-1063. [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura, H., M. Li, J. Zarycki, and J. U. Jung. 2001. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 757572-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholas, J. 2005. Human gammaherpesvirus cytokines and chemokine receptors. J. Interferon Cytokine Res. 25373-383. [DOI] [PubMed] [Google Scholar]
- 36.Nor, J. E., J. Christensen, D. J. Mooney, and P. J. Polverini. 1999. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am. J. Pathol. 154375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 40182-85. [DOI] [PubMed] [Google Scholar]
- 38.Reiter, E., and R. J. Lefkowitz. 2006. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17159-165. [DOI] [PubMed] [Google Scholar]
- 39.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarid, R., T. Sato, R. A. Bohenzky, J. J. Russo, and Y. Chang. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 3293-298. [DOI] [PubMed] [Google Scholar]
- 41.Seo, T., J. Park, D. Lee, S. G. Hwang, and J. Choe. 2001. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 756193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan, L., X. Qiao, E. Oldham, D. Catron, H. Kaminski, D. Lundell, A. Zlotnik, E. Gustafson, and J. A. Hedrick. 2000. Identification of viral macrophage inflammatory protein (vMIP)-II as a ligand for GPR5/XCR1. Biochem. Biophys. Res. Commun. 268938-941. [DOI] [PubMed] [Google Scholar]
- 43.Simonart, T., C. Degraef, J. C. Noel, D. Fokan, L. Zhou, O. Pradier, M. Ducarme, L. Schandene, J. P. Van Vooren, D. Parent, and M. Heenen. 1998. Overexpression of Bcl-2 in Kaposi's sarcoma-derived cells. J. Investig. Dermatol. 111349-353. [DOI] [PubMed] [Google Scholar]
- 44.Skurk, C., H. Maatz, H. S. Kim, J. Yang, M. R. Abid, W. C. Aird, and K. Walsh. 2004. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J. Biol. Chem. 2791513-1525. [DOI] [PubMed] [Google Scholar]
- 45.Sozzani, S., W. Luini, G. Bianchi, P. Allavena, T. N. Wells, M. Napolitano, G. Bernardini, A. Vecchi, D. D'Ambrosio, D. Mazzeo, F. Sinigaglia, A. Santoni, E. Maggi, S. Romagnani, and A. Mantovani. 1998. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood 924036-4039. [PubMed] [Google Scholar]
- 46.Spinetti, G., G. Bernardini, G. Camarda, A. Mangoni, A. Santoni, M. C. Capogrossi, and M. Napolitano. 2003. The chemokine receptor CCR8 mediates rescue from dexamethasone-induced apoptosis via an ERK-dependent pathway. J. Leukoc. Biol. 73201-207. [DOI] [PubMed] [Google Scholar]
- 47.Stine, J. T., C. Wood, M. Hill, A. Epp, C. J. Raport, V. L. Schweickart, Y. Endo, T. Sasaki, G. Simmons, C. Boshoff, P. Clapham, Y. Chang, P. Moore, P. W. Gray, and D. Chantry. 2000. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood 951151-1157. [PubMed] [Google Scholar]
- 48.Sun, Q., S. Zachariah, and P. M. Chaudhary. 2003. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-kappaB activation. J. Biol. Chem. 27852437-52445. [DOI] [PubMed] [Google Scholar]
- 49.Suster, S., C. Fisher, and C. A. Moran. 1998. Expression of bcl-2 oncoprotein in benign and malignant spindle cell tumors of soft tissue, skin, serosal surfaces, and gastrointestinal tract. Am. J. Surg. Pathol. 22863-872. [DOI] [PubMed] [Google Scholar]
- 50.Venetsanakos, E., A. Mirza, C. Fanton, S. R. Romanov, T. Tlsty, and M. McMahon. 2002. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp. Cell Res. 27321-33. [DOI] [PubMed] [Google Scholar]
- 51.Wang, H. W., T. V. Sharp, A. Koumi, G. Koentges, and C. Boshoff. 2002. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi's sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 212602-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 766185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]