Abstract
A structurally novel candidate microbicide, PPCM, which is formed from the reaction of d,l-mandelic acid with sulfuric acid, provides activity against human immunodeficiency virus (HIV) and herpes simplex virus (HSV) and is not cytotoxic. The objectives of the current studies were to comprehensively evaluate the activity of PPCM in cell and explant cultures, explore the possibility of combining PPCM with HIV-specific reverse transcriptase inhibitors, and evaluate the efficacy of a formulated gel against genital herpes in a murine model. PPCM inhibited infection by laboratory and clinical R5 and X4 clade B and clade C HIV strains in cell culture. Ectocervical and endocervical tissue explants exposed to HIV-1BaL in the presence of PPCM were protected (50% inhibitory concentrations [IC50] of 3.9 μg/ml for ectocervix and 3.1 μg/ml for endocervix), and transfer of virus to target T cells via migratory cells was significantly impaired (IC50 of 35.7 μg/ml for ectocervix and 54.6 μg/ml for endocervix). The drug also blocked infection by cell-associated virus. Combinations of PPCM with UC-781 or PMPA in vitro exhibited additive anti-HIV activity. PPCM was incorporated into stable, low-pH gel formulations at concentrations of 0.4% and 4%. Both gels prevented genital herpesvirus infection in mice, even when virus was introduced in human seminal plasma. The abilities of PPCM to inhibit primary HIV isolates, reduce infection by cell-associated virus, and transfer of HIV from migratory to T cells, combined with the complete protection provided by formulated gel against genital herpes, indicate that this drug is an excellent candidate for inclusion in a combination microbicide and would provide protection against both HIV and HSV.
The human immunodeficiency virus (HIV) and herpes simplex virus (HSV) pandemics continue unabated and pose a major public health threat. HIV has emerged as primarily a sexually transmitted infection (STI), with women bearing a disproportionate burden of disease worldwide. Condoms are effective, but social barriers limit their use. Clinical trials demonstrate that male circumcision significantly reduces the risk for HIV infection among men but may enhance transmission to their female partners in the immediate postprocedure time period. Moreover, social acceptance may limit its impact (19, 43). While vaccines may hold great promise as a prevention strategy, obstacles to development of effective and safe vaccines persist, as highlighted by the lack of protection that HIV vaccines in phase III trials have afforded (5, 36). Therefore, topical microbicides, a female-controlled strategy for preventing HIV and other STIs, may be crucial in stemming the pandemic.
Development of a safe and effective vaginal microbicide has proven difficult. The premature closure of several narrow-spectrum-microbicide human clinical trials underscores the challenges in microbicide development. The increase in HIV infection among women using nonoxynol-9 (N-9), Savvy (33), or cellulose sulfate highlights the need for improved preclinical evaluation of both the safety and the efficacy of candidate microbicides (15, 41). Specifically, efficacy studies must include a more extensive evaluation of the activities of candidate drugs against HIV isolates representing multiple clades in cell and explant culture systems reflective of the genital tract. The therapeutic success of combination systemic antiretroviral therapy suggests that a similar strategy may be necessary for prevention and supports prioritizing evaluation of candidate microbicides in combination. Advantages of this approach include the ability to combine drugs that target HIV at different stages of the viral life cycle, thereby reducing the risk of resistance. Additionally, combinations could provide activity against other sexually transmitted pathogens that fuel the HIV epidemic, most notably HSV (12, 18). Recent results suggest that HSV suppressive therapy fails to protect HSV-infected, HIV-susceptible individuals from HIV acquisition, highlighting the important role that topical microbicides may play in conferring protection against multiple pathogens (6).
The current studies were designed to further evaluate a structurally novel candidate microbicide, PPCM, which is formed from the reaction of d,l-mandelic acid with sulfuric acid. This compound was initially designated sulfuric acid-modified mandelic acid and has since been named PPCM by Yaso Biotechnologies, Inc., on the basis of further clarification of the chemical structure and synthesis (D. P. Waller, unpublished results). Although it has no sulfations or sulfonations, earlier studies indicated that this compound blocks the binding of HIV and HSV to cells by targeting the envelope glycoproteins gp120 and gB-2, respectively, and inhibits viral entry (21, 47). The current studies were undertaken to further define the extent of anti-HIV activity of PPCM in cell and explant culture models and to evaluate the anti-HIV activity of PPCM combined in vitro with the candidate microbicide antiretroviral agents tenofovir (PMPA {9-[R-2-(phosphonylmethoxy)propyl]-adenine monohydrate}) (1) and UC-781 (2). Formulated PPCM gels (0.4% and 4%) were also evaluated for the ability to prevent genital herpes in a murine model.
MATERIALS AND METHODS
Microbicides.
N-9 was purchased from Sigma-Aldrich, Ltd. (Poole, United Kingdom), as were all reagents used, unless stated otherwise. PPCM was synthesized by researchers at the Program for the Topical Prevention and Conception of Disease (TOPCAD) at Rush University (Chicago, IL). The 0.4% and 4% PPCM gels (and the matched placebo gel) were provided by Yaso Biotechnologies, Inc. (Coppell, TX). PMPA (tenofovir) was obtained from Gilead Sciences, Inc. (Foster City, CA), and UC-781 was obtained from Biosyn, Inc. (Philadelphia, PA).
Cell and virus cultures.
Cells and viruses were obtained from the AIDS reagent project, National Institute for Biological Standards and Control, Potters Bar, United Kingdom, unless stated otherwise. ME-180 cells, a cervical epithelial cell line (obtained from American Type Culture Collection, Manassas, VA), and TZM-bl cells, a HeLa cell line stably expressing CD4 and CCR5 and used for quantitative analysis of HIV-1 with luciferase as a reporter, were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete DMEM) (34). Vero cells and the T-cell lines PM-1 and H9 were cultured in RPMI 1640 supplemented like DMEM (complete RPMI). Jurkat-Tat-CCR5 cells, a T-cell line which has been transfected with the HIV-1 tat gene and the CCR5 coreceptor, rendering it permissive to X4 and R5 viruses, were provided by Quentin Sattentau (Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom) and cultured in complete RPMI supplemented with 250 μg/ml hygromycin B (for Tat selection) and 500 μg/ml Geneticin (for CCR5 selection). Raji cells stably expressing DC-SIGN (Raji/DC-SIGN cells) and negative-control cells were provided by V. N. Kewal Ramani (National Cancer Institute, Frederick, MD) and were cultured in complete RPMI (supplemented with 500 μg/ml geneticin for the transfected cells) (44). All cells were passaged every 3 to 4 days and cultured in a humidified incubator containing 5% CO2.
The primary HIV-1 isolates belonging to clades B and C, a gift from John P. Moore (Weill Medical College, Cornell University, New York, NY), were grown in peripheral blood mononuclear cells as described previously (28). The laboratory-adapted HIV-1 strains HIV-1RF (X4-utilizing strain) and HIV-1BaL (R5-utilizing strain) were grown in PM-1 cells and stored at −180°C after filtration through 0.2-μm filters (Millipore, MA). HSV-2 (G), a well-characterized laboratory strain, was grown and its titer determined on Vero cells.
Culture of mucosal tissue and infection with HIV-1 and HSV-2.
Cervical mucosal tissue was collected from premenopausal women undergoing therapeutic hysterectomies at St. George's, St. Helier's, and Kingston Hospitals (London, United Kingdom), and male genital mucosal tissue was collected from patients undergoing gender reassignment surgery at Charing Cross Hospital, London, United Kingdom, all with informed consent. Tissue was cut into explants of approximately 3 by 3 by 2 mm prior to culture and infection with 105 50% tissue culture infective doses (TCID50) of HIV-1 or 107 PFU/explant HSV-2 as previously described for cervical tissue and more recently established for male genital tissue (20, 28; L. Fischetti, S. M. Barry, T. J. Hope, and R. J. Shattock, submitted for publication). Explants used for infection included the epithelial layer and underlying stromal tissue and were from the penile glans or the ectocervical or endocervical areas of the cervix. In brief, explants were exposed to cell-free or cell-associated HIV-1BaL for 2 h in the presence or absence of different concentrations of PPCM in 200 μl complete RPMI. Cell-associated virus consisted of chronically infected PM-1 cells (1 × 106 cells/explant), which had been pretreated with 200 μg/ml mitomycin C for 1 h. After removal of the viral inoculum and compound by extensive washing with phosphate-buffered saline (PBS), explants were incubated overnight at 37°C. Migratory cells (MCs) present in the overnight cultures were collected and cocultured with 4 × 104 PM-1 cells. Both tissue explants and cocultures were transferred to new 96-well plates and incubated at 37°C. At 10 days postinfection, culture supernatants were collected and viral replication was assessed using a p24 enzyme-linked immunosorbent assay (ELISA; p24 antigen capture assay kit; NCI-Frederick Cancer Research and Development Centre, AIDS Vaccine Program).
For HSV infection, explants were exposed to HSV-2 (G) (107 PFU/explant) in the presence of different amounts of PPCM in a total volume of 200 μl complete RPMI for 2 h. After removal of unbound virus and compound by extensive washing, explants were cultured for 7 days in complete RPMI and fed with fresh medium every 2 to 3 days. Culture supernatants were harvested at 7 days postinfection and assessed for infectious HSV-2 by a plaque assay with ME-180 cells.
Luciferase assay for detection of HIV-1 infection.
TZM-bl cells were exposed to 103 TCID50 of HIV-1 in the presence of various concentrations of PPCM alone or in combination with the reverse transcriptase (RT) inhibitors UC-781 and PMPA. Virus and drugs were left in culture for 48 h at 37°C and then removed by washing once with 200 μl PBS. Following cell lysis with luciferase cell culture lysis reagent (Promega, Southampton, United Kingdom), luciferase activity in lysates was determined as previously described (28).
Anti-HIV activity of PPCM in cell models.
Cell-free HIV-1 (BaL or RF) was captured to 96-well plates coated with a monoclonal antibody against human HLA-DR as previously described (17, 28). Unbound virus was removed by washing, and immobilized virus was treated with 100 μl of serial dilutions of PPCM for 1 h at 37°C. To assess direct virucidal activity, the compound was removed and the plates were washed four times with 200 μl PBS before addition of target cells (4 × 104 Jurkat-Tat-CCR5 cells per well). Alternatively, cells were added without removal of compound or, to assess cell protection, Jurkat-Tat-CCR5 cells (4 × 104 cells/well) were exposed to the same concentrations of compound in U-bottom 96-well plates and washed in the same way before transfer to plates with immobilized virus. Viral replication was assessed by measuring RT levels in culture supernatants at 7 days postinfection as described previously (34).
To evaluate the activity of PPCM against cell-associated HIV-1, PM-1 or H9 cells chronically infected with B clade isolate HIV-1RF, HIV-1IIIB, or HIV-1BaL or the clade C clinical isolate Za003/97 were treated with 200 μg/ml mitomycin C in complete medium for 1 h at 37°C. Infected cells were washed twice with 50 ml PBS, added to 96-well plates (500 cells/well), and incubated with various concentrations of PPCM for 1 h before addition of 4 × 104 Jurkat-Tat-CCR5 cells per well. Cocultures were incubated at 37°C for 5 days and culture supernatants collected and stored at −20°C prior to measurement of RT activity as before.
Blockade of HIV-1 binding to DC-SIGN-expressing cells and subsequent transfer to HIV targets.
To assess potential blockade of HIV-1 transmission to target cells via DC-SIGN, Raji cells (1 × 104 cells/well) expressing DC-SIGN (and negative-control cells) were exposed to different concentrations of PPCM or 200 μg/ml mannan for 1 h. HIV-1BaL or HIV-1RF (2 ng p24) was added and allowed to bind to the cells for 2 h. Nonbound virus and PPCM were washed from the cells three times with 200 μl PBS, and cells were cocultured with 8 × 104 Jurkat-Tat-CCR5 cells per well for 7 days. Culture supernatants were collected and stored at −20°C prior to measurement of RT activity. Alternatively, and to assess the abilities of compounds to prevent virus binding to DC-SIGN, Raji/DC-SIGN cells (1 × 105 cells/well) were exposed to different concentrations of compound or mannan for 1 h. Virus was added and allowed to bind as before, but this time, the amount of HIV-1RF was increased 10-fold. After 2 h of incubation, cells were washed as described above, and 100 μl PBS-1% Triton X-100 was added to each well to lyse the cells and any bound virus. Virus present in lysates was quantified by p24 ELISA.
Combination studies.
PPCM and RTIs were combined at 1:1 ratios in terms of 50% inhibitory concentrations (IC50) and treated as a single drug that was used at a range of dilutions in the viral inhibition assay (luciferase assay), in conformity with the model of Chou and Talalay (10). To determine the ratios of compound that should be used in these studies, IC50 values were determined following exposure of TZM-bl cells to each viral isolate in the presence of single drugs for 48 h. The PPCM/PMPA ratios were 1:13.213 and 1:0.015 for HIV-1RF and HIV-1BaL, respectively, whereas the PPCM/UC-781 ratios were 1:0.055 and 1:0.015 for the X4 and R5 viruses, respectively. In parallel, single drugs were tested at the same concentrations. The resulting inhibitory effects were calculated and introduced in Calcusyn software for determination of the combination index (CI) for each combination at different inhibitory concentrations (IC75 and IC90) (8).
HSV plaque assays.
ME-180 cells seeded in 24-well plates were exposed in duplicate to serial dilutions of culture supernatants from ectocervical explants infected with HSV-2 in the absence or presence of PPCM. After incubation for 1 h, the inoculum was removed and the cells were washed thrice and overlaid with fresh medium. Viral titers were determined by counting plaques at 48 h postinfection (22). Only wells in which the number of plaques ranged from 20 to 100 were used to calculate the viral titer.
Cytotoxicity studies.
To assess the potential toxicity of PPCM, human cervical cells (ME180), TZM-bl cells, Jurkat-Tat-CCR5 cells, TZM-bl cells, cervical stroma tissue, and penile glans tissue were exposed to different concentrations of compound for various durations that mimicked exposure during antiviral assays. The surfactant agent N-9 was used as a toxic control. Cell and tissue viability was determined by measuring tetrazolium salt (MTT) cleavage into a blue-colored product (formazan) by viable cells (39). Cells or explants were incubated with 100 or 200 μl complete RPMI (0.5 mg/ml)-MTT at 37°C for 3 h, respectively. The formazan salts were solubilized by addition of 100 μl 20% sodium dodecyl sulfate in 1:1 H2O-N,N-dimethylformamide (cells) or 1 ml methanol (explants), and viability was determined by measuring the optical density at 570 nm (690-nm reference) in a Synergy-HT plate reader. For tissue studies, this value was corrected for explant dry weight.
Murine studies.
Five days prior to infection, mice were treated with 2 mg/ml Depo-Provera. On day 0, the mice were treated with 30 μl of 0.4% or 4% PPCM gel or a matched placebo gel 15 min prior to challenge with 20 μl of HSV-2 (G) (1 × 105 PFU) diluted in PBS or in pooled human seminal plasma obtained from males at low risk for STI (32). Mice were monitored for signs of disease for 14 days postinfection on a 0- to 4-point scale: 0, no apparent infection; 1, slight redness of the vagina; 2, moderate redness and swelling of the vagina and surrounding tissue; 3, severe redness, swelling, and hair loss of the genital and surrounding tissues; and 4, genital ulceration with severe redness, swelling, and hair loss of the genital and surrounding tissues. Mice reaching stage 4 genital disease or exhibiting neurologic signs (hind limb paralysis) were euthanized.
RESULTS
PPCM inhibits HIV-1 infection in cell culture.
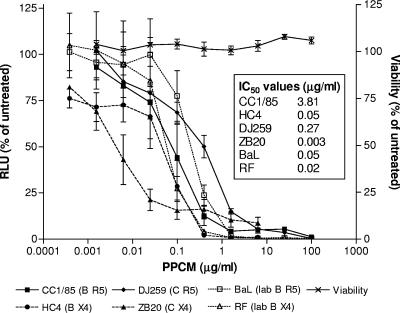
PPCM prevents infection of TZM-bl indicator cells by laboratory-adapted and primary clade C and B HIV isolates (Fig. 1). At a concentration of 100 μg/ml, a concentration that should be readily found in genital tract secretions following application of either a 0.4% or a 4% PPCM formulation (24), PPCM completely blocked infection by all isolates tested. No cytotoxicity was observed at any concentration tested.
FIG. 1.
PPCM inhibits HIV infection in cell culture. TZM-bl cells were infected with 103 TCID50 of each of the indicated HIV-1 strains in the presence or absence of various concentrations of PPCM. Virus and drug were left in culture for 48 h at 37°C, and infectivity was monitored by a luciferase assay. Cell viability was determined by an MTT assay following exposure to drug for 48 h. Results are means ± standard errors of the means obtained from three experiments conducted in triplicate. RLU, relative luciferase units.
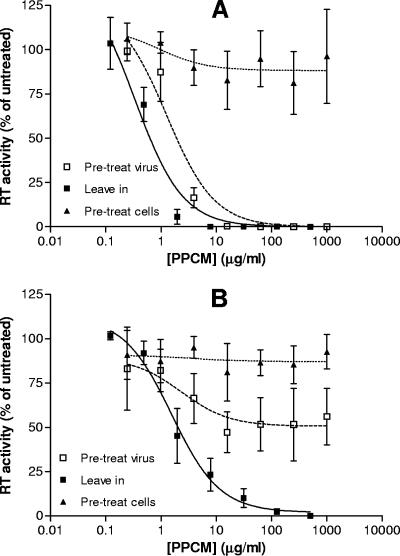
Additional in vitro studies were conducted to further define the mechanism of anti-HIV activity. Treatment of immobilized HIV-1RF (X4) with 15 μg/ml PPCM for 1 hour prior to addition of target cells was sufficient to completely inactivate viral particles before contact with target cells (IC50 = 1.82 ± 0.5 μg/ml), whereas when the compound was present for the duration of the assay, less than 8 μg/ml was needed to achieve complete inhibition (IC50 = 0.63 ± 0.3 μg/ml) (Fig. 2A). In contrast, pretreatment of HIV-1BaL (R5) with 1 mg/ml PPCM did not completely inactivate virions (IC50 > 1 mg/ml) (Fig. 2B). Nevertheless, when the compound was left in culture, a mean IC50 of 2.02 ± 0.5 μg/ml was obtained, indicating that although direct inactivation of the R5 virus is not readily achieved, the compound is still very effective at blocking attachment and/or fusion to target T cells. Little antiviral activity was observed when the target T cells were treated with PPCM and then washed to remove drug prior to viral exposure, suggesting that the drug needs to be present at the time of viral exposure. These data are consistent with earlier studies indicating that the drug primarily targets the virus and has greater antiviral activity against X4 than against R5 viruses (21).
FIG. 2.
PPCM targets HIV to prevent infection. The effects of PPCM on HIV-1RF (A) or HIV-1BaL (B) infectivity were compared under three different conditions: (i) presence of drug throughout the experiment (leave in), (ii) pretreatment of virus followed by removal of drug by washing (pre-treat virus), or (iii) treatment of target T cells with PPCM and then washing to remove drug prior to viral exposure (pre-treat cells). Results are presented as percent mean RT activities relative to levels for untreated cells and are means ± standard errors of the means obtained from four experiments conducted in six replicates.
PPCM blocks infection of target cells by cell-associated HIV-1.
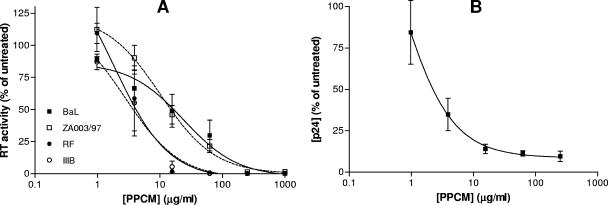
Both cell-free and cell-associated viruses may contribute to sexual transmission. To evaluate the activity of PPCM against cell-associated HIV-1, PM-1 or H9 cells chronically infected with B clade isolate HIV-1RF, HIV-1IIIB, or HIV-1BaL or the clade C clinical isolate Za003/97 were incubated with various concentrations of PPCM for 1 h and then cocultured with Jurkat-Tat-CCR5 cells. PPCM completely blocked infection by cell-associated virus, although greater activity was observed for X4 than for R5 strains (the IC50 values observed for BaL, Za008/93, RF, and IIIB were 7.16, 6.73, 2.32, and 1.90 μg/ml, respectively) (Fig. 3A). Furthermore, PPCM potently inhibited infection when ectocervical explants were challenged with cell-associated virus (mitomycin-treated PM-1 cells chronically infected with HIV-1BaL) in the presence of drug (Fig. 3B).
FIG. 3.
PPCM prevents infection by cell-associated HIV-1. (A) Cells chronically infected with different isolates of HIV-1 were treated with 200 μg/ml mitomycin C for 1 h at 37°C and with various concentrations of PPCM 1 h before addition of target Jurkat-Tat-CCR5 cells. Supernatants were collected at 5 days postinfection, and viral replication was assessed by determining RT activity. Results are expressed as percent RT activities relative to virus control levels and are means ± standard errors of the means for three independent experiments in which each condition was tested in six replicates. (B) Mitomycin-treated, infected T cells (PM-1) were added to ectocervical explants in the presence of PPCM. Cells and drug were removed by extensive washing after 2 h of incubation, and explants were cultured in complete medium for 10 days. Fifty percent of the culture medium was replaced every 2 to 3 days, and culture supernatants were analyzed for p24 content by ELISA. Results are means ± standard errors of the means for two independent experiments with different donors, and each condition was tested in triplicate.
PPCM inhibits HIV-1 infection of human ectocervical, endocervical, and penile tissues at concentrations that showed no tissue toxicity.
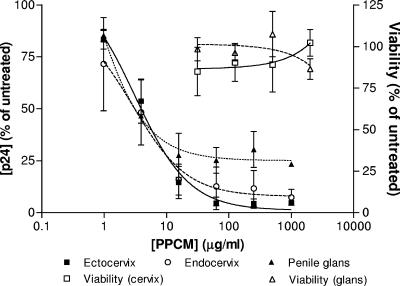
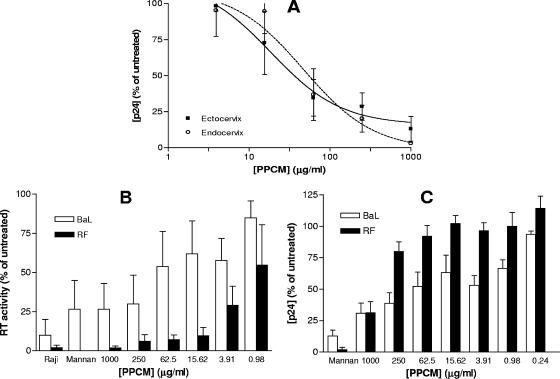
Explant cultures may provide a more rigorous evaluation of the antiviral activity of microbicides and offer the opportunity to examine the effects of the drugs on multiple cell types. As men will be exposed to microbicides and these products have the potential to be used by both women and men, it is important to also evaluate efficacy and safety in penile tissue. Ectocervical, endocervical, and penile explants were cultured in a nonpolarized manner and exposed to the R5 virus HIV-1BaL for 2 h in the presence or absence of a range of PPCM concentrations. An R5 isolate was selected for theses studies because R5 isolates predominate following sexual transmission (48). The compound inhibited infection of all three types of tissue in a dose-dependent manner (Fig. 4), with very similar IC50 values (3.9 μg/ml for ectocervix, 3.1 μg/ml for endocervix, and 3.1 μg/ml for penile glans). Notably, complete protection was not achieved in the penile model. No toxicity was observed on any of the tissues.
FIG. 4.
PPCM inhibits infection of human mucosal tissue. Ectocervical (squares, continuous line), endocervical (circles, dashed line), and penile (triangles, dotted line) explants were exposed to HIV-1BaL in the presence or absence of various concentrations of PPCM. Virus and PPCM were removed by extensive washing after 2 h. Following overnight incubation, explants were transferred to a new plate and cultured for 10 days. Fifty percent of the culture medium was replaced every 2 to 3 days, and culture supernatants were analyzed for p24 content by ELISA. Toxicity was assessed by an MTT assay following 24 h of exposure. Results are means ± standard errors of the means for three independent experiments (two for penile tissue) with different donors. Each condition was tested in triplicate.
PPCM prevents transfer of HIV from MCs to T cells.
MCs, which include immature dendritic cells (DCs) located in the epithelium and subepithelium have been previously described and play a critical role in the transmission of HIV-1 to target T cells in lymphoid tissue (23). Therefore, we examined the ability of PPCM to block the transmission of HIV-1BaL to permissive PM-1 cells via MCs emigrating from cervical explants that had been infected with HIV in the presence of PPCM (Fig. 5A). Transmission via cells migrating from ectocervix was greatly reduced, with an IC50 of 35.7 μg/ml; however, slightly higher concentrations of drug were needed to block transfer of virus from MCs emanating from endocervical explants, with an IC50 of 54.6 μg/ml.
FIG. 5.
PPCM inhibits transfer of HIV-1 via DC-SIGN and cervical MCs. (A) Ectocervical (squares, continuous line) and endocervical (circles, dashed line) explants were exposed to HIV-1BaL in the presence or absence of various concentrations of PPCM. Virus and PPCM were removed by extensive washing after 2 h. Following overnight incubation, MCs were cocultured with 4 × 104 target T cells (PM-1) for 10 days. Culture supernatants were analyzed for p24 content by ELISA, and results are means ± standard errors of the means for three independent experiments with different donors. Each condition was tested in triplicate. (B and C) Raji/DC-SIGN cells were treated with various concentrations of PPCM or mannan (200 μg/ml) for 1 h before addition of HIV-1BaL (open bars) or HIV-1RF (solid bars). Cells were extensively washed after 2 h of incubation, and then, either the target Jurkat-Tat-CCR5 cells were added in order to amplify any transmission of virus, cultured for 7 days, and assessed for viral replication by determining RT activity (B) or the bound virus was lysed immediately and quantified by p24 ELISA (C). The amount of HIV-1RF added for the binding assay was 10-fold that of HIV-1BaL in order to achieve similar levels of bound virus in the absence of PPCM. Results are expressed as percent RT activities (B) or p24 antigen (C) relative to levels for the control, where no compound was added, and are means ± standard errors of the means for three independent experiments where each condition was tested in six replicates.
These observations could reflect the ability of PPCM to directly block binding of HIV to DCs or the ability to prevent the transfer of hijacked virus to target cells. To further evaluate this, we took advantage of a cell culture system with the B-cell-transfected cell line Raji/DC-SIGN. PPCM greatly reduced the transmission of HIV-1RF, but not HIV-1BaL, to Jurkat-Tat-CCR5 cells (Fig. 5B). Notably, PPCM had only modest inhibitory effects on capture of HIV-1RF by the Raji/DC-SIGN cells, suggesting that the compound rendered the bound virus incapable of being transferred, rather than competitively inhibiting binding of HIV to DC-SIGN (Fig. 5C). These data are consistent with a recent study demonstrating that transmission of HIV-1BaL via DCs was not completely blocked unless PPCM was present at the time of transfer to target cells (7).
Anti-HIV activity of PPCM in combination with UC-781 or PMPA.
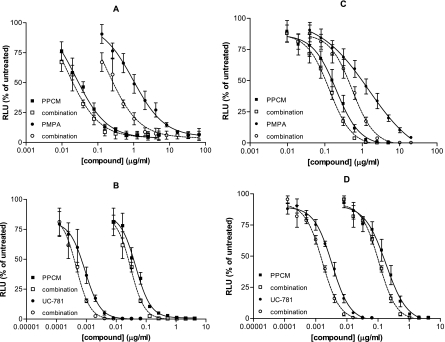
The therapeutic success of combination antiretroviral therapy systemically suggests that a similar approach may be necessary for effective microbicide development. Combining vaginal microbicides could inhibit HIV infection at multiple steps and reduce the risk of selection for resistant isolates. The optimal in vitro assays for evaluating combinations are not known. The multiple-drug effect analysis of Chou, based on the median-effect principle and the isobologram technique, has been used to analyze combined-drug effects, but for this model to be applicable, the drug ratio must be fixed (8, 10). This method plots dose-effect curves for each drug and for multiply diluted, fixed-ratio combinations of drug by using the median-effect equation. Using this approach, we exposed TZM-bl cells to HIV-1BaL or HIV-1RF in the presence of PPCM alone or combined with UC-781 and PMPA at fixed ratios as described above. Synergy was obtained when PPCM and PMPA were combined at concentrations that inhibited 75% and 90% of both R5 and X4 viruses in culture. However, when PPCM was combined with UC-781, we observed additive activity, and there was a suggestion of antagonism when the two drugs were combined at concentrations that inhibited 75% of the R5 isolate (Fig. 6 and Table 1).
FIG. 6.
PPCM inhibits HIV-1 infection in combination with RT inhibitors. TZM-bl indicator cells were exposed to HIV-1RF (A and B) or HIV-1BaL (C and D) in the presence or absence of various concentrations of PPCM alone or combined with different amounts of the RT inhibitors PMPA (A and C) and UC-781 (B and D). Virus and drug were left in culture for 48 h at 37°C, and infectivity was monitored by a luciferase assay. The effective concentrations for inhibition of HIV-1 replication by a compound alone and in combination with another compound are plotted in two curves for each drug. The shift between the two curves represents the dose reduction in the compound that can be used to achieve the effect of the single drug. Results are means ± standard errors of the means obtained from three independent experiments.
TABLE 1.
CIs obtained for combination of PPCM with UC-781 and PMPAa
| Combination | CI for:
|
|||
|---|---|---|---|---|
| HIV-1RF
|
HIV-1BaL
|
|||
| IC75 | IC90 | IC75 | IC90 | |
| PPCM-UC-781 | 1.03 | 0.78 | 1.15 | 1.06 |
| PPCM-PMPA | 0.91 | 0.83 | 0.72 | 0.52 |
TZM-bl cells were exposed to HIV-1 in the presence of PPCM alone or in combination with UC-781 or PMPA at a fixed ratio and a range of concentrations. Percent inhibition values were calculated relative to the control value for each concentration tested and were used for a computerized calculation of a CI by use of Calcusyn software. The calculation was based on the slope of the median-effect plot, which signifies the shape of the dose-effect curve, and the x intercept of the plot, which signifies the potency of each compound and each combination. CIs of <0.9 indicate synergy (i.e., greater than the additive effect expected when two agents are combined), CIs of 0.9 to 1.1 indicate nearly additive effects, and a CI of >1.1 indicates antagonism (i.e., less than the expected additive effect).
PPCM gel protects mice from genital herpes in both the presence and the absence of semen.
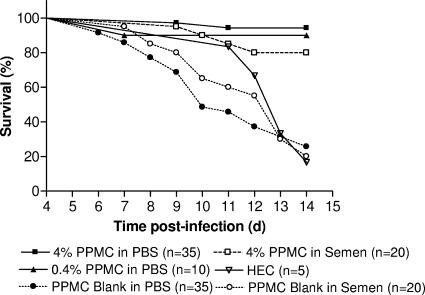
Prior studies have demonstrated that PPCM inhibits HSV infection primarily by binding to the viral envelope and inhibiting viral binding and entry (21). We have extended these in vitro observations and demonstrated that PPCM also blocks HSV infection of cervical explant cultures (Fig. 7) and in a murine model of genital herpes infection (Fig. 8). A stable, acid-buffered (pH 4.5 to 4.8) gel containing PPCM at two different concentrations, 0.4% and 4%, was produced and evaluated for efficacy in the murine model. Both active gels provided significant protection against HSV-2, with 94% survival and no signs of disease in 33/35 of the mice treated with a single dose of 4% gel. Control gel, which consisted of the GRAS excipients used in the active gel, protected only 9/35 (26%) of mice (Fig. 8). Moreover, the 4% gel retained activity when virus was introduced diluted in human semen and protected 16/20 (80%) mice. These findings differentiate PPCM from PRO 2000 or cellulose sulfate, as both of these drugs showed significant reductions in anti-HSV activity when virus was introduced in semen (32).
FIG. 7.
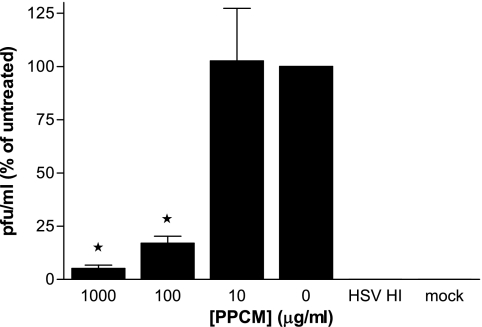
PPCM prevents HSV infection of cervical explants. Cervical explant cultures were exposed to 107 PFU/explant HSV-2 (G) in the absence or presence of the indicated concentration of drug. Heat-inactivated virus (HSV HI) was used as a control for input virus. Supernatants were collected at 7 days postinfection and assayed for HSV by plaque assays on ME180 cells. Results are presented as percentages of PFU formed in the presence of drug relative to the number of PFU formed in the presence of medium alone and are means ± standard errors of the means obtained from three experiments conducted in triplicate. Asterisks indicate statistical significance (P < 0.05).
FIG. 8.
PPCM protects mice challenged vaginally with HSV-2. Female BALB/c mice were pretreated with 30 μl of either 0.4% PPMC gel, 4% PPMC gel, or matched placebo gel and then 15 min later inoculated with 20 μl (1 × 105 PFU; 90% lethal dose) of HSV-2 (G) diluted in either PBS or semen. Results show survival rates pooled from four independent experiments (n = 5 to 10 mice/group/experiment). The 4% PPMC gel significantly protected the mice when virus was delivered in both PBS and semen (P < 0.0001 and P < 0.0004, respectively; log rank test). The 0.4% PPMC gel also afforded significant protection (P < 0.002). d, days.
DISCUSSION
Development of topical microbicide combinations that target both HIV and HSV may prove a powerful strategy for reducing HIV as epidemiological studies consistently demonstrate synergy between these two pathogens. This is particularly important in light of recent data indicating that acyclovir suppressive therapy does not protect against HIV acquisition in HSV-positive individuals at high risk for acquisition of HIV (6). PPCM is a structurally unique compound active against both viruses. PPCM inhibits primary clade B and clade C isolates at concentrations that would be predicted to be present in the genital tract following application of either a 0.4 or a 4% gel. For example, in a recent study, we found that the concentrations of PRO 2000 found in cervicovaginal lavage fluid 1 hour following application of a 0.5% gel ranged from ∼100 to 300 μg/ml (24). Moreover, in a different study, 25 μg/ml PRO 2000 was detected in cervicovaginal lavage samples collected 12 h following application of 4% gel (24, 26) The in vitro activity against clade C isolates is significant as clade C remains the most prevalent HIV strain worldwide and in Central and South Africa. Few published studies have documented the antiviral activity of microbicides against non-clade B isolates prior to the initiation of clinical trials, and in vitro studies have shown that Carraguard displays little or no activity against clade C isolates (14, 16, 27, 28). This lack of activity in vitro may have contributed to the lack of efficacy in the recently completed phase III Carraguard trial (E. Johansson, presented at the Microbicides 2008 conference, New Delhi, India, 26 February 2008). These disappointing results highlight the importance of extensive preclinical evaluation of candidate microbicides against HIV isolates from multiple clades.
Consistent with other anionic inhibitors of viral entry, PPCM is more active against X4 than against R5 isolates under each experimental system evaluated. This presumably reflects the ability of the anionic drug to bind irreversibly with the greater cationic charges in the V3 loop of gp120 in X4 than in R5 viruses (29, 38). In addition, it has been reported that polyanions also bind to the CD4-induced coreceptor binding site (42). This may explain why compounds belonging to this class inhibit R5 viruses more effectively when present at the time of attachment to the target cell. This differential activity suggests that X4 isolates could be inactivated by anionic polymers within the vaginal lumen, while R5 virus would require the compound to reach target cells within the mucosa with efficiency equal to that of the virus itself. However, despite the higher concentrations required, PPCM provided substantial protection against R5 viruses, particularly when the drug was present throughout the experiment and, most importantly, in the human explant model.
Early algorithms for preclinical assessment of candidate microbicides focused primarily on in vitro cell culture assays with cell-free virus. However, infection also may be initiated by cell-associated virus as well as by infected cells (25, 35). Similarly, successful sexual transmission of HIV-1 requires that the virus gain access to immune cells permissive for infection. MCs, which include immature DCs located in the genital tract epithelium and subepithelium, play a critical role in the transmission by sequestering the virus and then transferring HIV-1 to target T cells in lymphoid tissue (31). The current work demonstrates that PPCM inhibits infection by cell-associated virus and significantly reduces dissemination by MCs in explant culture systems. This reduction in MC-mediated transmission of HIV-1BaL was greater than that observed in a cell culture system where little protection was observed when DC-SIGN-expressing Raji cells were used, suggesting that the former results from inhibition of both cis- and trans-infection. Additionally, ongoing studies from our laboratory demonstrate that PPCM also blocks binding of HIV to human cervical epithelial cells and prevents subsequent transfer of the bound virus to T cells (P. M. M. Mesquita and B. C. Herold, unpublished results). Several studies have demonstrated that epithelial cells are capable of sequestering large amounts of HIV particles, possibly through engagement of syndecan proteoglycans, and that the sequestered virions remain infectious for days and can then be effectively transferred to immune target cells (3, 13, 37, 45). Together, these findings suggest that PPCM should be active in reducing sexual transmission of HIV.
A truly safe and effective microbicide is likely to require a combination of drugs that target different steps in the HIV life cycle and provide protection against other STIs known to facilitate HIV infection. The advantages of combinations have led to a change in oral preexposure prophylaxis trials, focusing on treatment with Truvada (a fixed dose combination of tenofovir disoproxil fumarate and emtricitabine) rather than monotherapy with tenofovir disoproxil fumarate alone. Whether resistance will be as great a problem with topically delivered microbicides as it is with oral therapy is not yet known, but there is a clear scientific rationale in support of a combination strategy. First, there is the potential for transmission of resistant HIV strains that may overcome a microbicide consisting of a single antiretroviral drug. Approximately 8 to 20% of all new HIV infections in North America and Western Europe are attributable to viruses that contain at least one drug resistance-associated mutation, and it is likely that this will increase over time in both developed and developing countries (40). An additional risk in the use of a single-drug microbicide is the potential for selection of drug resistance by a woman who uses an antiretroviral drug-based microbicide without knowing that she is infected or by an HIV-infected woman who uses a microbicide in an effort to protect her partner. Another distinct advantage of combination microbicides is the inclusion of a second drug that also targets other STIs, particularly HSV. Epidemiological studies consistently demonstrate a strong link between HSV-2 infection and the risk for HIV acquisition and transmission (18). The prevalence of HSV-2 infection among Africans with HIV ranges from 50 to 90% (30). Asymptomatic shedding is common and is associated with both a higher frequency and a larger amount of HIV-1 in genital secretions (30). In addition to the effect of HSV on HIV replication, genital herpes may facilitate HIV acquisition by disrupting the epithelial barrier, thereby increasing exposure of target cells to virus. Both clinical and subclinical rates of HSV-2 shedding are associated with the influx of activated CD4+ T cells into the genital mucosa (11).
Despite the clear scientific rationale for a combination approach to microbicides, development has been slow, in part because of the complexities of intellectual property conflicts and regulatory requirements. Recent efforts by the FDA to expedite the development of microbicide combinations include the requirement of limited bridging studies for cases where one of the active agents of a combination is being clinically evaluated as a microbicide. This should significantly accelerate the advancement of candidate combinations.
In the current studies, we demonstrated synergistic activity for PPCM combined with PMPA against R5 and X4 virus and at least additive activity for PPCM combined with UC781. It should be emphasized that the optimal in vitro assay for evaluating combinations is not established and in vitro synergy or additive activity is not a prerequisite for advancing a microbicide combination; combinations that are indifferent in vitro may prove beneficial in the clinical setting, where other factors, such as drug-resistant isolates, play a role.
Experience with systemic antiretroviral therapy suggests that PMPA induces less NRTI resistance than other drugs in its class, although a recent study found that HIV-1 subtype C viruses rapidly develop K65R resistance to PMPA in cell culture (4). Whether repeated exposure to vaginal PMPA could select for resistant viruses in women who use the gel and are HIV positive is not known. However, a combination of PMPA with PPCM could overcome this problem by blocking local HIV infection at distinct steps, thus decreasing the likelihood for selection of resistant viruses.
The anti-HSV and contraceptive activities of PPCM provide an additional benefit for a combination microbicides, as both UC-781 and PMPA are HIV specific and provide no contraception (46). Importantly, formulated PPCM protected mice from genital herpes, even when virus was introduced diluted in seminal plasma. We recently found that human seminal plasma interferes with the activity of PRO 2000 and cellulose sulfate, increasing by 100-fold the concentration required to inhibit 90% of the infection in the cell culture. The interference translated in vivo into a loss in protection in a murine model. The 2% PRO 2000 gel protected 100% of mice challenged intravaginally with HSV introduced in PBS, whereas only 55% of the mice were protected when virus was introduced in seminal plasma (32).
PPCM is not cytotoxic in vitro against cervical epithelial or immune cells (9, 21) and shows no cytotoxicity when cervical or penile glans explant tissue is used. PPCM gel is not cytotoxic toward lactobacilli, is not mutagenic, has low acute oral toxicity, and is safe in the rabbit vaginal irritation assay (47). Together, these studies support further development of PPCM, preferably in combination with an RT inhibitor, such as PMPA, or other HIV-specific drugs, as a topical microbicide.
Acknowledgments
This work was supported by grants from the National Institutes of Health, HD41763 (R.J.S. and B.C.H.) and R41 AI069659 (B.C.H.).
We acknowledge D. P. Waller, R. A. Anderson, and M. Frost for the gift of PPCM and its gel formulations and J. Moore for the primary HIV isolates.
Footnotes
Published ahead of print on 23 April 2008.
REFERENCES
- 1.Balzarini, J., S. Aquaro, C. F. Perno, M. Witvrouw, A. Holy, and E. De Clercq. 1996. Activity of the (R)-enantiomers of 9-(2-phosphonylmethoxypropyl)-adenine and 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem. Biophys. Res. Commun. 219337-341. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., H. Pelemans, S. Aquaro, C. F. Perno, M. Witvrouw, D. Schols, E. De Clercq, and A. Karlsson. 1996. Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol. Pharmacol. 50394-401. [PubMed] [Google Scholar]
- 3.Bobardt, M. D., U. Chatterji, S. Selvarajah, S. B. Van der, G. David, B. Kahn, and P. A. Gallay. 2007. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 81395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner, B. G., M. Oliveira, F. Doualla-Bell, D. D. Moisi, M. Ntemgwa, F. Frankel, M. Essex, and M. A. Wainberg. 2006. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS 20F9-F13. [DOI] [PubMed] [Google Scholar]
- 5.Cao, H., P. Kaleebu, D. Hom, J. Flores, D. Agrawal, N. Jones, J. Serwanga, M. Okello, C. Walker, H. Sheppard, R. El Habib, M. Klein, E. Mbidde, P. Mugyenyi, B. Walker, J. Ellner, and R. Mugerwa. 2003. Immunogenicity of a recombinant human immunodeficiency virus (HIV)-canarypox vaccine in HIV-seronegative Ugandan volunteers: results of the HIV Network for Prevention Trials 007 Vaccine Study. J. Infect. Dis. 187887-895. [DOI] [PubMed] [Google Scholar]
- 6.Celum, C., A. Wald, J. Hughes, J. Sanchez, S. Reid, S. Delancey-Moretlwe, F. Cowan, J. Fuchs, B. Koblin, and L. Corey. 2008. HSV-2 suppressive therapy for prevention of HIV acquisition: results of HPTN 039, abstr. 32LB. Abstr. 15th Conf. Retrovir. Oppor. Infect., 3 to 6 February 2008.
- 7.Chang, T. L., N. Teleshova, A. Rapista, M. Paluch, R. A. Anderson, D. P. Waller, L. J. Zaneveld, A. Granelli-Piperno, and M. E. Klotman. 2007. SAMMA, a mandelic acid condensation polymer, inhibits dendritic cell-mediated HIV transmission. FEBS Lett. 5814596-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, T. T., and T. C. Chou. 2000. Rational approach to the clinical protocol design for drug combinations: a review. Acta Paediatr. Taiwan 41294-302. [PubMed] [Google Scholar]
- 9.Cheshenko, N., M. J. Keller, V. MasCasullo, G. A. Jarvis, H. Cheng, M. John, J. H. Li, K. Hogarty, R. A. Anderson, D. P. Waller, L. J. Zaneveld, A. T. Profy, M. E. Klotman, and B. C. Herold. 2004. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob. Agents Chemother. 482025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 2227-55. [DOI] [PubMed] [Google Scholar]
- 11.Corey, L. 2007. Synergistic copathogens—HIV-1 and HSV-2. N. Engl. J. Med. 356854-856. [DOI] [PubMed] [Google Scholar]
- 12.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35435-445. [DOI] [PubMed] [Google Scholar]
- 13.Dezzutti, C. S., P. C. Guenthner, J. E. Cummins, Jr., T. Cabrera, J. H. Marshall, A. Dillberger, and R. B. Lal. 2001. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J. Infect. Dis. 1831204-1213. [DOI] [PubMed] [Google Scholar]
- 14.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 483834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doncel, G., and L. Van Damme. 2007. Update on the CONRAD cellulose sulfate trial, abstr. 106. Abstr. 14th Conf. Retrovir. Oppor. Infect., 25 to 28 February 2008.
- 16.Fernandez-Romero, J. A., M. Thorn, S. G. Turville, K. Titchen, K. Sudol, J. Li, T. Miller, M. Robbiani, R. A. Maguire, R. W. Buckheit, Jr., T. L. Hartman, and D. M. Phillips. 2007. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 349-14. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher, P., Y. Kiselyeva, G. Wallace, J. Romano, G. Griffin, L. Margolis, and R. Shattock. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 7911179-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman, E. E., H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, and R. J. Hayes. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2073-83. [DOI] [PubMed] [Google Scholar]
- 19.Gray, R. H., G. Kigozi, D. Serwadda, F. Makumbi, S. Watya, F. Nalugoda, N. Kiwanuka, L. H. Moulton, M. A. Chaudhary, M. Z. Chen, N. K. Sewankambo, F. Wabwire-Mangen, M. C. Bacon, C. F. Williams, P. Opendi, S. J. Reynolds, O. Laeyendecker, T. C. Quinn, and M. J. Wawer. 2007. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369657-666. [DOI] [PubMed] [Google Scholar]
- 20.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 745577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, M. Dzuzelewski, F. Francois, R. Morin, V. M. Casullo, R. A. Anderson, C. Chany, D. P. Waller, L. J. Zaneveld, and M. E. Klotman. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 7611236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 412776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, Q., I. Frank, V. Williams, J. J. Santos, P. Watts, G. E. Griffin, J. P. Moore, M. Pope, and R. J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 1991065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller, M. J., B. Zerhouni-Layachi, N. Cheshenko, M. John, K. Hogarty, A. Kasowitz, C. L. Goldberg, S. Wallenstein, A. T. Profy, M. E. Klotman, and B. C. Herold. 2006. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J. Infect. Dis. 19327-35. [DOI] [PubMed] [Google Scholar]
- 25.Krieger, J. N., R. W. Coombs, A. C. Collier, D. D. Ho, S. O. Ross, J. E. Zeh, and L. Corey. 1995. Intermittent shedding of human immunodeficiency virus in semen: implications for sexual transmission. J. Urol. 1541035-1040. [PubMed] [Google Scholar]
- 26.Lacey, C. J., A. Wright, J. N. Weber, and A. T. Profy. 2006. Direct measurement of in-vivo vaginal microbicide levels of PRO 2000 achieved in a human safety study. AIDS 201027-1030. [DOI] [PubMed] [Google Scholar]
- 27.Lu, H., Q. Zhao, G. Wallace, S. Liu, Y. He, R. Shattock, A. R. Neurath, and B. S. Jiang. 2006. Cellulose acetate 1,2-benzenedicarboxylate inhibits infection by cell-free and cell-associated primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 22411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madan, R. P., P. M. Mesquita, N. Cheshenko, B. Jing, V. Shende, E. Guzman, T. Heald, M. J. Keller, S. L. Regen, R. J. Shattock, and B. C. Herold. 2007. Molecular umbrellas: a novel class of candidate topical microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. J. Virol. 817636-7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 741948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagot, N., A. Ouedraogo, V. Foulongne, I. Konate, H. A. Weiss, L. Vergne, M. C. Defer, D. Djagbare, A. Sanon, J. B. Andonaba, P. Becquart, M. Segondy, R. Vallo, A. Sawadogo, P. Van de Perre, and P. Mayaud. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356790-799. [DOI] [PubMed] [Google Scholar]
- 31.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 795386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel, S., E. Hazrati, N. Cheshenko, B. Galen, H. Yang, E. Guzman, R. Wang, B. C. Herold, and M. J. Keller. 2007. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis. 1961394-1402. [DOI] [PubMed] [Google Scholar]
- 33.Peterson, L., K. Nanda, B. K. Opoku, W. K. Ampofo, M. Owusu-Amoako, A. Y. Boakye, W. Rountree, A. Troxler, R. Dominik, R. Roddy, and L. Dorflinger. 2007. SAVVY (C31G) gel for prevention of HIV infection in women: a phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS ONE 2e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 722855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quayle, A. J., C. Xu, K. H. Mayer, and D. J. Anderson. 1997. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J. Infect. Dis. 176960-968. [DOI] [PubMed] [Google Scholar]
- 36.Robertson, M., D. Mehrotra, D. Fitzgerald, A. Duerr, D. Casimiro, J. McElrath, D. Lawrence, and S. Buchbinder. 2008. Efficacy results from the STEP study (Merk V520 protocol 023/HVTN 502): a phase II test-of-concept trial of the MRKAd5 HIV-1 Gag/Pol/Nef trivalent vaccine, abstr. 88LB. Abstr. 15th Conf. Retrovir. Oppor. Infect., 3 to 6 February 2008.
- 37.Saidi, H., G. Magri, N. Nasreddine, M. Requena, and L. Belec. 2007. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology 35855-68. [DOI] [PubMed] [Google Scholar]
- 38.Shaunak, S., M. Thornton, I. Teo, B. Chandler, M. Jones, and S. Steel. 2003. Optimisation of the degree of sulfation of a polymer based construct to block the entry of HIV-1 into cells. J. Drug Target. 11443-448. [DOI] [PubMed] [Google Scholar]
- 39.Slater, T. F., B. Sawyer, and U. Straeuli. 1963. Studies on succinate-tetrazolium reductase systems. III. Points of coupling of four different tetrazolium salts. Biochim. Biophys. Acta 77383-393. [DOI] [PubMed] [Google Scholar]
- 40.Smith, D., N. Moini, R. Pesano, E. Cachay, H. Aiem, Y. Lie, D. Richman, and S. Little. 2007. Clinical utility of HIV standard genotyping among antiretroviral-naive individuals with unknown duration of infection. Clin. Infect. Dis. 44456-458. [DOI] [PubMed] [Google Scholar]
- 41.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360971-977. [DOI] [PubMed] [Google Scholar]
- 42.Vives, R. R., A. Imberty, Q. J. Sattentau, and H. Lortat-Jacob. 2005. Heparan sulfate targets the HIV-1 envelope glycoprotein gp120 coreceptor binding site. J. Biol. Chem. 28021353-21357. [DOI] [PubMed] [Google Scholar]
- 43.Wawer, M., G. Kigozi, D. Serwadda, F. Makumbi, F. Nalugoda, S. Watya, D. Buwembo, V. Ssempijja, L. Moulton, and R. Gray. 2008. Trial of male circumcision in HIV+ men, Rakai, Uganda: effects in HIV+ men and in women partners, abstr. 33LB. Abstr. 15th Conf. Retrovir. Oppor. Infect., 3 to 6 February 2008.
- 44.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 31817-23. [DOI] [PubMed] [Google Scholar]
- 45.Wu, Z., Z. Chen, and D. M. Phillips. 2003. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ cells: implications for mechanisms of sexual transmission. J. Infect. Dis. 1881473-1482. [DOI] [PubMed] [Google Scholar]
- 46.Zaneveld, L. J., D. P. Waller, R. A. Anderson, C. Chany, W. F. Rencher, K. Feathergill, X. H. Diao, G. F. Doncel, B. Herold, and M. Cooper. 2002. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate). Biol. Reprod. 66886-894. [DOI] [PubMed] [Google Scholar]
- 47.Zaneveld, L. J. D., R. A. Anderson, X.-H. Diao, D. P. Waller, C. Chany II, K. Feathergill, G. Doncel, M. D. Cooper, and B. Herold. 2002. Use of mandelic acid condensation polymer (SAMMA), a new antimicrobial contraceptive agent, for vaginal prophylaxis. Fertil. Steril. 781107-1115. [DOI] [PubMed] [Google Scholar]
- 48.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 2611179-1181. [DOI] [PubMed] [Google Scholar]