Abstract
The herpes simplex virus type 1 (HSV-1) portal is composed of a dodecamer of UL6 protein molecules whose incorporation into the capsid is mediated by interaction with the HSV-1 UL26.5 scaffold protein. Previous results with an in vitro capsid assembly assay demonstrated that nine amino acids (amino acids 143 to 151) of the UL26.5 protein are required for its interaction with UL6 and for incorporation of the portal complex into capsids. In the present study an HSV-1 mutant, bvFH411, was isolated and contained a deletion that removed the codons for UL26.5 amino acids 143 to 150. The mutant virus failed to produce infectious virus in noncomplementing cells, and only B capsids that contained only minor amounts of portal protein were made. These data corroborate our previous in vitro studies and demonstrate that amino acids 143 to 150 of UL26.5 are required for the formation of portal-containing HSV-1 capsids.
The herpes simplex virus type 1 (HSV-1) capsid is assembled in the nucleus as a precursor particle or procapsid that contains an outer icosahedral shell composed primarily of four viral structural proteins (VP5, VP19C, VP23, and VP26). Assembly of the procapsid requires a scaffolding protein that forms an inner core structure composed of ∼1,100 copies of the UL26.5 scaffold protein and ∼90 copies of the UL26 protein (2, 9). The UL26 and UL26.5 genes are expressed as 3′-coterminal transcripts, with the promoter for the UL26.5 gene located within the coding region of UL26 (9). The UL26.5 open reading frame (encoding 329 amino acids) overlaps and is in frame with the carboxyl-terminal end of the HSV-1 UL26 open reading frame (encoding 635 amino acids). The UL26 gene encodes a protease that cleaves itself and the UL26.5 protein at a position 25 amino acids from the C termini of these two proteins (5, 12, 13). Cleavage of the UL26 and UL26.5 proteins is not required for capsid assembly, but the cleavage event is essential for DNA encapsidation (6).
The scaffold aids in the assembly of the 125-nm outer shell structure. The scaffold is removed from the capsid when DNA is packaged in order to make room for the viral genome. The DNA enters the capsid through the portal vertex, which consists of 12 copies of the UL6 protein (15, 21). The portal is located at only one of the 12 capsid vertices (3, 4). The location of the portal at a unique vertex in the capsid suggests that there is a mechanism to control portal incorporation. Direct interaction of the scaffold with the portal has been demonstrated by in vitro experiments with purified proteins (16). In studies done using an in vitro capsid assembly assay, the interaction of the scaffold protein and portal proteins was found to be essential for incorporation of the portal into the capsid (14). More importantly, these studies found that in order for the portal to be incorporated into the capsid, it needs to be present when the assembly reaction is started (14). Delaying addition of the portal to the assembly reaction resulted in assembly of capsids that lacked a portal complex. Studies with deletion mutants demonstrate that amino acids 143 to 151 of the scaffold protein are critical for the formation of portal-containing capsids assembled in vitro (19). The results of these in vitro studies indicated that portal incorporation into the capsid was mediated by its specific interaction with the scaffolding protein. The goal of the current study was to demonstrate that this region of the scaffold protein is essential for producing infectious virus and for forming portal-containing capsids in HSV-1-infected cells.
An HSV-1 mutant was isolated that contained a deletion that removed sequences coding for amino acids 143 to 150 of the scaffold protein or amino acids 449 to 456 of UL26 (Fig. 1). The mutant was created using an infectious bacterial artificial chromosome (BAC) clone of the HSV-1 KOS genome (KOS-37) (7). The KOS BAC clone was transferred to GS1783 (an Escherichia coli strain containing an inducible ISceI gene, provided by G. Smith, Northwestern University Medical School), and mutagenesis was performed using the two-step bacteriophage λ Red-mediated homologous recombination system described by Tischer et al. (20). PCR was used to produce a construct that contained the kanamycin resistance gene (aphAI from plasmid pEPkan) with the forward primer 5′-ACTGCGACCAGGACGAACCGGACGCGGACTACGGCGCGCCGCGCGGGGTCGACTAGGGATAACAGGGTAATCGATTT-3′ and the reverse primer 5′GCCGCGCGCCGGGAGTCGACCCCGCGCGGCGCGCCGTAGTC CGCGTCCGGTTCGCCAGTGTTACAACCAATTAACC-3′.
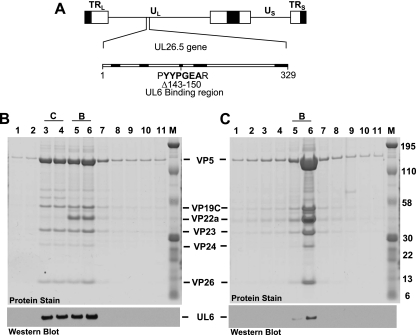
FIG. 1.
(A) Physical map of the HSV-1 genome showing the location of the UL26.5 gene, which encodes the 329-amino-acid VP22a scaffold protein. UL and US, long and short unique region sequences. The next line shows the location of the eight amino acids (amino acids 143 to 150) deleted from the scaffold mutant (bvFH411) that previously were shown to be essential for interaction with the UL6 portal protein. Regions that are conserved in the scaffold proteins of alphaherpesviruses are in bold. (B and C) Nuclear lysates of Vero cells infected at an MOI of 5 with KOS (B) or bvFH411 (C) were subjected to sucrose gradient sedimentation, and the protein composition for each gradient fraction was determined by SDS-PAGE (stained gel). Lane 1 is the bottom and lane 11 is the top of the gradient. Gradient fractions that contained C and B capsids are indicated (there was no A capsid band present in KOS gradient). The positions of the capsid proteins (major capsid protein, VP5; triplex proteins, VP19C and VP23; scaffold protein, VP22a; protease, VP24; smallest capsid protein, VP26) are indicated. Molecular mass standards are visible in lane M (kDa). In the bottom panels, gradient fractions were analyzed by SDS-PAGE followed by immunoblotting for UL6 using the 1C9 antibody (16).
The PCR-generated kanamycin cassette was inserted into the UL26.5 gene of the BAC clone by homologous recombination, and a markerless deletion of UL26.5 codons 143 to 150, which corresponds to nucleotides 52156 to 52180 of the HSV-1 genome, was achieved following the procedure described by Tischer et al. (20). The deletion mutation in the KOS BAC DNA was confirmed by PCR analysis and DNA sequencing. The BAC DNA was isolated and converted to infectious HSV-1 by transfection into C32 cells, a UL26/UL26.5 complementing cell line (18). The resulting virus, bvFH411, was plaque purified, and a large stock was made in C32 cells. The plating efficiency of bvFH411 was tested on C32 and Vero cells. The titer of the UL26.5 mutant was 7 × 107 on C32 cells and 3 × 105 on Vero cells. The low levels of wild-type virus in the mutant stocks is due to recombination that occurs between homologous sequences present in the mutant viral genome and the wild-type sequences in the C32 cell line. To demonstrate that the virus that plaques on Vero cells is due to wild-type virus, we picked a plaque from both Vero cells and C32 cells and did PCR analysis to determine if the UL26.5 gene contained the deletion that removed the codons for amino acids 143 to 151. The PCR analysis confirmed that the virus isolated from C32 cells contained the deletion while the virus isolated from Vero cells did not (data not shown). These results demonstrated that codons 143 to 150 of the UL26.5 gene are important for virus replication in noncomplementing cells.
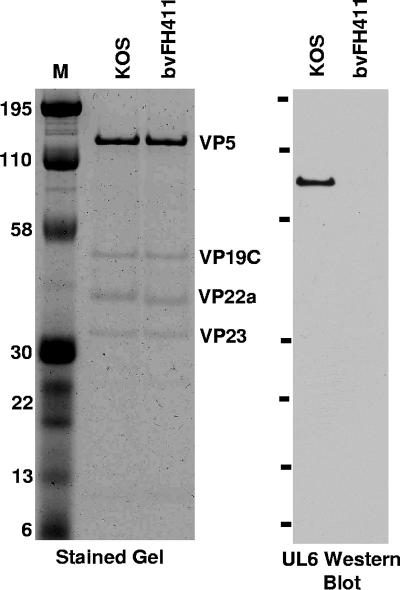
Studies with the in vitro capsid assembly assay showed that the scaffold protein missing amino acids 143 to 151 was capable of forming intact B capsids with no obvious morphological differences from capsids formed with the wild-type scaffold protein (19). However, the B capsids formed with the mutant scaffold protein did not incorporate the portal protein. To further examine the different capsid forms made in infected cells, nuclear lysates were prepared from Vero cells infected (multiplicity of infection [MOI] of 5) with KOS or bvFH411. The lysates were subjected to 20 to 60% sucrose gradient centrifugation, and capsids were viewed as visible light-scattering bands (10). In KOS-infected Vero cells, capsids with B and C bands being the most prominent were observed, while lysates of bvFH411-infected Vero cells contained only B capsids (data not shown). To determine whether the portal protein was present in these capsids, the sucrose gradient fractions (0.75 ml/fraction) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining to detect capsid proteins or by Western blot analysis to detect the UL6 protein (Fig. 1). The KOS gradient showed that the UL6 portal protein was found only in the fractions that contained B and C capsids, with each of these fractions containing similar amounts of UL6 (Fig. 1B). This would be expected, since the amount of UL6 (12 copies/capsid) is the same in all capsid forms (15, 21). The gradient from cells infected with the UL26.5 mutant contained only B capsids (Fig. 1C). UL6 was found to be present in the B capsid fractions but at a much lower level than in the capsids isolated from KOS-infected cells (Fig. 1B). To directly compare the amounts of UL6 present in the B capsids isolated from KOS and the UL26.5 mutant, equal amounts of capsids were separated on an SDS-polyacrylamide gel (Fig. 2). The stained gel demonstrated that similar amounts of capsids were loaded for both KOS and bvFH411. In addition, this gel showed that the scaffold protein (VP22a) from the UL26.5 mutant was slightly smaller than the wild-type scaffold protein due to the deletion of amino acids 143 to 150. The capsids produced with the wild-type scaffold showed a significant amount of UL6 associated with the B capsid fractions, while the capsids produced by the scaffold mutant showed no detectable portal protein. These observations indicate that, similar to what was found with the in vitro capsid assembly assay; the deletion of the portal binding domain of the scaffold protein prevents portal incorporation into capsids.
FIG. 2.
Comparison of the UL6 contents in B capsids isolated from KOS- and bvFH411-infected Vero cells. Equal amounts of capsids were separated by SDS-PAGE, and the gel was either stained or immunoblotted using the UL6 antibody 1C9 (16). Molecular mass standards are visible in lane M (kDa).
The open reading frame for the UL26 protease overlaps that for the scaffold protein, and the UL26 protease shares much of the scaffold's amino acid sequence. The proteolytic activity of the UL26 protein is critical for DNA packaging (9). Because the deletion is within a region common to both proteins, the deletion of amino acids 143 to 151 from UL26.5 also removes amino acids 449 to 456 from UL26. The deletion did not appear to alter the UL26 protein or its proteolytic function, since both the cleaved scaffold protein (VP22a) and the cleaved form of the UL26 protein (VP24) were present in the B capsids isolated from bvFH411 Vero cells (Fig. 1C).
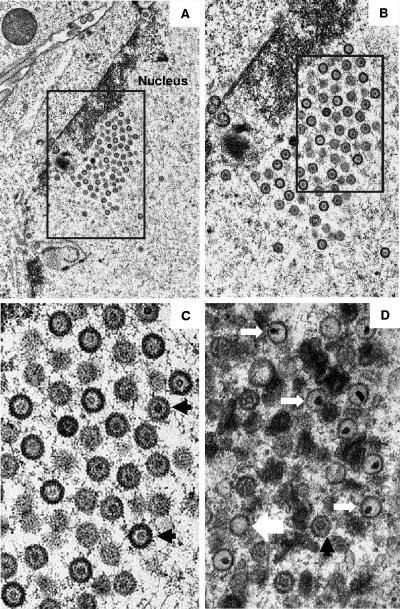
Thin sections of Vero cells infected with the UL26.5 deletion virus were examined by transmission electron microscopy to determine the type of capsids present (Fig. 3). The nuclei were found to contain capsids which had electron-translucent cores (B capsids). No dense-core capsids (C capsids) or empty capsids (A capsids) were observed in these cells (Fig. 3A to C), and there were no enveloped capsids detected in the cytoplasm (micrographs not shown). In contrast, when Vero cells were infected with wild-type KOS virus, an abundance of DNA-containing capsids was observed (Fig. 3D). The data demonstrate that deletion of residues 143 to 150 of the UL26.5 protein blocks DNA packaging but does not interfere with the assembly of B capsids.
FIG. 3.
Electron micrographs showing thin sections of Vero cells infected with bvFH411 (A to C) and KOS (D) viruses. The boxed area in panel A is shown at higher magnification in panel B, and similarly, the boxed area in panel B is shown enlarged in panel C. The large white, black, and small white arrows indicate representative A, B, and C capsids, respectively. Note that while cells infected with bvFH411 contain many A and B capsids (A to C), DNA-containing capsids (C capsids) are found only in KOS-infected cells (D).
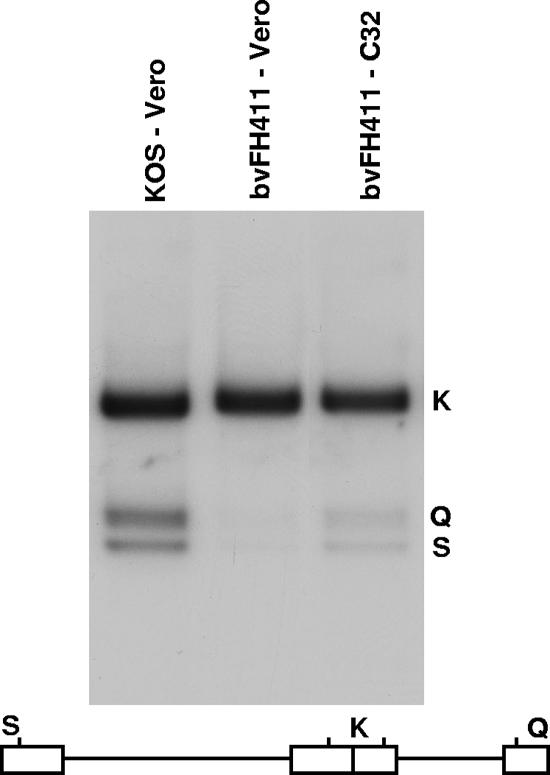
To determine whether viruses making portal-deficient capsids were impaired in DNA cleavage, total cell DNA was isolated from Vero or C32 cells infected with either KOS or bvFH411 and subjected to Southern blot analysis. Viral DNA replication generates concatemers that are cleaved into unit-length molecules and packaged into virions. The encapsidated viral DNA contains free chromosomal termini, whereas the nonpackaged DNA does not. The presence of chromosomal ends can be monitored by Southern blot analysis of total infected-cell DNA digested with BamHI and probed with the HSV-1 BamHI K fragment (10). Only cleaved viral DNA with free chromosomal ends will give rise to the terminal BamHI Q and S fragments, while concatemeric DNA gives rise only to the joint-spanning BamHI K fragment. The bvFH411 mutant was found to produce viral DNA when grown on either Vero or C32 cells, as assessed by the presence of the joint K fragment, but the Q and S fragments were present when bvFH411 was grown on the complementing C32 cells (Fig. 4). These results demonstrate that portal-containing capsids are essential for the cleavage of replicated viral DNA and are consistent with previous studies done with a UL6 null virus (11, 17).
FIG. 4.
Processing of viral DNA. Vero or C32 cells were infected with the indicated virus at an MOI of 5 PFU per cell. Total infected-cell DNA was isolated at 16 h postinfection, digested with BamHI, and subjected to Southern blot analysis using the BamHI K joint fragment (32P labeled) as a probe. Scanned images of the autoradiograph obtained from the Southern blots are shown. The locations of the BamHI K joint fragment and the two end fragments, BamHI-Q and -S, in the HSV-1 genome are shown at the bottom.
The results with this HSV-1 scaffold mutant, bvFH411, corroborate our previous in vitro studies demonstrating that removal of a 9-amino-acid region within the HSV-1 scaffold protein is sufficient to prevent portal incorporation into capsids. HSV-1 capsid assembly is a complex process requiring the highly ordered interaction of numerous viral proteins in order to form a capsid that is competent for DNA packaging (2). The importance of a scaffold-portal complex for the assembly of portal-containing capsids was first reported with bacteriophages where scaffold mutations resulted in the assembly of portal-negative capsids (1, 8). Such observations support the view that the formation of a scaffold-portal complex is required for assembly of portal-containing capsids. One of the critical steps in the assembly process is the incorporation of the portal complex at a single capsid vertex. Although the mechanism for incorporation of a single portal into the HSV-1 capsid has not been established, the identification of a specific region within the scaffolding protein that is important for this process establishes that the scaffold is an essential component in this process. Moreover, our results are consistent with earlier observations, using an in vitro capsid assembly assay, demonstrating the necessity for the formation of a scaffold-portal complex in the assembly of portal-containing capsids (16).
Finally, while this paper was under review, a related paper reporting on an HSV-1 mutant containing a deletion of amino acids 143 to 151of the HSV-1 scaffold protein was published. Similar conclusions about the role of this region in portal incorporation into capsids were reported (22).
Acknowledgments
We thank Klaus Osterrieder and David Leib for BAC reagents, Greg Smith for the GS1783 bacterial strain, Prashant Desai for the C32 cell line, and Shelley Cockrell for assistance with the DNA cleavage studies.
This work was supported by Public Health Services grants AI060836 (to F.L.H.) and AI41644 (to J.C.B.) from the National Institutes of Health.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Bazinet, C., and J. King. 1985. The DNA translocating vertex of dsDNA bacteriophage. Annu. Rev. Microbiol. 39109-129. [DOI] [PubMed] [Google Scholar]
- 2.Brown, J., M. A. McVoy, and F. L. Homa. 2002. Packaging DNA into herpesvirus capsids, p. 111-155. In A. H. E. Bogner (ed.), Structure-function relationships of human pathogenic viruses. Kluwer Acacdemic/Plenum Publishers, New York, NY.
- 3.Cardone, G., D. C. Winkler, B. L. Trus, N. Cheng, J. E. Heuser, W. W. Newcomb, J. C. Brown, and A. C. Steven. 2007. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology 361426-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, J. T., M. F. Schmid, F. J. Rixon, and W. Chiu. 2007. Electron cryotomography reveals the portal in the herpesvirus capsid. J. Virol. 812065-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiIanni, C. L., D. A. Drier, I. C. Deckman, P. J. McCann, 3rd, F. Liu, B. Roizman, R. J. Colonno, and M. G. Cordingley. 1993. Identification of the herpes simplex virus-1 protease cleavage sites by direct sequence analysis of autoproteolytic cleavage products. J. Biol. Chem. 2682048-2051. [PubMed] [Google Scholar]
- 6.Gao, M., L. Matusick-Kumar, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann, 3rd, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 683702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gierasch, W. W., D. L. Zimmerman, S. L. Ward, T. K. Vanheyningen, J. D. Romine, and D. A. Leib. 2006. Construction and characterization of bacterial artificial chromosomes containing HSV-1 strains 17 and KOS. J. Virol. Methods 135197-206. [DOI] [PubMed] [Google Scholar]
- 8.Greene, B., and J. King. 1996. Scaffolding mutants identifying domains required for P22 procapsid assembly and maturation. Virology 22582-96. [DOI] [PubMed] [Google Scholar]
- 9.Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7107-122. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson, J. G., K. Yang, J. D. Baines, and F. L. Homa. 2006. Linker insertion mutations in the herpes simplex virus type 1 UL28 gene: effects on UL28 interaction with UL15 and UL33 and identification of a second-site mutation in the UL15 gene that suppresses a lethal UL28 mutation. J. Virol. 8012312-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamberti, C., and S. K. Weller. 1996. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology 226403-407. [DOI] [PubMed] [Google Scholar]
- 12.Liu, F., and B. Roizman. 1993. Characterization of the protease and other products of amino-terminus-proximal cleavage of the herpes simplex virus 1 UL26 protein. J. Virol. 671300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, F. Y., and B. Roizman. 1991. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J. Virol. 655149-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcomb, W. W., F. L. Homa, and J. C. Brown. 2005. Involvement of the portal at an early step in herpes simplex virus capsid assembly. J. Virol. 7910540-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 7510923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomb, W. W., D. R. Thomsen, F. L. Homa, and J. C. Brown. 2003. Assembly of the herpes simplex virus capsid: identification of soluble scaffold-portal complexes and their role in formation of portal-containing capsids. J. Virol. 779862-9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel, A. H., F. J. Rixon, C. Cunningham, and A. J. Davison. 1996. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology 217111-123. [DOI] [PubMed] [Google Scholar]
- 18.Person, S., and P. Desai. 1998. Capsids are formed in a mutant virus blocked at the maturation site of the UL26 and UL26.5 open reading frames of herpes simplex virus type 1 but are not formed in a null mutant of UL38 (VP19C). Virology 242193-203. [DOI] [PubMed] [Google Scholar]
- 19.Singer, G. P., W. W. Newcomb, D. R. Thomsen, F. L. Homa, and J. C. Brown. 2005. Identification of a region in the herpes simplex virus scaffolding protein required for interaction with the portal. J. Virol. 79132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tischer, B. K., J. von Einem, B. Kaufer, and N. Osterrieder. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. BioTechniques 40191-197. [DOI] [PubMed] [Google Scholar]
- 21.Trus, B. L., N. Cheng, W. W. Newcomb, F. L. Homa, J. C. Brown, and A. C. Steven. 2004. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J. Virol. 7812668-12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, K., and J. D. Baines. 2008. Domain within herpes simplex virus 1 scaffold proteins required for interaction with portal protein in infected cells and incorporation of the portal vertex into capsids. J. Virol. 825021-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]