Abstract
Histone mRNA levels are cell cycle regulated, and a major regulatory mechanism is restriction of stem-loop binding protein (SLBP) to S phase. Degradation of SLBP at the end of S phase results in cessation of histone mRNA biosynthesis, preventing accumulation of histone mRNA until SLBP is synthesized just before entry into the next S phase. Degradation of SLBP requires an SFTTP (58 to 62) and KRKL (95 to 98) sequence, which is a putative cyclin binding site. A fusion protein with the 58-amino-acid sequence of SLBP (amino acids 51 to 108) fused to glutathione S-transferase (GST) is sufficient to mimic SLBP degradation at late S phase. Using GST-SLBP fusion proteins as a substrate, we show that cyclin A/Cdk1 phosphorylates Thr61. Furthermore, knockdown of Cdk1 by RNA interference stabilizes SLBP at the end of S phase. Phosphorylation of Thr61 is necessary for subsequent phosphorylation of Thr60 by CK2 in vitro. Inhibitors of CK2 also prevent degradation of SLBP at the end of S phase. Thus, phosphorylation of Thr61 by cyclin A/Cdk1 primes phosphorylation of Thr60 by CK2 and is responsible for initiating SLBP degradation. We conclude that the increase in cyclin A/Cdk1 activity at the end of S phase triggers degradation of SLBP at S/G2.
Progression through the cell cycle is driven by a class of protein kinases, the cyclin-dependent kinases (cdk's), which are sequentially activated as cells progress from one cell cycle stage to the next. In particular, progress into S phase requires the activation of cyclin E/Cdk2, ultimately resulting in the initiation of DNA replication, and progression through mitosis requires the activation of cyclin B/Cdk1 (cdc2), resulting in nuclear envelope breakdown and chromosome condensation (27). Cyclin A/Cdk2 activity is required for continued progression through S phase (36). The targets of the cdk's are activated or inactivated by phosphorylation. A second critical regulatory mechanism for cell cycle progression is targeted proteolysis of key protein regulators (13). These include the cyclin subunits of the cdk's, critical proteins in initiation of DNA replication, and proteins responsible for maintaining chromosome pairing (6).
While much is known about the events that must occur for cells to transit from G1 to S phase and for cells to enter and exit mitosis, much less is known about the molecular events at the end of S phase, as cells progress from S to G2 phase. S phase is characterized by replication of the chromosomes, and at the same time DNA is replicated, histone proteins must be synthesized to provide histones to assemble the newly replicated chromatin. Histone mRNAs are cell cycle regulated, and their expression is restricted to S phase. The metazoan replication-dependent histone mRNAs are the only eukaryotic cellular mRNAs that are not polyadenylated. Instead, they end in a conserved stem-loop sequence (18). Since the replication-dependent histone genes lack introns, the only processing reaction required for histone mRNA biosynthesis is cleavage of the nascent transcript to form the 3′ end of the mRNA (18).
The 3′ end of histone mRNAs is a major cis element responsible for the coordinated regulation of histone mRNA levels (17). The 3′ end is bound by the stem-loop binding protein (SLBP), which is required for both histone pre-mRNA processing and histone mRNA translation (18). The processing of histone pre-mRNA is cell cycle regulated, as is the stability of histone mRNAs (11). SLBP is also a cell cycle-regulated protein, accumulating only in S-phase cells (34), and regulation of SLBP levels is an important component of the cell cycle regulation of histone mRNA. SLBP is rapidly degraded at the end of S phase as a result of phosphorylation of two threonines in an SFTTP sequence (amino acids 58 to 62). Proline 62 is also required for SLBP degradation (37), as is a KRKL sequence (amino acids 95 to 98) that is a consensus cyclin binding site (1-3, 5, 30) thought to primarily interact with the S-phase cyclins A and E (16).
We report here that cyclin A/Cdk1 initiates SLBP degradation at the end of S phase by phosphorylating Thr61. Subsequent to that phosphorylation, CK2 phosphorylates T60, and the doubly phosphorylated SLBP is targeted for degradation. Cyclin A/Cdk1 activity is cell cycle regulated and is the major activity in late-S-phase cells that phosphorylates Thr61 of SLBP. We propose that cyclin A/Cdk1 is activated near the end of S phase, resulting in the degradation of SLBP and downregulating histone mRNA synthesis. It is likely that cyclin A/Cdk1 phosphorylates other targets at the same time, during the transition from S phase to G2 phase.
MATERIALS AND METHODS
Construct preparation.
For bacterial expression, the SBLP fragment (51 to 108) was subcloned into the PGEX2T vector just after the glutathione S-transferase (GST) tag using a PCR-generated insert with EcoRI and BamHI sites. For expression in HeLa cells, the GST-fused SLBP fragment was subcloned into a Myc-tagged pcDNA3 vector (Invitrogen) by PCR-generated insertion with XhoI and KpnI sites. All site-directed mutations were made according to the QuickChange site-directed mutagenesis protocols (Stratagene) using appropriate complementary oligonucleotides ranging from 30 to 50 nucleotides in length. All constructs were verified by DNA sequencing.
Transfection and selection of stable cell lines.
HeLa cell plasmid transfections were done with Lipofectamine (GibcoBRL) according to the manufacturer's protocol. Two micrograms of each plasmid was used with each six-well plate. For selection of stably transfected cells, the cells were replated onto a fresh 10-cm plate 24 h after transfection with medium containing 1 mg/ml G418. The medium containing G418 was changed every 5 days to remove dead cells. Cells were kept under selection until separate colonies could be observed by eye on the plates, and these stably transfected cells (at least 20 colonies per plate) were pooled together. To maintain the stably transfected cells, 200 μg of G418 per ml was kept in the medium and was removed just prior to synchronization.
Cell culture and synchronization.
HeLa cells were grown in Dulbecco modified Eagle medium plus 10% fetal bovine serum and penicillin-streptomycin. Cells were synchronized at the G1/S border by a double-thymidine block. Cells were plated at a low density 24 h before the start of the synchronization protocol, when cells were treated with 2 mM thymidine for 18 h for the initial block. After the initial block, cells were washed with phosphate-buffered saline, released into fresh medium for 9 h, and then blocked again with 2 mM thymidine for 16 h to arrest all the cells at the beginning of S phase. The cells were released into fresh medium after the thymidine was washed out, and lysates were prepared from the cells collected at various time points after the release. The cells progressed through G2 and mitosis synchronously. The cell cycle profile of the cells was determined by propidium iodide staining and flow cytometry analysis at the University of North Carolina (UNC) Flow Cytometry facility.
Lysate preparation and in vitro kinase assays.
Cells were collected at the indicated time points after release from a double-thymidine block, resuspended in lysis buffer (50 mM β-glycerophosphate, 20 mM NaF, 1.5 mM EGTA, 0.05% NP-40 with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 500 μM Na3VO4), and sonicated four times on ice (800 μl per 10-cm dish). Extracts were clarified by centrifugation for 10 min at 14,000 rpm at 4°C. In vitro kinase assays were performed in a 30 μl reaction mix (supplemented with a final 10 mM concentration of MgCl2 and 5 μM concentration of ATP) with the corresponding lysate (1 to 2 mg/ml protein; 10 μg protein) and 1 μg of substrate (GST-SLBP fragment or H1) in lysis buffer and 8 μCi of [γ-32P]ATP. In each reaction, immunoprecipitates on beads (from 80 to 100 μg lysate), the supernatant from the immunoprecipitates, or the lysate directly was used as a kinase source as indicated. The reactions were incubated at 30°C for 30 min and stopped by adding 2× sodium dodecyl sulfate (SDS) loading buffer. Samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and each gel stained with Coomassie, dried, analyzed by autoradiography, and quantified with a PhosphorImager.
Recombinant kinases.
Baculovirus-expressed recombinant cyclin A/Cdk2, cyclin B/Cdk1, and cyclin A/Cdk1 were obtained from Cell Signaling. Bacterially expressed recombinant casein kinase 2 (CK2) (alpha and beta subunits) was purchased from New England Biolabs. With cdk's and sequential phosphorylation experiments, kinase reactions were carried out in the kinase buffer described above. For phosphorylation with CK2 alone, the commercial buffer provided by NEB was used.
Analysis of phosphorylation sites.
Synthetic peptides corresponding to residues 54 to 68 of SLBP (54RRPESFTTPEGPKPR68), with the T at position 61 unmodified or phosphorylated and the same peptide with a phosphothreonine at Thr61[TphosTP], were synthesized. These were incubated with casein kinase II in the presence of [γ-32P]ATP and the amount of phosphorylation determined by binding to DEAE-cellulose followed by scintillation counting. The phosphorylation reaction was also carried out with unlabeled ATP and the peptides analyzed by mass spectrometry (matrix-assisted laser desorption ionization [MALDI]) using an ABI4800 TOF/TOF mass spectrometer.
Following phosphorylation of the recombinant GST-SLBP proteins with recombinant cyclin A/Cdc2 and/or CK2, the proteins were analyzed by mass spectrometry using electrospray as previously described (8). The sites of phosphorylation were confirmed by digesting GST-SLBP with trypsin and analyzing the peptides on an ABI4800 TOF/TOF mass spectrometer.
RNA interference.
Downregulation of Cdk1 expression was obtained by transfection of chemically synthesized small interfering RNA (siRNA) using oligofectamine 2000 transfection according to the manufacturer's protocol. Chemically synthesized siRNAs were obtained from Dharmacon (Lafayette, CO) and had the following sequences of the top strand: 5′AAGGGGUUCCUAGUACUGCAAdTdT3′ and 5′GGUCCGGCUCCCCCAAAUGdTdT3′ (control, C2). A portion of cells were lysed in a buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 8), 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor mixture (Roche), and 0.5% NP-40 and analyzed by Western blotting. The remaining cells were fixed with 70% ethanol, stained with propidium iodide, and analyzed for their DNA content by flow cytometry with a FACscan instrument and the Summit software (Cytomation, Inc.).
Antibodies and inhibitors.
SLBP was detected with an antibody raised against the C-terminal 13 amino acids of the protein (32). Cyclin A and cyclin B antibodies used in Western blots and immunoprecipitation experiments were purchased from Neomarkers (Feremont, CA) and SantaCruz (Santa Cruz, CA), respectively. Cdk1 and Cdk2 antibodies were a kind gift from Yue Xiong (UNC). All the kinase inhibitors tested were purchased from Calbiochem (La Jolla, CA).
RESULTS
A cyclin/cdk is required for degradation of SLBP at S/G2 transition.
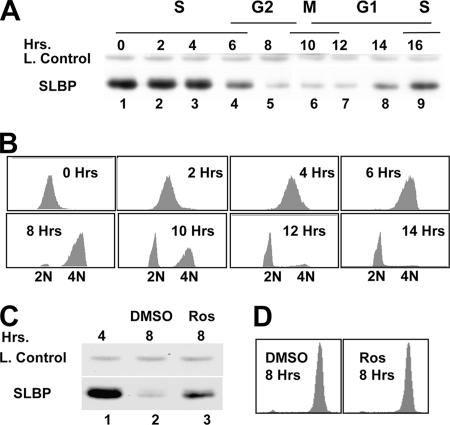
SLBP expression is limited to S phase (34), and at the end of S phase, SLBP is rapidly degraded (37) (Fig. 1A). Previously we have shown that SLBP degradation requires phosphorylation of two threonine residues (Thr60 and Thr61) by two different kinases (37). Further, we have shown that along with Thr60 and Thr61, Pro62 and a downstream KRKL sequence are required for SLBP degradation at the end of S phase (37). The observation that Thr61 is a possible cdk phosphorylation site and KRKL is a putative cyclin binding site suggested that a cyclin/cdk was involved in SLBP degradation by phosphorylating Thr61. To test this possibility, we examined the affects of roscovitine, a known Cdk1/Cdk2 inhibitor, on SLBP degradation at S/G2 in HeLa cells. At the end of S phase, the level of SLBP remained higher in cells treated with roscovitine than in dimethylsulfoxide (DMSO)-treated control cells (Fig. 1C). Fluorescence-activated cell sorting (FACS) analysis confirmed that both cell populations successfully completed S phase and accumulated at G2 without a significant difference in their cell cycle profile at the time of collection (Fig. 1D). Since roscovitine is specific to Cdk1/Cdk2 (7, 20), we hypothesized that one or both of these cdk's was involved in SLBP degradation, possibly by phosphorylating Thr61. Several other kinase inhibitors were tested by adding them to cells in mid-S phase. However, none of these inhibitors, including other inhibitors of proline-dependent kinases, inhibited the degradation of SLBP at the end of S phase (Table 1).
FIG. 1.
S/G2 degradation of SLBP is roscovitine sensitive. (A) Western blot analysis of SLBP levels in HeLa cells synchronized by a double-thymidime block and collected at the indicated time points after the release. (B) FACS analysis of the cells collected at indicated times after the release. (C) Western blot analysis of SLBP levels in HeLa cells which were treated with either dimethyl sulfoxide (DMSO, lane 2) or 20 μM roscovitine (Ros, lane 3) in DMSO 4 h after release from the double-thymidine block and collected four more hours later. (D) FACS analysis of both Ros- and DMSO-treated cells at the time of collection.
TABLE 1.
Effects of kinase inhibitors on SLBP degradation
| Inhibitor | Target(s)a | Stable SLBP at G2 |
|---|---|---|
| Roscovitine | Cdk1, Cdk2 | Yes |
| Wortmannin | PI 3-kinase | No |
| PD 98059 | MEK 1 | No |
| SB 202190 | p38 MAPKs | No |
| SB 203580 | p38 MAPKs | No |
| Rapamycin | mTOR | No |
| TBB | CK2 | Yes |
| DMAT | CK2 | Yes |
MAPK, mitogen-activated protein kinase.
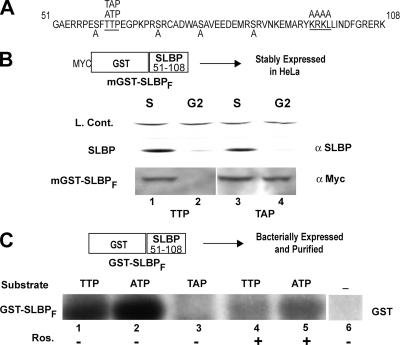
In order to determine whether phosphorylation of these two residues and the presence of the KRKL sequence were sufficient to trigger SLBP degradation, we designed a Myc-tagged GST protein fused to amino acids 51 to 108 of an SLBP fragment (mGST-SLBPF) in which all of the serines and threonines except Thr60 and Thr61 were mutated to alanine. We constructed similar genes encoding mutation of Thr60, Thr61, and the KRKL sequence to alanine. We stably expressed the wild type and Thr61A mGST-SLBPF in HeLa cells and then synchronized the cells by a double-thymidine block. Chimeric wild-type mGST-SLBPF was degraded at G2 in parallel with endogenous SLBP (Fig. 2B, lane 1 and lane 2). mGST-SLBPF with the Thr61A mutation was stable during G2 phase (Fig. 2B, lanes 3 and 4). This result confirmed that mGST-SLBPF was degraded by a mechanism similar to that with full-length SLBP, since mutation of Thr61 to alanine in full-length SLBP also prevented SLBP degradation at the end of S phase (37).
FIG. 2.
A fragment of SLBP fused to GST is sufficient to mimic S/G2 regulation of SLBP. (A) The SLBP fragment (amino acids 51 to 108) sufficient for S/G2 regulation is shown with the regions required for late-S-phase degradation underlined. The Ser/Thr residues that have been changed to alanine are shown by A below the wild-type sequence. Mutations that have been introduced in different constructs are indicated above the wild-type sequence. (B) HeLa cells stably expressing the Myc-GST-SLBP fragment (mGST-SLBPF) with wild-type TTP or TAP were synchronized by a double-thymidine block and collected at S or G2 phase. The levels of mGST-SLBPF and endogenous SLBP were determined by Western blot analysis using anti-Myc and anti-SLBP (α-Myc and α-SLBP) antibodies. (C) In vitro kinase assays with bacterially expressed GST-SLBPF (TTP, ATP, and TAP) or GST alone were performed in the presence of [γ-32P]ATP, using lysate from late-S-phase cells. The effect of roscovitine (Cdk1/Cdk2 inhibitor; 10 μM) on phosphorylation was also determined. Samples were analyzed by SDS-PAGE, and the phosphorylation level of each substrate was determined by autoradiography.
We next expressed the GST-SLBP fragment (51 to 108 amino acids) in bacteria and used the recombinant GST-SLBPF protein as a substrate for in vitro kinase assays. We also constructed and expressed similar GST fusion proteins containing TAP or ATP in place of the TTP (amino acids 60 to 62) sequence or a mutant with the KRKL sequence changed to four alanines (KRKL/4A). We prepared an extract from late-S-phase cells (6 h after release from a double-thymidine block) and tested this extract as a source of kinase activity. The extract phosphorylated the wild-type GST-SLBPF and the ATP mutant (Fig. 2C, lanes 1 and 2) but not the TAP mutant or GST protein (Fig. 2C, lanes 3 and 6). The activity was sensitive to roscovitine, demonstrating that this phosphorylation was mediated by Cdk1 or Cdk2 (Fig. 2C, lanes 4 and 5). Moreover, these results suggested that Thr61 was the only site in the GST-SLBP fragment phosphorylated by the kinase(s) in this lysate.
Phosphorylation of Thr61 increases in lysates from late-S, G2-phase cells.
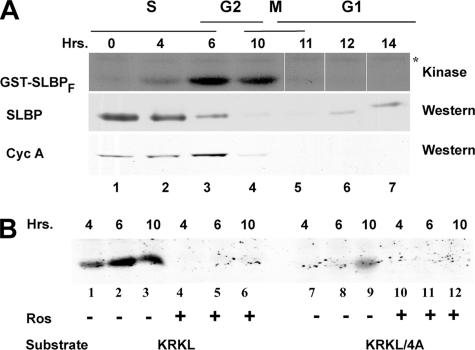
To determine whether the kinase activity responsible for the Thr61 phosphorylation was regulated during the cell cycle, we performed in vitro kinase assays using the GST-SLBPF protein as a substrate and lysates from HeLa cells isolated at different time points in the cell cycle as the kinase source. We tested extracts prepared from cells at different times after release from the thymidine block. The lysates prepared from G1/S (0 h), mid-S phase (4 h), S/G2 (6 h), M/G1 (10 or 11 h), and early and mid-G1 (12 and 14 h) were tested with the wild-type GST-SLBPF substrate, and we detected peak phosphorylation at late S phase (S/G2) overlapping the point where SLBP degradation started (Fig. 3A). We did not detect significant phosphorylation of this substrate using lysates from G1- or G1/S-phase cells. Note that there is an endogenous protein phosphorylated by kinases in the extract (Fig. 3A) which is present at similar levels in all lysates (also see Fig. 4B). Since we have previously shown that in vivo the KRKL region is required for both Thr61 phosphorylation and SLBP degradation at S/G2, we tested the phosphorylation profile of KRKL/4A GST-SLBPF. In the absence of the KRKL sequence, we could not detect significant phosphorylation in extracts from S and S/G2 cells (Fig. 3B, lanes 7 and 8), whereas there was a small amount of phosphorylation detected in the M/G1-phase lysate (Fig. 3B, lane 9). Addition of roscovitine to the in vitro kinase assays abolished the phosphorylation of the SLBP fragment and the residual phosphorylation seen in M/G1-phase extracts on the KRKL/4A mutant, suggesting that all the Thr61 phosphorylation, including the residual phosphorylation of the KRKL/4A mutant in the M/G1-phase extract, was mediated by a cyclin/Cdk (Fig. 3B).
FIG. 3.
Lysates from late-S-phase cells phosphorylate GST-SLBPF in a KRKL-dependent and roscovitine-sensitive manner. (A) In vitro kinase assays were performed in the presence of [γ-32P]ATP, using lysates from HeLa cells collected at indicated time points after the double-thymidine block (top row). Equal amounts of protein from total cell lysates were used in each lane. Samples were analyzed by SDS-PAGE, and the phosphorylation level of the substrate was determined by autoradiography (top row). The levels of SLBP and cyclin A were determined by Western blot analysis using anti-SLBP and anti-cyclin A (CycA) antibodies (middle and bottom rows). The asterisk indicates a closely migrating protein in the lysate whose phosphorylation level does not show significant change throughout the cell cycle. (B) The effects of roscovitine (10 μM) and the KRKL region on phosphorylation of mGST-SLBPF were analyzed. The GST-SLBPF protein with either the wild-type KRKL region or the 4A mutation was used as a substrate (indicated below the panel).
FIG. 4.
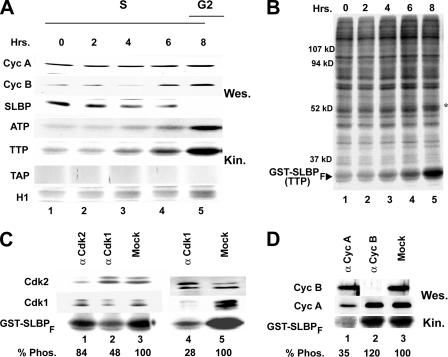
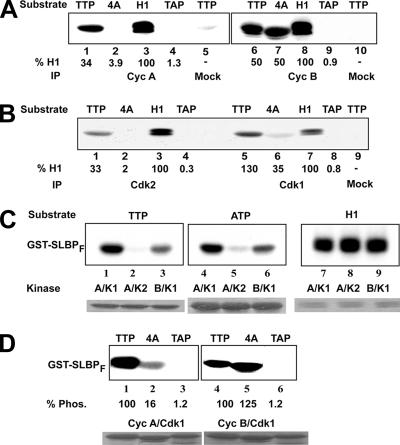
Phosphorylation in lysates from late-S-phase cells is on Thr61. (A) In vitro kinase assays with the indicated form of mGST-SLBPF (ATP, TTP, or TAP) or histone H1 protein were performed in the presence of [γ-32P]ATP, using equal amounts of lysate from HeLa cells collected at indicated time points after a double thymidine block (rows 4, 5, 6, and 7). Samples were analyzed by SDS-PAGE, and the phosphorylation level of each substrate was determined by autoradiography (rows 4, 5, 6, and 7). Levels of cyclin A (Cyc A), cyclin B (Cyc B), and the SLBP protein in the lysates were examined by Western blot analysis using corresponding antibodies (rows 1, 2, and 3). (B) An autoradiograph of the entire gel is shown for the experiment in panel A, row 5. The asterisk indicates an endogenous protein that is phosphorylated in parallel with GST-SLBPF. The positions of molecular mass markers are shown. (C) Late-S-phase HeLa cell lysates were immunodepleted with anti-Cdk1 (lanes 2), anti-Cdk2 (lane 1), or protein A beads (lanes 3 and 5) or double immunodepleted with anti-Cdk1 (lane 5). The levels of Cdk2 (top) and Cdk1 (middle) in each lysate were determined by Western blot analysis with corresponding antibodies. The autoradiogram of the in vitro kinase assay using the depleted extracts and GST-SLBPF as a substrate is shown at the bottom. (D) In vitro kinase assay using late-S-phase HeLa cell lysates immunodepleted with anti-cyclin A (lane 1), anti-cyclin B (lane 2), or protein A beads (lane 3). The level of cyclin B (top) or cyclin A (middle) remaining in each lysate was determined by Western blot (Wes.) analysis with corresponding antibodies. The autoradiogram of the in vitro kinase assay (Kin.) using the depleted extracts and GST-SLBPF as a substrate is shown at the bottom. The phosphorylation level (Phos.) of each substrate in panels C and D was quantified using a PhosphorImager and indicated as a percentage below the autoradiograph.
We then performed similar experiments at 2-h intervals after release from the thymidine block, repeating the same kinase assays with different time points throughout S phase. We detected phosphorylation peaking at late S phase (S/G2) on wild-type GST-SLBPF and the ATP mutant but not the TAP mutant (Fig. 4A), showing that the phosphorylation we detected occurred on Thr61. Interestingly, although phosphorylation on Thr61 significantly increased at S/G2, there was no significant increase in the phosphorylation of H1, indicating that in this experiment the cells had not reached the point in G2 where cyclin B/Cdk1 activity rapidly increases. We analyzed incorporation of 32PO4 into all the extract proteins, and there was similar total phosphorylation activity in the extract from each time point (Fig. 4B). There were similar total kinase activities in all the lysates, and the patterns of phosphorylated proteins were similar in all the lysates. There was one band which was phosphorylated in parallel with the GST-SLBPF substrate which might be an endogenous substrate for the Thr61 kinase (Fig. 4B, lanes 4 and 5).
Cyclin A/Cdk1 phosphorylates SLBP on Thr61.
In order to determine whether Cdk1 or Cdk2 was the major kinase responsible for the late-S-phase phosphorylation of Thr61, we performed immunodepletion experiments using late-S-phase lysates and Cdk1 or Cdk2 antibodies. In the case of Cdk2 depletion, although most of the Cdk2 was removed (Fig. 4C, lane 1), there was no significant difference in Thr61 phosphorylation. On the other hand, removal of even 40 to 50% of the Cdk1 from the lysate (Fig. 4C, lane 2) significantly reduced the phosphorylation on Thr61, suggesting that Cdk1 was the major kinase that is responsible for the phosphorylation detected in vitro. Mock depletion using the protein A beads alone had no effect on the kinase activity (Fig. 4C, lanes 3 and 5). We further reduced the amounts of Cdk1 in the extract by subjecting the extract to sequential depletion with two treatments with the anti-Cdk1 antibody. This treatment resulted in a further reduction of kinase activity using the GST-SLBPF substrate (Fig. 4C, lane 4) with no decrease in the amount of the Cdk2 protein.
We used antibodies against cyclin A and cyclin B to deplete these proteins and their associated kinases (Fig. 4D). Depletion of cyclin A resulted in a substantial reduction of kinase activity using the GST-SLBPF substrate (Fig. 4D, lane 1), while depletion of cyclin B (Fig. 4D, lane 2) had no effect. These results suggest that cyclin A/Cdk1 might be the kinase responsible for phosphorylating Thr61 of SLBP.
To determine the abilities of different cyclin/cdk's to phosphorylate SLBP on Thr61, we immunoprecipitated cyclin A and cyclin B complexes and Cdk2 or Cdk1 from late-S-phase lysates and tested their ability to phosphorylate the wild-type TTP, TAP, and KRKL/4A GST-SLBPF proteins using histone H1 as a control substrate. With these substrates, we detected phosphorylation only on GST-SLBPF containing TTP but not on the TAP GST-SLBPF, consistent with the fact we detect phosphorylation of SLBP only on Thr61. In order to determine the cyclin partner, we immunoprecipitated cyclin A/cdk's and cyclin B/cdk's and tested these kinase complexes for their ability to phosphorylate the GST-SLBPF proteins. Only cyclin A/cdk, but not cyclin B/cdk, required the KRKL region to phosphorylate Thr61 (Fig. 5A, lanes 1, 2, 6, and 7), indicating that cyclin B/Cdk1 is not likely to be the kinase responsible for Thr61 phosphorylation in vivo. Note that there is no phosphorylation of KRKL/4A GST-SLBPF detected in the late-S-phase lysates (Fig. 3B), although there is in the immunoprecipitates (Fig. 5A, lane 7). There is a much higher kinase concentration in the immunoprecipitates than in the lysates, and we presume that at the higher concentration the cyclin B immunoprecipitates can phosphorylate KRKL/4A GST-SLBPF.
FIG. 5.
The KRKL region is required for cyclin A/Cdk1 to phosphorylate Thr61. (A) Cyclin A (Cyc A) (lanes 1 to 4) and cyclin B (Cyc B) (lanes 6 to 9) immunoprecipitates (IP) from late-S-phase HeLa cell lysate were used in in vitro kinase assays in the presence of [γ-32P]ATP. Phosphorylation on the GST-SLBPF proteins TTP (lanes 1, 5, 6, and 10), KRKL/4A (lanes 2 and 7), TAP (lanes 4 and 9), and histone H1 (lanes 3 and 8) was detected by autoradiography. The phosphorylation level of each substrate was quantified using a PhosphorImager. For each set of reactions, the activity relative to that of histone H1 (set at 100) is given. (B) Cdk2 (lanes 1 to 4) or Cdk1 (lanes 5 to 8) immunoprecipitates from late-S-phase HeLa cell lysate were used in in vitro kinase assays in the presence of [γ-32P]ATP. Phosphorylation of GST-SLBPF proteins TTP (lanes 1, 5, and 9), KRKL/4A (lanes 2 and 6), and TAP (lanes 4 and 8), and of histone H1 (lanes 3 and 7) was detected by autoradiography. The phosphorylation level of each substrate was quantified by a PhosphorImager and normalized against the level of histone H1 phosphorylation. In the mock lanes (panel A, lanes 5 and 10, and panel B, lane 9), immunoprecipitations were done with just protein A beads. (C) Recombinant cyclin A/Cdk1 (lanes 1, 4, and 7), cyclin A/Cdk2 (lanes 2, 5, and 8), or cyclin B/Cdk1 (lanes 3, 6, and 9) were incubated with GST-SLBPF TTP (lanes 1 to 3) or ATP (lanes 4 to 6) or histone H1 (lanes 7 to 9) and [γ-32P]ATP. The proteins were resolved by gel electrophoresis and the phosphorylated proteins detected by autoradiography. The stained gel is shown below the lane. The experiment shown corresponds to a single gel and corresponding film image, with the assays with the different substrates separated by several lanes. (D) Recombinant cyclin A/Cdk1 (lanes 1 to 3) or cyclin B/Cdk1 (lanes 4 to 6) was incubated with GST-SLBPF TTP (lanes 1 and 4), KRKL/4A (lanes 2 and 5), or TAP (lanes 3 and 6). The proteins were resolved by gel electrophoresis and the phosphorylated (Phos.) proteins detected by autoradiography and quantified with a PhosphorImager. The activity was set at 100 for TTP with a particular kinase. The stained gel is shown below the lane. The experiment shown corresponds to a single gel and corresponding film image, with the assays with the different kinases separated by several lanes.
We also used antibodies against Cdk2 or Cdk1 to immunoprecipitate the cyclin/Cdk2 complexes and the cyclin/Cdk1 complexes from the same extracts and tested their ability to phosphorylate GST-SLBP on Thr61. As a control, we determined the ability of the immunoprecipitates to phosphorylate histone H1. The Cdk2 immunoprecipitates phosphorylated the SLBP fragment only 1/3 as efficiently as they phosphorylated histone H1 (Fig. 5B, lanes 1 and 3). All of this activity was dependent on the KRKL sequence. In contrast, the Cdk1 immunoprecipitates phosphorylated the SLBP fragment better than they phosphorylated histone H1 (Fig. 5B, lanes 5 and 7). The majority of this activity was dependent on the KRKL sequence (Fig. 5B, lane 6). When we normalized the ability of these Cdk immunoprecipitates to phosphorylate Thr61 to histone H1, we found that the Cdk1 immunoprecipitates phosphorylated GST-SLBPF three to four times more efficiently than the Cdk2 immunoprecipitates, further suggesting that a cyclin/Cdk1 is the kinase that phosphorylates SLBP on Thr61 and triggers its degradation at late S phase.
The Cdk2 complexes present in the cell are cyclin A/Cdk2 and cyclin E/Cdk2. Since there is a constant high level of cyclin A/Cdk2 activity during S phase and there is little Thr61 phosphorylation activity in the corresponding extracts (Fig. 4A), it is unlikely that cyclin A/Cdk2 is responsible for the phosphorylation of Thr61. The levels of cyclin E and cyclin E/Cdk2 activity are maximal in early S phase and low at late S phase (9), suggesting that this kinase complex is not responsible for the Thr61 kinase activity. The Cdk1 complexes that could be present in the cell are cyclin A/Cdk1 and cyclin B/Cdk1, and cyclin B/Cdk1 is the only known cyclin B/cdk complex.
We tested the abilities of recombinant cyclin A/Cdk2, cyclin A/Cdk1, and cyclin B/Cdk1 in amounts that showed similar histone H1 kinase activity (Fig. 5C, lanes 7 to 9) to phosphorylate the GST-SLBPF fusion protein (Fig. 5C). Cyclin A/Cdk1 phosphorylated the GST-SLBPF proteins on Thr61 (Fig. 5C, lanes 1 and 4), while cyclin A/Cdk2 had very little activity toward these substrates (Fig. 5C, lanes 2 and 5). Cyclin B/Cdk1 also phosphorylated the GST-SLBPF substrate on Thr61 (Fig. 5C, lanes 3 and 6), although not as effectively as cyclin A/Cdk1.
We then tested whether the activities of the recombinant kinases were dependent on the KRKL sequence, which is essential for SLBP degradation (37). Recombinant cyclin A/Cdk1 phosphorylated GST-SLBPF six times better than it phosphorylated the GST-SLBPF KRKL mutant (Fig. 5D, lanes 1 and 2). In contrast, cyclin B/Cdk1 phosphorylated both GST-SPBP fusion proteins equally well. Thus, all the data are consistent with cyclin A/Cdk1 being the kinase responsible for phosphorylating SLBP Thr61 at the end of S phase and triggering SLBP degradation.
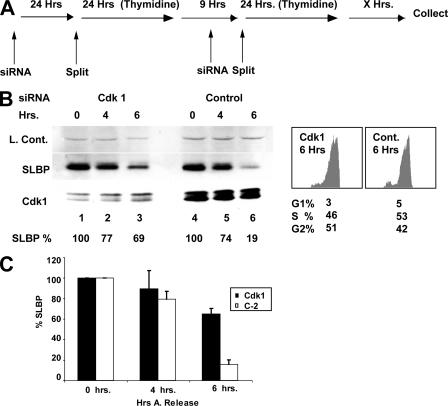
RNAi knockdown of Cdk1 results in stabilization of SLBP.
To further examine the role of Cdk1, we knocked down Cdk1 expression in synchronized HeLa cells using Cdk1-specific RNA interference (RNAi) (Fig. 6). Using the strategy shown in Fig. 6A, where siRNA treatment was combined with a double-thymidine block, we have been able to partially knock down Cdk1 while maintaining the ability to synchronize the cells. Control cells were treated with the same siRNA protocol using a control, C2 siRNA (C2) (31). The levels of the Cdk1 protein were reduced by approximately 50% by the RNAi treatment (Fig. 6B, cf. lanes 1 to 3 with lanes 4 to 6). At 6 h after release from the thymidine block, the cells were in late S phase. There was less SLBP degradation at late S phase when Cdk1 was knocked down compared to results for the control cells (Fig. 6B, lanes 3 and 6). The amount of SLBP remaining was normalized to a loading control, and the results of two experiments are averaged in Fig. 6C. FACS analysis of these cells showed that cells progressed through S phase and the cell cycle distribution of the cells was comparable in the treated and control cells (Fig. 6B, right).
FIG. 6.
Cdk1 knockdown inhibits late-S-phase SLBP degradation. (A) Outline of experimental setup for combining RNAi treatment with the synchronization procedure. (B) HeLa cells were transfected with Cdk1 siRNA (lanes 1 to 3) or a control siRNA (Cont.) (lanes 4 to 6), C2, followed by synchronization with a double thymidine block. Cells were collected at the indicated time points after the release from the double thymidine block. The levels of Cdk1 and SLBP were determined by Western blot analysis with corresponding antibodies. A cross-reacting band detected by the SLBP antiserum serves as a loading control. FACS analysis of the 6-h time point from cells in panel B with percentage values for each cell cycle phase indicated is shown at the right. (C) The effects of the RNAi treatment on SLBP levels, determined by densitometry of the Western blots from two independent experiments, were averaged.
Timing of SLBP degradation does not depend on Thr60 phosphorylation.
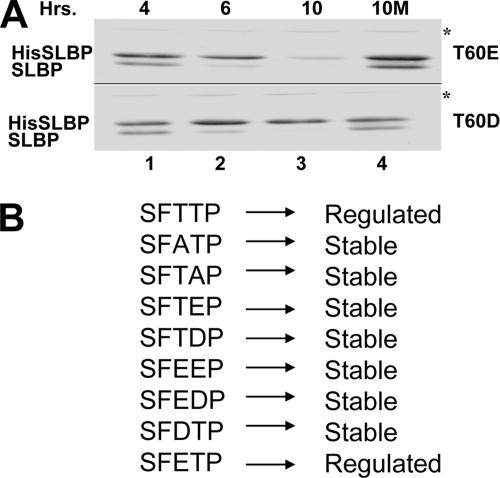
Previously we showed that changing either Thr60 or Thr61 to alanine stabilized SLBP at G2 (37). We previously tested some of the possible changes of Thr60 and Thr61 to acidic amino acids, but all of the changes we tested stabilized SLBP (37). Specifically, we could not mimic the phosphorylation on Thr61 by substitution with an acidic amino acid, and the SFEEP mutant was also stable (37). We constructed additional mutants in an attempt to mimic the phosphorylation of Thr60 and created the SFEDP, SFDTP, and SFETP mutants. We then selected populations of cells stably expressing the mutant SLBP proteins at a level similar to that for wild-type SLBP. The SFDTP mutant was stable at the end of S phase, indicating that the aspartic acid at position 60 did not mimic the phosphothreonine (Fig. 7A, bottom). In contrast, the SFETP mutant was regulated properly (Fig. 7A, top) and was degraded in parallel with wild-type SLBP. This was the only phosphorylation mutant that mimicked the wild-type protein (Fig. 7B). Treatment of the cells at 4 h after release with the proteosome inhibitor MG132 stabilized endogenous SLBP as well as the SFETP SLBP mutant protein (Fig. 7, lane 4). This result demonstrated that the glutamic acid at position 60 was capable of mimicking this phosphothreonine and must be able to target SLBP to the proteosome when Thr61 is also phosphorylated. Since SFETP SLBP was expressed at normal levels during S phase and then degraded, we conclude that phosphorylation of Thr61 by cyclin A/Cdk1 is essential for proper timing of the degradation of SLBP. In support of this, it has been shown that cyclin A/Cdk1 is activated at the end of S phase (21, 22). Thus, the mutagenesis data taken together with our biochemical results implicate cyclin A/Cdk1 as the kinase that phosphorylates Thr61 of SLBP, and this phosphorylation triggers degradation of SLBP at the end of S phase.
FIG. 7.
Changing Thr60 to Glu mimics phosphorylation of Thr60. (A) The first threonine, T60, in the SFTTP motif was changed to either glutamic acid (top) or aspartic acid (bottom) and stably expressed in HeLa cells as His-tagged SLBP (HisSLBP). Cells were synchronized by a double thymidine block and collected at the indicated hours after release. Levels of exogenous mutated His-tagged SLBP and endogenous SLBP were determined by Western blot analysis using SLBP antibody. The cells in lane 4 (10 M) were treated with MG132 (proteosome inhibitor) at 4 h after release and harvested at 10 h. (B) Summary of the effect of different mutations in the TTP motif on G2 SLBP degradation. “Regulated” indicates they showed a G2 degradation profile similar to that of endogenous SLBP.
CK2 phosphorylates Thr60, and phosphorylation is primed by phosphorylation of Thr61.
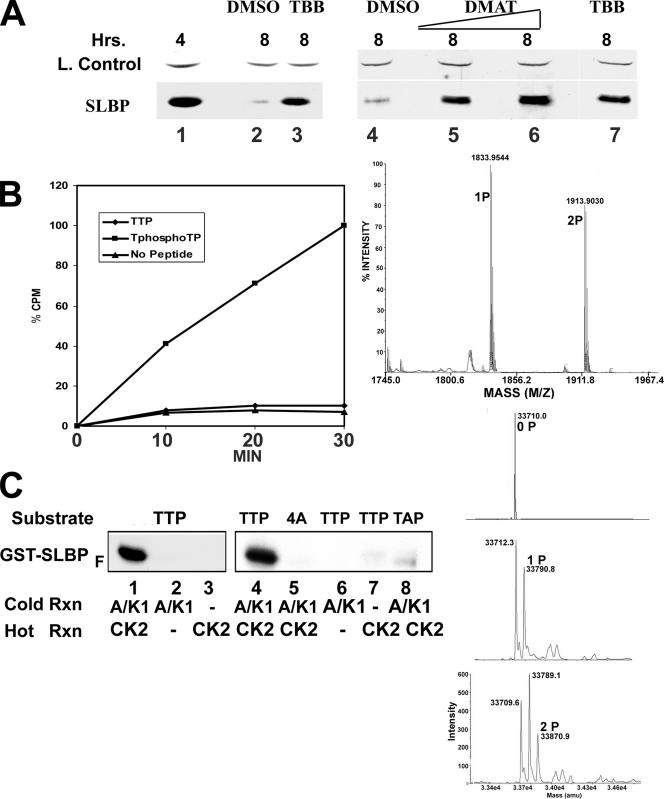
Analysis of the sequence around the SFTTPE sequence in SLBP (http://cbs.dtu.dk/services/NetPhosK) suggested that CK2 might phosphorylate T60. We tested the involvement of CK2 as a possible Thr60 kinase by using two different specific inhibitors of CK2, 4,5,6,7-tetrabromo-2-azabenzimidazole (TBB) and 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT). Treatment of cells with either TBB or DMAT at late S phase resulted in stabilization of SLBP in G2, and the degree of stabilization was dependent on the inhibitor concentration (Fig. 8A). Since CK2 phosphorylates serines or threonines which are often adjacent to a negative charge (acidic amino acids or a phosphorylated amino acid), we tested two synthetic peptide substrates that contained amino acids 54 to 68 of SLBP, one of which had a phosphothreonine at the equivalent of Thr61 and one which had a threonine at this position. The unphosphorylated substrate was not phosphorylated by CK2, but the phosphorylated substrate was actively phosphorylated (Fig. 8B), suggesting that phosphorylation of Thr61 primed the ability of CK2 to phosphorylate Thr60. We subjected the phosphorylated peptide to mass spectrometry analysis using a MALDI-time-of-flight spectrometer, and about 40% of the phosphorylated peptide was phosphorylated in a second site in 30 min (Fig. 8B, right). We have confirmed that a significant amount of the detected phosphorylation is on Thr60 by analysis of phosphoamino acids by thin-layer chromatography (data not shown).
FIG. 8.
CK2 phosphorylates Thr60 of SLBP. (A) Western blot analysis of SLBP levels in HeLa cells which were treated 4 h after release from a double thymidine block (at late S phase) and collected 4 h later at G2 with either DMSO (lanes 2 and 4) or different concentrations of two different CK2 inhibitors, TBB (75 μM [lane 3] or 50 μM [lane 7]) or DMAT (20 μM [lane 5] or 30 μM [lane 6]). The loading control is a cross-reacting band detected by the SLBP antisera. Lane 1 is a Western blot of cells 4 h after release prior to treatment. (B) The synthetic peptide TTP (54RRPESFTTPEGPKPR68) or the same peptide with a phosphothreonine at Thr 61 (TphosTP) was incubated with recombinant CK2 and [γ-32P]ATP, the peptides were bound to DEAE-paper, and the amount of the phosphorylated peptide was determined by liquid scintillation counting. The results are the averages from two experiments. On the right is analysis of the peptide by MALDI-TOF after 30 min of incubation with CK2. (C) The indicated GST-SLBPF substrates (above each lane) were incubated with unlabeled ATP and cyclin A/Cdk1 (lanes 1, 2, 4, 6, and 8) or buffer (lanes 3 and 7) overnight at 30°C. After the first incubation, CK2 (lanes 1, 3 to 5, 7, and 8) or buffer (lanes 2 and 6) was added together with [γ-32P]ATP and incubation continued for 30 min. The proteins were resolved by gel electrophoresis and the phosphorylated GST-SLBPF protein detected by autoradiography. GST-SLBPF was also incubated with unlabeled ATP and cyclin A/Cdk1 followed by CK2. GST-SLBPF was analyzed by electrospray ionization mass spectrometry, and the deconvoluted spectra of GST-SLBPF are shown (right, top), as is GST-SLBPF after incubation with cyclin A/Cdk1 (right, middle) and after incubation with cyclin A/Cdk1 followed by incubation with CK2 (right, bottom). The new peaks in the middle and bottom panels correspond to addition of one and two phosphates, respectively. Rxn, reaction.
To demonstrate that cyclin A/Cdk1 could prime phosphorylation by CK2 on Thr60 in the context of the region of SLBP that targets SLBP degradation, we carried out sequential phosphorylation reactions using our GST-SLBPF fusion proteins as substrates. The first reaction was carried out in the presence of unlabeled ATP in the presence or absence of recombinant cyclin A/Cdk1, and then a second incubation was carried out with radiolabeled ATP in the presence or absence of recombinant CK2. After 20 h of incubation at 30°C, cyclin A/Cdk1 was inactivated (Fig. 8C, lane 2), and incubation of the substrate only with CK2 and radiolabeled ATP resulted in no significant phosphorylation (Fig. 8C, lane 3). There was substantial phosphorylation of GST-SLBPF that was dependent on the addition of CK2 and prior incubation with cyclin A/Cdk1 (Fig. 8C, lane 1). Phosphorylation by CK2 was dependent on both the cyclin binding site in GST-SPBPF (Fig. 8C, lane 5) and the presence of Thr61 in the substrate (Fig. 8C, lane 8).
We confirmed these results by analysis of the GST-SLBP fragments by mass spectrometry. Using ESI (electrospray ionization) to determine the molecular weight of the GST-SLBP fragments, we showed that incubation with cyclin A/Cdk1 resulted in addition of one phosphate to GST-SLBP and subsequent incubation with CK2 resulted in addition of a second phosphate. The phosphorylation sites were confirmed as T61 and T60 by analysis by mass spectrometry on a MALDI TOF/TOF spectrometer(data not shown).
We have identified the two kinases responsible for phosphorylation of Thr60 and Thr61 that result in degradation of SLBP. Cyclin A/Cdk1 phosphorylates Thr61 at the end of S phase, and this phosphorylation is necessary for subsequent phosphorylation by CK2 on Thr60. Thus, activation of cyclin A/Cdk1 is responsible for the timing of SLBP degradation at the end of S phase.
DISCUSSION
The molecular events that occur at late S phase or the S/G2 transition, at the completion of chromosome replication, are poorly understood. Here we describe a cell cycle signaling mechanism that corresponds to the end of S phase where activation of cyclin A/Cdk1 results in an inhibition of histone mRNA biosynthesis by triggering the degradation of the stem-loop binding protein, the only known cell cycle-regulated protein in histone mRNA metabolism.
Thr60 and Thr61 phosphorylation mediates SLBP degradation at S/G2.
We have previously shown that phosphorylation of Thr60 and Thr61 is required for SLBP degradation at S/G2. Along with those threonines, the adjacent Pro62 and a downstream putative cyclin binding site (KRKL) are required for the Thr61 phosphorylation and SLBP degradation. Using stable expression of GST-SLBPF fragments containing only 57 amino acids, including both threonines and the KRKL region, but with all other possible phosphoacceptor sites (Thr and Ser) changed to alanine, we have shown that phosphorylation on Thr60 and Thr61 within this fragment constitutes a sufficient phosphorylation signal for SLBP degradation at the end of S phase.
Cyclin A/Cdk1 triggers SLBP degradation at S/G2.
The TP motif and requirement of a downstream KRKL region which is a putative cyclin binding site (RXL) suggested the involvement of a cyclin/Cdk in SLBP degradation by phosphorylation of Thr61. In support of this idea, we found that treatment of HeLa cells with roscovitine, a Cdk1/Cdk2 inhibitor, at late S phase inhibited the degradation of SLBP at S/G2. Furthermore, using our GST-SLBPF substrate for in vitro kinase assays, we showed that Thr61 kinase activity was detected only in late-S-phase lysates and the activity was roscovitine sensitive, supporting the idea that a cyclin/cdk is the Thr61 kinase.
Among the possible cyclin/cdk's that could phosphorylate GST-SLBPF based on the pattern of Thr kinase activity, we conclude cyclin A/Cdk1 is likely the Thr61 kinase. This result is supported by immunodepletion (Fig. 4) and immunoprecipitation (Fig. 5A and B) experiments in vitro, studies with recombinant cyclin A/Cdk1, cyclin A/Cdk2, and cyclin B/Cdk1 (Fig. 5C and D), and Cdk1 knockdown experiments in vivo (Fig. 6). Strikingly, cyclin A/Cdk1 but not cyclin A/Cdk2 phosphorylates T61 in vitro. Cyclin B/Cdk1 is also capable of phosphorylating T61 in vitro, but the activity is independent of the cyclin binding motif, KRKL, that is required for degradation of SLBP in vivo. These results suggest that the Cdk1 kinase can recognize T61 while Cdk2 cannot.
Cyclin A/Cdk1 kinase activity is important at the end of S phase.
There is an increase in cyclin A/Cdk1 activity at the end of S phase, and it increases prior to the activation of cyclin B/Cdk1 (21, 22). This pattern of activity is distinct from that of cyclin A/Cdk2, which is active throughout S phase and increases only slightly during G2 (22, 24, 26). Although there is high cyclin A/Cdk2 activity throughout S phase, both the in vitro Thr61 kinase activity in cell lysates and the SLBP degradation in vivo do not occur until the S/G2 border. Thus, the Thr61 phosphorylation profile we detected correlates well with cyclin A/Cdk1 activity which increases toward late S phase, with a gradual increase toward the point where it peaks at G2 (21, 22).
In vitro, cyclin A/Cdk2 is capable of phosphorylating SLBP on T61 at about 10% of the level observed with cyclin A/Cdk1. Cyclin B/Cdk1 is also capable of phosphorylating SLBP on T61, although this phosphorylation is not dependent on the KRKL sequence, which is required for SLBP degradation in vivo and for phosphorylation by either cyclin A kinase. In S-phase extracts where cyclin A/Cdk2 activity is high, we did not observe significant phosphorylation of T60. It is possible that cyclin A/Cdk2 phosphorylates SLBP at a low rate and that this accounts for the relatively short half-life (about 2 h) observed for SLBP normally (34). It is also possible that cyclin A/Cdk2 does not phosphorylate SLBP in vivo, possibly because it is sequestered by other substrates. Based on the stability of SLBP through mitosis when the KRKL sequence is mutated (37), it is likely that cyclin B/Cdk1 does not phosphorylate SLBP in vivo during mitosis, although it is more active than cyclin A/Cdk2 in vitro. These differences most likely reflect the differences (particularly the relatively high kinase and substrate concentrations) between the in vivo and in vitro situations.
Several substrates for cyclin A/Cdk1 have been found, and many of these are phosphorylated at the end of S phase or in G2. One substrate is Orc1, which is phosphorylated in G2/M, blocking its binding to chromatin, and this phosphorylation is part of the mechanism that prevents rereplication of DNA (14). Another substrate is the Flap endonuclease Fen1, which is phosphorylated by cyclin A/Cdk1 in late S phase, resulting in its dissociation from PCNA and likely playing a role in preventing further DNA replication (12). A third target of cyclin A/Cdk1 is the CCAAT displacement factor (CDP)/Cux transcription factor. It is phosphorylated by cyclin A/Cdk1 in G2 phase, and this phosphorylation blocks DNA binding and hence transcription activation activity (28). One target gene which is downregulated as a result is DNA polymerase α (29). The CDP/Cux transcription factor can also be phosphorylated by cyclin A/Cdk2, but this phosphorylation is on a different site and has no affect on the transcription activation activity (29). Cyclin A/Cdk1 also participates in anaphase promoting complex regulation, helping keep the anaphase promoting complex inactive in late S phase and early G2 (21).
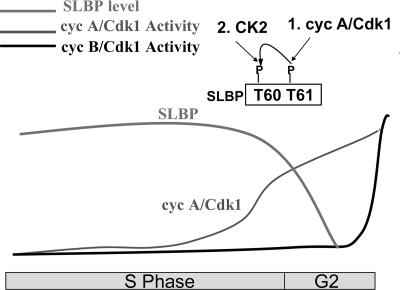
All of these substrates are involved in events that occur at the end of S phase and in early G2, particularly in downregulating the ability of cells to replicate DNA. The phosphorylation of SLBP by cyclin A/Cdk1 results in the subsequent degradation of SLBP and effectively downregulates histone mRNA biosynthesis. Thus, increasing cyclin A/Cdk1 activity at the end of S phase results in downregulation of both DNA replication and histone synthesis (Fig. 9).
FIG. 9.
Model for triggering SLBP degradation at S/G2. In contrast to cyclin B/Cdk1 activity, which increases rapidly at G2/M, there is a gradual increase in cyclin A/Cdk1 activity at the end of S phase that reaches a sufficient level at the S/G2 border to phosphorylate Thr61 of SLBP, and this primes subsequent phosphorylation of Thr60 by CK2 to mark SLBP for degradation.
A final known substrate of cyclin A/Cdk1 is CDC25B phosphatase, which is also targeted for degradation by phosphorylation by cyclin A/Cdk1 and is not phosphorylated by cyclin B/Cdk1 (4). This is the only other known substrate which is degraded as a result of phosphorylation by cyclin A/Cdk1. The biological role of this phosphorylation of CDC25B is not clear, but it may provide a mechanism for fine-tuning the activity of cyclin A/Cdk1.
Phosphorylation of Thr61 likely triggers SLBP degradation at the end of S phase.
The results with the substitution of acidic amino acids for Thr60 strongly suggest that the phosphorylation of Thr61 is the event that determines the timing of SLBP degradation. Only one of the possible acidic amino acid substitutions, SFETP, resulted in an SLBP that was appropriately regulated. Since in this mutant SLBP, Thr60 phosphorylation was not regulated (since we replaced this amino acid with E), we can conclude that Thr61 phosphorylation plays a role in determining the timing of SLBP degradation at G2. The failure of the SFEEP mutant to mimic the degradation signal is likely due to a failure of the E3 ligase to recognize SFEEP. It is also possible that the E3 ubiquitin ligase is cell cycle regulated and is part of the timing mechanism. According to our model, however, a critical component of the timing mechanism is an increase in the cyclin A/Cdk1 activity at the end of S phase, resulting in phosphorylation of SLBP at Thr61 and initiating SLBP degradation.
Phosphorylation of Thr60 by CK2 requires phosphorylation of Thr61 by cyclin A/Cdk1.
We did not detect phosphorylation of Thr60 in cell lysates in our experiments, either as a roscovitine-resistant phosphorylation of the wild-type SLBP fragment or as phosphorylation of the TAP mutant, raising the possibility that phosphorylation of Thr60 requires prior phosphorylation of Thr61. Treatment of cells in late S phase with CK2 inhibitors resulted in stabilization of SLBP at G2 (Fig. 8A), which led us to explore the possible role of CK2 in SLBP phosphorylation. Sequential phosphorylations dependent on an initial phosphorylation event are common (25), and the CK2 consensus target sequence S/T(−)X(−) (19, 35), which has a requirement for nearby negative charges, can be created by priming phosphorylations by other kinases (19). SLBP also has a conserved acidic amino acid at position +3 (Fig. 2A). However, replacement of the glutamic acid (E63) with alanine does not prevent degradation of SLBP (M. M. Koseoglu, unpublished data). Clearly some phosphorylation of Thr60 can occur in the absence of Thr61 phosphorylation, since the KRKL mutant SLBP is detectably phosphorylated in vivo on Thr60 alone without Thr61 phosphorylation (37), although we couldn't quantify the level of this phosphorylation.
CK2 is a constitutively active Ser/Thr kinase which is ubiquitously expressed throughout the cell cycle (15, 19). It is a highly pleiotropic kinase which is involved in several cellular events, including transcription, mRNA processing, translation, and several aspects of cell cycle. CK2 activity is necessary for both the G1/S and G2/M transition in yeast (10) and mammalian cells (23, 33). CK2 activity is involved in Wee1 degradation in G2, and this occurs at a similar time to that of SLBP degradation (33). In both cases a cyclin/Cdk1 primes phosphorylation by CK2, resulting in production of a phosphodegron which mediates degradation.
Our in vitro sequential phosphorylation analysis, along with the in vivo effect of CK2 inhibition on SLBP stability, suggests CK2 is the Thr60 kinase involved in SLBP degradation at the end of S phase. Although all the in vitro data support a role for CK2 in phosphorylating Thr60, we can't totally rule out the possibility that there is a different kinase that does this in vivo and that CK2 has an indirect effect on SLBP stability.
Conclusions.
SLBP is the sole protein factor involved in histone mRNA processing which is not present at cell cycle phases other than S phase. Along with the regulation of histone mRNA transcription and stability, cell cycle regulation of SLBP is one of the mechanisms that limit histone mRNA biosynthesis and thus histone production to S phase. Phosphorylation of SLBP by cyclin A/Cdk1 and CK2 is one of the mechanisms that effectively shuts down histone mRNA biosynthesis at the end of S phase. When DNA replication is completed, histone mRNA is rapidly degraded, and this does not require SLBP degradation (37). There are likely a number of proteins degraded at the end of S phase, and it is possible that cyclin A/Cdk1 may phosphorylate a number of other proteins at this time to inhibit chromosome replication.
Acknowledgments
This work was supported by NIH grants GM29832 (to W.F.M.) and GM69976 (to L.M.G.).
We thank Cell Signaling for kindly providing recombinant cyclin A/Cdk1. We thank Viorel Mecanu at the UNC Proteomics Facility for the mass spectrometry analysis.
Footnotes
Published ahead of print on 19 May 2008.
REFERENCES
- 1.Adams, P. D., X. T. Li, W. R. Sellers, K. B. Baker, X. H. Leng, J. W. Harper, Y. Taya, and W. G. Kaelin, Jr. 1999. Retinoblastoma protein contains a C-terminal motif that targets it for phosphorylation by cyclin-cdk complexes. Mol. Cell. Biol. 191068-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, P. D., W. R. Sellers, S. K. Sharma, A. D. Wu, C. M. Nalin, and W. G. Kaelin, Jr. 1996. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol. Cell. Biol. 166623-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archambault, V., N. E. Buchler, G. M. Wilmes, M. D. Jacobson, and F. R. Cross. 2005. Two-faced cyclins with eyes on the targets. Cell Cycle 4125-130. [DOI] [PubMed] [Google Scholar]
- 4.Baldin, V., C. Cans, M. Knibiehler, and B. Ducommun. 1997. Phosphorylation of human CDC25B phosphatase by CDK1-cyclin A triggers its proteasome-dependent degradation. J. Biol. Chem. 27232731-32734. [DOI] [PubMed] [Google Scholar]
- 5.Brown, N. R., M. E. Noble, J. A. Endicott, and L. N. Johnson. 1999. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat. Cell Biol. 1438-443. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, D. J. 2002. Proteolysis and the cell cycle. Cell Cycle 1233-234. [DOI] [PubMed] [Google Scholar]
- 7.De Azevedo, W. F., S. LeClerc, L. Meijer, L. Havlicek, M. Strnad, and S. H. Kim. 1997. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur. J. Biochem. 243518-526. [DOI] [PubMed] [Google Scholar]
- 8.Dominski, Z., X. Yang, C. S. Raska, C. S. Santiago, C. H. Borchers, R. J. Duronio, and W. F. Marzluff. 2002. 3′ end processing of Drosophila histone pre-mRNAs: requirement for phosphorylated dSLBP and coevolution of the histone pre-mRNA processing system. Mol. Cell. Biol. 226648-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulic, V., E. Lees, and S. I. Reed. 1992. Association of human cyclin E with a periodic G1-S phase protein kinase. Science 2571958-1961. [DOI] [PubMed] [Google Scholar]
- 10.Glover, C. V., III. 1998. On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 5995-133. [DOI] [PubMed] [Google Scholar]
- 11.Harris, M. E., R. Böhni, M. H. Schneiderman, L. Ramamurthy, D. Schümperli, and W. F. Marzluff. 1991. Regulation of histone mRNA in the unperturbed cell cycle: evidence suggesting control at two posttranscriptional steps. Mol. Cell. Biol. 112416-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henneke, G., S. Koundrioukoff, and U. Hubscher. 2003. Phosphorylation of human Fen1 by cyclin-dependent kinase modulates its role in replication fork regulation. Oncogene 224301-4313. [DOI] [PubMed] [Google Scholar]
- 13.Hoyt, M. A. 1997. Eliminating all obstacles: regulated proteolysis in the eukaryotic cell cycle. Cell 91149-151. [DOI] [PubMed] [Google Scholar]
- 14.Li, C. J., A. Vassilev, and M. L. DePamphilis. 2004. Role for Cdk1 (Cdc2)/cyclin A in preventing the mammalian origin recognition complex's largest subunit (Orc1) from binding to chromatin during mitosis. Mol. Cell. Biol. 245875-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 3691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loog, M., and D. O. Morgan. 2005. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434104-108. [DOI] [PubMed] [Google Scholar]
- 17.Lüscher, B., C. Stauber, R. Schindler, and D. Schümperli. 1985. Faithful cell-cycle regulation of a recombinant mouse histone H4 gene is controlled by sequences in the 3′-terminal part of the gene. Proc. Natl. Acad. Sci. USA 824389-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzluff, W. F. 2005. Metazoan replication dependent histone mRNAs: a unique class of RNA polymerase II transcripts. Curr. Opin. Cell Biol. 17274-280. [DOI] [PubMed] [Google Scholar]
- 19.Meggio, F., and L. A. Pinna. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17349-368. [DOI] [PubMed] [Google Scholar]
- 20.Meijer, L., and E. Raymond. 2003. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc. Chem. Res. 36417-425. [DOI] [PubMed] [Google Scholar]
- 21.Mitra, J., G. H. Enders, J. Azizkhan-Clifford, and K. L. Lengel. 2006. Dual regulation of the anaphase promoting complex in human cells by cyclin A-Cdk2 and cyclin A-Cdk1 complexes. Cell Cycle 5661-666. [PubMed] [Google Scholar]
- 22.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepperkok, R., P. Lorenz, W. Ansorge, and W. Pyerin. 1994. Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. J. Biol. Chem. 2696986-6991. [PubMed] [Google Scholar]
- 24.Pines, J., and T. Hunter. 1990. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 346760-763. [DOI] [PubMed] [Google Scholar]
- 25.Roach, P. J. 1991. Multisite and hierarchal protein phosphorylation. J. Biol. Chem. 26614139-14142. [PubMed] [Google Scholar]
- 26.Rosenblatt, J., Y. Gu, and D. O. Morgan. 1992. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc. Natl. Acad. Sci. USA 892824-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez, I., and B. D. Dynlacht. 2005. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 16311-321. [DOI] [PubMed] [Google Scholar]
- 28.Santaguida, M., Q. Ding, G. Berube, M. Truscott, P. Whyte, and A. Nepveu. 2001. Phosphorylation of the CCAAT displacement protein (CDP)/Cux transcription factor by cyclin A-Cdk1 modulates its DNA binding activity in G(2). J. Biol. Chem. 27645780-45790. [DOI] [PubMed] [Google Scholar]
- 29.Santaguida, M., and A. Nepveu. 2005. Differential regulation of CDP/Cux p110 by cyclin A/Cdk2 and cyclin A/Cdk1. J. Biol. Chem. 28032712-32721. [DOI] [PubMed] [Google Scholar]
- 30.Schulman, B. A., D. L. Lindstrom, and E. Harlow. 1998. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl. Acad. Sci. USA 9510453-10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner, E. J., and M. A. Garcia-Blanco. 2002. RNAi-mediated PTB depletion leads to enhanced exon definition. Mol. Cell 10943-949. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Z.-F., M. L. Whitfield, T. I. Ingledue, Z. Dominski, and W. F. Marzluff. 1996. The protein which binds the 3′ end of histone mRNA: a novel RNA-binding protein required for histone pre-mRNA processing. Genes Dev. 103028-3040. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe, N., H. Arai, J. Iwasaki, M. Shiina, K. Ogata, T. Hunter, and H. Osada. 2005. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc. Natl. Acad. Sci. USA 10211663-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield, M. L., L.-X. Zheng, A. Baldwin, T. Ohta, M. M. Hurt, and W. F. Marzluff. 2000. Stem-loop binding protein, the protein that binds the 3′ end of histone mRNA, is cell cycle regulated by both translational and posttranslational mechanisms. Mol. Cell. Biol. 204188-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wojda, I. 2000. Protein kinase CKII. Postepy Biochem. 46148-153. (In Polish.) [PubMed] [Google Scholar]
- 36.Yam, C. H., T. K. Fung, and R. Y. Poon. 2002. Cyclin A in cell cycle control and cancer. Cell Mol. Life Sci. 591317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng, L.-X., Z. Dominski, X. Yang, P. Elms, C. S. Raska, C. H. Borchers, and W. F. Marzluff. 2003. Phosphorylation of SLBP on two threonines triggers degradation of SLBP, the sole cell cycle-regulated factor required for regulation of histone mRNA processing, at the end of S phase. Mol. Cell. Biol. 231590-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]