Abstract
Copper is an essential cofactor of two mitochondrial enzymes: cytochrome c oxidase (COX) and Cu-Zn superoxide dismutase (Sod1p). Copper incorporation into these enzymes is facilitated by metallochaperone proteins which probably use copper from a mitochondrial matrix-localized pool. Here we describe a novel conserved mitochondrial metallochaperone-like protein, Cmc1p, whose function affects both COX and Sod1p. In Saccharomyces cerevisiae, Cmc1p localizes to the mitochondrial inner membrane facing the intermembrane space. Cmc1p is essential for full expression of COX and respiration, contains a twin CX9C domain conserved in other COX assembly copper chaperones, and has the ability to bind copper(I). Additionally, mutant cmc1 cells display increased mitochondrial Sod1p activity, while CMC1 overexpression results in decreased Sod1p activity. Our results suggest that Cmc1p could play a direct or indirect role in copper trafficking and distribution to COX and Sod1p.
Mitochondria require a large variety of chemical elements to perform biological functions such as respiration and free radical scavenging. Among these elements, copper is an essential redox metal used as a cofactor by critical enzymes, including mitochondrial cytochrome c oxidase (COX) and Cu-Zn superoxide dismutase (Sod1p).
COX is a multimeric metalloenzyme that contains two copper metal centers which are involved in the catalytic activity of the enzyme (37). The CuA site contains two copper atoms and is located in subunit 2 of COX (Cox2p). A third copper atom, referred to as CuB, is associated with the heme A group of heme a3 in subunit 1 (Cox1p). Sod1p contains one copper atom paired with one zinc atom at its catalytic site, and although the enzyme is present mostly in the cytoplasm, 1 to 5% of the total Sod1p is localized in the mitochondrial intermembrane space (IMS) (36). The exact pathways of copper trafficking to mitochondria and incorporation into these two enzymes are not fully understood.
Copper metalation of COX and Sod1p enzymes occurs within the mitochondrial IMS and requires the assistance of evolutionarily conserved enzyme-specific copper chaperones. Sod1p copper insertion requires the specific copper chaperone Ccs1p (12). A portion of Ccs1p is localized to the mitochondrial IMS and is required to metalate Sod1p, as this protein is imported into mitochondria without its copper cofactor (15, 36).
COX copper metalation is more complicated. It involves the IMS chaperone Cox17p (19), a small hydrophilic protein which binds copper(I) ions (7) through a CCXC metal binding motif. Cox17p also contains a twin CX9C structural motif. While the first cysteine in the CX9C motif is part of the copper binding motif, the remaining three conserved cysteines are not important for Cox17p copper binding function as shown by mutational analyses of the yeast protein (21). However, very recent structural studies on human COX17 have shown that the two adjacent cysteines in the CCXC domain are actually the copper binding cysteines (3). Cox17p transfers copper ions to two additional chaperones, Sco1p (20) and Cox11p (8, 22), that facilitate copper insertion into the COX CuA and CuB active sites, respectively. These proteins are anchored to the mitochondrial inner membrane through a transmembrane α-helix and expose their copper binding sides in the IMS, where copper transfer occurs (7, 9).
Two homologues of Cox17p are present in the IMS; these are the small soluble proteins Cox19p and Cox23p, which also contain twin CX9C metal binding motifs and are required for COX assembly (6, 30). Although Cox23p does not physically interact with Cox17p in a stable complex, recent data suggested that they function in a common pathway with Cox17p acting downstream of Cox23p (6). Cox19p could be part of the same copper distribution pathway. The recombinant form of Cox19p was reported to bind copper, a function for which only the amino (C30)- and carboxyl (C62)-terminal cysteines in the twin CX9C domains seem to be important (33).
The CX9C twin motifs present in Cox17p, Cox19p, and Cox23p are critical for the import of these proteins into the mitochondrial IMS through the recently described Mia40p pathway (27). After passage through the mitochondrial outer membrane TOM channel, these proteins are covalently trapped by Mia40p via disulfide bridges. Mia40p also contains cysteine residues, which are oxidized by the sulfhydryl oxidase Erv1p, and thus functions in a disulfide relay system that catalyzes the import of proteins into the IMS by an oxidative folding mechanism (27). The in vivo redox state of Cox17p, Cox19p, and Cox23p is not completely understood, but reduction of the cysteines could be required for metal binding as has been recently proposed (31), suggesting the existence of a mechanism of cysteine reduction in the IMS after the oxidative folding pathway. Additionally, Mia40p has been shown to affect Sod1p import into the IMS, probably in an indirect way by modulating Ccs1p import (27).
The copper used for COX and Sod1p metalation is provided from a matrix-localized pool consisting of a low-molecular-weight nonproteinaceous copper complex (11). However, the identities of key proteins in the copper trafficking process remain elusive. How copper is delivered to mitochondria and by which predicted mitochondrial membrane copper permease(s), how copper is transported from the matrix pool to the copper chaperones located in the IMS, and how the trafficking of copper toward COX and Sod1p is regulated are some of the open questions remaining.
In our search for novel protein candidates that can perform these functions, we used a two-step analysis of yeast proteins with unknown function in the Saccharomyces Genome Database website. We attempted to identify mitochondrial proteins involved in respiration and with sequences containing domains suggestive of metal binding and that are conserved in higher eukaryotes. One candidate resembled the copper chaperones Cox17p, Cox19p, and Cox23p in containing a twin CX9C domain. It is encoded by the open reading frame YKL137W and codes for a protein we have termed Cmc1p (CX9C mitochondrial protein required for full expression of COX). We show that yeast Cmc1p is an inner membrane-bound IMS protein that is present in high-molecular-weight complexes. Recombinant Cmc1p is capable of binding Cu(I) in a thiolate coordination, an ability shared with Cox17p and Cox19p. A human Cmc1p homologue also localizes to mitochondria in HeLa cells. Ablation of yeast CMC1 results in defective COX assembly and increased levels of active mitochondrial Sod1p compared to those in a wild-type strain. Our data therefore suggest that Cmc1p could be involved in regulating copper metalation of COX, affecting the levels of active mitochondrial Sod1p.
MATERIALS AND METHODS
Strains and media.
Most of the work was carried out with the wild-type strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and the isogenic Δcmc1 mutant strain in which the open reading frame YKL137W had been deleted by insertion of a kanamycin cassette. The strains, created by the Saccharomyces Genome Database project at Stanford University, CA (18), were obtained from Open Biosystems (Huntsville, AL), and the mutation in the Δcmc1 strain was confirmed by PCR-based tests. The kanamycin cassette and flanking sequences of the CMC1 gene in the Δcmc1 strain were PCR amplified, and the amplicon was transformed into a W303-1A wild-type strain (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) to create a W303Δcmc1 mutant strain. All S. cerevisiae strains used are listed in Table S1 in the supplemental material. The growth medium compositions have been described elsewhere (28) and are given in the supplemental material.
Characterization of yeast mitochondrial respiratory chain.
Mitochondria were prepared from strains grown in medium containing 2% galactose according to the method of Faye et al. (14) except that Zymolyase 20T (ICN Biochemicals Inc., Aurora, OH) instead of Glusulase was used for the conversion of cells to spheroplasts. Mitochondria prepared from the different strains were assayed polarographically for KCN-sensitive NADH oxidase using a Clark-type polarographic oxygen electrode from Hansatech Instruments (Norfolk, United Kingdom) at 24°C as described previously (4). Respiration was also assayed in whole cells in the presence of galactose or ethanol-glycerol (EG). The specific activities reported were corrected for KCN-insensitive respiration. Mitochondria were subsequently used for spectrophotometric assays carried out at 24°C to measure KCN-sensitive COX activity and antimycin A-sensitive NADH cytochrome c reductase activity as described previously (4). Total mitochondrial cytochrome spectra were obtained as reported previously (38). Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and quantification of COX was performed as described previously (29, 34). The sedimentation properties of Cmc1p-hemagglutinin (Cmc1p-HA) in sucrose gradients were analyzed as described previously (5). A detailed explanation of all the methods used is reported in the supplemental material.
Expression and purification of recombinant Cmc1p from Escherichia coli.
A sequence containing the CMC1 gene was cloned using NdeI and BamHI restriction enzymes into the pET15b protein expression vector (Novagen, Madison, WI), which provided a thrombin-cleavable N-terminal His6 affinity tag. The protein expression vector was transformed into the BL21 Star protein expression strain of E. coli (Invitrogen, Carlsbad, CA). Cells were initially grown overnight in 5 ml of Luria broth (LB) containing carbenicillin (50 μg/ml) at 37°C. Starter cultures were then diluted into 500 ml of minimal phosphate medium with antibody, grown with shaking (250 rpm) at 37°C to an optical density of 0.7, and then induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. The cells were harvested by centrifugation at 3,500 × g for 20 min and subsequently lysed by resuspension in 10 ml lysis buffer (50 mM Tris-HCl [pH 7.5], 300 mM NaCl, 50 mM imidazole, 1 mM β-mercaptoethanol, 20% glycerol, 0.1% [vol/vol] Triton X-100, 1 mM benzamidine, 2 mM phenylmethylsulfonyl fluoride) containing additional DNase I (0.1 mg/ml) per gram of cells. The cells were lysed using the EmulsiFlex-C3 high-pressure homogenizer (Avestin, Inc., Ottawa, Ontario, Canada), and particulates were removed from the lysate by centrifugation for 20 min at 14,000 rpm in an SS-34 Sorvall rotor.
The soluble E. coli lysate containing the His6-CMC1 recombinant fusion construct was directly loaded by FPLC (1 ml/min) onto a 5 ml size Ni2+ Sepharose HiTrap HP affinity column (Amersham, Piscataway, NJ) equilibrated at 4°C in [50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 50 mM imidazole, 1 mM β-mercaptoethanol, 20% glycerol, 0.1% vol/vol Triton X-100]. The column was subsequently washed until the absorbances at 254 nm and 280 nm returned to baseline. The His6-CMC1construct was eluted by increasing the imidazole concentration from 50 to 500 mM at 1 ml/min for 20 min. Chromatographic fractions (1 ml) containing the designated fusion protein were detected by absorbance at 280 nm and analyzed by sodium dodecyl sulfate (SDS)-PAGE. Fractions containing recombinant protein were pooled and incubated with 1 unit per mg of thrombin for 2 h at room temperature to cleave off the His6 tag. After the incubation with thrombin, separation of cleaved and noncleaved recombinant protein was performed by passing the sample on a 5-ml Ni2+-Sepharose HiTrap HP affinity column (Amersham) and collecting the flowthrough. The resultant sample was subjected to gel exclusion chromatography for buffer exchange into 50 mM Tris-HCl (pH 7.5)-300 mM NaCl-1 mM β-mercaptoethanol and subsequently concentrated and loaded on a Hiload Superdex 75 (Amersham) gel filtration column equilibrated in the same buffer. High-purity Cmc1p was subsequently collected from the appropriate fractions and concentrated as needed.
Preparation of Apo-Cmc1p, copper reconstitution, and fluorescence analyses.
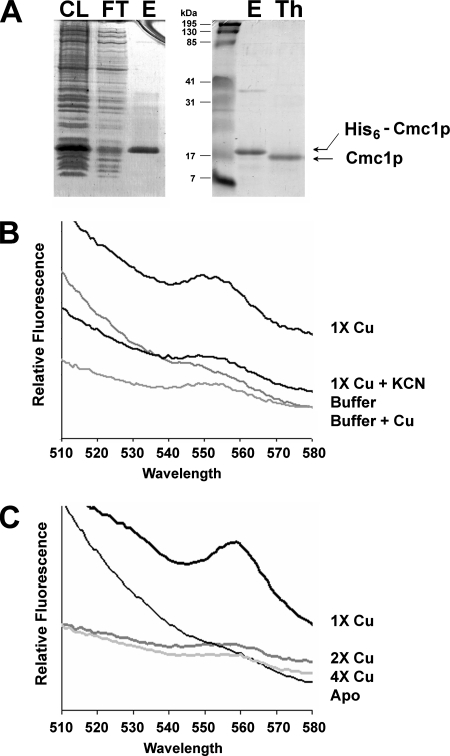
Apoprotein preparation and copper reconstitution were performed as described previously (13). Briefly, the purified Cmc1p protein (20 μM) was denatured and reduced in 200 mM Tris (pH 8.6), 6 M guanidine-HCl, and 100 mM dithiothreitol overnight at room temperature. The protein was subsequently desalted on Sephadex G-25 in 0.02N HCl. For reconstitution studies, the apoprotein was mixed with 0, 20, 40, or 80 μM of a copper(I) solution freshly prepared by dissolving CuCl in degassed 0.01 M HCl containing 4% NaCl. The apoprotein-copper mixture was then neutralized by the addition of sodium acetate to pH 6.5. The copper reconstitution experiments were performed under anaerobic conditions. In some experiments, the Cu(I) chelator KCN was added to the reconstituted protein at a final concentration of 5 mM.
The apo- and Cu-reconstituted proteins were analyzed with a Photon Technologies International Quantum Master fluorescence spectrophotometer (Birmingham, NJ) essentially as described previously (33). Following excitation at 310 nm, we monitored fluorescence emission from 510 to 580 nm. Excitation and emission slit widths were 3 nm and 20 nm, respectively. All experiments were done in triplicate.
In-gel SOD activity.
To quantify Sod1p activity in whole cells and in isolated mitochondria, we used an in-gel assay as described previously (16) and as detailed in the supplemental material. To quantify the superoxide dismutase (SOD) activity staining gel, the images were digitalized and densitometric analyses performed using the histogram function of the Adobe Photoshop program.
Miscellaneous procedures.
Standard procedures used for DNA cloning, bacterial and yeast transformation, protein concentration measurements, and Western blot analyses are described in the supplemental material.
Statistical analysis.
Most experiments (enzymatic and respiratory assays and cell growth curves) were done at least in triplicate. Data are presented as means ± standard deviations (SD) of absolute values or percentages of control values. The values obtained for wild-type and cmc1 mutant strains for the different parameters studied were compared by the Student t test. A P value of <0.05 was considered significant.
RESULTS
CMC1 is required for full expression of respiration and mitochondrial COX.
The open reading frame YKL137W found on chromosome XI was deleted as part of the Saccharomyces cerevisiae gene deletion project in the wild-type BY4741 background and shown to produce a growth defect in nonfermentable carbon sources (18). We named the YKL137W gene CMC1 for reasons stated below. We have confirmed that deletion of CMC1 in the BY4741 background (BYΔcmc1) results in an impaired ability to grow on solid and liquid media containing EG as respiratory carbon sources (Fig. 1A). To exclude the possibility that this phenotype was strain specific, we constructed a W303-1A strain carrying a null cmc1 allele. This strain (W303Δcmc1) displays respiratory growth impairment similar to but slightly more stringent than that of BYΔcmc1 (data not shown). In both backgrounds, the respiratory defect was only partial, because some growth of the mutants on EG-containing media was observed after 2 to 3 days of growth at 30°C.
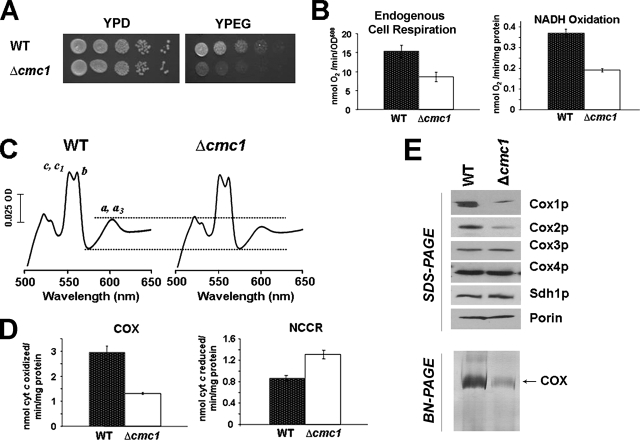
FIG. 1.
Characterization of a cmc1 null mutant. (A) The respiration-competent wild-type strain BY4741 (WT) and a strain carrying a null allele of cmc1 (Δcmc1) were grown overnight in liquid YPD medium. Tenfold serial dilutions of the two strains were plated on solid YPD or YPEG medium and incubated at 30°C. Pictures were taken after 3 days of incubation. (B) Respiratory properties of cmc1 mutant cells. KCN-sensitive endogenous cell respiration was measured polarographically in the presence of galactose. Oxygen consumption was also assayed in mitochondria prepared from the BY4741 wild-type strain and the Δcmc1 null mutant in the presence of NADH as the substrate as described in Materials and Methods. The bars indicate the means ± SD from at least three independent sets of measurements. (C) Total mitochondrial cytochrome spectra. Mitochondria from the wild-type strain BY4741 and the Δcmc1 null mutant were extracted at a protein concentration of 5 mg/ml with potassium deoxycholate under conditions that quantitatively solubilize all the cytochromes (39). Difference spectra of the reduced (sodium dithionite) versus oxidized (potassium ferricyanide) extracts were recorded at room temperature. The absorption bands corresponding to cytochromes a and a3 have maxima at 603 nm; the maxima for cytochrome b and for cytochromes c and c1 are 560 and 550 nm, respectively. (D) Mitochondrial respiratory chain enzyme spectrophotometric measurements in isolated mitochondria. COX and NADH cytochrome c reductase (NCCR) were measured as described in Materials and Methods. (E) Steady-state concentrations of COX subunits estimated by Western blot analyses of proteins separated by 12% Tris-glycine SDS-PAGE are shown in the upper panel. In the lower panel, extracts of 100 μg of mitochondrial proteins, prepared as described in Materials and Methods, were loaded on a BN-polyacrylamide gel. The gel was stained for COX activity. With the conditions used, only the monomeric COX is detected.
The capacity of the mutant BYΔcmc1 strain to respire was measured polarographically. KCN-sensitive endogenous cell respiration measured in whole cells and oxygen consumption measured in isolated mitochondria using NADH as an electron donor revealed the respiratory capacity of Δcmc1 to be ∼40% of wild-type levels (Fig. 1B). To ascertain whether the origin of the respiratory defect stemmed from a defect in the respiratory chain enzymes, we analyzed the oxidized minus reduced total mitochondrial cytochrome spectra. Comparison of the wild-type and mutant spectra revealed no apparent difference in the levels of cytochrome b, c, or c1. However, cytochromes a and a3 were less abundant in the Δcmc1 mutant than in the wild-type strain, suggesting a COX impairment (Fig. 1C). This defect was confirmed by measuring COX activity from isolated mitochondria, which indicated a ∼38% residual activity in the mutant compared to the isogenic wild type (Fig. 1D). The Δcmc1 W303-1A strain produced a similar but more pronounced phenotype (data not shown). In both backgrounds, the Δcmc1 mutant showed a significant increase of approximately 1.5 times in NADH-cytochrome c reductase activity over the wild type, which probably represents a compensatory mechanism for the COX deficiency (Fig. 1D). The oligomycin-sensitive ATPase activity in the mutant was similar to that in the wild type (data not shown).
Steady-state levels of Cox1p and Cox2p were decreased in the mutant. This is most likely due to the rapid turnover of the catalytic core subunits of the enzyme when the assembly process is hindered. No major difference was detected in the more stable nuclearly encoded subunits such as Cox4p (Fig. 1E, top panel). Steady-state levels of proteins from other respiratory complexes (Sdh1p) were not affected (Fig. 1E). The steady-state levels of COX subunits 1 and 2 correlated well with the steady-state levels of functionally assembled COX as indicated by isolated protein complexes separated in a BN gel and stained for COX activity (Fig. 1E, bottom panel). We also found that in in vivo pulse experiments, the amount of newly synthesized Cox1p was less in the Δcmc1 mutant (see Fig. S1 in the supplemental material). This phenotype likely results from a down-regulation of Cox1p synthesis when normal COX assembly is compromised, as has been reported for most COX assembly mutants (5).
CMC1 codes for a mitochondrial inner membrane protein facing the IMS.
To explore the cellular localization of Cmc1p, we used strains expressing tagged versions of the protein. An integrative plasmid expressing a CMC1 gene fused to a C-terminal HA tag fully complements the respiration-deficient phenotype of the Δcmc1 strain (Fig. 2A), and thus this strain is suitable for protein localization studies.
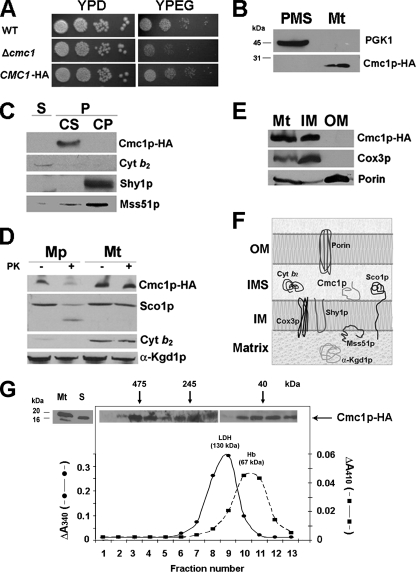
FIG. 2.
Mitochondrial localization of Cmc1p-HA. (A) Expression of an HA-tagged version of Cmc1p complements the respiratory growth defect of Δcmc1 mutant. The respiration-competent wild-type strain BY4741 (WT), a strain carrying a null allele of cmc1 (Δcmc1), and the mutant strain with a plasmid expressing HA-tagged Cmc1p (CMC1-HA) were grown overnight in liquid YPD medium. Tenfold serial dilutions of the two strains were plated on solid YPD or YPEG medium and incubated at 30°C. Pictures were taken after 3 days of incubation. (B) Cmc1p-HA is a mitochondrial protein. Mitochondria (Mt) and the postmitochondrial supernatant (PMS) fraction were isolated from the CMC1-HA strain. Samples of the two fractions corresponding to 40 μg of protein were separated by SDS-PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose, and probed with antibodies against HA (Cmc1p-HA) or the cytosolic marker 3-phosphoglycerate kinase subunit 1 (Pgk1p). (C) Cmc1p-HA is an extrinsic membrane protein. Soluble (S) and membrane-bound (P) mitochondrial proteins were separated from 40 μg of total mitochondria by brief gentle sonication followed by centrifugation at 35,000 rpm for 30 min. at 4°C. The pellet (P) was resuspended in 0.6 M sorbitol-20 mM HEPES buffer containing 0.1 M Na2CO3 [pH 11] and 50 mM EDTA and incubated on ice for 30 min. Centrifugation at 35,000 rpm for 30 min at 4°C allows the separation of the extrinsic proteins present in the supernatant (CS) from the intrinsic proteins in the pellet (CP). The different samples were loaded on a 10% Tris-Tricine gel, and Western blotting was performed against cytochrome b2, HA (Cmc1p-HA), Shy1p, and Mss51p. (D) Cmc1p-HA is a membrane protein facing the IMS. Four aliquots of 40 μg of mitochondrial protein were pelleted and resuspended in buffer containing either 20 mM HEPES or 0.6 M sorbitol-20 mM HEPES. One aliquot in each buffer was supplemented with final concentration of 100 μg/ml proteinase K (PK) and incubated on ice for 60 min. The reaction was stopped with 2 mM phenylmethylsulfonyl fluoride. Mitochondria (Mt) and mitoplasts (Mp) were recovered by centrifugation at 40,000 rpm for 15 min at 4°C. The pelleted fraction was resuspended in gel buffer and loaded on a 10% Tris-Tricine gel. Western blots were probed with antibodies against Cmc1p-HA, Sco1, porin, cytochrome b2, and α-ketoglutarate dehydrogenase. (E) Cmc1p is an inner mitochondrial membrane protein. Isolated mitochondria were fractionated into inner and outer membranes by sonication plus sucrose gradient sedimentation as described previously (24). Whole mitochondria (M), inner membranes (IM), and outer membranes (OM) were loaded on a 10% Tris-Tricine gel, and the levels of porin (outer membrane marker), Cox3p (inner membrane marker), and Cmc1p-HA were detected by immunoblotting. (F) Cartoon depicting the submitochondrial localization and topology of Cmc1p and other mitochondrial proteins used as references. (G) Sedimentation properties of Cmc1p-HA in a linear 7 to 20% sucrose gradient were analyzed as explained in Materials and Methods. The gradient was calibrated with hemoglobin (Hb) (67 kDa) and lactate dehydrogenase (LDH) (130 kDa). Following centrifugation, the gradient was collected in 13 equal fractions. Each fraction was assayed for Hb by absorption at 410 nm and for LDH activity by measuring NADH-dependent conversion of pyruvate to lactate. The distribution of Cmc1p was assayed by Western blot analysis. The mass of Cmc1p was determined from the positions of the respective peaks relative to those of the markers.
Cell fractionation and Western blot analyses enabled us to find Cmc1p-HA in the mitochondrial fraction but not in the cytoplasmic fraction (Fig. 2). Although the predicted size of Cmc1p is ∼13 kDa, an antibody against HA detected a protein of ∼20 kDa in mitochondria but not in the postmitochondrial supernatant fraction (Fig. 2B). The protein was absent in the Δcmc1 strain (data not shown). Even though Cmc1p is largely hydrophilic (Fig. 3A), disruption of mitochondria from the CMC1-HA strain by sonic irradiation failed to solubilize the protein, since it was found exclusively in the pellet fraction (Fig. 2C). Further treatment of the pellet with alkaline carbonate completely solubilized Cmc1p-HA, suggesting a peripheral association with the membrane. The solubility properties of Cmc1p-HA in these experiments contrasted with the lack of extraction of Shy1p, an intrinsic inner membrane protein with two transmembrane domains. Other controls consisted of the peripherally inner membrane-associated Mss51p protein, which faces the matrix and is only partially extracted in these conditions, and the soluble IMS cytochrome b2, which was recovered in the supernatant fraction (Fig. 2C). These results demonstrate that Cmc1p-HA is a mitochondrial protein loosely associated with membranes.
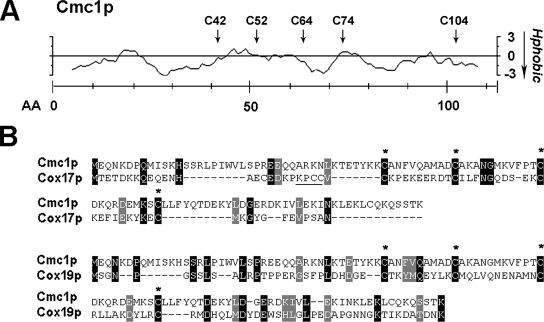
FIG. 3.
Homology of Cmc1p with other proteins involved in copper delivery to COX. (A) Hydropathy plot of Cmc1p obtained by using the algorithm of Kyte and Doolittle (23). The positions of cysteine residues in the Cmc1p sequence are shown by arrows. (B) Protein alignments of Cmc1p and other proteins with conserved CX9C twin motifs, Cox17p and Cox19p, required for COX assembly and previously shown to bind Cu(I). Conserved cysteines are indicated by asterisks. The known copper binding cysteines in Cox17p (3) are underlined.
The submitochondrial membrane localization and topology of Cmc1p-HA were probed by testing its sensitivity to proteinase K digestion in intact mitochondria and in mitoplasts prepared by hypotonic swelling of mitochondria. Cmc1p-HA was protected against proteinase K in intact mitochondria but not in mitoplasts (Fig. 2D). Similarly, Sco1p, an inner membrane protein previously shown to face the IMS (7) was digested in the mitoplasts but not in mitochondria (Fig. 2D). As expected, the hypotonic conditions used to disrupt the outer membrane resulted in the loss of cytochrome b2. Instead, α-ketoglutarate dehydrogenase, a soluble matrix protein, was protected against proteinase K in mitochondria and mitoplasts (Fig. 2D). These results indicate that Cmc1p is membrane bound and faces the IMS. To discern to which membrane Cmc1p-HA was bound, we sonicated mitochondria and separated inner and outer membranes by sucrose gradient fractionation. Porin and Cox3p were used as markers of the outer and inner mitochondrial membranes, respectively, to test the purity of the fractions. Like Cox3p, Cmc1p-HA is found exclusively in the inner membrane fraction (Fig. 2E). As depicted in Fig. 2F, we concluded that Cmc1p-HA is a mitochondrial protein loosely associated with the inner membrane and facing the IMS.
To further explore the submitochondrial localization of Cmc1p, a strain carrying a tandem affinity purification (TAP)-tagged version of the CMC1 gene was obtained from Open Biosystems. This strain had growth properties similar to those of the null cmc1 mutant strain at 30°C in media containing nonfermentable carbon sources (data not shown), indicating that the tagged protein was not functional, probably due to the large ∼20-kDa TAP tag. However, the TAP-tagged protein was imported and localized in the mitochondrial IMS as a soluble protein (data not shown). The TAP tag prevented the anchoring of the protein into the inner membrane, which could contribute to making it nonfunctional.
Cmc1p occurs in high-molecular-weight complexes.
The peripheral association of Cmc1p with the inner membrane could indicate that it is a component of a higher structure anchored in the membrane. To determine the native molecular weight of Cmc1p, we first empirically established the mildest conditions needed to extract the protein from the mitochondrial membranes in order to preserve any possible protein-protein interactions. For efficient extraction, detergent and salt were required at concentrations of 0.4% lauryl maltoside and 200 mM KCl (see Fig. S2 in the supplemental material). Subsequently, mitochondrial extracts were loaded onto 7 to 20% sucrose gradients to determine the sedimentation properties of Cmc1p-HA. The gradients were calibrated with two standards, 67-kDa hemoglobin and 130-kDa lactate dehydrogenase. After fractionation, the samples were separated in a Tris-Tricine gel and the sedimentation properties of Cmc1p-HA were analyzed by Western-blot analysis (Fig. 2G). The ∼20-kDa Cmc1p-HA protein was detected in three complexes with estimated masses of 40 kDa, 245 kDa, and greater than 475 kDa (Fig. 2G). Although the 40- and 475-kDa species are clearly more abundant in our mitochondrial extracts, the exact proportion of total Cmc1p-HA in each high-molecular-weight species and its significance remain to be established. The protein compositions of these high-molecular-weight complexes are currently under investigation. Initial pull-down experiments using HA-Sepharose 4B have failed to coprecipitate some possible candidates that could interact with Cmc1p, such as Cox17p, Cox11p, and Sco1p (data not shown), suggesting that these proteins do not stably interact with Cmc1p.
Suppression of the Δcmc1 mutant by copper.
The homology of Cmc1p with Cox17p and similar copper chaperones required for COX assembly (Fig. 3B) suggested that Cmc1p could also be part of the same pathway for copper delivery to COX. We investigated whether copper supplementation to the medium and/or overexpression of these assembly factors could alleviate the Δcmc1 respiratory growth defect. The supplementation of rich EG medium with 2.5 and 5 mM CuSO4 did partially rescue the growth defect of the mutant (Fig. 4A).
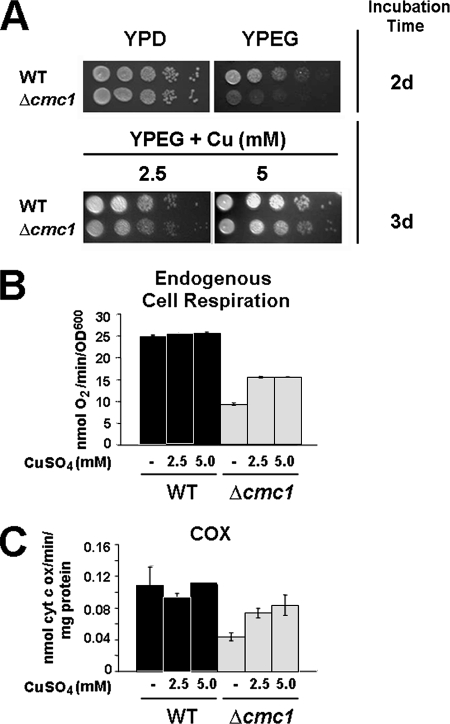
FIG. 4.
Copper supplementation alleviates respiratory deficiency in a cmc1 mutant. (A) Wild-type (WT) and Δcmc1 cells were serially diluted 10-fold and then spotted on YPD and YPEG plates and on YPEG plates supplemented with 2.5 and 5 mM CuSO4. Pictures were taken after the indicated time of incubation. (B) Cells were grown overnight on respiratory medium supplemented with 2.5 or 5 mM CuSO4. Cells were then harvested, and KCN-sensitive endogenous cell respiration was measured polarographically as described in Materials and Methods. (C) Cells grown as for panel B were harvested, converted to spheroplasts by treatment with Zymolyase, washed, and disrupted by two freezing-thawing cycles. COX activity was measured spectrophotometrically as oxidation of cytochrome c as described in Materials and Methods. The bars indicate the means ± SD from at least three independent sets of measurements.
To examine the impact of copper supplementation on Δcmc1 respiratory growth, we measured whole-cell respiration from cells grown in rich EG medium in the presence of copper. While additional copper had no significant effect on wild-type respiration, in Δcmc1 cells it induced a significant increase from 35% to 60% of wild-type values (Fig. 4B). The copper suppression effect on Δcmc1 cell respiration resulted from an increase in COX activity from 35% to 65% of wild-type values (Fig. 4C).
Overexpression of COX copper assembly factors, including Cox11p, Sco1p, Cox17p, Cox19p, Cox23p, and the mostly uncharacterized Pet191p, was not effective in restoring respiratory growth of the Δcmc1 strain at 30°C even when copper was supplemented to the medium (data not shown). Reciprocally, overexpression of CMC1 in strains carrying null alleles of sco1, cox11, cox17, cox19, cox23, and pet191 did not rescue the respiratory defect of these strains even when the medium was supplemented with copper (data not shown).
Cmc1p is not required for the mitochondrial import of other copper chaperones carrying a twin CX9C domain.
The twin CX9C motif present in Cmc1p is typical of systems involved in cysteine redox reactions within the mitochondrial IMS (17). Mitochondrial copper chaperones essential for COX assembly such as Cox17p and Cox19p also contain a twin CX9C motif and are imported and assembled by a disulfide relay system involving the Mia40p machinery, another CX9C containing protein (27). Cmc1p could act as a regulator/sorter of the import and stabilization of these Mia40 substrates involved in COX assembly. Western blot analyses showed that Cox17p accumulates at wild-type levels in Δcmc1 mitochondria, similar to the case for other Mia40-independent IMS proteins such as Sco1p and cytochrome c (data not shown). These results argue against a role for Cmc1p in regulating the Mia40p import system.
Recombinant Cmc1p has the ability to bind copper(I).
Cmc1p could perform its role in COX biogenesis by directly or indirectly affecting copper trafficking toward the COX copper metallochaperone Cox17p. We have tested in vitro the copper binding capacity of recombinant Cmc1p. Recombinant Cmc1p was purified (Fig. 5A), denatured, and reduced as explained in Materials and Methods. To determine the ability of ApoCmc1p to bind Cu(I) as previously described for Cox17p (7) and Cox19p (33), we performed copper reconstitutions experiments under anaerobic conditions. Spectroscopic analyses of the reconstituted Cu(I)Cmc1p protein revealed a fluorescence peak near 560 nm with excitation at 310 nm (Fig. 5B). These properties are consistent with Cu(I)-thiolate coordination as observed in CuCox17p and CuCox19p (33). The emission is significantly abolished by treatment with the Cu(I) chelator KCN (Fig. 5B). No emission peak was detected when the reconstitution assays were performed under aerobic conditions. Titration of apoCmc1p with Cu(I) resulted in Cu(I)Cmc1p formation, as judged by fluorescence emission that was maximal with equimolar amounts of Cu(I) and protein (Fig. 5C). The addition of excess (2 times and 4 times) Cu(I) resulted in attenuation in the observed emission (Fig. 5C) as previously reported for Cox19p (33), probably due to a disruption of the protein structure upon binding of excess Cu(I), thus increasing solvent accessibility to the Cu(I)-thiolate bonds as previously proposed (33).
FIG. 5.
Purification of recombinant Cmc1p and reconstitution of CuCmc1p. (A) Recombinant His6-Cmc1p was affinity purified using a combination of metal affinity and size exclusion chromatography as described in Materials and Methods. Subsequent fractions were analyzed by SDS-PAGE and Coomassie blue staining. CL, cell lysate; FT, flow through the metal column; E, pool of elution fractions; TH, thrombin-cleaved recombinant Cmc1p. (B) Cu(I) fluorescence of CuCmc1p. An excitation of 310 nm exhibits an emission peak with a maximum at ∼560 nm, indicative of Cu(I)-thiolate complexes that are not solvent accessible. The addition of 5 mM KCN, a Cu(I) chelator, to CuCmc1p significantly eliminates the observed emission. (C) Titration of reduced apoCmc1p with Cu(I). Maximal emission is obtained at one equivalent of copper.
The results obtained with the copper reconstitution experiments with recombinant Cmc1p allowed us to conclude that this protein has the ability to bind Cu(I) and warrant future experiments, out of the scope of this paper, to investigate the role in copper binding of the different cysteine residues present in Cmc1p.
Mitochondrial Cu-Zn SOD activity is increased in the Δcmc1 mutant.
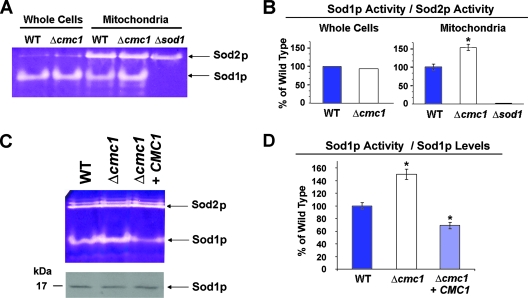
COX and Sod1p have been proposed to use the same mitochondrial matrix copper pool for their metalation (10). How copper is directed from the matrix to the respective COX and Sod1p metallochaperones located in the IMS remains unknown. Cmc1p could be involved in such a trafficking role from its location on the IMS face of the inner mitochondrial membrane. To explore this possibility, SOD activity was assayed by native gel electrophoresis and nitroblue tetrazolium staining. Our results revealed that the total cellular Sod1p activity was not different in the mutant and the wild type (Fig. 6A). However, Δcmc1 cells have a significant increase in the mitochondrial fraction of Sod1p activity compared to the wild type (Fig. 6A and B), despite no increase in the steady-state levels of the protein (Fig. 6C). Sod2p activity is not affected by the cmc1 deletion and was used to normalize the Sod1p activity values (Fig. 6B). Similar results were obtained when the activities were normalized by the amount of cellular or mitochondrial proteins loaded from the corresponding samples (data not shown) or when normalized by the steady-state levels of Sod1p (Fig. 6C). Interestingly, overexpression of CMC1 in a Δcmc1 strain resulted in a significant decrease in mitochondrial Sod1p activity, down to approximately 64% of wild-type values (Fig. 6C and D). We can envision that Cmc1p could act in a pathway controlling the trafficking of copper toward COX and thus indirectly affecting Sod1p metalation.
FIG. 6.
Mitochondrial Sod1p activity depends upon Cmc1p levels. (A) SOD activity in whole cells and in isolated mitochondria from the indicated strains was measured in an in-gel assay as described in Materials and Methods. WT, wild type. (B) Quantification of the SOD activity staining gel in panel A. The images were digitalized and densitometry performed using the histogram function of the Adobe Photoshop program. (C) SOD activity in mitochondria isolated from wild-type, Δcmc1 mutant, and CMC1-overexpressing cells (upper panel). The same samples were solubilized in SDS sample buffer and separated on a 12% polyacrylamide gel. Mitochondrial Sod1p and porin, a loading control, were visualized by immunoblotting (lower panel). (D) Quantification of the experiment in panel C was performed as for panel B by including data from three independent experiments. *, P < 0.01.
Cmc1p is conserved from yeasts to humans.
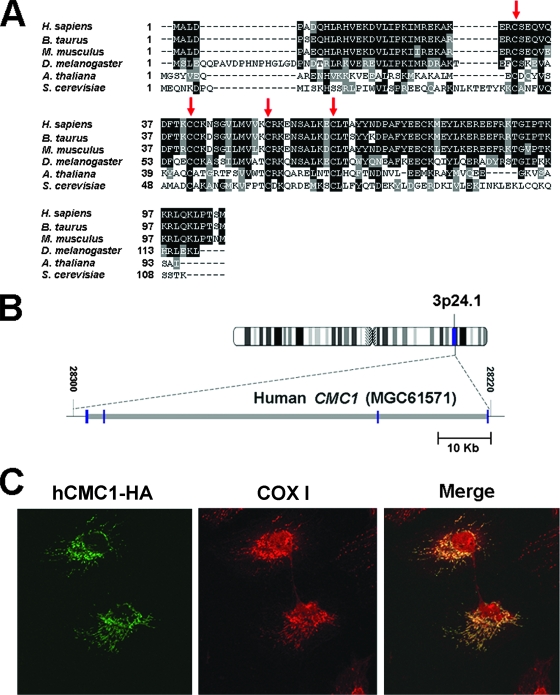
COX biogenesis factors containing the twin CX9C motif, including Cox17p, Cox19p, Cox23p, and Pet191p, are conserved from yeasts to humans (35). Similarly, the S. cerevisiae Cmc1p has homologues in mammals, fruit flies, plants, and other fungi. All homologues contain the signature conserved twin CX9C motif as indicated in Fig. 7A. A human cDNA clone (IMAGE 6163781) codes for a putative homologue of Cmc1p and was obtained for further studies. The cDNA sequence was mapped to locus MGC61571 on chromosome 3p24. The human gene, here referred to as hCMC1, consists of four exons separated by three large introns (Fig. 7B).
FIG. 7.
Properties and expression of the human gene homologue of yeast CMC. (A) CMC1 is conserved across kingdoms, from yeast to human. A sequence alignment of human (Homo sapiens), bovine (Bos taurus), mouse (Mus musculus), fruit fly (Drosophila melanogaster), and plant (Arabidopsis thaliana) homologues of S. cerevisiae CMC1 is shown. Red arrows indicate conserved cysteine residues. (B) Localization and genomic structure of the hCMC1 gene based on the result of a BLAST search of the human genome for homology to the hCMC1 cDNA sequence. The blue and gray bars depict the exon and intron regions, respectively. (C) Subcellular localization of hCmc1p in human HeLa cells. Cells were transiently transfected with a cDNA coding for an HA-tagged hCmc1p. The protein was visualized by indirect immunofluorescence using fluorescently conjugated antibodies to human Cox I (red) and to the HA epitope of hCMC1p-HA (green). A merged image is shown on the right.
The subcellular location of hCmc1p was studied by transient expression of the protein tagged with a C-terminal HA epitope in human HeLa cells. Immunohistochemical assays for the HA epitope in the transformed cells showed a typical mitochondrial network pattern that colocalized with subunit 1 of COX (Fig. 7C).
DISCUSSION
Copper trafficking from the mitochondrial matrix pool to the IMS chaperones involved in metal delivery to COX and Sod1p is not fully understood. In this study, Cmc1p was identified as a novel twin CX9C conserved protein able to bind Cu(I) whose role is required for full expression of COX and affects the levels of functional mitochondrial Sod1p.
We report that CMC1 codes for a COX biogenesis factor. Significantly, unlike most COX assembly-defective strains of yeast, which display a complete absence of COX, cmc1 null mutants produce 20 to 40% fully assembled and functional COX, depending on the genetic background. Only null mutants of cox5a, encoding a major isoform of the Cox5p structural subunit (32); shy1, encoding a COX assembly factor (25); and cox23, encoding a protein involved in copper transfer to COX (6), were previously shown to retain some residual COX activity. Our observation suggests that Cmc1p either has a redundant function or catalyzes the function of another protein.
Cmc1p is proposed to be relevant to mitochondrial copper trafficking and transfer to COX. Several lines of evidence support these possibilities. (i) The COX assembly and respiratory defects of cmc1 mutant strains are rescued by exogenous copper supplementation, as previously shown for cox17 mutants (19) and cox23 mutants overexpressing COX17 (6). (ii) Cmc1p shares homology with Cox17p and other IMS proteins containing twin CX9C structural motifs required for COX assembly, including Cox19p and Cox23p. These are soluble proteins, but at least Cox17p and Cox19p are known to be functional when tethered to the inner membrane (26, 33). Cmc1p is instead an extrinsic protein bound to the IMS face of the inner mitochondrial membrane. The possibility that Cmc1p has overlapping functions with these proteins is unlikely, as overexpression of the known COX copper chaperones (Cox17p, Cox19p, Cox23p, Cox11p, and Sco1p) does not result in any suppression of the respiratory growth defect in the Δcmc1 cells. Reciprocally, Cmc1p overexpression did not rescue the respiratory growth defect of mutants deficient in any of the mentioned COX copper chaperones even when excess copper was present in the medium. (iii) Recombinant Cmc1p has the ability to bind Cu(I) in a thiolate coordination, a property shared with Cox17p (19) and Cox19p (33).
Copper coordination in the different twin CX9C IMS proteins does not necessarily involve the motif cysteine residues. At least this seems to be the case for Cox17p. Cox17p is fully reduced in the cytoplasm. It enters the IMS and is oxidized by Mia40p, forming two disulfide bonds involving the cysteines in the CX9C motifs. The resulting Cox17p2S-S is retained within the IMS and constitutes the functional species competent for binding Cu(I) (3). Structural studies have recently shown that in human Cu(I)COX172S-S the Cu(I) is coordinated by the sulfurs of the two adjacent cysteines in the conserved CCXC domain (3). Mutagenesis analyses of the yeast Cox17p protein had previously shown that the last cysteine in the domain, which is the first of the CX9C structural motifs, was also functionally important in vivo (21). This suggested that a substitution in that position affects import and/or retention within the IMS and formation of a functional protein. The presence of a lysine adjacent to the Cox17p copper binding site (KXCC), resembles the copper binding region of the cytosolic metallochaperone Atx1p (1). This residue could be important for stabilizing copper binding (2) and modulating copper transfer (40). The copper coordination in Cox19p must be different than that in Cox17p, because it does not contain cysteine residues outside the twin CX9C motifs. Mutagenesis analyses of cysteines in Cox19p have shown that these residues are important for Cu(I) binding as well as for the Cox19p in vivo function in COX assembly (33). Interestingly, Cox19p isolated from the yeast mitochondrial IMS was found largely in the reduced state, suggesting that redox control of cysteines in Cox19p is central for its function (33). Yeast Cmc1p contains four cysteine residues in twin CX9C motifs (C42C52 and C64C74) and an additional cysteine in its C terminus at position 104, noticeably in a KXC domain (Fig. 3B) reminiscent of the Cox17p copper binding site. The relevance of all these residues for copper coordination in Cmc1p and in vivo function of the protein as well as the redox state of the protein in the IMS are currently under investigation in our laboratory. Elucidation of these features will help us to understand how Cmc1p performs its function.
The presence of a growing number of mitochondrial IMS CX9C proteins relevant for COX assembly is intriguing. As an obvious hypothesis, they could be part of the same pathway facilitating a transfer of copper to COX. Further data concerning the dynamics of in vivo redox state changing and metal binding of Cmc1p and other CX9C proteins will be required prior to understanding their specific roles in the IMS.
Cmc1p is an inner membrane-bound protein part of larger complexes. Cmc1p could interact with inner membrane proteins (e.g., an unidentified copper transporter) to directly or indirectly facilitate the distribution of copper from the matrix pool (11) to the COX copper chaperones. A role for Cmc1p linked to regulation of copper distribution from the matrix copper pool to COX would be expected to affect the copper delivery to Sod1p, a copper-containing enzyme in the IMS. Remarkably, mitochondrial Sod1p activity but not the protein steady-state level was significantly increased in cmc1 mutant cells and significantly decreased in CMC1-overexpressing cells. These observations could fit in a model in which Cmc1p is involved in facilitating copper trafficking from the proposed matrix copper pool to the IMS and subsequently to COX. In the absence of Cmc1p, copper flow toward COX would be limited and the metal more readily available for Sod1p.
In conclusion, in this work we have described Cmc1p as a new COX biogenesis factor whose role additionally affects mitochondrial Sod1p activity. The impact of these enzymes in aerobic energy production and free radical scavenging underscores the biological relevance of Cmc1p function. Cmc1p homologues are absent in bacteria but exist in other fungi, plants, and animals, including humans. Supporting the functional equivalence of the S. cerevisiae and human homologues, HA-tagged human Cmc1p colocalizes with a mitochondrial COX marker in human HeLa cells. The human gene must be considered a new candidate when screening for mutations responsible for mitochondrial disorders associated with COX deficiency.
Supplementary Material
Acknowledgments
We thank Flavia Fontanesi, Carlos Moraes, Tina Wenz, and Francisca Díaz for critically reading the manuscript. We thank Alexander Tzagoloff for providing antibodies against Cox17p and Sco1p, Bernard D. Lemire (University of Alberta, Edmonton, Canada) for providing anti-Sdh1p antibody, and Valeria Cullotta (Johns Hopkins University) for providing anti-Sod1p antibody.
This research was supported by National Institutes of Health research grant GM071775A (to A.B.) and by a research grant from the Muscular Dystrophy Association (to A.B.).
Footnotes
Published ahead of print on 28 April 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Arnesano, F., L. Banci, I. Bertini, F. Cantini, S. Ciofi-Baffoni, D. L. Huffman, and T. V. O'Halloran. 2001. Characterization of the binding interface between the copper chaperone Atx1 and the first cytosolic domain of Ccc2 ATPase. J. Biol. Chem. 27641365-41376. [DOI] [PubMed] [Google Scholar]
- 2.Arnesano, F., L. Banci, I. Bertini, D. L. Huffman, and T. V. O'Halloran. 2001. Solution structure of the Cu(I) and apo forms of the yeast metallochaperone, Atx1. Biochemistry 401528-1539. [DOI] [PubMed] [Google Scholar]
- 3.Banci, L., I. Bertini, S. Ciofi-Baffoni, A. Janicka, M. Martinelli, H. Kozlowski, and P. Palumaa. 2008. A structural-dynamical characterization of human Cox17. J. Biol. Chem. 2837912-7920. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos, A., D. Korr, and A. Tzagoloff. 2002. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh's syndrome. EMBO J. 2143-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrientos, A., A. Zambrano, and A. Tzagoloff. 2004. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 233472-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros, M. H., A. Johnson, and A. Tzagoloff. 2004. COX23, a homologue of COX17, is required for cytochrome oxidase assembly. J. Biol. Chem. 27931943-31947. [DOI] [PubMed] [Google Scholar]
- 7.Beers, J., D. M. Glerum, and A. Tzagoloff. 1997. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J. Biol. Chem. 27233191-33196. [DOI] [PubMed] [Google Scholar]
- 8.Carr, H. S., G. N. George, and D. R. Winge. 2002. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J. Biol. Chem. 27731237-31242. [DOI] [PubMed] [Google Scholar]
- 9.Carr, H. S., A. B. Maxfield, Y. C. Horng, and D. R. Winge. 2005. Functional analysis of the domains in Cox11. J. Biol. Chem. 28022664-22669. [DOI] [PubMed] [Google Scholar]
- 10.Cobine, P. A., L. D. Ojeda, K. M. Rigby, and D. R. Winge. 2004. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 27914447-14455. [DOI] [PubMed] [Google Scholar]
- 11.Cobine, P. A., F. Pierrel, M. L. Bestwick, and D. R. Winge. 2006. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem. 28136552-36559. [DOI] [PubMed] [Google Scholar]
- 12.Culotta, V. C., L. W. Klomp, J. Strain, R. L. Casareno, B. Krems, and J. D. Gitlin. 1997. The copper chaperone for superoxide dismutase. J. Biol. Chem. 27223469-23472. [DOI] [PubMed] [Google Scholar]
- 13.Dameron, C. T., D. R. Winge, G. N. George, M. Sansone, S. Hu, and D. Hamer. 1991. A copper-thiolate polynuclear cluster in the ACE1 transcription factor. Proc. Natl. Acad. Sci. USA 886127-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faye, G., C. Kujawa, and H. Fukuhara. 1974. Physical and genetic organization of petite and grande yeast mitochondrial DNA. IV. In vivo transcription products of mitochondrial DNA and localization of 23 S ribosomal RNA in petite mutants of Saccharomyces cerevisiae. J. Mol. Biol. 88185-203. [DOI] [PubMed] [Google Scholar]
- 15.Field, L. S., Y. Furukawa, T. V. O'Halloran, and V. C. Culotta. 2003. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J. Biol. Chem. 27828052-28059. [DOI] [PubMed] [Google Scholar]
- 16.Flohe, L., and F. Otting. 1984. Superoxide dismutase assays. Methods Enzymol. 10593-104. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel, K., D. Milenkovic, A. Chacinska, J. Muller, B. Guiard, N. Pfanner, and C. Meisinger. 2007. Novel mitochondrial intermembrane space proteins as substrates of the MIA import pathway. J. Mol. Biol. 365612-620. [DOI] [PubMed] [Google Scholar]
- 18.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418387-391. [DOI] [PubMed] [Google Scholar]
- 19.Glerum, D. M., A. Shtanko, and A. Tzagoloff. 1996. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 27114504-14509. [DOI] [PubMed] [Google Scholar]
- 20.Glerum, D. M., A. Shtanko, and A. Tzagoloff. 1996. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J. Biol. Chem. 27120531-20535. [DOI] [PubMed] [Google Scholar]
- 21.Heaton, D., T. Nittis, C. Srinivasan, and D. R. Winge. 2000. Mutational analysis of the mitochondrial copper metallochaperone Cox17. J. Biol. Chem. 27537582-37587. [DOI] [PubMed] [Google Scholar]
- 22.Hiser, L., M. Di Valentin, A. G. Hamer, and J. P. Hosler. 2000. Cox11p is required for stable formation of the Cu(B) and magnesium centers of cytochrome c oxidase. J. Biol. Chem. 275619-623. [DOI] [PubMed] [Google Scholar]
- 23.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157105-132. [DOI] [PubMed] [Google Scholar]
- 24.Manthey, G. M., B. D. Przybyla-Zawislak, and J. E. McEwen. 1998. The Saccharomyces cerevisiae Pet309 protein is embedded in the mitochondrial inner membrane. Eur. J. Biochem. 255156-161. [DOI] [PubMed] [Google Scholar]
- 25.Mashkevich, G., B. Repetto, D. M. Glerum, C. Jin, and A. Tzagoloff. 1997. SHY1, the yeast homolog of the mammalian SURF-1 gene, encodes a mitochondrial protein required for respiration. J. Biol. Chem. 27214356-14364. [DOI] [PubMed] [Google Scholar]
- 26.Maxfield, A. B., D. N. Heaton, and D. R. Winge. 2004. Cox17 is functional when tethered to the mitochondrial inner membrane. J. Biol. Chem. 2795072-5080. [DOI] [PubMed] [Google Scholar]
- 27.Mesecke, N., N. Terziyska, C. Kozany, F. Baumann, W. Neupert, K. Hell, and J. M. Herrmann. 2005. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 1211059-1069. [DOI] [PubMed] [Google Scholar]
- 28.Myers, A. M., L. K. Pape, and A. Tzagoloff. 1985. Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 42087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nijtmans, L. G., N. S. Henderson, and I. J. Holt. 2002. Blue Native electrophoresis to study mitochondrial and other protein complexes. Methods 26327-334. [DOI] [PubMed] [Google Scholar]
- 30.Nobrega, M. P., S. C. Bandeira, J. Beers, and A. Tzagoloff. 2002. Characterization of COX19, a widely distributed gene required for expression of mitochondrial cytochrome oxidase. J. Biol. Chem. 27740206-40211. [DOI] [PubMed] [Google Scholar]
- 31.Palumaa, P., L. Kangur, A. Voronova, and R. Sillard. 2004. Metal-binding mechanism of Cox17, a copper chaperone for cytochrome c oxidase. Biochem. J. 382307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poyton, R. O., and P. V. Burke. 1992. Oxygen regulated transcription of cytochrome c and cytochrome c oxidase genes in yeast. Biochim. Biophys. Acta 1101252-256. [PubMed] [Google Scholar]
- 33.Rigby, K., L. Zhang, P. A. Cobine, G. N. George, and D. R. Winge. 2007. Characterization of the cytochrome c oxidase assembly factor Cox19 of Saccharomyces cerevisiae. J. Biol. Chem. 28210233-10242. [DOI] [PubMed] [Google Scholar]
- 34.Schagger, H., and K. Pfeiffer. 2000. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 191777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solans, A., A. Zambrano, and A. Barrientos. 2004. Cytochrome c oxidase deficiency: from yeast to human. Preclinica 2336-348. [Google Scholar]
- 36.Sturtz, L. A., K. Diekert, L. T. Jensen, R. Lill, and V. C. Culotta. 2001. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 27638084-38089. [DOI] [PubMed] [Google Scholar]
- 37.Tsukihara, T., H. Aoyama, E. Yamashita, T. Tomizaki, H. Yamaguchi, K. Shinzawa-Itoh, R. Nakashima, R. Yaono, and S. Yoshikawa. 1995. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science 2691069-1074. [DOI] [PubMed] [Google Scholar]
- 38.Tzagoloff, A., A. Akai, and R. B. Needleman. 1975. Assembly of the mitochondrial membrane system. Characterization of nuclear mutants of Saccharomyces cerevisiae with defects in mitochondrial ATPase and respiratory enzymes. J. Biol. Chem. 2508228-8235. [PubMed] [Google Scholar]
- 39.Tzagoloff, A., A. Akai, R. B. Needleman, and G. Zulch. 1975. Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J. Biol. Chem. 2508236-8242. [PubMed] [Google Scholar]
- 40.Wernimont, A. K., D. L. Huffman, A. L. Lamb, T. V. O'Halloran, and A. C. Rosenzweig. 2000. Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat. Struct. Biol. 7766-771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.