Abstract
GA binding protein (GABP) is a ubiquitously expressed Ets family transcription factor that consists of two subunits, GABPα and GABPβ. GABPα binds to DNA, and GABPβ heterodimerizes with GABPα and possesses the ability to transactivate target genes. Our previous studies using GABPα-deficient mice revealed that GABPα is required for the development of both T and B cells. Two splice variants of GABPβ are generated from the Gabpb1 locus and differ in their carboxy-terminal lengths and sequences. The longer isoform (GABPβ1L) can homodimerize and thus form α2β2 tetramers depending on the gene context, whereas the shorter isoform (GABPβ1S) cannot. In this study, we generated mice that are deficient in GABPβ1L but that retain the expression of GABPβ1S. Surprisingly, GABPβ1L−/− mice had normal T- and B-cell development, and mature T and B cells showed normal responses to various stimuli. In contrast, targeting both GABPβ1L and GABPβ1S resulted in early embryonic lethality. Because of its incapability of forming homodimers, GABPβ1S has been suspected to have a dominant negative role in regulating GABP target genes. Our findings argue against such a possibility and rather suggest that GABPβ1S has a critical role in maintaining the transcriptional activity of the GABPα/β complex.
Ets family transcription factors have diverse functions in development, differentiation, apoptosis, and oncogenesis (15, 31). More than 30 Ets factors have been described, and all of them contain a conserved DNA binding Ets domain of approximately 85 amino acids in length. The Ets domain assumes a winged helix-loop-helix configuration and binds preferentially to a purine-rich consensus DNA sequence containing GGAA/T. GA binding protein (GABP) is the only Ets factor that functions as obligate multimeric proteins and consists of two unrelated subunits, GABPα and GABPβ (26). GABPα harbors the Ets domain close to its C terminus and is thus responsible for DNA binding. GABPβ cannot bind DNA but has transactivation activities. The interaction of these two subunits is mediated by the C terminus of GABPα, including the ETS domain, and the ankyrin repeats at the N terminus of GABPβ.
GABP was originally identified in studies of viral gene transcription (36, 37), but it is now known to regulate genes that control many basic cellular functions such as cellular respiration in mitochondria (30), protein components of ribosomes (10), and cell cycle progression (14, 32). A recent study using GABPα-deficient fibroblasts demonstrated that GABPα is required for reentry into the cell cycle by regulating the expression of genes that are required for DNA synthesis (such as thymidylate synthase) and the degradation of cyclin-dependent kinase inhibitors (such as S-phase kinase-associated protein) (41). The observation that the inactivation of both Gabpa alleles resulted in death prior to implantation highlights its essential roles for early embryogenesis (25). In addition to these basic cellular functions, GABP regulates tissue-specific target genes, such as those encoding the nicotinic acetylcholine receptor subunits δ and ɛ in neuromuscular synapses (7, 17). Recent studies revealed that GABPα can recruit the histone acetyltransferase p300 to the acetylcholine receptor ɛ subunit promoter for its activation in subsynaptic nuclei (24). However, contradictory results on the effect of disrupting Gabpa alleles on the formation and function of neuromuscular junction were recently reported (16, 22). In the immune system, GABP has been reported to increase transcription from the interleukin-2 (IL-2) enhancer (1) and the Fas promoter (19) in T cells. In B cells, Pax5 can recruit GABP to the immunoglobulin (Ig) α promoter, forming a ternary complex (8, 20). We have demonstrated that GABP is critical for the expression of the IL-7 receptor α chain (IL-7Rα) in T cells (38), and this can at least in part explain the defective thymocyte development due to the GABPα deficiency (39; our unpublished observations). In addition, the loss of GABPα expression caused severe defects in B-cell development and humoral responses (39).
Most recent GABP studies have focused on the DNA binding subunit GABPα. The most studied GABPβ is encoded by the Gabpb1 gene, which gives rise to two alternatively spliced isoforms, GABPβ1L and GABPβ1S (Fig. 1A). These two isoforms share 332 identical amino acids at their N termini but differ in their C-terminal lengths and sequences. The N terminus of each GABPβ1 isoform contains four ankyrin repeats that mediate heterodimerization with GABPα, and both isoforms were found to heterodimerize with GABPα with similar affinities (34). The N-terminal region also contains the nuclear localization signal (amino acids 243 to 317), and thus, both GABPβ1L and GABPβ1S can be targeted to the nucleus together with GABPα (28). In contrast to these common features, GABPβ1L has a longer C-terminal tail (50 amino acids), which is encoded entirely by exon 9. The GABPβ1L C terminus contains an array of hydrophobic residues that adopt a leucine zipper-like structure (36) and can thus form homodimers. Depending on the gene context where two Ets motifs are adjacent or brought into proximity, an α2β2 GABP tetramer complex can be formed (4, 29, 36). In contrast, the C terminus of GABPβ1S has only 15 amino acids (Fig. 1A) and is encoded by sequences immediately downstream of exon 8. GABPβ1S cannot form homodimers, as it lacks the leucine zipper-like structure (18, 29). The role of the long C terminus of GABPβ1 in its transcriptional activation activity has been controversial. In an in vitro assay using Drosophila Schneider cells, the GABPα/GABPβ1S heterodimer only weakly activated transcription, at approximately 1/10 of the level of a GABPα/GABPβ1L heterodimer (29). In contrast, when fused to a GAL4 DNA binding domain, both GABPβ1L and GABPβ1S were equally proficient in activating transcription in transfected cells (11). In this study, we have investigated the role of GABPβ1 in the immune system by targeting the longer isoform alone and both isoforms together by homologous recombination.
FIG. 1.
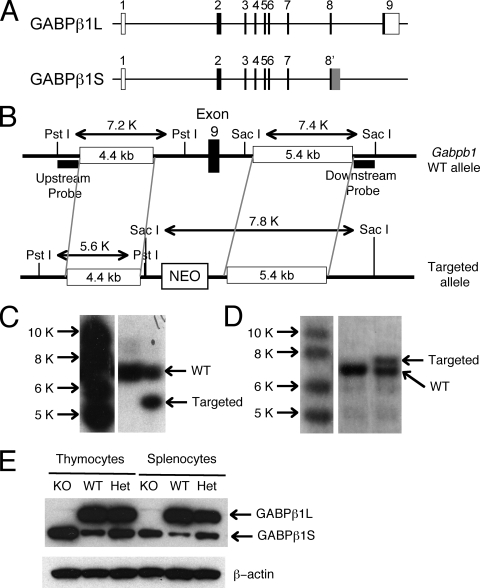
Generation of GABPβ1L-deficient mice. (A) Gabpb1 gene structure and different exon usages in two GABPβ isoforms by alternative splicing. The Gabpb1 gene contains nine exons based on data available at the UCSC Genome Bioinformatics website (http://genome.ucsc.edu). When the transcript from the Gabpb1 gene is spliced to generate the short GABPβ1S isotype, exon 9 is not used, but additional sequences downstream of exon 8 (gray box in exon 8′) are used instead. The sizes of exons are not drawn to scale. Open rectangles, noncoding exons; filled rectangles, coding sequences. (B) Schematic view of the targeting strategy. In the targeted allele, exon 9 was replaced with a neomycin (NEO) cassette. Key restriction enzyme sites and probes used in Southern blotting are indicated. (C) Identification of ES cells with correct homologous recombination. Genomic DNA was extracted from ES cells that were electroporated with the targeting construct, digested with PstI, and probed with an upstream probe by Southern blotting. (D) Identification of germ line-transmitted offspring. Chimeric mice resulting from blastocyst injection of ES cells were bred to C57BL/6 mice for germ line transmission. Genomic DNA was extracted from the tail samples of the progenies, digested with SacI, and probed with a downstream probe by Southern blotting. (E) Absence of GABPβ1L expression in knockout (KO) mice. Total cell lysates were isolated from thymocytes and splenocytes of the indicated genotypes and Western blotted with an antiserum recognizing both long and short forms of GABPβ1. β-Actin was detected as an equal loading control. Het, heterozygote.
MATERIALS AND METHODS
Generation of GABPβ1L knockout mice.
To specifically eliminate the expression of GABPβ1L, we deleted exon 9 in the Gabpb1 gene in the germ line. We amplified 4.4-kb and 5.4-kb DNA fragments, which were located upstream and downstream, respectively, of exon 9, from 129 embryonic stem (ES) cell genomic DNA using the Expand Long Template PCR system (Roche). These fragments were cloned upstream and downstream of a neomycin-resistant gene cassette in a gene target vector, pLoxp II (23) (Fig. 1B). PstI and SacI sites were inserted between the upstream fragment and the neomycin cassette to facilitate the identification of clones with correct homologous recombination after electroporation of the targeting construct into ES cells. The wild-type (WT) allele yields a 7.2-kb PstI fragment when Southern blotted with the upstream probe, whereas the targeted allele was detected as a 5.6-kb fragment (Fig. 1C). A downstream probe detected a 7.4-kb SacI fragment on the WT allele but a 7.8-kb fragment on the targeted allele (Fig. 1B and D).
ES cells with the expected homologous recombination were injected into C57BL/6 blastocysts, and the resulting chimeric mice were mated with C57BL/6 mice to achieve germ line transmission. The heterozygous offspring were then interbred to generate knockout mice. All mice were handled in accord with NIH and AAALAC guidelines and under an animal study protocol approved by the NHLBI Animal Care and Use Committee and the University of Iowa Institutional Animal Care and Use Committee. Listeria monocytogenes-infected mice were housed in accordance with biosafety regulations.
Generation of GABPβ1 knockout mice and timed mating.
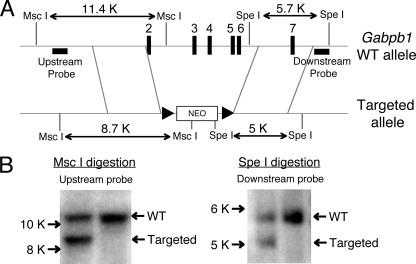
To ablate the expression of both GABPβ1L and GABPβ1S, we deleted exons 2 to 6, which encode the ankyrin repeats of both isoforms. We PCR amplified 3.7-kb and 3.1-kb DNA fragments that are upstream of exon 2 and downstream of exon 6, respectively, from 129 ES cells. These fragments were placed to flank the neomycin-resistant gene cassette in pLoxp II by standard molecular cloning methods (see Fig. 6A). The WT allele yields an 11.4-kb MscI fragment when Southern blotted with an upstream probe, whereas the targeted allele was detected as a 8.7-kb fragment (see Fig. 6B, left). A downstream probe detected a 5.7-kb SpeI fragment on the WT allele, versus a 5-kb fragment on the targeted allele (see Fig. 6B, right). ES cells with the expected homologous recombination were used in blastocyst injections, and upon germ line transmission, the heterozygous offspring were bred or subjected to timed matings for the production of homozygous offspring or embryos (39).
FIG. 6.
Targeting strategy to eliminate the expression of both GABPβ1L and GABPβ1S. (A) Schematic of the targeting strategy. In the targeted allele, exons 2 to 6 were replaced with a neomycin (NEO) cassette. Key restriction enzyme sites and probes used in Southern blotting are indicated. The neomycin cassette contains each of the MscI and SpeI sites, and size changes of MscI and SpeI digestion in the WT and targeted alleles are also indicated. (B) Identification of ES cells with correct homologous recombination. Genomic DNA was extracted from ES cells that were electroporated with the targeting construct, digested with either MscI (left) or SpeI (right), and probed with up- or downstream probes as marked in A, respectively.
Western blotting.
Whole-cell extracts were prepared from thymocytes and splenocytes as described previously (40). Lysate protein (30 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and immunoblotted with an antiserum recognizing the N termini of both GABPβ1L and GABPβ1S (38).
EMSAs and in vitro translation.
The preparation of nuclear extracts from preactivated WT or GABPβ1L−/− T cells and electrophoretic mobility shift assays (EMSAs) were performed as described previously (38). An oligonucleotide spanning the Ets binding site in the IL-7Rα promoter was labeled by [γ-32P]ATP and used as a probe in EMSAs (38). GABPβ2 cDNA was amplified from murine T-cell RNA by reverse transcriptase PCR, cloned into a pCR4 vector with a T7 promoter (Invitrogen), and sequenced. The GABPα, GABPβ1L, and GABPβ2 proteins were produced by in vitro translation using the TNT coupled reticulocyte lysate system (Promega) as described previously (38) and used in EMSAs.
Immunization and ELISAs.
To determine the immune response to a T-independent antigen, mice were immunized intraperitoneally with 100 μg trinitrophenyl 52 [TNP(52)]-AECM (aminoethyl-carboxyl-methyl)-Ficoll (Biosearch Technologies), and the sera were collected 8 days after immunization. The sera were subjected to 12 1:2 serial dilutions, and TNP-specific Igs were measured by enzyme-linked immunosorbent assays (ELISAs). In brief, high-binding plates (Immulux; Dynex) were coated with 20 μg/ml of TNP(38)-bovine serum albumin (BSA) (Bioresearch Technologies) to absorb antigen-specific Igs, which were then detected with biotin-conjugated rat anti-mouse antibodies specific for murine IgM and IgG3. Visualization of the antigen-antibody complexes was done with avidin-horseradish peroxidase and TMB (3,3′,5,5′-tetramethylbenzidine) substrate (BD PharMingen), and the absorbance at 450 nm was read using an ELX800 microplate reader (Bio-TEK). Linear absorbance readings were observed within one particular range of serum dilutions, and these absorbance readings were used to compare antigen-specific Ig titers in WT and GABPβ1L−/− mice.
To evaluate the immune response to a T-dependent antigen, mice were injected intraperitoneally with 100 μg of TNP(30)-keyhole limpet hemocyanin (KLH) (Biosearch Technologies) mixed with Imject Alum (1:1 volume; Pierce) and boosted with the same regimen on day 21. Sera were collected on days 7, 14, and 28 after the initial immunization. To determine the kinetics of antibody responses to the antigen, the levels of TNP-specific IgM and IgG1 in sera collected on different days were measured by ELISAs as described above except that the plates were coated with 6 μg of TNP(38)-BSA. For measuring affinity maturation, IgG1 levels in sera at days 14 and 28 were also determined with ELISAs by coating the plates with low-density hapten, i.e., TNP(4)-BSA. Absorbance values in a linear range were used to calculate an affinity maturation index, which is defined as the ratio of absorbance on TNP(4)-BSA-coated plates to the absorbance on TNP(38)-BSA-coated plates (33).
Proliferation assay.
Splenic T and B cells were purified by negative selection using EasySep T- and B-cell enrichment kits (StemCell Technology), respectively, and the purity of isolated cells was approximately 95%. T cells (2 × 105 cells/well) were stimulated with plate-bound anti-CD3 (1 μg/ml) in the absence or presence of anti-CD28 (1 μg/ml). B cells (2 × 105 cells/well) were stimulated with 5 μg/ml anti-IgM μ chain (Jackson ImmunoResearch Laboratories), 2.5 μg/ml anti-CD40 (BD PharMingen), 2.5 μg/ml lipopolysaccharide (LPS) (Sigma), or 5 μg/ml CpG oligonucleotides (OPN1862; Invivogen). The cells were cultured in 96-well plates in triplicate for each condition for 60 h and pulsed with 1 μCi [3H]thymidine (Perkin-Elmer) for the last 12 h of culture. The radioactivity incorporated into cells was collected on a UniFilter-96 apparatus (GF/B; Perkin-Elmer) with a cell harvester and was counted using a TopCount.NXT microplate scintillation and luminescence counter (Packard).
L. monocytogenes infection and detection of antigen-specific T cells.
WT and GABPβ1L−/− mice were infected with 5 × 106 attenuated L. monocytogenes cells expressing ovalbumin (OVA) as described previously (13). Forty-two days after the initial immunization, the mice were challenged with 7 × 105 virulent L. monocytogenes cells expressing OVA to determine the secondary T-cell responses. Antigen-specific CD4 and CD8 T cells were detected by intracellular staining for gamma interferon (IFN-γ) as described previously (2). Total splenocytes were isolated from immunized mice and incubated with 5 μM listeriolysin O190-201 (LLO190-201) (NEKYAQAYPNVS) peptide to stimulate CD4 cells or with 200 nM OVA257-264 (SIINFEKL) peptide to stimulate CD8 cells in the presence of brefeldin for 6 h. The cells were surface stained, fixed, permeabilized, and intracellularly stained for IFN-γ expression. The total number of antigen-specific T cells per spleen was calculated by multiplying the frequency of CD4+ or CD8+/Thy1.2+/IFN-γ+ cells after stimulation with a specific peptide by the total number of splenocytes. The number of cells producing cytokine in the absence of peptide was subtracted.
Flow cytometry.
Single-cell suspensions were prepared from thymuses, spleens, and bone marrow and stained with fluorochrome-conjugated antibodies, as described previously (39). Peritoneal cells were collected by washing the peritoneal cavity with phosphate-buffered saline. All fluorochrome-conjugated antibodies were obtained from BD PharMingen or eBiosciences.
RESULTS AND DISCUSSION
Deletion of exon 9 in the Gabpb1 gene eliminated the expression of GABPβ1L.
To investigate if the longer C terminus of GABPβ1L has an essential nonredundant role in the immune system, we generated GABPβ1L−/− mice by deleting exon 9 from the germ line (Fig. 1B). ES cells with the expected homologous recombination were obtained (Fig. 1C) and injected into blastocysts. The resulting chimeras were bred with C57BL/6 mice to achieve germ line transmission (Fig. 1D). In contrast to extreme early embryonic lethality caused by a GABPα deficiency (25), GABPβ1L−/− mice were born at a normal Mendelian ratio and were viable. GABPβ1L−/− mice had reduced body weights (83.9% ± 10.1% of the weights of WT littermate controls [n = 12]; P < 0.05 by t test) except that these mice had weak hind limbs, suggesting potential defects in neuron and/or muscle functions. However, pathology analysis of cerebrum, cerebellum, spinal cord, ganglia, and surrounding skeletal muscles did not reveal any apparent abnormalities. In this study, we have focused on the immune system. We prepared cell lysates from thymocytes and splenocytes and Western blotted them with an antiserum recognizing the N termini of both GABPβ1L and GABPβ1S. GABPβ1L is the predominant isoform in WT thymocytes and splenocytes (Fig. 1E). Targeting exon 9 in the Gabpb1 locus in GABPβ1L−/− mice completely abrogated the expression of GABPβ1L, whereas the expression of the GABPβ1S protein was increased (Fig. 1E); we hypothesize that this resulted from the increased availability of the shorter transcripts to the translation machinery. Concomitantly, the heterozygous mice also showed moderately increased levels of GABPβ1S in thymocytes and splenocytes (Fig. 1E).
Normal T-cell development in the absence of GABPβ1L.
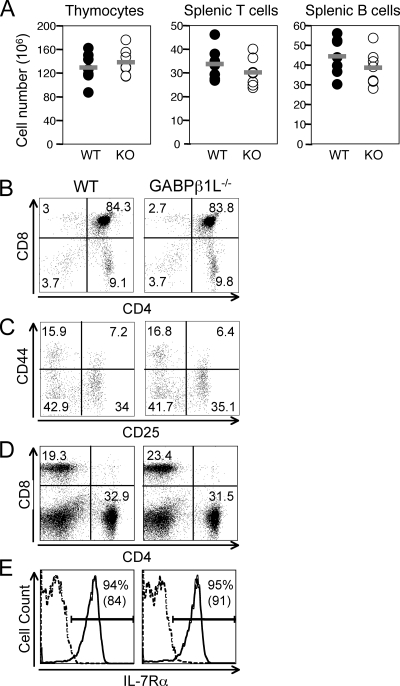
T cells develop in a specialized environment in the thymus, with the most immature thymocytes being double negative (DN) for CD4 and CD8 expression. DN thymocytes then develop into CD4 and CD8 double-positive cells, which in turn give rise to CD4+ or CD8+ single-positive cells after vigorous positive and negative selection (27). Compared with WT controls, thymic cellularity in GABPβ1L−/− mice was not diminished (Fig. 2A). Staining of thymocytes with anti-CD4 and anti-CD8 antibodies revealed that DN, double-positive, CD4+, and CD8+ populations were not altered in GABPβ1L−/− mice (Fig. 2B), suggesting that T-cell development is not affected by the loss of GABPβ1L expression. DN thymocytes can be further divided to four developmental stages based on CD25 and CD44 expression, i.e., DN1 (CD44+ CD25−), DN2 (CD44+ CD25+), DN3 (CD44− CD25+), and DN4 (CD44− CD25−). All four DN subsets appeared at comparable frequencies between WT and GABPβ1L−/− mice (Fig. 2C), indicating that the absence of GABPβ1L does not perturb early T-cell development. In the periphery, splenic CD4+ and CD8+ T cells in GABPβ1L−/− mice were produced at a normal ratio (Fig. 2D), although the total numbers of splenic T cells were slightly reduced (Fig. 2A), consistent with the reduced body sizes of GABPβ1L−/− mice. Previously, we demonstrated that the GABP complex directly regulates IL-7Rα expression in T cells (38). However, the level of expression of the IL-7Rα protein on GABPβ1L−/− CD4+ and CD8+ T cells was relatively normal (Fig. 2E and data not shown for CD8 cells), suggesting that GABPβ1L is dispensable for IL-7Rα expression.
FIG. 2.
Normal T-cell development in the absence of GABPβ1L. (A) Cellularity in lymphoid organs. Thymocytes and splenocytes were isolated from WT and knockout (KO) mice. Splenocytes were stained with anti-CD3 and anti-B220 antibodies. The percentages of CD3+ and B220+ populations were used to calculate T- and B-cell numbers, respectively. The differences between WT and knockout cells were not statistically significant by a Student's t test. (B) T-cell development in the thymus. Thymocytes were stained with anti-CD4 and anti-CD8, and each population is shown as the percentage of total thymocytes. (C) Early thymocyte development. DN thymocytes were further fractionated by the expression of CD25 and CD44, and each population is shown as a percentage of DN cells. (D) Splenic CD4 and CD8 cells. Splenocytes were stained for CD4 and CD8 expression, and each population is shown as the percentage of total splenocytes. (E) IL-7Rα expression on CD4 splenic T cells. The percentages of CD4 cells expressing IL-7Rα and the mean fluorescence intensities (in parentheses) are shown. Dashed line, isotype control. All data are representative of at least four independent analyses of more than six animals of each genotype.
Normal B-cell development in the absence of GABPβ1L.
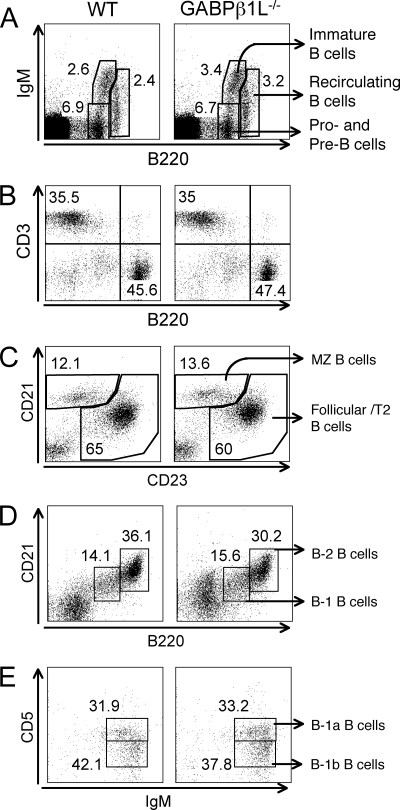
Next, we examined B-cell development in GABPβ1L−/− mice. In the bone marrow, B220+ IgM− cells include both pro-B and pre-B lymphocytes, which develop into immature B cells (B220med IgM+) and recirculating B cells (B220high IgM+) (12). All three populations in the bone marrow of GABPβ1L−/− mice were detected at percentages similar to those observed for WT controls (Fig. 3A). In the periphery, the absolute numbers of splenic B cells in GABPβ1L−/− mice were slightly decreased (Fig. 2A), perhaps due to the reduced body sizes of these mice. However, the frequencies of total splenic B cells, marginal-zone B cells (CD21high CD23dim), and follicular B cells (CD23bright) were comparable between WT and GABPβ1L−/− mice (Fig. 3B and C).
FIG. 3.
Normal B-cell development in the absence of GABPβ1L. (A) Early B-cell development in the bone marrow. Bone marrow cells were isolated and stained with anti-B220 and IgM. Each population is presented as a percentage of the total number of bone marrow cells. (B) Peripheral T and B cells. Splenocytes were isolated and stained with anti-CD3 and anti-B220. The percentages of T and B cells in the spleen are shown. (C) Marginal-zone (MZ) and follicular B cells. B220+ splenic B cells were further fractionated based on CD21 and CD23 expression. Each population is shown as a percentage of B220+ cells. (D) B-1 and B-2 B cells in the peritoneal cavity. Peritoneal cells were collected and stained with anti-B220 and anti-CD21. Each population is presented as a percentage of gated lymphocytes. (E) B-1a and B-1b B cells in the peritoneal cavity. Peritoneal cells were stained with anti-IgM and anti-CD5 in addition to anti-B220 and anti-CD21. Each population is presented as a percentage of B220+ CD21low B1 B cells. All data are representative of at least four independent analyses of more than six animals of each genotype.
Mature B cells are also present in the peritoneal cavity, providing a first defense against bacterial pathogens (3). In contrast to B-2 B cells, which are B220bright CD21high, the peritoneal B-1 B cells show a B220+ CD21med phenotype. Both B-1 and B-2 B cells were detected in the peritoneal cavities of GABPβ1L−/− mice at a frequency relatively similar to that observed in WT mice (Fig. 3D). Based on CD5 expression, B220+ CD21med B-1 B cells can be further fractionated to two subsets, B-1a (IgM+ CD5+) and B-1b (IgM+ CD5−) (21), and these subsets were present at comparable frequencies in WT and GABPβ1L−/− mice (Fig. 3E). These data collectively suggest that the loss of GABPβ1L expression did not substantially perturb B-cell development in the bone marrow, further maturation in the spleen, and the production of B-1 B cells in the peritoneal cavity.
Normal T-cell responses in the absence of GABPβ1L.
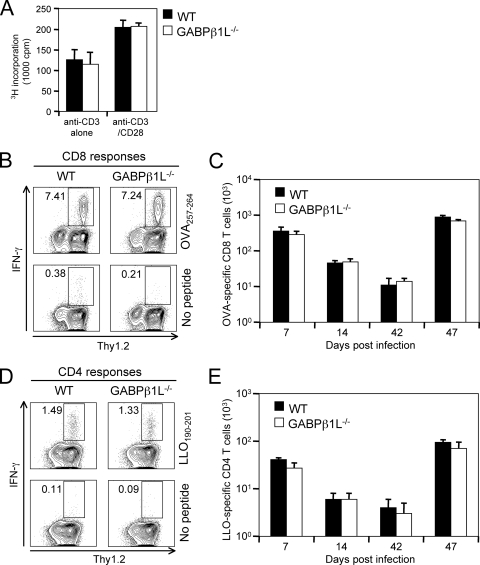
To determine if mature T cells that developed in the absence of GABPβ1L are functional, we isolated splenic T cells by negative selection and stimulated them with plate-bound anti-CD3 in the presence or absence of anti-CD28. Both WT and GABPβ1L−/− T cells responded and proliferated similarly (Fig. 4A). To determine the T-cell responses in vivo, we infected GABPβ1L−/− and littermate control mice with an attenuated strain of L. monocytogenes expressing OVA. We tracked OVA257-264-specific CD8 T cells by intracellular staining for IFN-γ in the presence or absence of peptide stimulation. As shown in Fig. 4B, WT and GABPβ1L−/− mice showed similar frequencies of OVA-specific CD8 cells on day 7 postinfection (p.i.), and this translated into similar total numbers of antigen-specific T cells in the spleen (Fig. 4C). In addition, we observed similar numbers of antigen-specific T cells at the contraction phase (day 14 p.i.) and memory phase (day 42 p.i.) during the primary response. To evaluate the secondary response of antigen-specific memory CD8 T cells, we challenged the infected mice with virulent L. monocytogenes cells expressing OVA on day 42 p.i. and determined T-cell responses 5 days later. The results are summarized in Fig. 4C and show similar CD8 T-cell responses between WT and GABPβ1L-deficient mice at all time points examined. We also evaluated the CD4 T-cell responses to LLO190-201 at different time points after infection with actA mutant L. monocytogenes cells expressing OVA and rechallenge with virulent L. monocytogenes cells expressing OVA and found similar potencies and kinetics of CD4 responses in both WT and GABPβ1L−/− mice (Fig. 4D and E). These data collectively indicate that the loss of GABPβ1L expression does not interfere with normal CD4 and CD8 T-cell responses to foreign antigens.
FIG. 4.
T-cell responses are similar in WT and GABPβ1L-deficient mice. (A) T-cell proliferation in vitro. Splenic T cells were purified by negative selection and stimulated with plate-bound anti-CD3 alone (1 μg/ml) or in combination with anti-CD28 (1 μg/ml). The proliferation assay was performed in triplicate for each condition with 2 × 105 purified T cells in each well. The cells were activated for 48 h, pulsed with 1 μCi [3H]thymidine, and harvested 12 h later. (B) Detection of antigen-specific CD8 T cells on day 7 p.i. The mice were infected with 5 × 106 actA mutant L. monocytogenes cells expressing OVA, and 7 days later, splenocytes were harvested and cultured in the presence or absence of 200 nM OVA257-264 peptide for 6 h. The cells were stained with anti-CD8 and Thy1.2, followed by intracellular staining of IFN-γ. The percentage of IFN-γ+ cells in the CD8+ population is shown. (Bottom) Background staining in the absence of peptide stimulation. Data are representative of data for at least six mice of each phenotype. (C) Kinetics of CD8 T-cell responses during primary and secondary infection. Antigen-specific T cells were detected as described above (B) on days 7, 14, and 42 p.i. A group of mice of 42 days after primary infection was challenged with 7 × 105 virulent L. monocytogenes cells expressing OVA, and the CD8 T-cell response was determined on day 5 after the secondary infection. Each time point is the average ± standard deviation of data for at least six mice. (D) Detection of antigen-specific CD4 T cells on day 7 p.i. Mice were infected as described above (B), and the splenocytes were stimulated with 5 μM LLO190-201 peptide. The percentage of IFN-γ+ cells in the CD4+ T-cell population is shown. (E) Kinetics of CD4 T-cell responses during primary and secondary infection. Mice were infected as described above (C), and antigen-specific CD4 T cells were detected as described above (D) at various time points.
Normal B-cell responses in the absence of GABPβ1L.
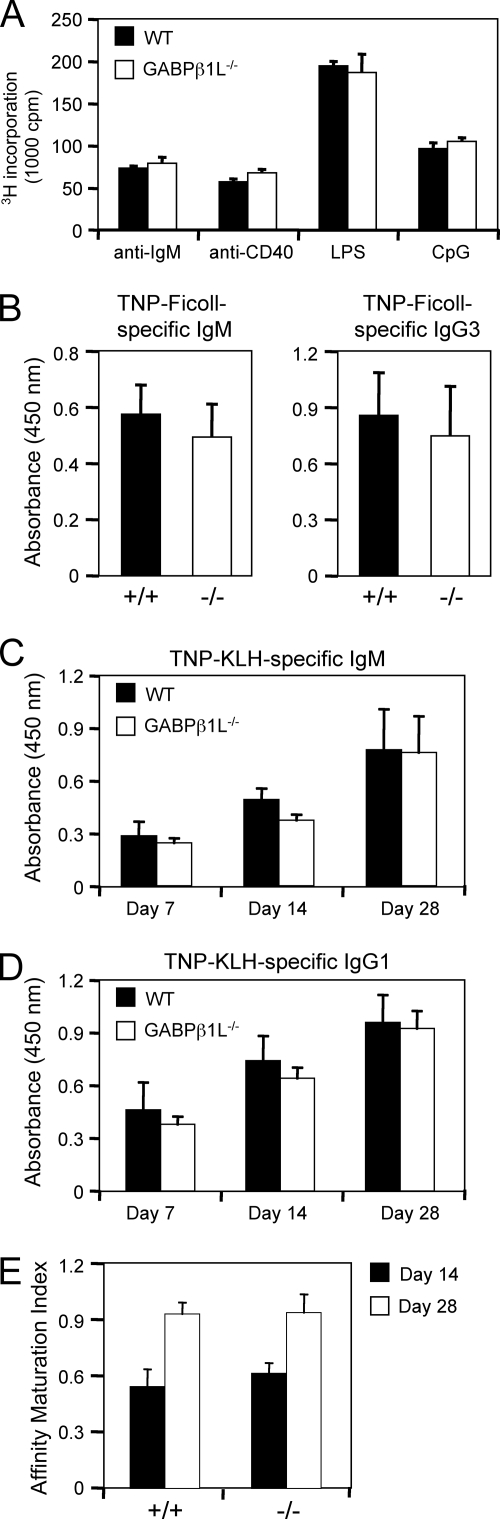
To determine if the ablation of GABPβ1L expression results in alterations in B-cell responses, we activated splenic B cells with various stimuli and measured their proliferative responses. We used anti-IgM to ligate the B-cell receptor, anti-CD40 to stimulate via CD40, LPS to stimulate via Toll-like receptor 4, and CpG nucleotides to stimulate via Toll-like receptor 9. All of these stimuli elicited comparable levels of proliferation between WT and GABPβ1L−/− B cells (Fig. 5A). To evaluate B-cell responses and T-and B-cell interactions in an in vivo setting, we immunized mice with TNP-Ficoll, a T-independent antigen, and TNP-KLH, a T-dependent antigen, and measured antigen-specific Ig levels. GABPβ1L−/− mice can produce TNP-Ficoll-specific IgM and IgG3. Although the mean Ig levels were slightly lower than those of WT littermate controls on average, this difference was not statistically significant by a Student's t test (Fig. 5B) and perhaps is explained by the small reduction in total splenic B-cell numbers in GABPβ1L−/− mice (Fig. 2A).
FIG. 5.
B-cell responses are similar in WT and GABPβ1L-deficient mice. (A) B-cell proliferation in response to various stimuli. Splenic B cells were purified by negative selection and stimulated with anti-IgM (5 μg/ml), anti-CD40 (2.5 μg/ml), LPS (2.5 μg/ml), or CpG (5 μg/ml). Incorporation of [3H]thymidine was determined as described in the legend of Fig. 4A. Data are representative of at least three independent experiments. (B) Humoral responses to a T-independent antigen. Mice were immunized with 100 μg of TNP-Ficoll, and sera were collected 1 week later. Levels of TNP-Ficoll-specific IgM and IgG3 were measured by ELISAs using TNP(38)-BSA-coated plates. Results are representative of two independent experiments with three to four animals of each genotype used in each experiment. (C and D) Immune responses to a T-dependent antigen. Mice were immunized with 100 μg of TNP-KLH mixed with alum and boosted with the same regimen 21 days after the initial immunization. Sera were collected on days 7, 14, and 28 postimmunization, and levels of TNP-KLH-specific IgM (C) and IgG1 (D) were measured by ELISAs using TNP(38)-BSA-coated plates. (E) Affinity maturation of TNP-KLH-specific IgG1. Binding of TNP-specific IgG1 to a low-density hapten, TNP(4)-BSA, was determined for sera at day 14 and day 28 obtained from TNP-KLH-immunized mice. The ratio of binding to TNP(4)-BSA to binding to TNP(38)BSA was used as an affinity maturation index. Results are averages ± standard deviations of data from four mice of each genotype.
With the T-dependent antigen TNP-KLH, we collected sera on days 7, 14, and 28 after initial immunization with a boost on day 21. TNP-KLH-specific IgM and IgG1 were detected on day 7, and the levels increased on day 14 and further increased on day 28 postimmunization in both WT and GABPβ1L−/− mice (Fig. 5C and D), indicating that both the kinetics of B-cell responses and the formation of B memory are not perturbed in the absence of GABPβ1L; small mean differences were not statistically significant. An important feature of humoral memory is the affinity maturation of Igs, which can be assessed by comparing their binding capacities to those of low-density and high-density haptens (35). TNP-specific IgG1 in sera from WT mice at day 14 showed the expected low binding to low-density hapten compared with that to high-density hapten, whereas TNP-specific IgG1 in sera on day 28 manifested markedly stronger binding to the low-density hapten, as indicated by the higher affinity maturation index (Fig. 5E). The affinity maturation in GABPβ1L−/− mice was comparable to that in WT mice (Fig. 5E), indicating that GABPβ1L is dispensable in this process.
Targeting both GABPβ1L and GABPβ1S resulted in early embryonic lethality.
Previous studies demonstrated that GABPβ1L and GABPβ1S can heterodimerize with GABPα with similar efficiencies. The lack of apparent phenotypes in GABPβ1L−/− mice suggests that the loss of GABPβ1L might be compensated for by GABPβ1S. Exons 1 to 6 in the Gabpb1 gene encode the N-terminal ankyrin repeats shared by both GABPβ1L and GABPβ1S. To eliminate the expression of both GABPβ1L and GABPβ1S, we targeted exons 2 to 6 in the Gabpb1 allele (Fig. 6A and B). However, no live-born mice were homozygous for the targeted alleles, and in addition, no homozygous embryos at embryonic day 12.5 were found (Table 1), indicating an early embryonic lethality in the absence of both GABPβ1L and GABPβ1S. These results suggest that the Gabpb1 gene is nonredundant and that the expression of at least one splice isoform is required for normal embryogenesis. The C-terminal domain in GABPβ1L that is absent in GABPβ1S has been assumed to contain transactivation activity; nevertheless, our observations suggest that GABPβ1S can still support the activation/suppression of GABP target genes. This is at least true for those GABP target genes that are critical for embryogenesis. It remains to be determined if this is the case in immune cells and awaits tissue-specific targeting of the two GABPβ1 isoforms.
TABLE 1.
Embryonic lethality of Gabpb1 targeted mice
| Age of mice | No. of litters | Total no. of embryos | No. of embryos with genotype:
|
||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | |||
| Embryonic day 12.5 | 5 | 33 | 15 | 18 | 0 |
| 1 day | 3 | 19 | 8 | 11 | 0 |
| 3 wk | 6 | 44 | 12 | 32 | 0 |
Possible contribution of another GABPβ isoform, GABPβ2.
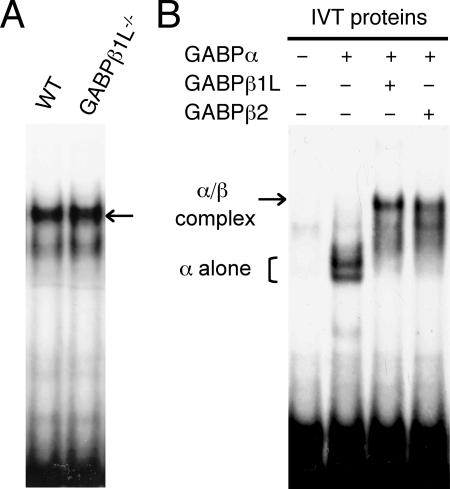
Because of the early embryonic lethality in the absence of both GABPβ1L and GABPβ1S, we could not directly evaluate the roles of GABPβ1S in T and B cells and thus cannot exclude the possibility that the loss of GABPβ1L could be compensated for by a third GABPβ isoform, GABPβ2, which is encoded by the Gabpb2 gene on chromosome 3 (6). The GABPβ2 protein is 414 amino acids long, with its N-terminal ankyrin repeats (amino acids 1 to 130) sharing 87% identity with the GABPβ1 isoforms. GABPβ2 also has a long C-terminal tail, with its 317 to 366 amino acid residues sharing 70% identity with the C terminus of GABPβ1L and also adopting a leucine zipper-like structure. Since its initial cloning, GABPβ2 has not been studied, and its function is completely unknown. Previously, we demonstrated that both GABPβ1L and GABPβ1S can heterodimerize with GABPα and bind to an Ets motif in the IL-7Rα promoter (38). In an EMSA using a probe containing the IL-7Rα promoter Ets motif and its flanking sequence, we detected a major protein-DNA complex with nuclear extract from preactivated WT T cells (Fig. 7A), and interestingly, the complex was not diminished when GABPβ1L-deficient nuclear extract was used. The complex formed with GABPβ1L−/− nuclear extract may contain GABPβ2 in addition to GABPβ1S. To investigate whether the heterodimer of GABPα and GABPβ2 can bind to the Ets motif in the IL-7Rα promoter, we performed EMSAs using in vitro-translated GABP proteins. Consistent with our previous findings, GABPα alone can form fast-migrating complexes with the probe, and a larger complex was formed in the presence of both GABPα and GABPβ1L (Fig. 7B). When in vitro-translated GABPβ2 was used in place of GABPβ1L, the GABPα/GABPβ2 complex manifested migration similar to that with the GABPα/GABPβ1L complex (Fig. 7B). Thus, it is possible that GABPβ2 may also contribute to the regulation of GABP target genes in the presence or absence of other GABPβ isoforms.
FIG. 7.
The GABPα/GABPβ2 complex can bind to an Ets motif in the IL-7Rα promoter. (A) Formation of a protein/DNA complex in the presence and absence of GABPβ1L. Splenic T cells from WT or GABPβ1L−/− mice were purified by negative selection and preactivated and expanded as described previously (38). Nuclear extracts were prepared and used in EMSAs. The probe contains an Ets motif in the IL-7Rα promoter with flanking sequences. The major protein/DNA complex, indicated by an arrow, contains GABPs as characterized in our previous studies (38). (B) GABPs bind to an Ets motif in the IL-7Rα promoter. The cDNAs of GABPα, GABPβ1L, and GABPβ2 were each separately cloned 3′ to the T7 promoter in plasmids and used in a reticulocyte lysate in vitro translation system to produce the respective proteins. The in vitro-translated (IVT) proteins were used in EMSAs as above (A) in the indicated combinations.
Complexity of GABPβ functions.
The GABPα/β complex has been shown to have versatile roles in regulating basic cellular functions and tissue-specific functions (9, 26, 31). Gene-targeting studies of the DNA binding GABPα subunit have revealed its critical roles during embryogenesis (25), during the reentry of the cell cycle (41), and in synaptic function at the neuromuscular junction (22, 24). In the immune system, we and others showed that GABP critically regulates IL-7Rα expression (5, 38). Recently, we demonstrated that GABP is a key component of gene the regulatory network, programming B-lineage commitment and differentiation by directly regulating Pax5 gene expression (39). In contrast to extensive studies on GABPα, the roles of the GABPβ subunit in vivo have not been investigated. Our observations that the loss of GABPβ1L does not perturb lymphocyte development revealed functional redundancy among GABPβ isoforms. In terms of the regulation of the Il7r gene, both GABPβ1S and GABPβ2 are capable of heterodimerizing with GABPα and bind to an Ets motif in the Il7r promoter. In an analysis using a synthetic peptide or recombinant protein, it was demonstrated that the C terminus of GABPβ2 not only can mediate the formation of GABPβ2 homodimers but also can mediate the heterodimerization of GABPβ2 with GABPβ1L (6). For GABP target genes that require an α2β2 heterotetramer, GABPβ1L and GABPβ2 appear to have redundant roles; however, it is noteworthy that GABPβ2 alone cannot compensate for the loss of both GABPβ1L and GABPβ1S during early embryogenesis. This suggests that products from the Gabpb1 and Gabpb2 genes also have nonredundant functions. The generation of GABPβ2-deficient and GABPβ1L- and GABPβ2-double-deficient mice will help to elucidate distinct and redundant roles of these isoforms.
If the leucine zipper-like structure in GABPβ1L or GABPβ2 was uniquely required for the transactivation of all GABP target genes, one would expect that GABPβ1S, which lacks this structure, would function as a dominant negative molecule and that GABPβ1L-deficient mice may thus show phenotypes similar to those of GABPα-deficient mice. Nevertheless, GABPβ1L−/− mice were grossly normal, with no apparent defects in the development and function of both T and B cells. Thus, it seems likely that GABPβ1S can cooperate with GABPα to form a functional complex rather than act as a dominant negative protein. This is further corroborated by the observation that embryos lacking both GABPβ1L and GABPβ1S are not viable. Taken together, our studies reveal unrecognized functional complexities of GABPβ isoforms, which may convey fine regulation of GABP activities and thus GABP target genes during embryogenesis and in lymphocyte development and function.
Acknowledgments
We thank Rosanne Spolski for critical reading of the manuscript. We thank Renee Cao and Eileen Sweezer at the Gene Targeting Core Facility, University of Iowa, for their excellent technical assistance in generating GABPβ1 knockout mouse.
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, NIH, and the Department of Microbiology, University of Iowa.
Footnotes
Published ahead of print on 21 April 2008.
REFERENCES
- 1.Avots, A., A. Hoffmeyer, E. Flory, A. Cimanis, U. R. Rapp, and E. Serfling. 1997. GABP factors bind to a distal interleukin 2 (IL-2) enhancer and contribute to c-Raf-mediated increase in IL-2 induction. Mol. Cell. Biol. 174381-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badovinac, V. P., and J. T. Harty. 2000. Intracellular staining for TNF and IFN-gamma detects different frequencies of antigen-specific CD8(+) T cells. J. Immunol. Methods 238107-117. [DOI] [PubMed] [Google Scholar]
- 3.Berland, R., and H. H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20253-300. [DOI] [PubMed] [Google Scholar]
- 4.Brown, T. A., and S. L. McKnight. 1992. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 62502-2512. [DOI] [PubMed] [Google Scholar]
- 5.Dekoter, R. P., B. L. Schweitzer, M. B. Kamath, D. Jones, H. Tagoh, C. Bonifer, D. A. Hildeman, and K. J. Huang. 2007. Regulation of the interleukin-7 receptor alpha promoter by the Ets transcription factors PU.1 and GA-binding protein in developing B cells. J. Biol. Chem. 28214194-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Brousse, F. C., E. H. Birkenmeier, D. S. King, L. B. Rowe, and S. L. McKnight. 1994. Molecular and genetic characterization of GABP beta. Genes Dev. 81853-1865. [DOI] [PubMed] [Google Scholar]
- 7.Duclert, A., N. Savatier, L. Schaeffer, and J. P. Changeux. 1996. Identification of an element crucial for the sub-synaptic expression of the acetylcholine receptor epsilon-subunit gene. J. Biol. Chem. 27117433-17438. [DOI] [PubMed] [Google Scholar]
- 8.Fitzsimmons, D., W. Hodsdon, W. Wheat, S. M. Maira, B. Wasylyk, and J. Hagman. 1996. Pax-5 (BSAP) recruits Ets proto-oncogene family proteins to form functional ternary complexes on a B-cell-specific promoter. Genes Dev. 102198-2211. [DOI] [PubMed] [Google Scholar]
- 9.Gallant, S., and G. Gilkeson. 2006. ETS transcription factors and regulation of immunity. Arch. Immunol. Ther. Exp. (Warsaw) 54149-163. [DOI] [PubMed] [Google Scholar]
- 10.Genuario, R. R., and R. P. Perry. 1996. The GA-binding protein can serve as both an activator and repressor of ribosomal protein gene transcription. J. Biol. Chem. 2714388-4395. [DOI] [PubMed] [Google Scholar]
- 11.Gugneja, S., J. V. Virbasius, and R. C. Scarpulla. 1995. Four structurally distinct, non-DNA-binding subunits of human nuclear respiratory factor 2 share a conserved transcriptional activation domain. Mol. Cell. Biol. 15102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy, R. R., and K. Hayakawa. 2001. B cell development pathways. Annu. Rev. Immunol. 19595-621. [DOI] [PubMed] [Google Scholar]
- 13.Haring, J. S., X. Jing, J. Bollenbacher-Reilley, H. H. Xue, W. J. Leonard, and J. T. Harty. 2008. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J. Immunol. 1802855-2862. [DOI] [PubMed] [Google Scholar]
- 14.Hauck, L., R. G. Kaba, M. Lipp, R. Dietz, and R. von Harsdorf. 2002. Regulation of E2F1-dependent gene transcription and apoptosis by the ETS-related transcription factor GABPγ1. Mol. Cell. Biol. 222147-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu, T., M. Trojanowska, and D. K. Watson. 2004. Ets proteins in biological control and cancer. J. Cell. Biochem. 91896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaworski, A., C. L. Smith, and S. J. Burden. 2007. GA-binding protein is dispensable for neuromuscular synapse formation and synapse-specific gene expression. Mol. Cell. Biol. 275040-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koike, S., L. Schaeffer, and J. P. Changeux. 1995. Identification of a DNA element determining synaptic expression of the mouse acetylcholine receptor delta-subunit gene. Proc. Natl. Acad. Sci. USA 9210624-10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMarco, K., C. C. Thompson, B. P. Byers, E. M. Walton, and S. L. McKnight. 1991. Identification of Ets- and notch-related subunits in GA binding protein. Science 253789-792. [DOI] [PubMed] [Google Scholar]
- 19.Li, X. R., A. S. Chong, J. Wu, K. A. Roebuck, A. Kumar, J. E. Parrillo, U. R. Rapp, R. P. Kimberly, J. W. Williams, and X. Xu. 1999. Transcriptional regulation of Fas gene expression by GA-binding protein and AP-1 in T cell antigen receptor. CD3 complex-stimulated T cells. J. Biol. Chem. 27435203-35210. [DOI] [PubMed] [Google Scholar]
- 20.Maier, H., R. Ostraat, S. Parenti, D. Fitzsimmons, L. J. Abraham, C. W. Garvie, and J. Hagman. 2003. Requirements for selective recruitment of Ets proteins and activation of mb-1/Ig-alpha gene transcription by Pax-5 (BSAP). Nucleic Acids Res. 315483-5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montecino-Rodriguez, E., and K. Dorshkind. 2006. New perspectives in B-1 B cell development and function. Trends Immunol. 27428-433. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary, D. A., P. G. Noakes, N. A. Lavidis, I. Kola, P. J. Hertzog, and S. Ristevski. 2007. Targeting of the ETS factor Gabpα disrupts neuromuscular junction synaptic function. Mol. Cell. Biol. 273470-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki, K., R. Spolski, C. G. Feng, C. F. Qi, J. Cheng, A. Sher, H. C. Morse III, C. Liu, P. L. Schwartzberg, and W. J. Leonard. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science 2981630-1634. [DOI] [PubMed] [Google Scholar]
- 24.Ravel-Chapuis, A., M. Vandromme, J. L. Thomas, and L. Schaeffer. 2007. Postsynaptic chromatin is under neural control at the neuromuscular junction. EMBO J. 261117-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ristevski, S., D. A. O'Leary, A. P. Thornell, M. J. Owen, I. Kola, and P. J. Hertzog. 2004. The ETS transcription factor GABPα is essential for early embryogenesis. Mol. Cell. Biol. 245844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosmarin, A. G., K. K. Resendes, Z. Yang, J. N. McMillan, and S. L. Fleming. 2004. GA-binding protein transcription factor: a review of GABP as an integrator of intracellular signaling and protein-protein interactions. Blood Cells Mol. Dis. 32143-154. [DOI] [PubMed] [Google Scholar]
- 27.Rothenberg, E. V., and T. Taghon. 2005. Molecular genetics of T cell development. Annu. Rev. Immunol. 23601-649. [DOI] [PubMed] [Google Scholar]
- 28.Sawa, C., M. Goto, F. Suzuki, H. Watanabe, J. Sawada, and H. Handa. 1996. Functional domains of transcription factor hGABP beta1/E4TF1-53 required for nuclear localization and transcription activation. Nucleic Acids Res. 244954-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawada, J., M. Goto, C. Sawa, H. Watanabe, and H. Handa. 1994. Transcriptional activation through the tetrameric complex formation of E4TF1 subunits. EMBO J. 131396-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarpulla, R. C. 2002. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim. Biophys. Acta 15761-14. [DOI] [PubMed] [Google Scholar]
- 31.Sharrocks, A. D. 2001. The ETS-domain transcription factor family. Nat. Rev. Mol. Cell Biol. 2827-837. [DOI] [PubMed] [Google Scholar]
- 32.Sowa, Y., Y. Shiio, T. Fujita, T. Matsumoto, Y. Okuyama, D. Kato, J. Inoue, J. Sawada, M. Goto, H. Watanabe, H. Handa, and T. Sakai. 1997. Retinoblastoma binding factor 1 site in the core promoter region of the human RB gene is activated by hGABP/E4TF1. Cancer Res. 573145-3148. [PubMed] [Google Scholar]
- 33.Stunz, L. L., L. K. Busch, M. E. Munroe, C. D. Sigmund, L. T. Tygrett, T. J. Waldschmidt, and G. A. Bishop. 2004. Expression of the cytoplasmic tail of LMP1 in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity 21255-266. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, F., M. Goto, C. Sawa, S. Ito, H. Watanabe, J. Sawada, and H. Handa. 1998. Functional interactions of transcription factor human GA-binding protein subunits. J. Biol. Chem. 27329302-29308. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi, Y., P. R. Dutta, D. M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187885-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson, C. C., T. A. Brown, and S. L. McKnight. 1991. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science 253762-768. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe, H., T. Wada, and H. Handa. 1990. Transcription factor E4TF1 contains two subunits with different functions. EMBO J. 9841-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue, H. H., J. Bollenbacher, V. Rovella, R. Tripuraneni, Y. B. Du, C. Y. Liu, A. Williams, J. P. McCoy, and W. J. Leonard. 2004. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat. Immunol. 51036-1044. [DOI] [PubMed] [Google Scholar]
- 39.Xue, H. H., J. Bollenbacher-Reilley, Z. Wu, R. Spolski, X. Jing, Y. C. Zhang, J. P. McCoy, and W. J. Leonard. 2007. The transcription factor GABP is a critical regulator of B lymphocyte development. Immunity 26421-431. [DOI] [PubMed] [Google Scholar]
- 40.Xue, H. H., D. W. Fink, Jr., X. Zhang, J. Qin, C. W. Turck, and W. J. Leonard. 2002. Serine phosphorylation of Stat5 proteins in lymphocytes stimulated with IL-2. Int. Immunol. 141263-1271. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Z. F., S. Mott, and A. G. Rosmarin. 2007. The Ets transcription factor GABP is required for cell-cycle progression. Nat. Cell Biol. 9339-346. [DOI] [PubMed] [Google Scholar]