Abstract
Increased fetal hemoglobin (Hb F; α2γ2) production in adults can ameliorate the clinical severity of sickle cell disease and β-thalassemia major. Thus, understanding the regulation of γ-globin gene expression and its silencing in adults has potential therapeutic implications. We studied a father and son in an Iranian-American family who had elevated Hb F levels and found a novel T-to-G transversion at nucleotide (nt) −567 of the HBG2 promoter. This mutation alters a GATA-1 binding motif to a GAGA sequence located within a previously identified silencing element. DNA-protein binding assays showed that the GATA motif of interest is capable of binding GATA-1 transcription factor in vitro and in vivo. Truncation analyses of the HBG2 promoter linked to a luciferase reporter gene revealed a negative regulatory activity present between nt −675 and −526. In addition, the T-to-G mutation at the GATA motif increased the promoter activity by two- to threefold in transiently transfected erythroid cell lines. The binding motif is uniquely conserved in simian primates with a fetal pattern of γ-globin gene expression. These results suggest that the GATA motif under study has a functional role in silencing γ-globin gene expression in adults. The T-to-G mutation in this motif disrupts GATA-1 binding and the associated repressor complex, abolishing its silencing effect and resulting in the up-regulation of γ-globin gene expression in adults.
During normal human fetal development, the predominant hemoglobin is fetal hemoglobin (Hb F) (α2γ2). From the third trimester of gestation forward, there is a gradual silencing of expression of γ-globin genes (HBG2, encoding Gγ-globin; HBG1 encoding Aγ-globin), accompanied by a reciprocal increase in β-globin gene (HBB) expression. Six to 9 months after birth, Hb A (α2β2) forms more than 95% of the total hemoglobin, while Hb F levels are normally at less than 1% throughout life.
Hb F inhibits the polymerization of sickle hemoglobin (Hb S) (4). Increased production of γ-globin chains reduces the globin chain imbalance in β-thalassemia major due to markedly decreased or absent β-globin chain synthesis (19). As a consequence, augmented γ-globin gene expression or Hb F production can ameliorate the clinical severity of these common hereditary disorders. It is therefore important to understand the regulation of γ-globin gene expression and its silencing.
Rare genetic mutations cause the syndrome of hereditary persistence of fetal hemoglobin (HPFH) (24). Several of these are large deletions removing both HBD (encoding δ-globin) and HBB. In addition, there are at least 15 known single-nucleotide polymorphisms (SNPs) in either the HBG2 or HBG1 promoters, resulting in up-regulation of γ-globin gene expression in adults. These SNPs are found between nt −202 and −110, and some of them modify transcription factor binding sites.
We studied a father and son in an Iranian-American family who had moderately elevated Hb F levels. A novel T-to-G SNP at nt −567 upstream of the HBG2 promoter was found in them, but not in other family members. This mutation alters a GATA-1 binding motif to the GAGA sequence. Additional studies have suggested that this GATA motif is likely to play a significant role in the silencing of γ-globin gene expression. The mutation of GATA to GAGA disrupts its silencing effect and is associated with increased γ-globin gene expression in affected adults.
MATERIALS AND METHODS
Hematology, hemoglobin, and globin gene mutation analyses.
Blood counts were done using a Sysmex XE hematology automated analyzer (Sysmex, Mundelein, IL). Hemoglobin analyses and measurements in the mother and her two sons were carried out with the Variant II cation high-performance liquid chromatography system (Bio-Rad, Hercules, CA). For the father, Hb F was determined by the alkali denaturation method and confirmed by isoelectric focusing. DNA was extracted from peripheral blood leukocytes by a standard phenol chloroform (50%/50% [vol/vol]) method. HBB, HBG2, and HBG1 were amplified by PCR and sequenced using the Big Dye Terminator Cycle Sequencing kit v3.1 (Applied Biosystems, California) (9). The α-globin genotype was analyzed by gap PCR (22).
Alignments of genomic sequences.
An alignment of the upstream region of human HBG2 with homologous sequences from 35 other vertebrates was extracted from precomputed alignments generated by the program TBA (2) as part of the ENCODE pilot project (12). The homolog in the horse was added based on recent genome-wide alignments generated with MultiZ (13), and the paralogous sequence from HBG1 was added by inspection. The reverse complements of the sequences from the reference genomes are displayed to maintain the conventional left-to-right orientation for transcription of the globin genes.
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from K562 and MEL cells using a kit obtained from Active Motif (Carlsbad, CA). Two double-stranded oligonucleotide probes were synthesized (Integrated DNA Technologies, Coralville, IA). Their HBG2 promoter-specific sequences were as follows: GATA567 (WT) probe, 5′-AGAACTTAAGAGATAATGGCCTAAA-3′; GATA567 (MUT) probe, 5′-AGAACTTAAGAGAGAATGGCCTAAA-3′.
The probes were end labeled with [γ-32P]dATP using T4 kinase, followed by gel purification using Bio-Spin 30 chromatography columns (Bio-Rad, Hercules, CA). The DNA-protein binding reaction was performed by mixing 5 μg nuclear extract protein and radiolabeled probes for 20 min, followed by polyacrylamide gel electrophoresis at 15 mA for 3 to 4 h at 25°C. The gel was dried and exposed to X-ray film. In competition experiments, unlabeled probes with 100-fold molar excess or as indicated were included in the reactions. Supershift experiments were carried out with anti-GATA-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
ELISA-based GATA competition assay.
An enzyme-linked immunosorbent assay (ELISA)-based GATA competition assay was performed using a TransAM GATA Family Transcription Factor Assay kit (Active Motif, Carlsbad, CA) with modification. Briefly, a 96-well plate on which oligonucleotides containing a GATA-1 consensus binding sequence (AGATAA) had been immobilized was used. MEL cell nuclear extract (Active Motif, Carlsbad, CA) as a source of GATA-1 protein was added to each well at 1 μg/well. Assays were performed in the absence or presence of 0.4 to 100 pmol of double-stranded oligonucleotide competitors that contained either a wild-type GATA or mutated GAGA site (underlined) at the HBG2 promoter under study: GG567WT oligo, 5′-CTAATGAGAACTTAAGAGATAATGGCCTAAAACCACAGAG-3′; GG567MU oligo, 5′-CTAATGAGAACTTAAGAGAGAATGGCCTAAAACCACAGAG-3′. After incubation for 1 h at room temperature with mild agitation, followed by washing, the GATA-1 protein bound to the oligonucleotides with which the plate was coated was detected by an anti-GATA-1 antibody and measured by horseradish peroxidase-conjugated antibody and colorimetric reagents.
ChIP assay.
Reagents for chromatin immunoprecipitation (ChIP) assays were obtained from the USB Corporation (Cleveland, OH), and assays were performed according to the manufacturer's protocol. Briefly, 5 × 106 K562 cells were used for each ChIP assay. The cells were exposed to 1% formaldehyde to cross-link DNA-protein complexes. The cell pellets were resuspended in lysis buffer with protease inhibitors and sonicated on ice three times for 10 seconds each time to generate sheared genomic-DNA fragments in lengths of approximately 1 kb. The lysates were then centrifuged for 10 min to remove debris and precleared with protein A-agarose beads. Antibody against GATA-1 (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the lysate for immunoprecipitation of DNA-GATA-1 complexes. After agitation at 4°C for 4 h, the immune complexes were collected with protein A-agarose beads, followed by washing with low- and high-salt immune complex wash buffers and Tris-EDTA buffer. The immune complexes were eluted with the elution buffer. The DNA-protein cross-links were reversed at 65°C overnight. DNA was recovered by phenol extraction and ethanol precipitation and resuspended in 50 μl of sterile water.
Immunoprecipitated DNA was analyzed for enrichment of targeted sequences by PCR assays. The primer pair P5/P6 (5′-TTGAAGTAAGGATTCAGTCTTATATTA-3′ and 5′-TTTGAATATACTCTCTGTGGTTTTAGGCC-3′) was used to confirm the binding of GATA-1 protein to the GATA motif at nt −569 to −566 of the HBG2 promoter. They generated an amplicon of 141 bp, which was subsequently sequenced for confirmation. Sheared genomic DNA was used as the PCR template for positive control. For negative control, the procedures were identical to those described above, except that anti-GATA-1 antibody was not added.
The primer pair P1/P2 (5′-CTGAGGCTTAGGGTGTGTGCCC-3′ and 5′-CACATTCTGTCTCAGGCATCC-3′) was used to confirm the binding of GATA-1 protein to the GATA motif in the HS2 core region, which was previously reported (8, 18). This served as the positive control for the ChIP assay.
Primer pairs P3/P4 (5′-CACACCCATCTCACAGATCCCC-3′ and 5′-CTTCCAGTTACTCACATAATAA-3′) and P7/P8 (5′-CACACATGGTTGTCTTCAGTTCTT-3′ and 5′-TCAAAACCCACATCTAACGTAAAC-3′) were used to amplify fragments upstream and downstream of the GATA motif at nt −569 to −566 of the HBG2 promoter, respectively. These sequences did not contain GATA motifs and served as negative controls for the intended ChIP assays.
Truncated Gγ-globin gene promoter constructs.
A 1,432-bp fragment of the Gγ-globin promoter (nt −1383 to +49 relative to the transcription start site) was amplified from the mother's genomic DNA by PCR and cloned into the pCR 2.1 vector using a TA cloning kit (Invitrogen, Carlsbad, CA). The KpnI/XhoI fragment was then inserted into the pGL3-basic plasmid (Promega, Madison, WI) to generate a reporter construct in which luciferase expression was driven by the human Gγ-globin gene promoter. The inserted promoter was sequenced and verified with the GenBank database (accession number U01317). Truncated Gγ-globin promoters were generated by PCR amplification using a common reverse primer beginning at nt +49 and forward primers beginning at nt −1383, −771, −675, −526, −330, and −167. They were subcloned into the pGL3-basic plasmid. All reporter constructs were sequenced to confirm their fidelity.
Site-directed mutagenesis.
The GATA-to-GAGA mutation at nt −567 or other positions was produced using the QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Similarly, the GATA-to-GATC mutation at nt −566 was constructed. All mutant plasmids were sequenced to ascertain their fidelity.
Transient-transfection and luciferase assays.
The cells to be transfected were seeded at 2 × 105/well in 24-well tissue-culture plates. Transfection was carried out with Lipofectamine LTX reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Briefly, 0.5 to 1 μg of DNA was added to 0.5 μl of Plus reagent (Invitrogen, Carlsbad, CA) in 100 μl of serum-free medium and incubated for 15 min, followed by adding 1.5 μl of Lipofectamine LTX. After incubation for an additional 15 min, the mixture was added to the cells and incubated for 36 h at 37°C. The transfected cells were collected, and total cell extracts were prepared in 1× passive lysis buffer. The Renilla expression vector pRL-SV40 (Promega, Madison, WI) was cotransfected for normalization of transfection efficiency. The dual-luciferase reporter system (Promega, Madison, WI) was used to measure luciferase and Renilla activities in the same sample. Each transfection experiment was performed with triplicate samples. The results are expressed as a percentage of the luciferase activity from either wild-type promoter constructs or pGL3 control plasmid.
Cell lines and culture conditions.
The K562 and HEL human erythroleukemia cell lines, the MEL mouse erythroleukemia cell line, and the human HeLa cell line were obtained from the American Type Culture Collection (Manassas, VA). They were grown at 37°C and 5% CO2 in RPMI 1640 or Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA).
RESULTS
Clinical case report.
Both parents and two sons of an Iranian-American family were referred to our laboratory for diagnosis of possible HPFH and either α- or β-thalassemia. They were clinically well. Their laboratory findings are summarized in Table 1. No β-globin gene mutation was found in any family member. The mother and her 13-year-old son were heterozygous for the rightward single α-globin gene deletion (−α3.7/α α), which could account for their borderline low erythrocyte mean corpuscular volume.
TABLE 1.
Clinical laboratory findings for an Iranian family with increased Hb F
| Family member | Age at diagnosis (yr) | Hb (g/dl) | MCV (fl)a | Hb A2 (%) | Hb F (%) | α-Globin genotype |
|---|---|---|---|---|---|---|
| Father | 52 | 15.7 | 82 | 2.5 | 10.2 | α α/α α |
| Mother | 44 | 14.1 | 77 | 3.0 | 0.7 | −α3.7/α α |
| Brother | 13 | 13.2 | 75 | 3.4 | 0.7 | −α3.7/α α |
| Proband | 9 | 13.8 | 75 | 3.3 | 5.9 | α α/α α |
MCV, mean corpuscular volume.
The father and his 9-year-old son had moderately elevated Hb F at 10.2% and 5.9%, respectively. There were SNP heterozygosities within their β-globin genes, indicating that both β-globin gene alleles were present and that father and son could not have harbored any of the large HPFH deletions. Known HPFH single-nucleotide mutations in HBG2 and HBG1 promoter regions, including the C-to-T polymorphism at nt −158 5′ to HBG2 (11), were not found.
Nucleotide sequencing of 1 kb upstream of HBG2 was carried out in all four family members. The only significant finding was a novel T-to-G transversion at nt −567 5′ of the HBG2 transcription start site present in the father and his 9-year-old son but not in the mother and her 13-year-old son (Fig. 1). This SNP altered a putative GATA-1 binding motif, AGATAA, to AGAGAA. This SNP was not found in over 300 other individuals of many different ethnic backgrounds who were referred to our diagnostic laboratory for ascertainment of possible hemoglobinopathy. These findings led us to further investigate the possible functional role of this putative GATA-1 binding motif in the silencing of the γ-globin gene expression.
FIG. 1.
Nucleotide sequences of HBG2 from nt −578 to −553. The β-like globin gene cluster on chromosome 11p is depicted at the top. (A) Sequences from the father and his 9-year-old son showing the T-to-G transversion at nt −567. (B) Normal sequence from the mother and her 13-year-old son.
Phylogenetic alignment of the putative GATA-1 binding motif.
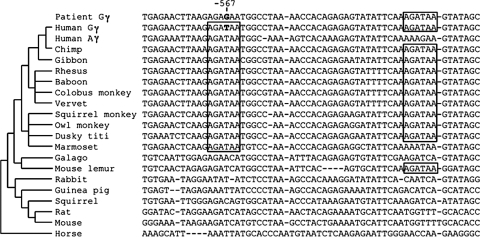
Two matches to the canonical WGATAR binding motif for GATA-1 are found in this region upstream of HBG2, one that contains the site of the SNP at nt −567 and the other 32 bp closer to the promoter (Fig. 2). The homologous region of HBG1 has only the motif corresponding to the one at nt −567. This AGATAA motif was present in the 11 simian primates examined (Fig. 2): humans, two apes, four Old World monkeys and four New World monkeys. It was not present in prosimian primates or four members of the glires clade (rabbit, guinea pig, squirrel, mouse, or rat) or in the perissodactyl horse. This restriction of conservation to the simian primates is informative, because the AGATAA motif corresponding to the one with the SNP is found only in species in which the γ-globin gene is expressed during fetal development. The other species (e.g., in the glires clade), in which the homologous gene is expressed during embryonic development, do not have the fetal-to-adult switch and do not have this motif in a homologous location. Thus, the presence of this GATA-1 binding motif is associated with the need for silencing of the γ-globin gene in adults.
FIG. 2.
Alignment of genomic sequences. An alignment of the upstream region of human HBG2 with homologous sequences of 35 other vertebrates was extracted from precomputed alignments generated by the program TBA as part of the ENCODE pilot project. See Materials and Methods.
The GATA motif at nt −569 to −566 of HBG2 is capable of binding GATA-1 transcription factor in vitro and in vivo.
To determine if the GATA motif of interest was capable of binding GATA-1 transcription factor, we carried out EMSAs. DNA-protein complex bands were observed when the wild-type oligonucleotide probe was incubated with the nuclear extract of either K562 or MEL cells (Fig. 3A and B). Competition with excess nonradiolabeled probe ablated binding activity in a dose-dependent manner. However, a probe with the mutant GAGA sequence did not compete out the binding of these complexes. The DNA-protein complex was supershifted with the addition of the anti-GATA-1 antibody (Fig. 3A). In addition, an ELISA-based GATA competition assay was developed. This assay confirmed that the oligonucleotide probe with a normal GATA motif competed for GATA-1 binding in a dose-dependent fashion but the mutant probe with the GAGA sequence did not (Fig. 3C). These results suggest that the GATA, but not the GAGA, motif binds GATA-1 protein in vitro.
FIG. 3.
EMSA of GATA-1 binding to the GATA motif at nt −580 to −556 of the HBG2 promoter. (A) Nuclear extracts from K562 cells (lanes 2 to 5) or MEL cells (lanes 7 to 10) were incubated with a 32P-end-labeled double-stranded oligonucleotide probe corresponding to the wild-type GATA motif. Competition experiments were carried out in the absence (lanes 2 and 7) or presence of a 100-fold molar excess of unlabeled wild-type GATA (lanes 3 and 8) or mutant GAGA (lanes 4 and 9) probe. Lanes 1 and 6 contained no nuclear protein in the incubation. The position of the DNA-GATA-1 complex is marked by an arrow. Note the supershifted DNA-GATA-1 bands (lanes 5 and 10) due to the addition of anti-GATA-1 antibody. Lanes 11 and 12, reference GATA probe (5′-CGCCGCAGAGATAAGGCACTGCC-3′) obtained from Active Motif (Carlsbad, CA) was incubated with K562 nuclear extract, with or without anti-GATA-1 antibody. (B) Competition assays were performed with increasing amounts of non-32P-labeled wild-type probe at 100-, 50-, and 25-fold molar excess (lanes 2 to 5 for K562 and lanes 6 to 9 for MEL nuclear protein). (C) ELISA-based GATA competition assays. The x axis shows the concentration of the competitor oligonucleotides added. The y axis shows the amount of GATA-1 bound, assessed by colorimetric readings at an optical density of 450 nm (see the text). This is one representative experiment of three similar experiments.
A ChIP assay was performed to examine the binding of GATA-1 protein in vivo (Fig. 4). There was significant enrichment of the HS2 core region containing a GATA motif among anti-GATA-1 immunoprecipitates compared with the total pool of fragmented genomic DNA (Fig. 4C, lanes P1 and P2) (8, 18). Similarly, enrichment of the GATA motif at nt −569 to −566 of the HBG2 promoter was observed (Fig. 4C, row P5-P6). Enrichment was not found in sequences not containing a GATA motif either upstream or downstream from the nt −567 site (Fig. 4C, rows P3-P4 and P7-P8). These results indicate that GATA-1 binds to the GATA motif at nt −569 to −566 of the HBG2 promoter in uninduced K562 cells.
FIG. 4.
ChIP assays were performed in K562 cells using anti-GATA-1 antibody as described in Materials and Methods. The nucleotide positions correspond to that in GenBank database accession number U01317. (A) A schematic representation of the human β-globin gene locus to show the relative positions of each set of PCR primer pairs (P1-P2, P3-P4, P5-P6, and P7-P8). See the text for details about these primer pairs. (B) Analysis of sonicated chromatin using 1.2% agarose gel electrophoresis. M, marker (size indicated in kb). (C) After fragmentation, sheared chromatin was immunoprecipitated with or without antibody (Ab) against GATA-1 and DNA purified and underwent PCR amplifications with different primer pairs. Lane 1, molecular weight marker (M); lane 2, no antibody was used during the immunoprecipitation step; lane 3, anti-GATA-1 antibody was used during the immunoprecipitation step; lane 4, fragmented genomic DNA was used as the PCR template. (D) The band intensities of PCR amplicons were measured by Scion Image Software. The results are expressed as mean plus standard deviation relative to the corresponding band intensity of the PCR amplicons, using fragmented genomic DNA as the template.
A negative regulatory element between nt −675 and −526 of the Gγ-globin gene promoter.
To identify possible silencing elements in the HBG2 promoter, a series of truncated HBG2 promoters linked to a luciferase reporter gene were constructed. They were transiently transfected into either K562 erythroid cells or HeLa cells (Fig. 5). The luciferase activities of these constructs in HeLa cells were very low, consistent with the HBG2 promoter fragments being erythroid cell specific. Deletion analyses revealed that the promoter region between nt −330 and −167 appeared to have a negative effect upon HBG2 promoter activity. At least three known human single-nucleotide HPFH mutations reside in this region of HBG2. Five other known single-nucleotide HPFH mutations reside in a comparable region of HBG1 (24). The luciferase activity of the construct with the nt −675 promoter amounted to two-thirds (68%) of the activity of the construct with the nt −526 promoter (Fig. 5). These results suggested that a lesser negative regulatory region was also present between nt −675 and −526. The GATA motif under study is located within this element.
FIG. 5.
Truncation analyses of the HBG2 promoter. The promoter/reporter gene constructs with different lengths of HBG2 promoter are depicted on the left. The relative luciferase activities of these constructs compared to that of the pGL3-control plasmid were studied in transiently transfected K562 and HeLa cells. All experiments were done in triplicate. The luciferase activity of the −675 construct differs from the activity of the −526 construct (P value, 0.038 by paired Student t test), but not from the activity of the −771 construct (P value, 0.120).
The GATA motif at nt −569 to −566 functions as a silencer of the HBG2 promoter.
HBG2 promoters from nt −1383 to +49, with or without the nt −567 T-to-G mutation, linked to a luciferase reporter gene were constructed and transiently transfected into K562, HEL, MEL, and HeLa cells. In transfected K562, HEL, and MEL cells, the constructs with the T-to-G mutation at nt −567 showed a 2.5-fold increase (P < 0.05) in transcription activity compared to the wild-type construct (Fig. 6). The luciferase activity in transfected nonerythroid HeLa cells was low, and there was no difference between the wild-type and mutant promoters.
FIG. 6.
T-to-G transversion at nt −567 increases the transcription activity of the HBG2 promoter in erythroid cells. The T-to-G transversion at nt −567 was made by site-directed mutagenesis within the 1,383-bp-long HBG2 promoter/luciferase reporter plasmid (pGL3−1383/+49). The wild-type (pGL3−1383/+49, WT) and the mutated (pGL3−1383/+49, MUT) plasmids were transiently transfected into K562, HEL, MEL, and HeLa cells. The luciferase activity relative to that of the wild-type promoter/reporter plasmid is shown on the y axis. The results are expressed as mean plus standard deviation; n = 3. The asterisks indicate significant differences from the wild-type plasmid; P < 0.01.
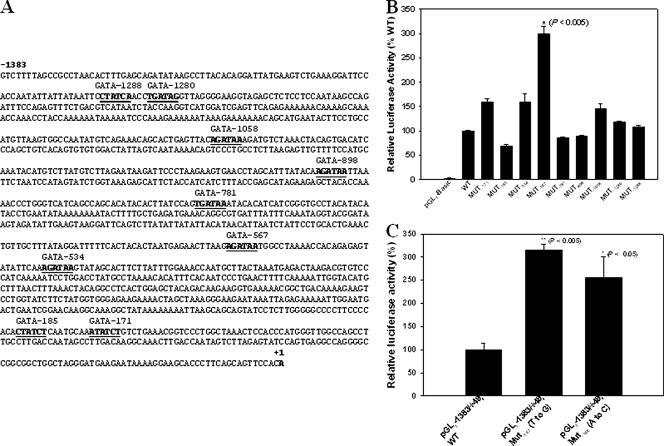
There are nine GATA consensus sequences within 1.4 kb upstream of HBG2 (Fig. 7A). Seven of these are restricted to primates or the subgroup of simians or apes. Two are conserved more deeply; the GATA consensus sequence at −185 is present in all eutherian mammals that have a γ-globin gene ortholog, and the one at −898 is conserved in primates and some rodents. To explore possible functions of these GATA sites in HBG2 promoter activity, each GATA sequence was mutated to GAGA by site-directed mutagenesis. These mutant promoters were linked to a luciferase reporter gene and transiently transfected into K562 cells. Some mutations had little effect, and others caused significant increases or decreases in luciferase activity, but the construct with the T-to-G mutation at nt −567 effected the highest (>3-fold increase) up-regulation of the luciferase activity (Fig. 7B). Furthermore, a promoter construct with a mutation of GATA to GATC at nt −566 similarly up-regulated the luciferase activity (Fig. 7C). These results lend strong support to the hypothesis that GATA-1 binding to the GATA motif at nt −569 to −566 plays an important role in silencing γ-globin gene expression in adults.
FIG. 7.
Comparisons of mutated GATA sequences in HBG2 promoter activity in transiently transfected K562 cells. (A) Nucleotide sequences from the transcription start site, nt −1383, to +1 of the human HBG2 promoter. Nine GATA sites (−1288, −1280, −1058, −898, −781, −567, −534, −185, and −171) are underlined and in boldface. (B) Transcription activities of wild-type (WT) and mutated (MUT) plasmids with a T-to-G substitution at each individual GATA site were studied in transiently transfected K562 cells. The relative luciferase activity was calculated as for Fig. 5. The results are expressed as mean plus standard deviation (SD); n = 3. The asterisk indicates a highly significant difference from the wild-type plasmid; P < 0.005. (C) The transcription activities of wild-type and mutated plasmids with either GATA to GAGA (middle bar) or GATA to GATC (right bar) at the GATA site under investigation. These plasmids were transiently transfected to K562 cells, and the luciferase results are expressed as relative activity (mean plus SD; n = 3) calculated in the same way as for Fig. 6.
DISCUSSION
During human embryonic and fetal development, there are two switches of HBB-like globin gene expression, from HBE (encoding embryonic ɛ-globin) to HBG2 and HBG1 during the first trimester and from HBG2 and HBG1 to HBB near birth, corresponding to the switches from embryonic hemoglobins to Hb F and from Hb F to Hb A. At least two models have been proposed to account for these developmental changes: an autonomous silencing mechanism and a competition model between neighboring globin genes for interaction with the locus control region (LCR) (20). A silencing element for ɛ-globin gene expression was identified from nt −467 to −182 upstream of the HBE transcription start site, mediated through GATA-1 and YY1 protein binding (10, 15-17). More recent experiments using transgenic mice containing a human HBB cluster yeast artificial chromosome suggested that additional silencing elements and multiple protein complexes effect down-regulation of HBE expression during development (14).
Down-regulation of HBG2 and HBG1expression is thought to result primarily from competition for the LCR and its associated proteins between HBB and HBG2 and HBG1 promoters (1, 3, 6). In transgenic mice containing an LCR linked to either HBG2, HBG1, or HBB alone, these genes were expressed in both fetal and adult mice. However, when the construct contained both HBG2 or HBG1 and HBB linked together, proper developmental expression of these genes was observed. This competition model is further supported by the observation that patients who have deletions of the HBB promoters usually have elevated Hb A2 or Hb F (23).
In the index cases for our study, both the father and his 9-year old son had moderately elevated Hb F and a novel T-to-G transversion in nt −567 of HBG2, which changed a GATA motif to a GAGA sequence. It was previously shown in transgenic mice containing a μLCR cassette linked to truncated HBG1 promoter fragments that there was a putative silencing element located between nt −730 and −382 (21). Given that there is high homology between the promoters of HBG1 and HBG2 and that the GATA motif of interest found in our family is present within this putative silencing element, we decided to investigate further the possible functional effect of this GATA motif and its mutated form.
Further support for a role for this AGATAA motif in silencing of the γ-globin genes comes from a phylogenetic analysis. This region does not appear to be under strong purifying selection in a wide range of mammals, but the AGATAA motif corresponding to nt −567 is present and has resisted any substitutions in 10 other simian primates that share the fetal pattern of expression of γ-globin genes found in humans. In contrast, no match to the GATA-1 binding site motif is found in the homologous segments of nonsimians. Thus, the presence of the motif is restricted to the mammals that express the γ-globin gene during fetal life, followed by silencing in adults.
The GATA motif under study binds GATA-1 protein in vitro as shown by EMSA and also in K562 cells in vivo by ChIP assay. Promoter truncation analyses suggest that there is a silencing element in the HBG2 promoter between nt −675 and −526. Mutation of the GATA motif from nt −569 to −566 to GAGA or to GATC, but not other GATA sequences, within 1.4 kb of the HBG2 promoter resulted in two- to threefold increases in the promoter activity when transiently transfected into K562, HEL, or MEL erythroid cell lines. Taken together, our clinical observations, along with phylogenetic alignment, promoter mutation and truncation studies, and previous transgenic mouse experimental results, are consistent with the hypothesis that the GATA motif under study is at least partly responsible for effecting the down-regulation of γ-globin gene expression in adults.
Harju-Baker and colleagues reported the same conclusion based on studies with transgenic mice containing a human HBB cluster yeast artificial chromosome (6a). These authors mutated the GATA motif at the HBG1 promoter to GAGA and found that HBG1 was expressed in adult transgenic mice, in contrast to the wild-type construct. GATA-1 interacts with FOG-1, which can recruit and bind to the NuRD repressor complex to effect transcription repression (7). Harju-Baker and colleagues have provided experimental evidence to support this model to account for the silencing effect of HBG1 expression mediated by the GATA motif.
During the course of our clinical studies, we searched for the T-to-G transversion in DNA samples from 134 Iranian individuals. Only one person, a woman, was found to have this specific transversion (data not shown), but her Hb F level was within the normal range. This observation reaffirms that the regulation of γ-globin gene expression and Hb F production is multifactorial and likely combinatorial and that the silencing effect of the GATA motif, as shown in our study, is but one of many genetic and nongenetic factors that can impact Hb F production in adults (5).
Acknowledgments
We sincerely thank Catherine J. Wu and Mehrdad Mohseni for making DNA samples from Iranian subjects available for us to study.
This work was supported in part by 1U54 HL 708819, R01 DK069646, and R01 DK065806.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Behringer, R. R., T. M. Ryan, R. D. Palmiter, R. L. Brinster, and T. M. Townes. 1990. Human γ- to β-globin gene switching in transgenic mice. Genes Dev. 4380-389. [DOI] [PubMed] [Google Scholar]
- 2.Blanchette, M., W. J. Kent, C. Riemer, L. Elnitski, A. F. Smit, K. M. Roskin, R. Baertsch, K. Rosenbloom, H. Clawson, E. D. Green, D. Haussler, and W. Miller. 2004. Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res. 14708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enver, T., N. Raich, A. J. Ebens, T. Papayannopoulou, F. Costantini, and G. Stamatoyannopoulos. 1990. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature 344309-313. [DOI] [PubMed] [Google Scholar]
- 4.Frenette, P. S., and G. F. Atweh. 2007. Sickle cell disease: old discoveries, new concepts, and future promise. J. Clin. Investig. 117850-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibney, G. T., C. I. M. Panhuysen, J. C. C. So, E. S. K. Ma, S. Y. Ha, C. K. Li, A. C. W. Lee, C. K. Li, H. L. Yuen, Y. L. Lau, D. M. Johnson, J. J. Farrell, A. B. Bisbee, L. A. Farrer, M. H. Steinberg, L. C. Chan, and D. H. K. Chui. 2008. Variation and heritability of Hb F and F-cells among β-thalassemia heterozygotes in Hong Kong. Am. J. Hematol. 83458-464. [DOI] [PubMed] [Google Scholar]
- 6.Harju, S., P. A. Navas, G. Stamatoyannopoulos, and K. R. Peterson. 2005. Genome architecture of the human β-globin locus affects developmental regulation of gene expression. Mol. Cell. Biol. 258765-8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Harju-Baker, S., F. C. Costa, H. Fedosyuk, R. Neades, and K. R. Peterson. 2008. Silencing of γ-globulin gene expression during adult definitive erythropoiesis mediated by GATA-1-FOG-1-Mi2 complex binding at the −566 GATA site. Mol. Cell. Biol. 283101-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong, W., M. Nakazawa, Y. Y. Chen, R. Kori, C. R. Vakoc, C. Rakowski, and G. A. Blobel. 2005. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 242367-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horak, C. E., M. C. Mahajan, N. M. Luscombe, M. Gerstein, S. M. Weissman, and M. Snyder. 2002. GATA-1 binding sites mapped in the β-globin locus by using mammalian chIp-chip analysis. Proc. Natl. Acad. Sci. USA 992924-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau, Y. L., L. C. Chan, Y. Y. A. Chan, S. Y. Ha, C. Y. Yeung, J. S. Waye, and D. H. K. Chui. 1997. Prevalence and genotypes of α- and β-thalassemia carriers in Hong Kong: implications for population screening. N. Engl. J. Med. 3361298-1301. [DOI] [PubMed] [Google Scholar]
- 10.Li, Q. L., C. Clegg, K. Peterson, S. Shaw, N. Raich, and G. Stamatoyannopoulos. 1997. Binary transgenic mouse model for studying the trans control of globin gene switching: evidence that GATA-1 is an in vivo repressor of human ɛ gene expression. Proc. Natl. Acad. Sci. USA 942444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, Q., K. Abel, O. Sripichai, J. Whitacre, V. Angkachatchai, W. Makarasara, P. Winichagoon, S. Fucharoen, A. Braun, and L. Farrer. 2007. β-Globin gene cluster polymorphisms are strongly associated with severity of HbE/β0-thalassemia. Clin. Genet. 72497-505. [DOI] [PubMed] [Google Scholar]
- 12.Margulies, E. H., G. M. Cooper, G. Asimenos, D. J. Thomas, C. N. Dewey, A. Siepel, E. Birney, D. Keefe, A. S. Schwartz, M. Hou, J. Taylor, S. Nikolaev, J. I. Montoya-Burgos, A. Loytynoja, S. Whelan, F. Pardi, T. Massingham, J. B. Brown, P. Bickel, I. Holmes, J. C. Mullikin, A. Ureta-Vidal, B. Paten, E. A. Stone, K. R. Rosenbloom, W. J. Kent, G. G. Bouffard, X. Guan, N. F. Hansen, J. R. Idol, V. V. Maduro, B. Maskeri, J. C. McDowell, M. Park, P. J. Thomas, A. C. Young, R. W. Blakesley, D. M. Muzny, E. Sodergren, D. A. Wheeler, K. C. Worley, H. Jiang, G. M. Weinstock, R. A. Gibbs, T. Graves, R. Fulton, E. R. Mardis, R. K. Wilson, M. Clamp, J. Cuff, S. Gnerre, D. B. Jaffe, J. L. Chang, K. Lindblad-Toh, E. S. Lander, A. Hinrichs, H. Trumbower, H. Clawson, A. Zweig, R. M. Kuhn, G. Barber, R. Harte, D. Karolchik, M. A. Field, R. A. Moore, C. A. Matthewson, J. E. Schein, M. A. Marra, S. E. Antonarakis, S. Batzoglou, N. Goldman, R. Hardison, D. Haussler, W. Miller, L. Pachter, E. D. Green, and A. Sidow. 2007. Analyses of deep mammalian sequence alignments and constraint predictions for 1% of the human genome. Genome Res. 17760-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, W., K. Rosenbloom, R. C. Hardison, M. Hou, J. Taylor, B. Raney, R. Burhans, D. C. King, R. Baertsch, D. Blankenberg, S. L. Kosakovsky Pond, A. Nekrutenko, B. Giardine, R. S. Harris, S. Tyekucheva, M. Diekhans, T. H. Pringle, W. J. Murphy, A. Lesk, G. M. Weinstock, K. Lindblad-Toh, R. A. Gibbs, E. S. Lander, A. Siepel, D. Haussler, and W. J. Kent. 2007. 28-Way vertebrate alignment and conservation track in the UCSC Genome Browser. Genome Res. 171797-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navas, P. A., Q. Li, K. R. Peterson, and G. Stamatoyannopoulos. 2006. Investigations of a human embryonic globin gene silencing element using YAC transgenic mice. Exp. Biol. Med. 231328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raich, N., C. H. Clegg, J. Grofti, P.-H. Roméo, and G. Stamatoyannopoulos. 1995. GATA1 and YY1 are developmental repressors of the human ɛ-globin gene. EMBO J. 14801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raich, N., T. Enver, B. Nakamoto, B. Josephson, T. Papayannopoulou, and G. Stamatoyannopoulos. 1990. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science 2501147-1149. [DOI] [PubMed] [Google Scholar]
- 17.Raich, N., T. Papayannopoulou, G. Stamatoyannopoulos, and T. Enver. 1992. Demonstration of a human ɛ-globin gene silencer with studies in transgenic mice. Blood 79861-864. [PubMed] [Google Scholar]
- 18.Reddy, P. M. S., and C. K. J. Shen. 1991. Protein-DNA interactions in vivo of an erythroid-specific, human β-globin locus enhancer. Proc. Natl. Acad. Sci. USA 888676-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rund, D., and E. Rachmilewitz. 2005. β-Thalassemia. N. Engl. J. Med. 3531135-1146. [DOI] [PubMed] [Google Scholar]
- 20.Stamatoyannopoulos, G. 2005. Control of globin gene expression during development and erythroid differentiation. Exp. Hematol. 33259-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatoyannopoulos, G., B. Josephson, J.-W. Zhang, and Q. Li. 1993. Developmental regulation of human γ-globin genes in transgenic mice. Mol. Cell. Biol. 137636-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan, A. S. C., T. C. Quah, P. S. Low, and S. S. Chong. 2001. A rapid and reliable 7-deletion multiplex polymerase chain reaction assay for α-thalassemia. Blood 98250-251. [DOI] [PubMed] [Google Scholar]
- 23.Weatherall, D. J., and J. B. Clegg. 2001. The thalassaemia syndromes, 4th ed., p.151. Blackwell Science, Oxford, United Kingdom.
- 24.Wood, W. G. 2001. Hereditary persistence of fetal hemoglobin and δβ thalassemia, p. 356-388. In M. H. Steinberg, B. G. Forget, D. R. Higgs, and R. L. Nagel (ed.), Disorders of hemoglobin: genetics, pathophysiology, and clinical management. Cambridge University Press, Cambridge, United Kingdom.