Abstract
Cancer cells in their respective microenvironments must endure various growth-constraining stresses. Under these conditions, the cancer cell-derived factors are thought to modulate the signaling pathways between cell growth and dormancy. Here, we describe a cancer cell-derived regulatory system that modulates the phosphatidylinositol 3′-kinase (PI3K)-Akt pathway under serum deprivation stress. Through the use of biochemical purification, we reveal that cancer cell-secreted insulin-like growth factor 1 (IGF-1) and clusterin, an extracellular stress protein, constitute this regulatory system. We show that secreted clusterin associates with IGF-1 and inhibits its binding to the IGF-1 receptor and hence negatively regulates the PI3K-Akt pathway during serum deprivation. This inhibitory function of clusterin appears to prefer IGF-1, as it fails to exert any effects on epidermal growth factor signaling. We demonstrate furthermore that the constitutive activation of oncogenic signaling downstream of IGF-1 confers insensitivity to the inhibitory effects of clusterin. Thus, the interplay between cancer cell-derived clusterin and IGF-1 may dictate the outcome of cell growth and dormancy during tumorigenic progression.
The process of cell proliferation requires the integration of various upstream signaling pathways. Physical contact with neighboring cells (e.g., contact inhibition) and limitation to environmental cues, such as growth factors, are key physiological mechanisms that restrict normal cell growth. Oncogenic transformation due to the accumulation of genetic lesions results in a loss of these properties, leading to uncontrolled cell growth. However, during tumorigenic progression, cancer cells continuously encounter various growth-constraining conditions, such as hypoxia, acidosis, and nutritional deprivation (1, 15, 16, 33). Under these conditions, cancer cells must modulate their signaling pathways to balance between cell growth and dormancy (1, 40). The cellular mechanisms and genetic components involved in this process are not clearly defined. One mechanism might be that cancer cell-derived factors support their own growth and influence neighboring cells to induce a favorable microenvironment (4, 6, 35, 36). A recent report that cancer cells facilitate the expansion of highly proliferative stromal fibroblasts, which in turn promotes tumor progression, supports this view (23). This finding highlights the importance of cancer cell-derived paracrine factors in the initiation of signaling events that eventually lead to a favorable environment for tumor growth. Identification of these factors and cognate signaling pathways is important to the interference of the cross talk between cancer cells and the microenvironment.
Many signaling receptors, such as growth factor, cytokine, and G protein-coupled receptors, rely on membrane-associated phosphatidylinositol-3,4,5-triphosphate (PIP3), a product of phosphatidylinositol 3′-kinase (PI3K), to elicit a wide variety of cellular responses (8, 13, 47). PI3K and its major downstream kinase, Akt, play key roles in many aspects of tumorigenesis, such as cellular proliferation, survival, and migration (2, 19). Constitutive activation of the PI3K-Akt pathway is closely associated with cancer cell resistance to chemotherapeutic agents. Deactivation of this pathway has been shown to increase the efficacy of many anticancer drugs, targeting a wide range of cellular components (2, 19, 21). While the cellular targets and processes elicited by the PI3K-Akt pathway are well established, the mechanism by which cancer cells modulate this pathway in response to various growth-constraining conditions is less defined. Uncovering the regulatory systems and genetic factors involved in this process will shed new light on the mechanisms of chemoresistance and tumorigenic progression.
As a first step to understanding the underlying cellular events, we investigated the extracellular regulation of the PI3K-Akt pathway in response to growth factor deprivation, a physiologically relevant growth-constraining condition, using HeLa cells as a model system for epithelial cancer cells. The initial biochemical assay identified a cancer cell-derived regulatory activity. We clarified the fact that this regulatory activity consisted of both a positive activator(s) regulated by the PI3K-Akt pathway itself and a negative factor(s). Using biochemical purification, we revealed that cancer cell-derived clusterin and insulin-like growth factor 1 (IGF-1) constituted this regulatory activity. By a combination of loss-of-function and gain-of-function studies, we demonstrated that clusterin negatively regulated the PI3K-Akt pathway through the attenuation of IGF-1, a major growth factor secreted by serum-starved cancer cells. We showed that the activation of oncogenic signaling conferred insensitivity to the inhibitory effects of clusterin. The interplay between cancer cell-derived clusterin and IGF-1 may provide a molecular framework with which to further dissect the complex relationships between cancer cells and their environments.
MATERIALS AND METHODS
Cell culture and stable cell lines.
HEK293 and HeLa cells and their derivative cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM). NB4 cells were cultured in RPMI medium. For routine maintenance, all cell lines were cultured in medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin under 5% CO2. Transfection was done using Lipofectamine 2000 (Invitrogen) according to manufacturer's guidance. To generate variant HeLa cells expressing a PIP3-binding pleckstrin homology (PH) domain fused to enhanced green fluorescence protein (PH-EGFP), the plasmid containing PH-EGFP driven by a chicken beta-globin promoter was cotransfected with pcDNA3.1 (Invitrogen). The transfected cells were selected for their resistance to G418 (2 mg/ml) for 2 weeks, and the PH-EGFP-expressing clones were isolated by using fluorescence microscopy. For the HEK293 vector or HEK293-Ras cells, HEK293 cells were transfected with the pBabe or pBabe-H-Ras (V12) vector (26). The stable cell lines were selected and propagated in the presence of puromycin (5 μg/ml).
Reagents and antibodies.
The human and zebrafish clusterins in the pCMV-SPORT6 vector were obtained from Openbiosystems. The clusterin short hairpin RNA (shRNA) plasmids were purchased from Upstate (pKD-clusterin-v3) and Openbiosystems (catalog no. RHS1764-9690776). Monoclonal clusterin antibody (B-5; catalog no. sc-5289) and polyclonal clusterin antibody (H-330, catalog no. sc-8354) were from Santa Cruz Biotechnology; alpha-actinin and actin antibodies were from Sigma; phosphotyrosine antibody (catalog no. 4G10) was from Millipore; monoclonal IGF-1 (catalog no. CBL52) was from Chemicon International; IGF-1 receptor (catalog no. MAB391) antibody was from R & D Systems; horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies were from Amersham Biosciences; all other antibodies were purchased from Cell Signaling Technology. All chemicals, unless specified otherwise, were obtained from Calbiochem.
Immunoblotting, immunodepletion, and immunoprecipitation.
For the Western blotting assay for adherent cells, cells were washed in phosphate-buffered saline (PBS), and preheated 1× NuPAGE lithium dodecyl sulfate (LDS) buffer (Invitrogen) was added directly to the plate (150 μl per 35-mm dish). Lysates were collected and boiled for 10 min in the presence or absence of β-mercaptoethanol. For cells grown in suspension, the cell pellet was made by centrifugation and washed in PBS and resuspended in 50 μl of PBS (per 1 million cells). An equal volume of preheated 2× NuPAGE LDS buffer was added, and the lysates were boiled. All protein samples were briefly sonicated before they were separated on NuPAGE 4 to 12% bis-Tris gels (Invitrogen). For conditioned medium (CM), each sample was centrifuged for 15 min, and an aliquot of the medium was mixed with 4× NuPAGE LDS buffer and boiled prior to electrophoresis. After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes (Millipore) that were blotted with the primary antibodies. Except for the phospho-Akt (T308) antibody (5% bovine serum albumin in Tris-buffered saline-Tween [TBS-T] buffer), all of the primary and the horseradish peroxidase-conjugated secondary antibodies were incubated in 5% nonfat dry milk (Bio-Rad) in TBS-T. The signal was detected using an ECL Plus Western blotting detection system (Amersham). For immunodepletion, 10 μl of 5 M NaCl was added to 500 μl of HeLa cell CM, and 5 μg of monoclonal clusterin or control antibody was added. After samples were incubated overnight at 4°C with gentle rotation, 30 μl of protein G/A-agarose slurry (Oncogene) was added, and samples were incubated for 3 h at 4°C. After the mixtures were centrifuged, the supernatants were checked for their abilities to activate Akt in NB4 cells. For immunoprecipitation, HeLa cells grown overnight in 60-mm culture dishes were serum starved for 30 min and then incubated with the intact or the heat-treated CM (2 ml) for 20 min. Cells were washed in PBS and lysed in ice-cold lysis buffer (10 mM Tris-HCl [pH 7.6], 1 mM EDTA, 150 mM NaCl, 1% NP-40) containing cocktails of protease inhibitors (Roche) and protein phosphatase inhibitors (Sigma). The lysates were cleared by centrifugation and incubated overnight with 2 μg of EGF receptor or IGF-1 β receptor. Twenty microliters of protein G/A-agarose slurry was added and incubated for an additional 3 h. After samples were washed three times with the lysis buffer at 4°C, the immunoprecipitates were resolved on NuPAGE 4 to 12% bis-Tris gels and analyzed for the phosphotyrosine level.
Biochemical purification and protein identification.
HeLa cells grown overnight in 100-mm dishes were washed once with PBS and incubated in 10 ml of serum-free DMEM for 6 h. The medium was replaced with fresh serum-free medium (10 ml per plate), and cells were incubated for another 36 to 48 h before the collection step. A total of 1 liter of the CM was used for two independent purifications. After centrifugation, the CM was loaded onto a column packed with High Q (Bio-Rad) anion-exchange resin (10 ml of bed volume). The flowthrough fraction was loaded onto a butyl-S-Sepharose column (Amersham Biosciences), the flowthrough fraction was collected, and 0.1 N HCl was added dropwise to adjust the pH to 4.5. The pH-adjusted sample was loaded onto a High S (Bio-Rad) column (5 ml of bed volume) preequilibrated with acidic PBS buffer (pH 4.5). The column was washed three times with acidic PBS buffer. The bound activity was eluted (5 ml per fraction) by a pH gradient (5.0 to 8.0) in PBS buffer. For the activity assay, 200 μl of each fraction was diluted with an equal volume of serum-free DMEM and heat treated at 65°C for 20 min. One million preserum-starved NB4 cells were incubated in the heat-treated fractions for 30 min. After cells were centrifuged, the cell pellet was briefly washed in PBS and lysed directly by boiling in 1× LDS buffer. The active fractions were concentrated with a Centriprep 10K concentrator column (Amicon) and resolved on NuPAGE 4 to 12% bis-Tris gels (Invitrogen). The protein bands of interest were cut from the Coomassie-stained gel. In-gel digestion of gel bands, matrix-assisted laser desorption ionization (MALDI) mass spectrometry analysis, and protein identification were done by the Taplin biological mass spectrometry facility at Harvard Medical School.
Concentration of protein and removal of chemicals.
Protein concentration was done using Centriprep centrifugal filter devices (Amicon). The filter membrane was rinsed twice in 15 ml of PBS by centrifugation for 30 min at 2,000 × g. Typically, 10 ml of CM was added to each device. The concentration process (centrifugation and removal of filtrate) was repeated until the volume of retentate reached 1 ml. To remove the chemical inhibitors in the CM used in Fig. 2A, the concentration process was repeated twice with serum-free medium.
FIG. 2.
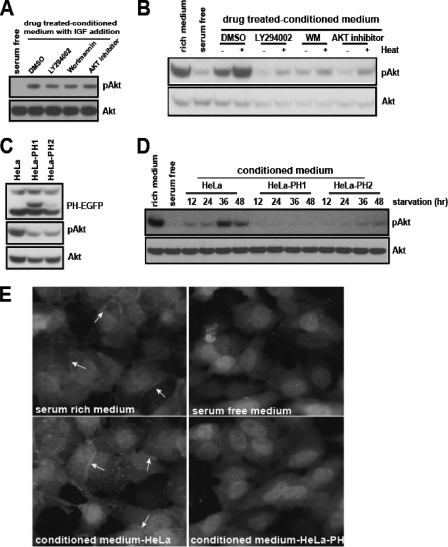
The PI3K-Akt signaling pathway mediates the secretion of Akt-activating activity. (A) HeLa cells were cultured in serum-free medium for 24 h in the presence of dimethyl sulfoxide (DMSO), LY294002 (5 μM), wortmannin (WM, 0.1 μM), or Akt inhibitor (3.6 μM). The CM was filtered through a Centriprep-10K column to remove the residual chemicals (see Materials and Methods). The filtered CM was mixed with IGF-1 (1 ng/ml) and tested for the removal of residual drug. (B) Each CM sample from the experiment described in the legend to panel A was untreated or heat treated, and its ability to activate Akt was assayed in HeLa cells. (C) The whole-cell lysates of HeLa cells or HeLa-PH cells grown in serum-rich medium were analyzed for phospho-Akt level. (D) The serum-free CM from HeLa, HeLa-PH1, or HeLa-PH2 cells was collected at 12-h intervals and tested for its ability to activate Akt. (E) The CM from HeLa or HeLa-PH cells was added to HeLa-PH cells that had been serum starved for 12 h. After the HeLa-PH cells were incubated for 30 min, they were fixed in 3% paraformaldehyde, and the membrane translocation of PH-EGFP was examined using fluorescence microscopy (20× objective). Arrows indicate the membrane translocation of PH-EGFP.
Neutrophil isolation and transmigration assay.
The isolation of mouse bone marrow neutrophils and the transmigration assay were done essentially as described previously (44).
MTT assay for cell proliferation.
HEK293 cells (4 × 104 cells) were plated on a 24-well plate in triplicate. Each well contained 250 μl of 1% or 2.5% FBS DMEM mixed with 250 μl of the control or the clusterin-containing HEK293T-CM. Cells were cultured for 3 days, and then 50 μl of 5 mg/ml MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] solution (dissolved in PBS and filtered through a 0.2-μm nylon filter) was added to each well and incubated at 37°C for 2 h. After the medium was removed, 900 μl of isopropanol with 0.1 M HCl was added to dissolve crystals. The solution was transferred into 1.5-ml Eppendorf tubes and centrifuged (14,000 rpm for 5 min). Absorbance of the supernatant was measured at a wavelength of 570 nm, with background subtraction at 650 nm.
Antibody neutralization and ELISA.
All antibodies were diluted in PBS (0.1 mg/ml). For IGF-1 receptor neutralization, NB4 cells (1.0 × 106) grown overnight in serum-rich medium were collected and washed once with PBS and incubated in the presence of different amounts of antibodies in serum-free medium (0.5 ml) for 30 min. Cells were collected and incubated with the heat-treated HeLa cell-CM for 30 min. For IGF-1 neutralization, HeLa cell-CM was heat treated and incubated with the control or IGF-1 antibody for 1 h at 4°C and then for 30 min at room temperature. Each CM sample was assayed in NB4 cells. The enzyme-linked immunosorbent assay (ELISA) for IGF-1 was carried out according to the manufacturer's instructions (Quantikine, human IGF-1; R & D Systems). To determine the IGF-1 level in HeLa cell-CM, the intact or heat-treated CM (100 μl) collected at different time points was analyzed along with the serially diluted IGF-1 standard. For the recovery of IGF-1, purified IGF-1 (2 ng) was incubated with the control or clusterin-containing HEK293T cell-CM (100 μl) for 1 h on ice, and then the amount of IGF-1 was determined by ELISA.
IGF-1 binding and pull-down experiments.
The human and zebrafish clusterin clones in the pCMV-SPORT6 vector were subcloned into the pcDNA3.1/TOPO-V5-HIS vector (Invitrogen) by PCR. The plasmid was transfected into HEK293T cells, and serum-free CM was collected. CM was incubated with purified IGF-1 (200 ng/ml) for 1 h on ice, and then Ni-nitrilotriacetic acid (NTA)-agarose beads (Qiagen) were added (50 μl/ml CM), and the solution was incubated overnight in a cold room with gentle rotation. The beads were then washed three times in PBS with imidazole (20 mM). After the final wash in PBS, the beads were boiled in 1× LDS buffer without a reducing agent and analyzed for IGF-1 by Western blotting. For IGF-1 coimmunoprecipitation and ELISA detection, HEK293 cells (5 × 106) were serum starved for 24 h and washed once with ice-cold PBS and then stimulated for 5 min at room temperature with the control or the clusterin-containing CM that had been preincubated with IGF-1 (50 ng/ml) on ice for 1 h. The cells were washed once with ice-cold PBS and lysed for 30 min on ice in the lysis buffer {40 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.3% CHAPS [3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate] with protease inhibitor cocktails }. The cells were collected in a tube and sonicated briefly before debris was cleared by centrifugation. The cleared lysates were preincubated with the rabbit control or the IGF-1 β receptor antibody (10 μg/ml of lysate) for 1 h, and then protein A/G beads were added, and the samples were incubated overnight with gentle rotation. The immune complex was washed three times with ice-cold PBS and resuspended in 100 μl of PBS. The immune complex was heat treated at 75°C for 30 min and then analyzed for the presence of IGF-1 by ELISA.
RESULTS
Serum-starved HeLa cells secrete an Akt-activating activity.
A number of signaling receptors rely on the PI3K-Akt pathway to control a wide range of cellular responses. However, how this pathway is regulated under cellular stress conditions is not well defined. We hypothesized that cancer cells under growth constraint conditions secrete soluble factors to modulate the PI3K-Akt pathway. To identify such regulatory factors, we established a biochemical system. We chose serum starvation as one of the paradigms for cellular stress. It is known that cellular stresses caused by serum deprivation cross talk with hypoxic responses (3, 11, 51), analogous to that in in vivo cancer cells. Also, excluding other serum factors allowed us to identify the cancer cell-derived signaling modulators.
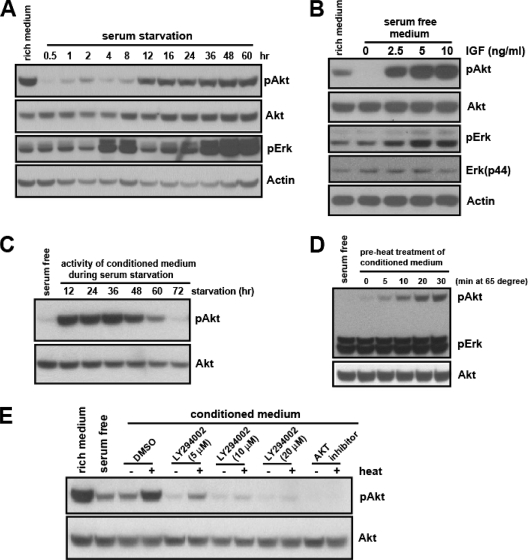
Akt is recruited onto the plasma membrane through its specific binding to PIP3, a product of PI3K, and is activated by phosphorylation; thus, Akt phosphorylation has been used widely as an indicator of PI3K-Akt pathway activation (8, 9, 32, 39, 46). HeLa cells grown in serum-rich medium were replenished with serum-free medium, and the level of phospho-Akt was examined at the indicated time points. Interestingly, the profile of the phospho-Akt exhibited a biphasic nature, that is, the lowest level appeared at first, followed by a gradual increase, and moderate levels were sustained at later time points (Fig. 1A). While the kinetics and levels of reactivation were slightly variable between experiments, the biphasic nature of phospho-Akt was highly reproducible (Fig. 1A and see Fig. S1A in the supplemental material). The initial deactivation (dephosphorylation) of Akt was rapid and came about within one-half hour of serum removal (see Fig. S1B in the supplemental material). The presence of growth factors, such as IGF, completely prevented this initial deactivation (Fig. 1B). Under the identical conditions, the initial deactivation of phospho-Erk was not obvious, and its response to IGF was much less robust than that of phospho-Akt (Fig. 1A and B and see Fig. S1A in the supplemental material).
FIG. 1.
Serum-starved HeLa cells secrete an Akt-activating activity. (A) HeLa cells grown in serum-rich medium (10% FBS in DMEM) overnight were replenished with serum-free medium, and the phospho-Akt (S473) and phospho-Erk (Thr202/Tyr204) from the whole-cell lysates were examined at the indicated time points. (B) HeLa cells grown overnight in serum-rich medium were replenished with serum-free medium or serum-free medium with the indicated amount of IGF-1 for 20 min. The whole-cell lysates were analyzed for phospho-Akt and phospho-Erk. (C) The CM from serum-starved HeLa cells was collected every 12 h. Each CM sample was added to a new batch of HeLa cells that had been serum deprived for 30 min. After cells were incubated for 30 min, the ability of each CM to increase the level of phospho-Akt activity was determined. (D) The serum-free HeLa cell CM (36 h) was heat treated at 65°C for the indicated times, and cooled to room temperature for 30 min. The Akt-activating activity of heat-treated CM was analyzed as described in the legend to panel C. (E) The Akt-activating activity of untreated or heat-treated HeLa cell CM was determined in the presence of the indicated amounts of LY294002 or Akt inhibitor (7.2 μM).
The reactivation of Akt following the initial deactivation could be due to restimulation by an extracellular Akt activator(s) secreted by the starved HeLa cells. If this is the case, the serum-free culture medium in which HeLa cells were grown (the CM) should contain the Akt-activating activity. To test this possibility, the serum-free CM from HeLa cells was collected at 12-h intervals for 3 days, and the ability to activate Akt (i.e., to prevent the initial deactivation upon serum removal) was examined in a new batch of cells. The serum-free CM, indeed, contained the Akt-activating activity. This activity was increased up until 36 h, followed by a gradual decrease, leading to an almost complete lack of activity by 72 h (Fig. 1C).
To better understand the molecular nature of the secreted Akt-activating activity, we first determined its physical properties. Interestingly, the preheating treatment at 65°C significantly enhanced the Akt-activating activity, while it had no effect on the level of phospho-Erk (Fig. 1D). To assess the molecular weight, we applied the CM to a filtration membrane designed to separate macromolecules based on their size. The activity was found to be bigger than 50 kDa, even after the preheating treatment (see Fig. S1C and S1D in the supplemental material). The activation of Akt by either the heat-treated or the untreated CM was blocked in the presence of Akt or PI3K inhibitor, suggesting that this activity acts upstream of PI3K (Fig. 1E).
The PI3K-Akt signaling pathway mediates the secretion of Akt-activating activity.
The expression of signaling ligands and antagonists is often under the control of their cognate signaling pathways. To determine if the level of Akt-activating activity in HeLa-CM could be regulated by the PI3K-Akt pathway, we treated HeLa cells with a sublethal amount of PI3K or an Akt inhibitor. Residual chemicals from the CM were removed by filtration (see Materials and Methods), and their absence from the CM was confirmed (Fig. 2A). Each CM sample was then tested for Akt-activating activity. Compared with the control CM, the drug-treated CM showed a significantly reduced activity level (Fig. 2B). To substantiate this finding, we generated variant HeLa cells with compromised PI3K signaling. The elevation of PIP3, a product of PI3K, and its recognition by various cellular proteins are the key steps for the PI3K-elicited cellular processes. We reasoned that the ectopic expression of the PIP3-binding PH domain would sequester the endogenous PIP3, hence attenuating the PI3K signaling. We generated two independent stable HeLa cell lines expressing a PH domain of Akt fused to EGFP, HeLa-PH1 and HeLa-PH2. As expected, compared to the wild-type HeLa cells, the HeLa-PH cells showed a reduced basal level of phospho-Akt (Fig. 2C). When the CM from the variant HeLa cells was tested for secreted Akt-activating activity, the variant CM, unlike wild-type HeLa-CM, showed no activity (for HeLa-PH1) or a much reduced activity (for HeLa-PH2) level at all time points (Fig. 2D). The lack of Akt-activating activity in the CM from HeLa-PH cells (HeLa-PH-CM) was indeed due to its inability to activate the PI3K, as it failed to induce the membrane translocation of PH-EGFP, a process mediated by PIP3 (Fig. 2E). Together with the previous results using chemical inhibitors, these findings suggest that the secretion and/or synthesis of Akt-activating activity is mediated by the PI3K-Akt signaling pathway.
Serum-starved HeLa cells also secrete a negative regulator of PI3K-Akt pathway.
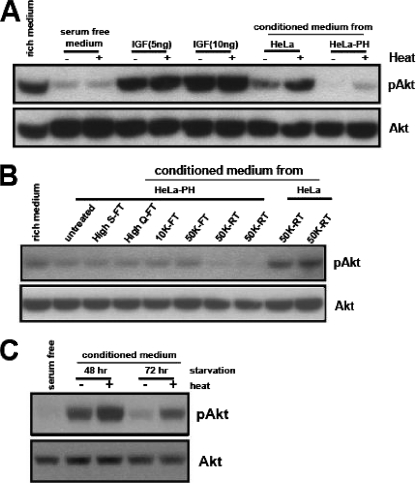
Heat activation is a unique feature of the HeLa cell-secreted PI3K-Akt-activating activity. We found that a preheating treatment of purified growth factors, such as IGF, had no differential effect (Fig. 3A). One mechanism that may explain this finding is the presence of a heat-sensitive negative regulator. The heat treatment may lead to conformational changes of this regulator and thus suppress its inhibitory effect on Akt activation. Supporting this idea, the CM from HeLa-PH cells, which do not secrete the Akt-activating activity, further decreased the basal level of phospho-Akt under serum-free conditions. The preheating treatment of CM from HeLa-PH cells abolished this inhibitory effect (Fig. 3A). Consistent with this finding, the concentrated CM from HeLa-PH cells reduced the level of phospho-Akt in HeLa cells, even under serum-rich conditions (Fig. 3B). The PI3K-Akt-activating activity in the CM from wild-type HeLa cells was reduced at later time points (72 h) (Fig. 1C). The preheating treatment of this CM significantly recovered the Akt-activating activity (Fig. 3C), further indicating the presence of a heat-sensitive negative regulator.
FIG. 3.
Serum-starved HeLa cells also secrete a negative regulator of the PI3K-Akt pathway. (A) Serum-free medium, IGF-1, or CM from HeLa or HeLa-PH cells was untreated or heat treated, and their abilities to activate Akt were tested. (B) The CM from HeLa-PH or its column fraction was added to HeLa cells grown in serum-rich medium (20% volume of the medium) and incubated for 30 min prior to analysis for phospho-Akt. For the Centriprep column retentate (RT), the CM was concentrated with the Centriprep 10K or 50K filter column. Each concentrate (10×) was added directly to HeLa cells grown in serum-rich medium. (C) The HeLa cell CM collected after 48 h or 72 h of serum starvation was untreated or heat treated, and its ability to activate Akt was assayed.
Biochemical purification identifies clusterin as a protein associated with the PI3K-Akt-activating activity.
We found that the enhancement of Akt-activating activity by heat treatment was retained after pretreating CM under high-salt, reducing, and both basic and acidic conditions (data not shown), indicating that the negative and positive regulators may exist in a complex. Therefore, we decided to biochemically purify them by following the Akt-activating activity. We have found that several other cancer cell types could also respond to the secreted Akt-activating activity from HeLa-CM (data not shown). For this biochemical purification, we utilized NB4 cells (an acute promyelocytic leukemic cell line) to measure the Akt-activating activity. NB4 cells were cultured in suspension; therefore, the contribution of Akt regulation by the cell adhesion-related processes was minimized. In addition, unlike HeLa cells, very little reactivation of basal phospho-Akt was found in NB4 cells during serum deprivation, making the background level consistently very low in each assay. To trace the Akt-activating activity level that can be enhanced by heat treatment, each fraction was treated at 65°C for 20 min and tested for its ability to increase the phospho-Akt level in serum-starved NB4 cells (see methods in the supplemental material). We showed that the Akt-activating activity existed in the flowthrough fractions of the hydrophobic (butyl-S-Sepharose) and anion-exchange columns (High Q). This activity could be retained on the cation-exchange (High S) column and recovered by elution with a pH gradient (see Fig. S2A, B, and C in the supplemental material). We then attempted to identify the proteins from this intermediate purification by mass spectrometry analysis.
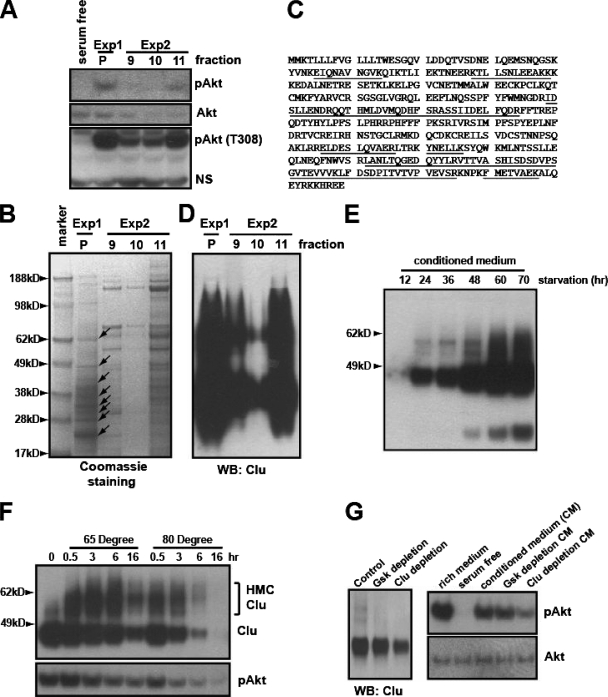
The final fractions from two independent purifications were tested for Akt-activating activity. Consistent with the previous observation that this activity acts upstream of the PI3K pathway (Fig. 1E and 2E), phosphorylation of both Akt-T308 and Akt-S473 was enhanced by the purified activity (Fig. 4A). Using the Coomassie staining of these fractions, we identified eight bands that appeared to be correlated with the activity (Fig. 4B, enriched in the E1-P and E2-fraction 11 but not in the E2 fractions 9 and 10; see also Fig. S2C in the supplemental material), and these were subjected to MALDI analysis. Intriguingly, one of the proteins identified, clusterin, was detected in all eight samples, with a high percentage of peptide coverage (Fig. 4C). The Western blotting analysis confirmed a broad range of clusterin immunoreactivity that corresponded to the analyzed bands (Fig. 4D).
FIG. 4.
Biochemical purification identified clusterin as a protein associated with the PI3K-Akt-activating activity. (A) The active fractions eluted from the High S column from two independent purification experiments (see Fig. S3 in the supplemental material) were mixed (1:1) with serum-free medium. After heat treatment, their ability to phosphorylate S473 and T308 of Akt was assayed in NB4 cells. Exp1 and Exp2 indicate two independent purification experiments. P indicates the pooled activity from the first purification. NS indicates a nonspecific band. (B) The corresponding fractions were concentrated with a Centriprep 10K column. Proteins were resolved on a NuPAGE 4 to 12% gel stained with Coomassie blue. Arrows indicate the protein bands analyzed by MALDI mass spectrometry. (C) Human clusterin peptides identified by MALDI analysis are underlined. (D) Western blotting (WB) of the corresponding fractions, using C-terminal-specific monoclonal clusterin antibody. (E) Serum-free CM from HeLa cells collected at the indicated time points was analyzed for clusterin. (F) CM from HeLa cells was heat treated for the indicated times, and proteins were analyzed with the C-terminal-specific clusterin antibody. The bracket indicates the high-molecular-weight complexes induced by heat treatment (top). Akt-activating activity of the corresponding CM was tested in NB4 cells (bottom). (G) CM was immunodepleted with the protein G/A beads alone (control), the control antibody (monoclonal anti-GSK), or the C-terminal-specific clusterin antibody. The supernatant was analyzed for clusterin (at left). Their abilities to activate Akt were tested in NB4 cells (at right).
Clusterin is a multifunctional protein involved in various biological processes, including tumorigenesis (17, 41), although the underlying mechanisms are still poorly characterized. Its function as an extracellular chaperon and its regulation by cellular stresses prompted us to evaluate this protein further. First, we found that clusterin was accumulated in the CM during serum starvation (Fig. 4E). Since the Akt-activating activity in CM can be enhanced by heat treatment, we determined if heat treatment could affect the conformation of clusterin. The heat treatment led to a high-molecular-weight complex of clusterin, indicating a structural change(s) (Fig. 4F). Longer periods of treatment at a higher temperature (80°C over 3 h) led to its degradation in a manner corresponding to the loss of Akt-activating activity (Fig. 4F). When the High S-bound activity was subjected to elution by a fine gradient of pH, clusterin was coeluted with the Akt-activating activity (see Fig. S2D in the supplemental material). In addition, the immunodepletion of clusterin from the CM led to a reduced Akt-phosphorylating activity, indicating that clusterin is associated with the Akt-activating activity (Fig. 4G).
Clusterin is a secreted negative regulator of the PI3K-Akt signaling pathway.
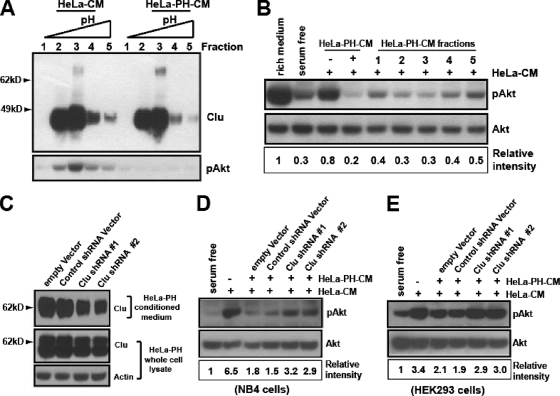
We showed that serum-starved HeLa cells secrete both positive and negative regulators of the PI3K-Akt pathway (Fig. 3). Clusterin is associated with the Akt-activating activity, but whether it acts as a positive or a negative regulator needs to be determined. First, we took advantage of those HeLa-PH cells whose CM lacked the Akt-activating activity (Fig. 2C and D). Equal amounts of CM from wild-type HeLa (HeLa-CM) and HeLa-PH-CM were subjected to the High S column, and bound proteins were eluted by a pH gradient. The level of clusterin in each fraction of HeLa-PH-CM was not different from that of corresponding fractions of HeLa-CM. However, all fractions from HeLa-PH-CM lacked the Akt-activating activity when they were tested in NB4 cells (Fig. 5A), indicating that clusterin by itself does not function as an Akt-activating factor.
FIG. 5.
Clusterin is a secreted negative regulator of the PI3K-Akt signaling pathway. (A) The High S column-bound proteins from HeLa cell CM (HeLa-CM) or HeLa-PH cells (HeLa-PH-CM) were eluted by the pH gradient. The eluted fractions were analyzed for clusterin (top), and the corresponding fractions were tested for their ability to activate Akt in NB4 cells (bottom). (B) HeLa-PH-CM inhibits the Akt activation elicited by HeLa-CM. HeLa-PH-CM and its fractions shown in panel A were mixed (1:1) with HeLa-CM and tested for their abilities to inhibit HeLa-CM-elicited Akt activation in NB4 cells. (C) The shRNA-mediated knockdown of clusterin in HeLa-PH1 cells is shown. HeLa-PH1 cells were transfected with the empty vector, the control shRNA vector, or two different clusterin shRNA vectors, and then the serum-free CM (24 h) and the whole-cell lysates of each transfectant were analyzed for clusterin expression. (D and E) Each HeLa-PH-CM was mixed (1:1) with HeLa-CM and tested as described in the legend to panel B for the ability to inhibit HeLa-CM-mediated Akt phosphorylation in NB4 or HEK293 cells. The representative Western blotting of at least three independent experiments is shown.
Previously, we observed that concentrated HeLa-PH-CM inhibited Akt phosphorylation (Fig. 3B). Therefore, we examined whether HeLa-PH-CM could inhibit the Akt-activating activity present in HeLa-CM. In the presence of equal amounts of HeLa-PH-CM, Akt phosphorylation by HeLa-CM was strongly inhibited, and, moreover, HeLa-PH-CM fractions from the High S column also reduced Akt phosphorylation in a manner that correlated with its clusterin level (Fig. 5B). This finding indicates the possibility that clusterin may play an inhibitory function. If this is the case, then the reduction of clusterin in HeLa-PH-CM should attenuate its ability to inhibit HeLa-CM-mediated Akt activation. To test this possibility, we knocked down the endogenous clusterin in HeLa-PH cells by RNA interference (Fig. 5C), and then the CM from these cells was tested for its ability to inhibit the Akt phosphorylation by HeLa-CM. We found that a significant reduction of clusterin in HeLa-PH cells, in which prosurvival IGF-1 signaling was compromised, led to apoptosis under serum-deprived conditions (see Fig. S7 in the supplemental material). Thus, we relied on a moderate knockdown that did not cause apoptosis. Compared to CM from empty vector- or control shRNA-expressing HeLa-PH cells, the CM from the clusterin-shRNA-expressing HeLa-PH cells manifested a reduced inhibitory activity when tested in both the NB4 (Fig. 5D) and the HEK293 cells (Fig. 5E), strongly indicating a negative role for clusterin.
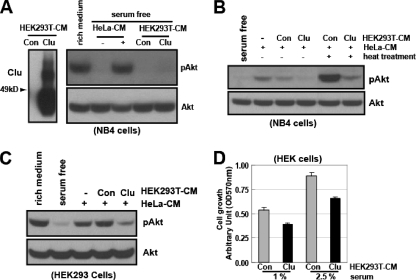
We then asked if the ectopic expression of clusterin could confer a similar inhibitory activity in a heterologous system. For this end, we used HEK293T cells, which almost completely lack endogenous clusterin and do not secrete Akt-activating factors during serum starvation (Fig. 6A). We transfected HEK293T cells with the empty or clusterin-expressing (Clu) vector, and the serum-free CM was collected. Neither the vector control nor clusterin-containing HEK293T-CM showed any effects on the Akt phosphorylation when tested with NB4 cells (Fig. 6A). However, compared with the control CM, the CM from clusterin-transfected cells (HEK293T-Clu) markedly inhibited the Akt phosphorylation induced by HeLa-CM when tested with both NB4 cells and HeLa cells (Fig. 6B and C).
FIG. 6.
Ectopic expression of clusterin inhibits PI3K-Akt activation and cell proliferation. (A) The empty control (Con) vector or the clusterin-expressing plasmid (Clu) was transfected into HEK293T cells. The resulting serum-free CM (30 h) fractions were analyzed for clusterin expression (left) and their abilities to affect the Akt phosphorylation in NB4 cells (right). (B) The Con- or Clu-containing HEK293T-CM samples were mixed (1:1) with HeLa-CM and left untreated or were heat treated and tested for their abilities to affect HeLa-CM-mediated Akt phosphorylation in NB4 cells. (C) Effects of clusterin on HeLa-CM-elicited Akt phosphorylation in HEK293 cells. (D) The Con- or Clu-containing HEK293T-CM was mixed (1:1) with different amounts of serum and added to HEK293 cells in triplicate. Cell proliferation was measured by MTT assay after 72 h. Results are the means ± standard errors of three independent experiments.
Activation of the PI3K-Akt pathway promotes cell proliferation (31, 42). To determine if clusterin could exert a sustained inhibitory effect on the PI3K-Akt pathway, we examined its effects on cell proliferation. The same number of HEK293 cells were plated using the reduced serum conditions (1% and 2.5%) in the presence of control or clusterin-containing HEK293T-CM and cultured for 3 days. As revealed by the relative cell proliferation, compared to that of the control, the presence of clusterin led to a 28 to 30% reduction in cell proliferation (Fig. 6D). Taken together, these data support a role for clusterin in the negative regulation of the PI3K-Akt pathway.
Clusterin inhibits the IGF-1- but not the EGF-mediated signaling pathway.
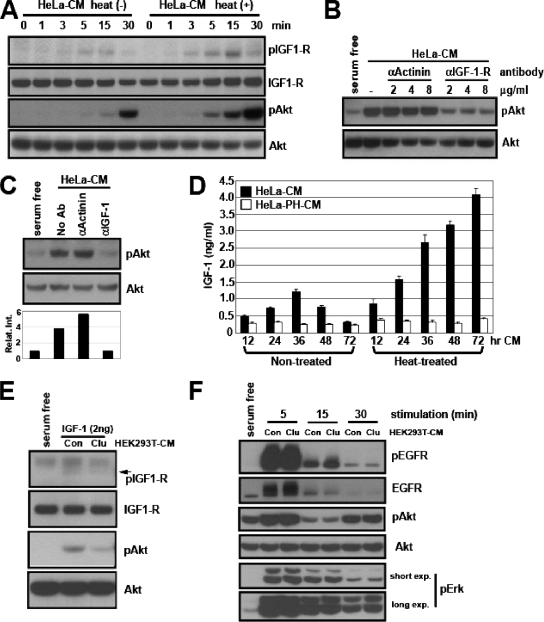
Next, we decided to identify the Akt activator(s) that is inhibited by clusterin. First, we found that the heat-treated HeLa-CM did not enhance G protein-coupled receptor signaling as determined by Akt activation and chemotactic migration of mouse peripheral neutrophils (see Fig. S3A and B in the supplemental material). We then checked for the growth factor receptor signaling. NB4 cells lack both the PDGF and the EGF receptors (data not shown). This finding led us to examine IGF receptor signaling. Using a phosphorylation-specific antibody, we determined the kinetics of IGF-1 receptor activation by HeLa-CM with or without heat treatment. HeLa-CM activated the IGF-1 receptor, and its activation preceded that of Akt phosphorylation. Importantly, the heat treatment of HeLa-CM significantly enhanced the activation of the IGF receptor (Fig. 7A). Next, we asked if the activation of the IGF receptor by HeLa-CM was responsible for Akt activation. For this purpose, we took advantage of a neutralizing antibody against the IGF-1 receptor (27). Preincubation of NB4 cells with the IGF-1 receptor-neutralizing antibody, but not the control antibody, markedly inhibited the Akt activation by the heat-treated HeLa-CM (Fig. 7B). Activation of the IGF-1 receptor strongly indicates the presence of its ligands, such as IGF-1, in HeLa-CM. However, its activation could be also due to cross talk between signaling receptors (12, 25). Therefore, we determined if similar neutralization of IGF-1 could affect the Akt-activating activity of HeLa-CM. The inhibitory activity of the IGF-1-neutralizing antibody has been well documented (34). We also confirmed that this antibody blocked the activity of both purified and serum IGF-1 (see Fig. S3C in the supplemental material). The IGF-1-neutralizing antibody, but not the control antibody, almost completely abolished Akt activation by heat-treated HeLa-CM (Fig. 7C). Next, we determined if IGF-1 was indeed secreted by HeLa cells during serum starvation. We showed that serum-free CM from HeLa-PH cells lacked Akt-activating activity (Fig. 2D). Therefore, we measured the amount of IGF-1 in HeLa-CM and HeLa-PH-CM with or without heat treatment, by ELISA. The results showed that IGF-1 was indeed present in HeLa-CM and its level peaked at 36 h and decreased thereafter. Importantly, the heat treatment significantly increased the detectable level of IGF-1, and the amount of IGF-1 detected in 72-h HeLa-CM was comparable (slightly higher) to that detected in 36-h HeLa-CM (Fig. 7D). As expected, very little IGF-1 was detected in untreated and heat-treated HeLa-PH-CM (Fig. 7D), confirming that the lack of IGF-1 was responsible for its inability to activate Akt.
FIG. 7.
Clusterin inhibits the IGF-1-mediated, but not the EGF-mediated, signaling pathway. (A) Serum-starved NB4 cells were incubated with the untreated [heat(−)] or heat-treated [heat(+)] HeLa-CM for the indicated times, and the activation of the IGF-1 receptor and Akt was analyzed in whole-cell lysates. (B) NB4 cells were preincubated with different amounts of control or IGF-1 receptor-neutralizing antibody in serum-free medium and then incubated with heat-treated HeLa-CM for 30 min before the HeLa-CM was analyzed for phospho-Akt level. (C) The IGF-1-neutralizing antibody blocked the HeLa-CM-elicited Akt activation. The heat-treated HeLa-CM was preincubated with the control or IGF-1-neutralizing antibody, and then the ability to activate Akt was tested in NB4 cells. (D) Secretion of IGF-1 by serum-starved HeLa cells. The levels of IGF-1 in untreated or heat-treated CM collected from HeLa cells or HeLa-PH cells at different time points of serum starvation were measured by ELISA. (E) Functional inhibition of IGF-1 by clusterin. The purified IGF-1 was preincubated with the control (HEK293T-Con-CM) or clusterin-containing CM (HEK293T-Clu-CM) and assayed for the activation of IGF-1 receptor and Akt in NB4 cells. (F) The purified EGF (5 ng/ml) was preincubated with the control or clusterin-containing CM as described in the legend to panel E and assayed for the activation of the EGF receptor, Akt, and Erk in serum-starved HeLa cells at the indicated time points.
We showed that ectopic clusterin inhibited the Akt-activating activity of HeLa-CM (Fig. 6B and C), indicating that clusterin may attenuate IGF-1 function. To test this possibility, we preincubated the purified IGF-1 with the control or the clusterin-containing HEK293T-CM and then compared their activity levels. Consistent with our prediction, the presence of clusterin led to a functional inhibition of IGF-1, as revealed by a decreased activation of the IGF-1 receptor and Akt (Fig. 7E). To further determine if the inhibitory function of secreted clusterin is preferential for IGF-1 or if it functions generally to inhibit other growth factors, we examined its effects on EGF signaling.
We found that the preheating treatment of HeLa-CM failed to enhance the activity of the EGF receptor, while it markedly increased that of the IGF-1 receptor (see Fig. S3D in the supplemental material). Next, we determined if clusterin could affect the activity of purified EGF. Within 5 min, the stimulation of starved HeLa cells with EGF led to a robust activation of the EGF receptor, Akt, and Erk (see Fig. S3E in the supplemental material). Unlike clusterin's inhibitory function toward IGF-1 (Fig. 7E and Fig. 8A), at all time points, the presence of clusterin had little effect on the activation of the EGF receptor and its downstream components (Fig. 7F).
FIG. 8.
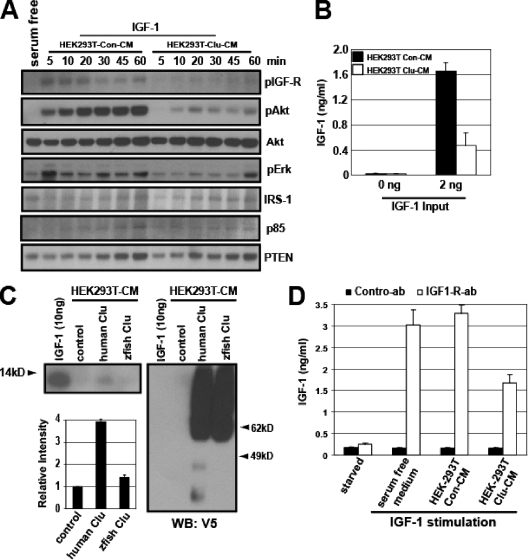
Clusterin associates with IGF-1 and inhibits its binding to the IGF-1 receptor. (A) The time course analysis of IGF-1-induced Akt activation in the presence or absence of clusterin. The purified IGF-1 (2 ng/ml) was preincubated with the control (Con) or clusterin-containing (Clu) CM and treated in NB4 cells for the indicated times and assayed for the IGF-1 signaling components. (B) The purified IGF-1 was treated as described in the legend to panel A, and the amount was determined by ELISA. (C) The HEK293T-CM collected after transfection with the control or the C-terminal His6-tagged human or zebrafish (zfish) clusterin vector was preincubated with purified IGF-1 and pulled down with Ni-NTA agarose beads and analyzed for IGF-1. (D) Serum-starved HEK293 cells were stimulated; purified IGF-1 was treated as described in the legend to panel A and then immunoprecipitated with IGF-1b receptor. The immune complex was analyzed for IGF-1 by ELISA.
Clusterin associates with IGF-1 and inhibits its binding to the IGF-1 receptor.
To gain insight into the mechanism by which clusterin attenuates IGF-1 function, we first determined its effects on the kinetics of IGF-1-induced Akt activation in NB4 cells. Upon IGF-1 stimulation, the phosphorylation of IGF-1 receptor peaked at 5 min, gradually decreased over the next 40 min, and then increased over the next 15 min. The later increase (at 60 min) could be due to an elevation of the IGF-1 receptor itself. The level of pAkt gradually increased for 20 min and remained the same thereafter. The presence of clusterin markedly inhibited the activation of both the IGF-1 receptor and Akt at all time points, indicating that it directly attenuates IGF-1 function rather than perturbs its signaling kinetics (Fig. 8A). The level of phospho-Erk was also reduced in the presence of clusterin, although not as significantly as that of phospho-Akt. The levels of downstream IGF-1 signaling components, such as IRS-1, p85, and PTEN, of the control were not noticeably different than those of the clusterin-containing CM (Fig. 8A).
Clusterin was copurified with the Akt-activating activity, which contains IGF-1 (Fig. 4 and see Fig. S2E in the supplemental material), suggesting that they may exist in a complex. To determine if clusterin could form a complex with IGF-1 in vitro, we employed two independent approaches. First, we incubated the purified IGF-1 with the control or the clusterin-containing CM and determined if clusterin could affect the accessibility of IGF-1. We found that preincubation with clusterin reduced the amount of IGF-1 detected by ELISA (Fig. 8B). Next, we performed a biochemical pull-down experiment. We found that, under a nonreducing condition, the purified IGF-1 could be readily detected by Western blotting (see Fig. S4A in the supplemental material). For the pull-down experiment, the C-terminally tagged (V5 and His6) clusterin was expressed in HEK293T cells. As a control, we used the zebrafish clusterin, which shares less than 40% identity with human clusterin but is readily glycosylated and secreted when expressed in HEK293T cells (see Fig. S4B and C in the supplemental material). The purified IGF-1 was incubated with the control or the human clusterin- or the zebrafish clusterin-containing CM and pulled down with Ni-NTA beads. This experiment revealed that the purified human IGF-1 could form a complex with human clusterin but not with zebrafish clusterin (Fig. 9C). Next, we determined if clusterin prevents binding of IGF-1 to its receptor. For this end, serum-starved HEK293 cells were stimulated with IGF-1 that had been preincubated with control or clusterin-containing CM and then immunoprecipitated with the nonneutralizing IGF-1 receptor antibody. Given the fact that the purified IGF-1 is heat stable (Fig. 3A), we heat treated the immunocomplex to release the bound IGF-1, and its level was measured by ELISA. Consistent with the previous results, the presence of clusterin reduced the amount of IGF-1 bound to its receptor (Fig. 8D). Together, these results suggest that clusterin attenuates IGF-1 signaling by interfering with its binding to the receptor.
FIG. 9.
Oncogenic signaling leads to insensitivity to clusterin-mediated inhibition. (A and B) The control (Con)- or clusterin (Clu)-containing HEK293T-CM was mixed with HeLa-CM (1:1), and samples were tested for their abilities to affect Akt phosphorylation in HEK293 and HEK293T cells. (C) The Con- or Clu-containing HEK293T-CM was mixed with HeLa-CM in the presence of the indicated amounts of LY294002 and then tested for their effects on Akt phosphorylation in HEK293T cells. (D) The Con- or Clu-containing HEK293T-CM was mixed (1:1) with HeLa-CM and tested for the ability to affect HeLa-CM-mediated Akt phosphorylation in HEK293-Ras or HEK293-vector cells. (E) Whole-cell lysates of serum-starved HeLa, HEK293, HEK293T, or HEK-Ras and their corresponding CM fractions were tested for Akt-activating activity in NB4 cells.
Oncogenic signaling leads to insensitivity to clusterin-mediated inhibition.
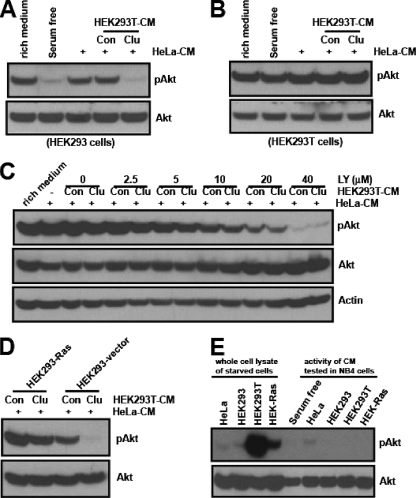
Our data show that clusterin plays negative roles in the PI3K-Akt pathway through the inhibition of IGF-1 function. If this is the primary mechanism, then constitutive activation of downstream components of IGF-1 receptor should confer insensitivity to clusterin. To investigate this possibility, we examined the effects of clusterin on cell lines containing the impaired IGF-1 receptor signaling. Consistent with previous reports (14, 38), we found that compared to the level of PI3K activation found with wild-type HEK293 cells, HEK293T cells in which simian virus 40 large T (SV40T) antigen is overexpressed had a high level of PI3K activation, as demonstrated by a prominent augmentation of PIP3-mediated membrane translocation of PH-EGFP (see Fig. S5A in the supplemental material). Unlike that in wild-type HEK293 cells, clusterin failed to affect Akt phosphorylation in HEK293T cells (Fig. 9A and B). This insensitivity was not caused by a “high basal level of phospho-Akt” per se, as lowering the basal level of phospho-Akt by cotreatment with LY294002 still failed to manifest any additional inhibitory effects (Fig. 9C).
To determine whether any other oncogenic events could induce a similar insensitivity to clusterin, we transfected HEK293 cells with an empty vector or an oncogenic form of H-Ras, H-Ras(V12). The oncogenic H-Ras(V12) protein constitutively elicits downstream signaling pathways, including that of the PI3K, independently of upstream receptor tyrosine kinases. In the control HEK293 vector, clusterin almost completely blocked HeLa-CM-mediated Akt phosphorylation. In contrast, in HEK293-Ras cells, clusterin showed only a marginal inhibitory effect (Fig. 9D). The inhibition of H-Ras protein in these cells, with a farnesyl transferase inhibitor, significantly restored their sensitivity to clusterin (see Fig. S5B in the supplemental material). To determine if this insensitivity was due to the uncontrolled activation of downstream IGF-1 signaling or to transformed cells secreting other factors that acted upstream of Akt, the serum-free CM from these cells were tested for their abilities to activate Akt. Compared with HeLa or wild-type HEK293 cells, the basal levels of phospho-Akt in both HEK293T and HEK-Ras cells were much higher under serum deprivation conditions. However, unlike HeLa-CM, the CM from HEK or its transformed counterparts failed to activate Akt when tested with NB4 cells. These results suggest that oncogenic activation downstream of IGF-1 signaling can lead to insensitivity to clusterin-mediated control without the secretion of other activating factors.
Synthesis of clusterin during serum deprivation is independent of IGF-1 signaling.
It has been reported that IGF-1 receptor signaling regulates clusterin expression (10), and we showed that clusterin was secreted during serum deprivation (Fig. 4E). We therefore determined if IGF-1 signaling could affect the synthesis and/or secretion of clusterin during serum deprivation. Clusterin undergoes several steps of posttranslational modifications, including glycosylation, proteolytic cleavage, and disulfide bond formation (28, 41). The precursor and mature (to-be-secreted) forms could be differentiated easily by treatment with the reducing agents that disrupt the intermolecular (between α and β chains) disulfide bonds present only in the mature form. We analyzed the whole-cell lysates of HeLa cells grown in serum-rich or serum-free medium in the presence or absence of a reducing agent. Both the total and the secreted clusterin levels were found to be elevated during serum starvation (see Fig. S6A in the supplemental material), and the time course analysis further revealed that the level of the proteolytically processed (mature) form could be increased within 12 h of serum deprivation (see Fig. S6B in the supplemental material). This initial elevation in protein level appeared not to be due to its transcripts, which began to increase 24 h after serum deprivation (data not shown). It has been reported that there are two isoforms of clusterin (nuclear and secreted) present inside cells (41). To determine which isoform was accumulated during serum deprivation, HeLa cells were treated with tunicamycin, an inhibitor of N glycosylation. This treatment revealed a nonglycosylated precursor form of clusterin (approximately 55 kDa) with a concomitant loss of the 62-kDa precursor form, indicating that only the secreted (or nonnuclear) isoform was present during serum deprivation (see Fig. S6C in the supplemental material). Next, we determine if the perturbation of IGF-1 signaling could affect the level of clusterin during serum deprivation. The addition of IGF-1 or IGF-1 receptor-neutralizing antibody failed to influence the level of clusterin (see Fig. S6D and E in the supplemental material). We then determined if restimulation of starved cells with serum or IGF-1 could affect its level. Restimulation with serum led to a reduction in the clusterin level within 2 h, but IGF-1 failed to exert any effects at later time points and higher concentrations (see Fig. S6F in the supplemental material). These results showed that the synthesis of clusterin during serum deprivation is independent of IGF-1 signaling. Reduction of the clusterin level within 2 h after serum restimulation suggests that its level could be controlled by protein translation and/or turnover. To test this possibility, we treated HeLa cells grown under serum-rich or -starved conditions with cycloheximide and examined the level of clusterin over a time course. Within 2 h of treatment, only a small fraction (approximately 10%) of the precursor form was detected under both serum-rich and -starved conditions. Intriguingly, while around 35% of the mature form was detected under serum-rich conditions (see Fig. S6G in the supplemental material), a significant fraction (75%) of the mature form still remained under serum-starved conditions, indicating a posttranslational regulation of clusterin.
DISCUSSION
The ability of cancer cells to modulate the cellular signaling pathways in accordance with the changing local environment represents one of the molecular mechanisms underlying the progression of malignancy and resistance to anticancer drugs. The PI3K-Akt pathway serves as a converging point for many upstream signaling receptors. However, the cellular mechanisms that modulate this pathway under growth-constraining conditions are not clearly defined. In the present study, we show that serum-deprived cancer cells secrete a PI3K-Akt-regulating activity that consists of both positive and negative factors. Via biochemical purification, we reveal that cancer cell-derived clusterin and IGF-1 constitute this regulatory activity. We demonstrate furthermore that clusterin negatively modulates the PI3K-Akt pathway through inhibition of IGF-1, a major growth factor secreted by serum-starved cancer cells, thus identifying a novel function for clusterin.
Previous studies with clusterin, including overexpression and knockdown in cancer cells, implicated it in different aspects of the tumorigenic process (5, 10, 29, 41, 49). Clusterin exists in an intracellular form and an extracellular form, and its functional roles in biological processes have been enigmatic. Difficulties in delineating its function as an intracellular or an extracellular factor may explain many of its promiscuous functions. The two isoforms (nuclear and secreted) of clusterin were shown to play opposing functions for cell survival, in which the nuclear form was proapoptotic, and the secreted form was prosurvival (30, 41). We confirmed that during serum deprivation in HeLa cells, only the secreted form of clusterin could be found (see Fig. S6A to C in the supplemental material), and its knockdown in HeLa-PH cells, in which prosurvival IGF-1 signaling was compromised, led to apoptosis (see Fig. S7 in the supplemental material). This suggests that the secretory forms, while they are still inside the cell, play a prosurvival function, in agreement with other studies (41). Relatively few studies have focused on the extracellular functions of clusterin in relation to tumorigenesis. Nevertheless, several experimental results identified its inhibitory roles in cell proliferation (5, 45, 50), which is consistent with our finding (Fig. 6D). For instance, it has been shown that recombinant human clusterin suppressed epithelial cell proliferation, and a high incidence of papilloma was found in clusterin-null mice in a chemical-induced skin carcinogenesis model (45). However, the underlying mechanism remains unknown. This is partly due to its capacity to bind to a wide range of molecules, including lipids and protein aggregates. Thus, clusterin is thought to play promiscuous functions (41), often resulting in conflicting results (18, 48). Despite this view, we found that the inhibitory effect of clusterin on the PI3K-Akt pathway could be attributed to its preferential attenuation of IGF-1, not to other growth factors such as EGF (Fig. 7).
Cancer cells under stress conditions may secrete many autocrine and/or paracrine factors that feed into the PI3K-Akt pathway to support cell growth and survival (4, 6, 24, 36). Surprisingly, however, we found that the inhibition of IGF-1 or its receptor alone almost completely abolished the Akt activation induced by CM from the serum-starved cancer cells (Fig. 7B and C). We showed that, under identical serum deprivation conditions, HeLa cells with an impaired PI3K-Akt pathway failed to secrete the Akt-activating activity (Fig. 2D) and IGF-1 (Fig. 7), identifying a crucial role for this pathway in producing its own upstream activator. Whether this finding can be extended to other cancer cell types and different stress conditions needs to be determined. Nevertheless, this result further explains why the deactivation of the PI3K-Akt pathway could be so effective in managing human cancers.
While the presence of IGF-1 in the tumor microenvironment is known (37), its secretion by serum-starved cancer cells has not been readily observed. One potential reason might be its biological accessibility. For example, we showed that, as measured by ELISA, the levels of IGF-1 in serum-free CM were marginal, especially at later time points (i.e., 72 h compared to 36 h). However, as revealed by ELISA following heat treatment, this was not due to a lack of its secretion (Fig. 7D). Instead, this result demonstrates that a large portion of secreted IGF-1 exists in a nonaccessible complex, indicating an important role for extracellular factors in modulating the bioavailability of IGF-1 (37). The current study identifies a novel role for cancer cell-derived clusterin in this process.
Extracellular clusterin manifests a growth suppressive function (Fig. 6D). Why would tumor cells secrete a factor that could potentially suppress their own growth? The level of clusterin is tightly associated with various cellular stress responses (7, 41). Therefore, its secretion may reflect the cellular adaptive responses to endure adverse environmental conditions (i.e., by suppressing its own growth and that of surrounding cells). In a mouse model of prostate cancer, it has been shown that epithelial cancer cells initiate and promote the clonal expansion of stromal fibroblasts that lack the p53 tumor suppressor gene (23), indicating that the cancer cell-derived factors initially impose selective pressures (i.e., antiproliferation) on neighboring cells. The presence of clusterin in the tumor microenvironment can contribute to such selective pressures. For example, the availability of glucose is often limited in the tumor microenvironment (16), and its uptake, a process mediated by the PI3K-Akt pathway (13, 22), can be a deciding factor for cell proliferation. Under this condition, those cells with a higher resistance (or insensitivity) to clusterin would gain growth advantages and thus would be positively selected. Our demonstration that constitutive activation of oncogenic signaling led to insensitivity to clusterin is consistent with this idea (Fig. 9).
The cellular adaptive response to environmental stresses is recognized as an important mechanism that facilitates tumorigenic progression (20, 43). We identified an extracellular regulatory system for the PI3K-Akt pathway in cancer cells under serum deprivation stress. We demonstrated that cancer cell-derived clusterin, an extracellular stress protein, and IGF-1 constituted this regulatory system, thus providing a genetic tool with which to better understand the complex signaling interplays between cancer cells and their environment.
Supplementary Material
Acknowledgments
We thank Leslie Silberstein, James Campbell, John Manis, and Li Cai for helpful discussions.
H. Luo is supported by NIH grants NS052200 and GM076084 and a research scholar grant from the American Cancer Society.
Footnotes
Published ahead of print on 5 May 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alt-Holland, A., W. Zhang, A. Margulis, and J. A. Garlick. 2005. Microenvironmental control of premalignant disease: the role of intercellular adhesion in the progression of squamous cell carcinoma. Semin. Cancer Biol. 1584-96. [DOI] [PubMed] [Google Scholar]
- 2.Bader, A. G., S. Kang, L. Zhao, and P. K. Vogt. 2005. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer 5921-929. [DOI] [PubMed] [Google Scholar]
- 3.Baek, J. H., J. E. Jang, C. M. Kang, H. Y. Chung, N. D. Kim, and K. W. Kim. 2000. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene 194621-4631. [DOI] [PubMed] [Google Scholar]
- 4.Barcellos-Hoff, M. H., and S. A. Ravani. 2000. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 601254-1260. [PubMed] [Google Scholar]
- 5.Bettuzzi, S., F. Scorcioni, S. Astancolle, P. Davalli, M. Scaltriti, and A. Corti. 2002. Clusterin (SGP-2) transient overexpression decreases proliferation rate of SV40-immortalized human prostate epithelial cells by slowing down cell cycle progression. Oncogene 214328-4334. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmick, N. A., E. G. Neilson, and H. L. Moses. 2004. Stromal fibroblasts in cancer initiation and progression. Nature 432332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjork, J. K., and L. Sistonen. 2006. Clustering of heat-shock factors. Biochem. J. 395e5-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26657-664. [DOI] [PubMed] [Google Scholar]
- 9.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 2961655-1657. [DOI] [PubMed] [Google Scholar]
- 10.Criswell, T., M. Beman, S. Araki, K. Leskov, E. Cataldo, L. D. Mayo, and D. A. Boothman. 2005. Delayed activation of insulin-like growth factor-1 receptor/Src/MAPK/Egr-1 signaling regulates clusterin expression, a pro-survival factor. J. Biol. Chem. 28014212-14221. [DOI] [PubMed] [Google Scholar]
- 11.Das, B., H. Yeger, R. Tsuchida, R. Torkin, M. F. Gee, P. S. Thorner, M. Shibuya, D. Malkin, and S. Baruchel. 2005. A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1alpha through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Res. 657267-7275. [DOI] [PubMed] [Google Scholar]
- 12.Desbois-Mouthon, C., W. Cacheux, M. J. Blivet-Van Eggelpoel, V. Barbu, L. Fartoux, R. Poupon, C. Housset, and O. Rosmorduc. 2006. Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib. Int. J. Cancer 1192557-2566. [DOI] [PubMed] [Google Scholar]
- 13.Engelman, J. A., J. Luo, and L. C. Cantley. 2006. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7606-619. [DOI] [PubMed] [Google Scholar]
- 14.Fei, Z. L., C. D'Ambrosio, S. Li, E. Surmacz, and R. Baserga. 1995. Association of insulin receptor substrate 1 with simian virus 40 large T antigen. Mol. Cell. Biol. 154232-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatenby, R. A., E. T. Gawlinski, A. F. Gmitro, B. Kaylor, and R. J. Gillies. 2006. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 665216-5223. [DOI] [PubMed] [Google Scholar]
- 16.Gatenby, R. A., and R. J. Gillies. 2004. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4891-899. [DOI] [PubMed] [Google Scholar]
- 17.Gleave, M., and K. N. Chi. 2005. Knock-down of the cytoprotective gene, clusterin, to enhance hormone and chemosensitivity in prostate and other cancers. Ann. N. Y. Acad. Sci. 10581-15. [DOI] [PubMed] [Google Scholar]
- 18.Han, B. H., R. B. DeMattos, L. L. Dugan, J. S. Kim-Han, R. P. Brendza, J. D. Fryer, M. Kierson, J. Cirrito, K. Quick, J. A. Harmony, B. J. Aronow, and D. M. Holtzman. 2001. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat. Med. 7338-343. [DOI] [PubMed] [Google Scholar]
- 19.Hanada, M., J. Feng, and B. A. Hemmings. 2004. Structure, regulation and function of PKB/AKT: a major therapeutic target. Biochim. Biophys. Acta 16973-16. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 10057-70. [DOI] [PubMed] [Google Scholar]
- 21.Hennessy, B. T., D. L. Smith, P. T. Ram, Y. Lu, and G. B. Mills. 2005. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 4988-1004. [DOI] [PubMed] [Google Scholar]
- 22.Hill, M. M., S. F. Clark, D. F. Tucker, M. J. Birnbaum, D. E. James, and S. L. Macaulay. 1999. A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 197771-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill, R., Y. Song, R. D. Cardiff, and T. Van Dyke. 2005. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 1231001-1011. [DOI] [PubMed] [Google Scholar]
- 24.Hurbin, A., J. L. Coll, L. Dubrez-Daloz, B. Mari, P. Auberger, C. Brambilla, and M. C. Favrot. 2005. Cooperation of amphiregulin and insulin-like growth factor-1 inhibits Bax- and Bad-mediated apoptosis via a protein kinase C-dependent pathway in non-small cell lung cancer cells. J. Biol. Chem. 28019757-19767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurbin, A., L. Dubrez, J. L. Coll, and M. C. Favrot. 2003. Inhibition of apoptosis by amphiregulin via an insulin-like growth factor-1 receptor-dependent pathway in non-small cell lung cancer cell lines. Ann. N. Y. Acad. Sci. 1010354-357. [DOI] [PubMed] [Google Scholar]
- 26.Jo, H., R. Zhang, H. Zhang, T. A. McKinsey, J. Shao, R. D. Beauchamp, D. W. Ballard, and P. Liang. 2000. NF-kappa B is required for H-ras oncogene induced abnormal cell proliferation and tumorigenesis. Oncogene 19841-849. [DOI] [PubMed] [Google Scholar]
- 27.Karey, K. P., and D. A. Sirbasku. 1988. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 484083-4092. [PubMed] [Google Scholar]
- 28.Lakins, J., S. A. Bennett, J. H. Chen, J. M. Arnold, C. Morrissey, P. Wong, J. O'Sullivan, and M. Tenniswood. 1998. Clusterin biogenesis is altered during apoptosis in the regressing rat ventral prostate. J. Biol. Chem. 27327887-27895. [DOI] [PubMed] [Google Scholar]
- 29.Lau, S. H., J. S. Sham, D. Xie, C. H. Tzang, D. Tang, N. Ma, L. Hu, Y. Wang, J. M. Wen, G. Xiao, W. M. Zhang, G. K. Lau, M. Yang, and X. Y. Guan. 2006. Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene 251242-1250. [DOI] [PubMed] [Google Scholar]
- 30.Leskov, K. S., D. Y. Klokov, J. Li, T. J. Kinsella, and D. A. Boothman. 2003. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J. Biol. Chem. 27811590-11600. [DOI] [PubMed] [Google Scholar]
- 31.Liang, J., J. Zubovitz, T. Petrocelli, R. Kotchetkov, M. K. Connor, K. Han, J. H. Lee, S. Ciarallo, C. Catzavelos, R. Beniston, E. Franssen, and J. M. Slingerland. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 81153-1160. [DOI] [PubMed] [Google Scholar]
- 32.Luo, H. R., H. Hattori, M. A. Hossain, L. Hester, Y. Huang, W. Lee-Kwon, M. Donowitz, E. Nagata, and S. H. Snyder. 2003. Akt as a mediator of cell death. Proc. Natl. Acad. Sci. USA 10011712-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma, Y., and L. M. Hendershot. 2004. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2851-65. [DOI] [PubMed] [Google Scholar]
- 34.Maiorano, E., A. Ciampolillo, G. Viale, P. Maisonneuve, A. Ambrosi, V. Triggiani, E. Marra, and E. Perlino. 2000. Insulin-like growth factor 1 expression in thyroid tumors. Appl. Immunohistochem. Mol. Morphol. 8110-119. [DOI] [PubMed] [Google Scholar]
- 35.Mueller, M. M., and N. E. Fusenig. 2004. Friends or foes: bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4839-849. [DOI] [PubMed] [Google Scholar]
- 36.Orimo, A., P. B. Gupta, D. C. Sgroi, F. Arenzana-Seisdedos, T. Delaunay, R. Naeem, V. J. Carey, A. L. Richardson, and R. A. Weinberg. 2005. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121335-348. [DOI] [PubMed] [Google Scholar]
- 37.Pollak, M. N., E. S. Schernhammer, and S. E. Hankinson. 2004. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 4505-518. [DOI] [PubMed] [Google Scholar]
- 38.Prisco, M., F. Santini, R. Baffa, M. Liu, R. Drakas, A. Wu, and R. Baserga. 2002. Nuclear translocation of insulin receptor substrate-1 by the simian virus 40 T antigen and the activated type 1 insulin-like growth factor receptor. J. Biol. Chem. 27732078-32085. [DOI] [PubMed] [Google Scholar]
- 39.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 3071098-1101. [DOI] [PubMed] [Google Scholar]
- 40.Shachaf, C. M., and D. W. Felsher. 2005. Tumor dormancy and MYC inactivation: pushing cancer to the brink of normalcy. Cancer Res. 654471-4474. [DOI] [PubMed] [Google Scholar]
- 41.Shannan, B., M. Seifert, K. Leskov, J. Willis, D. Boothman, W. Tilgen, and J. Reichrath. 2006. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 1312-19. [DOI] [PubMed] [Google Scholar]
- 42.Shin, I., F. M. Yakes, F. Rojo, N. Y. Shin, A. V. Bakin, J. Baselga, and C. L. Arteaga. 2002. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat. Med. 81145-1152. [DOI] [PubMed] [Google Scholar]
- 43.Spencer, S. L., R. A. Gerety, K. J. Pienta, and S. Forrest. 2006. Modeling somatic evolution in tumorigenesis. PLoS Comput. Biol. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian, K. K., Y. Jia, D. Zhu, B. T. Simms, H. Jo, H. Hattori, J. You, J. P. Mizgerd, and H. R. Luo. 2007. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood 1094028-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas-Tikhonenko, A., I. Viard-Leveugle, M. Dews, P. Wehrli, C. Sevignani, D. Yu, S. Ricci, W. el-Deiry, B. Aronow, G. Kaya, J. H. Saurat, and L. E. French. 2004. Myc-transformed epithelial cells down-regulate clusterin, which inhibits their growth in vitro and carcinogenesis in vivo. Cancer Res. 643126-3136. [DOI] [PubMed] [Google Scholar]
- 46.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2489-501. [DOI] [PubMed] [Google Scholar]
- 47.Watton, S. J., and J. Downward. 1999. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9433-436. [DOI] [PubMed] [Google Scholar]
- 48.Wehrli, P., Y. Charnay, P. Vallet, G. Zhu, J. Harmony, B. Aronow, J. Tschopp, C. Bouras, I. Viard-Leveugle, L. E. French, and P. Giannakopoulos. 2001. Inhibition of post-ischemic brain injury by clusterin overexpression. Nat. Med. 7977-979. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, H., J. K. Kim, C. A. Edwards, Z. Xu, R. Taichman, and C. Y. Wang. 2005. Clusterin inhibits apoptosis by interacting with activated Bax. Nat. Cell Biol. 7909-915. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, W., L. Janulis, I. I. Park, and C. Lee. 2002. A novel anti-proliferative property of clusterin in prostate cancer cells. Life Sci. 7211-21. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, W., J. Chen, X. Cong, S. Hu, and X. Chen. 2006. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 24416-425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.