Abstract
Despite the wealth of information available on the biochemical functions and our recent findings of its roles in genome stability and cancer avoidance of the structure-specific flap endonuclease 1 (FEN1), its cellular compartmentalization and dynamics corresponding to its involvement in various DNA metabolic pathways are not yet elucidated. Several years ago, we demonstrated that FEN1 migrates into the nucleus in response to DNA damage and under certain cell cycle conditions. In the current paper, we found that FEN1 is superaccumulated in the nucleolus and plays a role in the resolution of stalled DNA replication forks formed at the sites of natural replication fork barriers. In response to UV irradiation and upon phosphorylation, FEN1 migrates to nuclear plasma to participate in the resolution of UV cross-links on DNA, most likely employing its concerted action of exonuclease and gap-dependent endonuclease activities. Based on yeast complementation experiments, the mutation of Ser187Asp, mimicking constant phosphorylation, excludes FEN1 from nucleolar accumulation. The replacement of Ser187 by Ala, eliminating the only phosphorylation site, retains FEN1 in nucleoli. Both of the mutations cause UV sensitivity, impair cellular UV damage repair capacity, and decline overall cellular survivorship.
Flap endonuclease 1 (FEN1) represents a unique class of structure-specific 5′ nucleases that possess three distinct nuclease activities: FEN activity, nick-specific exonuclease (EXO) activity, and gap-dependent endonuclease (GEN) activity (18, 37, 56). Unlike endonucleases that recognize a specific DNA sequence, FEN1 recognizes a specific DNA structure, independent of the DNA sequence. Specifically, FEN1 recognizes a branched DNA structure consisting of a single unpaired 3′ nucleotide (3′ flap) overlapping with a variable-length region of 5′ single-stranded DNA (5′ flap) (27, 29). These “double-flap” or “overlap-flap” structures result from DNA polymerase and/or helicase activity that displaces damaged DNA or RNA primers, creating a 5′ single-stranded DNA flap. The newly synthesized DNA and the displaced region compete for base pairing with the template strand, resulting in the formation of the double-flap structure (53). FEN1 cleaves this substrate precisely after the first base pair that precedes the 5′ flap to remove the single-stranded DNA 5′ flap and create a nicked DNA product ready for ligation (27, 29, 66). This FEN activity-driven reaction is most likely critical for RNA primer removal during the maturation of Okazaki fragments and long-patch DNA base excision repair (33, 34, 42, 44). However, under the circumstances in which the ligase is not able to compete for the nick substrate, the FEN1 nuclease will transfer its reaction mode from FEN to EXO and continue to remove the nucleotides from the 5′ end, generating a single-stranded region (gap) (2, 24). This gap is an ideal substrate for the newly discovered third activity of the FEN1 nuclease (GEN). The same nuclease is able to make another transition to nick the single-strand gap region, causing DNA double-strand breaks. This concerted action of EXO and GEN has been proven to occur only under specific circumstances, such as a result of DNA damage during the S phase of the cell cycle and during the resolution of interstrand DNA cross-links and hairpin structures due to trinucleotide repetitive sequences as well as DNA fragmentation during cellular apoptosis (49, 58, 70).
Due to the essential roles of FEN1 in DNA replication/repair and apoptosis, the deletion of FEN1 in Saccharomyces cerevisiae (rad27) results in a high level of sensitivity to DNA damage reagents such as UV irradiation and methyl methane sulfonate, a strong mutator phenotype, and conditional lethality (52, 61). The complete removal of FEN1 activities via homozygous knockout causes early embryonic lethality in mice (35). Recently, we generated a transgenic mouse that specifically eliminates the EXO and GEN activities but retains FEN activity via the knock-in of a point mutation, E160D. The FEN1 deficiency caused a strong mutator phenotype and a retardation of apoptotic DNA fragmentation and subsequent chronic inflammation. Approximately 60% of the mice developed lung adenoma in a late life stage (69). These data highlight the importance of FEN1 functions in cells and qualify it as a tumor suppressor gene (20).
The multiple and seemingly contradictory functions, from the maturation of DNA replication in a dividing cell to DNA fragmentation, in an apoptotic cell make one wonder how FEN1's involvement in different pathways is controlled. Three mechanisms have been proposed in a recent review by Shen et al. (57): (i) the formation of proper protein-protein complexes, (ii) posttranslational modifications, and (iii) cellular compartmentalization. To date, at least 20 proteins involved in several different pathways have been reported to interact with FEN1 (57). Permission for FEN1 to fit into an appropriate complex might be the first gatekeeper. For example, GEN-induced double-strand DNA breaks are very toxic to the genome in a normal cell, and GEN activity is accordingly low. It is only when interstrand DNA cross-links are introduced that the Werner syndrome protein (WRN) forms a complex with FEN1 and specifically activates the GEN activity (70). In contrast, proliferating cell nuclear antigen (PCNA) interacts with FEN1 and stimulates the FEN activity for RNA primer removal and the cleavage of long-patch base excision repair intermediates (16). The disruption of the FEN1/PCNA interaction results in DNA replication defects, pulmonary hypoplasia, pancytopenia, and newborn lethality in mice (69).
FEN1 is a target for at least two posttranslational modifications in vivo: acetylation (14, 19) and phosphorylation (21). Cdk1-cyclin A phosphorylates FEN1 at Ser187 in late S phase (21). The in vitro Cdk1-cyclin A phosphorylation of FEN1 has been shown to reduce the endonuclease and exonuclease activities of FEN1 without affecting DNA binding (21). In addition, the phosphorylation of FEN1 abolishes PCNA binding (21). Serine phosphorylation is hypothesized to be one of the cell cycle regulatory mechanisms of FEN1 activities (21, 70). Ser187 is the only identified residue to be phosphorylated (21). In the late S phase, phosphorylation may be used to block FEN1's nuclease activities and its recruitment to the DNA replication site by PCNA. Therefore, this phosphorylation may contribute to ceasing the replication machinery, thereby ensuring the exit from S phase (21).
Recently, phosphorylation and acetylation have been shown to regulate the subcellular or subnuclear localization of many proteins, including those involved in DNA metabolic pathways (3, 12, 17, 30, 32, 45, 60, 68). For example, tyrosine phosphorylation is critical for the localization of Rad52, a homologous recombination factor, to DNA repair foci (32). On the other hand, serine phosphorylation of the transcription factor FOXO4 by protein kinase B (45) regulates its nuclear export. Acetylation also plays an active role in the subcellular or subnuclear localization of some proteins. The acetylation of WRN, for example, determines its translocation from the nucleolus to nucleoplasmic DNA replication foci upon UV irradiation (3).
In the last decade, we and several other groups have defined three biochemical activities of FEN1 and revealed its roles in different DNA metabolism pathways as well as its relationship to various phenotypes observed in yeast (57). However, we have yet to investigate the details of the dynamic localization of the enzyme in response to DNA damage and different cell cycle phases, which may be an important cellular mechanism for regulating its in vivo activities in mammalian cells. We previously reported that FEN1 localizes into the nucleus in response to DNA damage and cell cycle phases (51). In the current work, for the first time, we have observed that FEN1 is superaccumulated in nucleoli and plays a role in the stability of ribosomal DNA (rDNA). Upon UV irradiation, FEN1 is phosphorylated at Ser187 and moves out of nucleoli. The replacement of the phosphorylation site Ser187 with an alanine impairs the translocation of the nuclease from the nucleolus to the nuclear plasma and reduces its capability for genomic repair and cellular survival.
MATERIALS AND METHODS
Cell culture and transient transfection.
HeLa cells were cultured in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) supplemented with penicillin-streptomycin and 10% fetal bovine serum. Plasmid DNAs were transfected into HeLa cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Immunofluorescence microscopy.
HeLa cells were cultured on 12-mm coverslips (Fisher Scientific) until reaching 50% confluence. For fixation, the coverslips were washed with warm phosphate-buffered saline (PBS) twice, and the cells were fixed in methanol at −20°C for 30 min. The coverslips were then permeabilized with ice-cold acetone for 15 s and incubated with Image-iT FX signal enhancer (Invitrogen, Carlsbad, CA) for 30 min in a humid environment. The coverslips were rinsed with PBS and incubated with primary antibodies (anti-FEN1, anti-c23, or anti-c-myc at 4 μg/μl in PBS) for 1 h at room temperature in a humid environment. After three 5-min washes with PBS, the coverslips were incubated with goat anti-rabbit Alexa Fluor 488 or goat anti-mouse Alexa Fluor 568 antibody (10 μg/ml in PBS; Invitrogen, Carlsbad, CA) in the dark at room temperature for 1 h and subsequently incubated with 200 ng/ml DAPI (4′,6′-diamidino-2-phenylindole) in PBS in the dark for 10 min and washed twice for 5 min each with PBS. The coverslips were then placed onto a drop of Slowfade Gold antifade reagent (Invitrogen, Carlsbad, CA) and immobilized by nail polish. The signals were visualized and recorded by use of an Olympus IX81 fluorescence microscope or a Zeiss LM510 confocal microscope.
Protein expression and purification.
The construction of the protein expression vectors encoding His6-tagged wild-type FEN1 and the E178A mutant was previously described (70). The plasmids for the expression of the His6-tagged FEN1 S187A and S187D mutant proteins were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Primers for mutagenesis are as follows: S187AF (5′-GCCTCACCTTCGGCGCCCCTGTGTAATGC-3′), S187AR (5′-GCATTAGCACAGGGGCGCCGAAGGTGAGGC-3′), S187DF (5′-TGCCTCACCTTCGGCGACCCTGTGCTAATGCG-3′), and S187DR (5′-CGCATTAGCACAGGGTCGCCGAAGGTGAGGCA-3′). The pET28b vectors containing the wild-type and mutant genes were transformed into Escherichia coli BL21 cells for overexpression. Protein expression was performed as previously described (70) except that the IPTG (isopropyl-β-d-thiogalactopyranoside) induction step was carried out at 30°C to avoid the formation of inclusion bodies. All purification steps were carried out at 4°C. To purify His6-tagged proteins, the harvested cells (150 ml of culture) were lysed in 3 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl [pH 8.0]) containing 1 mM phenylmethylsulfonyl fluoride and 1 mg/ml lysozyme. After incubation on ice for 30 min, the cell lysate was sonicated until clear. The cell lysate was subsequently centrifuged at 18,000 × g for 30 min. The supernatant was then loaded onto PrepEase columns (USB Corporation, Cleveland, OH). His6-tagged proteins were eluted according to the manufacturer's instructions. Protein purity was determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and the concentration was quantified using the Bradford assay kit (Bio-Rad Laboratories, Hercules, CA).
Preparation of nuclear extract.
Crude nuclear extracts were prepared from cultured HeLa cells essentially as previously described (64). Harvested cells were washed twice with cold PBS and resuspended in 0.5 ml of hypotonic buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol) containing protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN), followed by incubation on ice for 10 min. Next, NP-40 was added to a final concentration of 0.5%, and the cells were vortexed for 10 s. The nuclear pellet was obtained by centrifugation at 3,000 × g for 20 s, followed by the addition of 150 μl buffer B (20 mM HEPES, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25% glycerol) containing protein inhibitors to resuspend the nuclear pellet. The samples were then incubated on ice with vigorous agitation for 30 min. The nuclear extracts (supernatants) were recovered by centrifugation for 10 min at 10,000 × g at 4°C.
Purification of nucleoli.
Nucleoli were purified by using a procedure described previously by Andersen et al. (1). The crude nuclear pellet was prepared as described above. Next, the nuclear pellet was resuspended in 3 ml of 0.25 M sucrose containing 10 mM MgCl2 and protease inhibitors, followed by a spin at 1,200 × g for 10 min through a 0.88 M sucrose cushion (4 ml) containing 0.05 mM MgCl2 and protease inhibitors. The purified nuclear pellet was resuspended in 3 ml of 0.34 M sucrose containing 0.05 mM MgCl2 and sonicated on ice for several bursts of 30 s with 5-min intervals. Nucleoli were then purified from the resulting homogenate by centrifugation at 2,000 × g for 20 min through a 0.88 M sucrose cushion (4 ml) containing 0.05 mM MgCl2 and protease inhibitors. The supernatant containing the nucleoplasmic fraction devoid of nucleoli was harvested for further analyses. The pellet contained purified nucleoli and was resuspended in 0.34 M sucrose containing 0.05 mM MgCl2 for further analyses.
Immunoprecipitation and Western blotting.
To detect the phosphorylation level of FEN1, crude nuclear extract, nucleoplasma, or nucleolus lysate, prepared as previously described, were incubated with anti-phosphorylated serine antibody (Millipore)-linked protein A-Sepharose beads in a binding buffer containing 50 mM Tris-HCl (pH 8.0), 0.1% NP-40, 150 mM NaCl, and protease inhibitors. Sepharose beads were washed five times with the same binding buffer, followed by boiling for 5 min in an SDS-PAGE sample buffer. Sepharose bead-captured proteins were separated by 4 to 15% gradient SDS-PAGE. Western blotting was performed using anti-FEN1 antibody (GTX70185; Genetex, San Antonio, TX).
FEN1 nuclease activity assay.
The cleavage of DNA substrates by FEN1 was determined under the same conditions as those previously published (69). Briefly, 32P-labeled DNA substrates were incubated with purified FEN1 in a buffer solution containing 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, and 5 mM MgCl2. The reactions were carried out at 37°C for 40 min and were terminated with stop solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol). The product and substrate were then separated by 15% denaturing polyacrylamide gel electrophoresis and visualized by autoradiography.
2-D gel analysis.
All yeast strains were grown in yeast extract-peptone-dextrose medium at 30°C to an optical density at 600 nm of 0.6 to 1.0 for two-dimensional (2-D) gel analysis. Genomic DNA was prepared, and the purified DNA was digested with BglII. Benzoylated-naphthoylated-DEAE-cellulose was used to enrich branched DNA molecules (65). A 2-D gel analysis was performed as demonstrated in previous studies (6).
Expression of FEN1 and its mutants in yeast cells and UV damage survivorship.
A yeast isogenic RAD27 deletion strain was constructed as previously described (61). This strain was based on the RDKY2672 wild-type yeast strain (MATa ura3-52 his3Δ200 trip1Δ63 leu2Δ1 ade2Δ1 ade8 lys2-Bgl hom3-10). Human E178A, S187A, and S187D FEN1 mutant cDNAs were expressed in RDKY2608 (Δrad27) using yeast expression vector pRS314 as described previously (61). UV sensitivity was measured as the survival rate of yeast cells upon exposure to UV irradiation as described previously (52). Experiments were repeated at least three times independently.
RESULTS
FEN1 is superaccumulated in nucleoli in HeLa cells.
Many DNA repair/replication proteins are dynamically localized into different subnuclear compartments for their efficient functions (4, 59). We previously observed that FEN1 localizes to the nucleus in response to DNA damage and phases of the cell cycle (51). Via high-resolution immunofluorescence confocal microscopic experiments, we found that the FEN1 protein localizes to the nucleus, forming bright foci. These foci had an obvious condensed structure when viewed under the light microscope and are possibly the nucleoli. To confirm our observation, we stained HeLa cells with an antibody against nucleolin (c23), a phosphoprotein that specifically localizes to the nucleoli (39). FEN1 is colocalized with nucleolin (Fig. 1A and B). More than 1,000 cultured cells were randomly counted, and approximately 91% of those cells exhibited visible FEN1 superaccumulation in the nucleoli. We also evaluated the endogenous WRN localization. WRN localized exclusively to the nucleolus, consistent with data from a previous report (3). We fractionated the nucleoli from the nuclei (Fig. 1B), probed the extracted proteins using anti-FEN1 and anti-WRN antibodies, and found that the FEN1 protein is present in both nucleoli and nuclei, whereas WRN is present only in nucleoli (Fig. 1C).
FIG. 1.
FEN1 is superaccumulated in nucleoli. (A) FEN1 is colocalized with c23 in nucleoli. HeLa cells were stained with anti-FEN1 (α-FEN1) and anti-c23 (α-c23) antibodies as described in Materials and Methods. (B) Nucleoli were purified from cells and stained by anti-FEN1 and anti-c23 antibodies. (C) The purity of isolated nucleoli was confirmed by Western blotting using anti-FEN1 and anti-WRN (α-WRN) antibodies. BF, bright field; NLO, nucleolar lysate; NE, nuclear extract.
Nucleolar FEN1 has a role in maintenance of rDNA stability.
Localized nucleolar FEN1 could be the stored form of the enzyme that migrates to the nuclear plasma as needed. It could also be the active form, playing a role in the maintenance of rDNA stability. We have used the well-established yeast system to test this hypothesis. In yeast, the rDNA occurs as ∼150 tandem repeats of a 9.1-kb sequence on chromosome XII. Each rDNA repeat contains two transcribed regions (35S and 5S rRNA) and two nontranscribed spacers (NTS1 and NTS2). NTS1 houses the polar replication fork barriers (RFBs) (130 bp), and NTS2 contains an origin of replication, an autonomously replicating sequence (ARS) (7, 38). When replication initiates from the active ARS, the replication forks moving in the same direction as 5S rRNA transcription are blocked at the RFB, whereas the fork progressing in the direction of 35S rRNA transcription is allowed to pass through the RFB (7, 38). Thus, the RFB imposes polar replication arrest in rDNA units containing an active ARS.
To study spontaneous replication pausing and recombination in rDNA at the RFB, we used neutral-neutral 2-D agarose gel electrophoresis (8, 65). We used three yeast strains to examine if there is an accumulation of stalled DNA replication forks (bubble structures near the RFB), the wild type, the Δrad27 mutant, and the E176A rad27 knock-in mutant, which has defects in EXO activity and lacks GEN activity. Indeed, we found a considerable increase in the amount of RFB in rDNA regions of the Δrad27 and E176A mutants compared to those in wild-type yeast cells (Fig. 2), implying that FEN1 is important for the maintenance of rDNA stability and that the EXO and GEN activities are involved in this particular function. We also observed a noticeable amount of converged forks, which occur when two forks collide at the RFB, in the E176A strain (Fig. 2). The level of converged forks has been shown to correlate with the extent of the RFB in rDNA regions (65).
FIG. 2.
FEN1 mutation causes stalled replication forks in rDNA regions of yeast cells. (A) Schematic of the 2-D gel pattern of the rDNA replication fork. Arrows show directions of first-dimension (1D) and second-dimension (2D) electrophoresis. (B) 2-D gel analysis of rDNA RFB. Genomic DNA was isolated from wild-type (wt) and E176A and rad27 deletion (Δrad27) mutant yeast strains and resolved by 2-D agarose electrophoresis. rDNA regions were detected by a 32P-labeled rDNA probe (65). Arrows indicate converged forks (CF) and RFBs. (C) Relative quantification of the stalled DNA replication forks shown in B. The intensity of the RFB spot (indicated by arrow) was divided by the intensity of the 1N spot and is reported as the “ratio of RFB to 1N.”
FEN1 translocates out of nucleoli upon UV irradiation.
As a protein involved in DNA repair, FEN1 is particularly important in the resolution of UV-induced DNA interstrand cross-links (70). We sought to determine how the FEN1 protein species in nucleoli responds to UV-induced DNA damage. HeLa cells were treated by UV irradiation (120 J/m2) and fixed after various recovery times. At 30 min after UV irradiation, approximately 25% of the FEN1 translocated to nuclear plasma (Fig. 3 and 4B). At 2 to 4 h after UV irradiation, most of the FEN1 translocated out of the nucleoli, and FEN1/nucleolin colocalization disappeared. At 12 to 16 h after UV irradiation, the nucleolar localization of FEN1 was recovered (Fig. 3). UV irradiation also induced the translocation of FEN1 out of nucleoli in HMCB cells (see Fig. S1 in the supplemental material).
FIG. 3.
UV treatment drives FEN1 out of nucleoli. The cells were washed twice with warm PBS (pH 7.4) and treated with UV-C (120 J/m2) using a UV Stratalinker 1800 apparatus (Stratagene, La Jolla, CA) with the lids removed. After irradiation, the cells were supplied with complete medium and harvested at the indicated times. Cell immunofluorescence staining was performed as described in Materials and Methods. The dynamic curve of the translocation of FEN1 out of nucleoli is quantitated in Fig. 4B.
FIG. 4.
UV-induced phosphorylation and translocation of FEN1 in HeLa cells. (A) FEN1 phosphorylation was induced by UV in nuclear extracts. HeLa cells were exposed to 120 J/m2 UV-C and harvested after the different times indicated. Cell nuclear extracts were prepared as described in Materials and Methods. Phosphorylated (P) FEN1 was pulled down by anti-serine phosphorylation antibody, followed by detection with anti-FEN1 antibody. The total nuclear extract protein concentrations of different samples were determined by a Bradford assay and confirmed by Western blotting using anti-lamin A antibody. As controls, purified FEN1 was incubated with 1 mM ATP in the absence or presence of CDK2/CycE, which phosphorylates FEN1 in vitro (21). Nonphosphorylated or phosphorylated FEN1 was pulled down with antiserine antibody and detected by anti-FEN1 antibody. (B) Dynamic curve of UV-induced phosphorylation and translocation of FEN1. The top (the dynamic curve of phosphorylated FEN1 in nuclear extracts upon UV treatment) is based on the image quantification above (A). The bottom (the percentage of FEN1-positive nucleoli in a microscope field) is based on data from multiple experiments as described in the legend to Fig. 3. (C) Distribution of total and phosphorylated FEN1 in nucleus plasma and nucleoli upon UV treatment. Nucleoli and nucleus plasma were isolated as described in Materials and Methods. For the total FEN1 assay, samples of 5 μg of total protein were used for a Western blot, followed by detection with anti-FEN1 antibody. For the detection of phosphorylated FEN1, nucleus plasma and nucleolar lysates (250 μg total proteins) were pulled down by antiphosphorylation antibody and then detected by anti-FEN1 antibody in a Western blotting assay. Detection of lamin A and c23 proteins served as an internal control for nuclear plasma and nucleoli, respectively.
UV irradiation increases the phosphorylation of FEN1.
Next, we asked what event drives FEN1 to migrate out of the nucleoli in response to UV damage. We tested whether phosphorylation is important in this process. HeLa cells were treated by UV irradiation (120 J/m2) and were harvested after various recovery times, similarly to what we have done with the experiments described in the legend of Fig. 3. The nuclear extract was immunoprecipitated with anti-serine phosphorylation antibody and detected with anti-FEN1 antibody by Western blot analysis. The specificity of the anti-serine phosphorylation antibody was validated using the purified recombinant FEN1 protein and the in vitro-phosphorylated FEN1 protein (Fig. 4A). The fraction of phosphorylated FEN1 increased with UV irradiation after 2, 4, and 8 h. At 8 h, the level of phosphorylated FEN1 peaked and then began to decrease, returning to the basal level by the 16-h time point (Fig. 4A and B). Anti-lamin A antibody was used as the control to measure the protein level of nuclear extracts at different time points (Fig. 4A). In the meantime, the number of FEN1-positive nucleoli started to decrease at 30 min after UV exposure (Fig. 3B and 4B). At 8 or 12 h, a minimum number of the nucleoli could be observed but returned to a normal maximum number at 16 h, corresponding to the basal level of phosphorylated FEN1 (Fig. 4B).
Next, we further examined the nuclear compartmental distribution changes in the total and phosphorylated fractions of FEN1 under conditions of UV exposure and found that while the total amount of FEN1 increased, it decreased in nucleoli. Similarly to the pattern of the total FEN1 level, the level of phosphorylated FEN1 increased in nuclear plasma, while it decreased in nucleoli (Fig. 4C). This implies that phosphorylated FEN1 translocates from the nucleoli to participate in DNA repair in response to UV irradiation.
Mutation of Ser187 to Asp diminishes the nucleolar localization of FEN1.
Early work indicated that there is only one in vivo phosphorylation site, serine at amino acid residue position 187 (21). The phosphorylation of Ser187 of FEN1 in late S phase is suggested to be related to the exit from the S phase of the cell cycle (21). We hypothesized that this same phosphorylation modification drives FEN1 from the nucleolus to the nuclear plasma to join the DNA damage repair foci for repair reactions. If that is the case, we should be able to alter this site in two ways. First, we can eliminate it or replace it with an alanine and determine if the elimination of the phosphorylation on the FEN1 protein will impair its translocalization from the nucleoli. Alternatively, we can replace it with an aspartate, mimicking a constant phosphorylation status (10, 11, 23, 25, 41, 62), to determine whether the FEN1 protein is no longer superaccumulated in the nucleoli. To test this, we made S187A and S187D mutants and transiently expressed them in HeLa cells. Figure 5 shows the localization of transiently expressed wild-type FEN1 and the S187D and S187A mutants in HeLa cells. The exogenously expressed FEN1 proteins were tagged with the c-myc peptide and thus were able to be distinguished from endogenous protein (Fig. 5A). Transiently expressed wild-type FEN1 in more than 95% of HeLa cells was localized in the nucleus and exhibited stronger nucleolar accumulation, as seen with endogenous FEN1. The transiently expressed S187A FEN1 protein in approximately 97% of cells had the same pattern of localization as wild-type FEN1. However, the S187D mutation apparently lost its ability for nucleolar enrichment. Less than 5% of cells had nucleolar superaccumulation of mutant FEN1 (Fig. 5B).
FIG. 5.
Mutation of Ser187 to Asp diminishes the nucleolar localization of FEN1. Wild-type (wt) FEN1 and the S187A and S187D mutants were cloned into vector pIRESneo with a c-myc tag and transfected into HeLa cells. (A) The expression of exogenous FEN1 was determined by using an anti-FEN1 antibody. (B) The localization of exogenous FEN1 in cells was detected by an anti-c-myc antibody (α-c-myc) as described in Materials and Methods.
Impairment of FEN1 nucleolar translocation decreases cell DNA repair capacity and survivorship.
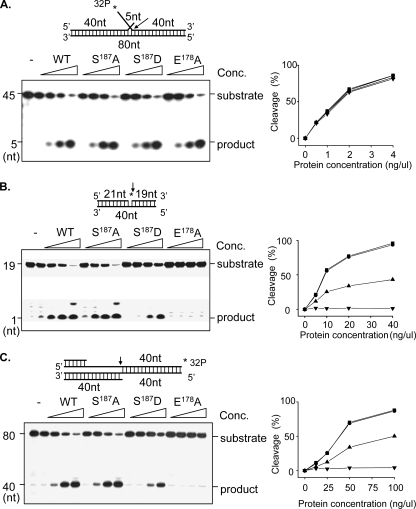
To test if the point mutation of Ser187 to Ala or Asp would affect enzyme activities or not, we purified S187A and S187D recombinant proteins, along with the wild type and the previously described E178A mutant (70), and analyzed the nuclease activity profiles of each of the proteins. In Fig. 6, we demonstrated that neither S187A nor S187D altered the FEN activity. The S187A mutation did not change the EXO or GEN activities either, while S187D exhibited severe defects in EXO as well as GEN activities.
FIG. 6.
Activities of wild-type (WT) and mutant FEN1 on double-flap, nick, and gap substrates. DNA substrates were labeled with 32P as indicated. Labeled substrates (1 pmol) were incubated with various amounts of purified wild-type or mutant FEN1s at 37°C for 20 min. (A) FEN activity on 5′-labeled double-flap substrate. (B) EXO activity on 5′-labeled nick substrate. (C) GEN activity on 3′-labeled gap substrate. In each panel, the top left shows the schematic structure of the corresponding DNA substrates. The bottom left shows the image of PAGE separating the DNA substrate and the cleavage products. The graph on the right-hand side represents the percentage of cleavage of DNA substrates at different enzyme concentrations. Symbols: ▪, wild type; •, S187A mutant; ▴, S187D mutant; ▾, E178A mutant. nt, nucleotides.
Human wild-type FEN1 and all three point mutation genes were transformed into a yeast rad27 null mutant strain, RDKY2608, using pRS314 as a yeast expression vector (70). The empty vector was also transformed into the wild-type (RDKY2672) and rad27 null mutant strains to be used as control strains. As we previously reported (50), human FEN1 has the ability to replace the UV resistance functions in a rad27 null mutant and exhibits behavior similar to that of the yeast wild-type strain. However, neither E178A (70) nor S187D cells were able to survive upon exposure to UV irradiation, similar to the rad27 null mutant cells. This is most likely because both E178A and S187D had severe defects in EXO and GEN activities, as demonstrated in our biochemical assays. Interestingly, the S187A mutation, a point mutation which failed to translocate from nucleoli to the nuclear plasma, without any defect in its nuclease activities, had severe defects in UV resistance. It displayed an intermediate phenotype between the wild-type and null mutant strains, implying that FEN1 nucleolar translocation contributes to UV DNA damage repair.
DISCUSSION
The observation that FEN1 is enriched in nucleoli is a novel finding. The nucleolus is the site for the processing of rRNAs and for their assembly into ribosomes. Not bound by a membrane, like other cellular organelles, the nucleolus is a large aggregate of macromolecules, including the rRNA genes themselves (rDNA), precursor rRNAs, mature rRNAs, rRNA-processing enzymes, snoRNPs, ribosomal protein subunits, and partly assembled ribosomes (26). Over 340 different proteins have been identified in the human nucleolus by proteomic analysis, including FEN1 (1, 55). The rDNA in nucleoli is organized as a cluster of tandem repetitive sequences. Due to its importance in ribosome biogenesis, the nucleotide sequence of this cluster needs to be maintained with a high degree of fidelity, which presumably requires the tight control of DNA replication, transcription, and repair. Many DNA replication and repair factors have been shown to localize into nucleoli, including WRN (43), BLM (67), XPG (5, 48), Rad52 (9), the Slx1-Slx4 endonuclease complex (9), and Mus81 (15). These proteins have been suggested to reside in nucleoli to participate in rDNA transcription (WRN, BLM, and XPG), homologous recombination (Rad52 and Slx1-Slx4), or DNA repair (Mus81). It is likely that FEN1, which is involved in the resolution of stalled replications (70) and homologous recombination (31), also serves a function in nucleoli.
We postulate that FEN1 may be important in maintaining the stability of rDNA replication forks based on both our own observations that FEN1 is enriched in the nucleoli of HeLa cells with a considerable increase in the presence of stalled DNA forks (Fig. 1 and 2) as well as three observations concerning the stability of rDNA replication. Firstly, Zou and Rothstein previously indicated that defects of the primase subunit cause an instability of rDNA replication forks (71). Since the defects of FEN1 during RNA primer removal result in the collision of DNA replication forks (61), we hypothesize that FEN1 may play a role at the site of rDNA replication forks similar to that at DNA replication forks. Secondly, rDNA possesses abundant tandem repeats, which may provide opportunities for the displaced RNA primers to align with downstream repeated sequences to form bubble structures. We have found that such bubble structures can be resolved by FEN1 when forming a complex with WRN (40) such that FEN1 could potentially play a role in the stability of the rDNA tandem repeats. Lastly, within rDNA repeats in the nucleolus, RFBs naturally occur in almost all eukaryotic organisms and stall replication forks (22). Studies reported previously by Weitao et al. showed that replication pausing and Holliday junction formation are accompanied by double-strand break formation at or near the RFB in yeast rDNA (65). The bubble structures that are cleaved by the GEN activity of FEN1 (70) resemble such stalled replication forks. The deficiency of the GEN activity resulting from the E176A FEN1 mutation causes an accumulation of RFB, likely due to a decreased capacity to resolve stalled replication forks in rDNA. Together, these pieces of evidence lead us to propose that FEN1 in the nucleoli processes or resolves stalled replication forks of rDNA at or near the RFBs, using the concerted action of the EXO and GEN activities, similar to what we observed with FEN1's role in the resolution of the hairpin structures derived from trinucleotide repetitive sequences (49).
Nucleoli are also likely to be utilized as a reservoir for the FEN1 protein to function in the nucleus, which explains the translocation of FEN1 out of nucleoli upon UV irradiation. UV irradiation generates DNA damage and possibly stalls DNA replication forks, which occurs at sites all over the chromosome in addition to rDNA sites. FEN1 is translocated out of nucleoli to participate in the rescue of stalled DNA replication forks and is translocated back to nucleoli after damage is repaired (Fig. 3 and 4). UV-induced phosphorylation on its unique site, Ser187, signals the protein to translocate itself from the nucleolus to the nuclear plasma for its possible functions in UV resistance. The disruption of the phosphorylation site retains the protein inside nucleoli and reduces cellular capacity in nuclear genomic repair (Fig. 5 to 7).
FIG. 7.
The S187D mutation is sensitive to UV irradiation. (A) Serial dilutions (10-fold each) of the same number of log-phase cells from the same culture were inoculated onto each of two yeast extract-peptone-dextrose plates with and without UV treatment. The plates were incubated at 30°C for 3 days. Wild-type (wt) and mutant strains are indicated. (B) The survival rate was measured as the survival of cells exposed to the UV irradiation over that of cells without exposure.
Initially, the UV resistance capacity of the FEN1 nuclease was attributed to its possible involvement in the nucleotide excision repair (NER) pathway, serving as a backup mechanism for XPG nuclease (44). However, a double deletion of rad27 (hFEN1) and rad2 (hXPG) in yeast cells did not result in a synergistic negative impact on the mutant cellular capacity of UV resistance (50). To date, there is no evidence available to indicate that FEN1 interacts with any NER component protein. With the discovery of GEN activity, we proposed that FEN1 introduces DNA double-strand breaks when the DNA replication forks are stalled by UV cross-links and therefore induces recombination repair (70). FEN1 plays an important role in the rescue of UV-induced stalling of replication forks but is not directly involved in nucleotide excision repair (70). This is consistent with the relatively slow kinetics of UV-induced phosphorylation and nucleolus export of FEN1 (Fig. 3 and 4). It has been shown that the repair of UV-induced damage by the NER pathway is relatively fast (<10 min) (46), while other repair events, including the rescue of UV-induced stalled replication forks, may require several hours (13). Alternatively, the relocalization of phosphorylated FEN1 out of nucleoli to eliminate FEN1 activity for rDNA replication may be important for the UV-induced S-phase arrest, allowing time to repair UV damages.
Several nuclease complexes have been reported to be involved in the resolution of stalled DNA replication forks induced by UV irradiation or chemicals such as camptothecin, including the Slx1-Slx4 endonuclease complex (9), the Mus81/MMS4 complex (15), topoisomerase III (28), the DNA2 helicase/nuclease (65), Metnase (36, 54), and XPF-ERCC1 (47, 63). This leads one to question, why are there so many nucleases involved in the resolution of stalled DNA replication forks? Recent findings have revealed that these endonucleases did not overlap with each other in the resolution of stalled DNA replication forks. They either work in different complexes or recognize different configurations of substrates. For example, Metnase functions on a supercoiled DNA structure, recognizing and cleaving the 5′-terminal inverted repeat element (36, 54). The Mus81/MMS4 complex cuts the leading template strand adjacent to the replication fork barrier to generate a double-strand break (28), while the GEN activity of FEN1, in complex with WRN, makes a nick on the lagging template strand near the replication fork barrier (70). Other nucleases, such as Slx1-Slx4, XPF-ERCC1, DNA2, and topoisomerase III, are likely responsible for the resolution of Holliday junctions (9), which also lead to break-induced recombination repair.
Supplementary Material
Acknowledgments
We acknowledge Brian Armstrong and his staff for their assistance in immunofluorescence microscopic experiments. We thank V. Chavez and L. D. Finger for critical review of the manuscript.
This work was supported by NIH grant R01 CA085344 to B.S.
Footnotes
Published ahead of print on 28 April 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Andersen, J. S., C. E. Lyon, A. H. Fox, A. K. Leung, Y. W. Lam, H. Steen, M. Mann, and A. I. Lamond. 2002. Directed proteomic analysis of the human nucleolus. Curr. Biol. 121-11. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, C. J., A. F. Wahl, B. Shen, M. S. Park, and R. A. Bambara. 1996. Mechanism of tracking and cleavage of adduct-damaged DNA substrates by the mammalian 5′- to 3′-exonuclease/endonuclease RAD2 homologue 1 or flap endonuclease 1. J. Biol. Chem. 27129624-29631. [DOI] [PubMed] [Google Scholar]
- 3.Blander, G., N. Zalle, Y. Daniely, J. Taplick, M. D. Gray, and M. Oren. 2002. DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J. Biol. Chem. 27750934-50940. [DOI] [PubMed] [Google Scholar]
- 4.Block, G. J., C. H. Eskiw, G. Dellaire, and D. P. Bazett-Jones. 2006. Transcriptional regulation is affected by subnuclear targeting of reporter plasmids to PML nuclear bodies. Mol. Cell. Biol. 268814-8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradsher, J., J. Auriol, L. Proietti de Santis, S. Iben, J. L. Vonesch, I. Grummt, and J. M. Egly. 2002. CSB is a component of RNA pol I transcription. Mol. Cell 10819-829. [DOI] [PubMed] [Google Scholar]
- 6.Brewer, B. J., and W. L. Fangman. 1987. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell 51463-471. [DOI] [PubMed] [Google Scholar]
- 7.Brewer, B. J., and W. L. Fangman. 1988. A replication fork barrier at the 3′ end of yeast ribosomal RNA genes. Cell 55637-643. [DOI] [PubMed] [Google Scholar]
- 8.Brewer, B. J., D. Lockshon, and W. L. Fangman. 1992. The arrest of replication forks in the rDNA of yeast occurs independently of transcription. Cell 71267-276. [DOI] [PubMed] [Google Scholar]
- 9.Coulon, S., E. Noguchi, C. Noguchi, L. L. Du, T. M. Nakamura, and P. Russell. 2006. Rad22Rad52-dependent repair of ribosomal DNA repeats cleaved by Slx1-Slx4 endonuclease. Mol. Biol. Cell 172081-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean, A. M., and D. E. Koshland, Jr. 1990. Electrostatic and steric contributions to regulation at the active site of isocitrate dehydrogenase. Science 2491044-1046. [DOI] [PubMed] [Google Scholar]
- 11.Dean, A. M., M. H. Lee, and D. E. Koshland, Jr. 1989. Phosphorylation inactivates Escherichia coli isocitrate dehydrogenase by preventing isocitrate binding. J. Biol. Chem. 26420482-20486. [PubMed] [Google Scholar]
- 12.Elias, B., A. Laine, and Z. Ronai. 2005. Phosphorylation of MdmX by CDK2/Cdc2(p34) is required for nuclear export of Mdm2. Oncogene 242574-2579. [DOI] [PubMed] [Google Scholar]
- 13.Essers, J., W. Vermeulen, and A. B. Houtsmuller. 2006. DNA damage repair: anytime, anywhere? Curr. Opin. Cell Biol. 18240-246. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich-Heineken, E., G. Henneke, E. Ferrari, and U. Hubscher. 2003. The acetylatable lysines of human Fen1 are important for endo- and exonuclease activities. J. Mol. Biol. 32873-84. [DOI] [PubMed] [Google Scholar]
- 15.Gao, H., X. B. Chen, and C. H. McGowan. 2003. Mus81 endonuclease localizes to nucleoli and to regions of DNA damage in human S-phase cells. Mol. Biol. Cell 144826-4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gary, R., K. Kim, H. L. Cornelius, M. S. Park, and Y. Matsumoto. 1999. Proliferating cell nuclear antigen facilitates excision in long-patch base excision repair. J. Biol. Chem. 2744354-4363. [DOI] [PubMed] [Google Scholar]
- 17.Gay, F., D. Calvo, M. C. Lo, J. Ceron, M. Maduro, R. Lin, and Y. Shi. 2003. Acetylation regulates subcellular localization of the Wnt signaling nuclear effector POP-1. Genes Dev. 17717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington, J. J., and M. R. Lieber. 1994. Functional domains within FEN-1 and RAD2 define a family of structure-specific endonucleases: implications for nucleotide excision repair. Genes Dev. 81344-1355. [DOI] [PubMed] [Google Scholar]
- 19.Hasan, S., M. Stucki, P. O. Hassa, R. Imhof, P. Gehrig, P. Hunziker, U. Hubscher, and M. O. Hottiger. 2001. Regulation of human flap endonuclease-1 activity by acetylation through the transcriptional coactivator p300. Mol. Cell 71221-1231. [DOI] [PubMed] [Google Scholar]
- 20.Henneke, G., E. Friedrich-Heineken, and U. Hubscher. 2003. Flap endonuclease 1: a novel tumour suppresser protein. Trends Biochem. Sci. 28384-390. [DOI] [PubMed] [Google Scholar]
- 21.Henneke, G., S. Koundrioukoff, and U. Hubscher. 2003. Phosphorylation of human Fen1 by cyclin-dependent kinase modulates its role in replication fork regulation. Oncogene 224301-4313. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez, P., L. Martin-Parras, M. L. Martinez-Robles, and J. B. Schvartzman. 1993. Conserved features in the mode of replication of eukaryotic ribosomal RNA genes. EMBO J. 121475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinchliffe, K. A., A. Ciruela, A. J. Letcher, N. Divecha, and R. F. Irvine. 1999. Regulation of type IIalpha phosphatidylinositol phosphate kinase localisation by the protein kinase CK2. Curr. Biol. 9983-986. [DOI] [PubMed] [Google Scholar]
- 24.Huang, L., J. A. Rumbaugh, R. S. Murante, R. J. Lin, L. Rust, and R. A. Bambara. 1996. Role of calf RTH-1 nuclease in removal of 5′-ribonucleotides during Okazaki fragment processing. Biochemistry 359266-9277. [DOI] [PubMed] [Google Scholar]
- 25.Huang, W., and R. L. Erikson. 1994. Constitutive activation of Mek1 by mutation of serine phosphorylation sites. Proc. Natl. Acad. Sci. USA 918960-8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt, T., and J. Wilson. 2002. Molecular biology of the cell, 4th ed. Garland Science Publishing, New York, NY.
- 27.Kaiser, M. W., N. Lyamicheva, W. Ma, C. Miller, B. Neri, L. Fors, and V. I. Lyamichev. 1999. A comparison of eubacterial and archaeal structure-specific 5′-exonucleases. J. Biol. Chem. 27421387-21394. [DOI] [PubMed] [Google Scholar]
- 28.Kaliraman, V., J. R. Mullen, W. M. Fricke, S. A. Bastin-Shanower, and S. J. Brill. 2001. Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev. 152730-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao, H. I., L. A. Henricksen, Y. Liu, and R. A. Bambara. 2002. Cleavage specificity of Saccharomyces cerevisiae flap endonuclease 1 suggests a double-flap structure as the cellular substrate. J. Biol. Chem. 27714379-14389. [DOI] [PubMed] [Google Scholar]
- 30.Kardinal, C., M. Dangers, A. Kardinal, A. Koch, D. T. Brandt, T. Tamura, and K. Welte. 2006. Tyrosine phosphorylation modulates binding preference to cyclin-dependent kinases and subcellular localization of p27Kip1 in the acute promyelocytic leukemia cell line NB4. Blood 1071133-1140. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi, K., Y. Taniguchi, A. Hatanaka, E. Sonoda, H. Hochegger, N. Adachi, Y. Matsuzaki, H. Koyama, D. C. van Gent, M. Jasin, and S. Takeda. 2005. Fen-1 facilitates homologous recombination by removing divergent sequences at DNA break ends. Mol. Cell. Biol. 256948-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitao, H., and Z. M. Yuan. 2002. Regulation of ionizing radiation-induced Rad52 nuclear foci formation by c-Abl-mediated phosphorylation. J. Biol. Chem. 27748944-48948. [DOI] [PubMed] [Google Scholar]
- 33.Klungland, A., and T. Lindahl. 1997. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1). EMBO J. 163341-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klungland, A., I. Rosewell, S. Hollenbach, E. Larsen, G. Daly, B. Epe, E. Seeberg, T. Lindahl, and D. E. Barnes. 1999. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. USA 9613300-13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kucherlapati, M., K. Yang, M. Kuraguchi, J. Zhao, M. Lia, J. Heyer, M. F. Kane, K. Fan, R. Russell, A. M. Brown, B. Kneitz, W. Edelmann, R. D. Kolodner, M. Lipkin, and R. Kucherlapati. 2002. Haploinsufficiency of flap endonuclease (FEN-1) leads to rapid tumor progression. Proc. Natl. Acad. Sci. USA 999924-9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, S. H., M. Oshige, S. T. Durant, K. K. Rasila, E. A. Williamson, H. Ramsey, L. Kwan, J. A. Nickoloff, and R. Hromas. 2005. The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc. Natl. Acad. Sci. USA 10218075-18080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieber, M. R. 1997. The FEN-1 family of structure-specific nucleases in eukaryotic DNA replication, recombination and repair. Bioessays 19233-240. [DOI] [PubMed] [Google Scholar]
- 38.Linskens, M. H., and J. A. Huberman. 1988. Organization of replication of ribosomal DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 84927-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lischwe, M. A., R. L. Richards, R. K. Busch, and H. Busch. 1981. Localization of phosphoprotein C23 to nucleolar structures and to the nucleolus organizer regions. Exp. Cell Res. 136101-109. [DOI] [PubMed] [Google Scholar]
- 40.Liu, R., J. Qiu, L. D. Finger, L. Zheng, and B. Shen. 2006. The DNA-protein interaction modes of FEN-1 with gap substrates and their implication in preventing duplication mutations. Nucleic Acids Res. 341772-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maciejewski, P. M., F. C. Peterson, P. J. Anderson, and C. L. Brooks. 1995. Mutation of serine 90 to glutamic acid mimics phosphorylation of bovine prolactin. J. Biol. Chem. 27027661-27665. [DOI] [PubMed] [Google Scholar]
- 42.Maga, G., G. Villani, V. Tillement, M. Stucki, G. A. Locatelli, I. Frouin, S. Spadari, and U. Hubscher. 2001. Okazaki fragment processing: modulation of the strand displacement activity of DNA polymerase delta by the concerted action of replication protein A, proliferating cell nuclear antigen, and flap endonuclease-1. Proc. Natl. Acad. Sci. USA 9814298-14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marciniak, R. A., D. B. Lombard, F. B. Johnson, and L. Guarente. 1998. Nucleolar localization of the Werner syndrome protein in human cells. Proc. Natl. Acad. Sci. USA 956887-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsumoto, Y. 2001. Molecular mechanism of PCNA-dependent base excision repair. Prog. Nucleic Acid Res. Mol. Biol. 68129-138. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki, H., A. Ichino, T. Hayashi, T. Yamamoto, and U. Kikkawa. 2005. Regulation of intracellular localization and transcriptional activity of FOXO4 by protein kinase B through phosphorylation at the motif sites conserved among the FOXO family. J. Biochem. (Tokyo) 138485-491. [DOI] [PubMed] [Google Scholar]
- 46.Meldrum, R. A., W. S. Meaking, and C. W. Wharton. 1994. The kinetics and mechanism of repair of UV induced DNA damage in mammalian cells. The use of ‘caged’ nucleotides and electroporation to study short time course events in DNA repair. Nucleic Acids Res. 221234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park, C. H., T. Bessho, T. Matsunaga, and A. Sancar. 1995. Purification and characterization of the XPF-ERCC1 complex of human DNA repair excision nuclease. J. Biol. Chem. 27022657-22660. [DOI] [PubMed] [Google Scholar]
- 48.Park, M. S., J. A. Knauf, S. H. Pendergrass, C. H. Coulon, G. F. Strniste, B. L. Marrone, and M. A. MacInnes. 1996. Ultraviolet-induced movement of the human DNA repair protein, Xeroderma pigmentosum type G, in the nucleus. Proc. Natl. Acad. Sci. USA 938368-8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parrish, J. Z., C. Yang, B. Shen, and D. Xue. 2003. CRN-1, a Caenorhabditis elegans FEN-1 homologue, cooperates with CPS-6/EndoG to promote apoptotic DNA degradation. EMBO J. 223451-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu, J., M. X. Guan, A. M. Bailis, and B. Shen. 1998. Saccharomyces cerevisiae exonuclease-1 plays a role in UV resistance that is distinct from nucleotide excision repair. Nucleic Acids Res. 263077-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu, J., X. Li, G. Frank, and B. Shen. 2001. Cell cycle-dependent and DNA damage-inducible nuclear localization of FEN-1 nuclease is consistent with its dual functions in DNA replication and repair. J. Biol. Chem. 2764901-4908. [DOI] [PubMed] [Google Scholar]
- 52.Reagan, M. S., C. Pittenger, W. Siede, and E. C. Friedberg. 1995. Characterization of a mutant strain of Saccharomyces cerevisiae with a deletion of the RAD27 gene, a structural homolog of the RAD2 nucleotide excision repair gene. J. Bacteriol. 177364-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynaldo, L. P., A. V. Vologodskii, B. P. Neri, and V. I. Lyamichev. 2000. The kinetics of oligonucleotide replacements. J. Mol. Biol. 297511-520. [DOI] [PubMed] [Google Scholar]
- 54.Roman, Y., M. Oshige, Y. J. Lee, K. Goodwin, M. M. Georgiadis, R. A. Hromas, and S. H. Lee. 2007. Biochemical characterization of a SET and transposase fusion protein, Metnase: its DNA binding and DNA cleavage activity. Biochemistry 4611369-11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scherl, A., Y. Coute, C. Deon, A. Calle, K. Kindbeiter, J. C. Sanchez, A. Greco, D. Hochstrasser, and J. J. Diaz. 2002. Functional proteomic analysis of human nucleolus. Mol. Biol. Cell 134100-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen, B., J. Qui, D. J. Hosfield, and J. A. Tainer. 1998. Flap endonuclease homologues identified in archaebacteria exist as independent proteins. Trends Biochem. Sci. 23171-173. [DOI] [PubMed] [Google Scholar]
- 57.Shen, B., P. Singh, R. Liu, J. Qiu, L. Zheng, L. D. Finger, and S. Alas. 2005. Multiple but dissectible functions of FEN-1 nucleases in nucleic acid processing, genome stability and diseases. Bioessays 27717-729. [DOI] [PubMed] [Google Scholar]
- 58.Singh, P., L. Zheng, V. Chavez, J. Qiu, and B. Shen. 2007. Concerted action of exonuclease and Gap-dependent endonuclease activities of FEN-1 contributes to the resolution of triplet repeat sequences (CTG)n- and (GAA)n-derived secondary structures formed during maturation of Okazaki fragments. J. Biol. Chem. 2823465-3477. [DOI] [PubMed] [Google Scholar]
- 59.Stein, G. S., S. K. Zaidi, C. D. Braastad, M. Montecino, A. J. van Wijnen, J. Y. Choi, J. L. Stein, J. B. Lian, and A. Javed. 2003. Functional architecture of the nucleus: organizing the regulatory machinery for gene expression, replication and repair. Trends Cell Biol. 13584-592. [DOI] [PubMed] [Google Scholar]
- 60.Thevenet, L., C. Mejean, B. Moniot, N. Bonneaud, N. Galeotti, G. Aldrian-Herrada, F. Poulat, P. Berta, M. Benkirane, and B. Boizet-Bonhoure. 2004. Regulation of human SRY subcellular distribution by its acetylation/deacetylation. EMBO J. 233336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tishkoff, D. X., N. Filosi, G. M. Gaida, and R. D. Kolodner. 1997. A novel mutation avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell 88253-263. [DOI] [PubMed] [Google Scholar]
- 62.Trautwein, C., C. Caelles, P. van der Geer, T. Hunter, M. Karin, and M. Chojkier. 1993. Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature 364544-547. [DOI] [PubMed] [Google Scholar]
- 63.Tsodikov, O. V., J. H. Enzlin, O. D. Scharer, and T. Ellenberger. 2005. Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF-ERCC1. Proc. Natl. Acad. Sci. USA 10211236-11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wadman, I. A., H. Osada, G. G. Grutz, A. D. Agulnick, H. Westphal, A. Forster, and T. H. Rabbitts. 1997. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 163145-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weitao, T., M. Budd, L. L. Hoopes, and J. L. Campbell. 2003. Dna2 helicase/nuclease causes replicative fork stalling and double-strand breaks in the ribosomal DNA of Saccharomyces cerevisiae. J. Biol. Chem. 27822513-22522. [DOI] [PubMed] [Google Scholar]
- 66.Xie, Y., Y. Liu, J. L. Argueso, L. A. Henricksen, H.-I. Kao, R. A. Bambara, and E. Alani. 2001. Identification of rad27 mutations that confer differential defects in mutation avoidance, repeat tract instability, and flap cleavage. Mol. Cell. Biol. 214889-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yankiwski, V., R. A. Marciniak, L. Guarente, and N. F. Neff. 2000. Nuclear structure in normal and Bloom syndrome cells. Proc. Natl. Acad. Sci. USA 975214-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao, L. J., T. Subramanian, Y. Zhou, and G. Chinnadurai. 2006. Acetylation by p300 regulates nuclear localization and function of the transcriptional corepressor CtBP2. J. Biol. Chem. 2814183-4189. [DOI] [PubMed] [Google Scholar]
- 69.Zheng, L., H. Dai, M. Zhou, M. Li, P. Singh, J. Qiu, W. Tsark, Q. Huang, K. Kernstine, X. Zhang, D. Lin, and B. Shen. 2007. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat. Med. 13812-819. [DOI] [PubMed] [Google Scholar]
- 70.Zheng, L., M. Zhou, Q. Chai, J. Parrish, D. Xue, S. M. Patrick, J. J. Turchi, S. M. Yannone, D. Chen, and B. Shen. 2005. Novel function of the flap endonuclease 1 complex in processing stalled DNA replication forks. EMBO Rep. 683-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou, H., and R. Rothstein. 1997. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell 9087-96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.