Abstract
Objective
Use the meta-analytic approach to examine the effects of aerobic exercise on total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) in children and adolescents.
Study design
Randomized controlled trials which were limited to aerobic exercise ≥4 weeks in children and adolescents 5–19 years of age.
Results
Twelve outcomes representing 389 subjects were available for pooling. Using random-effects modeling, a trend for statistically significant decreases of 12% was found for TG (X̄ ± S.E.M., −11.0 ± 6.1 mg/dl; 95% CI, −22.8–0.8 mg/dl) with no statistically significant changes for TC, HDL-C, and LDL-C. Decreases in LDL-C were associated with increased training intensity (r = −0.89; 99% CI, −0.99 to −0.04) and older age (r = −0.90; 99% CI, −0.99 to −0.25) while increases in HDL-C were associated with lower initial HDL-C (r = −0.75; 99% CI, −0.94 to −0.80). Statistically significant decreases in TG were observed in overweight/obese subjects with a trend for increases in HDL-C (TG, X̄ ± S.E.M., −23.9 ± 7.0 mg/dl; 95% CI, −37.6 to −10.1 mg/dl; HDL-C, X̄ ± S.E.M., 4.0 ± 2.3 mg/dl; 95% CI, −0.5–8.5 mg/dl).
Conclusions
Aerobic exercise decreases TG in overweight/obese children and adolescents.
Keywords: Children, Adolescents, Exercise, Cholesterol, Lipids, Meta-analysis, Systematic review
1. Introduction
The American Academy of Pediatrics has concluded that epidemiologic and experimental evidence has shown that elevated cholesterol levels during childhood and adolescence increases the risk for future coronary heart disease from elevated cholesterol levels as adults, although the precise risk is unknown [1]. One approach for maintaining optimal cholesterol levels in children and adolescents is exercise, a low-cost, nonpharmacologic intervention that is available to the vast majority of children and adolescents [2]. Unfortunately, previous randomized controlled trials that have examined the effects of aerobic exercise on lipids and lipoproteins in children and/or adolescents have led to conflicting results with only 25% of total cholesterol (TC) outcomes, 33% of high-density lipoprotein (HDL-C) outcomes, 22% of low-density lipoprotein outcomes (LDL-C), and 9% of triglyceride (TG) outcomes reported as statistically significant [3–14]. However, counting the number and percentage of statistically significant results is a method that has been shown to be less valid than the meta-analytic approach [15], an approach that has not been previously used to examine the effects of aerobic exercise on lipids and lipoproteins in children and adolescents. Therefore, the purpose of this study was to use the meta-analytic approach to examine the effects of aerobic exercise on lipids and lipoproteins in children and adolescents.
2. Methods
2.1. Data sources
Studies were retrieved via (1) electronic database searches (MEDLINE, EMBASE, Current Contents, Sport Discus, Dissertation Abstracts International), (2) cross-referencing from original and review articles, (3) hand searching, and (4) expert review of our reference list (Dr. Bernard Gutin, personal communication). The two major key words used in our searches included exercise and cholesterol. We used a broad versus focused search strategy in order minimize the inability to locate studies that might meet our inclusion criteria.
2.2. Study selection
The inclusion criteria for this study were as follows: (1) randomized controlled trials, (2) aerobic exercise of at least 4 weeks as the intervention (no diet intervention), (3) children and adolescents 5–19 years of age, (4) studies published in journal, dissertation, or Master's thesis format, (5) studies published in the English-language, (6) studies published and indexed between January 1, 1955 and January 1, 2005, and (7) assessment of one or more of the following lipids and lipoproteins in the apparently fasting state: TC, HDL-C, LDL-C, TG. We limited our studies to randomized controlled trials because it is the only way to control for confounders that are not known or measured as well as the observation that non-randomized controlled trials tend to overestimate the effects of healthcare [16,17]. Multiple publication bias (the tendency for data on the same subjects to be reported more than once in different studies) was avoided by inspecting each study for duplication. We chose 1955 as the starting date because it appears to have been the first time that an exercise and lipid and lipoprotein intervention study was conducted and published [18]. We did not include studies from foreign language journals because of cost as well as our concern about the potential for error in the translation and interpretation of findings. All studies were selected by both authors. Discrepancies were resolved by consensus.
2.3. Data abstraction
Coding sheets that could hold 368 items from each study were developed. All studies were coded by both authors, independent of each other. Discrepancies were resolved by consensus. Cohen's kappa [19] for inter-rater agreement prior to correcting discrepant items was 0.91.
2.4. Statistical analysis
Changes in TC, HDL-C, LDL-C and TG were calculated by taking the change outcome difference (final minus initial) between the exercise and control groups (exercise minus control) for each group from each study. In order to enhance interpretation for a wider range of readers, we reported all lipid and lipoprotein data in milligrams per deciliter (mg/dl) versus millimoles. All pooled outcomes were weighted by the inverse of the variance. Ninety-five percent confidence intervals (95% CIs) were used to establish the significance of our findings. A random-effects model, which controls for statistical heterogeneity, was used for all analyses [20]. These same approaches were used in the analysis of our secondary outcomes, i.e., changes in body weight, percent body fat, and maximum oxygen consumption (VO2max in ml kg−1 min−1). Publication bias was examined using the regression approach of Egger et al. [21]. Cumulative meta-analysis, ranked by year, was performed in order to examine the consistency of our results over time [22]. Study quality, ranging from a low of 0 to a high of 5, was assessed using a previously developed index that has been shown to be both valid and reliable [23]. Sensitivity analysis was conducted by deleting each study from the model once in order to see if any one study had a statistically significant influence on our overall results. In addition, we examined the sensitivity of our lipid and lipoprotein outcomes by limiting our analysis, based on the availability of data, to (1) studies conducted in the United States (country bias) [24], (2) studies published in peer-reviewed journals (publication bias) [25], (3) studies in which all subjects were overweight and/or obese, (4) studies in which all subjects had Type 1 diabetes, (5) studies that reported no exercise at least 24 h prior to lipid assessment, and (6) studies that reported no prior participation in the physical activity intervention. Based on the availability of data, we also conducted simple, least squares regression analyses (method of moments approach) [26] in order to examine the association between changes in our primary outcomes (TC, HDL-C, LDL-C, and TG) and study quality, age, initial lipid and lipoprotein levels, initial percent body fat, changes in body weight, percent body fat, and VO2max in ml kg−1 min−1, as well as the length, frequency, intensity, duration, and total minutes of training. We were unable to conduct any type of multiple regression analysis because of missing data for different variables from different studies. Because of the large number of simple regression tests conducted, we used 99% confidence intervals to establish the statistical significance of our results.
Descriptive statistics are reported as mean ± standard deviation (X̄ ± S.D.) while primary and secondary outcomes are reported as mean ± standard error of the mean (X̄ ± S.E.M.). Study quality was reported using the median. Data were analyzed using Stata SE (version 8.2) [27] and SPSS (version 13.0) [28].
3. Results
3.1. Study characteristics
Of the more than 3000 studies reviewed, a total of 12 met our criteria for inclusion [3–14]. All of the studies appeared to use an analysis-by-protocol approach in the analysis of their data [3–14], with one study apparently having no dropouts [10]. A total of 25 groups (13 exercise, 12 usual care controls) representing initial and final assessment in 389 subjects (211 exercise, 178 control) were included in the analysis. The percentage of subjects that started the study but were not available for follow-up assessment ranged from 0 to 65% in the exercise groups (X̄ ± S.D., 16 ± 21%) and 0–61% in the control groups (X̄ ± S.D., 16 ± 19%). Study quality ranged from 1 to 2 (median = 2).
3.2. Subject characteristics
Initial characteristics of the subjects are shown in Table 1. Seven of the studies (58%) included both males and females [3–6,8–10], four were limited to males [7,11–13], and one was limited to females [14]. For those studies that reported information on race and ethnicity, one reported that subjects included Asians, Hispanics, and Whites [7], another reported the inclusion of Asians, Blacks, and Whites [8], and another reported that all subjects were White [11]. Three studies reported that all subjects were prepubescent [5,9,12] while another reported that all subjects were postpubescent [3]. Four studies reported that none of the subjects were taking any type of medication(s) [6,7,11,13]. Another four studies reported that none of the subjects smoked [3,6,7,12] while one reported that none consumed alcohol [7]. Seven studies reported that none of the subjects participated in any prior physical activity that was similar to the intervention [3,4,6–8,12,13]. One study reported dietary habits during the study that may have affected changes in lipids and lipoproteins [5]. Two studies reported that all subjects were overweight or obese [8,10] while another two reported that all subjects had Type 1 diabetes [5,6].
Table 1.
Initial characteristics of subjects
| Variable | N | Exercise | N | Control | ||
|---|---|---|---|---|---|---|
| X̄ ± S.D. | Range | X̄ ± S.D. | Range | |||
| Age (years) | 12 | 11 ± 3 | 8–16 | 11 | 12 ± 3 | 9–16 |
| Height (cm) | 13 | 148 ± 13 | 128–169 | 12 | 150 ± 12 | 133–174 |
| Body weight (kg) | 13 | 47 ± 15 | 27–75 | 12 | 49 ± 14 | 31–74 |
| Body fat (%) | 7 | 27 ± 12 | 15–45 | 6 | 27 ± 14 | 16–45 |
| VO2max (ml kg−1 min−1) | 9 | 42 ± 12 | 22–59 | 8 | 40 ± 11 | 23–61 |
| TC (mg/dl) | 12 | 163 ± 17 | 133–198 | 11 | 167 ± 22 | 137–213 |
| HDL-C (mg/dl) | 12 | 48 ± 11 | 38–67 | 11 | 46 ± 9 | 33–65 |
| LDL-C (mg/dl) | 9 | 95 ± 17 | 61–121 | 8 | 101 ± 16 | 71–125 |
| TG (mg/dl) | 11 | 81 ± 24 | 50–121 | 10 | 82 ± 18 | 55–122 |
Notes: N, number of groups reporting data; X̄ ± S.D., mean ± standard deviation; Range represents group ranges; BMI, body mass index; VO2max, maximum oxygen consumption; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
3.3. Lipid assessment characteristics
Lipids and lipoproteins were assessed in the morning after an overnight fast that ranged between 8 and 12 h (X̄ ± S.D., 11.7 ± 0.7 h). The number of hours that exercise was avoided prior to the assessment of lipids and lipoproteins ranged from 24 to 72 h (X̄ ± S.D., 31.2 ± 16.1 h) for the four studies that reported this information [3,6,12,13].
3.4. Training program characteristics
For those studies that reported data, the length of training ranged from 5 to 16 weeks (X̄ ± S.D., 10.7 ± 3.2), frequency from 3 to 5 times per week (X̄ ± S.D., 3.7 ± 0.8), intensity from 44% to 90% of VO2max (X̄ ± S.D., 74.7 ± 11.8), and duration from 20 to 60 min per session (X̄ ± S.D., 35.0 ± 12.5). Total minutes of training (length × frequency × duration) ranged from 480 to 2560 min (X̄ ± S.D., 1482.5 ± 679.4). Compliance, defined as the percentage of exercise sessions attended, ranged from 80 to 100% (X̄ ± S.D., 91.5 ± 7.4). While a variety of supervised aerobic activities were used, the most common included walking, jogging, stationary cycling, and various movements to music.
3.5. Primary and secondary outcomes
3.5.1. Primary outcomes
A description of our lipid and lipoprotein results, adjusted for control group changes, is shown in Table 2 as well as Figs. 1–4. While no statistically significant changes were observed for TC, HDL-C, or LDL-C, there was a trend for statistically significant decreases of approximately 12% for TG. Cumulative meta-analysis, ranked by year, showed that results have continued to remain nonsignificant throughout the years for TC, HDL-C, and LDL-C. However, there has been a trend over the years for changes in TG to approach statistical significance. With each study deleted from the model once, results remained statistically nonsignificant for TC, HDL-C, and LDL-C. However, changes in TG were statistically significant when the study by Blessing et al. (X̄ ± S.E.M., −12.5 ± 4.9 mg/dl; 95% CI, −23.7 to −1.3 mg/dl) [3] and Ferguson et al. (X̄ ± S.E.M., −7.0 ± 3.4 mg/dl; 95% CI, −0.3 to −13.6 mg/dl) [8] were deleted from the model as well as when the high-intensity training group in the study by Savage et al. [12] was deleted (X̄ ± S.E.M., −12.8 ± 3.4 mg/dl; 95% CI, −0.3 to −25.3 mg/dl). When limited to subjects who were overweight or obese [8,10] a statistically significant reduction in TG was found (X̄ ± S.E.M., −23.9 ± 7.0 mg/dl; 95% CI, −37.6 to −10.1 mg/dl), as was a trend for statistically significant increases in HDL-C (X̄ ± S.E.M., 4.0 ± 2.3 mg/dl; 95% CI, −0.5−8.5 mg/dl). Similar to our overall results, there was a trend for statistically significant reductions in TG when we limited our analysis to the two studies in which all subjects had Type 1 diabetes (X̄ ± S.E.M., −9.4 ± 5.6 mg/dl; 95% CI, −20.4−1.6 mg/dl) [5,6] but not when studies were limited to those in which there was no prior participation in the physical activity intervention (X̄ ± S.E.M., −14.2 ± 8.4 mg/dl; 95% CI, −30.5−2.2 mg/dl) [3,4,6–8,12]. Statistically significant decreases in TC were found when results were limited to those studies that reported that subjects refrained from exercise for at least 24 h prior to the assessment of lipids (X̄ ± S.E.M., −8.4 ± 3.1 mg/dl; 95% CI, −14.5 to −2.3 mg/dl) [3,6,12,13]. No other statistically significant or clinically relevant differences were observed for any of our other sensitivity analyses (95% CI crossed 0 for all).
Table 2.
Primary and secondary outcomes
| Variable | N | X̄ ± S.E.M. | 95% CI |
|---|---|---|---|
| Primary outcomes (mg/dl) | |||
| TC | 12 | −0.6 ± 2.0 | −4.4 to 3.3 |
| HDL-C | 12 | −1.4 ± 1.7 | −4.8 to 1.9 |
| LDL-C | 9 | 1.2 ± 2.8 | −4.3 to 6.7 |
| TG | 11 | −11.0 ± 6.1 | −22.8 to 0.8 |
| Secondary outcomes | |||
| Body weight (kg) | 10 | 0.31 ± 0.3 | −0.31 to 0.92 |
| Body fat (%) | 6 | −2.0 ± 0.5 | −3.0 to −1.1a |
| VO2max (ml kg−1 min−1) | 9 | 3.0 ± 0.9 | 1.2 to 4.8a |
N, number of groups reporting data; X̄ ± S.E.M., mean ± standard error of the mean; CI, confidence interval; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; VO2 max, maximum oxygen consumption.
Significantly different from zero (0).
Fig. 1.
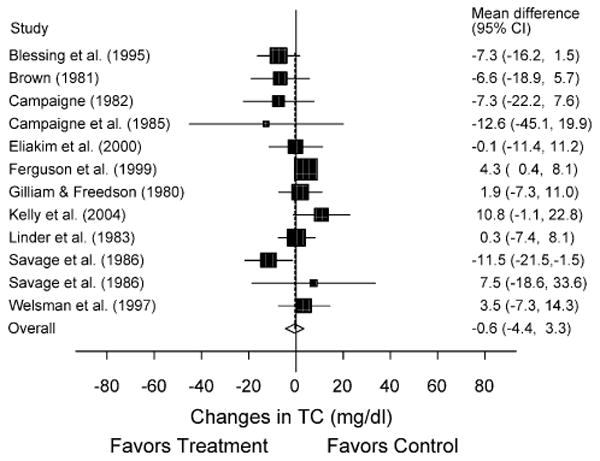
Forest plot for changes in TC. The black boxes, sized relative to random-effects weighting, represent the mean change in TC for each study while the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in TC across all listed studies while the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Fig. 4.
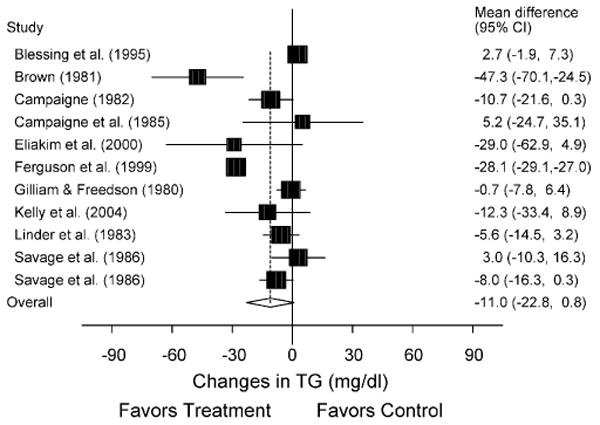
Forest plot for changes in TG. The black boxes, sized relative to random-effects weighting, represent the mean change in TG for each study while the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in TG across all listed studies while the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Greater increases in HDL-C were associated with lower initial levels of HDL-C (r = −0.75; 99% CI, −0.94 to −0.80). In addition, greater decreases in LDL-C were associated with older age (r = −0.90; 99% CI, −0.99 to −0.25) as well as higher levels of training intensity (r = −0.89; 99% CI, −0.99 to −0.04). No other statistically significant relationships were observed (99% CI crossed 0 for all).
3.5.2. Secondary outcomes
As can be seen in Table 2, there were no statistically significant changes in body weight. However, there was a statistically significant reduction of 7% in percent body fat as well as a statistically significant increase of 7% for VO2max in ml kg−1 min−1.
4. Discussion
The overall results of this study suggest that short-term aerobic exercise does not improve TC, HDL-C, and LDL-C in children and adolescents (all 95% CIs crossed zero). However, there was a trend for statistically significant reductions in TG which were independent of changes in body weight and percent body fat. Similar results were found when we limited our analysis to studies in which all subjects had Type 1 diabetes [5,6]. Reductions in TG did reach statistical significance when three studies were independently deleted from the model [3,8,12] as well as when studies were limited to overweight and obese subjects [8,10]. The antagonistic results for TG in relation to the Ferguson et al. study [8] most likely reflect the differential weighting of this study across the two different types of analyses. In addition to our TG findings, there was also a trend for statistically significant increases in HDL-C in overweight and obese subjects. The statistically significant reductions in TG among overweight and obese children and adolescents may be especially important given the increasing prevalence of overweight and obesity among children, adolescents, and adults [29,30], as well as the fact that elevated TG levels are an independent risk factor for coronary heart disease in adults [31]. The former notwithstanding, our analysis was limited to only two studies in which all children and adolescents were overweight or obese [8,10]. Given the above, it would appear plausible to suggest that a need exists for additional randomized controlled trials in this population.
We found several potentially important relationships in this study. These included (1) an increase in HDL-C with lower initial levels of HDL-C, (2) greater decreases in LDL-C with older age, and (3) greater decreases in LDL-C with higher levels of training intensity. While these results are interesting, they should be considered exploratory in nature. Furthermore, these findings, like any correlation that deviates from 1, could be a statistical (regression toward the mean) versus physiological phenomenon [32].
With the exception of changes in TG, our findings for TC, HDL-C, and LDL-C were generally unimpressive. Overall, our findings are consistent with previous narrative reviews on this topic. For example, Linder and Durant concluded that intervention studies have failed to demonstrate that aerobic exercise alters lipid and lipoprotein levels in children and adolescents [33] while Haskell concluded that most aerobic training studies in children and adolescents did not result in any changes in lipids and lipoproteins [34]. In addition, Vaccaro and Mahon [35] as well as Despres et al. [36] concluded that because of conflicting information from intervention studies, no recommendation could be made regarding the feasibility of exercise for improving lipids and lipoproteins in children and adolescents. More recently, Tolfrey et al. concluded that the majority of intervention studies have suggested that regular aerobic exercise has little, if any, influence on the lipid and lipoprotein levels of children and adolescents [37]. The strength of our study over previous narrative reviews was the use of a more quantitative approach (meta-analysis) in order to provide more definitive information regarding the magnitude of change in selected lipids and lipoproteins as a result of aerobic exercise in children and adolescents as well as an examination of potential associations between changes in lipids and lipoproteins and selected characteristics. The strength of our meta-analysis over the individual studies themselves provided the opportunity to increase estimates of treatment effectiveness as well as power for our primary endpoints [38]. For example, since TG have high day-to-day variability, the added power of our meta-analysis may have uncovered the important relationship that we observed between aerobic exercise and changes in TG.
While our overall changes in lipids and lipoproteins among children and adolescents were generally modest, aerobic exercise should almost always be recommended because of the numerous other benefits that can be derived from such. For example, the fact that we found statistically significant decreases in percent body fat as well as increases in VO2max in ml kg−1 min−1 lends support for the importance of aerobic exercise for the overall health of children and adolescents. In addition, participation in aerobic exercise improves overall cardiovascular risk and may prevent risk factor acquisition over time [39]. More specifically, the benefits of aerobic exercise may be the result of its independent protective effect on event rates and inflammatory markers as well as its usefulness in the prevention of the multiple risk phenotype or metabolic syndrome and hypertension. These positive benefits should be obtainable by adhering to the recent physical activity guidelines for children and adolescents as set forth by the United States Department of Health and Human Services and the United States Department of Agriculture [40]. This includes participation in at least 60 min of moderate intensity physical activity on most days of the week, preferably daily.
While the results of our meta-analysis provide important information, they need to be considered with respect to the following limitations. First, a common problem with all reviews, meta-analytic or otherwise, is missing data. For example, we were unable to conduct any type of multiple regression analysis because of missing data for different variables from different studies. Consequently, we chose to conduct a number of simple regression tests in order to maximize the amount of data that was available. However, as a result of this approach, we were unable to control for potential confounders such as the potential for differential changes in dietary behavior between exercise and control groups. In addition, we increased our risk for conducting a Type I error despite using a more stringent confidence level (99%).
Less than half the studies (46%) reported data on compliance of the subjects to the exercise protocol. Since this could affect changes in lipids and lipoproteins, it is strongly suggested that future studies include this information. In addition, only four studies reported that subjects refrained from exercise for at least 24 h prior to the assessment of lipids [3,6,12,13]. Since this may affect lipid and lipoprotein levels, it would appear plausible to suggest that future studies have subjects refrain from exercise for at least 24 h prior to lipid assessment and that the number of hours that exercise is avoided be reported.
Ideally, many of the limitations of our current meta-analysis could be overcome by conducting an individual-patient data (IPD) versus summary means meta-analysis. However, this must be countered with the difficulty in obtaining IPD from investigators. For example, in our previous meta-analytic research dealing with the effects of exercise on bone mineral density in adults we were only able to obtain IPD from approximately 29 of 76 (38%) eligible studies [41].
In conclusion, the results of our study suggest that aerobic exercise decreases TG in overweight and obese children and adolescents. In addition, decreases in LDL-C are associated with increased training intensity and older age while increases in HDL-C are associated with lower initial HDL-C. However, these associations need to be tested further in large, well-designed, randomized controlled trials.
Fig. 2.
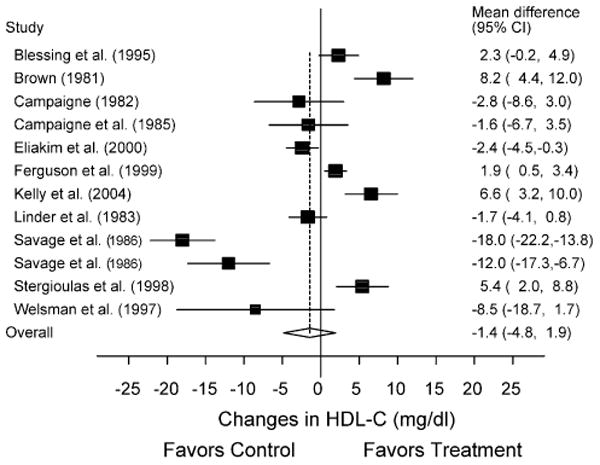
Forest plot for changes in HDL-C. The black boxes, sized relative to random-effects weighting, represent the mean change in HDL-C for each study while the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in HDL-C across all listed studies while the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Fig. 3.
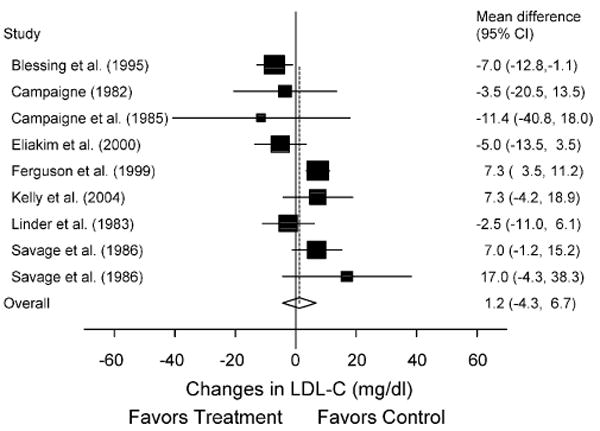
Forest plot for changes in LDL-C. The black boxes, sized relative to random-effects weighting, represent the mean change in LDL-C for each study while the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in LDL-C across all listed studies while the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Acknowledgments
The authors would like to thank Bernard Gutin, Ph.D., Medical College of Georgia, for reviewing our reference list. This study was supported by a grant from the National Institutes of Health-National Heart, Lung and Blood Institute, Award #R01-HL069802 (G.A. Kelley, Principal Investigator).
References
- 1.American Academy of Pediatrics. Cholesterol in childhood (RE9805) Pediatrics. 1998;101:141–7. [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. NIH Publication No 91–2732. National Institutes of Health; 1991. National cholesterol education program: report of the expert panel on blood cholesterol levels in children and adolescents. [Google Scholar]
- 3.Blessing DL, Keith RE, Williford HN, et al. Blood lipid and physiological responses to endurance training in adolescents. Pediatr Exerc Sci. 1995;7:192–202. [Google Scholar]
- 4.Brown MK. The effects of diet and exercise on selected coronary risk factors in children. Provo, Utah: Brigham Young University; 1981. dissertation. [Google Scholar]
- 5.Campaigne BN. The effects of a physical activity program on children ages 5 to 11 years with insulin-dependent diabetes mellitus. The University of Michigan; 1982. dissertation. [Google Scholar]
- 6.Campaigne BN, Landt KW, Mellies MJ, et al. The effects of physical training on blood lipid profiles in adolescents with insulin-dependent diabetes mellitus. Phys Sports Med. 1985;13:83–9. doi: 10.1080/00913847.1985.11708949. [DOI] [PubMed] [Google Scholar]
- 7.Eliakim A, Makowski GS, Brasel JA, et al. Adiposity, lipid levels, and brief endurance training in nonobese adolescent males. Int J Sports Med. 2000;21:332–7. doi: 10.1055/s-2000-3779. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson MA, Gutin B, Le NA, et al. Effects of exercise training and its cessation on components of the insulin resistance syndrome in obese children. Int J Obes Relat Metab Disord. 1999;22:889–95. doi: 10.1038/sj.ijo.0800968. [DOI] [PubMed] [Google Scholar]
- 9.Gilliam TB, Freedson PS. Effects of a 12-week school physical fitness program on peak VO2, body composition and blood lipids in 7 to 9 year old children. Int J Sports Med. 1980;1:73–8. [Google Scholar]
- 10.Kelly AS, Wetzsteon RJ, Kaiser DR, et al. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr. 2004;145:731–6. doi: 10.1016/j.jpeds.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Linder CW, DuRant RH, Mahoney OM. The effect of physical conditioning on serum lipids and lipoproteins in white male adolescents. Med Sci Sports Exerc. 1983;15:232–6. [PubMed] [Google Scholar]
- 12.Savage MP, Petratis MM, Thomson WH, et al. Exercise training effects on serum lipids of prepubescent boys and adult men. Med Sci Sports Exerc. 1986;18:197–204. [PubMed] [Google Scholar]
- 13.Stergioulas A, Tripolitsioti A, Messinis D, et al. The effects of endurance training on selected coronary risk factors in children. Acta Paediatr. 1998;87:401–4. doi: 10.1080/08035259850156986. [DOI] [PubMed] [Google Scholar]
- 14.Welsman JR, Armstrong N, Withers S. Responses of young girls to two modes of aerobic training. Br J Sports Med. 1997;31:139–42. doi: 10.1136/bjsm.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges LV, Olkin I. Vote-counting methods in research synthesis. Psychol Bull. 1980;88:359–69. [Google Scholar]
- 16.Sacks HS, Chalmers TC, Smith H. Randomized versus historical controls for clinical trials. Am J Med. 1982;72:233–40. doi: 10.1016/0002-9343(82)90815-4. [DOI] [PubMed] [Google Scholar]
- 17.Schulz KF, Chalmers I, Hayes R, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 18.Mann GV, Teel K, Hayes O, et al. Exercise in the disposition of dietary calories: regulation of serum lipoprotein and cholesterol levels in human subjects. N Engl J Med. 1955;253:349–55. doi: 10.1056/NEJM195509012530901. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–20. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 20.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple graphical test. Br Med J. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. J Clin Epidemiol. 1995;48:45–57. doi: 10.1016/0895-4356(94)00106-z. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Vickers A, Goyal N, Harland R, et al. Do certain countries produce only positive results? A systemtaic review of controlled trials. Control Clin Trials. 1998;19:159–66. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 25.Sterling TD, Rosenbaum WL, Weinkam JJ. Publication decisions revisited: the effect of the outcome of statistical tests on the decision to publish and vice versa. Am Stat. 1995;49:108–12. [Google Scholar]
- 26.Overton RC. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychol Meth. 1998;3:354–79. [Google Scholar]
- 27.Stata/SE 8.2 for Windows. College Station, TX: Stata Corporation LP; 2005. [Google Scholar]
- 28.SPSS 13.0 for Windows. SPSS, Inc.; 2004. [Google Scholar]
- 29.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999−2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999−2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 31.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Regression towards the mean. BMJ. 1994;308:1499. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linder CW, DuRant RH. Exercise, serum lipids, and cardiovascular disease—risk factors in children. Pediatr Clin North Am. 1982;29:1341–54. doi: 10.1016/s0031-3955(16)34284-5. [DOI] [PubMed] [Google Scholar]
- 34.Haskell WL. The influence of exercise on the concentrations of triglyceride and cholesterol in human plasma. Exerc Sport Sci Rev. 1984;12:205–44. [PubMed] [Google Scholar]
- 35.Vaccaro P, Mahon AD. The effects of exercise on coronary heart disease risk factors in children. Sports Med. 1989;8:139–53. doi: 10.2165/00007256-198908030-00002. [DOI] [PubMed] [Google Scholar]
- 36.Despres JP, Bouchard C, Malina RM. Physical activity and coronary heart disease risk factors during childhood and adolescence. Exerc Sport Sci Rev. 1990;18:243–61. [PubMed] [Google Scholar]
- 37.Tolfrey K, Jones AM, Campbell IG. The effect of aerobic exercise training on the lipid-lipoprotein profile of children and adolescents. Sports Med. 2000;29:99–112. doi: 10.2165/00007256-200029020-00003. [DOI] [PubMed] [Google Scholar]
- 38.Sacks HS, Berrier J, Reitman D, et al. Meta-analysis of randomized controlled trials. N Engl J Med. 1987;316:450–5. doi: 10.1056/NEJM198702193160806. [DOI] [PubMed] [Google Scholar]
- 39.Carnethon MR, Gidding SS, Nehgme R, et al. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290:3092–100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Department of Agriculture, editor. U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2005. 6th. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 41.Kelley GA, Kelley KS, Tran ZV. Retrieval of individual patient data for an exercise meta-analysis. Am J Med Sports. 2002;4:350–4. [Google Scholar]


