Abstract
Immunoblotting to analyze low molecular weight proteins like calmodulin and metallothioneins is challenging and requires modifications for reproducible detection. Human globin chains are 17 kDa proteins and are not detectable by conventional immunoblotting using nitrocellulose membranes. Here we describe an immunoblotting method using nitrocellulose membranes that allows quantitative analyses of globin chains. While previous studies demonstrated that the fixation of blotted membranes with glutaraldehyde improves immunodetection of low molecular weight proteins, we found that the detection sensitivity for human globins is increased markedly by the fixation with paraformaldehyde, but not glutaraldehyde. This immunoblotting procedure facilitates studies of post-transcriptional mechanisms for globin gene expression.
Expression of human β-like globin genes is believed to be controlled primarily at the level of gene transcription [1]. Much effort has been directed towards investigating the mechanisms by which the expression of β-like globin mRNAs is regulated in erythroid cells, where various molecular techniques have been used [2]. Globin chain synthesis was examined by incubating erythroid precursors with 3H leucine followed by triton acid urea gel electrophoresis analysis and fluorography [3]. Globin chain analysis was also performed by high performance liquid chromatography [4].
The application of immunoblotting to analyze the expression of human globin chains in primary erythroid cells has been limited. This is partly because appropriate antibodies against human globin chains have not been available until recently. Second, globins are low molecular weight proteins and may not be efficiently immobilized on blotting membranes. Indeed, we were unable to detect the bands of human globins by conventional immunoblotting using nitrocellulose membranes.
Our studies found that the critical steps for efficient immunodetection of human globin proteins include a short electrotransfer time and fixation of the proteins onto nitrocellulose membranes with paraformaldehyde. Although glutaraldehyde was used to immobilize other low molecular weight proteins onto blotting membranes [5, 6], human globin chains were not immobilized in a reproducible manner on nitrocellulose membranes by this chemical. The immunoblotting procedure described here allows the consistent detection of human globin chains on nitrocellulose and PVDF membranes and has high signal to noise ratios.
Total cellular extracts were prepared from nucleated erythroblasts that were cultured as described [7]. Nucleated erythroblasts (1 to 5 × 106 cells) were washed twice with 1 × phosphate buffered saline (PBS) and suspended with 50 to 200 μl of 1 × RIPA buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.2, 0.1% SDS, 1.0% Triton X-100, 1% sodium deoxycholate, 5 mM EDTA) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) supplemented by 1 mM PMSF, 0.1% SDS, and 10% (v/v) protein phosphatase inhibitor cocktail Set IV (EMD Chemicals, Gibbstown, NJ, USA). Total cellular extracts were obtained by spinning at 14,000 × g for 15 min at 4 °C.
Immunoblotting, including sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transfer, was performed as described previously [8]. Briefly, 0.1 to 10 μg of total cellular extracts were separated on 12 % SDS polyacrylamide gels (Mini-PROTEAN 3, 7.0 × 8.3 cm × 1.5 mm, Bio-Rad, Hercules, CA, USA). Electrotransfer was performed in a cold room at 30 volts using Mini Trans-Blot Cell (Bio-Rad) to either nitrocellulose membranes (0.2 μm) or Immuno-blot PVDF membranes (0.2 μm) (Bio-Rad); the transfer buffer was 25 mM Tris base, 192 mM glycine, 20% methanol (nitrocellulose) or 10% methanol (PVDF). Prior to electrotransfer, nitrocellulose membranes were soaked with the transfer buffer for 15 minutes. PVDF membranes were treated with 100% methanol for 15 seconds, transferred to a container of distilled water for 2 minutes, and then soaked with the transfer buffer for 15 minutes. After electrotransfer, nitrocellulose membranes were treated at room temperature for 30 minutes with either 1 × PBS containing 0.4 % paraformaldehyde or 2.5 % glutaraldehyde (Sigma Chemicals, St. Louis, MO). PVDF membranes were soaked at room temperature for 30 minutes with either 100 % methanol or 1 × PBS containing 0.4 % paraformaldehyde or 2.5 % glutaraldehyde.
Antibodies against human globin chains that were used in this study were obtained from Santa Cruz Biotechnology: α-globin (sc-31110), β-globin (sc-21757), and γ-globin (sc-21756). All membranes were blocked with TBS-T (20 mM Tris-HCl, pH 7.6, 154 mM NaCl, 0.1% Tween 20) containing 5 % non-fat dry milk (Bio-Rad) for 2 hours at room temperature. Membranes were then incubated overnight at 4 °C with primary antibody diluted in 5 % non-fat dry milk in TBS-T. Dilution ratios of primary antibodies were: 1:500 for α-globin, 1:1000 for β-globin, and 1:1000 for γ-globin. Membranes were washed 3 times with 1 × TBS-T. Secondary antibodies conjugated with horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology. Membranes were then incubated at room temperature for 2 hours with HRP-conjugated secondary antibody in 1 × TBS-T containing 5 % non-fat dry milk; the dilution ratio was 1: 5000. Signals for protein bands were visualized by SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL, USA) according to the protocol provided by the supplier.
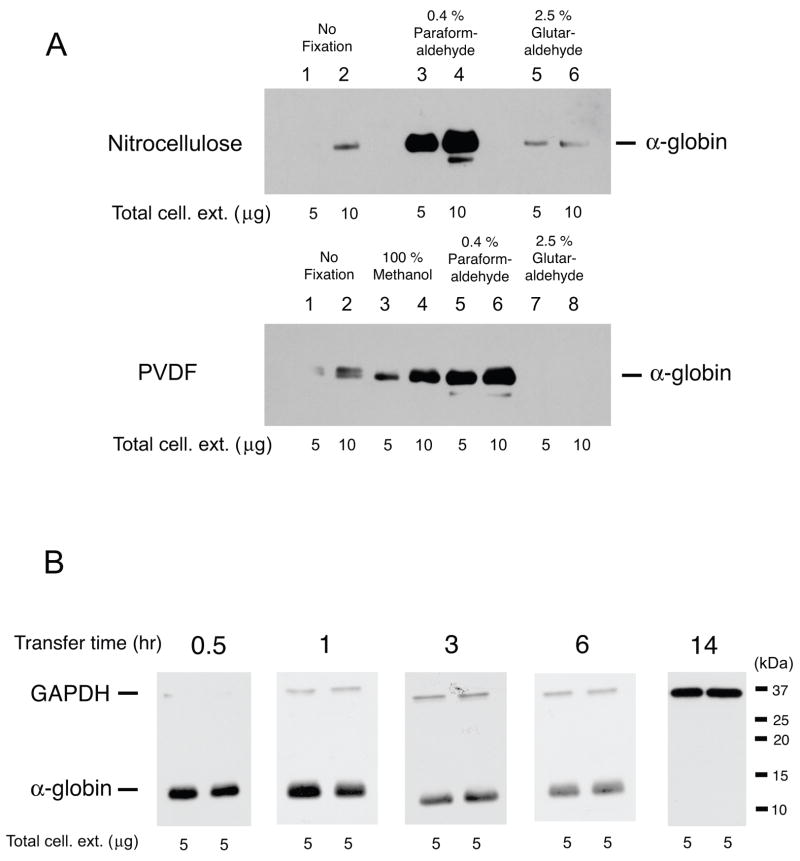
We tested both nitrocellulose membranes and PVDF membranes with or without fixation by chemicals. Initially, we performed conventional immunoblotting with a nitrocellulose membrane and overnight electrotransfer at 30 volts without chemical fixation. Only very faint signals were detected for the α-globin chain on the membrane (Fig. 1A upper panel lanes 1 & 2); detection was not successful when 5% BSA was used in place of 5% non-fat dry milk. Previous studies showed that glutaraldehyde fixation improved detection of low molecular weight proteins [5, 6]. We next soaked nitrocellulose membranes with 2.5% glutaraldehyde at room temperature for 30 minutes after electrotransfer, but the signals were not significantly enhanced (Fig. 1A upper panel lanes 5 & 6). Similarly, no appreciable signals were detected on PVDF membranes treated with 2.5% glutaraldehyde (Fig. 1A lower panel lanes 7 & 8). We noticed that PBS solution containing glutaraldehyde developed a brown color within 10 to 15 minutes, suggesting that glutaraldehyde is degraded quickly, which may affect the fixation of proteins on membranes. Next we employed paraformaldehyde to efficiently fix proteins on nitrocellulose membranes; this chemical is used for fixing hematopoietic cells [9]. We treated a nitrocellulose membrane with 0.4 % paraformaldehyde at room temperature for 30 minutes and the signals were markedly enhanced (Fig. 1A upper panel lanes 3 & 4). Similarly, the signals for the α-globin chain on PVDF membranes were substantially enhanced by the fixation with 0.4 % paraformaldehyde, while fixation with 100 % methanol modestly improved the signal intensity (Fig. 1A lower panel lanes 3 to 6). These results suggest that paraformaldehyde, which is less toxic than glutaraldehyde, is useful for immunoblotting of low molecular weight proteins.
Figure 1.
Optimization of immunoblotting conditions to detect human globin chains. A. Comparison of nitrocellulose membranes (top panel) and PVDF membranes (lower panel). Amounts of total cellular extracts that were loaded onto 12 % SDS gels are shown at the bottom of panels. After electrotransfer for 30 min at 30 volts, nitrocellulose membranes were treated as follows: lanes 1 & 2, no fixation; 3 & 4, 0.4 % paraformaldehyde; 5 & 6, 2.5 % glutaraldehyde. PVDF membranes were treated as follows: 1 & 2, no fixation; 3 & 4, 100 % methanol; 5 & 6, 0.4 % paraformaldehyde; 7 & 8, 2.5 % glutaraldehyde. Membranes were incubated with anti-human α-globin antibody (1: 500 dilution) followed by the treatment of HRP-conjugated anti-goat antibody (1: 5000 dilution). Blotted membranes were exposed to X-ray films for 3 minutes.
B. Effects of electrotransfer time on immobilization of human globin chains onto nitrocellulose membranes. Five μg of total cellular extracts were loaded onto each lane of 12 % SDS gels and electrophoresed at 150 volts for 50 minutes. Electrotransfer of globin chains to nitrocellulose membranes was then performed at 30 volts for specific lengths of time that was shown at the top of the gels. Blotted membranes were soaked with 1 × PBS containing 0.4 % paraformaldehyde for 30 minutes. Chemiluminescent signals for human α-globin were detected as described above. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Blots were exposed to X-ray films for 10 seconds except for the membrane that was electrotransfered for 14 hours, which was exposed to an X-ray film for 30 minutes to ensure that no signals were detected for α-globin. Similar results were obtained when PVDF membranes were used.
We next examined an optimal transfer time for human α-globin to membranes. The electrotransfer times were varied from 30 minutes to overnight (14 hours), and the nitrocellulose membranes were then soaked in 1 × PBS containing 0.4 % paraformaldehyde for 30 minutes. Ponceau S staining of gels and membranes confirmed efficient transfer to both nitrocellulose and PVDF after electrotransfer. The results are shown in Fig. 1B. The highest signal intensity of human α-globin was seen on the blotted membrane with the transfer time of 30 minutes, while the control protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (~35 kDa) was undetectable. The signal intensities for α-globin decreased as a function of the electrotransfer time. It is of note that the signal for α-globin is undetectable on a membrane for which electro-transfer was carried out overnight at 30 volts. Thus, optimal electrotransfer time may vary depending on the size of target proteins. Importantly, these results also suggest that the binding of human globin chains to blotting membranes is unstable and that these proteins may dissociate from the membrane matrix after prolonged electrotransfer.
To compare the sensitivity of the immunoblotting procedure described here with those of mRNA analysis methods such as primer extension and RNase protection assay, we examined the detection limits by using total cellular extracts. As shown in Fig. 2, our immunoblotting procedure detected as little as 0.1 to 5 μg of total cellular extracts on nitrocellulose membranes. Since about 100 μg of total cellular extracts are obtained from 1 million of primary erythroblasts, our immunoblotting procedure requires 1,000 to 50,000 cells for each assay. In contrast, primer extension and the RNase protection assay are performed using 0.1 to 1 microgram of total RNA [2, 10]. Since our experiments indicate that about 2 to 4 micrograms of total RNA are recovered from 1 million of primary erythroblasts, 25,000 to 500,000 cells are needed to perform one assay of these mRNA analysis methods, suggesting that the sensitivity of our immunoblotting procedure may be comparable to those of radioactive mRNA analysis methods.
Figure 2.

Sensitivity of immunoblotting for human globin chains. Total cellular extracts (0.1 to 10 μg) were separated in 12 % SDS gels and proteins were electrotransfered to nitrocellulose membranes at 30 volts for 30 minutes. After soaking with 1 × PBS containing 0.4 % paraformaldehyde for 30 minutes, membranes were incubated with primary antibodies (dilution ratios: α-globin 1:500, β-globin 1:1000, and γ-globin 1:1000) and appropriate secondary antibodies (dilution ratios: 1:5000 for α-, β-, and γ-globins). Blots were exposed to X-ray films for the following lengths of time: α-globin, 1 minute; β-globin and γ-globin, 10 seconds.
Reprobing blotted membranes with distinct antibodies facilitates studies with gene expression. We found that a 30 minute incubation of blotted membranes with conventional reprobing buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS and 100 mM β-mercaptoethanol) at room temperature completely erased all signals.
In this study, we report an immunoblotting procedure that enables us to detect human globin chains with simple modifications; short electro-transfer time and fixation with paraformaldehyde. Although Too et al. previously reported the fixation of neuropeptides to gelatin-coated nitrocellulose membranes for immunodetection [11], our studies found that gelatin coating does not necessarily enhance the immobilization of human globin chains on nitrocellulose membranes. This suggests that the fixation of human globin chains on nitrocellulose membranes by paraformaldehyde is simple and efficient for immunodetection. Both nitrocellulose and PVDF membranes treated with paraformaldehyde allowed us to reprobe them at least 5 times with distinct antibodies without loss of sensitivity. Compared to triton acid urea gel electrophoresis followed by fluorography, which takes one to two weeks to complete, and high performance liquid chromatography that requires costly equipment, the immunoblotting procedure shown here is much simpler and inexpensive and may assist us in the investigation of post-transcriptional mechanisms for globin gene expression.
Acknowledgments
This work was supported by National Institutes of Health grants DK61806 and HL73452 (T.I.). We thank Dr. Nicola Conran for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Stamatoyannopoulos G. Hypersensitive site 5 of the human beta locus control region functions as a chromatin insulator. Blood. 1994;84:1399–1401. [PubMed] [Google Scholar]
- 3.Weinberg RS, Goldberg JD, Schofield JMA, Lenes L, Styczynski R, Alter BP. Switch from fetal to adult hemoglobin is associated with a change in progenitor cell population. J Clin Invest. 1983;71:785–794. doi: 10.1172/JCI110832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 5.Van Eldik LJ, Wolchok SR. Conditions for reproducible detection of calmodulin and S100 beta in immunoblots. Biochem Biophys Res Commun. 1984;124:752–759. doi: 10.1016/0006-291x(84)91022-2. [DOI] [PubMed] [Google Scholar]
- 6.Mizzen CA, Cartel NJ, Yu WH, Fraser PE, McLachlan DR. Sensitive detection of metallothioneins-1, -2 and -3 in tissue homogenates by immunoblotting: a method for enhanced membrane transfer and retention. Journal of Biochemical & Biophysical Methods. 1996;32:77–83. doi: 10.1016/0165-022x(95)00044-r. [DOI] [PubMed] [Google Scholar]
- 7.Kuroyanagi Y, Kaneko Y, Muta K, Park BS, Moi P, Ausenda S, Cappellini MD, Ikuta T. cAMP differentially regulates g-globin gene expression in erythroleukemic cells and primary erythroblasts through c-Myb expression. Biochem Biophys Res Commun. 2006;344:1038–1047. doi: 10.1016/j.bbrc.2006.03.203. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Kuroyanagi Y, Terui K, Moi P, Ikuta T. Negative regulation of g-globin gene expression by cAMP-dependent pathway in erythroid cells. Exp Hematol. 2004;32:244–253. doi: 10.1016/j.exphem.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL, Warner NL. Paraformaldehyde fixation of hematopoietic cells for quantitative flow cytometry (FACS) analysis. J Immunol Methods. 1981;47:25–30. doi: 10.1016/0022-1759(81)90253-2. [DOI] [PubMed] [Google Scholar]
- 10.Ikuta T, Papayannopoulou T, Stamatoyannopoulos G, Kan YW. Globin gene switching. In vivo protein-DNA interactions of the human beta-globin locus in erythroid cells expressing the fetal or the adult globin gene program. J Biol Chem. 1996;271:14082–14091. doi: 10.1074/jbc.271.24.14082. [DOI] [PubMed] [Google Scholar]
- 11.Too CK, Murphy PR, Croll RP. Western blotting of formaldehyde-fixed neuropeptides as small as 400 daltons on gelatin-coated nitrocellulose paper. Anal Biochem. 1994;219:341–348. doi: 10.1006/abio.1994.1274. [DOI] [PubMed] [Google Scholar]