Abstract
Resting blood pressure in children and adolescents can track into adulthood. The purpose of this study was to use the meta-analytic approach to examine the effects of exercise on resting systolic and diastolic blood pressure in children and adolescents. Twelve randomized, controlled trials representing 16 outcomes in 1266 subjects met the inclusion criteria. Reductions in blood pressure were approximately 1% and 3% for resting systolic and diastolic blood pressures, respectively. However, random-effects modeling using 5000 bootstrap confidence intervals revealed that neither result was statistically significant (systolic, X̄±SEM=−1±2; 95% bootstrap confidence intervals=−2 to 0 mm Hg; diastolic, X̄±SEM=−2±1; 95% bootstrap confidence intervals=−3 to 0 mm Hg). The results of this study suggest that short-term exercise does not appear to reduce resting systolic and diastolic blood pressure in children and adolescents. However, a need exists for additional studies, especially in hypertensive children and adolescents.
It has been suggested that blood pressure levels during childhood and adolescence track into adulthood.1 One possible intervention that has particular appeal is exercise, a low-cost nonpharmacologic intervention that is available to most children and adolescents. However, randomized, controlled trials on this topic have led to less than overwhelming results,2–15 with only 26% and 32% of the outcomes from these studies reporting statistically significant decreases in resting systolic and diastolic blood pressure. One of the possible reasons for the lack of statistically significant results may have been the small number of sample sizes for some of these studies. Meta-analysis is a quantitative approach in which the major goal is to combine the results of individual studies in order to arrive at an overall conclusion about a body of research. This approach is especially appropriate when the number of studies is small and/or the number of subjects that can be enrolled in any one study is small.16 To date, we are not aware of any meta-analytic work in which the effects of exercise on resting systolic and diastolic blood pressures in children and adolescents have been examined. Therefore, the purpose of this study was to use the meta-analytic approach to examine the effects of exercise on resting systolic and diastolic blood pressure in children and adolescents.
Methods
Data Sources
Studies were retrieved via the following methods: 1) computerized literature searches (MEDLINE, Embase, Current Contents, Sport Discus, Dissertation Abstracts International); 2) review of reference lists from retrieved articles; and 3) review of retrieved articles by two experts on exercise and blood pressure (Dr. James Hagberg and Dr. Douglas Seals).
Study Selection
Inclusion criteria for this study were as follows: 1) randomized, controlled trials; 2) exercise intervention lasting at least 8 weeks; 3) children and adolescents under 21 years of age as subjects; 4) journal articles, dissertations, and masters' theses published in the English-language literature between January, 1966 and December, 1999; and 5) changes in resting systolic and diastolic blood pressures assessed. For studies that included multiple groups; we only included those groups that met our inclusion criteria. We did not include studies published in foreign language journals because of the potential error in the translation and interpretation of findings. Missing primary outcome data in the form of summary means for each group from each study were requested from the authors. Multiple publication bias was avoided by examining each study in order to ensure that the data from the same subjects were not included in more than one study.
Data Extraction
Coding sheets that could hold up to 231 items were developed and utilized in this investigation. All studies were coded by the first two authors, independently of each other. The authors then met and reviewed every item for accuracy and consistency. Discrepancies were resolved by consensus. The major categories of variables coded included: 1) study characteristics; 2) physical characteristics of subjects; 3) blood pressure assessment characteristics; and 4) exercise program characteristics.
Statistical Analysis
Primary and Secondary Outcomes
The primary outcomes in this study were changes in resting systolic and diastolic blood pressures. Since all but one were parallel trials, changes in blood pressure were calculated as the difference (exercise minus control) of the changes (final minus initial) in these mean values. Since most studies did not report variances for net changes in blood pressure either alone or separately for each group, estimated variances for each group (exercise and control) were calculated from variances at baseline and final measurement, using previously developed methods.17 Pooled effect sizes were calculated by assigning weights equal to the inverse of the total variance for net changes in resting blood pressure. For studies that included multiple groups, outcomes were treated as independent data points. Because of the small sample size in this investigation, bootstrap resampling, a nonparametric procedure, was used to generate 95% bootstrap confidence intervals (BCIs) around mean effect size changes in resting blood pressure.18 The number of iterations chosen for each BCI was 5000.19 If the 95% BCI included zero (0.00), it was concluded that there was no statistically significant effect of exercise on resting blood pressure. Heterogeneity of net changes in resting systolic and diastolic blood pressure was examined using the Q statistic and a p value of 0.10, since this statistic tends to suffer from low power.20 A random-effects model was used for all analyses.20
Publication bias (the tendency for studies that yield statistically significant results to be submitted and published) was examined using a recently developed rank-based data augmentation technique that formalizes the use of funnel plots and estimates and adjusts for the number of missing studies as well as the outcomes of the missing studies.21,22
To examine the influence of each study on the overall results, analyses were also performed with each study deleted from the model once. Study quality was examined using a previously developed questionnaire that has been shown to have both face validity and reliability (r=0.77). This questionnaire assesses bias pertaining to randomization, blinding, and withdrawals/dropouts. The number of points possible ranged from 0 (worst score) to 5 (best score).23
Secondary outcomes (changes in body weight and body mass index [kg/m2)], percent body fat, maximum oxygen consumption [mL/kg-1/min-1)], and resting heart rate) were examined using the same methods as those for examining net changes in resting systolic and diastolic blood pressure.
Mockrator Analysis
For categorical variables, subgroup analyses were performed using analysis of variance-like procedures for meta-analysis.20 Net changes in resting systolic and diastolic blood pressures were examined with data partitioned according to source (journal vs. other), country (United States vs. other), gender (males vs. females), age (children vs. adolescents), diet (change vs. no change), type of blood pressure instrument used (electronic vs. manual, mercury vs. random zero), position of blood pressure assessment (sitting vs. supine), hypertensive vs. not hypertensive, as reported by authors, improvements in cardiovascular fitness (yes vs. no), and type of exercise program (aerobic vs. strength). Because of a lack of information, we were unable to conduct any type of analysis in relation to obesity, smoking, alcohol, previous physical activity, drugs, and blinding. Randomization tests (5000 iterations) were used to determine the significance level for between-group differences, while 95% BCIs were generated from 5000 bootstrap samples. The significance level for all subgroup analyses was set at p≤0.05.
To examine the influence of continuous variables on changes in resting systolic and diastolic blood pressure, simple least squares regression models calculated with each effect size weighted by the reciprocal of its variance were used.20 The following continuous variables were regressed against changes in resting systolic and diastolic blood pressures: study quality, percent dropout, percentage of males in the studies, initial blood pressure levels, age, height, body weight, body mass index (kg/m2), initial maximum oxygen consumption (mL/kg-1/min-1), initial resting heart rate, changes in body weight, changes in body mass index (kg/m2), changes in maximum oxygen consumption (mL/kg-1/min-1), changes in resting heart rate, rest period before the assessment of resting blood pressure, length, frequency, intensity (percentage of maximum oxygen consumption in mL/kg-1/min-1), and duration of training, total minutes of training (length × frequency × duration), and compliance, defined as the percentage of exercise sessions that the subjects attended. Insufficient data were available to examine the association between changes in resting blood pressure and initial percent fat, changes in percent fat, and the rest period between multiple blood pressure assessments. We were unable to conduct any type of multiple regression analysis because of missing data for selected variables. All analyses in this investigation were based on summary means vs. individual patient data from included studies.
The significance level for all regression analyses was set at p≤ 0.05.
We used an independent t test to examine any potential differences between study quality for those studies published in journals vs. those published as dissertations and masters' theses. The significance level for this test was set at p≤ 0.05. Descriptive statistics are reported as X̄±SD, while changes in primary and secondary outcomes are reported as X̄±SEM of original values. Ninety-five percent BCIs for primary and secondary outcomes were based on 5000 bootstrap iterations. Bonferroni adjustments were not made because of the increased risk of a type II error.
Results
Study Characteristics
Fourteen studies met the criteria for inclusion2–15; however, we were unable to obtain necessary data from two studies,14,15 leaving us with a total of 12 studies for this analysis (percent loss that met our inclusion criteria=14%). A general description of the characteristics of the included studies is shown in Table I. There were 32 groups (16 exercise, 16 control) and 1266 subjects (649 exercise, 617 control) in which pre- and post-assessment of resting blood pressure took place. The minimum and maximum numbers of subjects were eight and 142 for the exercise groups (X̄±SD=41±38) and eight and 136 subjects for the control groups (X̄±SD=39±36). For those studies that reported data, percent dropout, defined as the number of subjects that did not complete the study, ranged from 0%–28% in the exercise groups (X̄±SD=6%±9%) and 0%–19% in the control groups (X̄±SD=8%±7%). Eight studies were published in refereed journals,2,4–6,9,10,12,13 three as dissertations,3,7,11 and one as a master's thesis.8 Nine studies were conducted in the United States,2,3,5–9,11,12 two in Australia,4,13 and one in Denmark.10 Study quality ranged from 1–4 (X̄±SD=2±1), with no differences found between those studies published in journals vs. those published as dissertations and masters' theses (p=0.28).
Table I.
Characteristics of Included Studies
| Reference | Study Design | Exercise Intervention | BP Assessment Methods |
|---|---|---|---|
| Blessing et al.2 | RCT: 25 children (15 M, 10 F) ages 13–18 years randomly selected from a residential school for visual impairments, assigned to an exercise group and matched for age, sex, and race with a random pool of 25 normally sighted controls (standard physical education) from a nearby school | 5-Minute warm-up, 40 minutes of endurance exercise (stationary cycling, treadmill walking/jogging) reaching 90% of maximum heart rate, and 5-minute cool-down, 3 times per week for 16 weeks | Not available |
| Brown3 | RCT: 40 girls and boys ages 10–14 years, assigned to either an exercise-only group, diet-only group, both a diet and exercise group, or a control group | 5–10-Minute warm-up, 20–40 minutes endurance exercise (jogging/walking on an outdoor track, active games) 5 times per week for 12 weeks | Sphygmomanometer in sitting position after 5 minutes of quiet rest; DBP recorded at onset of 4th phase of Korotkoff sounds; average of 3 trials recorded for both SBP and DBP |
| Dwyer et al.4 | RCT: 500 M and F aged 10 years, assigned to either a fitness, skill, or control (standard physical education) group | Fitness group: 14 weeks game activities that raised the heart rate 3 times per week for 75 minutes per day (15 in early morning, 60 during the day) | Standard mercury column sphygmomanometer in sitting position after 5-minute rest; DBP recorded at 5th Korotkoff sound; all measurements made with assessor blinded to group assignment |
| Ewart et al.5 | RCT: 99 African American 9th-grade girls assigned to either an exercise or control (standard physical education) group | 18 Weeks exercise (Project Heart aerobics classes), 50 minutes per session | Average of 6 trials taken in sitting position after 15-minute rest; all measurements made with assessor blinded to group assignment |
| Faigenbaum et al.6 | RCT: 25 M and F aged 8–12 years, assigned to either an exercise or a control group | 8 Weeks weight training 2 times per week for 35 minutes per session, consisting of 3 sets of 10–15 repetitions of 5 exercises at 50%–100% of 10-repetition maximum | One measurement using manual sphygmomanometer in sitting position after a few minutes' rest |
| Friday7 | RCT: 178 M aged 14–19 years, assigned to aerobic exercise/rational stage-directed hypnotherapy, aerobic exercise/basketball, rational stage-directed hypnotherapy, or no-treatment control | Aerobic exercise/rational stage-directed hypnotherapy group: aerobic exercises using 30-minute YMCA PULSE, protocol; aerobic exercise/basketball group: aerobic exercises using the 30-minute YMCA PULSE + 15 minutes of basketball; both groups exercised 3 times per week for 8 weeks | Not available |
| Gallagher8 | RCT: 16 M aged 14–17 years, assigned to either an exercise or control condition after weight and height matching | 8 Weeks weight training, 3 times per week for ≈45 minutes. Each session consisted of 2 sets of ≈10 repetitions of 9 exercises at 60% of ≥1 repetition maximum. | Lowest of 3 measures using aneroid sphygmomanometer in supine position after 15 minutes' rest; DBP recorded at the 5th Korotkoff sound |
| Gutin & Owens9 | Crossover RCT: 81 obese 7–11-year-old M and F who participated in both an exercise and control condition | 16 weeks aerobic exercise: 20 minutes treadmill, cycle, and ski machine, 20 minutes high-energy games, 5 days per week for 40 minutes per session; average intensity, 157 beats per minute | Average of the last 3 of 5 measures, taken at 1-minute intervals with a DINAMAP* automated monitor in the supine position after 10-minute rest |
| Hansen et al.10 | RCT: 132 normotensive and hypertensive M and F aged 7–11 years, assigned to either an exercise or a control (standard physical education) group | 32 weeks organized games, gymnastics, and other exercises, 3 times per week for 50 minutes per session (10 minute warm-up, 40 minutes activity) at greater than 70% of maximal heart rate for 35 minutes per session | One measurement taken with a Hawksley** random-zero sphygmomanometer in sitting position after 5-minute rest; DBP recorded at onset of 4th phase of Korotkoff sounds |
| Jones11 | RCT: 188 3rd and 4th grade M and F, assigned to either an exercise or control (standard physical education) group | 12 Weeks aerobic exercise, 5 days per week for 30 minutes per session | 9 Measurements (3/day over a 3-day period) with standard mercury sphygmomanometer in sitting position after at least 5-minutes rest; DBP recorded at onset of 4th phase of Korotkoff sounds and 5th Korotkoff phase; all measurements made with assessor blinded to group assignment |
| Linder et al.12 | RCT: 50 M aged 11–17 years, assigned to either an exercise or control group | 8 Weeks aerobic exercise (walking and jogging) 3 days per week at 80% of maximal heart rate for 30 minutes per session; also, soccer or rugby 1 day per week for 60 minutes | Not available |
| Vandongen et al.13 | RCT: 1147 M and F aged 10–12 years, assigned to one of 5 interventions (fitness, fitness + school nutrition, school-based nutrition, school + home nutrition, home-based nutrition) or a control group | Approximately 36 weeks fitness activity 5 times per week for 15 minutes per session | Average of 3 measures taken at 1-minute intervals with a DINAMAP 1846 SX/P automatic BP recorder in sitting position after 5-minute rest |
Only those groups that met our inclusion criteria were included in our analysis.
YMCA PULSE=Young Men's Christian Association People Using Life Saving Exercises; BP=blood pressure; RCT=randomized, controlled trial; SBP=systolic blood pressure; DBP=diastolic blood pressure; M=males; F=females;
DINAMAP, Critikon, Tampa, FL;
Hawksley, Lancing, West Sussex, UK
Subject Characteristics
Initial subject characteristics for the exercise and control groups are shown in Table II. Initial resting systolic blood pressure ranged from approximately 95–120 mm Hg in both the exercise and control groups, while resting diastolic blood pressure ranged from 56–78 mm Hg. For those studies that reported information on gender, seven included both males and females,2,3,6,9–11,13 three were limited to males,7,8,12 and one was limited to females.5 For the six studies that reported information on race, two included subjects that were black and white,5,8 while one each included subjects that were either black, white, and Asian,9 black, white, Hispanic, and Asian,11 or white.12 Another study reported that subjects were from the following groups: Australian, British, Italian, and Greek, as well as others.4 One study reported that subjects were not taking any drugs that could affect blood pressure,12 while another reported that none of the subjects smoked cigarettes.2 For the six studies that reported information on diet, all reported that there were no statistically significant or clinically important changes during the study.2,3,7,9,12,13 Two studies reported that the subjects had not engaged in any vigorous physical activity prior to the study.2,9 One study reported that subjects were classified as being at either stage 1 or 2 of the Tanner stages of maturational development,6 while another reported that changes in maturational development (changes in Tanner stage classification) occurred in some of the exercise and control group subjects during the study.10 Another study included only subjects classified as obese.9 None of the studies reported information on the use of alcohol.
Table II.
Initial Characteristics of Subjects
| Variable | No. | Exercise | No. | Control |
|---|---|---|---|---|
| Age (years) | 7 | 12±3 | 7 | 12±3 |
| Height (cm) | 8 | 154±14 | 8 | 153±14 |
| Weight (kg) | 8 | 53±13 | 8 | 51±13 |
| BMI (kg/m2) | 11 | 22±3 | 11 | 21±3 |
| Fat (%) | 2 | 22±3 | 2 | 20±1 |
| Vo2max (mL/kg-1/min-1) | 4 | 50±3 | 4 | 48±5 |
| RHR (bpm) | 5 | 79±5 | 5 | 79±6 |
| SBP (mm Hg) | 16 | 108±7 | 16 | 108±6 |
| DBP (mm Hg) | 16 | 67±7 | 16 | 68±7 |
No. indicates number of groups reporting data.
BMI=body mass index; Vo2max=maximum oxygen consumption; RHR=resting heart rate; bpm=beats/minute; SBP=systolic blood pressure; DBP=diastolic blood pressure
Blood Pressure Assessment Characteristics
Six studies reported using a manual sphygmomanometer,3,4,6,8,10,11 while two reported using an electronic one.9,13 Two studies reported that they used a mercury sphygmomanometer4,11 and one each reported the use of either a random-zero10 or aneroid8 sphygmomanometer. Seven studies reported the assessment of resting blood pressure in the sitting position3–6,10,11,13 while two assessed blood pressure in the supine position.8,9 For those studies that reported such information, the number of measures taken during the assessment of resting blood pressure at each phase ranged from one to nine.3,5,6,8–11,13 Assessment at each phase (baseline and final) took place on both single and multiple days. The rest period before the assessment of blood pressure ranged from 5–15 minutes.3–5,6,8–11,13 The three studies that reported information on the rest period between multiple assessments reported a rest period of approximately 1 minute.9,11,13 Two studies reported the assessment of resting diastolic blood pressure at the onset of the fourth phase of Korotkoff sounds,3,10 two used the fifth,4,8 and one used both.11 One study reported that resting blood pressure was assessed approximately 72 hours after the last exercise session.8 Three studies reported that the person responsible for the assessment of resting blood pressure was blinded to group assignment.4,5,11
Training Program Characteristics
Length of training in the studies ranged from 8–36 weeks (X̄±SD=20±11 weeks), frequency from two to five times per week (X̄±SD=4±1 times per week), and duration from 10–75 minutes per session (X̄±SD=36±15 minutes per session). Total minutes of training (length × frequency × duration) ranged from 560–5250 minutes (X̄±SD=2302±1326 minutes). For those studies in which aerobic activity was the primary intervention, intensity (maximum oxygen consumption in mL/kg-1/min-1) ranged from approximately 58%–90% (X̄±SD=73%±9%) of each subject's individually determined maximum oxygen consumption. For those studies in which resistance training was the primary intervention, one6 reported that subjects performed three sets of 10–15 repetitions of seven different exercises, with approximately 1 minute of rest between sets, while another8 reported that subjects performed two sets of 10 repetitions of nine different exercises at approximately 60% of 1 repetition maximum. Ten of the studies used an intervention that was primarily aerobic in nature2–5,7,9–13 while the other two used a resistance training protocol.6,8 For those studies that used an aerobic-type intervention, modalities included walking, jogging, aerobic dance, and cycling, as well as a variety of other activities. Compliance, defined as the percentage of exercise sessions that the subjects attended, ranged from 65% to approximately 97% (X̄±SD=85%±11%).
Primary and Secondary Outcomes
Baseline and final values for resting systolic and diastolic blood pressures from each study are shown in Table III, while individual outcomes for changes in resting systolic and diastolic blood pressure are shown in Figures 1 and 2, respectively. Decreases of approximately 1% and 3% were observed for resting systolic and diastolic blood pressure, respectively. However, random-effects modeling using 5000 bootstrap iterations resulted in nonsignificant findings for both resting systolic (Figure 1) and diastolic (Figure 2) blood pressures. When examined for potential publication bias, changes in both resting systolic and diastolic blood pressures remained stable (systolic, X̄±SEM=−1±2 mm Hg; 95% BCI=−2 to 0 mm Hg; diastolic, X̄±SEM=−2±1 mm Hg; 95% BCI=−3 to 1 mm Hg). With each study deleted from the model once, changes in resting systolic blood pressure ranged from 0±1 mm Hg (95% BCI=−1 to 0 mm Hg) to −1±2 mm Hg (95% BCI=−3 to 0 mm Hg). For resting diastolic blood pressure, changes ranged from −1±2 mm Hg (95% BCI=−2 to 1 mmHg) to −3±1 mm Hg (95% BCI=−3 to 0 mm Hg). For resting diastolic blood pressure, there was also a consistent pattern of nonsignificant reductions, with smaller reductions observed for more recent vs. earlier studies.
Table III.
Initial and Final Blood Pressure Results
| Systolic (mm Hg) | Diastolic (mm Hg) | ||||||
|---|---|---|---|---|---|---|---|
| Study | Group | n | Initial X̄±SD | Final X̄±SD | n | Initial X̄±SD | Final X̄±SD |
| Blessing et al.2 | EX | 25 | 111±16 | 108±17 | 25 | 68±10 | 67±9 |
| CON | 24 | 112±4 | 111±16 | 24 | 70.1±9 | 69±10 | |
| Brown3 | EX | 10 | 110±1 | 107±2 | 10 | 75±2 | 69±1 |
| CON | 9 | 104±3 | 101±3 | 9 | 70±2 | 68±3 | |
| Dwyer et al.4 | EX | 142 | 102±10 | 101±NA | 142 | 63±9 | 60±NA |
| CON | 136 | 103±10 | 102±NA | 136 | 62±10 | 61±NA | |
| Ewart et al.5 | EX | 44 | 120±6 | 114±6 | 44 | 58±7 | 57±7 |
| CON | 44 | 120±8 | 116±8 | 44 | 60±7 | 59±7 | |
| Faigenbaum et al.6 | EX | 14 | 112±4 | 111±4 | 14 | 78±4 | 78±6 |
| CON | 9 | 109±5 | 108±5 | 9 | 78±3 | 76±5 | |
| Friday7 | EX | 34 | 110±13 | 115±12 | 34 | 70±12 | 72±9 |
| CON | 30 | 114±10 | 117±9 | 30 | 73±11 | 75±10 | |
| Gallagher8 | EX | 8 | 114±10 | 117±7 | 8 | 73±3 | 74±5 |
| CON | 8 | 109±6 | 114±7 | 8 | 73±4 | 74±5 | |
| Gutin & Owens9 | EX | 36 | 108±11 | 108±10 | 36 | 58±7 | 60±7 |
| CON | 39 | 107±12 | 109±11 | 39 | 59±6 | 60±5 | |
| Hansen et al.10 | EX-normotensive boys | 18 | 101±13 | 97±9 | 18 | 68±8 | 68±7 |
| EX-normotensive girls | 17 | 102±6 | 98±8 | 17 | 66±5 | 65±6 | |
| EX-hypertensive boys | 17 | 113±11 | 107±7 | 17 | 77±7 | 74±7 | |
| EX-hypertensive girls | 15 | 116±11 | 115±10 | 15 | 76±10 | 77±6 | |
| CON-normotensive boys | 17 | 108±9 | 109±9 | 17 | 73±7 | 73±5 | |
| CON-normotensive girls | 16 | 106±7 | 105±5 | 16 | 68±8 | 72±4 | |
| CON-hypertensive boys | 16 | 114±10 | 116±10 | 16 | 77±7 | 79±6 | |
| CON-hypertensive girls | 16 | 114±9 | 114±10 | 16 | 76±10 | 79±7 | |
| Jones11 | EX | 90 | 95±6 | 98±7 | 90 | 56±6 | 58±7 |
| CON | 90 | 95±7 | 97±7 | 90 | 56±7 | 58±6 | |
| Linder et al.12 | EX | 21 | 104±14 | 102±15 | 21 | 62±10 | 65±11 |
| CON | 18 | 99±8 | 101±11 | 18 | 62±11 | 63±8 | |
| Vandongen et al.13 | EX-boys | 81 | 104±8 | 101±8 | 81 | 61±6 | 56±6 |
| EX-girls | 77 | 104±11 | 101±8 | 77 | 61±6 | 56±5 | |
| CON-boys | 78 | 107±8 | 103±11 | 78 | 64±6 | 58±6 | |
| CON-girls | 67 | 106±8 | 103±11 | 67 | 61±5 | 58±2 | |
EX=exercise; CON=control; NA=not available
Figure 1.
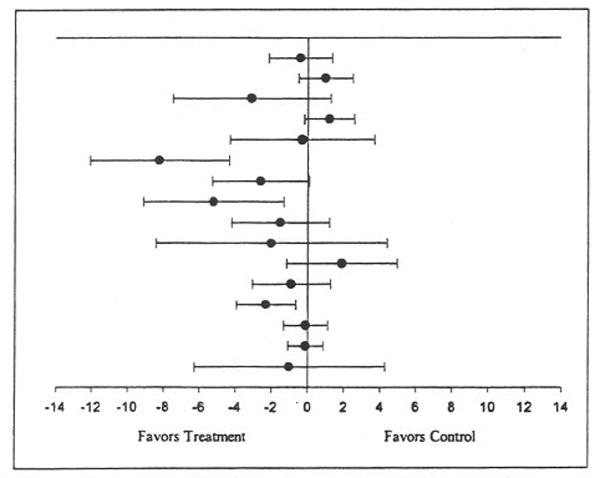
Ladder plot for changes in resting systolic blood pressure. Black circles (•) represent the change outcome for each group, while the bars that pass through the circles represent the 95% confidence intervals for each change outcome. The overall mean change was not statistically significant (X̄±SEM = −1±2; 95% bootstrap confidence interval = −2 to 0 mm Hg).
Figure 2.
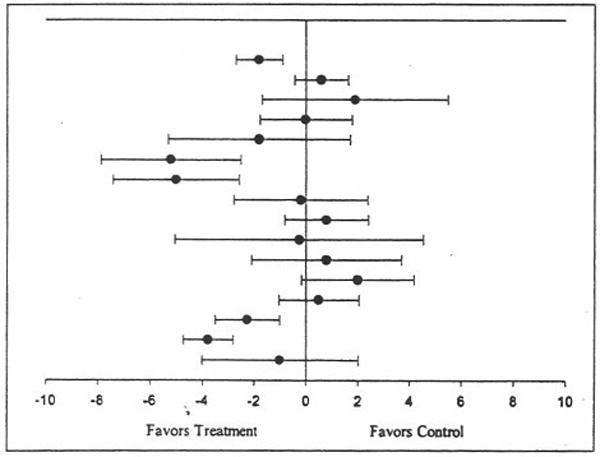
Ladder plot for changes in resting diastolic blood pressure. Black circles (•) represent the change outcome for each group, while the bars that pass through the circles represent the 95% confidence intervals for each change outcome. The overall mean change was not statistically significant (X̄±SEM = −2±1; 95% bootstrap confidence interval = − 3 to 0 mm Hg).
For secondary outcomes, a statistically significant increase of approximately 6% was found for maximum oxygen consumption (X̄±SEM=3±3 mL/kg-1/min-1; 95% BCI=2–4 mL/kg-1/min-1), while a statistically significant decrease of approximately 4% was found for resting heart rate (X̄±SEM=−3±6 beats per minute; 95% BCI=−6 to −1 beats per minute). No statistically significant changes were observed for body weight, body mass index (kg/m2), or percent body fat.
Moderator and Regression Analyses
No statistically significant or clinically important differences were found for changes in resting systolic and diastolic blood pressures when data were partitioned by selected categorical variables, including type of training (aerobic vs. resistance training) and gender. Regression analyses revealed an inverse association between initial body mass index and changes in resting systolic blood pressure, with higher body mass indexes resulting in greater reductions in systolic blood pressure (R= 0.61; SEM=0.13; p=0.05). No statistically significant or clinically important associations were observed for changes in resting diastolic blood pressure.
Discussion
The overall results of this study suggest that exercise has little effect on reducing resting systolic and diastolic blood pressures in children and adolescents. The lack of significant findings in this study occurred irrespective of statistically significant increases in maximum oxygen consumption and decreases in resting heart rate. In addition, since most of the studies did not appear to use any type of blinding in the assessment of resting blood pressure, one might have expected reductions in favor of the exercise groups as a result of nonblinding. However, this was not the case.
Despite the fact that meta-analysis can improve outcomes of treatment effectiveness when the number of studies is small and/or the number of subjects that can be enrolled in any one study is small, our results are consistent with the majority of randomized, controlled trials on this topic.2–15 One of the possible reasons for the lack of statistically significant reductions in resting blood pressure may have to do with the fact that the majority of subjects enrolled in these studies were classified as normotensive by the investigators. For example, for both resting systolic and diastolic blood pressures, only two of the 16 outcomes in this study were derived from groups classified as hypertensive. A recent narrative review on this topic concluded that there was little evidence that exercise reduced resting blood pressure in normotensive adolescents, but that aerobic exercise consistently reduced resting blood pressure in hypertensive adolescents.24 Thus, our nonsignificant findings between hypertensives and normotensives may have been the result of the small number of hypertensive outcomes included in this analysis. However, we also did not find any statistically significant association between initial resting blood pressure and changes in resting systolic and diastolic blood pressures. Furthermore, the conclusions derived from the previously mentioned review included trials of many different types (randomized, nonrandomized, cross-sectional, etc.), while we limited our analysis to randomized, controlled trials only. Since children with higher blood pressure levels may be at greater risk for cardiovascular problems as adults, it would appear plausible to suggest that future randomized, controlled trials on this topic include and limit their data analysis to children and adolescents classified as hypertensive. If normotensive subjects are included in future research, it would seem appropriate to intervene in this population group from the standpoint of preventing an increase in resting blood pressure over time. Consequently, future studies on normotensive children and adolescents should be conducted over a longer period of time than those included in our analysis.
Our finding of an association between initial body mass index and changes in resting systolic blood pressure would suggest that children and adolescents with a greater body mass index would experience larger reductions in resting systolic blood pressure. However, given the large number of statistical tests conducted in this study, this result may have been nothing more than the play of chance. This is especially true given the fact that no similar associations were observed for either body weight or percent body fat. In contrast, the relatively small sample size could have underestimated these associations and the subsequent importance of whether exercise might be a useful intervention in overweight subjects.
Another possible reason for the lack of statistically significant results may relate to the difficulty in obtaining a true sedentary control group in children and adolescents. For example, five of the studies in this investigation reported that subjects assigned to the control condition received standard physical education,2,4,5,10,11 and we would suspect that other studies included in this analysis also had a similar control condition but did not report this type of information. In addition, children and adolescents tend to be more physically active than adults,25 thereby making it more difficult to find a sedentary sample for assignment to an exercise and control condition. Furthermore, in our opinion, it would be inappropriate to request that children and adolescents who are already physically active become inactive. However, despite the lack of a true sedentary control group for most of these studies, statistically significant and positive exercise-induced changes were observed for maximum oxygen consumption and resting heart rate. This would suggest that children and adolescents assigned to the exercise groups received significantly more physical activity than subjects assigned to the control condition. It is important to note however, that it is possible that the statistically significant decreases in resting heart rate could have arisen from different accommodating effects between exercise and control groups because the exercise groups may have been seen more often and been more at ease at follow-up. However, if this were the case, one would have also expected to see similar changes in resting blood pressure for the exercise groups.
Finally, despite the fact that we found no statistically significant relationship between changes in resting blood pressure and length of training, it should be pointed out that the longest intervention study in our analysis was 36 weeks. It would appear plausible to suggest that exercise training over a longer period of time in both hypertensive and normotensive children and adolescents might confer benefits to resting blood pressure not observed in shorter-term studies. Future studies may also want to focus on females, particularly adolescent females, since there is convincing evidence to suggest that females are less active then males, especially during adolescence.25 In conclusion, the results of this study suggest that short-term exercise does not reduce resting systolic and diastolic blood pressure in children and adolescents. However, a need exists for additional studies, especially in hypertensive children and adolescents.
Acknowledgments
This study was supported by a grant from the National Institutes of Health, Heart, Lung, and Blood Institute Award #2R01HL56893 (G.A. Kelley, Principal Investigator).
The authors would like to thank James Hagberg, PhD, University of Maryland at College Park, and Douglas Seals, PhD, University of Colorado at Boulder, for reviewing our reference list and coding sheet.
References
- 1.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine study. Pediatrics. 1984;84:633–641. [PubMed] [Google Scholar]
- 2.Blessing DL, Keith RE, Williford HN, et al. Blood lipid and physiological responses to endurance training in adolescents. Pediatr Exerc Sci. 1995;7:192–202. [Google Scholar]
- 3.Brown MK. The Effects of Diet and Exercise on Selected Coronary Risk Factors in Children [dissertation] Provo, UT: Brigham Young University Press; 1981. [Google Scholar]
- 4.Dwyer T, Coonan WE, Leitch DR, et al. An investigation of the effects of daily physical activity on the health of primary school students in South Australia. Int J Epidemiol. 1983;12:308–313. doi: 10.1093/ije/12.3.308. [DOI] [PubMed] [Google Scholar]
- 5.Ewart CK, Rohm Young D, Hagberg JM. Effects of school-based aerobic exercise on blood pressure in adolescent girls at risk for hypertension. Am J Public Health. 1998;88:949–951. doi: 10.2105/ajph.88.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faigenbaum AD, Zaichkowsky LD, Westcott WL, et al. The effects of twice-a-week strength training program on children. Pediatr Exerc Sci. 1993;5:339–346. [Google Scholar]
- 7.Friday WW. Physiological, Psychological, and Behavioral Effects of Aerobic Exercise and Cognitive Experiential Therapy on Juvenile Delinquent Males [dissertation] Columbus, OH: Ohio State University; 1987. [Google Scholar]
- 8.Gallagher JS. The Chronic Effects of a Weight Training Program on Blood Pressure in Adolescent Males [master's thesis] Philadelphia, PA: Temple University; 1987. [Google Scholar]
- 9.Gutin B, Owens S. Role of exercise intervention in improving body fat distribution and risk profile in children. Am J Hum Biol. 1999;11:237–247. doi: 10.1002/(SICI)1520-6300(1999)11:2<237::AID-AJHB11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Hansen HS, Froberg K, Hyldebrandt N, et al. A controlled study of eight months of physical training and reduction of blood pressure in children: the Odense schoolchild study. BMJ. 1991;303:682–685. doi: 10.1136/bmj.303.6804.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones TM. Regular Aerobic Exercise and Blood Pressure in Elementary School Children: A Randomized Clinical Trial [dissertation] Chapel Hill, NC: University of North Carolina at Chapel Hill; 1988. [Google Scholar]
- 12.Linder CW, DuRant RH, Mahoney OM. The effect of physical conditioning on serum lipids and lipoproteins in white male adolescents. Med Sci Sports Exerc. 1983;15:232–236. [PubMed] [Google Scholar]
- 13.Vandongen R, Jenner DA, Thompson C, et al. A controlled evaluation of a fitness and nutrition intervention program on cardiovascular health in 10- to 12-year old children. Prev Med. 1995;24:9–22. doi: 10.1006/pmed.1995.1003. [DOI] [PubMed] [Google Scholar]
- 14.Stephens MB, Wentz SW. Supplemental fitness activities and fitness in urban elementary school classrooms. Fam Med. 1998;30:220–223. [PubMed] [Google Scholar]
- 15.Adeniran SA, Toriola AL. Effects of different running programmes on body fat and blood pressure in schoolboys aged 13–17 years. J Sports Med Phys Fitness. 1988;28:267–273. [PubMed] [Google Scholar]
- 16.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. 2nd. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 17.Follmann D, Elliot P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 18.Efron B, Tibshirani R. An Introduction to the Bootstrap. London, UK: Chapman and Hall; 1993. [Google Scholar]
- 19.Zhu W. Making bootstrap statistical inferences: a tutorial. Res Q Exerc Sport. 1997;68:44–55. doi: 10.1080/02701367.1997.10608865. [DOI] [PubMed] [Google Scholar]
- 20.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- 21.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Alpert BS, Wilmore JH. Physical activity and blood pressure in adolescents. Pediatr Exerc Sci. 1994;8:361–380. [Google Scholar]
- 25.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]


