Abstract
Conjugated linoleic acid (CLA) is a dietary chemopreventive agent that induces apoptosis in the mammary adipose vascular endothelium and decreases mammary brown adipose tissue (BAT) and white adipose tissue (WAT). To determine onset and extent of stromal remodeling, we fed CD2F1/Cr mice diets supplemented with 1 or 2 g/100 g mixed CLA isomers for 1–7 wk. BAT loss, collagen deposition, and leukocyte recruitment occurred in the mouse mammary fat pad, coincident with an increase in parenchymal-associated mast cells in mice fed both levels of CLA. Feeding experiments with purified isomers (0.5 g/100 g diet) demonstrated that these changes were induced by trans-10, cis-12 CLA (10,12-CLA), but not by cis-9, trans-11 CLA (9,11-CLA). This stromal remodeling did not require tumor necrosis factor (TNF)-α, a major cytokine in mast cells, as TNF-α null mice demonstrated collagen deposition, increased leukocytes, and BAT loss in the mammary fat pad in response to 10,12-CLA. To test the hypothesis that mast cells recruited in response to 10,12-CLA were required for stromal remodeling, Steel mice (WBB6F1/J-kitW/kitW-V), which lack functional mast cells, were examined for their stromal response to 10,12-CLA. Both wild-type and Steel mice showed a significantly increased leukocytic adipose infiltrate, collagen deposition, and decreased adipocyte size, although BAT was maintained in Steel mice. These results demonstrate that 10,12-CLA induces an inflammatory and fibrotic phenotype in the mouse mammary gland stroma that is independent of TNF-α or mast cells and suggest caution in the use of 10,12-CLA for breast cancer chemoprevention.
Introduction
The mammary gland is a complex tissue composed of epithelium embedded in a stromal matrix containing fibroblasts, adipocytes, blood vessels, and lymphatics, as well as a variety of infiltrating leukocytes (1,2). Although most cancers of the breast arise in the epithelium, the stroma has potential to alter both the progression or suppression of epithelial cancers [reviewed in (3)]. The local stromal environment has been found to influence mammary epithelial morphogenesis (4-8), hormonal responsiveness (9), and functional differentiation (6) in vivo. Stromal cell populations undergo changes in abundance and phenotype during the extensive postnatal development that occurs in the mammary gland during pregnancy and lactation (10,11). The hormone-dependent stromal plasticity of the adult mammary gland may reflect a potential readiness of the stroma to be modified, in a positive fashion, by chemopreventive agents.
In addition to adipocytes and fibroblasts, other cells have the potential to modify the epithelium directly, or indirectly, through the stroma. Peripheral blood-derived leukocytes (PBL),6 including lymphocytes, monocytes, and polymorphonuclear leukocytes [(PMN), which include eosinophils, neutrophils, and granulocytes], can infiltrate both the adipose stroma (the fat tissue occupying the regions between ducts and lobules) and the parenchymal stroma (the sheath of fibrocellular stroma that surrounds the ductal and lobular epithelium). Tissue resident leukocytes, such as macrophages and mast cells, may also play a pivotal part in augmenting this infiltration by secreting cytokines, such as tumor necrosis factor (TNF)-α, which recruit PBL (12). Cooperation between tissue resident leukocytes (mast cells and macrophages) and PBL (PMN and lymphocytes) in mammary gland remodeling has been previously demonstrated; macrophages and eosinophils are required for the terminal branching that is essential for full epithelial development (13). Like macrophages, mast cells are abundant mammary gland resident cells. However, unlike macrophages, mast cells are proliferation competent and contain large abundant granules of concentrated bioactive compounds, including leukocyte recruiting cytokines (eotaxin, tryptase) (14,15), vascular remodeling and vascular permeability factors (vascular endothelial growth factor, heparin, TNF-α, histamine) that stimulate fibroblast proliferation and extracellular matrix deposition (16), and enzymes such as matrix metalloproteinases. All of these can potentially contribute to mammary stromal remodeling (17).
Stromal remodeling has been documented to occur in the presence of several chemopreventive compounds, including conjugated linoleic acid (CLA) [reviewed in (3)]. CLA is a family of fatty acids containing 18 carbons with 2 double bonds in a conjugated configuration. Of the potential isomers, cis-9, trans-11-CLA (9,11-CLA) is the dominant isomer occurring naturally in ruminant-derived food products and trans-10, cis-12-CLA (10,12-CLA), although a minor component in the diet, is present in dietary supplements at a 1:1 ratio with 9,11-CLA [reviewed in (18)]. Multiple studies have demonstrated the chemopreventive effects of CLA on mammary carcinogenesis in rats (18-20). Although CLA has also been found to directly affect the mammary epithelial cell by decreasing the proliferation and increasing apoptosis in organoids in primary culture (21), CLA may mediate some of its chemopreventive effects through its induction of mammary stromal remodeling (22). These changes include decreased unilocular adipocyte size, induction of apoptosis of the adipose vasculature and subsequent loss of brown adipose tissue (BAT), and decreased white adipose tissue (WAT) (22). The stromal effects are largely mediated through 10,12-CLA, although 9,11-CLA induces a less profound decrease in unilocular adipocyte diameter (23). The objective of this study was to test the hypothesis that mast cells or mast cell-derived cytokines such as TNF-α (a cytokine implicated in acute PBL recruitment) are required for mammary gland stromal remodeling induced by CLA.
Materials and Methods
Animals and diets
Expt. 1: Effect of mixed CLA isomers on mammary gland remodeling
Six-wk-old CD2F1/Cr female mice (National Cancer Institute, Frederick Cancer Research Facility, Biological Testing Branch, Frederick, MD) were fed semisynthetic AIN-76A diets containing 5 g/100 g corn oil, without or with supplementation with 1 or 2 g/100 g CLA, as described previously (22,23). CLA was obtained from Nu-Chek Prep., and contained 85–88% 9,11- and 10,12-CLA at an ~1:1 ratio, as well as trace amounts of other isomers. The mice were killed by cervical dislocation 1, 3, 5, or 7 wk after initiation of diet and the mammary glands were fixed in formalin for paraffin embedding. A total of 120 mice were used, with 10 mice per diet and time point.
Expt. 2: Effect of purified CLA isomers on mammary gland remodeling
A second group of 6-wk-old female CD2F1/Cr mice was used to determine isomer-specific effects of CLA. Eight mice per group were fed AIN 76A diets, supplemented with 0, 0.5, or 1 g/100 g of either 9,11-CLA or 10,12-CLA. The purified CLA isomers were obtained from Natural ASA and were of >90% purity. Mice were killed by cervical dislocation at 3 d, 7 d, or 7 wk after initiation of diet.
Expt. 3: Comparison of effects of dietary 10,12-CLA on mammary gland remodeling in TNF-α null and wild-type mice
To determine the TNF-α requirement for mammary gland remodeling, we used 7th generation C57BL/6-background TNF-α null mice and their 7th generation TNF-α wild-type controls. These mice were originally obtained from Dr. George Kollias (Alexander Fleming Biomedical Sciences Research Center, Vari, Greece) at the C57BL/6 sixth generation and were bred in house. The treatment groups were as follows: TNF+/+ mice fed the AIN-76A diet supplemented with 0 or 0.5g/100 g 10,12-CLA (n=3 or 4 per group, respectively); and TNF−/− mice fed the AIN-76A diet supplemented with 0 or 0.5 g/100 g 10,12-CLA (n = 3 per group). Diets were fed for 7 d, and then the mice were killed by cervical dislocation. Abdominal mammary glands were fixed in formalin for paraffin embedding.
Expt. 4: Comparison of effects of dietary 10,12-CLA on mammary gland remodeling in Steel and wild-type mice
To determine the mast cell requirement for mammary gland remodeling, we compared mammary glands from wild-type (WBB6F1/J) and Steel mice (WBB6F1/J-kitw/kitw-v) (Jackson Labs). Mice were fed the AIN-76A diet without or with 0.5 g/100 g 10,12-CLA for 7 d (n = 3), prior to killing by cervical dislocation. Abdominal mammary glands were fixed in formalin for paraffin embedding.
Mice were housed in accordance with the standards set by the NIH and the Roswell Park Cancer Institute Animal Care and Use Committee. All mice consumed food and water ad libitum. The rooms where the animals were kept were air-conditioned and humidity controlled, with light cycles of 12 h of light, and 12 h of darkness.
Mast cell staining and analysis
To analyze mast cell recruitment, we stained paraffin sections (5 μm thick) using the May-Grunwald Giemsa staining protocol, which causes the cell nuclei to stain blue, the cytoplasm to stain pink, and the mast cells to stain purple with magenta-stained granules. Mast cell abundance was evaluated under a microscope using a 20× field of view (200× total magnification). The following items were analyzed: 1) mast cells per duct; 2) mast cells per lobule; 3) ductules per lobule; and 4) mast cells per lobular ductule. At least 10 ducts per mammary gland were analyzed.
Masson’s trichrome staining
Collagen was visualized in paraffin sections using the revised Masson’s trichrome staining protocol, with Light Green dye rather than Aniline Blue dye (24). Using this procedure, we could distinguish multiple cellular and tissue structures, with collagen and reticulin staining greenish-blue [blue using the original aniline blue protocol (24)], nuclei staining black, and cytoplasm, keratin, muscle fibers, and intercellular fibers staining from red to pink.
Adipocyte diameter
Hematoxylin and eosin-stained paraffin sections were prepared from the number 4 mammary glands of mice killed 7 d after dietary intervention. Nine fields of unilocular fat per mouse were photographed under 20× objective magnification under bright field illumination using a Hitachi KPD-50 camera connected to an Olympus BX40 microscope. The maximum vertical and horizontal diameters of 15–20 randomly selected adipocytes per field (each completely contained within the photographic field) were measured. The results were then averaged to obtain the mean value of adipocyte diameter.
Leukocyte infiltration of the WAT
The cellular infiltration of the WAT was quantified by assessing the number of nuclei per 40× (Fig. 2, Table 6) or 20× objective field (Table 5). Because the WAT in the mammary gland is normally composed of very large unilocular adipocytes (with a mean diameter >50 μm) and interspersed with a sparse capillary network, there is usually a very small number of nuclei per field of unilocular fat. When stromal remodeling events such as involution occur, leukocyte infiltration is seen in the mouse mammary fat pad (2). The degree of cellular infiltration above background can be easily assessed by counting the number of nuclei in a microscopic field. Epithelial and endothelial nuclei were excluded. In Expt. 2, mammary glands from 8 mice per dietary treatment were examined, with 10–12 fields per section analyzed, for a minimum of 95 fields quantified per treatment group. Although it is possible to clearly distinguish some cells in cross-section unequivocally as polymorphonuclear leukocytes, because of the cross-sectional nature of the material being evaluated (5-μm sections), we considered it more accurate to use the total number of nuclei per field as a surrogate index for leukocyte infiltration. In Expt. 3, 18–24 fields from 3–4 mice per group were quantified and, in Expt. 4, 18–27 fields from 3 mice per group were quantified.
FIGURE 2.

Isomer- and dose-dependence of the effects of CLA on extracellular matrix deposition and PMN recruitment to the mammary fat pad in CD2F1/Cr mice (Expt. 2). Grayscale image of Masson’s trichrome-stained sections showing collagen deposition in the WAT at wk 1 in response to the 10,12 isomer of CLA (C, arrow), and at 7 wk of feeding (arrow in F indicates collagen deposition). Arrowheads indicate PMN. Images were photographed with a 20× objective. The change in cellular recruitment to the WAT at d 7 in response to CLA isomers is shown quantitatively (G). Bars indicate means ± SEM (n = 8 mice per group, 95–96 fields per mouse). Means without a common letter differ, P < 0.05.
TABLE 6.
10,12-CLA induces only partial mammary gland stromal remodeling in mast-cell negative Steel mice (WBB6F1/J-kitw/kitw-v (Expt. 4)1
| Variable | Genotype | Control | 10,12-CLA |
|---|---|---|---|
| Nuclei, n/40× WAT field | WBB6F1/J | 19.4 ± 1.4a | 66.8 ± 12.9c |
| WBB6F1/J-kitw/kitw-v | 32.9 ± 3.1b | 87.4 ± 19.7c | |
| WAT adipocyte diameter, μm | WBB6F1/J | 86.8 ± 3.0a | 79.4 ± 3.4c |
| WBB6F1/J-kitw/kitw-v | 79.3 ± 0.4b | 74.2 ± 2.2c | |
| Fields with BAT, % | WBB6F1/J | 34.5 ± 4.7a | 20.5 ± 6.9b |
| WBB6F1/J-kitw/kitw-v | 36.0 ± 4.3a | 30.1 ± 2.8a |
Values are means ± SEM (n = 3). Means for a variable without a common superscript letter differ, P < 0.05.
TABLE 5.
10,12-CLA induces PMN recruitment and decreases BAT in C57BL/6 TNF null mice (Expt. 3)1
| Variable | Genotype | Control | 10,12-CLA |
|---|---|---|---|
| Nuclei, n/20× WAT field | C57BL/6-TNF+/+ | 72.6 ± 4.4a | 170.8 ± 30.9b |
| C57BL/6-TNF−/− | 66.8 ± 6.5a | 168.2 ± 17.8b | |
| Fields with BAT, % | C57BL/6-TNF+/+ | 35.7 ± 5.2a | 0 ± 0b |
| C57BL/6-TNF−/− | 33.0 ± 9a | 5.25 ± 4.03b* |
Values are means ± SEM (n = 3, except *: n = 4). Means for a variable without a common letter differ, P < 0.05.
Analysis of BAT abundance
The evaluation of percentage fields containing BAT was performed as described (23). Briefly, mammary BAT was defined as regions of multilocular fat cells with a central nucleus, supported by an abundant capillary network. All adipose stromal fields present in the paraffin section were evaluated for the presence or absence of BAT under a 20× objective. The number of fields examined were as follows. Expt. 3: n = 155, n = 125, n = 220, n = 175 fields analyzed for TNF+/+ control, TNF+/+ 10,12-CLA, TNF−/− control, TNF−/− 10,12-CLA, respectively (3 mice per group); Expt. 4: n =174, n =130, n =178, n =129 fields analyzed for control wild-type, control 10,12-CLA, Steel control, and Steel 10,12-CLA groups, respectively (n = 3 mice per group, except for Steel 10,12-CLA, where there were n = 4 mice per group).
Statistics
Means for each mouse were calculated for statistical analysis. Data were analyzed using Minitab software as follows. For single variables, we performed 1-way ANOVA, followed by the following post hoc tests: Expt. 1, for weights and mast cell numbers, compared with control, Dunnett’s test; for mast cell number, leukocyte infiltration, % BAT, and WAT diameter, Dunn’s method; and for comparison of percentage of trichrome positive WAT fields, Tukey test. Fisher’s test was performed to assess difference between levels of CLA. To examine interactions between 2 variables [Expt. 1, Table 2: effects of diet and time on mast cell number; Expts. 3 and 4, effects of genotype and diet on stromal remodeling (Tables 5 and 6)], we performed 2-way ANOVA, followed by Chi-square test. Differences with P < 0.05 were considered significant. Values in the text are means ± SEM.
TABLE 2.
Effect of mixed CLA isomers on the number of mast cells in the parenchymal stroma surrounding ducts in CD2F1/Cr mouse mammary glands (Expt. 1)1
| CLA, g/100 g |
|||
|---|---|---|---|
| Time | 0 | 1 | 2 |
| wk | n/40× duct-containing field | ||
| 1 | 0.20 ± 0.05 | 0.46 ± 0.09 | 0.64 ± 0.30 |
| 3 | 0.44 ± 0.14a | 1.19 ± 0.30*b | 1.82 ± 0.52b |
| 5 | 0.45 ± 0.10*a | 1.67 ± 0.29b | 1.72 ± 0.18b |
| 7 | 0.97 ± 0.25*a | 1.33 ± 0.23a,b | 2.06 ± 0.44b |
Values are means ± SEM (n = 10, except values with *: n = 9). Means in a row with superscripts without a common letter differ, P < 0.05.
Results
CLA feeding induces collagen deposition and leukocyte recruitment
Feeding mixed CLA isomers to mice (Expt. 1) did not affect body weight until wk 5 of feeding, when a modest, but significant, decrease occurred (Table 1). In contrast, CLA feeding induced dramatic alterations in the mouse mammary glands, including thickening of the fibrocellular stroma surrounding mammary ducts, as seen by Masson’s trichrome staining of paraffin sections (Fig. 1B,C, arrow; greenish-blue staining indicates collagen), compared with mice fed the control diet (Fig. 1A, arrow). Stimulation of collagen deposition was not limited to the parenchymal compartment, as deposition of collagen in the WAT also occurred (Fig. 1E,F, arrows). WAT is a depot which is normally poor in intercellular interstitial matrix (Fig. 1D), although adipocytes are surrounded by a closely apposed basement membrane. CLA also induced collagen deposition in the BAT (Fig. 1H,I, arrows).
TABLE 1.
Effect of feeding mixed CLA isomers on body weight of CD2F1/Cr mice (Expt. 1)1
| CLA, g/100 g |
|||
|---|---|---|---|
| Time | 0 | 1 | 2 |
| wk | g | ||
| 0 | 19.8 ± 0.3 | 19.9 ± 0.2 | 19.8 ± 0.2 |
| 1 | 21.1 ± 0.4 | 20.0 ± 0.5 | 19.5 ± 0.5 |
| 3 | 21.1 ± 0.2 | 20.2 ± 0.2 | 20.6 ± 0.2 |
| 5 | 22.4 ± 0.4a | 20.8 ± 0.2b | 21.0 ± 0.3b |
| 7 | 23.5 ± 0.7a | 21.2 ± 0.4b | 21.7 ± 0.5b |
Values are means ± SEM (n = 10, except 0 wk: n = 40). Means in a row with superscripts without a common letter differ, P < 0.05.
FIGURE 1.

Deposition of interstitial matrix in response to feeding CD2F1/Cr mice with mixed isomers of CLA (Expt. 1). Accumulation of collagen in the mammary glands was visualized as greenish-blue staining, using Masson’s trichrome, and can be seen in the fibrocellular stroma surrounding ducts (A-C, arrows), in the WAT (E,F, arrows), and in the BAT (H,I, arrows) of mice fed diets supplemented with 1 or 2 g/100 g mixed CLA isomers for 7 wk. Infiltrating cells in the adipose tissue are indicated by arrowheads. Magnification bar, 50 μm.
Within and adjacent to this region of collagen deposition, cellular infiltration occurred in the adipose compartments (Fig. 1E,F, arrowheads). The WAT is normally a paucicellular environment, consisting of adipocytes and stromal vascular stem cells interlaced with blood vessels, and lymphatics. The cellular infiltrate consisted largely of PMN, which are defined morphologically as small round cells with condensed chromatin and a multi-lobed nucleus.
10,12-CLA, but not 9,11-CLA, induces PMN infiltration and collagen deposition in the WAT
To determine which isomer of CLA was responsible for collagen deposition and PMN recruitment to the mammary fat pad, we fed CD2F1/Cr mice purified isomers of CLA and the mice were analyzed for changes in the fat pad at early (d 7) and later (wk 7) time points after CLA supplementation was initiated (Expt. 2). No alterations in body weight occurred except at wk 7, and then only in the group receiving the highest dose of 10,12-CLA (1 g/100 g), which showed a modest but significant decrease (23). CLA induced deposition of fibrillar collagen in the white fat as early as 7 d after diet initiation in mice fed 0.5% 10,12-CLA (Fig. 2C, arrow), but not in those fed the control diet (Fig. 2A), or the diet supplemented with 9,11-CLA (Fig. 2B). These differences were maintained at wk 7 (Fig. 2D–F).
To quantify the changes in collagen deposition, we examined trichrome-stained serial paraffin sections. We evaluated WAT staining only (and ignored the interstitial protein clearly associated with trabeculae, large blood vessels, or surrounding the epithelium), and found the percentage of trichrome-positive WAT fields was 21.5 ± 2.9% (SEM), 16.5 ± 2.6% and 79.5 ± 2.9% in the control, 9,11-CLA, and 10,12-CLA groups. Both the 9,11-CLA group and the 10,12-CLA group differed from the control group (P < 0.05, n = 4 mice per group).
Isomer-specific effects of 10,12-CLA were also seen in the induction of PMN recruitment to the mammary WAT (Fig. 2C and F, arrowheads). 10,12-CLA supplementation significantly and dose-dependently increased leukocyte recruitment as early as d 7 (Fig. 2G). Leukocyte recruitment in the mice fed the 9,11-CLA supplemented diet was similar to that of the control group.
CLA feeding rapidly induces an increase in mast cells associated with the mammary parenchymal stroma
Feeding mixed CLA isomers for 7 wk (Expt. 1) specifically increased mast cell staining in the parenchymal-associated stroma rather than the adipose stroma, with that in the fibrocellular stroma surrounding mammary ducts and lobules (Fig. 3, Tables 2 and 3) significantly greater than that in mice fed the control diets (Fig. 3A). As early as 1 wk after initiating dietary supplementation, the number of mast cells in the fibrocellular stroma surrounding each duct was greater in mice fed either the 1 g/100 g or 2 g/100 g mixed isomer CLA-supplemented diets than in those fed the control diet (Table 2). Although this CLA-dependent increase was maintained over the 7 wk feeding, a dose-dependent effect of CLA occurred only at wk 5 (Table 2, and Fig. 3).
FIGURE 3.
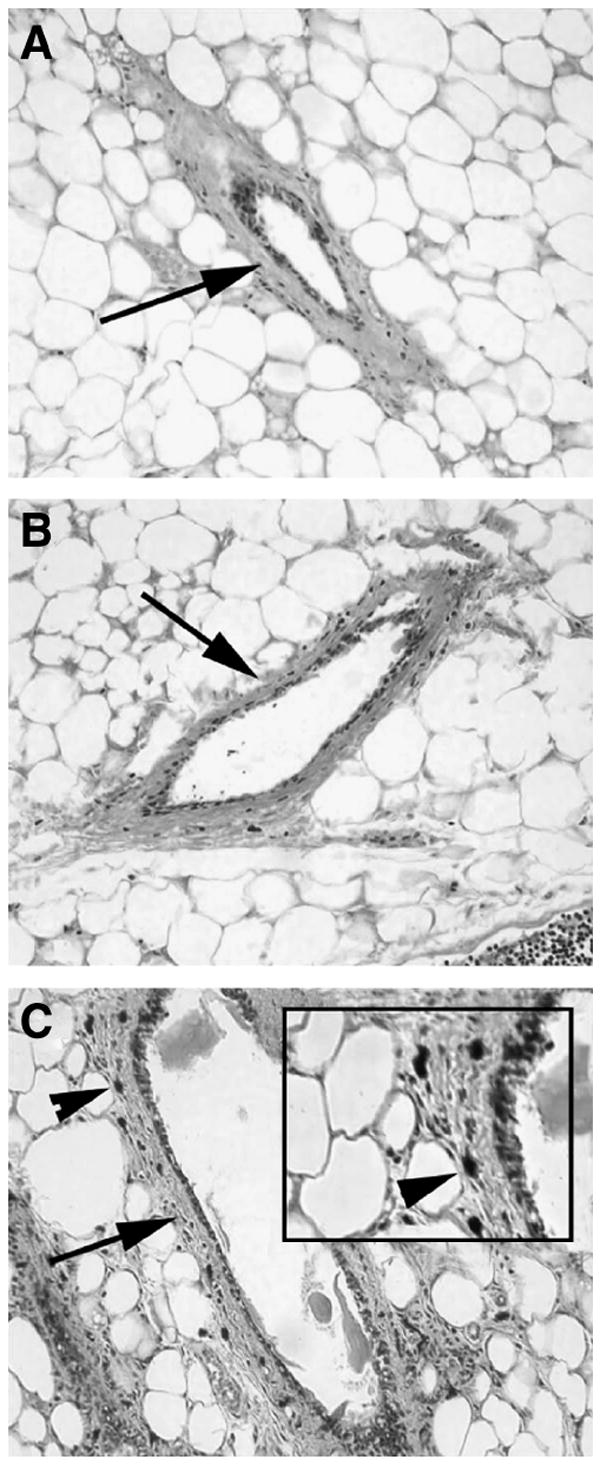
CLA induces an increase in duct-associated mast cells in the mammary gland of C2DF1/Cr (Expt. 1). May-Grunwald Giemsa staining of paraffin sections revealed the presence of abundant large cells containing basophilic granules (arrowheads, cells stained dark blue but shown here in grayscale as black) associated with the fibrocellular stroma surrounding mammary ducts in mice fed CLA (C, arrowhead). Duct of a mouse fed control diet (A). Duct of mouse fed 1 g/100 g mixed CLA isomers for 7 wk (B). Duct of mouse fed 2 g/100 g mixed CLA isomers for 7 wk (C). All images were photographed using a 20× objective. The inset in C is a 2-fold magnification of the area around the arrowhead. Arrows indicate the fibrocellular stroma surrounding ducts.
TABLE 3.
Effect of mixed CLA isomers on the number of mast cells in the parenchymal stroma surrounding lobules in CD2F1/Cr mouse mammary glands (Expt. 1)1
| CLA, g/100 g |
|||
|---|---|---|---|
| Time | 0 | 1 | 2 |
| wk | n/lobular ductule in a 40× field | ||
| 1 | 0.19 ± 0.05a | 0.19 ± 0.04a | 0.46 ± 0.14b |
| 3 | 0.32 ± 0.08a | 0.58 ± 0.17a | 1.14 ± 0.25b |
| 5 | 0.26 ± 0.10a | 0.78 ± 0.10b | 1.07 ± 0.18b |
| 7 | 0.60 ± 0.25 | 0.78 ± 0.19 | 1.09 ± 0.20 |
Values are means ± SEM (n = 10). Means in a row with superscripts without a common letter differ, P < 0.05.
Independent of diet, there were time-dependent increases in the background numbers of mast cells associated with the fibrocellular stroma (Table 2, first column). The number of mast cells per duct increased from wk 1 to wk 3 of diet treatment (P < 0.05) and, in fact, was almost doubled in the control dietary group, a time period which corresponds to an age of 7–9 wk. A smaller increase occurred in mice at later time points, suggesting that the greatest age-dependent increase in mast cells is associated with ductal growth during the peripubertal period. There was no interaction between diet and time.
To determine whether mast cell changes were also present in the lobular parenchymal stroma, we assessed the number of mast cells associated with the lobular stroma and expressed data per lobular ductule to compensate for the different degrees of lobular development during the estrous cycle (25). Similar to its effects on the ductal stroma, CLA significantly increased mast cell number in the lobular stroma (Table 3).
10,12-CLA is the isomer responsible for the recruitment of mast cells
The ability of CLA to recruit mast cells into the ductal stroma was isomer specific (Table 4, Expt. 2). No change occurred in the mammary glands of mice fed 9,11-CLA at any time point examined. However, ductal mast cell number in the parenchymal stroma was significantly increased at 7 wk in mice fed the diet supplemented with 1 g/100 g 10,12-CLA. Mast cells also accumulated in association with the vascular extracellular matrix (data not shown).
TABLE 4.
Effect of 9,11-CLA and 10,12-CLA on the number of mast cells in the parenchymal stroma surrounding ducts in CD2F1/Cr mouse mammary glands (Expt. 2)1
| 9,11-CLA, g/100 g diet |
10,12-CLA, g/100 g diet |
||||
|---|---|---|---|---|---|
| Time | 0 | 0.5 | 1 | 0.5 | 1 |
| n/40× duct-containing field | |||||
| 3 d | 0.30 ± 0.11 | 0.62 ± 0.09 | 0.31 ± 0.16 | 0.37 ± 0.08 | 0.42 ± 0.15 |
| 7 d | 0.48 ± 0.12 | 0.64 ± 0.39 | 0.45 ± 0.16 | 0.41 ± 0.08 | 0.89 ± 0.22 |
| 7 wk | 0.52 ± 0.15a | 0.34 ± 0.14a | 0.45 ± 0.16a | 0.97 ± 0.10b | 2.00 ± 0.24c |
Values are means ± SEM (n = 8). Means in a row with superscripts without a common letter differ, P < 0.05.
Is TNF-α required for CLA-induced stromal remodeling?
To determine whether TNFα, an abundant cytokine in mast cells, was required for CLA-induced leukocyte recruitment and subsequent mammary gland remodeling, we fed TNF null and wild-type mice the control or 0.5 g/100 g 10,12-CLA-supplemented diets for 7 d (Expt. 3). TNF deficiency did not alter adipose leukocyte recruitment (Table 5), protein deposition in the interstitial matrix of the mammary fat pad, or mast cell infiltration (data not shown) in mice fed 10,12-CLA. However, loss of brown fat in the mammary gland was incomplete when compared with TNF wild-type mice, which showed a total loss of mammary BAT during the same time period (Table 5).
Does mammary gland remodeling require mast cells?
To determine whether an increase in mast cells was required for the stromal remodeling induced by 10,12-CLA, mast cell-negative Steel mice (WBB6F1/J-kitw/kitw-v) and wild-type (WBB6F1/J) mice were fed control or 0.5 g/100 g 10,12-CLA-supplemented diets for 7 d. The mammary glands were subsequently observed for stromal remodeling (Expt. 4). Similar to CD2F1/Cr mice, 10,12-CLA induced protein deposition in the WAT (Fig. 4, arrows) and leukocyte infiltration in the mammary glands (Fig. 4, arrowheads) of both wild-type and Steel mice. Interestingly, the baseline number of infiltrating cells was significantly greater and the starting adipocyte size significantly smaller in Steel mice than in control mice. Regardless, both Steel mice and their wild-type controls showed an increased leukocyte infiltration and a decrease in mean white adipocyte diameter in response to 10,12-CLA (Table 6). Feeding 10,12-CLA did not completely ablate BAT in the wild-type or Steel mice, but it did significantly decrease BAT in the wild-type mice (Table 6).
FIGURE 4.

The effect of 10,12-CLA on extracellular matrix deposition and PMN recruitment in the mammary fat pad of mast-cell negative Steel mice (WBB6F1/J-kitW/kitW-V) and their wild-type controls (WBB6F1/J) (Expt. 4). Analysis of mammary glands from mast-cell negative Steel mice after short-term CLA feeding (7 d with 0.5 g/100 g 10,12-CLA) revealed that interstitial matrix protein deposition and PMN recruitment occur in the absence of functional mast cells. Arrows indicate sites of protein accumulation; arrowheads show PMN.
Discussion
Breast cancer incidence varies widely in different countries. One possible factor is societal differences in consumption of foods containing cancer-protective compounds such as CLA. However, epidemiological studies vary in their conclusions about the effects of consumption of CLA-rich foods, or of levels of serum or tissue CLA, on breast cancer risk. Although a case-control study demonstrated a decreased risk of breast cancer in women with a high intake of dairy products rich in CLA (26), other studies have not confirmed this (27-29); this is probably due to the small range of CLA intake. Despite the varied conclusions of these epidemiological studies, animal studies have verified that dietary supplementation with purified CLA isomers is sufficient to inhibit mammary carcinogenesis [reviewed in (18)].
Possible mechanisms whereby CLA inhibits the development of mammary tumors include systemic immune or hormonal changes, as well as local changes in the mammary gland microenvironment. In terms of large scale changes in energy expenditure, body weight changes in response to CLA feeding were not observed until after 5 wk of feeding (Table 1); it is therefore unlikely that they contribute to the observed changes in the mammary stroma that occur shortly after diet initiation. Local effects on the mammary gland microenvironment include direct effects on the mammary epithelium (21,30), as well as protective changes within the stroma, including inhibition of angiogenesis and locally decreased vascular endothelial growth factor [reviewed in (18)]. This study demonstrates that these stromal vascular changes are accompanied by additional modifications, such as increased extracellular matrix protein deposition in association with both the parenchymal and adipose compartments of the mammary gland. By trichrome staining, this protein deposit was found to be rich in collagen, a normal component of the ductal interstitial matrix, but not the adipose stroma in human mammary gland (31).
The CLA-induced increase in matrix deposition in the mammary fat pad was specific to the 10,12-CLA isomer and was accompanied by a rapid increase in cells, including PMN, in the normally paucicellular fat pad. The resulting appearance of the mammary fat pad in 10,12-CLA–fed mice was, in fact, very similar to that observed in involution (1,2,32,33), both in the abundance of infiltrating leukocytes, and in the extracellular matrix deposition observed between individual adipocytes. However, in contrast to involution, in which mammary adipocytes increase in both size and abundance (1), the remodeling induced by 10,12-CLA is accompanied by a decrease in white adipocyte size and abundance (23).
The increase in PMN and extracellular matrix deposition seen in the mammary fat pad in response to 10,12-CLA suggests that fibrosis, a state in which fibroblasts increase both in number and matrix-secreting activity, occurred. This process can be induced by the activation of fibroblasts by inflammatory cells, such as PMN, one of the cell types that accumulates in the mammary fat pad in response to 10,12-CLA. The mechanisms whereby fibrosis might be induced by PMN include the elaboration of inflammatory cytokines, such as TNF, which has been shown to induce fibrosis (34). In fact, TNF-deficient mice have been shown to be resistant to the experimental induction of fibrosis in other systems (35). In our studies, TNF was not required for mammary adipose remodeling, as TNF null mice remained capable of PMN recruitment and decreased BAT. Despite the fact that the loss of mammary BAT in CLA-fed TNF null mice was incomplete, this was not significantly different from that in control mice fed 10,12-CLA.
Although both wild-type and Steel mice retained BAT after 7 d of feeding, CLA-fed Steel mice showed an attenuation of BAT ablation compared with their wild-type (WBB6F1/J) counterparts, suggesting that mast cells contribute to the diet-induced remodeling of BAT that occurs in the mouse mammary gland. It is also evident that strain background plays a role in the remodeling, because ablation of BAT (and its supporting vasculature) in response to 10,12-CLA is normally brisk and virtually complete within 7 d in other mouse models, including C57BL/6 TNF-control mice (in this study) and in a larger study with CD2F1/Cr mice (23).
The isomer-specific PMN recruitment and collagen deposition in the mammary fat pad was associated with additional collagen deposition in the sheath of interstitial matrix surrounding the epithelial compartment and recruitment of mast cells to both the lobular and ductal parenchymal stroma. Parenchymal stroma-associated mast cells can act to recruit PMN to the adjacent adipose compartment, via the secretion of a number of biological effectors, including cytokines such as IL-4, IL-5, and TNF, eicosanoids, platelet activating factors, proteoglycans such as heparin, and proteases (36). The ability of 10,12-CLA to induce some mammary adipose remodeling (PMN recruitment and collagen I deposition) in mast cell–deficient Steel mice suggests that mast cells do not play a critical role in decreased adipocyte size or PMN recruitment to the mammary fat pad.
The function of the increased mast cells seen in response to 10,12-CLA is not known. Although 10,12-CLA has been associated with decreased angiogenesis and apoptosis of the adipose vasculature (23), mast cells are usually associated with increased angiogenesis (37,38). The overall role of mast cells as an accelerator or inhibitor of breast cancer is still unknown. Their association with breast cancer induction is suggested by several studies, including increased incidence of breast cancer in women with high histamine levels (39,40) and growth inhibition by mast cell stabilizers (41). Furthermore, mast cells accumulate at sites of hyperplasia (42,43), and at the tumor-host interface (17,44), and this accumulation has been associated with increased risk of metastasis (45,46).
In contrast, an increased number of mast cells and increased histamine levels were associated with a favorable prognosis and enhanced survival in a human breast cancer study of 187 women (47). It is noteworthy that this study showed no correlation between mast cell number and inflammatory infiltrates (47), suggesting that the presence of mast cells is not sufficient to indicate their functional activation state. Moreover, in a study of 483 women, tumor-free lymph nodes were found to have higher numbers of mast cells than lymph nodes containing breast cancer metastases (48). However, a study with 424 patients found no significant difference in 10-y disease-free survival in patients with high vs. low mast cell numbers associated with their breast tumors (49).
The conflicting literature regarding the ability of mast cells to act in a chemoprotective or cancer-promoting manner in the breast during cancer induction and/or progression suggests that the effects of the tissue-resident mast cells may depend on other factors in the local breast microenvironment regulating their activation status, similar to the ability of macrophages to act in a chemoprotective or cancer-promoting manner [reviewed in (50)]. The observation that 10,12-CLA induces a seemingly inflammatory mammary phenotype, complete with leukocyte recruitment and fibrosis, is surprising, given the generally protective effects of this isomer in mammary and other tumor models. These results may suggest that caution is warranted against the ingestion of this specific isomer at the high doses that are potentially attainable through supplementation with synthetic (rather than dietary) sources.
Acknowledgments
We thank Mary Vaughan for technical assistance and John Fischer for critical reading of this manuscript.
Footnotes
Supported by NCI CA61763 (M.I.), AICR-02A112-REN (M.I), and by the shared resources of the NCI Cancer Center Support Grant CA 16056 to Roswell Park Cancer Institute.
Author disclosures: J. S. Russell, no conflicts of interest; S. O. McGee, no conflicts of interest; M. M. Ip, no conflicts of interest; D. Kuhlmann, no conflicts of interest; and P. A. Masso-Welch, no conflicts of interest.
Abbreviations used: BAT, brown adipose tissue; CLA, conjugated linoleic acid; 9,11-CLA, cis-9, trans-11-CLA; 10,12-CLA, trans-10, cis-12-CLA; PBL, peripheral blood-derived leukocytes; PMN, polymorphonuclear leukocyte; TNF, tumor necrosis factor; WAT, white adipose tissue.
Literature Cited
- 1.Masso-Welch PA, Darcy KM, Stangle-Castor NC, Ip MM. A developmental atlas of rat mammary gland histology. J Mammary Gland Biol Neoplasia. 2000;5:165–85. doi: 10.1023/a:1026491221687. [DOI] [PubMed] [Google Scholar]
- 2.Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–44. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- 3.Schedin P, Elias A. Multistep tumorigenesis and the microenvironment. Breast Cancer Res. 2004;6:93–101. doi: 10.1186/bcr772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kratochwil K. Organ specificity in mesenchyme induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- 5.Cunha GR, Young P, Hamamoto S, Guzman R, Nandi S. Developmental response of adult mammary epithelial cells to various fetal and neonatal mesenchymes. Epithelial Cell Biol. 1992;1:105–18. [PubMed] [Google Scholar]
- 6.Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–41. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 7.Sakakura T, Sakagami Y, Nishizuka Y. Persistence of responsiveness of adult mouse mammary gland to induction by embryonic mesenchyme. Dev Biol. 1979;72:201–10. doi: 10.1016/0012-1606(79)90111-8. [DOI] [PubMed] [Google Scholar]
- 8.Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol. 1982;91:202–7. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 9.Haslam SZ, Counterman LJ. Mammary stroma modulates hormonal responsiveness of mammary epithelium in vivo in the mouse. Endocrinology. 1991;129:2017–23. doi: 10.1210/endo-129-4-2017. [DOI] [PubMed] [Google Scholar]
- 10.Traurig HH. Cell proliferation in the mammary gland during late pregnancy and lactation. Anat Rec. 1967;157:489–504. [Google Scholar]
- 11.Knight CH, Peaker M. Development of the mammary gland. J Reprod Fertil. 1982;65:521–36. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–18. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 13.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–82. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman SI, Goetzl EJ, Austen KF. Preformed eosinophil chemotactic factor of anaphylaxis (ECF-A) J Immunol. 1974;112:351–8. [PubMed] [Google Scholar]
- 15.Walls AF, He S, Buckley MG, Jung K-S, Holgate ST, Shute JK, Cairns JA. Granulocyte recruitment by human mast cell tryptase. Int Arch Allergy Immunol. 1995;107:372–3. doi: 10.1159/000237039. [DOI] [PubMed] [Google Scholar]
- 16.Combs JW, Purnell DM. Functional characteristics of mast cells associated with rat mammary tumors induced by 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1973;50:1003–11. doi: 10.1093/jnci/50.4.1003. [DOI] [PubMed] [Google Scholar]
- 17.Dabbous MK, Walker R, Haney L, Carter LM, Nicolson GL, Woolley DE. Mast cells and matrix degradation at sites of tumour invasion in rat mammary adenocarcinoma. Br J Cancer. 1986;54:459–65. doi: 10.1038/bjc.1986.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip MM, Masso-Welch PA, Ip C. Prevention of mammary cancer with conjugated linoleic acid: Role of the stroma and the epithelium. J Mammary Gland Biol Neoplasia. 2003;8:103–18. doi: 10.1023/a:1025739506536. [DOI] [PubMed] [Google Scholar]
- 19.Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–5. [PubMed] [Google Scholar]
- 20.Ip C, Scimeca JA. Conjugated linoleic acid and linoleic acid are distinctive modulators of mammary carcinogenesis. Nutr Cancer. 1997;27:131–5. doi: 10.1080/01635589709514514. [DOI] [PubMed] [Google Scholar]
- 21.Ip MM, Masso-Welch PA, Shoemaker SF, Shea-Eaton WK, Ip C. Conjugated linoleic acid inhibits proliferation and induces apoptosis of normal rat mammary epithelial cells in primary culture. Exp Cell Res. 1999;250:22–34. doi: 10.1006/excr.1999.4499. [DOI] [PubMed] [Google Scholar]
- 22.Masso-Welch PA, Zangani D, Ip C, Vaughan MM, Shoemaker SF, Ramirez RA, Ip MM. Inhibition of angiogenesis by the cancer chemo-preventive agent conjugated linoleic acid. Cancer Res. 2002;62:4383–9. [PubMed] [Google Scholar]
- 23.Masso-Welch P, Zangani D, Ip C, Vaughan MM, Shoemaker SF, Oflazoglu McGee S, Ip MM. Isomers of conjugated linoleic acid differ in their effects on angiogenesis and survival of mouse mammary adipose vasculature. J Nutr. 2004;134:299–307. doi: 10.1093/jn/134.2.299. [DOI] [PubMed] [Google Scholar]
- 24.Vacca LL. Laboratory Manual of Histochemistry. New York: Raven Press; 1985. [Google Scholar]
- 25.Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5:211–25. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- 26.Aro A, Mannisto S, Salminen I, Ovaskainen ML, Kataja V, Uusitupa M. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr Cancer. 2000;38:151–7. doi: 10.1207/S15327914NC382_2. [DOI] [PubMed] [Google Scholar]
- 27.Chajès V, Lavillonnière F, Maillard V, Giraudeau B, Jourdan M-L, Sébédio J-L, Bougnoux P. Conjugated linoleic acid content in breast adipose tissue of breast cancer patients and the risk of metastasis. Nutr Cancer. 2003;45:17–23. doi: 10.1207/S15327914NC4501_2. [DOI] [PubMed] [Google Scholar]
- 28.Voorrips LE, Brants HAM, Kardinaal AFM, Hiddink GJ, Van den Brandt PA, Goldbohm RA. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: the Netherlands cohort study on diet and cancer. Am J Clin Nutr. 2002;76:873–82. doi: 10.1093/ajcn/76.4.873. [DOI] [PubMed] [Google Scholar]
- 29.McCann SE, Ip C, Ip MM, McGuire MK, Muti P, Edge SB, Trevisan M, Freudenheim JL. Dietary intake of conjugated linoleic acids and risk of premenopausal and postmenopausal breast cancer, Western New York Exposures and Breast Cancer Study (WEB Study) Cancer Epidemiol Biomarkers Prev. 2004;13:1480–4. [PubMed] [Google Scholar]
- 30.Park Y, Allen KGD, Shultz TD. Modulation of MCF-7 breast cancer cell signal transduction by linoleic acid and conjugated linoleic acid in culture. Anticancer Res. 2000;20:669–76. [PubMed] [Google Scholar]
- 31.Hartveit F. Mast cell association with collagen fibers in human breast stroma. Eur J Morphol. 1993;31:209–18. [PubMed] [Google Scholar]
- 32.Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia. 2002;7:163–76. doi: 10.1023/a:1020351919634. [DOI] [PubMed] [Google Scholar]
- 33.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy M-A, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanji AA, Zakim D, Rahemtulla A, Daly T, Miao L, Zhao SP, Khwaja S, Tahan SR, Dannenberg AJ. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol- induced liver disease in the rat. Hepatology. 1997;26:1538–45. doi: 10.1002/hep.510260622. [DOI] [PubMed] [Google Scholar]
- 35.Piguet PF, Kaufman S, Barazzone C, Muller M, Ryffel B, Eugster HP. Resistance of TNF/LTα double deficient mice to bleomycin-induced fibrosis. Int J Exp Pathol. 1997;78:43–8. doi: 10.1046/j.1365-2613.1997.d01-240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plaut M, Zimmerman EM. Allergy and mechanisms of hypersensitivity. In: Paul WE, editor. Fundamental Immunology. third. New York: Raven Press; 1993. pp. 1399–426. [Google Scholar]
- 37.Coussens L, Raymond W, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey G, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norrby K. Mast cells and de novo angiogenesis: Angiogenic capability of individual mast-cell mediators such as histamine, TNF, IL-8 and bFGF. Inflamm Res. 1997;46:S7–8. [PubMed] [Google Scholar]
- 39.Smith CJ, Leggett AM, Lefante JJ. Allergic etiology of benign fibrocystic changes of the breast. Med Hypotheses. 1987;24:21–8. doi: 10.1016/0306-9877(87)90043-0. [DOI] [PubMed] [Google Scholar]
- 40.Cole P, Elwood JM, Kaplan SD. Incidence rates and risk factors of benign breast neoplasms. Am J Epidemiol. 1978;108:112–20. doi: 10.1093/oxfordjournals.aje.a112594. [DOI] [PubMed] [Google Scholar]
- 41.Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer. 2003;107:159–63. doi: 10.1002/ijc.11340. [DOI] [PubMed] [Google Scholar]
- 42.Dunn MR, Montgomery PO. A study of the relationship of mast cells to carcinoma in situ of the uterine cervix. Lab Invest. 1957;6:542–6. [PubMed] [Google Scholar]
- 43.Farnoush A, Mackenzie IC. Sequential histological changes and mast cell response in skin during chemically-induced carcinogenesis. J Oral Pathol. 1983;12:300–6. doi: 10.1111/j.1600-0714.1983.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 44.Hartveit F. Mast cells and metachromasia in human breast cancer: their occurance, significance, and consequence: a preliminary report. J Pathol. 1981;134:7–11. doi: 10.1002/path.1711340103. [DOI] [PubMed] [Google Scholar]
- 45.Hartveit F, Thoresen S, Tangen M, Maartmann-Moe H. Mast cell changes and tumour dissemination in human breast carcinoma. Invasion Metastasis. 1981;4:146–55. [PubMed] [Google Scholar]
- 46.Thoresen S, Tangen M, Hartveit F. Mast cells in the axillary nodes of breast cancer patients. Diagn Histopathol. 1982;5:65–7. [PubMed] [Google Scholar]
- 47.Aaltomaa S, Lipponen P, Papinaho S, Kosma VM. Mast cells in breast cancer. Anticancer Res. 1993;13:785–8. [PubMed] [Google Scholar]
- 48.Horny H-P, Horst H-A. Frequency distribution of tissue mast cells and eosinophilic granulocytes in tumor-draining axillary and paracolic lymph nodes. J Cancer Res Clin Oncol. 1986;112:151–5. doi: 10.1007/BF00404399. [DOI] [PubMed] [Google Scholar]
- 49.Kashiwase Y, Morioka J, Inamura H, Yoshizawa Y, Usui R, Kurosawa M. Quantitative analysis of mast cells in benign and malignant breast lesions - Immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Int Arch Allergy Immunol. 2004;134:199–205. doi: 10.1159/000078766. [DOI] [PubMed] [Google Scholar]
- 50.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]


