Abstract
Background
The Venezuelan equine encephalitis (VEE) virus replicon system was used to produce virus-like replicon particles (VRP) packaged with a number of different VEE-derived glycoprotein (GP) coats. The GP coat is believed to be responsible for the cellular tropism noted for VRP and it is possible that different VEE GP coats may have different affinities for cells. We examined VRP packaged in four different VEE GP coats for their ability to infect cells in vitro and to induce both humoral and cellular immune responses in vivo.
Methodology/Principal Findings
The VRP preparations were characterized to determine both infectious units (IU) and genome equivalents (GE) prior to in vivo analysis. VRP packaged with different VEE GP coats demonstrated widely varying GE/IU ratios based on Vero cell infectivity. BALB/c mice were immunized with the different VRP based on equal GE titers and the humoral and cellular responses to the expressed HIV gag gene measured. The magnitude of the immune responses measured in mice revealed small but significant differences between different GP coats when immunization was based on GE titers.
Conclusions/Significance
We suggest that care should be taken when alternative coat proteins are used to package vector-based systems as the titers determined by cell culture infection may not represent accurate particle numbers and in turn may not accurately represent actual in vivo dose.
Introduction
Venezuelan equine encephalitis (VEE) virus is a member of the Togaviridae family within the Alphavirus genus. Alphaviruses have single-stranded, positive-sense, RNA genomes that are capped at the 5′ end and polyadenylated at the 3′ end. The viral nonstructural proteins are encoded for in the 5′ two-thirds of the genome and the structural proteins are encoded in the 3′ one-third of the genome. The nonstructural proteins are translated in cells directly from the capped input RNA, whereas the structural proteins are translated from a subgenomic RNA transcribed from a 26S promoter present on the full-length, negative-stranded, RNA replication intermediate (reviewed in [1]). A number of live attenuated VEE virus variants have been described [2]–[7]. One of these attenuated VEE viruses (V3014) was used to generate a replicon system that has been used as a vaccine vector to express a wide array of genes [8], [9]. Such recombinant replicons are generated by replacing the structural protein coding region with genes of interest (GOI) generating what is essentially a self-replicating mRNA. Because the replicon RNA does not contain the structural genes for VEE, it is a single-cycle, propagation-defective RNA and replicates only within the cell into which it is introduced. The replicon RNA can be packaged into virus-like replicon particles (VRP) by supplying the structural protein genes of VEE in trans [8].
VEE virus and VRP derived from the VEE replicon system have a demonstrated lymphotropism [1], [10]–[12]. This characteristic may, in part, explain the robust antigen-specific immune responses, both humoral and cellular, detected in animals immunized with VRP vaccines [8], [13]–[26]. The cell tropism of VEE virus (or VRP) is believed to be defined by the envelope proteins that are embedded in the membrane that forms the outer surface of the particles. Amino acid changes in the envelope glycoproteins (GP) of VEE viruses have been shown to affect the relative rate of virus penetration of cells in culture [2], relative heparan sulfate or hydroxylapatite column binding [7], [27], in-vivo serum virus clearance rates and overall virus attenuation in animal models [5], [7], [27]–[29]. In addition, single or multiple amino acid changes in the GP coat have been shown to delay VEE virus progression to, or spread beyond, the draining lymph node of footpad-inoculated mice, while wild type VEE virus progresses on into the serum and seeds multiple other organs [5], [30]. It is assumed that VRP packaged with mutant GP coats would maintain the same surface properties and cell tropisms of the parent VEE virus harboring the same GP mutation(s) [10]. Considering this, VRP packaged with mutant GP coats, which in the context of live VEE viruses demonstrate altered phenotypes, tropisms and different trafficking to and from draining lymph nodes, may be less immunogenic than VRP packaged with the wild type (V3000) VEE GP coat.
Previous experience using VRP packaged with two different VEE GP coats, V3000 (the wild type Trinidad donkey GP coat) and V3014, has suggested that different GP coats can induce different levels of immunogenicity in mice (Kamrud and Smith, unpublished data) possibly due to lower dendritic cell tropism of the V3014 GP coat [10]. We were interested in determining the effects of different GP coats, derived from several live VEE viruses, on the immunogenicity of single-cycle VRP in BALB/c mice. Here we report the relative immunogenicity (both cellular and humoral) in vivo of VRP packaged with GP coats from wild-type and three attenuated variants of VEE virus in BALB/c mice and additionally demonstrate that the GP coats impart variable in vitro Vero cell infectivity relative to one another.
Results
Quantitative reverse transcription PCR (RTqPCR) analysis of VRP genome copies
A replicon expressing the HIV gag gene was packaged with each the following VEE GP coats: V3014 GP, V3000 GP, V3042 GP and TC-83 GP. The GP genes were identified by the name of the VEE infectious clones from which they were derived; V3000, V3014, V3042 and TC-83. A list of the specific mutations found in each of the glycoprotein coats is shown in Table 1. Infectious titers were determined for each preparation of VRP on Vero cells and are referred to as infectious units (IU). The number of replicon genome equivalents (GE) was also determined for each VRP preparation by quantitative reverse transcription PCR (RTqPCR ) using primers specific for the nsP2 gene region. Determination of the number of GE in a VRP preparation is an estimate of the total number of physical particles. A summary of the IU and GE titers is shown in Table 2. VRP packaged with the V3000 and V3042 GP coats demonstrated much higher GE/IU ratios than VRP packaged with V3014 or TC-83 GP coats (Table 2). These data suggest that a large number of particles packaged with the V3000 and V3042 GP coats are not detected in a normal infectivity assay carried out in Vero cells.
Table 1. Structural protein coding region and location of glycoprotein mutations in different VEE viruses.
| VEE virus infectious clone | VEE glycoprotein (amino acid number) | Relative heparan sulfate binding | |||||
| E2 (120) | E2 (209) | E2 (239) | E2 (323) | E1 (81) | E1 (272) | ||
| V3000 | T | E | I | G | F | A | Weak |
| V3014 | T | K | N | G | F | T | Strong |
| TC-83 | R | E | N | E | F | A | Strong |
| V3042 | T | E | I | G | I | A | Weak |
Table 2. Comparison of genome equivalents.
| VEE GP coat | IU Titera | GE Titerb | GE/IU |
| V3014 | 4.90×109 | 2.22×1011 | 45 |
| TC-83 | 3.00×1010 | 6.92×1011 | 23 |
| V3000 | 3.90×108 | 1.40×1012 | 3590 |
| V3042 | 5.30×108 | 6.60×1011 | 1245 |
Infectious unit (IU) titer determined on Vero cells. IU/ml titer represented.
Genome equivalent (GE) titer determined by quantitative reverse transcription PCR. GE/ml titer represented.
Effect of NaCl concentration on Vero cell VRP infectivity
The V3014 and TC-83 GP coats contain heparan binding mutations at E2-209 and E2-120, respectively [8], [31]. To determine whether the high GE/IU ratios for V3000 and V3042 GP packaged VRP were due to different Vero cell binding affinities, alternative buffers were used during the cell adsorption step of the titration assay. Both the pH and NaCl concentration of the titration buffers were altered from what is normally found in the MEM medium (pH 7.4, 150 mM NaCl) used for sample dilution. Reducing the concentration of NaCl also changed the osmolality of the titration buffers so sucrose was added in place of NaCl to maintain the osmolality normally found in growth medium (∼270 mOsm). Results of the titration assays are summarized in Table 3. Reducing the NaCl concentration (from 150 mM to ≤3.25 mM) in the titration buffer resulted in a dramatic increase in IU titer for both the V3000 and V3042 GP packaged VRP. The majority of these particles therefore contain fully functional replicon RNAs but are differentially infectious in vitro. Little change was noted in apparent VRP titer between the different pH levels tested, suggesting that the NaCl concentration of the titration buffer was the most important variable responsible for this effect. A result of this increase in VRP titers was that the GE/IU ratios for V3000 and V3042 GP packaged VRP dropped 58 to 38 fold, respectively (Table 3). However, even with the more sensitive infectivity assay the GE/IU ratios still remained 5 to 10 times higher than the ratios for V3014 and TC-83 GP packaged VRP. The low NaCl concentration buffers did not increase the IU titers of V3014 or TC-83 GP packaged VRP (data not shown).
Table 3. Effect of pH and NaCl on Vero cell VRP titer.
| VEE GP coat | Titration buffer (pH) | IU/mlc | GEd/IU |
| V3000 | Media (7.4) | 1.5×108 | 9333 |
| V3000 | Phosphatea (7.0) | 6.3×109 | 222 |
| Phosphate (7.4) | 8.7×109 | 160 | |
| V3000 | Trisb (7.4) | 4.2×109 | 333 |
| V3000 | Tris (8.0) | 4.0×109 | 350 |
| V3042 | Media (7.4) | 5.7×107 | 11578 |
| V3042 | Phosphate (7.0) | 2.1×109 | 314 |
| V3042 | Phosphate (7.4) | 1.8×109 | 366 |
| V3042 | Tris (7.4) | 2.2×109 | 300 |
| V3042 | Tris (8.0) | 1.2×109 | 550 |
Phosphate: 20 mM sodium phosphate buffer + 5.5% sucrose at designated pH used to titrate VRP packaged with indicated VEE GP coat.
Tris: 20 mM trishydroxymethylaminomethane + 5.5% sucrose at designated pH used to titrate VRP packaged with indicated VEE GP coat.
Infectious unit (IU) titer determined on Vero cells.
Genome equivalent (GE) titer determined by quantitative reverse transcription PCR.
Effect of GP coat on VRP immunogenicity
Because VRP IU titer determined on Vero cells detected only a minority of the particles packaged with the V3000 or V3042 GP coats, quantitative RT- PCR was used to determine the relative GE titer for each preparation. In order to detect possible differences in immunogenicity imparted to VRP packaged with different VEE GP coats, a range of GE-based titers were used to immunize mice (Table 4). All of the VRP used in experiments (in vitro and in vivo) were purified using the same purification protocol. Sixteen mice were immunized at three week intervals with each of three different GE doses. Sera were collected one week after each immunization. Eight mice per group were sacrificed for T-cell analysis 7 days after the prime and the remaining animals were sacrificed 7 days after the boost. The results of GAG-specific ELISPOT and ELISA analysis are summarized in Figures 1, 2, 3. There was a dose dependent, antigen-specific, ELISPOT response detected 7 days after the priming immunization (Figure 1). Animals immunized with the V3000 GP packaged VRP showed higher numbers of spot forming cells (SFC) then animals immunized with VRP packaged with any of the other GP coats at the 4.5×107 GE dose tested. A similar result was noted at the 4.5×105 GE dose, with the exception of the V3042 GP packaged VRP which were not significantly different from V3000 GP packaged VRP (Figure 1). Differences were also noted at the lowest dose tested (4.5×103 GE), the V3042 GP packaged VRP demonstrated significantly more SFCs than V3014 or TC-83 GP packaged VRP (Figure 1). ELISA analysis of serum collected at this time point revealed that titers above background could not be detected (data not shown).
Table 4. VEE GP coat mouse immunogenicity study design.
| Group | VEE GP coat | GE dosea | IU doseb | # animals |
| 1 | V3014 | 4.5×107 | 1.00×106 | 16 |
| 2 | V3000 | 4.5×107 | 1.20×104 | 16 |
| 3 | TC-83 | 4.5×107 | 1.90×106 | 16 |
| 4 | V3042 | 4.5×107 | 3.60×104 | 16 |
| 5 | V3014 | 4.5×105 | 1.00×104 | 16 |
| 6 | V3000 | 4.5×105 | 1.20×102 | 16 |
| 7 | TC-83 | 4.5×105 | 1.90×104 | 16 |
| 8 | V3042 | 4.5×105 | 3.60×102 | 16 |
| 9 | V3014 | 4.5×103 | 1.00×102 | 16 |
| 10 | V3000 | 4.5×103 | 1.2 | 16 |
| 11 | TC-83 | 4.5×103 | 1.90×102 | 16 |
| 12 | V3042 | 4.5×103 | 3.6 | 16 |
Genome equivalent (GE) titer determined by quantitative reverse transcription PCR.
Infectious unit (IU) titer determined on Vero cells.
Figure 1. GAG-specific ELISPOT analysis post prime.
Splenocytes were isolated from individual animals and GAG specific gamma interferon ELISPOT assays were performed to determine the number of antigen-specific cytokine-secreting T cells. Error bars represent 1 standard error.
Figure 2. GAG-specific ELISPOT analysis post boost.
Splenocytes were isolated from individual animals and GAG specific gamma interferon ELISPOT assays were performed to determine the number of antigen-specific cytokine-secreting T cells. Error bars represent 1 standard error.
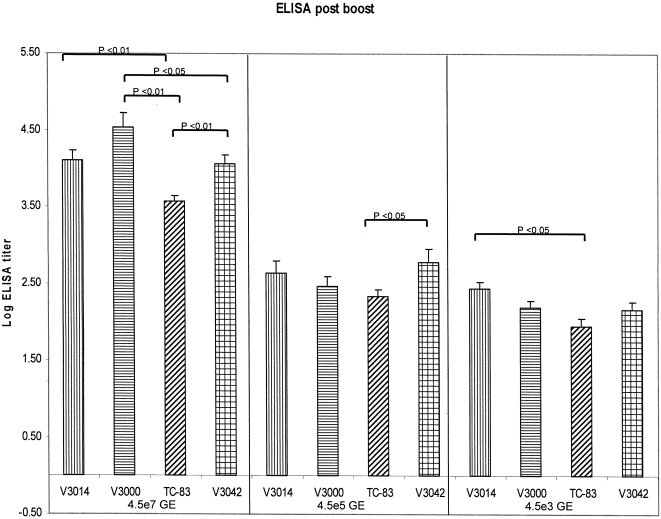
Figure 3. GAG-specific ELISA analysis post boost.
Sera was collected from individual animals 1 week after the booster vaccination and GAG-specific ELISA analysis was performed. Error bars represent 1 standard error.
ELISPOT analysis of samples collected after the boost revealed differences between GP coats at each GE dose, with V3042 GP packaged VRP inducing the highest level of SFCs at each dose tested (Figure 2). Only the ELISPOT responses detected in animals immunized with VRP packaged with the V3042 GP coat remained significant between different GE dosing groups (Figure 2). ELISA analysis of post-boost samples revealed a dose response effect between groups receiving different GE doses and some small but significant differences between GP coats largely in animals that received the highest VRP dose (Figure 3). The difference between the V3042 and TC-83 ELISA responses were mirrored at the 4.5×107 GE and 4.5×105 GE doses. The only other set of responses that remained significantly different between two different GE doses were V3014 and TC-83 in the 4.5×107 GE and 4.5×103 GE groups.
Discussion
VRP have been shown to induce a broad array of immune responses to the foreign gene product expressed by the replicon, including cytotoxic T lymphocytes (CTL), lymphoproliferative responses and neutralizing antibodies. Moreover, VRP have been shown to confer protection in animal models against a variety of diseases that require humoral and/or cellular effector mechanisms for protection [13]–[22], [32]–[35].
One of the reasons that VRP vaccines are so potent in their ability to induce immune responses may relate to their ability to target dendritic cells (DC), which are the most potent antigen-presenting cells of the immune system. The lymphotropic nature of VEE virus has been known for many years, but it has only recently been appreciated that VEE virus specifically infects DC in the draining lymph nodes of mice, and VRP target the same cells [10]. VRP also infect human DC in vitro [12]. Recent data have demonstrated the specific usage of DC SIGN and L-SIGN as attachment receptors for alphaviruses and may mechanistically explain their DC-targeting phenotype. In addition to lymphoid specificity, recent studies suggest that the immunogenicity of alphavirus replicon vaccines may be also be influenced by the activation of the innate immune system, via ribonuclease L or a double-stranded-RNA-dependent protein kinase (PKR), that occurs in cells infected with an alphavirus or alphavirus replicon [36]–[40]. Thus, the DC targeting and high protein expression that stimulate the adaptive immune responses, along with signaling of the innate immune system by dsRNA replicative intermediates, combine to make the VEE replicon system an attractive vaccine vector.
Although VRP have demonstrated ability to induce robust immune responses only VRP packaged in a few different VEE GP coats have been tested in animals. VRP packaged with the V3014 GP have been examined the most [13]–[22], [32]–[35]. Other studies have included VRP packaged with V3000 GP: [10], [41], [42], V3010 GP: [10] and V3533 GP: [10]. Because the VRP doses used in these studies did not take particle to infectious unit ratios into consideration it is not possible to compare the immune responses demonstrated between these studies. As such, a direct comparison of the immunogenicity of VRP packaged with different VEE GP coats has not been described previously.
Data presented here suggest that large differences in Vero cell infectivity exist between VRP packaged with the V3000 GP and V3042 GP coats compared to V3014 GP and TC-83 GP coats. This is perhaps not unexpected for V3000 GP packaged particles compared to V3014 GP and TC-83 GP packaged particles because of the methods used to produce these respective attenuated viruses. The V3014 GP mutations were identified in an attenuated VEE virus with rapid binding and penetration characteristics on BHK cells [2], [43] and the TC-83 GP mutations were identified in an attenuated VEE virus after multiple cell culture passages [31], [44]. Both GP coats have key attenuating mutations that map to the E2 coding region and confer a strong heparan sulfate-binding phenotype [7]. The heparan sulfate-binding capacity of the V3014 GP and TC-83 GP may explain why VRP packaged with these coats demonstrate a low GE/IU ratio based on Vero cell infectivity. It is well accepted that tissue culture adaptation of VEE virus leads to selection of heparan sulfate binding mutations in the GP coat [7], but attenuating mutations in the GP coat of VEE viruses unrelated to heparan sulfate binding have been identified [5], [28]. An example of this is the V3042 virus. The GP coat of V3042 virus has a Phe→Ile amino acid change at position 81 of the E1 protein [6], [28] and this GP coat does not impart a strong heparan sulfate binding phenotype. The lower heparan sulfate binding capacity of VRP packaged with the V3042 GP coat (and V3000 GP coat) likely contributes to a GE/IU ratio, determined on Vero cells, that is 5–10 times that of V3014 GP and TC-83 GP coat packaged VRP. The weak heparan sulfate interaction is supported by the apparent increase in IU titer (and resultant reduction in GE/IU ratio) noted when VRP titration was carried out in low NaCl concentration buffers. It is possible that the lower ion concentration in the titration buffers may have allowed weak heparan sulfate charge associations with these GP coats that would normally not be strong enough to facilitate cell binding and VRP entry.
We have previously explored the use of the V3000 GP coat to package VRP and attempted to determine whether this coat could impart higher immunogenicity to particles when compared to that of the V3014 GP coat. Those preliminary results suggested that V3000 GP packaged VRP induced more robust immune responses in mice than did V3014 GP packaged VRP, but differences in the methods used to purify the two VRP made interpretation of the results difficult (Kamrud and Smith unpublished results). To eliminate differences in VRP purification, that may affect immunogenicity, a uniform production process was used for all VRP used in this study. In addition, particle to infectious unit ratios, as estimated by measuring GEs, were measured by RTqPCR to control for differences in particle number that may not be evident when VRP titer determined on Vero cells was the sole method used to assess potency.
The greater than 100-fold difference in GE/IU ratios between V3000 GP and V3014 GP packaged VRP may, in part, explain the preliminary results alluded to above. To assist in our analysis, only immune responses shown to be statistically different between GP coats that occurred in at least two of the tested GE dosing groups were considered. With this limitation, the majority of differences that were found between GP coats were seen only at the T-cell level by γIFN ELISPOT. Interestingly, VRP packaged with the V3000 GP coat induced the highest ELISPOT results after the prime while VRP packaged with the V3042 GP coat induced the highest ELISPOT results after the boost. It is unclear at this time why this was the case. The ELISPOT data suggest that induction of T-cell responses may be more sensitive to differences in VEE GP coat than induction of B-cell responses based on ELISA. Clearly, VRP induction of T-cell responses occurred rapidly as GAG-specific γIFN secreting cells could be detected 7 days after the prime while a GAG-specific ELISA response could not. It is possible that the nature of the GAG antigen is the basis for the low post prime humoral immune response noted; VRP packaged with the different GP coats that express an alternative antigen should be tested in the same manner to explore this further. Post-boost, the GAG-specific ELISA analysis showed a dose response effect independent of the GP coat used to package the VRP. Both the V3014 and V3042 GP VRP demonstrated significantly higher ELISA responses when compared to TC-83 GP VRP at two different GE dose levels. These data suggest that VRP packaged with V3014 or V3042 GP coats may be better at inducing humoral immune responses than TC-83 GP packaged particles but they are no different than V3000 GP coated particles. Similar studies have been conducted using either VEE or Sindbis (SIN) replicon vectors packaged in homologous or heterologous GP coats, respectively [41]. The results from that study indicated that the highest humoral and cellular immune responses detected in immunized animals correlated with the VEE non-structural genes used in the animals, not with the VEE or SIN GP coats used to package the VRP [41]. Our data suggest that some of the VEE GP coats examined here impart large differences in Vero cell infection capacity which results in a significant under estimation of the actual particles present in those VRP preparations. When animals are immunized with VRP packaged with different GP coats based on genome equivalents rather than Vero cell infectious units those differences are not mirrored in BALB/c mice based on the immune responses detected. That is, those particles not detected by Vero cell-based infection assay (IU) appear to be functional in vivo in BALB/c mice. Finally, these data suggest that caution should be taken when examining alternative GP coats used to package vector-based vaccines (such as adenovirus, adeno-associated virus, vesicular stomatitis virus and alphavirus-based vectors) as differences in apparent immunogenicity imparted by the GP coat may be an artifact of the method/cell type used to determine IU titer and immunization dose.
Materials and Methods
Cells and media
VRP production and titration were conducted using a certified Vero cell line derived from a master cell bank prepared from cells obtained from the World Health Organization. Vero cells were grown in Minimum Essential Medium (MEM: Invitrogen, Carlsbad, CA) medium containing 5% fetal bovine serum (FBS, HyClone, Logan, UT), for expansion before electroporation. After electroporation Vero cells were seeded into OptiPro (Invitrogen) serum-free medium with 2 mM glutamine.
Construction of GAG replicon and GP helpers
The Du422 gag gene has been described previously [24]. The gag gene was cloned into an optimized replicon vector containing an Encephalomyocarditis virus (EMCV) IRES element and spacer sequence as described previously [9]. Site directed mutagenesis was carried out using a QuikChange® Site-Directed mutagenesis kit (Stratagene, La Jolla, CA) to introduce a G→A change at nucleotide position 3 (nt3A) at the 5′ end of the replicon RNA. The nucleotide 3 G→A mutation is a known VEE virus attenuating mutation [31] and was incorporated into the replicon to add an additional measure of safety to VRP generated with the V3000 GP coat. The nt3A, IRES-optimized, GAG replicon RNA was packaged with each of the different VEE GP coats analyzed in this study.
Helpers coding for the GP genes defined by the sequence of the VEE infectious clones V3000, V3014, V3042 and TC-83 were constructed. Construction of the V3014 GP helper, pHGP3014, has been described previously [8]. The V3000 GP gene was derived from the infectious clone of the wild type virulent Trinidad donkey strain of VEE [43]. The V3000 GP was cloned as a SpeI and SphI gene fragment into the V3014 GP helper plasmid digested with the same enzymes replacing the V3014 GP gene to generate the V3000 GP helper, pHGP3000. The TC-83 GP gene was digested from the full length infectious clone of VEE TC-83 virus [31] and cloned into a helper plasmid as described above to generate the TC-83 GP helper, pHGPTC-83. The V3042 GP helper was generated by introducing the E1-81 (Phe→Ile) mutation [28] into the pHGP3000 GP helper using site directed mutagenesis (Stratagene) as described above. The V3042 GP helper, pHGP3042, was sequenced to ensure that no errors were introduced during the mutagenesis reaction.
RNA transcription, electroporation, VRP production and VRP titration
VRP were produced by co-electroporating Vero cells with replicon RNA combined with capsid and GP helper RNAs (sometimes referred to as the split helper system). The methods used to in vitro transcribe replicon, capsid and GP RNA and electroporate the RNAs into Vero cells have been described previously [9]. The infectious titer of VRP was determined by immunofluorescence assay (IFA) using goat anti-VEE nsP2 specific polyclonal antiserum as the primary antibody and donkey anti-goat Alexa Fluor 488 (Invitrogen) as the secondary antibody on methanol fixed Vero cells using a Nikon Eclipse TE300 fluorescence microscope. VRP were serially diluted in MEM (5% FBS), dilutions were inoculated onto Vero cells and analyzed 18 hr post infection as described above by IFA to determine an initial IU/ml titer. For VRP titers determined in low NaCl titration buffers, each VRP preparation was diluted to within two orders of magnitude of the original IU/ml titer determined in MEM (5% FBS). The VRP were then diluted 1:10 in one of the low NaCl titration buffers (20 mM sodium phosphate + 5.5 % sucrose, pH 7.0 and pH 7.4 or 20 mM Tris + 5.5 % sucrose, pH 7.4 and pH 8.0) followed by serial 2 fold dilutions in the respective low NaCl titration buffers. The VRP dilutions were inoculated onto Vero cells and analyzed as described above by IFA to determine IU/ml titer. The VRP were tested for the presence of contaminating replication competent VEE (RCV) using two blind passages on Vero cells as described previously [9].
VRP purification
VRP were collected 18 hr post-electroporation, by removing the media and washing the cells with 0.4 M NaCl (salt wash). Some of the GP coats produced VRP that bound to Vero cells with less affinity than V3014 GP VRP, thereby resulting in a significant proportion of VRP in the media. In order to collect all of the VRP generated with each GP coat and to maintain a uniform purification method for all VRP the salt wash was combined with the media from electroporated cells and the pool concentrated 10 fold on a tangential flow filtration (TFF) system with 100,000 molecular weight cutoff Hydrosart membrane (Sartorius, Edgewood, NY). A 5 M NaCl /10 mM sodium phosphate pH 7.3 solution was added to the TFF retentate to produce a solution with a final sodium chloride concentration of 2 M. The solution was recirculated 5 minutes with the permeate closed and then the solution was concentrated to one half the original volume. The solution was diafiltered against 3 volumes of cold phosphate buffered saline containing 3 mM magnesium chloride. The permeate of the system was closed, 25,000 U of Benzonase (EM Science, NJ) was added and the system was allowed to recirculate for 5 min. The pump was stopped for 60 min and then restarted. A 5 M sodium chloride /10 mM sodium phosphate pH 7.3 solution was added to the TFF retentate to produce a solution with a final sodium chloride concentration of 2 M. The solution was recirculated 5 minutes with the permeate closed and then the solution was concentrated to one half the original volume. The solution was diafiltered against 3 volumes of cold 0.5 M sodium chloride/10 mM sodium phosphate pH 7.3. The TFF retentate was drained from the system, passed through a 0.2 µ filter and diluted 1:10 into 10 mM Tris buffer, pH 8.0. The diluted solution was loaded to a 3.5 mL Cellufine sulfate (Chisso, Japan) column at 200 cm/hr. The column was washed with 10 mM Tris, pH 8 followed by 10 mM sodium phosphate, pH 7.3. VRP were eluted with a step gradient to 1 M sodium chloride/10 mM phosphate, pH 7.3. Fractions were pooled based upon the A280 absorbance elution profile and the VRP titer in the pooled fractions was determined by IFA as described above.
Quantitative reverse transcription PCR (RTqPCR) analysis of VRP
To determine the number of genome equivalents present in each different GP coated VRP, a standard one-step RT-qPCR protocol was performed on an Applied Biosystems 7500 Fast Real Time PCR System sequence detection system. Amplification was detected by means of a fluorogenic probe designed to anneal to a region of the nsP2 gene on the replicon between the two primers. A 5′ reporter dye (6-FAM) and a 3′ quencher dye (BHQ-1) were attached to the probe. Proximity of the reporter and quencher dyes resulted in the suppression of reporter fluorescence prior to amplification. Upon successful amplification of the target region, the 5′ exonuclease activity of DNA polymerase released the reporter dye from the hybridized probe, resulting in a fluorescent signal. Purified VEE replicon RNA was used to generate a standard curve in the assay, and the fluorescent signal of each VRP sample was measured over forty PCR cycles and compared to the fluorescent signal of the standards to determine genome equivalents.
Vaccination of mice and sample collection
Female, 6–8 week old, BALB/c, mice (Charles River Laboratory, Kingston, NY) were immunized with VRP produced with the different GP coats based on genome equivalent (GE) titers. A summary of the GE titers, the respective Vero cell infectious titer and the number of animals immunized in each group for each dose are shown in Table 4. Mice were immunized at 0 and 3 weeks by subcutaneous (SC) injection into the rear footpad. GAG-specific antibody and T cell responses were monitored 1 week after each immunization. Blood was collected by retro-orbital bleeds for all groups. Eight (8) mice from each group were sacrificed 1 week after each immunization and splenocytes collected from individual animals for T-cell analysis.
ELISA and ELISPOT analysis
For ELISA analysis, dilutions of sera from GAG-VRP vaccinated mice were made into PBS containing 1% BSA and 0.05% Tween-20 beginning with 1:40 followed by two fold dilutions out to 1:2560. Pre-bleed samples were diluted to only 1:40. ELISA plates (Nunc, Rochester, NY), which had been coated with HIV GAG antigen (0.25 µg/well) in carbonate/bicarbonate buffer (Sigma-Aldrich, St. Louis, MO) overnight at 4°C, were incubated with 200 µl/well of blocking buffer (PBS, 3% BSA) at 30°C for 1 hour. Blocked plates were washed three times with 200 µl of PBS. Fifty µl of diluted sera was added to the plates in duplicate and incubated at 30°C for one hour. Plates were washed three times with 200 µl PBS. 100 µl of alkaline phosphatase-conjugated anti-mouse polyvalent immunoglobulin's (IgG, IgA, IgM) (Sigma) diluted in blocking buffer (1:500) was added to each well. Plates with secondary antibody were incubated for 1 hour at room temperature and then washed three times with 200 µl PBS. Plates were developed using Fast™ p-Nitrophenyl phosphate tablet sets (Sigma) and reading at a wavelength of 405. An OD405 of 0.2 or greater was considered positive.
Splenocytes were isolated from individual animals and GAG specific gamma interferon ELISPOT assays were performed to determine the number of antigen-specific cytokine-secreting T cells. This procedure has been described previously [45].
Acknowledgments
We thank Dr. Jennifer Ruiz for expert assistance in development of the low NaCl titration buffers.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: NIH grant 5U01AI53876-02
References
- 1.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston RE, Smith JF. Selection for accelerated penetration in cell culture coselects for attenuated mutants of Venezuelan equine encephalitis virus. Virology. 1988;162:437–443. doi: 10.1016/0042-6822(88)90484-9. [DOI] [PubMed] [Google Scholar]
- 3.Davis NL, Powell N, Greenwald GF, Willis LV, Johnson BJ, et al. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology. 1991;183:20–31. doi: 10.1016/0042-6822(91)90114-q. [DOI] [PubMed] [Google Scholar]
- 4.Davis NL, Brown KW, Greenwald GF, Zajac AJ, Zacny VL, et al. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212:102–110. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 5.Grieder FB, Davis NL, Aronson JF, Charles PC, Sellon DC, et al. Specific restrictions in the progression of Venezuelan equine encephalitis virus-induced disease resulting from single amino acid changes in the glycoproteins. Virology. 1995;206:994–1006. doi: 10.1006/viro.1995.1022. [DOI] [PubMed] [Google Scholar]
- 6.Turell MJ, Ludwig GV, Kondig J, Smith JF. Limited potential for mosquito transmission of genetically engineered, live-attenuated Venezuelan equine encephalitis virus vaccine candidates. Am J Trop Med Hyg. 1999;60:1041–1044. doi: 10.4269/ajtmh.1999.60.1041. [DOI] [PubMed] [Google Scholar]
- 7.Bernard KA, Klimstra WB, Johnston RE. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology. 2000;276:93–103. doi: 10.1006/viro.2000.0546. [DOI] [PubMed] [Google Scholar]
- 8.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, et al. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 9.Kamrud KI, Custer M, Dudek JM, Owens G, Alterson KD, et al. Alphavirus replicon approach to promoterless analysis of IRES elements. Virology. 2007;360:376–387. doi: 10.1016/j.virol.2006.10.049. Epub 2006 Dec 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald GH, Johnston RE. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol. 2000;74:914–922. doi: 10.1128/jvi.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson ED, Smith JF. Alphaviral-Based Strategies for the Immunotherapy of Cancer. In: Morse MA, Clay TM, Lyerly HK, editors. Handbook of Cancer Vaccines. Totowa, NJ: 2004. pp. 203–224. [Google Scholar]
- 12.Nishimoto KP, Laust AK, Wang K, Kamrud KI, Hubby B, et al. Restricted and selective tropism of a Venezuelan equine encephalitis virus-derived replicon vector for human dendritic cells. Viral Immunol. 2007;20:88–104. doi: 10.1089/vim.2006.0090. [DOI] [PubMed] [Google Scholar]
- 13.Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000;19:142–153. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 14.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75:11677–11685. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz-Cherry S, Dybing JK, Davis NL, Williamson C, Suarez DL, et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000;278:55–59. doi: 10.1006/viro.2000.0635. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Pushko P, Parker MD, Dertzbaugh MT, Smith LA, et al. Candidate vaccine against botulinum neurotoxin serotype A derived from a Venezuelan equine encephalitis virus vector system. Infect Immun. 2001;69:5709–5715. doi: 10.1128/IAI.69.9.5709-5715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Dyas BK, Nystrom SS, Lind CM, Smith JF, et al. Immune protection against staphylococcal enterotoxin-induced toxic shock by vaccination with a Venezuelan equine encephalitis virus replicon. J Infect Dis. 2002;185:1192–1196. doi: 10.1086/339677. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Hadjipanayis AG, Welkos SL. Venezuelan equine encephalitis virus-vectored vaccines protect mice against anthrax spore challenge. Infect Immun. 2003;71:1491–1496. doi: 10.1128/IAI.71.3.1491-1496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Groebner JL, Hadjipanayis AG, Negley DL, Schmaljohn AL, et al. Multiagent vaccines vectored by Venezuelan equine encephalitis virus replicon elicits immune responses to Marburg virus and protection against anthrax and botulinum neurotoxin in mice. Vaccine. 2006;24:6886–6892. doi: 10.1016/j.vaccine.2006.06.004. Epub 2006 Jun 6821. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JA, Bray M, Bakken R, Hart MK. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology. 2001;286:384–390. doi: 10.1006/viro.2001.1012. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JA, Hart MK. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol. 2001;75:2660–2664. doi: 10.1128/JVI.75.6.2660-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251:28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 23.Balasuriya UB, Heidner HW, Davis NL, Wagner HM, Hullinger PJ, et al. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine. 2002;20:1609–1617. doi: 10.1016/s0264-410x(01)00485-6. [DOI] [PubMed] [Google Scholar]
- 24.Williamson C, Morris L, Maughan MF, Ping LH, Dryga SA, et al. Characterization and selection of HIV-1 subtype C isolates for use in vaccine development. AIDS Res Hum Retroviruses. 2003;19:133–144. doi: 10.1089/088922203762688649. [DOI] [PubMed] [Google Scholar]
- 25.Davis NL, West A, Reap E, MacDonald G, Collier M, et al. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life. 2002;53:209–211. doi: 10.1080/15216540212657. [DOI] [PubMed] [Google Scholar]
- 26.Nelson EL, Prieto D, Alexander TG, Pushko P, Lofts LA, et al. Venezuelan equine encephalitis replicon immunization overcomes intrinsic tolerance and elicits effective anti-tumor immunity to the ‘self’ tumor-associated antigen, neu in a rat mammary tumor model. Breast Cancer Res Treat. 2003;82:169–183. doi: 10.1023/B:BREA.0000004373.09678.bb. [DOI] [PubMed] [Google Scholar]
- 27.Jahrling P, Heisey GB, Hesse RA. Evaluation of vascular clearance as a marker for virulence of alphaviruses: Disassociation of rapid clearance with low virulence of Venezuelan encephalitis virus strains in guinea pigs. Infection and Immunity. 1977;17:356–360. doi: 10.1128/iai.17.2.356-360.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis NL, Grieder FB, Smith J, Greenwald GF, Valenski ML, et al. A molecular genetic approach to the study of Venezuelan equine encephalitis virus pathogenesis. Archives of Virology Suppl. 1994;9:99–109. doi: 10.1007/978-3-7091-9326-6_11. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig GV, Turell MJ, Vogel P, Kondig JP, Kell WK, et al. Comparative neurovirulence of attenuated and non-attenuated strains of Venezuelan equine encephalitis virus in mice. Am J Trop Med Hyg. 2001;64:49–55. doi: 10.4269/ajtmh.2001.64.49. [DOI] [PubMed] [Google Scholar]
- 30.Aronson JF, Grieder FB, Davis NL, Charles PC, Knott T, et al. A single-site mutant and revertants arising in vivo define early steps in the pathogenesis of Venezuelan equine encephalitis virus. Virology. 2000;270:111–123. doi: 10.1006/viro.2000.0241. [DOI] [PubMed] [Google Scholar]
- 31.Kinney RM, Chang GJ, Tsuchiya KR, Sneider JM, Roehrig JT, et al. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′-noncoding region and the E2 envelope glycoprotein. J Virol. 1993;67:1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balasuriya UB, Heidner HW, Hedges JF, Williams JC, Davis NL, et al. Expression of the two major envelope proteins of equine arteritis virus as a heterodimer is necessary for induction of neutralizing antibodies in mice immunized with recombinant Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74:10623–10630. doi: 10.1128/jvi.74.22.10623-10630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis NL, Caley IJ, Brown KW, Betts MR, Irlbeck DM, et al. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74:371–378. doi: 10.1128/jvi.74.1.371-378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson JA, Hevey M, Bakken R, Guest S, Bray M, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 35.Johnston RE, Johnson PR, Connell MJ, Montefiori DC, West A, et al. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine. 2005;23:4969–4979. doi: 10.1016/j.vaccine.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 36.White LJ, Wang JG, Davis NL, Johnston RE. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J Virol. 2001;75:3706–3718. doi: 10.1128/JVI.75.8.3706-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol. 2000;74:3366–3378. doi: 10.1128/jvi.74.7.3366-3378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryman KD, Meier KC, Nangle EM, Ragsdale SL, Korneeva NL, et al. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J Virol. 2005;79:1487–1499. doi: 10.1128/JVI.79.3.1487-1499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery SA, Berglund P, Beard CW, Johnston RE. Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages. J Virol. 2006;80:7729–7739. doi: 10.1128/JVI.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ventoso I, Sanz MA, Molina S, Berlanga JJ, Carrasco L, et al. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 2006;20:87–100. doi: 10.1101/gad.357006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perri S, Greer CE, Thudium K, Doe B, Legg H, et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J Virol. 2003;77:10394–10403. doi: 10.1128/JVI.77.19.10394-10403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson JM, Whitmore AC, Konopka JL, Collier ML, Richmond EM, et al. Mucosal and systemic adjuvant activity of alphavirus replicon particles. Proc Natl Acad Sci U S A. 2006;103:3722–3727. doi: 10.1073/pnas.0600287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis NL, Willis LV, Smith JF, Johnston RE. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology. 1989;171:189–204. doi: 10.1016/0042-6822(89)90526-6. [DOI] [PubMed] [Google Scholar]
- 44.Berge TO, Banks IS, Tigertt WD. Attenuation of Venezuelan equine encephalomyelitis virus by in vitro cultivation in guinea pig heart cells. American Journal of Hygiene. 1961;73:209–218. [Google Scholar]
- 45.Reap EA, Dryga SA, Morris J, Rivers B, Norberg PK, et al. Cellular and humoral immune responses to alphavirus replicon vaccines expressing cytomegalovirus pp65, IE1, and gB proteins. Clin Vaccine Immunol. 2007;14:748–755. doi: 10.1128/CVI.00037-07. Epub 2007 Apr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]