Abstract
Sepsis induces extensive lymphocyte apoptosis, a process which may be beneficial to host survival by down-regulating the inflammatory response or, alternatively, harmful by impairing host defenses. To determine the beneficial vs. adverse effects of lymphocyte apoptosis in sepsis, we blocked lymphocyte apoptosis either by N-benzyloxycarbonyl-Val-Ala-Asp(O-methyl) fluoromethyl ketone (z-VAD), a broad-spectrum caspase inhibitor, or by use of Bcl-2 Ig transgenic mice that selectively overexpress the antiapoptotic protein Bcl-2 in a lymphoid pattern. Both z-VAD and Bcl-2 prevented lymphocyte apoptosis and resulted in a marked improvement in survival. z-VAD did not decrease lymphocyte tumor necrosis factor-α production. Considered together, these two studies employing different methods of blocking lymphocyte apoptosis provide compelling evidence that immunodepression resulting from the loss of lymphocytes is a central pathogenic event in sepsis, and they challenge the current paradigm that regards sepsis as a disorder resulting from an uncontrolled inflammatory response. Caspase inhibitors may represent a treatment strategy in this highly lethal disorder.
Keywords: apoptosis, shock, endotoxin, mortality, programmed cell death
Sepsis is the leading cause of death in many intensive care units (1). The Centers for Disease Control estimated that over 500,000 people develop sepsis and 175,000 die annually in the United States alone (1). Recently, apoptosis has been identified as an important cause of lymphocyte cell death in sepsis (2–4). Sepsis results in activation of numerous proinflammatory mediators, which may damage cells and result in organ injury. A major focus of sepsis research has been the development of antiinflammatory strategies, e.g., antiendotoxin or anticytokine agents (5). If a hyperinflammatory state exists, apoptosis may be beneficial to the host by eliminating lymphocytes that produce excessive proinflammatory cytokines (4, 5). Conversely, lymphocyte apoptosis may be harmful in sepsis by causing depletion of lymphocytes that are essential for defense against invading microorganisms (4, 5).
Numerous studies demonstrate the key role of the “executioner” cysteine–aspartate proteases (termed “caspases”) that are triggered in response to proapoptotic stimuli and that result in disassembly of the cell (6, 7). The decisive role of caspases in the cell death program has been demonstrated by studies in which pharmacologic blocking of caspase activation prevented apoptosis and improved organ function and/or survival in animal models of ischemia/reperfusion injury and meningitis (8–11).
Apoptosis is also modulated by the bcl-2 gene family, which contains both pro- and antiapoptotic members (12–14). The protein product Bcl-2 can prevent apoptotic cell death from a wide array of adverse stimuli, including hypoxia, serum and growth factor withdrawal, glucocorticoids, and ionizing irradiation, among others (15, 16).
The present experiments were performed to determine whether either caspase inhibition or Bcl-2 overexpression could prevent sepsis-induced lymphocyte apoptosis. Survival studies were also undertaken to show whether prevention of lymphocyte apoptosis could result in an improvement in survival in a clinically relevant animal model of sepsis. Investigation of potential mechanisms responsible for the antiapoptotic effect of Bcl-2 and N-benzyloxycarbonyl-Val-Ala-Asp(O-methyl) fluoromethyl ketone (z-VAD) were performed as well.
Herein, we show that use of a pan-caspase inhibitor or Bcl-2 overexpression prevents lymphocyte apoptosis in sepsis. Significantly, both therapies caused a marked improvement in survival as well. These findings suggest that (i) immunodepression resulting from the loss of lymphocytes is a key mechanism in sepsis, and (ii) caspase inhibitors may provide a strategy to improve outcome in sepsis.
Methods
Bcl-2-Immunoglobulin Transgenic Mice.
Generation of the bcl-2-Ig transgenic mice has been described previously (17). Briefly, the molecular consequences of the t(14;18)(q32;q21) chromosome were reconstructed by generating minigene constructs of the bcl-2-Ig gene found on the der(14) chromosome. bcl-2-Ig constructs were microinjected into the pronuclei of C57B6/J × C3H/He F1 fertilized eggs, and two-cell stage embryos were reimplanted into pseudopregnant ICR outbred females. The resultant bcl-2-Ig transgenics (B6C3F1) were backcrossed to B6C3F1 mice for over six generations. The tissue-specific expression of the bcl-2-Ig transgene was examined in a variety of organs, and all transgenic mice examined demonstrated a lymphoid pattern of expression revealing transcripts in spleen and thymus. In the present study, PCR of tail-snip DNA was performed to identify the human bcl-2 in transgenic mice. Tails from transgenic mice were positive for human bcl-2, whereas tails from wild-type control mice were negative (data not shown).
Immunohistochemical Staining for Human Bcl-2.
Paraffin-embedded slides were dewaxed and rehydrated. Endogenous peroxidase activity was blocked by incubating in 3% H2O2 in methanol for 20 min at room temperature. Antigen retrieval was performed by microwaving slides in 300 ml of 0.01 M citrate buffer (pH 6.0) for 10 min. A primary mouse anti-human mAb (PharMingen, catalog no. 65111A) was applied (17.5 μg/ml) at room temperature for 1 hr. After rinsing, a secondary rat anti-mouse Ab (PharMingen, catalog no. M031737) (10 μl/ml) was added at room temperature for 30 min. After rinsing, a strepavidin-biotin complex (VECTASTAIN ABC; Vector Laboratories) was applied at room temperature for 30 min. After rinsing, metal-enhanced diaminobenzidine (Pierce) was applied. Slides were counterstained with hematoxylin.
Sepsis Model: Cecal Ligation and Puncture (CLP).
Mice weighing 18–26 g (8–12 wk of age) were housed for at least 3 days before manipulations. The CLP model was used to induce intraabdominal peritonitis, as described previously (4). As described previously, blood cultures are positive for many aerobic and anaerobic Gram-positive and Gram-negative bacteria in CLP, but not sham-operated mice. Mice were anesthetized with halothane, and a midline abdominal incision was made. The cecum was mobilized, ligated below the ileocecal valve, and punctured twice with a 25-gauge needle. The abdomen was closed in two layers, and the mice were injected s.c. with 1.0 ml of 0.9% saline. Sham-operated mice were handled in the same manner, except the cecum was not ligated or punctured.
Survival Studies.
Bcl-2.
The methods for the survival studies have been described previously (18). Bcl-2 overexpressor mice and age and sex-matched B6C3F1 mice underwent CLP. Approximately 1 hr after CLP, mice received metronidazole (35 mg/kg) and cefriazone (50 mg/kg) by i.p. injection. Antibiotics were repeated every 12 hr for 48 hr and then discontinued. Mice were allowed free access to food and water.
z-VAD.
z-VAD was purchased from Enzyme Systems Products (Livermore, CA). z-VAD was dissolved in DMSO and then diluted in PBS (15% vol/vol). For z-VAD survival studies, female ND4 mice (18–25 g) (Harlan, Omaha, NE) were used. Thirty minutes after CLP, mice received metronidazole and ceftriazone. The dose and time of z-VAD administration relative to CLP are provided in Fig. 5.
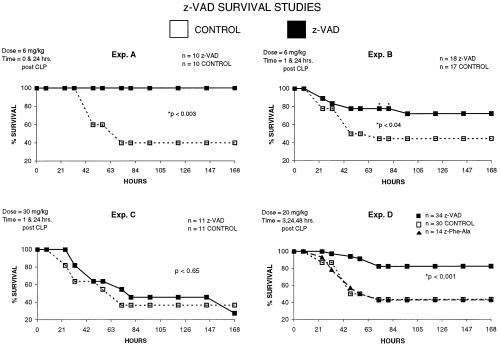
Figure 5.
The caspase inhibitor z-VAD improves survival in sepsis. A series of survival studies were performed in which ND4 mice underwent CLP and received antibiotics, as described in Methods. Next, mice received z-VAD or the diluent in which z-VAD was dissolved. The dose (mg/kg body weight) and time of administration of z-VAD are indicated in the upper left corner of each experiment. n = number of mice per group. (Exp. A) In this study, z-VAD was administered at the same time as CLP. Mice in the z-VAD group had improved survival; *, P < 0.003. (Exp. B) Mice received z-VAD (6 mg/kg) 1 hr after CLP. Mice treated with z-VAD had improved survival at 82–84 hr after CLP, compared with control mice; *, P < 0.04. (Exp. C) Mice received high-dose z-VAD (30 mg/kg) 1 hr after CLP. There was no difference in survival in the treated vs. untreated group. (Exp. D) Three separate studies were performed, and the results were combined. Mice received 20 mg/kg z-VAD or z-FA-FMK (z-Phe-Ala; a control noncaspase inhibitor that does have limited activity to block cathepsins) 1, 24, and 48 hr after CLP. A third group of mice (CONTROL) received the diluent for the z-VAD, i.e., DMSO in PBS (see Methods). Mice in the z-VAD-treated group had improved survival compared with both z-FA-FMK (P < 0.02) and control (P < 0.001).
N-Benzyloxycarbonyl-Phe-Ala-fluoromethyl ketone (z-FA-FMK).
A commonly employed negative control inhibitor, z-FA-FMK (19–21), was purchased from Enzyme Systems Products and was dissolved and given in the same manner as z-VAD. z-FA-FMK lacks aspartate in the P1 position and therefore does not block caspase activity (19–21). The results are shown in Fig. 5D.
Flow Cytometry.
Flow cytometry was performed as previously described (18). Briefly, tissue sections from thymi (10–20 mg) or spleens (100–200 mg) were gently glass-ground to dissociate the cells, which were washed twice in PBS with 1% BSA and 0.01% sodium azide. Red cells were lysed by hypotonic lysis in ice-cold ammonium chloride. The cells were resuspended in PBS, and the desired fluorescent indicator was added. The degree of apoptosis was quantified by using a commercially available kit with fluorescein-labeled annexin V (R & D Systems), as previously described (18). Mouse T and B cells were identified by using fluorescently labeled anti-mouse mAbs specific for the desired cell subtype (PharMingen). Identification of apoptotic cells and cell phenotyping were performed simultaneously on a FACSCalibur (Becton Dickinson).
Cytokine Analysis.
To determine the effect of z-VAD on cytokine production, isolated thymocytes were prepared and incubated in cell culture medium (RPMI 1640) at 37°C under 95% O2/5% CO2, as described above. z-VAD (100 ng/ml) was added to one group of cells. The thymocytes with or without z-VAD were stimulated with phorbol 12-myristate 13-acetate (5 ng/ml) and ionomycin (500 ng/ml). After 4 hr of incubation and stimulation, the supernatant was obtained. Tumor necrosis factor (TNF)-α was assayed in duplicate by ELISA using commercially available kits (Genzyme), as previously described (18). For each assay, a standard curve was generated and, based on replicates of the measured absorbance, demonstrated an average coefficient of variance of <10%.
Statistical Analysis.
Data reported are mean ± SEM. Data were analyzed with a statistical software program RS/1 from BBN Software Products (Cambridge, MA). Differences in group survival were analyzed by Fisher’s exact p test. All other data were analyzed by one-way ANOVA. Significance was accepted at P < 0.05.
Results
Sepsis Causes Extensive Apoptosis of T and B Cells.
Thymi and spleens were harvested 18–24 hr after CLP or sham surgery, and cells were isolated from these organs. Flow cytometry demonstrated that sepsis caused a marked increase in apoptosis of thymic T cells and splenic T and B cells in B6C3F1 mice (Fig. 1 A and B). In thymi, CD4, CD8, and doubly positive CD4+ and CD8+ T cells had increased apoptosis in sepsis. In spleens, sepsis increased lymphocyte apoptosis in T cells (CD3+); both CD4+ and CD8+ T cells were affected. Sepsis also increased splenic B cell (CD19) apoptosis.
Figure 1.
Bcl-2 prevents apoptosis in sepsis. Thymocytes or splenocytes from septic or sham-operated Bcl-2 transgenic or B6C3F1 mice were examined for apoptosis by flow cytometry and the apoptosis indicator annexin V. Different classes of T or B cells were determined by labeling cell markers, i.e., CD4, CD8, CD3, or CD19 with fluorophore-conjugated Abs from PharMingen. Sepsis increased apoptosis in T and B cells from thymi and spleens of B6C3F1 mice; *, P < 0.05 septic vs. sham. Although there was no protection from apoptosis in thymocytes from septic Bcl-2 transgenic mice, both T and B cells in spleens from septic Bcl-2 mice were protected against sepsis-induced apoptosis; *, P < 0.05 septic vs. sham, n = 5 sham and n = 7–8 septic for both Bcl-2 and B6C3F1 mice. The protection by Bcl-2 was consistent with the greater expression of Bcl-2 in spleen vs. thymus, as seen in immunohistochemical stains (see Fig. 2).
In Bcl-2 transgenic mice, thymocytes from septic mice had increased apoptosis compared with control mice (Fig. 1C). In contrast to thymocytes, in the spleen, both B cells (CD19+) and T cells (CD4+ and CD8+) were protected from sepsis-induced apoptosis and were not different from sham-operated mice (Fig. 1D).
Bcl-2 Protects Against Lymphocyte Apoptosis.
Immunohistochemical staining for human Bcl-2 protein was negative in sham-operated and septic non-Bcl-2-Ig transgenic mice (data not shown). Bcl-2 Ig transgenic mice demonstrated intense expression of human Bcl-2 in spleen (Fig. 2 Top) with a markedly decreased expression of Bcl-2 in thymus [similar to the original description of these transgenic mice (17)] (Fig. 2 Middle). In the spleen, Bcl-2 was expressed in both the B cell region (lymphoid follicle) and T cell region (periarteriolar lymphoid sheath).
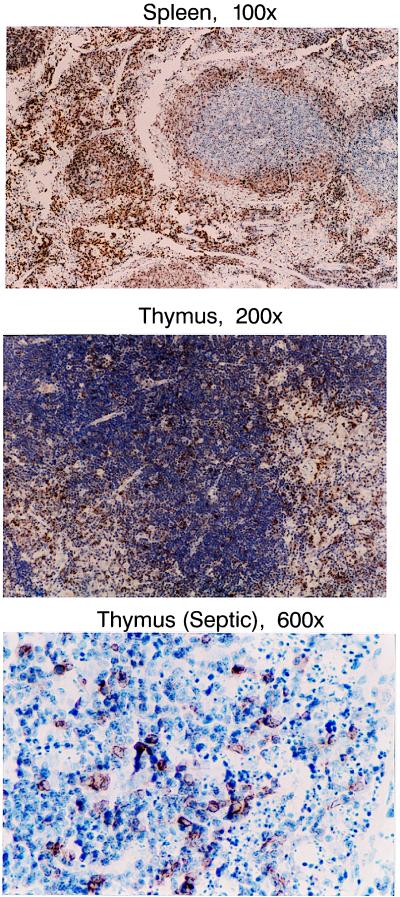
Figure 2.
Immunohistochemical staining for human Bcl-2 in thymi and spleens from Bcl-2 transgenic mice. Splenocytes that have a brown color (resulting from diaminobenzidine staining) are positive for human Bcl-2. Note the much greater staining in spleen vs. thymus in the Bcl-2 transgenic mice (Top vs. Middle). Although there was much less staining in thymi vs. spleens of Bcl-2 transgenics, the cells that were positive for Bcl-2 in thymi were resistant to sepsis-induced apoptosis. As is apparent in Bottom, none of the Bcl-2-positive cells in the septic thymus were apoptotic, i.e., none of the Bcl-2-positive cells had features of pyknosis or karyorrhexis (n = 10 thymi from septic Bcl-2-Ig transgenic mice)
Determination of the degree of apoptosis by flow cytometry and annexin-V labeling demonstrated significant sepsis-induced apoptosis in thymocytes and splenocytes of B6C3F1 but not Bcl-2 overexpressor mice. There was complete protection from apoptosis in both splenic B and T cells of Bcl-2 transgenic mice with sepsis (Fig. 1D). There was no demonstrable protection against apoptosis in thymocytes from Bcl-2 transgenic mice (Fig. 1C). To determine whether the lack of protection in thymocytes was the result of the lower level of expression of Bcl-2 in thymi vs. spleen in the Bcl-2 transgenics, light microscopic examination of thymi stained for Bcl-2 expression was performed. Light microscopic examination of thymi from septic Bcl-2 transgenic mice did confirm the antiapoptotic effect of Bcl-2 in thymocytes during sepsis by uniformly demonstrating that none of the thymocytes with apoptotic features of pyknosis or karyorrhexis were positively stained for Bcl-2 (n = 10 septic Bcl-2 transgenic mice) (Fig. 2 Bottom).
z-VAD Prevents Sepsis-Induced Apoptosis in Thymi and Spleens.
To reflect the clinical condition, the pan-caspase inhibitor z-VAD was administered 60 min after induction of sepsis by CLP. z-VAD prevented sepsis-induced lymphocyte apoptosis in both thymocytes and splenocytes in a dose-dependent fashion (Fig. 3).
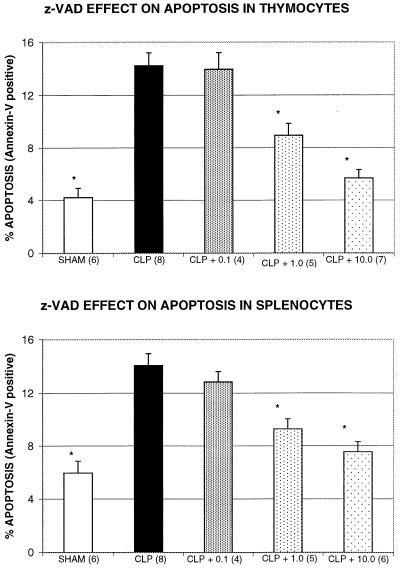
Figure 3.
z-VAD prevents apoptosis in thymocytes and splenocytes during sepsis. Female ND4 mice (Harlan) underwent CLP or sham surgery, as described in Methods. One hour after surgery, mice received z-VAD at 0.1, 1.0, or 10.0 mg/kg body weight. Apoptosis was evaluated 18–22 hr after CLP or sham surgery by annexin V labeling and flow cytometry of isolated thymocytes (Upper) or splenocytes (Lower), as described in Methods. Sham-operated mice and mice treated with 1.0 or 10.0 mg/kg body weight z-VAD were significantly different from untreated CLP mice; *, P < 0.05. The number of mice per group is indicated in parentheses.
z-VAD Did Not Block TNF-α Production.
TNF-α is an important mediator of sepsis and can induce apoptosis in certain cells (5). To determine whether the protective effect of z-VAD could be the result of decreased TNF production, isolated thymocytes were incubated with or without z-VAD and stimulated with phorbol myristate acetate + ionomycin. The supernatant was obtained 4 hr after stimulation, and TNF-α was assayed. TNF-α (pg/1 × 106 cells) in control thymocytes was 6.95 ± 0.26 (n = 6) and increased to 11.55 ± 0.36 (n = 6) with stimulation (P < 0.05). Cells incubated with z-VAD had an increase in TNF that was comparable to nontreated cells, i.e., 8.4 ± 0.46 (n = 6) prior to stimulation and 12.08 ± 0.96 (n = 6) after stimulation.
Overexpression of Bcl-2 Improves Survival in Sepsis.
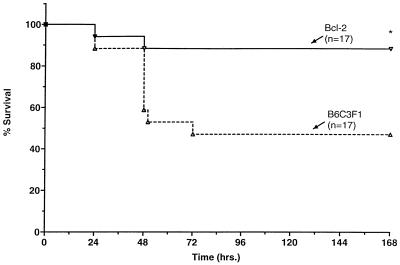
Bcl-2 transgenic mice (n = 17) and age- and sex-matched B6C3F1 mice (n = 17) underwent CLP to induce abdominal sepsis. Approximately 1 hr postsurgery, mice received antibiotics (metronidazole and cefriaxone) by i.p. injection, and survival was recorded daily. After 7 days, 47% of B6C3F1 mice were alive, whereas 88% of Bcl-2 transgenic mice survived (Fig. 4); this improvement in survival was statistically significant (P < 0.02).
Figure 4.
Overexpression of Bcl-2 improves survival in sepsis. Generation of the Bcl-2-Ig transgenic mice has been described previously (17) (see Methods). Bcl-2 overexpressor mice and age- and sex-matched B6C3F1 mice underwent CLP. One hour after CLP, mice received metromidazole and ceftriazone as antibiotic coverage (see Methods for details). Antibiotics were repeated every 12 hr for 48 hr, and survival was improved in Bcl-2 overexpressors vs. B6C3F1 mice; *, P < 0.02. n = number of mice in each group.
z-VAD Improves Survival in Sepsis.
The dose and time of administration of z-VAD were varied in a series of survival studies. Antibiotics were administered 30 min after CLP in all groups.
In experiment A (Fig. 5), z-VAD (6 mg/kg) was administered immediately and 24 hr after CLP. Control mice received the diluent for z-VAD [DMSO in PBS (15% vol/vol)] immediately after CLP and 24 hr later. After 7 days, there was a 40% survival in the control group vs. a 100% survival in the z-VAD group, and this difference was significant (P < 0.003).
In experiment B (Fig. 5), z-VAD (6 mg/kg) or the diluent for z-VAD was administered 1 hr and 24 hr after CLP. After 7 days, there was a 44.4% survival in the control vs. a 72.2% survival in the z-VAD-treated group. At 82–84 hr, the z-VAD-treated mice had a statistically improved survival compared with the control mice (P < 0.04).
In experiment C (Fig. 5), high-dose z-VAD (30 mg/kg) or the z-VAD diluent was administered 1 hr and 24 hr after CLP to the respective groups. There was no difference in survival between the two groups.
In experiment D (combined results of three separate experiments; Fig. 5), intermediate-dose z-VAD (20 mg/kg) or the diluent was administered 3, 24, and 48 hr after CLP. In one of the three studies, an additional group of mice received z-FA-FMK, which is the negative control for z-VAD (19–21). This compound has no effect on caspases. After 7 days, there was an 82.4% survival in the z-VAD group vs. a 43.3% survival in the diluent control group, and this difference was statistically significant (P < 0.001). There was a 42.9% survival in the z-FA-FMK-treated mice, which was not statistically different from the diluent control group.
In addition to differences in survival, there was a subjective distinction in the general appearance and activity of the z-VAD-treated mice vs. the control group. With the exception of the group of mice that received high-dose z-VAD, i.e., 30 mg/kg, the z-VAD-treated mice were more active within their cages, had less piloerection, and struggled more during drug injections. These physical differences were noted within a few hours postsurgery and persisted in the z-VAD-treated mice for the duration of the study.
Discussion
Sepsis accelerates lymphocyte apoptosis in both animals and patients. The present study is important because it shows that prevention of lymphocyte apoptosis by either of two completely independent methods, (i.e., caspase inhibition or Bcl-2 overexpression,) is associated with improved survival in our well-characterized murine model of sepsis. This work is in agreement with our previous related investigation (18), in which a different transgenic mouse line that selectively overexpressed Bcl-2 in T cells exhibited little or no T lymphocyte apoptosis and showed markedly improved outcome in CLP-induced sepsis. The present findings, which show that mice that selectively overexpress Bcl-2 in B cells also have improved survival in sepsis, demonstrate an important role for Ig-producing cells as well. Considered together, these series of experiments provide compelling evidence for the critical role of the lymphocyte in resolving severe infection. An effective host response to invading microorganisms must entail a coordinated interplay among the various immunologic cells, including B and T cells and macrophages (22, 23). For example, lymphocytes produce a host of cytokines that activate macrophages and vice versa. Furthermore, B cells as well as macrophages have an important role as antigen-presenting cells to T cells (24). The well-documented apoptosis of lymphocytes during sepsis occurs systemically, includes both B and T cells, (2–4, 25) and is extensive, involving ≈12–25% of lymphocytes in spleen and thymus (Fig. 1). Apoptotic cells undergo rapid phagocytosis, with the entire process lasting less than 2 hr in some cells (26). Therefore, it is likely that the percentage of lymphocytes that actually undergo apoptosis is underestimated by the present results. This significant decrease in the number of lymphocytes will impact multiple facets of the immunological response and may lead to uncontrolled infection and death.
The present findings call into question the current paradigm for much of contemporary sepsis research, which is based on the concept of organ injury resulting from an uncontrolled inflammatory response (5, 27, 28). Rather, the present results agree with work of Lederer et al. (29), who suggest that the major defect in sepsis is an impaired immunologic response to infection. The failure of many antiinflammatory-based therapeutic trials in sepsis, i.e., anti-endotoxin, anti-TNF, anti-IL-1, steroids, anti-platelet-activating factor, etc., to improve outcome in sepsis has led some investigators to question the hypothesis that the major defect in sepsis is uncontrolled inflammation (27, 30). Recent studies in which administration of the immune-enhancing cytokine interferon-γ improved outcome in septic patients support our contention that the major defect in sepsis is an inadequate immune response (31).
A growing body of data suggests that the present laboratory findings are applicable in the clinical arena. Castelino et al. (32) reported that, in hospitalized patients, decreased circulating lymphocytes (lymphopenia) were most frequently the result of sepsis and trauma. Cheadle et al. (33) showed that trauma patients developed lymphopenia that was maximal at day 3. The lowest numbers of lymphocytes occurred in trauma patients who developed infection or death. Rajan et al. (34) noted that patients in whom the circulating lymphocyte count was decreased for at least a 3-day duration had a greatly increased risk of nosocomial sepsis. In a prospective study of 20 patients who died of sepsis and multiple organ dysfunction, we demonstrated caspase 3 activation and focal apoptosis in ≈56% of spleens from septic patients (35). In some spleens, 6–25% of splenocytes were focally apoptotic. Minimal apoptosis was observed in spleens from patients who died of nonseptic causes. Additional indirect evidence of the extensive nature of sepsis-induced lymphocyte apoptosis in septic patients included a profound decrease in the number of circulating lymphocytes and a markedly decreased lymphocyte density in splenic white pulp (35). Together, these clinical investigations indicate that immunodepression associated with lymphocyte depletion is a marker of severe infection and organ dysfunction.
A second important implication of the present study is the potential for a therapeutic approach to sepsis. Both Bcl-2 overexpression and the broad-spectrum caspase inhibitor z-VAD improved survival. It is possible that lymphocytes may be protected from apoptosis in sepsis by administration of drugs that increase either Bcl-2 or other antiapoptotic Bcl-2 family members, such as Bcl-xL. Among the many effects of IL-2 is induction of Bcl-2 and Bcl-xL (36). IL-2 has been shown to improve survival in certain models of acute bacterial infection (37), and it is possible that IL-2’s protective effect is the result of prevention of lymphocyte apoptosis by production of antiapoptotic Bcl-2 family members.
In this context, there is a tremendous interest in the potential clinical use of caspase inhibitors because of their efficacy in a variety of clinical models, including ischemia/reperfusion injury in brain (8, 9) and liver (10), and in the prevention of neuronal cell death in bacterial meningitis (11). Of particular relevance to the present study is the report by Braun et al. (11), who noted neuroprotection by caspase inhibition in bacterial meningitis. Although no outcome studies were performed, Braun et al. showed that pneumococcal meningitis caused extensive apoptosis of hippocampal neurons, which was prevented by z-VAD (11). These investigators did not comment on lymphocyte apoptosis in their model. The CLP model employed in the present study is an extensively utilized model that is felt to be the most clinically relevant animal model of sepsis (38). In the CLP model, apoptosis occurs primarily in lymphocytes with a secondary but diminished involvement of gastrointestinal epithelial cells (4). No apoptosis occurs in brain, heart, liver, or kidney in the CLP model of sepsis. Importantly, z-VAD was effective in preventing lymphocyte apoptosis in sepsis even when administered after the onset of sepsis. There was improved survival in sepsis even after a 3-hr delay in administration of z-VAD. Thus, a therapeutic window exists in which caspase inhibitors may be effective after the onset of the disorder.
The protective effect of z-VAD on survival in sepsis was lost at the highest dose, i.e., 30 mg/kg. Although there is no ready explanation for this loss of efficacy at a high dose of z-VAD, it may be related to an unrecognized toxic or nonspecific effect of these compounds. In addition to blocking caspases, fluoromethyl ketone compounds such as z-VAD inhibit a variety of cathepsins that are involved in the inflammatory response (39, 40). Recent studies have shown that excessive blockade of the inflammatory response in sepsis may worsen outcome in the disorder (25). Thus, it is possible that z-VAD at high concentrations may impact other aspects of the host response to sepsis.
An interesting finding noted in the splenocytes of the Bcl-2 overexpressors was the decrease in apoptosis in the T cells (Fig. 1). Given that Bcl-2 was overexpressed only in B cells, it is surprising that the T cells would also be protected. We observed a similar phenomenon in Bcl-2 transgenic mice in which Bcl-2 overexpression in T cells was associated with decreased apoptosis in B cells as well (18). As we speculated in the previous studies (18), a potential paracrine effect may be responsible for the cross-cell protection. Su et al. (41) demonstrated that paracrine apoptosis occurred in Jurkat T cells and was mediated by shedding of Fas ligand. Therefore, if B cell apoptosis is decreased in the spleen, neighboring T cells may also be protected.
Cytokines such as TNF-α are important mediators of the septic response (28) and may induce apoptosis in certain cells. Therefore, one way in which z-VAD may be protective could be by reducing cytokine production in sepsis. The present results showing that z-VAD-treated cells do not make less TNF-α compared with untreated cells suggest that the protective effect of z-VAD is not related to blockade of TNF-α production, although effects on other proapoptotic cytokines are not excluded.
In summary, prevention of lymphocyte apoptosis in sepsis results in a marked improvement in survival. Immune depression resulting from the loss of lymphocytes may be the key factor in inability to survive sepsis. Caspase inhibitors may represent a therapeutic approach in the treatment of sepsis.
Acknowledgments
This work was supported by the National Institutes of Health Grants GM44118 and GM55194 and the Alan A. and Edith L. Wolff Foundation.
Abbreviations
- z-VAD
N-benzyloxycarbonyl-Val-Ala-Asp(O-methyl) fluoromethyl ketone
- CLP
cecal ligation and puncture
- z-FA-FMK
N-benzyloxycarbonyl-Phe-Ala fluoromethyl ketone
- TNF
tumor necrosis factor
References
- 1.Stone R. Science. 1994;264:365–367. doi: 10.1126/science.8153620. [DOI] [PubMed] [Google Scholar]
- 2.Wang S D, Huang K J, Lin Y S, Lei H Y. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 3.Ayala A, Herndon C, Lehman D, DeMaso C, Ayala C, Chaudry I. Shock. 1995;3:259–268. doi: 10.1097/00024382-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss R S, Swanson P E, Cobb J P, Jacobson A, Buchman T G, Karl I. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Bone R C. Crit Care Med. 1996;24:1125–1129. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 7.Thornberry N A, Lazebnik Y A. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 8.Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gomez-Isla T, Hyman B T, Moskowitz M A. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez I, Matsuura K, Ody C, Nagata S, Vassalli P. J Exp Med. 1996;184:2067–2072. doi: 10.1084/jem.184.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cursio R, Guggenheim J, Ricci J E, Crenesse D, Rostagno P, Maulon L, Saint-Paul M C, Ferrua B, Auberger A P. FASEB J. 1999;13:253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 11.Braun J S, Novak R, Herzog K-H, Bodner S M, Cleveland J L, Tuomanen E I. Nat Med. 1999;5:298–302. doi: 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 12.Korsmeyer S J, Shutter J R, Veis D J, Merry D E, Oltvai Z N. Cancer Biol. 1993;4:327–335. [PubMed] [Google Scholar]
- 13.Reed J C. Nature (London) 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 14.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 15.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–886. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 16.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 17.McDonnell T J, Deane N, Platt F M, Nunez G, Jaeger U, McKearn J P, Korsmeyer S J. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss R S, Swanson P E, Knudson C M, Chang K C, Cobb J P, Osborne D F, Zollner K M, Buchman T G, Korsmeyer S J, Karl I E. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 19.Sarin A, Wu M L, Henkart P A. J Exp Med. 1996;184:2445–2450. doi: 10.1084/jem.184.6.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pronk G J, Ramer K, Amiri P, Williams L T. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 21.McColl K S, He H, Zhong H, Whitacre C M, Berger N A, Distelhorst C W. Mol Cell Endocrinol. 1998;139:229–238. doi: 10.1016/s0303-7207(98)00051-3. [DOI] [PubMed] [Google Scholar]
- 22.Abbas A K, Lichtman A H, Prober J S. Cellular and Molecular Immunology. 3rd Ed. Philadelphia: Saunders; 1997. pp. 42–46. [Google Scholar]
- 23.Paul W E. Fundamental Immunology. 3rd Ed. New York: Raven; 1993. pp. 668–671. [Google Scholar]
- 24.Roitt I M. Essential Immunology. London: Blackwell Scientific; 1997. pp. 174–175. [Google Scholar]
- 25.Ayala A, Herndon C, Lehman D, Ayala C, Chaudry I H. Blood. 1996;87:4261–4275. [PubMed] [Google Scholar]
- 26.McCarthy N J, Evan G I. Curr Top Dev Biol. 1998;36:259–278. doi: 10.1016/s0070-2153(08)60507-4. [DOI] [PubMed] [Google Scholar]
- 27.Nasraway S A. Crit Care Med. 1999;27:427–430. doi: 10.1097/00003246-199902000-00054. [DOI] [PubMed] [Google Scholar]
- 28.Bernard G R. Crit Care Med. 1999;27:434–436. doi: 10.1097/00003246-199902000-00057. [DOI] [PubMed] [Google Scholar]
- 29.Lederer J A, Rodrick M L, Mannick J A. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Remick D G, Garg S J, Newcomb D E, Wollenberg G, Huie T K, Bolgos G L. Crit Care Med. 1998;26:895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 31.Docke W E, Randow F, Syrbe U, Krausch D, Asabullah K, Reinke P, Volk H C, Kox W. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 32.Castelino D J, McNair P, Kay T W H. Aust N Z J Med. 1997;27:170–174. doi: 10.1111/j.1445-5994.1997.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheadle W G, Pemberton R M, Robinson D, Livingston D H, Rodriguiz J L, Polk H C., Jr J Trauma. 1993;35:844–851. doi: 10.1097/00005373-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Rajan G, Sleigh J W. Intensive Care. 1997;23:1187. doi: 10.1007/s001340050482. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss R S, Swanson P E, Freeman B D, Tinsley K W, Cobb J P, Matuschak G M, Buchman T G, Karl I E. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Lord J D, McIntosh B C, Greenberg P D, Nelson B H. Shc J Immunol. 1998;161:4627–4633. [PubMed] [Google Scholar]
- 37.Goronzy J, Weyand C, Quan J, Fathman C G, O’Hanley P. J Immunol. 1989;142:1134–1138. [PubMed] [Google Scholar]
- 38.Deitch E A. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Esser R E, Watts L M, Angelo R A, Thornburg L P, Prior J J, Palmer J T. J Rheumatol. 1993;20:1176–1183. [PubMed] [Google Scholar]
- 40.Garcia-Calvo M, Peterson E P, Leiting B, Ruel R, Nicholson D W, Thornberry N A. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 41.Su X, Cheng J, Liu W, Liu C, Wang Z, Yang P, Zhou T, Mountz J D. J Immunol. 1998;160:5288–5297. [PubMed] [Google Scholar]