Abstract
The increase in excitatory outflow from the medial prefrontal cortex is critical to the development of sensitization to amphetamine. There is evidence that psychostimulant-induced changes in dopamine-GABA interactions are key to understanding the behaviorally sensitized response. The objective of this study was to characterize the effects of different amphetamine paradigms on the Fos activation of GABAergic interneurons that contain parvalbumin in the medial prefrontal cortex. Although a sensitizing, repeated regimen of amphetamine induced Fos in all cortical layers, only layer V parvalbumin-immunolabeled cells were activated in the infralimbic and prelimbic cortices. Repeated amphetamine treatment was also associated with a loss of parvalbumin immunoreactivity in layer V, but only in the prelimbic cortex. An acute amphetamine injection to naïve rats was associated with an increase in Fos, but in parvalbumin-positive neurons of the prelimbic cortex, where it was preferentially induced in layer III. These data indicate that distinct substrates mediate the response to repeated or acute amphetamine treatment. They also suggest that a sensitizing amphetamine regimen directs mPFC outflow, via changes in inhibitory neuron activation, towards subcortical centers important in reward.
Keywords: prelimbic cortex, infralimbic cortex, GABA, dopamine, Fos, behavioral sensitization
Repeated amphetamine (AMPH) exposure in humans has been associated with long-lasting changes in behavior (Robinson and Berridge, 2000). In experimental animals, such treatment progressively augments locomotion (behavioral sensitization), an effect that persists after treatment and can be measured by a drug challenge (Wolf, 1998). Sensitization to AMPH appears to require changes in dopamine and excitatory amino acid transmission in the medial prefrontal cortex (mPFC) and ventral tegmental area (VTA) (Wolf et al., 1995; Li and Wolf, 1997; Tzschentke and Schmidt, 2000; Wolf et al., 2003). Lesions of the mPFC block the development of sensitization, whether AMPH is repeatedly injected systemically or intra-VTA (Wolf et al., 1995; Li and Wolf, 1997; Cador et al., 1999; but see Tzschentke and Schmidt, 2000). AMPH sensitization also induces region-specific increases in mPFC Fos expression (Hedou et al., 2002), although the active neurons have yet to be fully identifed.
A large proportion of mPFC interneurons contain gamma-aminobutyric acid (GABA) and parvalbumin (PV) (Gabbott et al., 1997). These neurons provide the predominant local inhibitory input to pyramidal cells (Gabbott et al., 1997) through synaptic cartridges on proximal axons and contacts on cell bodies (Hendry et al., 1983; DeFelipe et al., 1989). Although the dendrites of PV-positive (+) interneurons are present in all layers, their axonal networks are densest close to the cell body and axons tend to travel in the same layer as the somata (Kawaguchi and Kubota, 1993). Mesocortical pathway stimulation activates these PV+, fast spiking interneurons (Tseng et al., 2006) possibly through dopamine-induced increases in GABAergic interneuron excitability (Gorelova et al., 2002; Tseng and O'Donnell, 2006). This means that PV+ cell activation can contribute to the silencing of pyramidal neuron activity in a layer-specific manner (Gonzalez-Islas and Hablitz, 2001; Gulledge and Jaffe, 2001; Seamans et al., 2001; Tseng and O'Donnell, 2004; Tseng et al., 2006). Following drug administration, there are data to implicate GABA transmission in the motor stimulant response (Karler et al., 1997; Bartoletti et al., 2005); however, no studies differentiate the regional or lamina-specific effects of inhibitory interneurons.
Dopaminergic terminals from VTA neurons form dense contacts onto interneurons in layers V and VI (Sesack et al., 1995) and excitatory afferents from limbic and thalamic regions innervate specific layers where they contact both PV+ interneurons and pyramidal cells (Gabbott et al., 2002; Rotaru et al., 2005; Gabbott et al., 2006). Since layer III pyramidal neurons connect the mPFC to other cortices, and layer V and VI pyramidal cells project primarily to subcortical targets (Jones, 1984; Gabbott et al., 2005), PV+ interneurons are ideally positioned to shape the receptive fields of pyramidal cells in a layer-specific manner.
Since the organization and connections of PV+ interneurons are not homogeneous, long-term neuroadaptations following AMPH exposure could differentially activate these neurons. Using Fos immunohistochemistry and the colocalization of PV immunoreactivity with Fos, we mapped the location and Fos induction of these GABAergic interneurons following acute and repeated AMPH administration.
Experimental Procedures
Animals, drugs, and behavior
All experiments were conducted in accordance with the Declaration of Helsinki and the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996 and approved by the Rosalind Franklin University Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Thirty-two male Sprague-Dawley rats (Harlan, Indianapolis, IN) were divided into 4 groups: Rats in groups 1 (repeated AMPH, n = 8) and 2 (repeated vehicle, n = 9) weighed 125-139 g at the start of experiments and those in groups 3 (acute AMPH, n = 10) and 4 (acute vehicle, n = 5) weighed 275-300 g, in order to match the final testing age of the rats in groups 1 and 2. All animals were handled for 2 days prior to the start of experiments. A repeated, intermittent injection schedule was selected that has previously been shown to induce robust behavioral sensitization and mPFC neuronal adaptations (Robinson and Camp, 1987; Paulson et al., 1991; Robinson and Kolb, 1999; Onn and Grace, 2000). The first group of rats received repeated D-amphetamine sulfate (AMPH in 0.9% saline, i.p.) injections: 3 mg/kg for 5 days, except for the first and last day when they received 1.5 mg/kg, and followed by 2 drug-free days and repeated for 3 weeks. The second group of rats was administered repeated saline injections (vehicle in the same volume) following the same procedure as for the first group. Behavioral analysis was conducted in a Plexiglas box equipped with two banks of infrared sensors that recorded movement and animal location (AccuScan Instruments, Inc., Columbus, OH, USA). On the first and last days of treatment, animals were habituated to the box, administered drug, and their movement recorded for 45 min. Locomotor sensitization was evaluated by comparing movement (total distance traveled) on the last injection day to that on the first day. Rats sensitized to AMPH and those administered saline were withdrawn for 3 weeks. All rats were handled daily for 5 days during the final week before euthanasia to avoid handling stress and its potential for Fos induction. On the final day, the AMPH-treated rats received a challenge injection of AMPH (1.5 mg/kg, i.p.) and vehicle-treated rats, a saline injection. Ninety minutes later, all animals were euthanized. The final two groups of rats were handled daily for 5 days before being administered a single injection of AMPH (1.5 mg/kg, i.p.) or saline, 90 min before euthanasia.
Animal perfusion and immunohistochemistry
Rats (n = 5 per group) were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with ice-cold 2.5% sucrose in 0.1 M phosphate buffered saline, followed by 4% buffered paraformaldehyde and 2.5% sucrose. All solutions were prepared as a single batch, and the duration of the perfusions was kept uniform throughout. The brains were removed, post-fixed for 2 h in the same fixative without sucrose, and transferred to 30% sucrose buffer overnight at 4°C. Coronal sections (70 μm) were cut on a freezing microtome. Sections through the mPFC were collected in 0.1 M phosphate buffer, cryoprotected, and stored at -20°C until processing.
Sections for light microscopy were blocked for 1 h in 10% normal horse serum and 0.1% Triton-X 100 (Tx), and then incubated for 48 h at 4° C in mouse anti-PV (1:2,000; Swant, Bellinzona, Switzerland). Sections were incubated for 2 h in biotinylated horse anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA) at room temperature (RT), followed by 1 h in avidin biotin complex (ABC Elite Kit, Vector Laboratories, Burlingame, CA). The sections were then reacted with the Vector SG substrate kit (Vector Laboratories), rinsed thoroughly, mounted from gelatin onto slides, dried, and dehydrated before coverslipping.
Sections for confocal microscopy were blocked for 3 h in 5% normal donkey serum and 0.1% Tx, followed by 72 h at 4° C in a cocktail of primary antisera containing rabbit anti-c-Fos (1:15,000; PC38; Calbiochem, San Diego, CA) and mouse anti-PV. The dilution of the mouse anti-PV sera was optimized to 1:5,000 for confocal microscopy. Sections were rinsed and blocked again for 3 h at RT in 5% normal donkey serum and 0.1% Tx, and then incubated for 2 h at RT in a second cocktail containing Cy3-conjugated donkey anti-rabbit, 1:500 (Jackson ImmunoResearch) and Cy2-conjugated donkey anti-mouse, 1:500. The sections were rinsed, mounted on poly-L-lysine slides, and coverslipped using a premixed mounting medium compatible with fluorescent microscopy and resistant to fluorescent quenching. Some sections were incubated without one of the primary or secondary antisera as controls. All solutions, including antibody preparations, were prepared as a single batch. Sections from all groups of animals were processed at the same time.
Image Analysis and Quantification
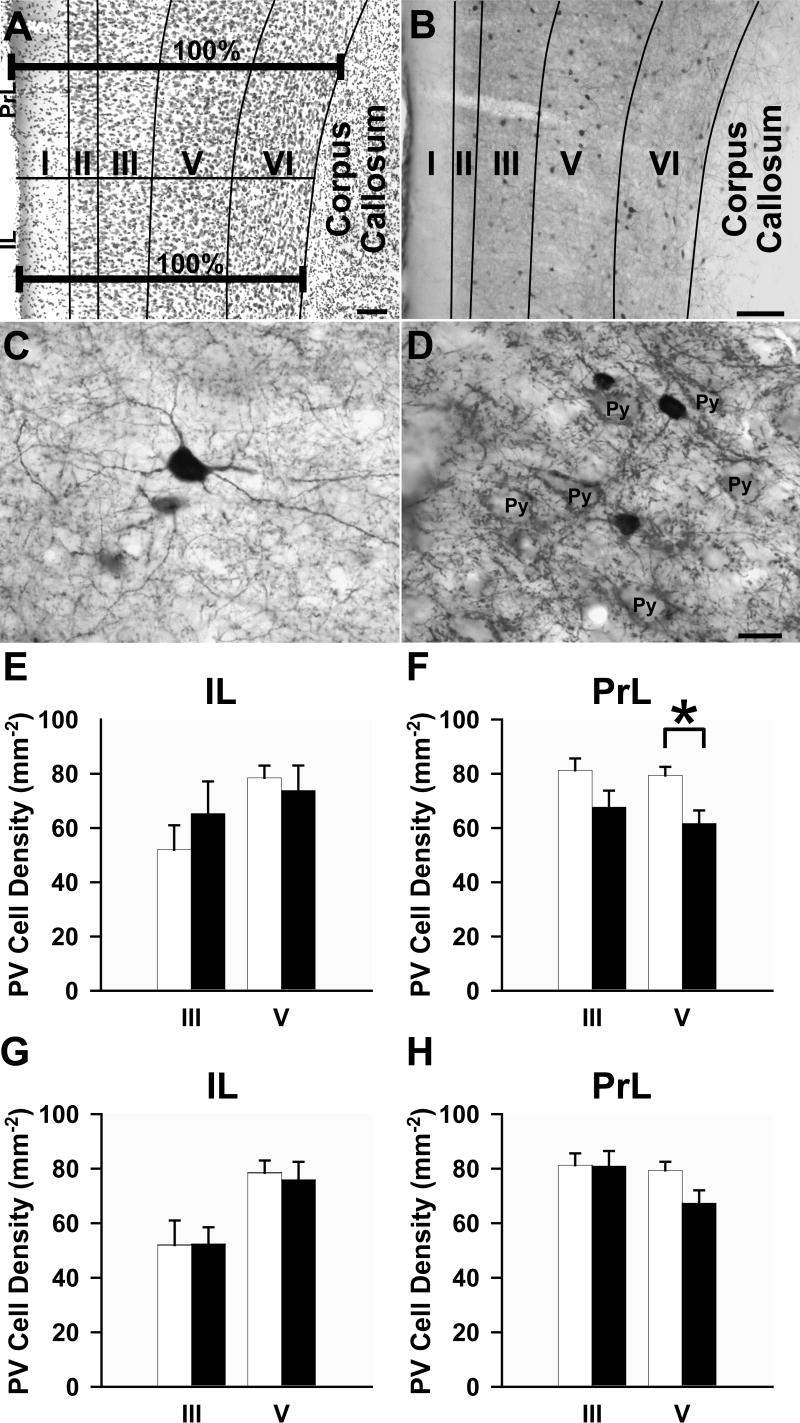
An investigator blind to the treatment groups captured images at 20X magnification on an Olympus Fluoview Series confocal laser-scanning microscope at 2 different rostrocaudal levels (from Bregma: 2.7 and 3.2 mm). The total area analyzed for the PrL was approximately 5.0 mm2 (average of 2.5 mm2 per section) and for the IL was approximately 1.88 mm2 (average of 0.94 mm2 per section). With the sequential-acquisition setting at a resolution of 1024 × 768 pixels using a z-series projection, the stacks were captured at 0.5 μm depth intervals to a total of 10 μm thickness. Images were then merged. Immunolabeled neurons in layers III and V of the prelimbic (PrL) and infralimbic (IL) cortices were captured using the appropriate laser settings. Single and dually labeled PV+ and Fos-immunoreactive cells were analyzed for each layer using the technique described by Gabbott and colleagues (1997). Briefly, the distance between the pial surface and the underlying white matter of the mPFC is assigned 100% of the width of the mPFC (Fig. 1A). This total width can be broken into the sum of the widths of each layer for each region analyzed: IL: I (17.8%), II (27.9%), III (46.6%), V (73.0%), VI (100%); PrL: I (17.4%), II (24.8%), III (44.6%), V (73.1%), VI (100%). Using this method, each layer was demarcated and layers III and V were individually analyzed (Fig. 1B). We pooled the data from 2 sections per animal and established a mean for each group.
Figure 1.
(A) Light micrograph illustrating the laminar distribution of Nissl stained neurons and glia of the mPFC using the method of delineation described by Gabbott and colleagues (1997). (B) Representative light micrograph showing laminar distribution of PV+ interneurons in the mPFC using this same method. (C) Light micrograph of a multipolar PV+ interneuron. Note the fine dendritic processes. (D) Light micrograph illustrating unstained pyramidal cells (Py) surrounded by PV+ punctae. (E-H) Bar graphs illustrating the density of PV+ interneurons in layers III and V of the (E,G) IL and (F,H) PrL areas of the mPFC. Open bars, vehicle; filled bars, (E,F) repeated or (G,H) acute AMPH administration. Scale bars: A and B, 100 μm, D (also valid for C), 15 μm. *p < 0.05, repeated AMPH group has a significantly lower density of neurons compared to vehicle.
We studied PV+ and Fos-immunoreactive densities through a 10 μm thickness of each section. Although the penetration of both these antibodies was good and the staining to this depth was uniform in all sections, the immunolabeling did not fully penetrate the sections, thereby precluding a stereological analysis of total cell number. Since the accuracy of density data is dependent upon the tissue volume being the same for all groups, we estimated the volume of the IL and PrL cortices using a Nikon E400 microscope equipped with a motorized stage in 3 axes and Stereo Investigator software (MicroBrightField, Inc; Williston, VT). After a random start, 10 sections (1 in 5 series) through the entire region were selected, stained with cresyl violet, and the volume calculated using a modified Cavalieri method (as described in Bothwell et al., 2001). The IL and PrL regions were identified using Nissl staining as a guide for area delineation (Krettek and Price, 1977; Jones, 1984; Paxinos and Watson, 2005).
Statistical Analysis
Paired Student's t-tests were used to compare within groups' locomotor activity on the first and last day of treatments and Student's t-tests to compare between groups' locomotor activity. For each region, a Student's t-test was used to compare the density of Fos-immunoreactive and PV+ cells and the ratios of dually labeled (Fos/PV+) neurons to total PV+ (single plus dual label) neurons between AMPH and vehicle groups.
Results
Locomotor sensitization
As expected, AMPH produced an increase in locomotor activity, and sensitization is evident with repeated treatment. There was a significant increase in the total distance traveled between the first and the last test session (difference: +1018 ± 182 cm, t = -5.594, p < 0.001, i.e. ∼52% greater than the first session) for animals treated repeatedly with AMPH. In contrast, locomotor activity for the vehicle treated rats did not differ between the first and the last sessions (difference: +85 ± 52 cm, t = -1.641, p = 0.116, i.e. ∼20% greater than the first session). The acute AMPH injection significantly enhanced basal locomotor activity over that for vehicle (difference: +1534 ± 153 cm, t = -7.733, p < 0.001, i.e. ∼456% greater than vehicle).
Laminar organization of PV+ interneurons
The PV+ interneurons and their processes were distributed uniformly rostral to caudal in the mPFC, as described by others (Gabbott et al., 1997). The dendrites and axons of PV+ cells were distributed throughout layers I-VI, although processes were much less evident in layer I than in the other layers (Figs. 1B-C). The immunoreactive punctae were also evident in all layers and often the boutons clearly delineated the outlines of pyramidal cell somata, especially in layer V (Fig. 1D). The density of PV+ neurons in layer V of the PrL, but not the IL, was significantly decreased following repeated AMPH treatment (Figs. 1E, F). The density of PV+ interneurons in the PrL and IL did not differ for any layer for rats treated acutely with AMPH compared to those receiving vehicle (Figs. 1G, H), and there was no significant difference between the acute and repeated vehicle groups for any layer. Moreover, we found no difference in the volumes of the PrL or IL for animals treated with AMPH compared to those injected with vehicle.
Lamina-specific induction of Fos
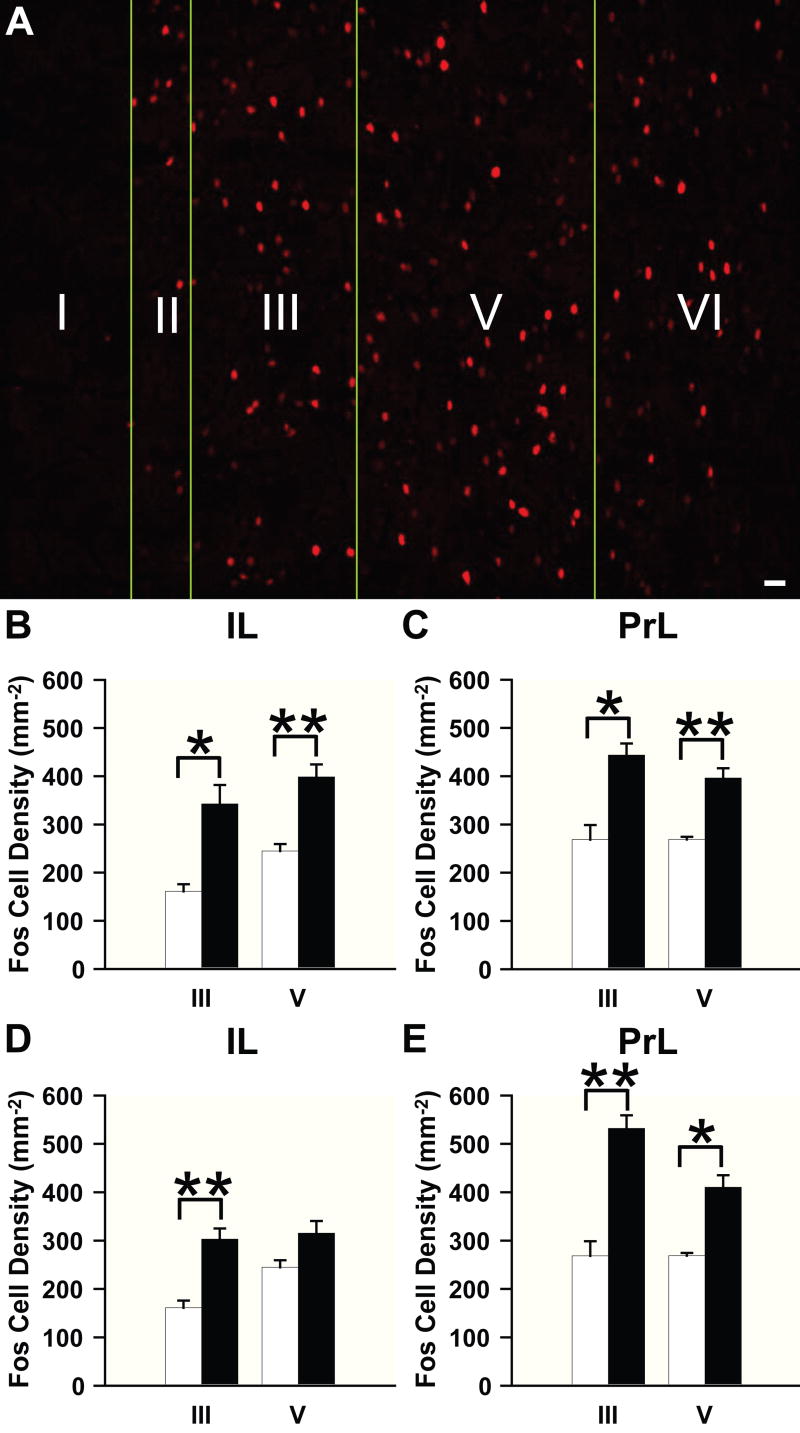
To evaluate whether the response to repeated or acute AMPH treatment induces Fos in a lamina-specific manner, we carried out a layer analysis of Fos immunoreactivity in the IL and PrL areas of the mPFC after drug challenge. Following either AMPH treatment, Fos-immunoreactive cells were evident in all layers, except layer I, in the IL and PrL cortices (Fig. 2A). When analyzed quantitatively, the density of Fos-immunoreactive cells was significantly increased in layers III and V of both IL and PrL areas after repeated AMPH treatment compared to vehicle (Figs. 2B, C). Animals acutely administered AMPH also showed a significant increase in Fos in layers III and V of the PrL and layer III of the IL, when compared to vehicle controls (Figs. 2D, E). There was no significant difference in laminar Fos expression between the acute and repeated vehicle groups.
Figure 2.
Effects of AMPH treatment on laminar Fos induction in the mPFC. (A) Representative confocal micrograph showing the laminar distribution of Fos-immunoreactive nuclei. (B-E) Bar graphs illustrating Fos-immunoreactive cell density in the (B,D) IL and (C,E) PrL areas of the mPFC. Open bars, vehicle; filled bars, (B,C) repeated or (D,E) acute AMPH administration. Scale bar: A, 10 μm. *p < 0.005, **p < 0.001, repeated and acute AMPH groups have a significantly higher density of cells compared to vehicle.
Laminar organization of PV/Fos colocalization
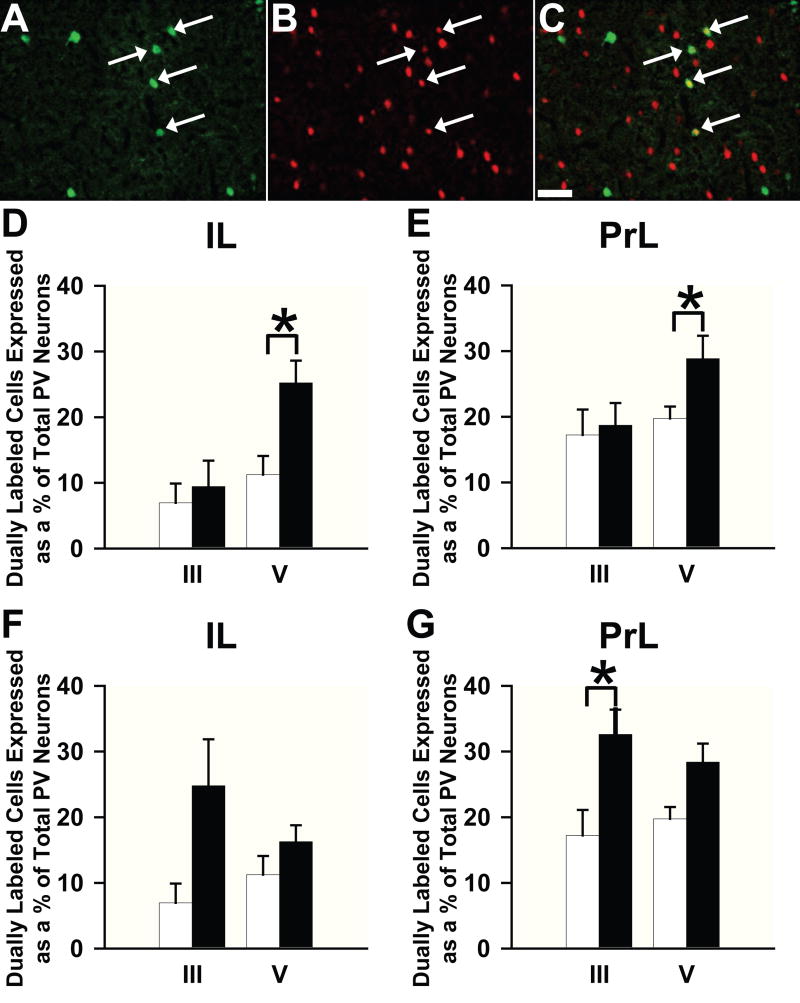
Although overall Fos was not induced in a layer-specific manner, we questioned whether Fos would be activated in the PV+ interneurons differentially by region or layer after either AMPH or vehicle treatment. We, therefore, evaluated cells that co-localized PV and Fos in the IL and PrL. We found that these co-labeled cells appeared in all layers of both regions. Figures 3A-C illustrate the typical pattern of co-localization of these proteins after repeated AMPH injection. Quantitative analysis revealed no differences between repeated AMPH- and vehicle-treated rats in the density (cells/mm2) of dually labeled cells. However, there was a trend (p = 0.08) towards an increase in the density of dually immunolabeled cells in layer V of the IL. Following acute AMPH treatment, there was a significant increase in the density of co-labeled (Fos + PV) neurons in layer III of the PrL (p < 0.05), but no difference between the groups for layer V of the PrL and layers III and V of the IL.
Figure 3.
Localization of Fos- immunoreactive and PV+ neurons using confocal microscopy: (A) PV+ interneurons (green), (B) Fos-immunoreactive cells (red), and (C) the merged image illustrating Fos and PV colocalization (white arrows). (D-G) Bar graphs illustrating the degree of colocalization of PV+ and Fos-immunolabeled neurons in the (D,E) IL and (F,G) PrL areas of the mPFC. Open bars, vehicle; filled bars, (D,E) repeated or (F,G) acute AMPH administration. Scale bar in C is valid for A and B and equals 50 μm. *p < 0.05, repeated AMPH treatment produces a significantly higher proportion of colocalized to total PV+ cells when compared to vehicle in layer V of both regions and acute AMPH treatment significantly increases this proportion when compared to vehicle in layer III of the PrL.
In order to establish whether Fos activation of PV+ neurons was affected by the change in PV immunoreactivity (see above), we analyzed the ratio of Fos-immunoreactive PV+ cells to all PV+ (single and dual labeled) neurons. When we compared rats that had been treated repeatedly with AMPH to those administered vehicle, we found that the proportion of dually labeled neurons was significantly increased in layer V, but not layer III, of both the IL and PrL cortices (Figs. 3D, E). When we compared rats that had been treated acutely with AMPH to those administered vehicle, we also found a significant increase in the proportion of dually immunolabeled cells in layer III, but not in layer V of the PrL, but found no difference in any layer of the IL (Fig. 3F-G).
Discussion
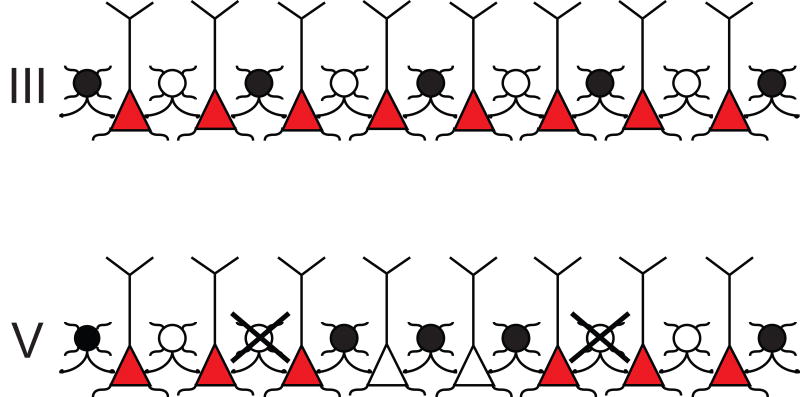
In the present study, we provide new evidence that an acute injection of AMPH to naïve rats induces Fos in layer III PV+ interneurons of the PrL cortex and a repeated AMPH regimen induces Fos in layer V PV+ interneurons in both the PrL and IL cortices. In addition, we found the repeated paradigm to be associated with a significant loss of PV+ immunoreactivity exclusively in layer V of the PrL cortex. These findings indicate that distinct neural substrates mediate the effects of these two AMPH treatment paradigms and suggest that a subpopulation of GABAergic interneurons can modulate pyramidal cell excitation in a layer-specific manner (Fig. 4A-B).
Figure 4.
A schematic diagram that summarizes the potential lamina-specific focusing of pyramidal cells by PV+/GABA inhibition in the PrL cortex after the repeated AMPH paradigm. Filled circles (•) indicate PV+ interneurons that contain Fos, whereas open circles (○) represent interneurons that show no Fos activation. Interneurons that have lost their PV immunoreactivity are illustrated as crossed (X) open circles. Red and empty triangles represent pyramidal neurons. It is well known that the PV+ interneurons provide a direct inhibition of pyramidal cells by synapsing onto their cell bodies or proximal axonal shafts in the same cortical layer (Hendry et al., 1983; DeFelipe et al., 1989; Kawaguchi and Kubota, 1993). Following repeated administration of AMPH, the density of co-labeled interneurons is unchanged in layers III and V. However, the proportion of activated PV+ neurons is increased in layer V due to the loss of PV-immunoreactivity. These data support a model in which the pattern of PV+ activation will presumably synchronize outflow from this layer, by silencing some pyramidal cells (empty triangles) and leaving others activated (red triangles).
There are data from electrophysiological studies that point to the GABAergic interneurons as critical mediators of dopamine-glutamate interactions in the mPFC (Seamans et al., 2001; Tseng and O'Donnell, 2004; Tseng et al., 2006) and, from other studies, that changes in GABA transmission are associated with the development of sensitization to cocaine or AMPH (Karler et al., 1997; Jayaram and Steketee, 2005). However, no investigation has shown a lamina-specific activation of inhibitory interneurons with acute or repeated exposure to drug. Such focusing of neural activity in a particular layer may explain how the behavioral consequences of repeated drug taking become reinforced (Sanchis-Segura and Spanagel, 2006).
The PV protein buffers intracellular calcium and maintains synaptic strength for the fast-spiking phenotype of this class of cortical interneurons (Caillard et al., 2000). Thus, the loss of PV immunoreactivity, specifically in layer V of the PrL cortex, suggests a change in the electrical response through decreased efficacy of this population following a repeated regimen of AMPH. Although our method of analysis cannot differentiate between a reduction in PV immunoreactivity below detection levels and a loss of neurons, either outcome would lead to a change in PV function.
As expected, the density of dually labeled (Fos/PV+) cells was significantly increased whenever the proportion of dually labeled to total PV+ cells was elevated (see acute and repeated results), with one important exception. Following repeated treatment, the density of co-labeled cells was unchanged compared to controls in layer V of the PrL, but the proportion of Fos-activated PV+ neurons increased significantly. Presumably, this was due to the decrease in the density of PV+ neurons. If the loss of PV attenuates inhibition on pyramidal cells, the net outflow from the deep layers could increase. Alternatively, the increase in dually labeled interneurons may compensate for the overall loss of PV+ neurons creating a net zero effect on inhibition in this region. However, given that there is an overall increase in Fos for the mPFC, a net zero change is unlikely. If the loss of PV immunoreactivity means that the proportion of Fos-activated PV+ neurons actually increases, as we found here, the overall effect will be an increase in inhibition on specific subsets of pyramidal neurons (Fig. 4). Our data are consistent with the findings of Homayoun and Moghaddam (2006), who provided direct physiological evidence for progressively more inhibition in the mPFC for rats engaged in a goal-directed behavior and treated repeatedly with AMPH.
The loss of PV immunoreactivity in the PrL but not IL after repeated drug treatment points to a regional vulnerability to this psychostimulant. Within the regions evaluated in the present study, previous work has shown that prenatal exposure to cocaine leads to the loss of the synaptic cartridges of the PV+ chandelier cells in the PrL region (Morrow et al., 2003), and animals subjected to cocaine-induced conditioned place preference (CPP) show a significant induction of Fos in the PrL but not the IL (Miller and Marshall, 2004). Thus, the pyramidal outflow from the PrL, which affects targets important for reward such as the nucleus accumbens and BLA (Vertes, 2004), is critical for the expression of CPP (Miller and Marshall, 2005) and possibly for sensitization to repeated AMPH administration (present results).
Dopamine can indirectly inhibit pyramidal cell activity in the mPFC (Penit-Soria et al., 1987; Pirot et al., 1992; Seamans et al., 2001; Gorelova et al., 2002; Tseng and O'Donnell, 2004; Tseng and O'Donnell, 2006) through increases in GABA activity and release (Grobin and Deutch, 1998; Gorelova et al., 2002; Tseng and O'Donnell, 2006). A drug challenge following long-term withdrawal from repeated psychostimulant treatment dramatically elevates the dopamine content in the mPFC (Williams and Steketee, 2005). Therefore, the activity of mPFC neurons that are not Fos-labeled may be reduced due to the effect of dopamine. However, despite dopamine's effects on interneuron excitability and the concurrent inhibition of pyramidal neuron activity, the present results point to an overall increase in Fos activity in response to an AMPH challenge, following long-term withdrawal from repeated AMPH treatment. This effect cannot be explained solely by Fos induction in PV+ interneurons. Electrophysiological data show a change in the pattern of firing for mPFC pyramidal neurons following repeated AMPH treatment (Onn and Grace, 2000; Peterson et al., 2000), and strong inhibition in the mPFC in response to a challenge injection after withdrawal from repeated AMPH (Homayoun and Moghaddam, 2006). Therefore, an alternative model that incorporates both these seemingly paradoxical increases in interneuron and pyramidal neuron activity should be considered (Fig. 4).
Current theories of cognitive functioning of the frontal cortex are based on the selective activation of small networks of pyramidal neurons (Rao et al., 1999). Changes in the pattern of interneuronal activity could suppress specific subsets of projection cells while leaving others active (Fig. 4). Altered interneuronal activity could restructure the pattern of pyramidal cell firing (Seamans and Yang, 2004). Dopaminergic stimulation of GABAergic neurons can “tune” the outputs of the mPFC by suppressing all but the most active pathways (Rao et al., 1999; Durstewitz et al., 2000) and synchronizing neuronal firing patterns (Kawaguchi, 2001; Tseng et al., 2006). This tuning results in a strengthened representation of the predominant stimuli that is resistant to distracters, therefore allowing the organism to focus on the task at hand. Thus, we conclude that glutamate-mediated plasticity that accompanies behavioral sensitization (Wolf, 2002) could be due, at least in part, to this GABA-mediated focusing of mPFC outflow.
Acknowledgments
The work was supported by a USPHS grant from the NIH, DA016662. We thank Dr. Kuei-Yuan Tseng for his critical reading of the manuscript and Mrs. Jennifer Jackolin for her technical assistance.
Abbreviations
- +
positive
- AMPH
D-amphetamine sulfate
- BLA
basolateral amygdala
- CPP
conditioned place preference
- GABA
gamma-aminobutyric acid
- IL
infralimbic
- mPFC
medial prefrontal cortex
- PrL
prelimbic
- PV
parvalbumin
- RT
room temperature
- Tx
Triton X-100
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartoletti M, Gubellini C, Ricci F, Gaiardi M. Baclofen blocks the development of sensitization to the locomotor stimulant effect of amphetamine. Behav Pharmacol. 2005;16:553–558. doi: 10.1097/01.fbp.0000179279.98029.e9. [DOI] [PubMed] [Google Scholar]
- Bothwell S, Meredith GE, Phillips J, Staunton H, Doherty C, Grigorenko E, Glazier S, Deadwyler SA, O'Donovan CA, Farrell M. Neuronal hypertrophy in the neocortex of patients with temporal lobe epilepsy. J Neurosci. 2001;21:4789–4800. doi: 10.1523/JNEUROSCI.21-13-04789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. D-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience. 1999;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A. 2000;97:13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Visualization of chandelier cell axons by parvalbumin immunoreactivity in monkey cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:2093–2097. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946:314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ. Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol. 2001;86:2911–2918. doi: 10.1152/jn.2001.86.6.2911. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Deutch AY. Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther. 1998;285:350–357. [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Multiple effects of dopamine on layer V pyramidal cell excitability in rat prefrontal cortex. J Neurophysiol. 2001;86:586–595. doi: 10.1152/jn.2001.86.2.586. [DOI] [PubMed] [Google Scholar]
- Hedou G, Jongen-Relo AL, Murphy CA, Heidbreder CA, Feldon J. Sensitized Fos expression in subterritories of the rat medial prefrontal cortex and nucleus accumbens following amphetamine sensitization as revealed by stereology. Brain Res. 2002;950:165–179. doi: 10.1016/s0006-8993(02)03034-2. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Houser CR, Jones EG, Vaughn JE. Synaptic organization of immunocytochemically identified GABA neurons in the monkey sensory-motor cortex. J Neurocytol. 1983;12:639–660. doi: 10.1007/BF01181528. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram P, Steketee JD. Effects of cocaine-induced behavioural sensitization on GABA transmission within rat medial prefrontal cortex. Eur J Neurosci. 2005;21:2035–2039. doi: 10.1111/j.1460-9568.2005.04000.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. Plenum Press; New York: 1984. pp. 521–553. [Google Scholar]
- Karler R, Bedingfield JB, Thai DK, Calder LD. The role of the frontal cortex in the mouse in behavioral sensitization to amphetamine. Brain Res. 1997;757:228–235. doi: 10.1016/s0006-8993(97)00221-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Distinct firing patterns of neuronal subtypes in cortical synchronized activities. J Neurosci. 2001;21:7261–7272. doi: 10.1523/JNEUROSCI.21-18-07261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Li Y, Wolf ME. Ibotenic acid lesions of prefrontal cortex do not prevent expression of behavioral sensitization to amphetamine. Behav Brain Res. 1997;84:285–289. doi: 10.1016/s0166-4328(96)00158-1. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Axo-axonic structures in the medial prefrontal cortex of the rat: reduction by prenatal exposure to cocaine. J Neurosci. 2003;23:5227–5234. doi: 10.1523/JNEUROSCI.23-12-05227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci. 2000;20:2332–2345. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam; Boston: 2005. [Google Scholar]
- Penit-Soria J, Audinat E, Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res. 1987;425:263–274. doi: 10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse. 2000;36:342–344. doi: 10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pirot S, Godbout R, Mantz J, Tassin JP, Glowinski J, Thierry AM. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience. 1992;49:857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 2:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. J Comp Neurol. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O'Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine Modulation of Prefrontal Cortical Interneurons Changes during Adolescence. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine- and cocaine-induced behavioural sensitization. Cereb Cortex. 2000;10:488–498. doi: 10.1093/cercor/10.5.488. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Time-dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology. 2005;48:51–61. doi: 10.1016/j.neuropharm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-D-aspartate antagonists. Neuroscience. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]