Abstract
Purpose/Objectives
To determine the effect of aerobic and strength resistance training and epoetin alfa (EPO) therapy on transfusions, stem cell collections, transplantation recovery, and multiple myeloma treatment response.
Design
Randomized clinical trial.
Setting
A myeloma research and therapy center in the south central United States.
Sample
135 patients with multiple myeloma, 120 evaluable.
Methods
Random assignment to exercise or usual care groups. All patients received EPO based on an algorithm. Aerobic capacity, using the six-minute walk test, was assessed prior to induction chemotherapy, prior to stem cell mobilization, and following stem cell collection for all patients and before and after transplantation for patients continuing in the study. Data analysis included analysis of variance to compare other outcome variables by groups.
Main Research Variables
Number of red blood cell and platelet transfusions during transplantation, number of attempts at and total number of days of stem cell collection, time to recovery after transplantation, and response to intensive therapy for multiple myeloma.
Findings
Recovery and treatment response were not significantly different between groups after transplantation. The exercise group had significantly fewer red blood cell transfusions and fewer attempts at stem cell collection. Serious adverse events were similar in each group.
Conclusions
Exercise with prophylactic EPO therapy reduces the number of RBC transfusions and attempts at stem cell collection for patients receiving intensive treatment for multiple myeloma.
Implications for Nursing
Exercise is safe and has many physiologic benefits for patients receiving multiple myeloma treatment.
Key Points.
Exercise in combination with epoetin alfa therapy reduced the number of transfusions and the number of attempts at stem cell collection while improving aerobic capacity.
Patients receiving chemotherapy and autologous peripheral blood stem cell transplantation can exercise safely.
Nurses should use exercise as an intervention to reduce the need for transfusions and improve stem cell collection.
At least 60% of patients with multiple myeloma are anemic (hemoglobin less than 12 g/dl) at diagnosis (Multiple Myeloma Research Foundation, 2002), and almost all become anemic during treatment, often requiring red blood cell (RBC) transfusions (Knight, Wade, & Balducci, 2004). Epoetin alfa (EPO) increases hemoglobin levels and reduces the need for RBC transfusions in diverse groups of patients with cancer-related anemia (Henry, 2005), particularly patients with multiple myeloma receiving chemotherapy treatment (Barlogie & Beck, 1993).
Evidence supports the benefit of exercise in managing fatigue during and after treatment for patients with breast cancer and other solid tumors (Courneya & Friedenreich, 1999; Galvao & Newton, 2005; Knols, Aaronson, Uebelhart, Fransen, & Aufdemkampe, 2005; Oldervoll, Kaasa, Hjermstad, Lund, & Loge, 2004; Schmitz et al., 2005; Stevinson, Lawlor, & Fox, 2004; Stricker, Drake, Hoyer, & Mock, 2004), as well as for those receiving hematopoietic stem cell transplantations, including patients with multiple myeloma (Strong, Karavatas, & Reicherter, 2006). Although limited by small sample size, results from a recent randomized controlled trial (Drouin et al., 2006) suggest that exercise increases hemoglobin levels. Women receiving radiation treatment for breast cancer (N = 20) were randomly assigned to aerobic exercise or to placebo stretching during the seven-week treatment period. Hemoglobin increased from 12.3 to 12.4 g/dl in the aerobic exercise group and decreased from 12.3 to 11.8 g/dl in the placebo stretching group (p = 0.009). Peak oxygen intake measures correlated to the final measures for hemoglobin (p = 0.013). The researchers concluded that moderate intensity aerobic exercise may be a safe and economical way to improve fitness and maintain erythrocytes during radiation treatment for breast cancer.
Because exercise increases hemoglobin levels, it also may improve stem cell collection during apheresis procedures. Exercise augments coagulation and fibrinolysis while maintaining the balance in the two processes (Li, He, Blomback, & Hjemdahl, 2007). Exercise has been shown to increase circulating activated platelets (Li et al.) and the change is partially explained by an increase in platelet counts induced by exercise (Ahmadizad, El-Sayed, & Maclaren, 2006; Aldemir & Kilic, 2005). However, no convincing evidence exists that suggests EPO therapy can provide the same benefits for patients with normal or near-normal hemoglobin levels as it does for patients with moderate-to-severe anemia (Steensma & Loprinzi, 2005), and it also is unclear whether EPO, with or without concurrent exercise therapy, improves tumor response to cancer therapy (Steensma & Loprinzi) or recovery after transplantation.
The purpose of the current study was to determine the affect of EPO therapy (short term versus long term) with and without a home-based individualized exercise program that incorporated aerobic and strength resistance training for patients being treated with high-dose chemotherapy and autologous peripheral-blood stem cell transplantation (PBSCT) for multiple myeloma. The endpoints for the study included the number of attempts at and total number of days of stem cell collection, number of RBC and platelet transfusions during the transplantation period, time-to-recovery after transplantation, and response to intensive therapy for multiple myeloma.
Methods
Subjects
The sample included patients newly diagnosed with multiple myeloma and eligible for treatment with an intensive treatment protocol that included tandem autologous PBSCT. Inclusion criteria for the study were a new diagnosis of multiple myeloma and an intensive treatment protocol with or without thalidomide. Patients at high risk for pathologic fractures or spinal cord compression were excluded. Other exclusion criteria were major psychiatric illness, microcytic or macrocytic anemia, uncontrolled hypertension, RBC transfusions within the previous two weeks, or EPO therapy within eight weeks prior to study enrollment. The institutional review board at the University of Arkansas for Medical Sciences approved the study.
For a fixed effects two-tailed analysis of variance (ANOVA) design with two levels of care (usual care and exercise) and two levels of duration (short and long term), a power analysis using G*Power 3.0.5 (Faul, Erdfelder, Lang, & Buchner, 2007) indicated that a sample size of 128 participants (64 per group) would have an actual power of 80.14% to detect a moderate partial η2 effect size of 0.06.
Research Design
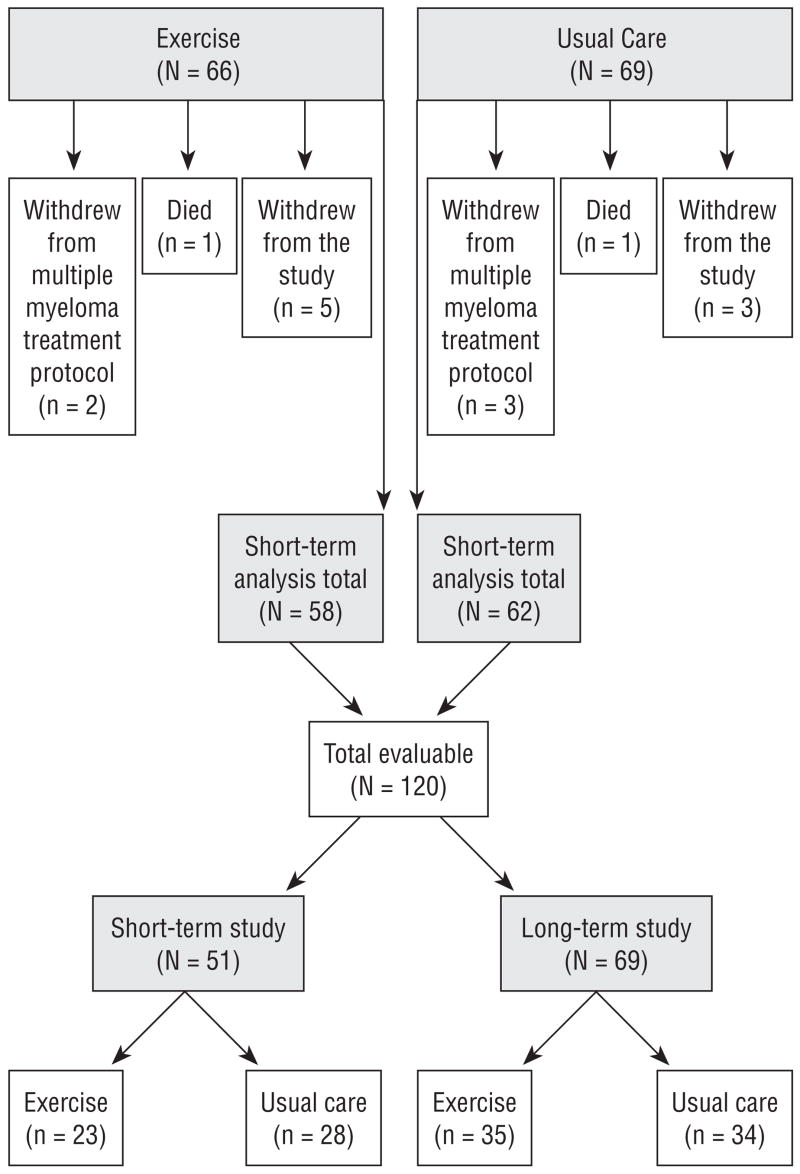
Patients (n = 135) who met eligibility criteria and provided informed consent were randomized to usual care (n = 69) or exercise (n = 66) groups as they were enrolled in the multiple myeloma treatment protocol. Patients were stratified by age (60 and younger versus older than 60) and whether or not they were taking thalidomide as they were randomized to exercise or usual care groups. A total of 120 patients completed the short-term section of the study and were evaluable. The reasons for attrition (n = 15, 11%) are illustrated in Figure 1.
Figure 1.
Enrollment, Attrition, and Group Assignment
Remaining patients (n = 120) were in the study for about 15 weeks, which included stem cell collection. The first 70 patients who met eligibility for long-term participation (i.e., response to EPO) continued in the study for an additional 15 weeks, which included the first transplantation. Nine patients had no response to EPO (four in the exercise group and five in usual care), and one eligible patient declined continued enrollment, leaving 69 patients to continue the study for about 15 more weeks.
Interventions
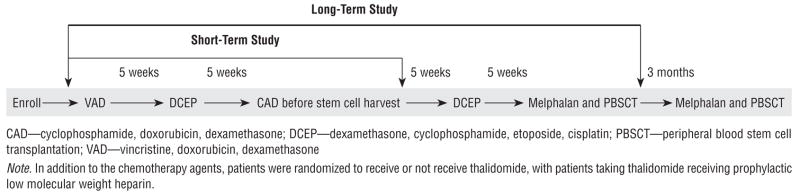
The intensive treatment protocol, called Total Therapy II, included induction chemotherapy with vincristine, doxorubicin, and dexamethasone (0.5 mg, 10 mg/m2, and 40 mg, respectively); dexamethasone, cyclophosphamide, etoposide, and cisplatin (40 mg, 400 mg/m2, 40 mg/m2, and 15 mg/m2, respectively); and cyclophosphamide, doxorubicin, and dexamethasone (750 mg/m2, 15 mg/m2, and 40 mg, respectively) for mobilization. The regimen was followed by stem cell collection and repeat treatment with DCEP and high-dose melphalan (200 mg/m2) followed with autologous PBSCT. The interval between each of the treatments was about five weeks. Patients received a second treatment of high-dose melphalan and a stem cell transplantation about three months after the first transplantation. Fifty percent of the patients were randomized to receive thalidomide (400 mg daily) during induction, after transplantation consolidation, and maintenance therapy. Patients randomized to thalidomide received prophylactic low molecular weight heparin (Barlogie, 2001). Outpatient treatment was planned and hospital admission was reserved for severe disease, treatment complications, or insurance reasons. Figure 2 shows the treatment protocol (Total Therapy II and duration of the study).
Figure 2.
Treatment Protocol for Total Therapy II Through the Duration of the Study
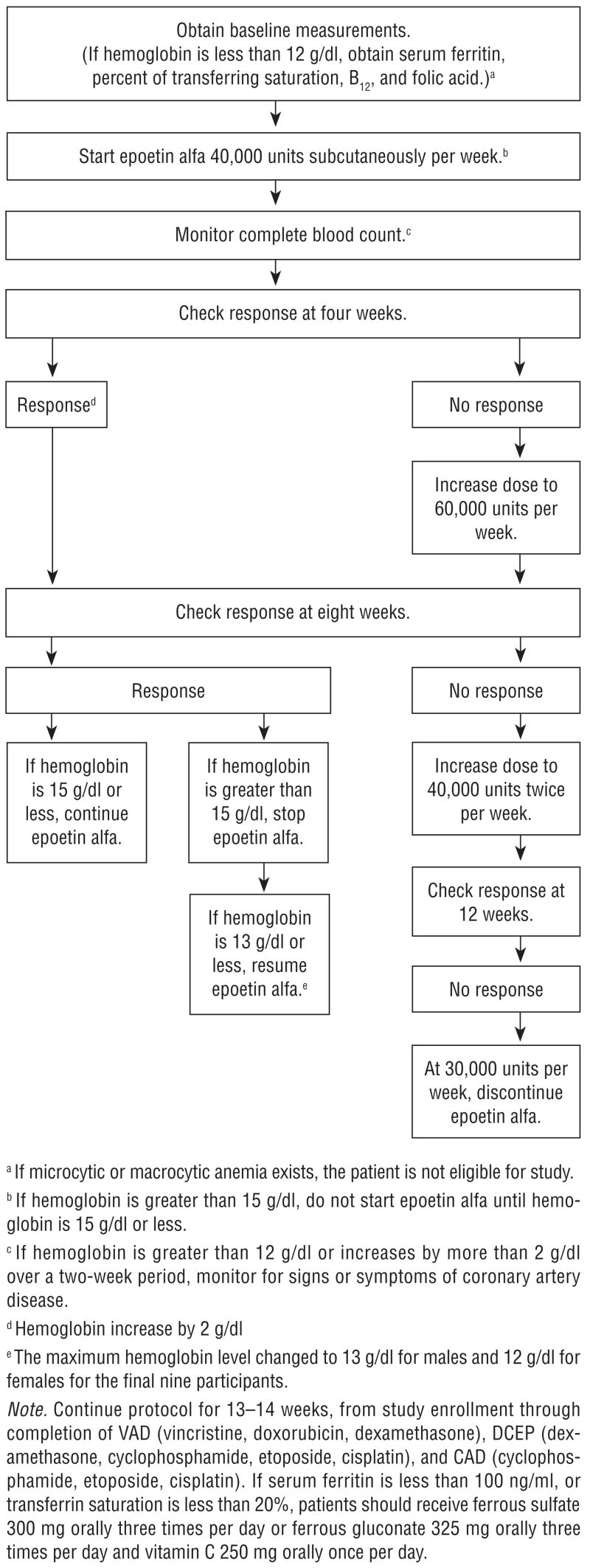
EPO was administered to the first 102 study participants according to an investigational algorithm that allowed hemoglobin levels to reach 15 g/dl before dose reduction or delay, and started before chemotherapy unless baseline hemoglobin was less than 15 g/dl, differing from the recommended hemoglobin parameters for EPO administration to patients who are receiving chemotherapy outside of a clinical trial setting (Engert, 2005; Ortho Biotech Products, 2005). The usual dose is 150 units/kg of body weight, three times per week, or 40,000 units weekly, with suggested target hemoglobin range of 10–12 g/dl (Engert; Ortho Biotech Products). The investigational therapy algorithm was used for all patients in the study in an attempt to alleviate the anemia caused by multiple myeloma and high-dose chemotherapy; therefore, it was easier to determine the effects of exercise. Figure 3 shows the investigational EPO therapy algorithm used for patients in the study. The protocol was continued for 13–15 weeks from study enrollment through completion of chemotherapy for all 111 patients in the study, and through first transplantation for the 69 patients who continued long term. If serum ferritin was less than 100 ng/ml or transferrin saturation less than 20%, patients were advised to take 300 mg of ferrous sulfate orally three times daily, or 325 mg of ferrous gluconate orally three times daily and 250 mg of vitamin C orally once daily.
Figure 3.
Investigational Epoetin Therapy Algorithm
Patients assigned to the usual care group were advised to follow the written exercise recommendations provided by their physician. The patients were generally advised to remain as active as possible and to try to walk 20 minutes per day. Patients assigned to the exercise group received an individualized exercise prescription, a set of color-coded exercise stretch bands with varying resistance, and a notebook and videotape illustrating the exercises. Strength resistance training was included to strengthen muscles so patients could improve the aerobic component of the exercise program (see Table 1).
Table 1.
Exercise Intervention Group Home-Based Individualized Exercise Program
| Component | Activity |
|---|---|
| Stretching | Performed daily for the hamstrings, shoulder rotators, calves, hip flexors, and triceps |
| Aerobics | Walking to tolerance (until tired) |
| Strength resistance training | Performed on alternate days:
Biceps curls with exercise stretch bands Triceps extension with chair push-ups Quadriceps strengthening with chair stand Hamstring strengthening while sitting or standing |
Measurements
Patients faxed weekly activity logs to investigators, which were used, along with periodic personal interviews, to assess compliance with the exercise program for patients in the exercise group and the exercise patterns of patients in the usual care group. Additional objective assessments of all patients included aerobic capacity, using the six-minute walk test (distance, in feet, walked on a level surface in six minutes) and the Borg Scale (Borg, 1998) to determine perceived exertion at each assessment. The six-minute walk test has been shown to be linearly related to maximum metabolic equivalents (r = 0.687, p < 0.001), supporting its validity and strong test retest reliability (intraclass correlation = 0.97) (Hamilton & Haennel, 2000). The Borg Scale is a simple method of subjectively rating exertion (American College of Sports Medicine, 2001; Borg; Sports Coach, 1997), and the rate of perceived exertion correlates with approximate heart rate. Measurements were obtained for each patient in the study at baseline (prior to induction chemotherapy), prior to stem cell mobilization, and following stem cell collection. Additional measurements were obtained before and after transplantation for each patient continuing the study through the first transplantation.
The numbers of RBC and platelet transfusions during the transplantation period were obtained from the University of Arkansas for Medical Sciences blood bank. All patients received transfusions from the blood bank during their transplantations, so it was an accurate source for data. Data on stem cell collection were obtained from the University of Arkansas for Medical Sciences apheresis laboratory. Response to intensive treatment for multiple myeloma was the criterion for evaluation of Total Therapy II, and endpoints were obtained from the Myeloma Institute for Research and Therapy database. Time-to-recovery after transplantation was defined as the number of days before white blood cell recovery (absolute neutrophil count of 2.0 or higher).
Data Analysis
Data analysis included descriptive statistics to show demographics and outcome variables of interest by groups; t test and chi-square to check for equivalence of groups for age, gender, and race; and ANOVA to compare outcome variables by groups. A significance level of p < 0.05 was used for all analyses.
Results
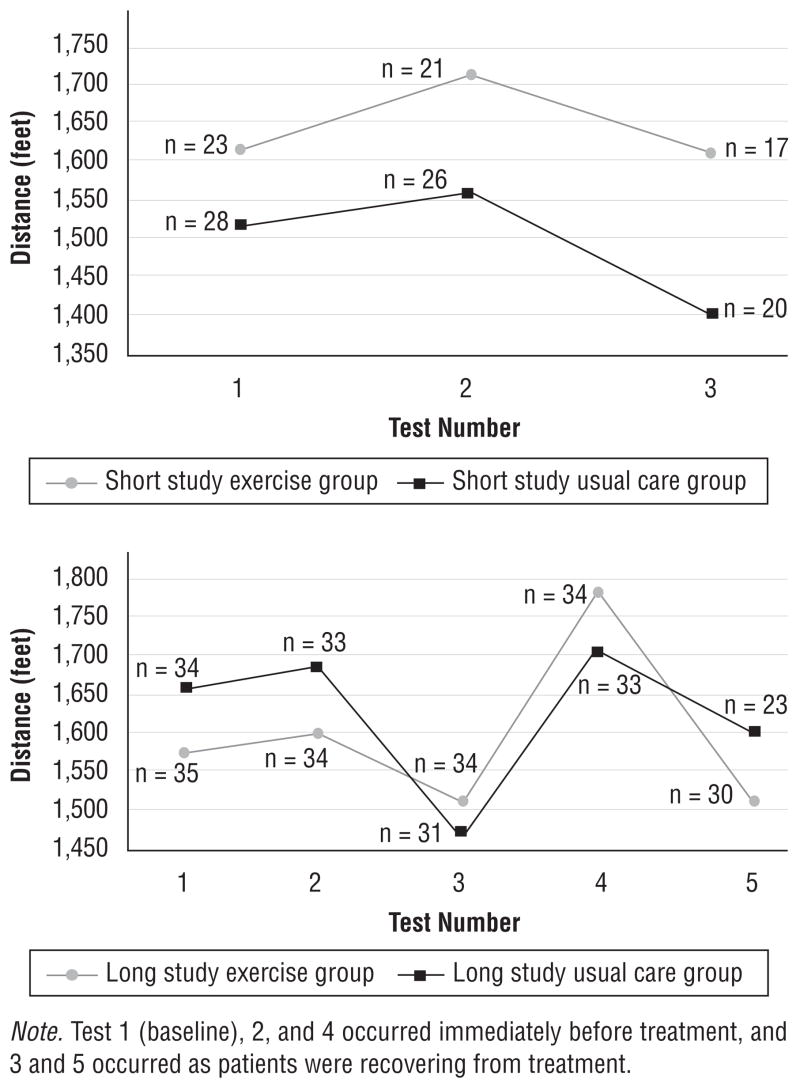
The groups were equivalent in age, gender, and race (see Table 2). Figure 4 provides a comparison, by groups, of the means for the walk test measurements and shows a trend toward better exercise performance by the exercise group. Aerobic capacity declined as treatment program intensity increased, but patients assigned to exercise had less of a decline. Additionally, more patients in the usual care group, compared to the exercise group, were unable to perform the walk tests as treatment progressed.
Table 2.
Demographic Characteristics
| Short Terma |
Long Termb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Exercise (N = 23)
|
Usual Care (N = 28)
|
Exercise (N = 35)
|
Usual Care (N = 34)
|
|||||
| Characteristic | n | % | n | % | n | % | n | % |
| Gender | ||||||||
| Male | 12 | 52 | 11 | 39 | 23 | 66 | 24 | 71 |
| Female | 11 | 48 | 17 | 61 | 12 | 34 | 10 | 29 |
| Race | ||||||||
| Caucasian | 20 | 87 | 25 | 89 | 31 | 89 | 32 | 94 |
| Other | 3 | 13 | 3 | 11 | 4 | 11 | 2 | 6 |
| Characteristic | X̄ | Range | X̄ | Range | ||||
|
| ||||||||
| Age (years) | 55 | 32–74 | 55 | 25–76 | ||||
In study through stem cell collection
In study through first transplantation
Figure 4.
Distance Walked in Six Minutes for Exercise and Usual Care Groups During Short- and Long-Term Participation
No significant differences between the groups in response to the intensive treatment protocol for multiple myeloma or between the groups recovering after transplantation were found. Hemoglobin levels during the transplantation period were higher for patients in the long-term group than those in the short-term group; however, hemoglobin levels were similar for the long-term and short-term groups at discharge after transplantation. The higher hemoglobin during the transplantation period was expected for the patients in the long-term group because they had received EPO, according to the investigational algorithm, up to the time of transplantation. All groups had equivalent baseline hemoglobin levels before chemotherapy (see Table 3).
Table 3.
Hemoglobin Levels Before Chemotherapy and During Transplantation Period
| Short Term (g/dl)
|
Long Term (g/dl)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise (N = 23)
|
Usual Care (N = 28)
|
Exercise (N = 35)
|
Usual Care (N = 34)
|
|||||||||
| Time | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI |
| Baseline | 11.6 | 1.7 | 10.9, 12.3 | 12.1 | 1.6 | 11.5, 12.8 | 11.7 | 1.8 | 11.1, 12.3 | 11.5 | 1.8 | 10.9, 12.1 |
| Before transplantation | 11.0 | 1.6 | 10.4, 11.6 | 10.8 | 1.5 | 10.2, 11.3 | 12.0 | 1.4 | 11.5, 12.5 | 12.0 | 1.6 | 11.5, 12.5 |
| Lowest during transplantation | 8.7 | 0.9 | 8.2, 9.1 | 8.5 | 0.9 | 8.1, 8.9 | 9.1 | 1.1 | 8.8, 9.5 | 9.2 | 1.2 | 8.9, 9.6 |
| Mean during transplantation | 10.4 | 0.9 | 9.8, 10.6 | 10.1 | 0.7 | 9.7, 10.4 | 10.8 | 1.1 | 10.5, 11.1 | 10.8 | 1.1 | 10.5, 11.2 |
| Highest during transplantation | 11.8 | 1.2 | 11.3, 12.3 | 11.8 | 1.0 | 11.3, 12.3 | 12.6 | 1.3 | 12.2, 13.0 | 12.7 | 1.4 | 12.3, 13.1 |
| Discharge | 10.6 | 1.1 | 10.1, 11.2 | 10.6 | 1.4 | 10.1, 11.1 | 11.0 | 1.3 | 10.6, 11.5 | 10.9 | 1.4 | 10.5, 11.4 |
CI—confidence interval; SD—standard deviation
The maximum EPO dose was similar for patients in the exercise and usual care groups. Dose adjustments occurred 5, 9, and 13 weeks into the study, and all escalations occurred before stem cell collection (see Table 4).
Table 4.
Maximum Dose of Epoetin Alfa (EP0)
| Maximum EPO Dosea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 40,000 Units
|
60,000 Units
|
80,000 Units
|
Total
|
||||||
| Group | n | % | n | % | n | % | n | % | |
| Exercise | 37 | 64 | 12 | 21 | 9 | 15 | 58 | 100 | |
| Usual care | 43 | 69 | 9 | 15 | 10 | 16 | 62 | 100 | |
| Total | 80 | 67 | 21 | 17 | 19 | 16 | 120 | 100 | |
Given according to investigational EPO therapy algorithm. Dose escalations occurred before stem cell collection.
Fewer attempts were made at stem cell collection for the exercise group compared with the usual care group (Bonferroni adjusted p < 0.025). Differences in hemoglobin level at the time of apheresis do not offer an explanation, with the mean hemoglobin level on the first day of apheresis similar for the two groups (10.8 g/dl [SD 1.3] for the exercise group and 10.9 g/dl [SD 1.2] for the usual care group).
A trend toward fewer RBC and platelet transfusions during the transplantation period occurred in the exercise group compared to the usual care group. When the nine patients who did not respond to EPO were excluded from the analysis, a statistically significant difference in RBC and platelet transfusions between the exercise and usual care groups was found (Bonferroni adjusted p < 0.025) (see Table 5). The number of attempts at and total number of days of stem cell collection also were significantly fewer for the exercise group compared to the usual care group (Bonferroni adjusted p < 0.025) (see Table 6).
Table 5.
Number of Transfusions During Transplantation Period
| Short Term (N = 51)
|
Long Term (N = 69)
|
Long Term Versus Short Term
|
Exercise Versus Usual Care
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise (N = 23) | Usual Care (N = 28) | Exercise (N = 35) | Usual Care (N = 34) | |||||||||||||
| Group | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | F | Partial η2 | F | Partial η2 |
| Total sample group | ||||||||||||||||
| Red blood cell transfusions | 1.8 | 2.2 | 0.8, 2.7 | 2.3 | 2.5 | 1.4, 3.1 | 1.0 | 1.3 | 0.2, 1.7 | 1.8 | 2.9 | 1.0, 2.6 | 2.26 | 0.019 | 2.57 | 0.022 |
| Platelet transfusions | 2.3 | 1.6 | 1.0, 3.6 | 3.1 | 3.2 | 1.9, 4.2 | 2.0 | 2.0 | 1.0, 3.1 | 3.6 | 4.5 | 2.6, 4.7 | 0.054 | 0.000 | 4.11* | 0.034 |
|
Short Term (N = 42)
|
Long Term (N = 69)
|
Long Term Versus Short Term
|
Exercise Versus Usual Care
|
|||||||||||||
|
Exercise (N = 19)
|
Usual Care (N = 23)
|
Exercise (N = 35)
|
Usual Care (N = 34)
|
|||||||||||||
| Group | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | F | Partial η2 | F | Partial η2 |
|
| ||||||||||||||||
| Minus those unresponsive to EPO | ||||||||||||||||
| Red blood cell transfusions | 1.8 | 2.2 | 0.8, 2.7 | 2.4 | 2.6 | 1.5, 3.3 | 1.0 | 1.3 | 0.2, 1.7 | 1.8 | 2.9 | 1.0, 2.6 | 0.97 | 0.009 | 5.15* | 0.046 |
| Platelet transfusion | 2.3 | 1.6 | 1.0, 3.6 | 3.5 | 3.4 | 2.2, 4.8 | 2.0 | 2.0 | 1.0, 3.1 | 3.6 | 4.5 | 2.6, 4.7 | 0.002 | 0.000 | 5.52* | 0.049 |
p < 0.05
CI—confidence interval; EPO—epoetin alfa; SD—standard deviation
Table 6.
Number of Attempts At and Total Days of Stem Cell Collection
| Short Term (N = 51)
|
Long Term (N = 69)
|
Long Term Versus Short Term
|
Exercise Versus Usual Care
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise (N = 23)
|
Usual Care (N = 28)
|
Exercise (N = 35)
|
Usual Care (N = 34)
|
|||||||||||||
| Group | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | F | Partial η2 | F | Partial η2 |
| Total group | ||||||||||||||||
| Attempts | 1.1 | 0.3 | 0.8, 1.3 | 1.4 | 0.8 | 1.2, 1.6 | 1.1 | 0.4 | 0.9, 1.3 | 1.3 | 0.6 | 1.1, 1.5 | 0.014 | 0.000 | 5.81* | 0.049 |
| Total days | 4.0 | 2.3 | 2.6, 5.3 | 5.3 | 4.0 | 4.1, 6.5 | 4.5 | 3.1 | 3.8, 6.0 | 4.9 | 3.1 | 3.8, 6.0 | 0.005 | 0.000 | 2.17 | 0.019 |
|
Short Term (N = 42)
|
Long Term (N = 69)
|
Long Term Versus Short Term
|
Exercise Versus Usual Care
|
|||||||||||||
|
Exercise (N = 19)
|
Usual Care (N = 23)
|
Exercise (N = 35)
|
Usual Care (N = 34)
|
|||||||||||||
| Group | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | X̄ | SD | 95% CI | F | Partial η2 | F | Partial η2 |
|
| ||||||||||||||||
| Minus those unresponsive to EPO | ||||||||||||||||
| Attempts | 1.1 | 0.2 | 0.8, 1.3 | 1.5 | 0.9 | 1.2, 1.7 | 1.1 | 0.4 | 0.9, 1.3 | 1.3 | 0.7 | 1.1, 1.5 | 0.11 | 0.001 | 7.6** | 0.068 |
| Total days | 3.6 | 1.8 | 2.2, 5.1 | 5.8 | 4.2 | 4.5, 7.1 | 4.5 | 3.1 | 3.4, 5.6 | 4.9 | 3.1 | 3.8, 6.0 | 0.006 | 0.000 | 4.24* | 0.039 |
p < 0.05;
p < 0.01
CI—confidence interval; EPO—epoetin alfa; SD—standard deviation
Serious Adverse Events
Overall, the incidence of serious adverse events (SAE) was similar between all groups. The most common SAE were fever, hyponatremia, pneumonia, hyperglycemia, deep vein thrombosis (DVT), infection, and neutropenia. Multiple myeloma, chemotherapy, and thalidomide are risk factors for DVT, and questions arise regarding whether EPO, particularly at higher-than-approved hemoglobin parameters for dosing, may be associated with an increased risk of DVT. With grade 3 or 4 DVT (National Cancer Institute, 2007), confirmed by Doppler scan, the incidence of DVT occurring between enrollment and transplantation was similar between the exercise and usual care groups and between patients taking or not taking thalidomide. DVT occurring in the upper extremities (n = 17) was related to a central venous catheter. DVT also was found to occur in the lower extremities (n = 12). Five patients had a pulmonary embolus, two of which were in the long-term study. The two patients were receiving thalidomide, with one assigned to exercise and one to usual care. The other three patients experiencing a pulmonary embolus were in the short-term study, with two receiving thalidomide—one assigned to usual care and one to exercise—and the other not receiving thalidomide and assigned to the exercise group. Of the patients with DVT, nine (36%) had a history of previous thromboembolism and 10 (40%) had a DVT preceded by hemoglobin of more than 13 g/dl. Four patients reported symptoms of DVT, but a Doppler scan could not confirm the diagnoses. One death in the long-term study was related to pneumonia. Table 7 shows the incidence of SAE and DVT by group.
Table 7.
Incidence of Serious Adverse Events (SAE) and Deep Vein Thrombosis (DVT)
| Short Terma |
Long Termb |
|||
|---|---|---|---|---|
| Adverse Event | Exercise(N = 23) | Usual Care (N = 28) | Exercise (N = 35) | Usual Care (N = 34) |
| 1 SAEc | 13 | 8 | 12 | 9 |
| More than 1 SAE | 2 | – | 3 | 5 |
| 1 DVTd | 6 | 4 | 6 | 5 |
| More than 1 DVT | – | 1 | 2 | 1 |
In study through stem cell collection
In study through first transplantation
P values for chi-squared test were not significant.
Grade 3 or 4; p value for chi-squared test was not significant.
Discussion
The current study’s findings suggest that aerobic and strength-resistance training combined with prophylactic EPO therapy benefits patients by reducing the number of RBC and platelet transfusions and the number of attempts at and total number of days of stem cell collection. Hemoglobin levels during the transplantation period were similar for the exercise and usual care groups. However, compared to the exercise group, the usual care group required significantly more RBC transfusions to maintain the same level of hemoglobin, suggesting that exercise had an effect on hemoglobin level. The findings in the current study are, therefore, consistent with others that report the benefit of exercise in raising hemoglobin levels in patients with cancer. The underlying mechanism to explain this benefit is unknown, but the greatest benefit seems to come from exercise in combination with EPO.
Based on the study results, EPO therapy acted with exercise to decrease the number of transfusions among patients receiving high-dose chemotherapy and transplantations as treatment for multiple myeloma. Duration of EPO administration is important in maintaining higher hemoglobin levels during the transplantation period. Patients in the current study who received EPO according to the study algorithm through their first transplantation had higher hemoglobin levels during the transplantation period than patients who were in the study only through stem cell collection. Even with EPO therapy, patients in the current study had an average hemoglobin level of 10.8 g/dl during the transplantation period.
The results also suggest that the investigational use of EPO as prophylactic therapy is beneficial for patients with normal or near-normal hemoglobin levels and patients with anemia. Unless baseline hemoglobin was greater than 15 g/dl, all patients in the study received EPO according to the study algorithm just before receiving chemotherapy. Starting EPO therapy before chemotherapy may help prevent chemotherapy-induced anemia, but several weeks of EPO therapy are needed before the benefit is seen (Ortho Biotech Products, 2005). Study results may not be applicable to patients with less myelosuppressive treatment regimens.
Erythropoiesis-stimulating agents (ESAs) were approved by the U.S. Food and Drug Administration (FDA) based on their effectiveness in reducing the need for RBC transfusions in patients receiving chemotherapy for cancer. The current approved labeling for ESAs, including an FDA Black Box Warning, recommends using the lowest dose necessary in patients receiving chemotherapy to avoid the need for blood transfusions. The FDA (2007) also advises that hemoglobin not exceed 12 g/dl. Health risks associated with the use of ESAs in the treatment of anemia for use in patients with advanced breast, head and neck, lymphoid, and non-small cell lung cancers include shortened overall survival or time-to-tumor progression and an increased incidence of thrombotic events (e.g., myocardial infarction, stroke, thrombosis) in patients receiving chemotherapy (FDA).
The incidence of DVT (21%) was high among patients in the current study. The risk of DVT is known to be high with cancer (Naess et al., 2007), with central venous catheters (Monreal et al., 2006), and with chemotherapy and thalidomide (Zangari et al., 2003), particularly with doxorubicin and thalidomide (Zangari et al., 2002). Additional studies are being conducted using medical record reviews to compare the incidence of DVT among patients in the current study with patients who were treated with the same high-dose chemotherapy and autologous PBSCT protocol but did not receive ESA therapy according to the current study’s algorithm.
The Centers for Medicare and Medicaid Services (CMS) recently limited coverage of ESAs to patients receiving chemotherapy with a hemoglobin level below 10 g/dl with symptomatic ischemia and who cannot be transfused, or below 9 g/dl in patients with cardiovascular disease (Purrough et al., 2007). The American Society of Clinical Oncology (ASCO) expressed concern over CMS’s national coverage that “makes it impossible for physicians to use these agents in a manner consistent with the FDA-approved prescription labeling for these agents,” and, at the request of CMS, ASCO submitted supporting clinical information (Bailes, 2007, p. 2). The FDA’s response was that FDA-approved labeling and the CMS coverage decision were generally consistent (FDA, 2007). CMS asked for new evidence to show that patients receiving chemotherapy for cancer require hemoglobin levels to be above 10 g/dl. ESA therapy is superior to transfusion therapy (Schnell, 2007).
Limitations
The current study is limited by the lack of transfusion criteria (i.e., hemoglobin level or other criteria used to determine whether a transfusion is needed). The analysis may have missed specific disease-related influences on hemoglobin levels. Patients with hemoglobin less than 8 g/dl received RBC transfusions according to the transplantation protocol. Some patients with hemoglobin values above 8 g/dl may have received transfusions because of symptoms or other clinical indications. Although randomization should have distributed disease risks equally for the exercise and usual care groups, patients were not randomly assigned to the short- and long-term groups. However, by taking the first eligible patients, similar numbers of patients from the exercise and usual care groups did take part in the long-term study. The hemoglobin level at which anemia requires transfusions is not well established (Bailes, 2007). Whether patients in the current study received transfusions because of a low hemoglobin level, symptoms, or both is unknown. Regardless of the reason, patients in the usual care group received more transfusions than patients in the exercise group to maintain a similar hemoglobin level.
Implications for Nursing
The results of the current study have important implications for oncology nurses. Although the risks and benefits of ESA therapy are not known, this study has determined that patients receiving high-dose chemotherapy and autologous PBSCT can exercise safely. Therefore, nurses should use exercise as an intervention that may help decrease the need for transfusions. Exercise may also help patients during stem cell collection.
Acknowledgments
This investigator-initiated study was funded by the National Institute of Nursing Research of the National Institutes of Health (grant R01 NR008937-01A1) and Ortho Biotech Clinical Affairs, LLC (protocol PR01-02-016). Anaissie is involved in grant/research support for Pfizer Inc., Astellas Pharma Inc., and Enzon Pharmaceuticals, Inc., and is a consultant for Pfizer Inc., Astellas, Amgen Inc., Enzon, and Gilead Sciences, Inc. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Forum or the Oncology Nursing Society.
References
- Ahmadizad S, El-Sayed MS, Maclaren DP. Responses of platelet activation and function to a single bout of resistance exercise and recovery. Clinical Hemorheology and Microcirculation. 2006;35(1–2):159–168. [PubMed] [Google Scholar]
- Aldemir H, Kilic N. The effect of time of day and exercise on platelet functions and platelet-neutrophil aggregates in healthy male subjects. Molecular and Cellular Biochemistry. 2005;280(1–2):119–124. doi: 10.1007/s11010-005-8238-8. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. Perceived exertion. 2001 Retrieved October 10, 2007, from http://www.acsm.org/AM/Template.cfm?Section=Search§ion=20014&template=/CM/ContentDisplay.cfm&ContentFileID=307.
- Bailes JS. Communication to M.O. Leavitt. 2007 Retrieved October 10, 2007, from http://www.esafacts.org/ASCO.pdf.
- Barlogie B. High-dose therapy and innovative approaches to treatment of multiple myeloma. Seminars in Hematology. 2001;38(2 Suppl 3):21–27. doi: 10.1016/s0037-1963(01)90091-5. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Beck T. Recombinant human erythropoietin and the anemia of multiple myeloma. Stem Cells. 1993;11(2):88–94. doi: 10.1002/stem.5530110203. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion (Borg rating of perceived exertion scale) 1998 Retrieved October 10, 2007, from http://www.cdc.gov/nccdphp/dnpa/physical/measuring/perceived_exertion.htm.
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: A literature review. Annals of Behavioral Medicine. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- Drouin JS, Young TJ, Beeler J, Byrne K, Birk TJ, Hryniuk WM, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer. 2006;107(10):2490–2495. doi: 10.1002/cncr.22267. [DOI] [PubMed] [Google Scholar]
- Engert A. Recombinant human erythropoietin in oncology: Current status and further developments. Annals of Oncology. 2005;16(10):1584–1595. doi: 10.1093/annonc/mdi307. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. GPower 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. Journal of Clinical Oncology. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Hamilton DM, Haennel RG. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. Journal of Cardiopulmonary Rehabilitation and Prevention. 2000;20(3):156–164. doi: 10.1097/00008483-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Henry DH. Epoetin alfa for the treatment of cancer- and chemotherapy-related anaemia: Product review and update. Expert Opinion on Pharmacotherapy. 2005;6(2):295–310. doi: 10.1517/14656566.6.2.295. [DOI] [PubMed] [Google Scholar]
- Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. American Journal of Medicine. 2004;116(Suppl 7A):11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- Li N, He S, Blomback M, Hjemdahl P. Platelet activity, coagulation, and fibrinolysis during exercise in healthy males: Effects of thrombin inhibition by argatroban and enoxaparin. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(2):407–413. doi: 10.1161/01.ATV.0000253906.19648.ac. [DOI] [PubMed] [Google Scholar]
- Monreal M, Munoz FJ, Rosa V, Romero C, Roman P, Di Micco P, et al. Upper extremity DVT in oncological patients: Analysis of risk factors. Data from the RIETE registry. Experimental Oncology. 2006;28(3):245–247. [PubMed] [Google Scholar]
- Multiple Myeloma Research Foundation. Myeloma treatments. 2002 Retrieved January 24, 2006, from http://www.multiplemyeloma.org/treatments/3.07.02.asp.
- Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: A population-based study. Journal of Thrombosis and Haemostasis. 2007;5(4):692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Common terminology criteria for adverse events. 2007 Retrieved October 10, 2007, from http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA, Loge JH. Physical exercise results in the improved subjective well-being of a few or is effective rehabilitation for all cancer patients? European Journal of Cancer. 2004;40(7):951–962. doi: 10.1016/j.ejca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Ortho Biotech Products. (2005). Procrit® (epoetin alfa) prescribing information [Package insert]. Ortho Biotech Products, L.P.
- Purrough S, Jacques L, Ciccanti M, Long K, Koller E, Feinglass S. Decision memo for erythropoiesis stimulating agents (ESAs) for non-renal disease indications. 2007 Retrieved October 10, 2007, from http://www.cms.hhs.gov/determinationprocess/downloads/id203d.pdf.
- Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- Schnell FM. Communications to S.E. Phurrough. 2007 Retrieved October 10, 2007, from http://www.legislink.com/site/DocServer/COA_ESA_Commentary_6-8-07.pdf.
- Sports Coach. Borg scale. 1997 Retrieved October 10, 2007, from http://www.brianmac.co.uk/borgscale.htm.
- Steensma DP, Loprinzi CL. Erythropoietin use in cancer patients: A matter of life and death? Journal of Clinical Oncology. 2005;23(25):5865–5868. doi: 10.1200/JCO.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: Systematic review of controlled trials. Cancer Causes and Control. 2004;15(10):1035–1056. doi: 10.1007/s10552-004-1325-4. [DOI] [PubMed] [Google Scholar]
- Stricker CT, Drake D, Hoyer KA, Mock V. Evidence-based practice for fatigue management in adults with cancer: Exercise as an intervention. Oncology Nursing Forum. 2004;31(5):963–976. doi: 10.1188/04.ONF.963-976. [DOI] [PubMed] [Google Scholar]
- Strong A, Karavatas S, Reicherter EA. Recommended exercise protocol to decrease cancer-related fatigue and muscle wasting in patients with multiple myeloma. Topics in Geriatric Rehabilitation. 2006;22(2):172–186. [Google Scholar]
- U.S. Food and Drug Administration. FDA public health advisory: Erythropoiesis-stimulating agents (ESAs) 2007 Retrieved October 10, 2007, from http://www.fda.gov/CDER/Drug/advisory/RHE2007.htm.
- Zangari M, Barlogie B, Thertulien R, Jacobson J, Eddleman P, Fink L, et al. Thalidomide and deep vein thrombosis in multiple myeloma: Risk factors and effect on survival. Clinical Lymphoma. 2003;4(1):32–35. doi: 10.3816/clm.2003.n.011. [DOI] [PubMed] [Google Scholar]
- Zangari M, Siegel E, Barlogie B, Anaissie E, Saghafifar F, Fassas A, et al. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: Implications for therapy. Blood. 2002;100(4):1168–1171. doi: 10.1182/blood-2002-01-0335. [DOI] [PubMed] [Google Scholar]