Abstract
Vasopressin (VP) secreted within the brain modulates neuronal function acting as a neurotransmitter. Based on the observation that VP prevented serum deprivation-induced cell death in the neuronal cell line, H32, which expresses endogenous V1 receptors, we tested the hypothesis that VP has anti-apoptotic properties. Flow cytometry experiments showed that 10nM VP prevented serum deprivation-induced cell death and annexin V binding. Serum deprivation increased caspase-3 activity in a time and serum concentration dependent manner, and VP prevented these effects through interaction with receptors of V1 subtype. The signaling pathways mediating the anti-apoptotic effect of VP involve mitogen activated protein (MAP) kinase and extracellular signal-regulated kinases (ERK), Ca2+/calmodulin dependent kinase (CaMK) and protein kinase C (PKC). Western blot analyses revealed time-dependent decreases of Bad phosphorylation and increases in cytosolic levels of cytochrome c following serum deprivation, effects which were prevented by 10nM VP. These data demonstrate that activation of endogenous V1 VP receptors prevents serum deprivation-induced apoptosis, through phosphorylation-inactivation of the pro-apoptotic protein, Bad, and consequent decreases in cytosolic cytochome c and caspase-3 activation. The data suggest that VP has anti-apoptotic activity in neurons and that VP may act as a neuroprotective agent in the brain.
Keywords: Vasopressin, V1a receptor, Apoptosis, MAPK/ERK, Bad, RSK
INTRODUCTION
Vasopressin (VP), produced mainly in magnocellular and parvocellular neurons of the hypothalamus, is an important neuropeptide involved in water conservation, blood pressure control and pituitary ACTH hormone secretion (1–3). In addition, VP secreted within the central nervous system (CNS), modulates neuronal function acting as a neurotransmitter. The functions of VP are mediated through membrane VP receptors belonging to the G protein-coupled membrane receptor (GPCR) superfamily (4). There are two major VP receptor subtypes, V1, which is coupled to calcium phospholipid dependent pathways, and V2, which is coupled to cAMP-dependent pathways. While V2 receptors are responsible for the effects of VP on water homeostasis in the kidney, V1 receptors mediate the effects of VP in other tissues, including the brain (5, 6). VP produced in the medial amygdala and the bed nucleus of the stria terminalis projects to the lateral septum and ventral hippocampal sites where VP acting through V1 VP receptors affects memory and behavior (7, 8). Previous studies showed that VP has trophic actions in a variety of cells and primary tissues including neurons (9). Such trophic actions of VP have been implicated in the mechanism by which VP facilitates learning and memory in the hippocampus (10). We have recently observed considerable amount of cell death in the neuronal cell line, H32, following overnight serum deprivation, but the effect was less evident when VP was present in the incubation medium (11). This observation suggested that VP protected H32 cells against serum-deprivation induced cell death and that VP may have protective properties.
The neuronal cell line H32 expresses functional V1 receptors (12) , mostly V1a and a small proportion of V1b receptors. We have shown that in addition to stimulation of PKC and CaMK dependent pathways, activation of these receptors transactivate epidermal growth factor receptors (EGFR) resulting in activation of MAPK/ERK (12). The MAPK/ERK pathway is involved in neuronal development, memory formation, synaptic plasticity and neuronal survival (13, 14). Stimulation of MAPK/ERK signaling pathway by growth factor receptors and GPCRs generally lead to a mitogenic and proliferative response (15). In particular, activation of MAPK/ERK transduces a survival signal in a number of systems (16). Thus, it is possible that activation of the MAP kinase pathway by VP could mediate neuroprotective effects. Since H32 cells contain endogenous V1 VP receptors, this cell line provides a good model for studying possible functions of VP in neurons and the mechanisms by which VP exerts its effects.
The objective of this study was to determine whether VP has anti-apoptotic effects on hypothalamic H32 neuronal cells, and to examine the signaling pathways and mechanisms involved in the effects of VP. We demonstrate that activation of endogenous V1 VP receptors by VP in H32 hypothalamic cells protects from serum deprivation-induced apoptosis, an effect which is mediated via phosphorylation-inactivation of the pro-apoptotic protein, Bad, and consequently decreases the release of cytochome c resulting in caspase-3 activation. The signaling pathways mediating this effect appear to involve the EGFR, MAPK/ERK, CaMK and PKC. This study suggests a novel action of VP in the brain as an anti-apoptotic and neuroprotective agent.
MATERIALS AND METHODS
Materials
Calphostin C, BIM, Gö 6983 and NK-93 were purchased from BIOMOL Research Lab. (Plymouth Meeting, PA); UO126, SL327, AG1478, SB203580 and H89 were from Calbiochem (San Diego, CA). Antibodies against phospho-Bad (Ser112), Bad, Phospho-p44/42 MAP Kinase (Thr202/Tyr204), p44/42 MAP Kinase, Phospho-RSK (Thr359/Ser363), RSK were purchased from Cell Signaling Technology™ (Beverly, MA); β-Tubulin antibody from Sigma (Saint Louis, MO). The non-peptide V1a VP receptor antagonist SR49059 and the V1b VP receptor antagonist SSR149415 were provided by Dr. Claudine Serradeil-Le Gal (Sanofi-Synthlab, Toulouse, France).
Cell culture and treatments
The hypothalamic cell line H32, provided by Dr Joachim Spiess, Goettingen, Germany, was cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Gaithersburg, MD) containing 10% fetal bovine serum (Life Technologies, Inc.), 10% horse serum and 1% penicillin/streptomycin (Life Technologies, Inc.). After 24h culture in 100mm plates (1.5×106 cell per plate), at 37°C, under 5% CO2/95% air, the medium was changed to serum-free medium containing 0.1% BSA, with or without VP. To determine the signaling pathways and receptor subtypes mediating the effect of VP, cells were incubated in the presence and absence of inhibitors. After incubation for the time periods indicated in results and figure legends, cells were processed for caspase-3 activity or Western blot analysis.
Plasmids and transfection
Wild type Bad and Bad S112/136A mutant, cloned into pcDNA3 vector, were provided by Dr G. Kulik (Wake Forest University School of Medicine, Winston-Salem, NC). Ribosomal S6 kinase 90 kDa (RSK) wild type, RSK1 dominant negative mutant (RSK1 K112/464R) and RSK2 dominant negative mutant (RSK2 KR100) were obtained from Dr. M. E. Greenberg (Harvard Medical School, Boston, MA). Transient transfection was performed in Opti-MEM I Reduced Serum Medium (Invitrogen) using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer’s recommendations. Cells were used after 24h transfection.
Flow cytometry assay
Cells were collected using trypsin-EDTA, centrifuged at 200 × g for 5 min, washed twice with ice-cold PBS and resuspended in 0.3 ml of PBS containing 2% FBS. Forward Scatter (FSC) and Side Scatter (SSC) of cells were acquired by a FACSCalibur flow cytometer (Becton Dickinson, CA) and analyzed by FlowJo software (TreeStar, San Jose, CA). FSC indicates cell size, and SSC is related to cell granularity or internal complexity. Living cells were gated based on cell optic characteristics (FSC and SSC).
FACS detection of apoptotic cells
The degree of apoptosis following serum deprivation and VP treatment was examined by FACS, based on the ability of fluorescence-labeled annexin V to bind phosphatidyl serine, which is translocated to the outer membrane layer during early apoptosis, and the capacity of amino-actinomycin D to bind to the nuclei of late apoptotic cells. H32 cells (2.5 × 105) were incubated with 5ul of annexin V-FITC (1 mg/ml) and 7-Amino-actinomycin D (7-AAD) (1 mg/ml) (BD Biosciences) for 15 minutes at room temperature, according to the manufacturer’s instructions, and immediately analyzed by flow cytometry as described above. This method allows discrimination of early apoptotic cells (annexin V+ /7-AAD−) and late apoptotic cells (annexin V+ /7-AAD+) (17). Early apoptotic cells (annexin V+ /7-AAD−) and late apoptotic cells (annexin V+/7-AAD+) were counted for total apoptosis.
Caspase-3 activity measurement
Caspase-3 activity was measured using a Caspase-3/CPP32 fluorometric protease assay kit (BioSource International, Inc., Camarillo, CA) according to the manufacturer's protocol. Briefly, cells were washed with PBS, centrifuged for 5 min at 800× g, the supernatant removed and the pellet resuspended in ice cold lysis buffer. After 20 min incubation at room temperature, samples were centrifuged at 16,000 × g for 10 min at 4 °C, and protein concentrations in the supernatants determined using BCA™ protein Assay (PIERCE, Rockford, IL). Aliquots containing 100µg of protein were incubated with substrate DEVD (Asp-Glue-Val-Asp)-AFC (7-amino-4-trifluoromethyl coumarin) for 90 min at 37 °C. Upon cleavage of the substrate by Caspase-3, free AFC, which emits a yellow-green fluorescence, was measured by using a FLUOStar OPTIMA microplate reader (BMG Labtechnologies Inc, Durham, NC), with a 405 nm excitation and 505 nm emission filter.
Cytosolic cytochrome c levels
The levels of cytosolic cytochrome c were measured using a Cytochrome c ELISA Kit (MBL, Watertown, MA). Briefly, H32 cells were cultured in 100mm culture flasks, serum deprived for 0, 0.5, 1, 2, 4 and 6h in the absence or in the presence of VP (10 nM). After treatment, the cells were harvested using trypsin-EDTA, spun down at 200 × g for 5 min, washed twice with ice-cold PBS and resuspended in 500 µL ice-cold homogenization buffer (10 mm Tris/HCl (pH 7.5), 0.3 m sucrose, 25 µg/mL aprotinin, 1 mm phenylmethylsulphonyl fluoride, and 10 µg/mL leupeptin). Cells were then homogenized on ice using a dounce homogenizer and centrifuged at 10 000 × g for 60 min at 4 °C. Protein concentrations in the supernatants (cytosolic fractions) were determined using BCA™ protein Assay (Pierce, Rockford, IL). Cytosolic cytochrome c level was detected using peroxidase conjuagted anti-cytochrome c polyclonal antibody, according to the manufacturer's instructions.
Western blot analysis
Western blot analysis was performed essentially as described previously (18). Briefly, cells were lysed with T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with proteinase and phosphate inhibitor cocktail (Sigma). Protein concentrations were determined by BCA™ Protein Assay (Pierce) and 20 µg of protein were loaded and separated in a 4–20% SDS-PAGE (Invitrogen,). Proteins were transferred from the gel to a polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Piscataway, NJ), incubated with 5% nonfat dried milk in Tri-buffered saline (TBS plus 0.1% Tween-20 (TBST)) for 1h and incubated with the antibodies at a 1:1,000 dilution overnight. After washing in TBST, membranes were incubated for 2h with peroxidase-linked anti-Rabbit IgG at a 1:10,000 dilution or anti-mouse IgG at a 1:5,000. β-tubulin was used to correct for protein loading. Detection of immunoreactive band was performed by using ECL Plus TM reagents (Amersham Pharmacia Biotech) and exposure to BioMax MR film (Kodak, Rochester, NY). Densitometric quantification of the immunoblots was performed by using the public domain NIH Image program (ImageJ 1.36b developed at the US National Institutes of Health, and available on the Internet at: http://rsb.Info.nih.gov/nih-image).
Data analysis
Statistical significance of the differences between groups was calculated by one-way analysis of variance (ANOVA), followed by Student-Newman-Keuls method for pairwise multiple comparisons. Statistical significance was set at p < 0.05. Data are presented as means ± standard error of the mean (SEM) from the values in the number of observations indicated in results or legends to Figures.
RESULTS
Serum deprivation causes cell death in H32 hypothalamic cells and VP promotes cell survival
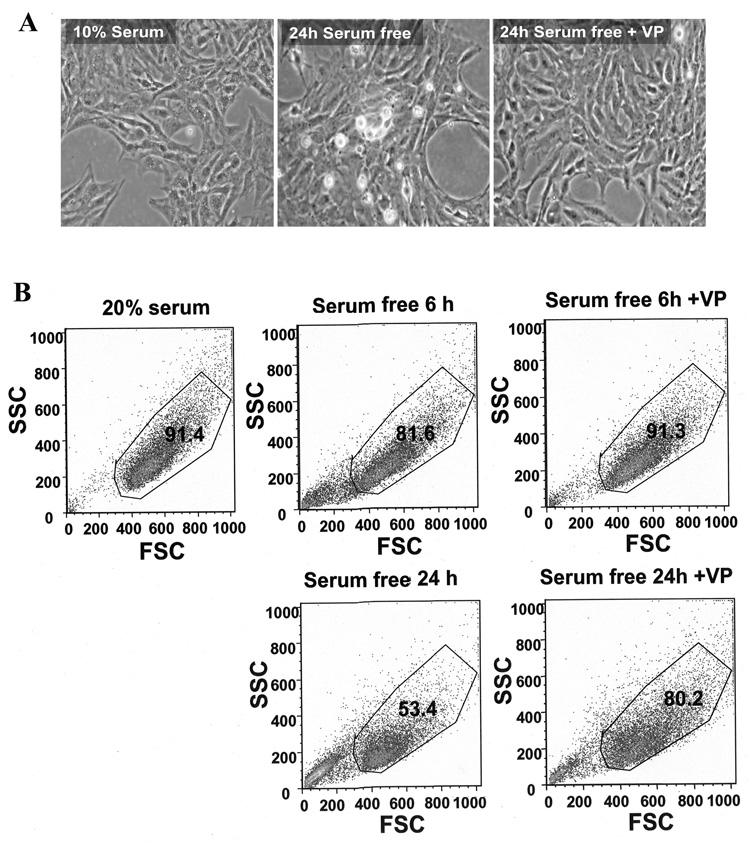
In initial experiments, incubation of H32 cells with serum-free medium for 24h caused morphological changes evident by light microscopy examination. In contrast to cells cultured in 10% serum, a large proportion of cells cultured in serum-free medium were retracted, rounded in shape, and some detached from the culture plate, suggesting cell death. In contrast, cells incubated in serum free medium in the presence of 10 nM VP appeared similar to those cultured with 10% serum (Fig. 1A). To quantify the effects of serum-deprivation on cell death and the protective action of VP, we used flow cytometry to analyze the living cell population selected by Forward Scatter (FSC) and Side Scatter (SSC). H32 neuronal cells cultured with 20% serum showed a 91.4% living cell population. Serum starvation for 6h and 24h reduced the living cell population to 81.6% and 53.4%, respectively, and VP (10 nM) reversed the changes at 6h (91.3%) and markedly reduced cell death after 24h serum deprivation (80.2%) (Fig. 1B).
Fig. 1.
Serum starvation induced cell death in H32 hypothalamic cells. (A) Light microscopy images of H32 hypothalamic cells following incubation with 10% serum, or serum starvation for 24h in the presence or in the absence of 10nM VP. (B) Flow cytometry analysis of the cell size (Forward Scatter [FSC]) and granularity (Side Scatter [SSC]) of H32 hypothalamic cells following incubation for 6 or 24 h with 20% serum, serum-free medium or serum-free medium plus 10nM VP. The gated population represents the percentage of living cells. Each plot is representative of three experiments with similar results.
Serum deprivation induces apoptosis and VP has anti-apoptotic effects
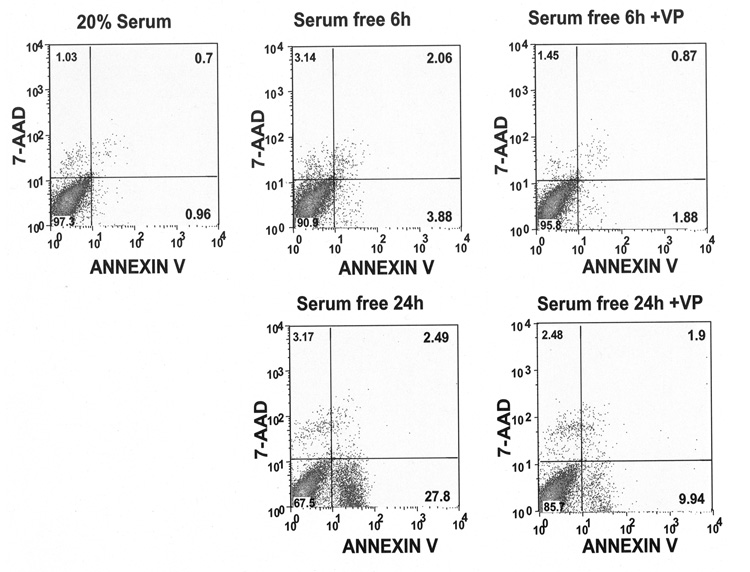
To confirm the above cell death was through apoptosis, H32 hypothalamic cells were dual labeled with FITC conjugated annexin V and 7-AAD, and subjected to FACS. Annexin V is a cell membrane marker for early stage apoptosis; 7-AAD stains the nucleus of apoptotic cells. As shown in Fig. 2 and Table 1, 6h serum starvation increased the percentage of annexin V-labeled cells (4.3% vs 0.9%, p<0.05), as well as the number of double-labeled (annexin V plus 7-AAD) cell population (6.9% vs 1.7%, p<0.05), indicating an increase in the proportion of cells undergoing early and late apoptosis. VP treatment reversed serum starvation-induced cell apoptosis (2.9% vs 6.9%, p<0.05). A larger increase in annexin V plus 7-AAD-labeled cells was detected after 24h serum starvation compared to the serum-incubated control (32.8% vs 1.7%, p<0.001). This effect was markedly reduced by addition of VP during serum deprivation (10.9% vs 32.8%, p<0.05) (Table 1, Fig 2). These data indicate that serum deprivation causes apoptotic cell death and that VP has anti-apoptotic actions.
Fig. 2.
VP protects H32 hypothalamic cells from serum deprivation-induced apoptotic cell death. FACS analysis of the percentage of H32 hypothalamic cells undergoing early (annexin V +/7-AAD− -labeled cells) and late apoptosis (annexin V +/7-AAD+-labeled cells) after 6 or 24 h incubation in 20% serum or serum-free medium without or with 10nM VP. The results were plotted as fluorescence intensity of annexin V as a function of fluorescence intensity of 7-AAD. The numbers in the corner of each square present the percentage of cells for annexin V −/7-AAD−-viable cells (the number in left low corner); annexin V +/7-AAD− early apoptotic cells (the number in right low corner); annexin V +/7-AAD+-lated apoptotic cells (the number in right high corner) and annexin V −/7-AAD+-broken cells (the number in left high corner). Plots are representative of the results in three different experiments.
Table 1.
Effect of serum deprivation and VP on apoptosis in H32 hypothalamic cells
| Early apoptotic cells (%) | Late apoptotic cells (%) | Total apoptotic cells (%) | |
|---|---|---|---|
| With 20% Serum | 0.92±0.12 | 0.82±0.23 | 1.74±0.19 |
| Serum-free for 6h | 4.26±1.14# | 2.61±0.92# | 6.87±1.12# |
| Serum-free for 6h + VP 10 nM | 1.97±0.45* | 0.89±0.56* | 2.86±0.50* |
| Serum–free for24h | 29.7±6.32### | 3.09±1.12# | 32.8±3.26### |
| Serum-free for 24h + VP 10 nM | 9.02±2.65*## | 1.88±0.75# | 10.9±1.79*## |
The percentage of H32 hypothalamic cells undergoing early (annexin V +/7-AAD− - labeled cells) and late apoptosis (annexin V +/7-AAD+-labeled) was measured by FACS as described in “Materials and Methods”. Early apoptotic cells plus late apoptotic cells equate to total apoptotic cells. The values are expressed as the mean ± S.E.M of three experiments.
p< 0.05, compared to corresponding serum-free group
p< 0.05
p< 0.01
p< 0.001 compared to corresponding with 20% serum group.
Effects of serum deprivation and VP on caspase-3 activity
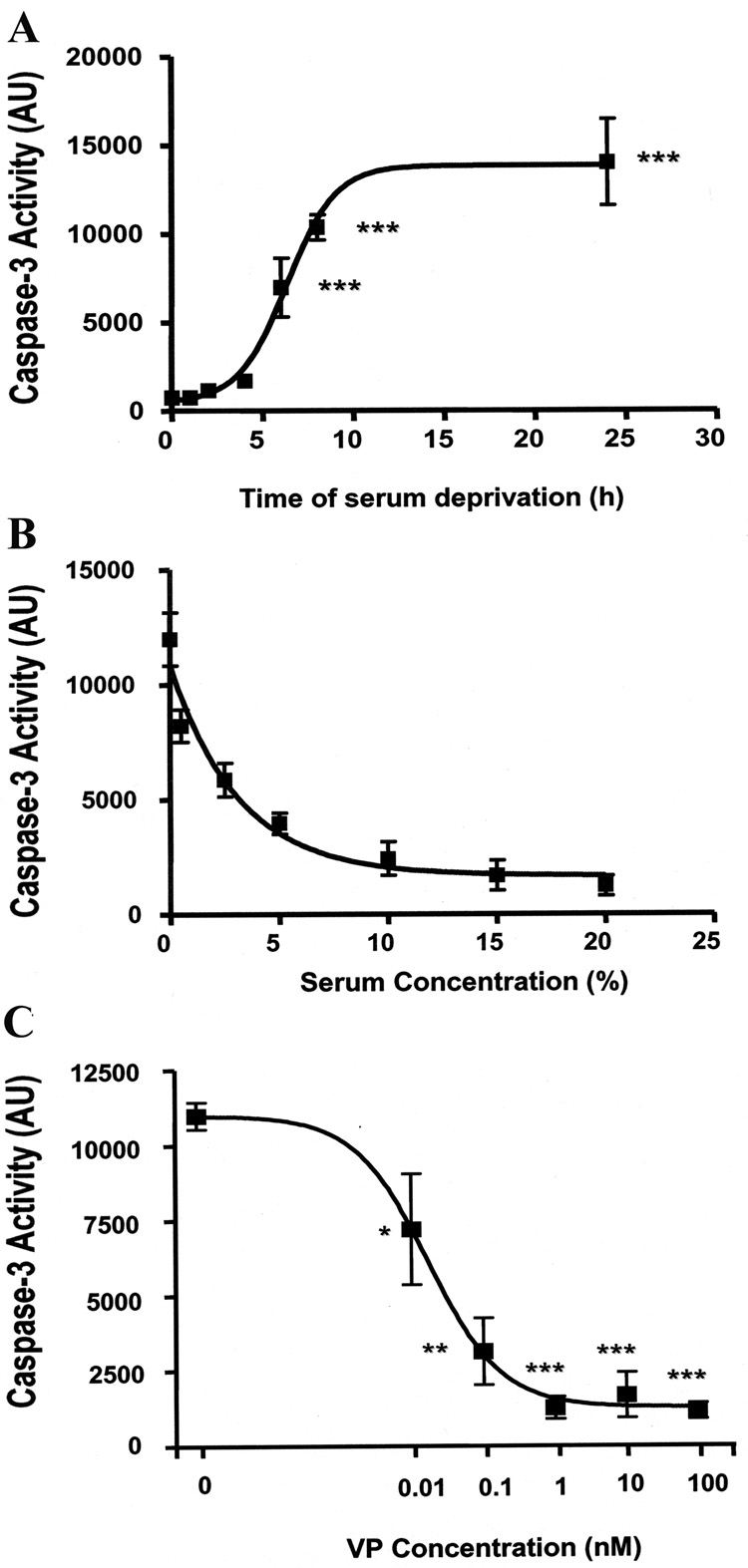
To further study the effects of serum deprivation on apoptosis, we examined the time course of the effect of serum deprivation on caspase-3 activity in H32 cells. As shown in Fig. 3A, incubation of H32 cells in serum free medium induced a time-dependent increase of caspase-3 activity, with levels increasing significantly at 6h serum starvation (P<0.001). Based on these data, all further experiments were performed under 6 h serum deprivation conditions. The effect of serum concentration on cell survival is shown in Fig. 3B. Control cells incubated in 20% serum displayed very low caspase-3 activity levels (~200 AU). Caspase-3 levels increased progressively with serum concentrations lower than 10%, reaching levels 6 times higher than controls after 6 h in the absence of serum. To determine whether VP has an effect on caspase-3 activity, H32 hypothalamic cells were serum deprived for 6h in the absence or presence of increasing doses of VP. As shown in Fig. 3C, VP decreased serum deprivation-induced caspase-3 activity with an IC50 of 0.02 nM and a maximal inhibitory concentration of 1 nM. A significant inhibition was already observed with 0.01 nM (P<0.01 nM vs basal). These data indicate that serum-deprivation activates the pro-apoptotic protein, caspase-3, and VP inhibits caspase-3 activity.
Fig. 3.
Serum deprivation induces caspase-3 activity and VP prevents this effect (A) Time course of caspase-3 activity following serum deprivation ***p<0.001, compared to basal caspase-3 activity at time point 0. (B) Serum concentration-dependence of induction of caspase-3 activity. H32 hypothalamic cells were incubated with increasing concentrations of serum (0 to 20%) for 6h. (C) Dose-response of the effect of VP on serum deprivation-induced caspase-3 activity. H32 hypothalamic cells were incubated under serum-free concentration for 6h with increasing dose of VP. Caspase-3 activity was determined as described in “Materials and Methods”. The values are expressed as the average + S.E.M of five experiments conducted in duplicate. * p< 0.05, ** p< 0.01 and ***p<0.001, compared to serum deprivation group.
The anti-apoptotic effect of VP is mediated via V1 VP receptors
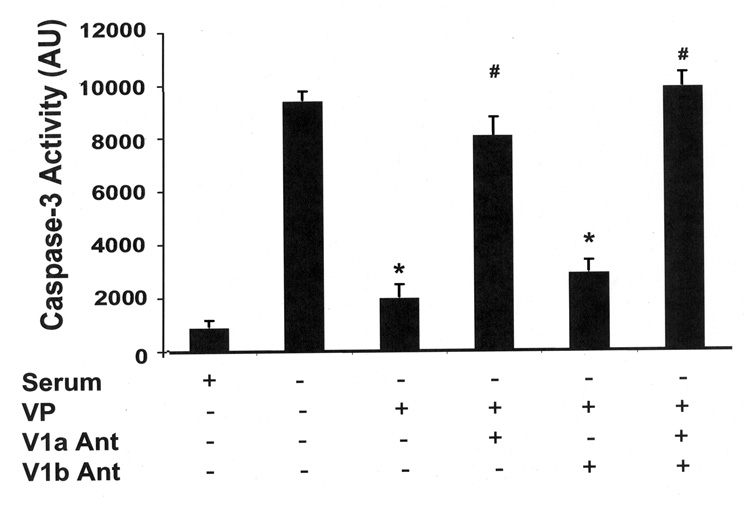
Previous studies have shown that H32 neuronal cells express endogenous V1a and V1b receptors (12). Thus, we used V1 receptor subtype-specific antagonists to determine which type of receptor mediates the anti-apoptotic effect of VP. As shown in Fig 4, the inhibition of VP on caspase-3 activity was significantly reduced by 88% in the presence of the V1a receptor antagonist, SR49059. In the presence of the non-peptide V1b receptor antagonist SSR149415, the inhibitory effect of VP on caspase-3 activity was blocked by only 8%. Moreover, the combination of both antagonists, SR49059 and SSR149415, completely abolished the inhibitory action of VP on caspase-3 activity. This result indicates that the protective action of VP is mediated by V1 VP receptors, mainly via V1a VP receptors which are predominant in these cells.
Fig. 4.
VP inhibits caspase-3 activation through V1 VP receptors. H32 hypothalamic cells were incubated in serum-free conditions for 6h with or without VP (10 nM). Aliquots of the selective V1a VP receptor antagonist SR49059 or the V1b VP receptor antagonist SSR149415 or vehicle were added 30 min before VP treatment. Caspase-3 activity was determined. The bars represent the mean ± S.E.M of three experiments conducted in duplicate. * p< 0.05, compared to serum-free group; # p< 0.05 compared to serum-free plus VP group.
Signaling transduction pathways mediating the anti-apoptotic effect of VP
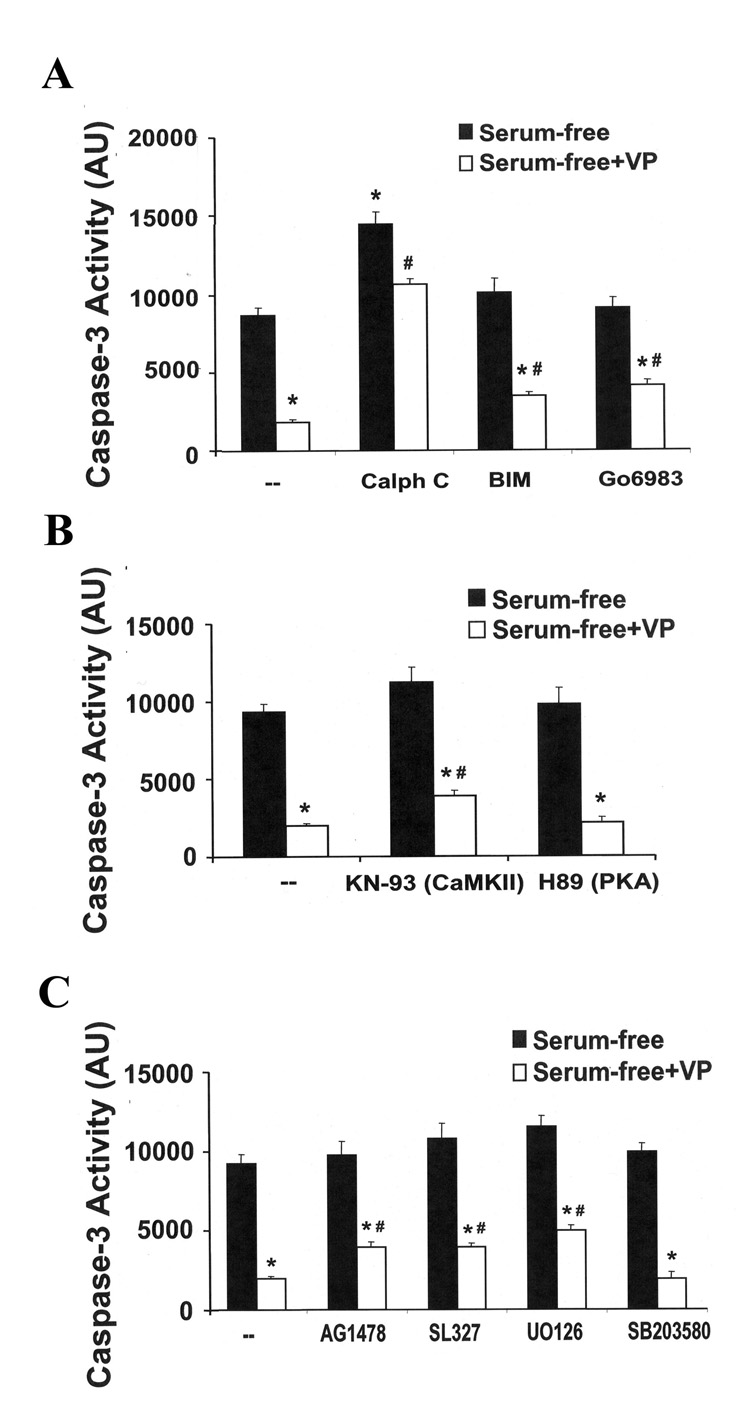
Since V1 VP receptors are coupled to phospholipase C (PLC), with consequent increases of intracellular Ca2+ and PKC activity (19), we examined the effect of the generic PKC inhibitors, calphostin C, BIM and Gö 6983, on the suppressive effect of VP on caspase-3 activity (Fig 5A). The PKC inhibitor calphostin C significantly exacerbated serum deprivation-induced caspase-3 activity, and completely prevented the inhibitory effect on VP on caspase-3 activity. On the other hand, BIM and Gö 6983 had no effect on their own and partially blocked the inhibitory effect of VP on serum deprivation-induced caspase-3 activity (26% and 29%, higher than levels in the presence of VP, respectively) (Fig. 5A). These results suggest that the anti-apoptotic effect of VP is at least in part mediated through the PKC pathway.
Fig. 5.
Signaling transduction pathways involved in the anti-apoptotic effect of VP. H32 hypothalamic cells were incubated in serum-free conditions for 6h in the presence or absence of 10 nM VP. (A) The PKC inhibitors, calphostin C (1 µM), BIM (100 nM), Gö6983 (1µM) were added 30 min before VP treatment. (B) The calcium calmodulin II inhibitor, KN-93 (10 µM), or the PKA inhibitor, H89 (1 µM), were added 30 min before VP treatment. (C) The EGF receptor inhibitor AG1478 (100 µM), the MEK inhibitors, SL327 (1µM) or U0126 (1µM), or the p38 MAP kinase inhibitor, SB203580 (10µM) were added 30 min before VP treatment. Caspase-3 activity was determined. The values are expressed as the mean ± S.E.M of four experiments conducted in duplicate. * p< 0.05, compared to serum-free group; # p< 0.05 compared to serum-free + VP group.
To determine the involvement of Ca2+ dependent calmodulin kinase, cells were subjected to serum deprivation with or without VP, in the presence and in the absence of the CaMKII inhibitor KN93. As shown in Fig 5B, 26% of the anti-apoptotic effect of VP was attenuated by co-incubation with KN-93 (p<0.05), suggesting partial involvement of the CaMK pathway. On the other hand, the anti-apoptotic effect of VP was refractory to the protein kinase A inhibitor, H89, which is consistent with the inability of VP to increase cAMP production.
Since we have previously shown that VP transactivates the EGF receptor causing activation of the MEK/ERK MAPK pathway (12), we examined the effect of the selective MEK inhibitors SL327 and U0126 on the protective effect of VP on serum deprivation-induced apoptosis. Both MEK inhibitors, SL327 and U0126, ablated about 35% of the inhibitory effect of VP on caspase-3 activity (Fig 5C). Similarly, about 32% of the inhibitory effect of VP on caspase-3 activity was attenuated by the EGFR inhibitor AG1478 (Fig 5C). In contrast, SB203580, a p38 MAPK inhibitor, had no effect on the inhibitory effect of VP on caspase-3 activity (Fig 5C). These results suggest the involvement of multiple signaling pathways, including MAPK/ERK, CaMK and PKC, in the anti-apoptotic effects of VP in H32 neuronal cells.
VP induces phosphorylation of the pro-apoptotic protein Bad and prevents cytochrome c release
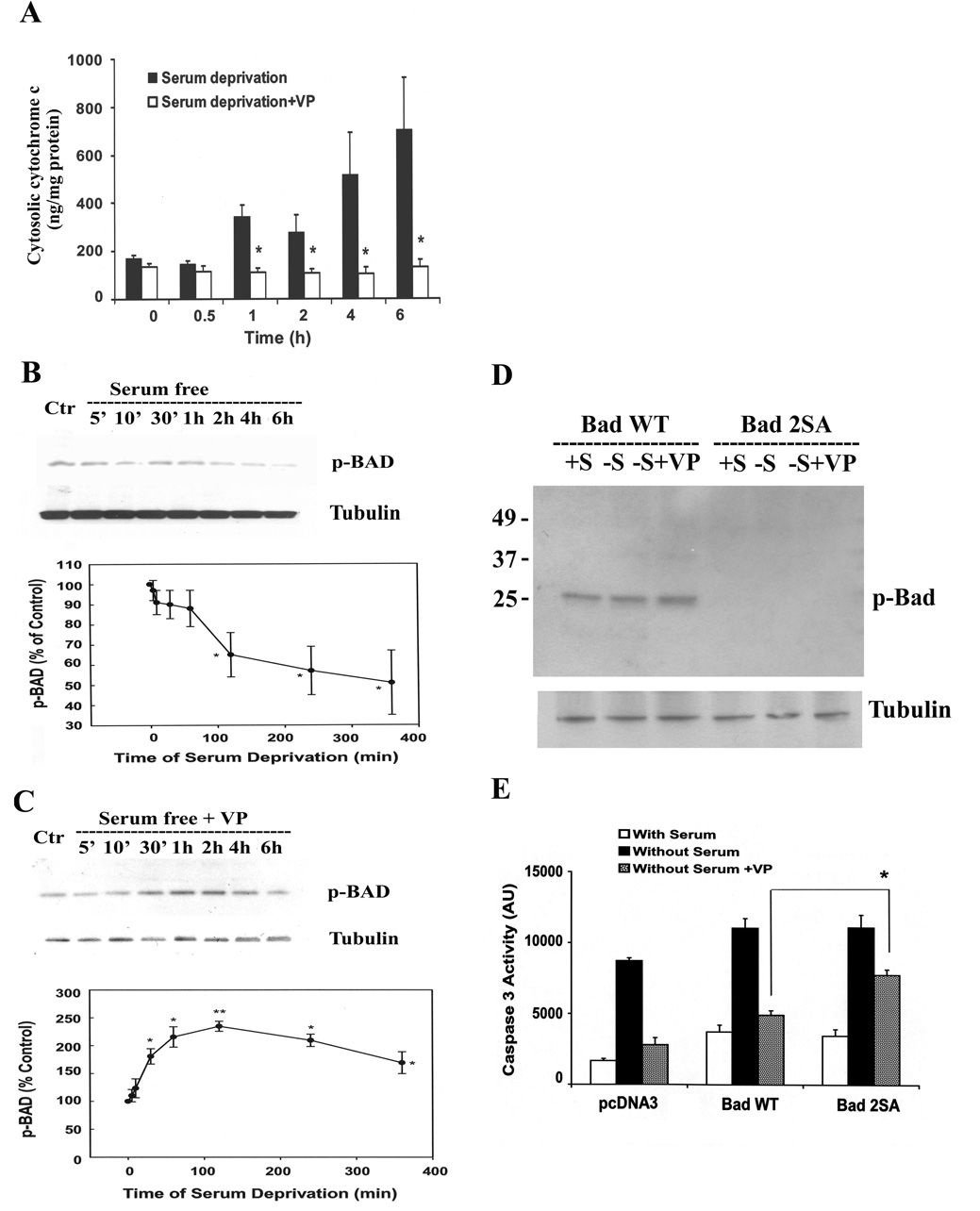
Release of mitochondrial cytochrome c to the cytosol is a critical step in the mechanism of serum deprivation-induced apoptotic cell death. To determine whether VP reduces the release of mitochondrial cytochrome c, we examined the levels of cytochome c in cytosol of H32 cells incubated under serum-free conditions in the presence or absence of 10 nM VP. As shown in Fig. 6A, serum deprivation induced a time-dependent increase of cytochrome c levels in the cytosol. Co-incubation of serum-deprived cells with VP completely prevented the increase in cytosolic cytochrome c at all time points.
Fig. 6.
VP induces the phosphorylation of pro-apoptotic protein, BAD, and prevents the release of cytochrome c form mitochondria. (A) The time course of cytosolic cytochrome c in the presence and the absence of 10nM VP. Cytochrome c levels were measured using an ELISA Kit as described in Methods. Bars represent the mean ± S.E.M of three experiments conducted in duplicate. * p< 0.05 and ** p< 0.01, significantly different form the corresponding serum deprivation group. Time course of BAD phosphorylation in the absence (B) or in the presence of 10 nM VP (C). Phosphorylation of BAD (p-Bad Ser112) was examined by Western blotting. Data are expressed as the mean ± S.E.M of three experiments. * p< 0.05, significantly different form the control group. PCDNA3 or Bad wild type (BAD WT) or Bad dominant negative mutant (Bad 2SA) was transfected into H32 cells. 24h after transfection, cells were incubated in 20% serum(+S) or serum-free medium(−S), or serum-free medium with 10nM VP(−S+VP). (D) Phosphorylation of BAD (Ser112) was examined by Western blotting, and (E) caspase-3 activity was determined as described in “Materials and Methods”. The bars represent the mean ± S.E.M of three experiments conducted in duplicate. * p< 0.05, compared to Bad WT Without Serum+VP group. .
To determine whether VP inhibits apoptosis by inducing inactivating phosphorylation of the pro-apoptotic protein, Bad, we examined phosphorylation levels of Bad in H32 cells subjected to serum deprivation in the presence or absence of VP. Western blot analysis using antibody against phospho-Bad(Ser112), revealed time-dependent decreases in phospho-Bad levels, with significant reduction by 2 h and a tendency for a further decrease up to 6 h (Fig. 6B). Co-incubation of the cells with 10nM VP prevented the inhibitory effect of serum deprivation on phospho-Bad(Ser112) levels, resulting in significant increases in Bad phosphorylation over the levels observed under nutrient deprivation. Phospho-Bad(Ser112) levels were significantly higher by 30 min, reached near maximal values by 1 h, and started a slow decline towards basal levels at 6 h (Fig 6C). To confirm the involvement of Bad in the anti-apoptotic effect of VP, we transfected H32 cells with wild type Bad or the phosphorylation inactive mutant in which Ser112 and 136 were replaced by alanine (Bad 2SA) serving as a dominant negative mutant of Bad. As shown in Fig 6D, transfection of BAD 2SA effectively prevented the effect of VP on Bad phosphorylation. Transfection of wild type Bad in H32 cells increased basal caspase-3 activity, but did not prevent the inhibitory effect of VP on serum deprivation-induced caspase-3 activation. In contrast, blockade of Bad phosphorylation by Bad 2SA transfection, markedly inhibited the ability of VP to prevent serum deprivation-induced caspase-3 activation, suggesting that Bad phosphorylation plays an important role mediating the anti-apoptotic effect of VP (Fig 6E).
Upstream signaling pathways involved in VP-induced Bad phosphorylation
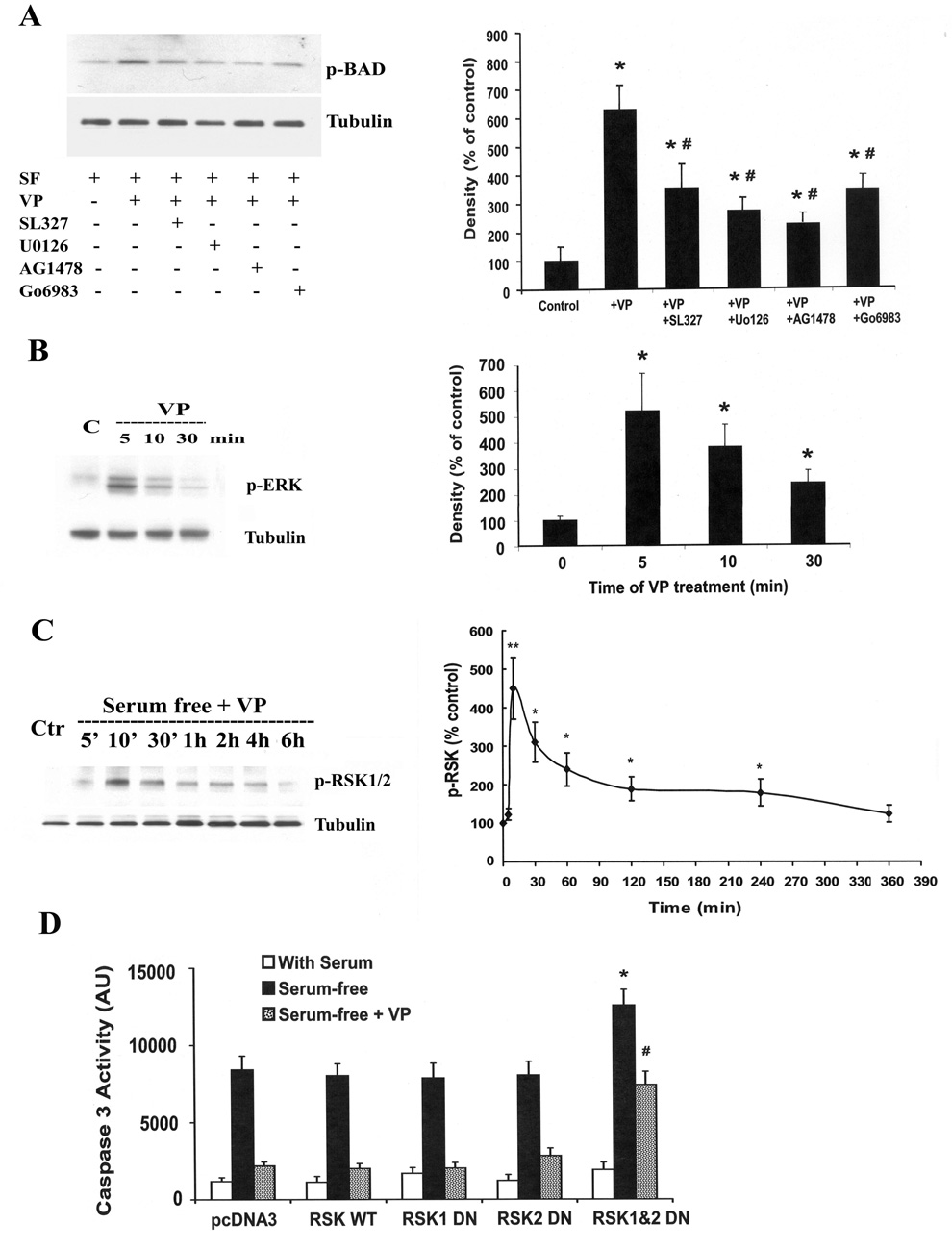
The upstream signaling pathways involved in VP-induced Bad phosphorylation were studied by examining the effect of VP on Bad phosphorylation in the presence of PKC, MEK and EGFR inhibitors. As shown in Fig. 7A, the MEK inhibitors SL327 and Uo126 significantly reduced VP-induced Bad phosphorylation by 53% and 67%, respectively. Similarly, the EGFR inhibitor AG1478 reduced VP-induced Bad phosphorylation by 70%. In addition, the generic PKC inhibitor Go6983 blocked ~55% of Bad phosphorylation induced by VP. Consistent with the involvement of the MAPK/ERK cascade, incubation of H32 cells with 10 nM VP markedly increased ERK phosphorylation from undetectable levels by 5 min. This increase of p-EKR gradually declined from 10 min to 30 min (Fig. 7B). Since phosphorylated ERK regulates Bad phosphorylation through activation of ribosomal 6 kinase (RSK) by phosphorylation at Thr359 and Ser363(20), we measured levels of phosphorylated RSK by western blot. As shown in Fig. 7C, VP caused a rapid and transient increase of p-RSK. Levels of p-RSK reached a maximum at 10 min of VP treatment, started a gradual decline by 30 min, to reach levels not significantly different from basal by 6 h. To confirm the involvement of RSK in mediating the anti-apoptotic effect of VP, the effect of VP on serum deprivation-induced caspase-3 activity was studied in H32 cells transfected with dominant negative mutants for RSK 1 and 2. As shown in Fig. 7D, the inhibitory effect of VP on serum deprivation-induced caspase-3 activity was unaffected by separated transfection of either RSK1 or 2 dominant negative mutants separately. In contrast, blockade of RSK 1 and 2 by co-transfection of RSK1 and RSK 2 mutants potentiated the effect of serum deprivation on caspase-3 activation, and reduced the inhibitory effect of VP on serum deprivation-induced caspase-3 activity by 57% (p<0.05).
Fig. 7.
Upstream signaling pathways involved in VP-induced BAD phosphorylation. (A) Effect of the ERK-MAP kinase inhibitors, SL327 (1µM) and UO126 (1µM), the EGF receptor inhibitor, AG1478 (100 µM) and the PKC inhibitor Go6983 (1µM) on VP-induced BAD phosphorylation. H32 cell incubated in serum-free medium for 2 h (control) were pre-incubated with the inhibitors for 30 min before addition of 10nM VP. Immunoblot analysis of phosphorylation of BAD (Ser112) was conducted as described in Method. For measurement of the effects of VP on ERK phosphorylation (B) and RSK phosphorylation (C), H32 hypothalamic cells were incubated in serum-free medium (SF) for 2h (control), before addition of 10 M VP. Cell extracts for Western blotting analysis of p-ERK and p-RSK were prepared at the time points indicated in the graphs. Data represent the mean and SEM of the results obtained in three experiments. * p< 0.05 and p<0.01, compared to serum-free (control) group, # p< 0.05 compared to serum-free + VP group. (D) PCDNA3 or RSK wild type (RSK WT) or RSK dominant negative mutants (RSK 1, RSK2 or RSK1&2) were transfected into H32 cells. 24h after treansfection, cells were incubated in 20% serum (With Serum), or serum-free medium (Serum-free), or serum-free medium with 10nM VP (Serum-free+VP). Caspase-3 activity was determined. The bars represent the mean ± S.E.M of three experiments conducted in duplicate. * p< 0.05, compared to RSK WT serum-free group, # p< 0.05 compared to RSK WT serum-free + VP group‥
DISCUSSION
This study demonstrates that VP has anti-apoptotic effects on neuronal H32 cells, an effect which is partially mediated by phosphorylation-inactivation of the pro-apoptotic protein Bad, and consequent inhibition of caspase-3 activation. VP secreted within the brain regulates a broad spectrum of behavioral and cognitive functions, such as learning and memory, by acting upon V1 receptors in the cerebral cortex, hippocampus and other limbic areas (7, 8, 21, 22). The present demonstration that VP has anti-apoptotic actions expands the potential range of actions of VP by providing supporting evidence for a role of the peptide as a neuroprotective agent. Studies in progress reveal that VP can partially prevent nutrient deprivation-induced cell death and caspase-3 activation in rat hippocampal cell cultures containing about 95% neurons (Chen and Aguilera, unpublished). It has been shown that VP inhibits the secretion of the pro-inflammatory cytokines, interleukin 1β and TNF from cultured astroglia (23). Although the latter effect could contribute to protection in some conditions, the present demonstration that VP has antiapoptotic actions in a neuronal cell line, suggest that VP can excert neuroprotection directly in neuron expressing V1 receptors (24–27).
The immortalized hypothalamic cell line, H32, used in this study, was originally characterized as a corticotrophin-releasing hormone expressing cell (28). This cell line expresses endogenous V1 receptors, predominantly of the V1a subtype and a small proportion of the V1b subtype (12, 29). This is consistent with the present demonstration that the protective effects of VP on H32 cells is largely blocked by the V1a antagonist and completely prevented by the combination of V1a and V1b antagonists. In keeping with the V1 receptor coupling to Gq/11 (4, 30, 31), incubation of H32 cells with VP causes activation of phosphatidylinositol specific PLC, with hydrolysis of phosphatidylinositol-4, 5-bisphosphate to IP3 and DAG. IP3 causes Ca2+ release of the endoplasmic reticulum and activation of CaMK, and DAG stimulates PKC activity. VP also transactivates the EGFR in these cells leading to stimulation of the MAPK/ERK pathway (12). Because of these characteristics, the cell line H32, provides an ideal model to study the effects of VP in neurons.
The changes in cell morphology and size, as well as annexin V and 7-AAD staining, clearly show that cells undergo apoptosis following serum deprivation and that VP has a protective effect. Moreover, the marked increases in caspase-3 activity indicate that serum deprivation induces apoptosis in H32 cells through the caspase pathway. An important pathway by which activation death receptors trigger the caspase cascade involves the pro-apoptotic protein Bad, and release of mitochondrial cytochome c (32, 33). In its dephosphorylated state, Bad is able to interact with and inactivate the anti-apoptotic proteins, Bcl-2 and Bcl-XL, resulting in release of mitochondrial cytochrome c. Phosphorylation of Bad at the serine residue 112 allows binding of Bad to the scaffolding protein 14-3-3, preventing its interaction with the anti-apoptotic proteins Bcl-2 and Bcl-XL, mitochondrial damage, and the release of cytochome c (34). Here we demonstrate that serum deprivation-induced caspase-3 activation and apoptosis in neurons involves dephosphorylation of the pro-apoptotic protein Bad, and that VP promotes cell survival at least partially through Bad phosphorylation. This was clear from the demonstration that VP phosphorylates Bad in serum-deprived H32 cells, and also from the ability of the Bad phosphorylation mutant to attenuate the protective action of VP. Multiple signaling pathways including, PKC, PKA and MAPK have been shown to mediate Bad phosphorylation (35). The present study provides strong evidence for the participation of the MAP kinase ERK/RSK pathway mediating VP induced neuroprotection. As demonstrated by the present experiments and previous reports (36–38), VP is a potent activator of the ERK/MAP kinase pathway. ERK phosphorylation is mediated by transactivation of the EGF receptor (12, 36). The present demonstration that EGF receptor and MEK inhibitors reduce the effects of VP on caspase-3 activation and Bad phosphorylation supports a role of this pathway on the neuroprotective actions of VP. The immediate downstream target of activated ERK within the apoptosis pathway is RSK, which is responsible for Bad phosphorylation (39). The present demonstration that transfection of the RSK dominant negative reduces the protective action of VP on caspase-3 activation provides further support for the involvement of this pathway in the anti-apoptotic actions of VP. On the other hand, the fact that MEK and EGFR inhibitors and the RSK dominant negative only partially blocked the inhibitory effect of VP on caspase-3 activity suggests that in addition to the MAPK/ERK/RSK pathway other signaling systems maybe involved in the anti-apoptotic actions of VP. In this regard, ongoing studies showing that the combination of a the MEK inhibitor and a PKC inhibitor prevents the stimulatory action of VP on Bad phosphorylation (J Chen and G Aguilera, unpublished).
It has been shown that PKC can mediate Bad phosphorylation and induce cell survival (40). For example, PKC activation promotes neuronal survival in sympathetic and sensory neurons and reduces serum-deprivation-induced death of cerebellar granule neurons (41, 42). Moreover, PKC inhibitors induce apoptosis in cortical and cerebellar granule neurons, as well as in neuronal cell lines (43). However, the effects of PKC are complex as previous studies suggest that different isoforms of PKC may have unique or even opposite effects on cell survival (44–46). In the present study, we showed that the effects of VP on both Bad phosphorylation and caspase-3 activity inhibition were partially blocked by the PKC inhibitors, BIM and Gö6983, suggesting a role for PKC signaling pathway on the protective effect of VP. Although the PKC inhibitor, calphostin C, totally prevented the protective effect of VP, it was a potent activator of caspase-3 activity on its own, rendering the data difficult to interpret. In this regard, it has been shown that calphostin C has apoptotic effects (47), which are independent on its ability to inhibit PKC (48). Since activation of PKCα by VP mediates transactivation of the ERK/MAP kinase pathway by VP in H32 cells (36), it is possible that the participation of PKC is mediated at least in part by ERK activation. However, the additive effect of the MEK and PKC inhibitors in preventing VP-induced Bad phosphorylation argue against this possibility and suggest that both pathways act independently. The exact involvement of PKC and potential differential effects of the diverse PKC isozymes on the protective effect of VP are currently under investigation.
An additional pathway to be considered is the serine/threonine kinase, PI3K/Akt pathway (49), which inactivates several pro-apoptotic molecules, including Bad, through phosphorylation of Ser136 (50). Studies based on the effect of PI3 kinase inhibitors suggest that VP can activate this pathway (27, 38). Experiments in our laboratory have shown that the PI3 kinase inhibitor, LY 294002 (10 µM), attenuated the effects of VP on caspase-3 activity (Chen and Aguilera, unpublished). However, attempts to elucidate the involvement of this pathway were inconclusive because of problems with high background using phospho-AKT and Ser136 Bad (which is specifically phosphorylated by PI3 kinase) antibodies for western blots in H32 cells. Further studies are clearly needed to determine whether the PI3K/Akt signaling pathway is involved in the protective effect of VP.
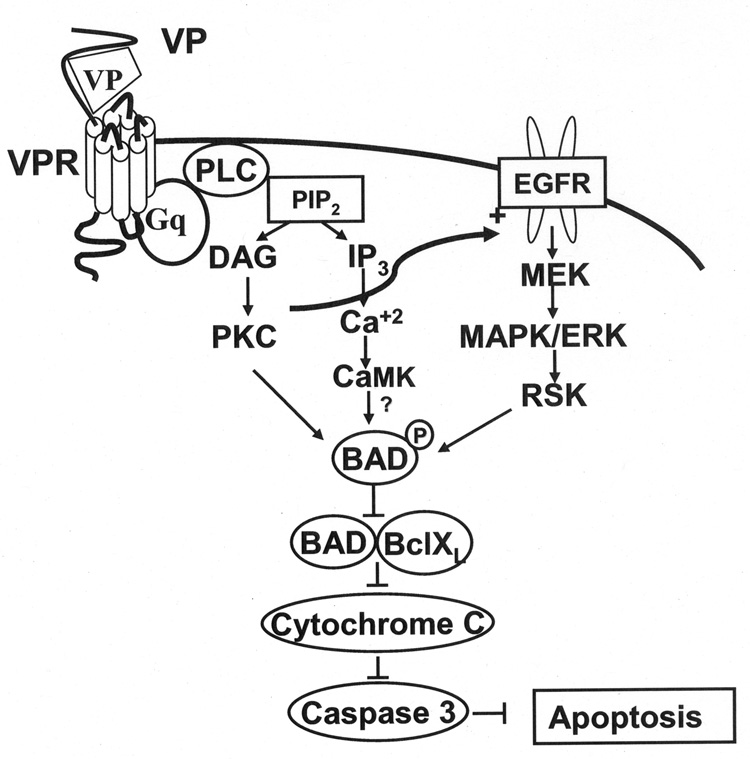
In summary, VP by acting upon V1 receptors protects neuronal cells from serum deprivation-induced apoptosis. Although the exact signaling pathways mediating the effect are unclear, the data clearly show that VP causes phosphorylation-inactivation of the pro-apoptotic protein Bad, preventing the release of mitochondrial cytochrome c and activation of the caspase cascade. This effect is partially mediated by activation of the ERK/MAP kinase/RSK pathway (Fig. 8). Since VP is released in the brain during stress conditions (51) and VP V1 receptors are present in neurons at important sites controlling behavior and learning (7, 8), it is likely that the peptide plays a role as a neuronal protective agent.
Fig. 8.
Diagram of the proposed mechanisms mediating the protective effect of VP on serum deprivation-induced apoptosis in H32 hypothalamic cells. Activation of phosphatidylinositol-specific PLC following binding of VP to V1 VP receptors, results in IP3 and DAG formation and consequent increases in intracellular Ca2+ and activation of CaMK and PKC. In addition, transactivation of EGFR subsequently activates MEK/ERK/RSK cascade, which in turn phosphorylates BAD causing its inactivation and preventing cytochrome c release, activation of the caspase cascade and apoptotic cell death.
Acknowledgement
This work was supported by the Intramural Research Program of National Institute of Child Health and Human Development, NIH. We would like to thank Dr. Baoying Liu (Laboratory of Immunology, NEI, NIH) for assistance in flow cytometry and FACS, and we thank Dr. Vincent Schram (Microscopy & Imaging Core, NICHD, NIH) for technical assistance in photograph capture under confocal microscope.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gilles G, Linton EA, Lowry PF. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 2.Rivier C, Rivier J, Mormade P, Vale WW. Studies of the nature of interaction between vasopressin and corticotropin releasing factor on adrenocorticotropin release in the rat. Endocrinology. 1984;115:882–886. doi: 10.1210/endo-115-3-882. [DOI] [PubMed] [Google Scholar]
- 3.Thibonnier M. Signal transduction of V1-vascular vasopressin receptors. Kidney Int. 1988;34:S52–S56. doi: 10.1016/0167-0115(92)90067-5. [DOI] [PubMed] [Google Scholar]
- 4.Peter J, Burbach H, Adan RA, Lolait SJ, van Leeuwen FW, Mezey E, Palkovits M, Barberis C. Molecular neurobiology and pharmacology of the vasopressin/oxytocin receptor family. Cell Mol Neurobiol. 1995;15:573–595. doi: 10.1007/BF02071318. [DOI] [PubMed] [Google Scholar]
- 5.Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Ostrowski NL, Lolait SJ, Bradley DJ, O'Carroll AM, Brownstein MJ, Young WS. Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992;131:533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 7.Alescio-Lautier B, Paban V, Soumireu-Moura B. Neuromodulation of memory in the hippocampus by vasopressin. Eur J Pharmacol. 2000;405:63–72. doi: 10.1016/s0014-2999(00)00542-2. [DOI] [PubMed] [Google Scholar]
- 8.Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- 9.Zachary I, Woll PJ, Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987;124:295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]
- 10.Brinton RD, Monreal AW, Fernandez JG. Vasopressin-induced neurotrophism in cultured hippocampal neurons via V1 receptor activation. J Neurobiol. 1994;25:380–394. doi: 10.1002/neu.480250404. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Volpi S, Aguilera G. Neuroprotective and antiapoptotic actions of vasopressin in the hypothalamic cell line H32. 89th Annunal Meeting of the Endocrine Society; Toronto. 2007. [Google Scholar]
- 12.Volpi S, Liu Y, Aguilera G. Vasopressin increases GAGA binding activity to the V1b receptor promoter through transactivation of the MAP kinase pathway. J Mol Endocrinol. 2006;36:581–590. doi: 10.1677/jme.1.01995. [DOI] [PubMed] [Google Scholar]
- 13.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 2000;268:14553–14556. [PubMed] [Google Scholar]
- 14.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 15.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 16.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 2000;268:14553–14556. [PubMed] [Google Scholar]
- 17.Lecoeur H, Ledru E, Prevost MC, Gougeon ML. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods. 1997;209:111–123. doi: 10.1016/s0022-1759(97)00138-5. [DOI] [PubMed] [Google Scholar]
- 18.Volpi S, Liu Y, Aguilera G. Vasopressin increases GAGA binding activity to the V1b receptor promoter through transactivation of the MAP kinase pathway. J Mol Endocrinol. 2006;36:581–590. doi: 10.1677/jme.1.01995. [DOI] [PubMed] [Google Scholar]
- 19.Birnbaumer M. Vasopressin receptors. Trends Endocrinol Metab. 2000;11:406–410. doi: 10.1016/s1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- 20.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 21.Landgraf R, Wotjak CT, Neumann ID, Engelmann M. Release of vasopressin within the brain contributes to neuroendocrine and behavioral regulation. Prog Brain Res. 1998;119:201–220. doi: 10.1016/s0079-6123(08)61571-x. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski NL, Lolait SJ, Young WS. Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Brinton RD. Suppression of proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha in astrocytes by a V1 vasopressin receptor agonist: a cAMP response element-binding protein-dependent mechanism. J Neurosci. 2004;24:2226–2235. doi: 10.1523/JNEUROSCI.4922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Doroshenko P, Cao XY, Irfan N, Coderre E, Kolaj M, Renaud LP. Vasopressin induces depolarization and state-dependent firing patterns in rat thalamic paraventricular nucleus neurons in vitro. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1226–R1232. doi: 10.1152/ajpregu.00770.2005. [DOI] [PubMed] [Google Scholar]
- 25.Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 27.Tyagi MG, Parthiban KV. Vasopressin mediates neuroprotection in mice by stimulation of V1 vasopressin receptors: influence of PI-3 kinase and gap junction inhibitors. Indian J Exp Biol. 2003;41:574–580. [PubMed] [Google Scholar]
- 28.Mugele K, Kugler H, Spiess J. Immortalization of a fetal rat brain cell line that expresses corticotropin-releasing factor mRNA. DNA Cell Biol. 1993;12:119–126. doi: 10.1089/dna.1993.12.119. [DOI] [PubMed] [Google Scholar]
- 29.Volpi S, Rabadan-Diehl C, Cawley N, Aguilera G. Transcriptional regulation of the pituitary vasopressin V1b receptor involves a GAGA-binding protein. J Biol Chem. 2002;277:27829–27838. doi: 10.1074/jbc.M201508200. [DOI] [PubMed] [Google Scholar]
- 30.Wange RL, Smrcka AV, Sternweis PC, Exton JH. Photoaffinity labeling of two rat liver plasma membrane proteins with [32P] gamma-azidoanilido GTP in response to vasopressin. J Biol Chem. 1991;266:11409–11412. [PubMed] [Google Scholar]
- 31.Jard S. Vasopressin receptors. A historical survey. Adv Exp Med Biol. 1998;449:1–13. [PubMed] [Google Scholar]
- 32.Goodsell DS. The molecular perspective: cytochrome C and apoptosis. Oncologist. 2004;9:226–227. doi: 10.1634/theoncologist.9-2-226. [DOI] [PubMed] [Google Scholar]
- 33.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 34.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 35.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Volpi S, Soh JW, Aguilera G. Activation of GAGA binding protein to the vasopressin V1b receptor promoter involves VP-induced MAP kinase phosphorylation though transactivation of the EGF receptor. 86th Annual Meeting of The Endocrine Society; New Orleans, Louisiana. 2004. [Google Scholar]
- 37.Ghosh PM, Bedolla R, Thomas CA, Kreisberg JI. Role of protein kinase C in arginine vasopressin-stimulated ERK and p70S6 kinase phosphorylation. J Cell Biochem. 2004;91:1109–1129. doi: 10.1002/jcb.10789. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh PM, Mikhailova M, Bedolla R, Kreisberg JI. Arginine vasopressin stimulates mesangial cell proliferation by activating the epidermal growth factor receptor. Am J Physiol Renal Physiol. 2001;280:F972–F979. doi: 10.1152/ajprenal.2001.280.6.F972. [DOI] [PubMed] [Google Scholar]
- 39.Fang X, Yu S, Eder A, Mao M, Bast RCJ, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 40.Tan Y, Ruan H, Demeter MR, Comb MJ. p90(RSK) blocks bad-mediated cell death via a protein kinase C-dependent pathway. J Biol Chem. 1999;274:34859–34867. doi: 10.1074/jbc.274.49.34859. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka S, Koike T. Activation of protein kinase C delays apoptosis of nerve growth factor-deprived rat sympathetic neurons through a Ca(2+)-influx dependent mechanism. Neurosci Lett. 2001 Nov 2;313(1–2):9–12. doi: 10.1016/s0304-3940(01)02193-0. 313:9–12. [DOI] [PubMed] [Google Scholar]
- 42.Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, Lindholm D. Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]
- 43.Heasley LE, Johnson GL. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989;264:8646–8652. [PubMed] [Google Scholar]
- 44.Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM. PKC-delta is an apoptotic lamin kinase. Oncogene. 2000;19:2331–2337. doi: 10.1038/sj.onc.1203555. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima T. Signaling cascades in radiation-induced apoptosis: roles of protein kinase C in the apoptosis regulation. Med Sci Monit. 2006;12:RA220–RA224. [PubMed] [Google Scholar]
- 46.Spitaler M, Wiesenhofer B, Biedermann V, Seppi T, Zimmermann J, Grunicke H, Hofmann J. The involvement of protein kinase C isoenzymes alpha, epsilon and zeta in the sensitivity to antitumor treatment and apoptosis induction. Anticancer Res. 1999;19:3969–3976. [PubMed] [Google Scholar]
- 47.Ozaki I, Tani E, Ikemoto H, Kitagawa H, Fujikawa H. Activation of stress-activated protein kinase/c-Jun NH2-terminal kinase and p38 kinase in calphostin C-induced apoptosis requires caspase-3-like proteases but is dispensable for cell death. J Biol Chem. 1999;274:5310–5317. doi: 10.1074/jbc.274.9.5310. [DOI] [PubMed] [Google Scholar]
- 48.Spinedi A, Oliverio S, Di Sano F, Piacentini M. Calpain involvement in calphostin C-induced apoptosis. Biochem Pharmacol. 1998;56:1489–1492. doi: 10.1016/s0006-2952(98)00169-5. [DOI] [PubMed] [Google Scholar]
- 49.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 50.del Peso L, González-García M, Page C, Herrera R, Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 51.Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci. 1996;16:7725–7732. doi: 10.1523/JNEUROSCI.16-23-07725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]