Abstract
The current incidence of HIV-1/AIDS affects around 7,000 pregnant women in the United States. When given during pregnancy, the nucleoside analog AZT significantly reduces maternal-fetal transmission. It has been previously shown that AZT is incorporated into DNA, where it causes mutations in the HPRT and TK genes. It also changes cell cycle gene expression, and induces S-phase arrest, micronuclei, chromosomal aberrations, sister chromatid exchanges, telomeric attrition, and other genotoxic effects in cultured cells. A predicted consequence of these events is genomic instability that together, with clastogenicity may contribute to the carcinogenic potency of AZT. Various aspects of genotoxicity are explored in this contribution seeking to understand the multiple effects of this antiretroviral agent in animal models and humans. This mini-review describes some of the experimental models used to elucidate the genotoxicity induced by antiretroviral therapy during human pregnancy. The use of diverse methods to detect biomarkers of exposure, such as an AZT-specific radioimmunoassay, micronuclei bearing intact chromosomes, and telomeric DNA attrition highlight the role of in-vitro models to elucidate exposure and risk. The relevance of the in vitro models is followed by the introduction of the role of the nucleoside analogs in transplacental carcinogenesis along with the description of a transplacental perfusion model and a transplacental carcinogenesis rodent model. In a more direct clinical application the use of AZT-DNA incorporation as a biomarker of exposure, in experiments conducted in vivo in Erythrocebus patas monkeys and in humans, addresses the possibility of elucidation of potential cancer risk in those infants exposed in utero.
Two relevant aspects of this contribution are the potential application of some of the models described in this mini-review, as diagnostic tools in antiretroviral-exposed populations, and the use of these models to understand the nature of the genotoxicities and minimize the undesirable side effects of the antiretroviral therapy.
Introduction
Patients infected with the human immunodeficiency virus (HIV-1) undergo extensive therapy with antiretroviral nucleoside analog drugs, among which zidovudine, (Retrovir®, 3’azido-3’-deoxythymidine, AZT) is used the most frequently and has become an essential component of the Highly Active Antiretroviral Therapy (HAART). The current incidence of HIV-1/AIDS affects around 7,000 pregnant women/year in the United States. When given during pregnancy, the nucleoside analog AZT significantly reduces maternal-fetal transmission. However, AZT is carcinogenic in rodents, producing 10%–22% incidences of vaginal squamous papillomas and carcinomas in adult mice [1, 2]. Moreover, transplacental administration of AZT to mice results in significant increases of tumors, in liver, lung and reproductive organs of the offspring, one year after exposure [3]. It has been previously shown that AZT is incorporated into DNA, where it causes mutations in the HPRT and TK genes. It also changes cell cycle gene expression, and induces S-phase arrest, micronuclei, chromosomal aberrations, sister chromatid exchanges, telomeric attrition, and other genotoxic effects in cultured cells [4]. A predicted consequence of these events is genomic instability that, together with clastogenicity, may contribute to the carcinogenic potency of AZT. Various aspects of genotoxicity are explored in this contribution seeking to understand the multiple effects of the drug in animal models and humans.
AZT is selectively incorporated at the telomeric ends and causes telomeric shortening
Zidovudine, (Retrovir®, 3′-azido-2′,3′-dideoxythymidine; AZT), is a nucleoside reverse transcriptase inhibitor (NRTI) designed to inhibit viral replication in HIVinfected patients.
Inhibitors of the HIV-1 reverse transcriptase can also become incorporated into nuclear and mitochondrial DNA [3–6] and act as chain terminators [7] by lacking the 3′-OH of the deoxyribose sugar (Figure 1 A), then becoming unable to extend the nascent DNA chain by forming a 5′ to 3′-phosphodiester bond with the following nucleotide (Figure 1 B–C). Nucleoside reverse transcriptase inhibitors are implicated as chemical mutagens and transplacental carcinogens [3, 4, 8–10] and some consequences of DNA incorporation have been reported and categorized as gene mutations, chromosomal mutations, and telomere shortening [4].
Figure 1.
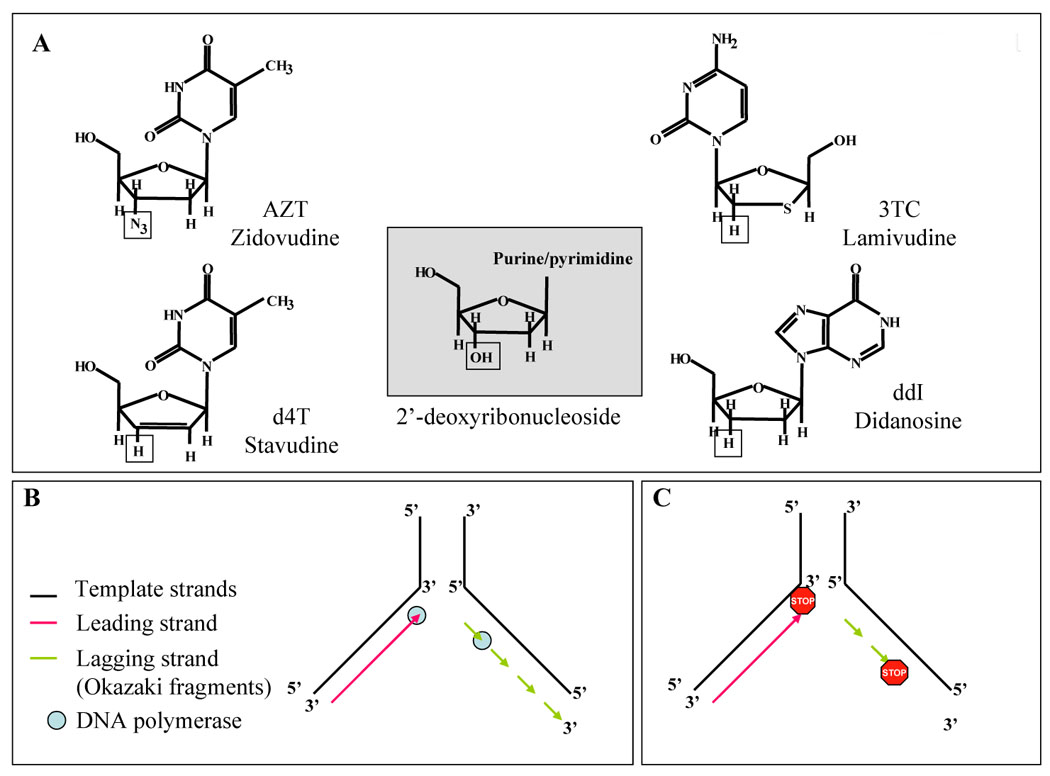
A. Structures of the commonly used nucleoside analogs. The hydroxyl group at the 3’ position in the 2’-deoxyribonucleoside (center box, square) is absent in the nucleoside analogs. AZT has an azido group; 3TC and ddI have hydrogen; and D4T has an unsaturated 2’,3’-dideoxyribose. B. DNA replication on a normal template. C. Mechanism of inhibition of DNA replication by insertion of a nucleoside analog that lack the 3’ hydroxyl, cannot form the phosphodiester link, and therefore causes chain termination. “STOP” indicates incorporated nucleoside analog.
In cultured cells, AZT becomes preferentially incorporated into the DNA of chromosomal ends or telomeres, causing irreversible telomeric shortening and the potential for premature senescence [3, 11–13]. In experiments utilizing CHO cells exposed to 800 µM AZT for 24 hours were performed to obtain metaphase spreads that in turn were incubated with anti-AZT antibodies (Sigma, St Louis MO). A preferential localization of AZT within the telomeric region of the chromosomes was observed [14].
Furthermore, telomeric DNA attrition determined by Southern blot experiments using a telomeric probe was described in monkeys and mice exposed transplacentally to the drug [3], and in HeLa cells exposed long term to the drug [13] (Figure 2). E.patas monkeys were administered 40.0 mg/day AZT in food for the last half of the gestational period, and a 24 mg/day of a second nucleoside, 3TC, during the last 10 weeks of gestation. Fetuses were taken at term by C-section and analysis of the telomeric length assessed by southern blot and compared with untreated control monkeys was performed in multiple tissues after DNA extraction. Telomeric shortening was observed in brain cortex and cerebellum (Figure 2 A and B) and in heart and lung (data not shown) [15].
Figure 2.
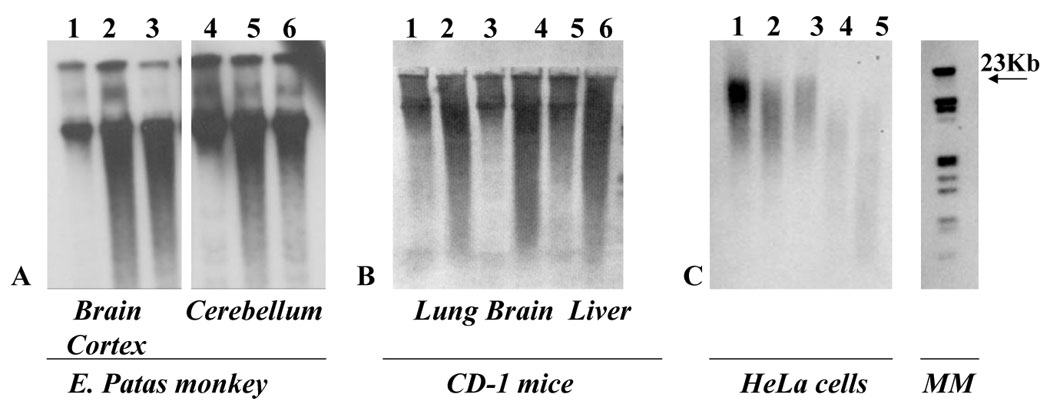
Southern blot images of A. Erythrocebus patas monkeys brain cortex and cerebellum DNA blotted with a telomeric probe. The 1st and 4th lanes correspond to an untreated monkey while the 2nd, 3rd, 5th and 6th lanes correspond to two different newborn monkeys, exposed to AZT AND 3TC in utero. Lanes 1 to 3: Brain Cortex, lanes 4 to 6 Cerebellum B. lanes 1, 3 and 5 lung, brain and liver DNA of control mice; lanes 2, 4, and 6 lung, brain and liver DNA of mice exposed to AZT in utero. C. Control DNA of He la cells (lanes 1 to 3), long term AZT exposed DNA (lanes 4 and 5). DNA size estimated using the molecular marker (MM) on the right. Shortened telomeres are evidenced by the larger smear indicating smaller fragments of DNA. Modified from [30] and [13]
CD1 mice were administered AZT during the last week of a three week gestation. At the end of that period, the pups were sacrificed, the organs of the same litter pooled, DNA extracted and Southern blots performed with a specific biotinylated telomeric probe. Shortening of the telomeres was observed in lung; brain and liver of AZT exposed mice compared with untreated controls (Figure 2 B). Additionally a progressive shortening of telomeric DNA, was demonstrated in HeLa cells exposed for 15 passages to AZT 800 µM (Figure 2 C). The shortening was not reversed after continuous growing for 25 passages in AZT free media [13].
Uncapped telomeres will confer a lack of stability to the chromosomes and allow for incorrect exchanges of chromosomal material among them in interphase. Once the mitosis occurs and the daughter cells separate during telophase, bridges of DNA could be seen as a consequence of the adhesiveness of the disrupted telomeres (Figure 3A).
Figure 3.
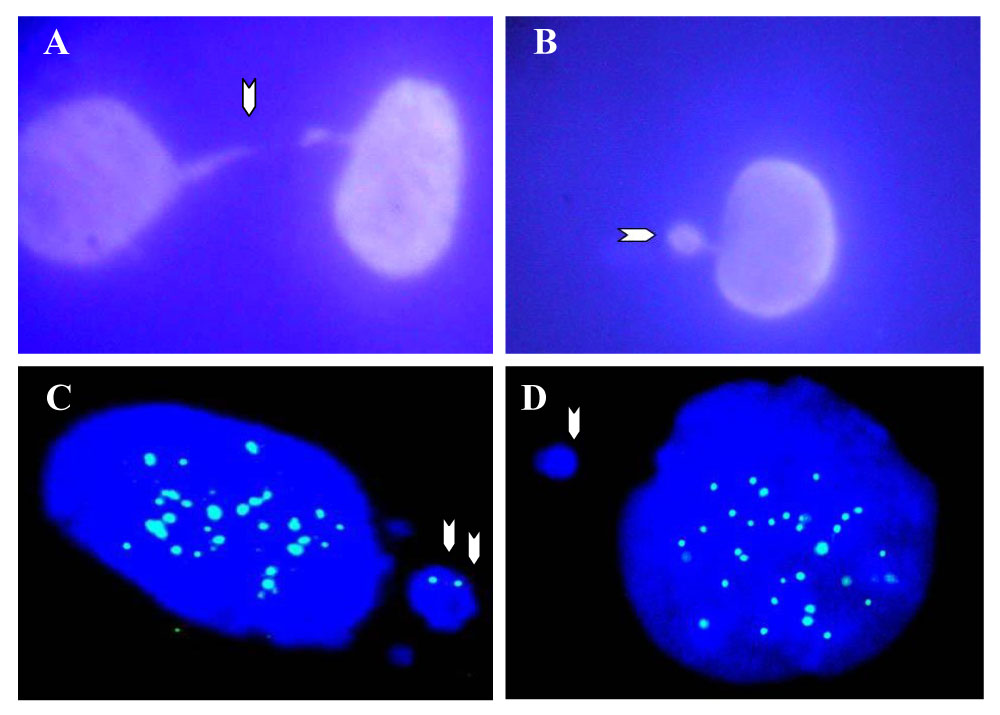
DAPI staining of A. Chromatin bridge (arrow) in AZT-exposed He La cell telophase, B. micronucleus (arrow) in an AZT treated He La cell. C.CREST-positive micronucleus (blue spots in both nucleus and micronucleus, arrows) and D. CREST-negative micronucleus with positive CREST signals in the nucleus of CHO cells exposed to AZT. Adapted from [31], unpublished data.
Chromatin bridges and micronuclei are a consequence of telomeric attrition
Typically, the material involved in the formation of chromatin bridges will remain lost in the cytoplasm of any or both of the daughter cells as a small mass known as a micronucleus (Figure 3B). Micronuclei differ in size and most of the time a large micronucleus bears intact chromosomes inside as demonstrated by the use of kinetochore specific antibodies able to detect centromeric regions of the chromosome.
Kinetochore-specific antibodies isolated from patients with the CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome, [16] were used to incubate normal human mammary epithelial cells (NHMEC) after exposure to AZT. The mentioned cells, obtained from mammoplasty tissue were exposed to 200 µM AZT for 24 hr. After harvesting and fixation, cells were incubated with the CREST antibody and stained with Alexa 488 and DAPI. Scoring of micronuclei bearing chromosomes, identified by a CREST positive signal micronuclei, as well as those negative for the CREST antibody (Figure 3 C–D) was achieved in 500 micronucleated cells. An increment in micronuclei bearing intact chromosomes was seen in human mammary, AZT-treated cells.
AZT is a transplacental carcinogen in mice
The genomic instability described plus reports of clastogenicity and mutagenicity induced by the agent, generated interest to address the human health impact of the use of the drug in human pregnancy. A mouse transplacental model was used to address the potential of the drug to cause cancer in offspring exposed for 1 week in utero and allowed to grow to adulthood without further exposure. Enhanced tumorigenicity was observed in multiple organs of mice exposed transplacentally to AZT [3, 17, 18]. This enhanced tumorigenicity was evidenced not only by increased tumor incidences, but also by shortened tumor latency, as AZT-induced neoplasms were found in CD-1 mouse offspring at one year of age [3] in the reproductive female system, lungs for male and female and lungs of the male (Figure 4 A). Diwan et al., [18] reported results from a two-year study in which they indicate finding reproductive organ tumors in mice transplacentally-exposed to AZT. Typically the strain of mice used in the study, CD-1 Swiss mice, has a very low if not absent tumor incidence in the reproductive system. Exposure to AZT, on the other hand, increased the incidence of mammary adenocarcinomas, ovarian, seminal vesicle and testicular tumors.
Figure 4.
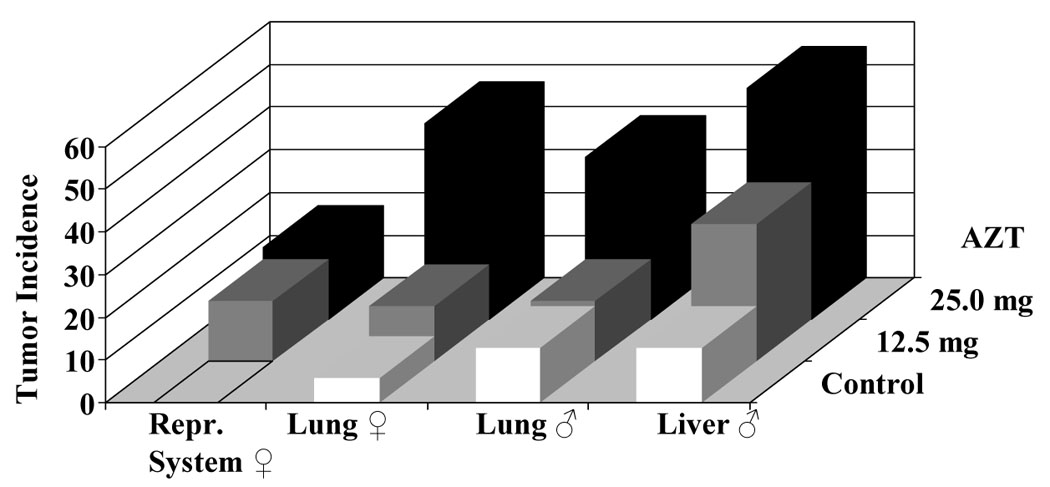
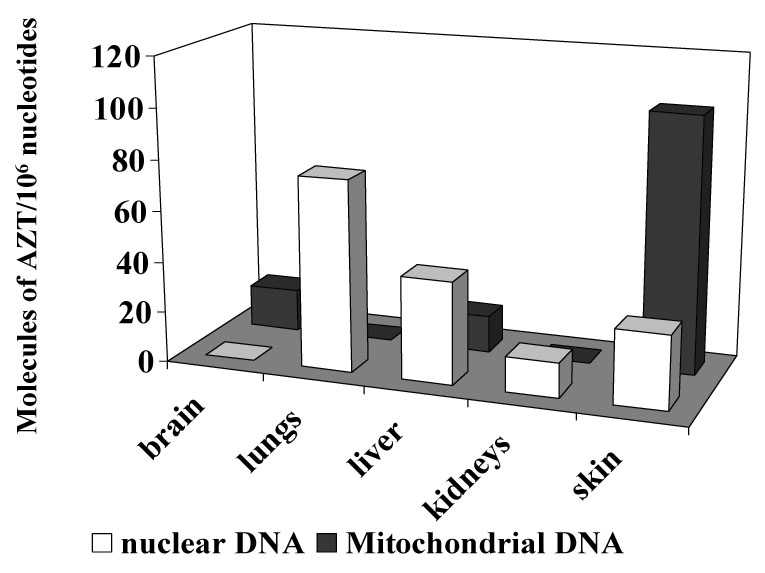
A. Tumor incidence induced by 12.5 mg/day (gray bars) and 25.0 mg/day (black bars) AZT in the reproductive system, lungs and liver of CD1 mice exposed in utero to AZT for one week. Adapted and modified from [3]. B. Molecules of AZT/106 nucleotides, in nuclear (white bars) and mitochondrial (dark bars) DNA from brain, lungs, liver kidneys and skin of mice exposed to 25.0 mg/day AZT during the last week of gestation, and sacrificed at birth.
Investigators performing transplacental bioassays also sought to establish molecular biomarkers of carcinogenesis by analysis of the incorporation of AZT into the DNA of multiple tissues of the offspring (Figure 4 B). Incorporation of AZT into nuclear and mitochondrial DNA of multiple organs pooled from each litter was determined by an AZT-specific radioimmunoassay (RIA) and values of incorporation reported in that study ranged from undetectable to up to 100 molecules of AZT/106 nucleotides [3]. The transplacental carcinogenicity of AZT was subsequently confirmed in rats and another strain of mice [19]
AZT incorporates into DNA of human placentas and infant blood DNA
In an attempt to determine if the genotoxicity of AZT and other nucleoside analogs would compromise human health, experiments using placental perfusion were achieved. The model encompassed the perfusion of fresh human placentas ex vivo with three AZT doses on the maternal side of the organ, with perfusates recovered on the fetal side at 15 min intervals for a period of 2 hours. At that time, the maternal and fetal concentrations reached equivalent levels [20] and incorporation of AZT into DNA of the placenta was measured by AZT-RIA. A dose response was observed in the incorporation of AZT into placental DNA from tissues obtained 120 min after the initial perfusion [20] (Figure 5).
Figure 5.
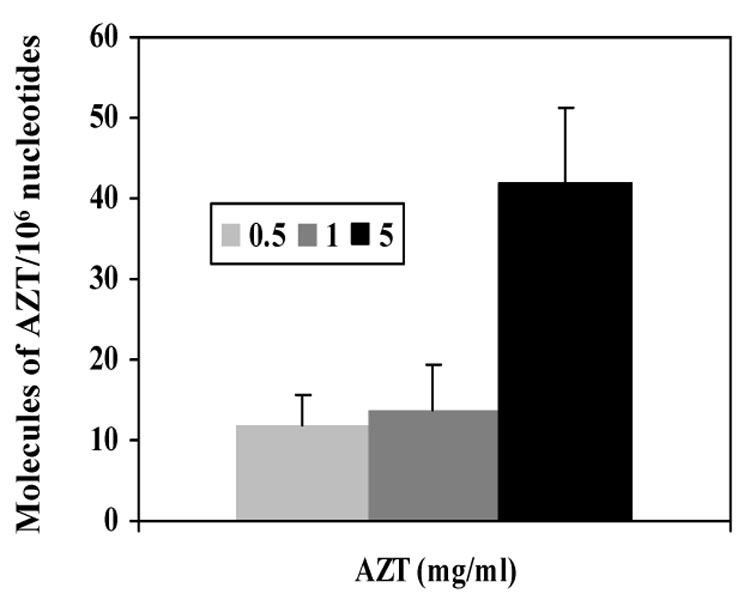
AZT-DNA incorporation in human placental tissues of ex vivo AZT perfused placenta, 120 min after perfusion. Light bar represents 0.5 mg/ml AZT, dark gray corresponds to the 1 mg/ml AZT dose and black bar represents the 5 mg AZT dose. Adapted and modified from [20]
The observations in monkeys prompted a parallel study in humans to investigate the human health impact of the use of nucleoside analogs during pregnancy. For this purpose, mother-infant pairs exposed to different regimens of highly active antiretroviral therapy (HAART) were studied. Peripheral blood leukocytes from the mother and umbilical cord blood leukocytes from the fetus were isolated and DNA extracted. AZT-RIA was performed on DNA extracted from the samples obtained at birth and the results are presented in Figure 6. Each point represents the average of three separate RIAs and the amount of AZT –DNA is expressed as molecules of AZT/106 nucleotides. There is a lack of correlation between the time of exposure, indicated in months on the abscissa, and the amount of AZT incorporated into the DNA among these samples. Additionally, no correlation between AZT-DNA incorporation in the mother and the infant was observed. Conversely, the data suggested an inverse correlation between the amount of AZT into DNA in the mother versus the infant. One more observation seen from these data is that the high incorporation of two infants with 0.3 months of exposure at birth is not matched by any longer period of exposure. On the light of new biochemical data collected in our laboratory, it is possible to speculate that infants exposed for long time to the analog developed a resistance mechanism that is not observed in those infants exposed for a short time. In vitro studies in lymphoblastoid cells indicated that the expression of the enzyme TK1, responsible for phosphorylation of AZT, is decreased at earlier passages of chronic treatments to be completely abolished later on [21]. Radioisotopic labeling of the substrate of the enzyme revealed that TK1 enzymatic activity is decreased as well (unpublished data). Patients exposed for long term also present a decrease on the TK 1 enzyme activity [22, 23]. Additionally, lack of expression of the active form of the enzyme has been seen in human mammary epithelial cells that do not incorporate AZT into the DNA (Olivero et al., TAAP, in press).
Figure 6.
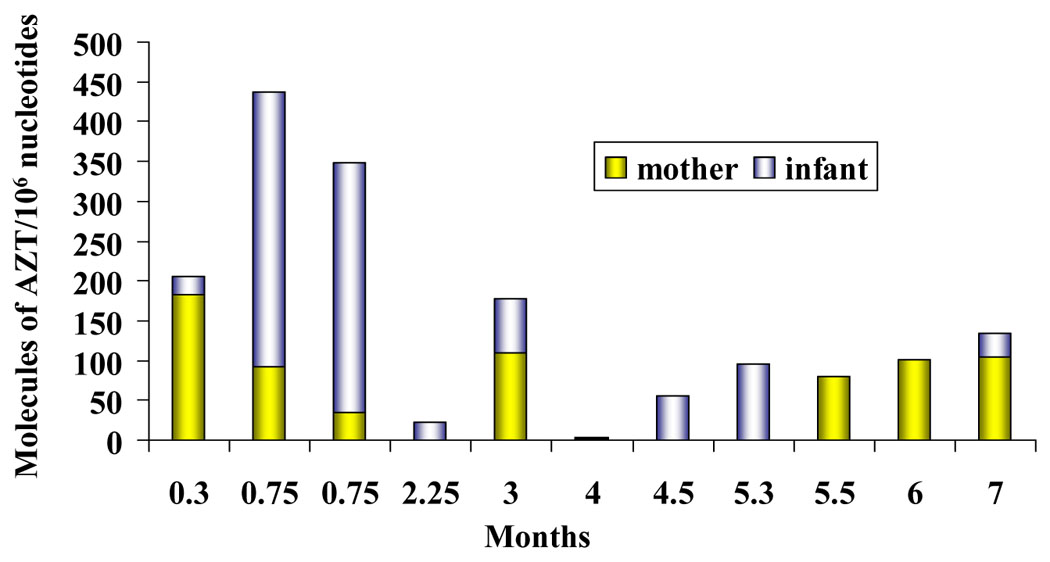
AZT-DNA incorporation in mother- (yellow columns) infant (gray columns) pairs. Each bar represents the amount of AZT into DNA of peripheral blood (maternal DNA) or cord blood (infant DNA) expressed in molecules of AZT/106 nucleotides. The length of exposure from 0.3 to 7 months is represented in the abscissa. Adapted and modified from [5]
Combined Therapy
While perinatal AZT monotherapy decreased HIV transmission rates from 25% to 8% [24, 25], administration of combination therapy using multiple agents, known as HAART, further reduced the rate of vertical transmission to ≤2% [26]. However the increased genotoxicity associated with the combination regimens remains unclear.
The highly active antiretroviral therapy (HAART) typically includes two nucleoside reverse transcriptase inhibitors combined with either a non-nucleoside reverse transcriptase inhibitor or a protease inhibitor. HAART is recommended for HIV-1 positive mothers, to decrease vertical transmission [27]. Although there is no clear knowledge of the direct or indirect consequences of exposure to this complex regimen in the human fetus, studies in cell culture and animal models suggest increased genotoxicity is induced by the combination. In vitro studies revealed a synergistic effect on the frequency of HPRT and TK mutants induced by the AZT-ddI combination as compared with single drug exposures [28]. Similarly, an additive effect was observed in the incorporation of AZT into DNA of E. patas monkeys exposed in utero to AZT and 3TC during the last period of their gestation [15]. The authors indicated that the DNA damage sustained by the fetuses exposed to both drugs is double than the ones exposed to a single compound. In that study, where monkeys were administered AZT and 3TC, incorporation of AZT into DNA of multiple tissues was achieved by a RIA, additionally, incorporation of 3TC into DNA was determined by a specific RIA. Incorporation of both nucleoside analogs was observed in the tissues, indicating a greater genotoxic potential.
With the benefits of HAART, many newborns are exposed in utero to multiple drugs and the use of monotherapy is rare in industrialized nations. An observation of the incorporation of AZT and 3TC into DNA of infants exposed in utero suggested that those infants exposed to combination therapy sustain more damage into their DNA as well [10]. Similarly to the studies in vitro, an enhancement in the frequency of mutations in the HPRT and GPA genes was observed in these cohorts. A preliminary study of multiplicative effects of combination therapy demonstrated an increased in the mutagenic frequency of the HPRT gene only in those children exposed to combination therapy when compared to AZT alone.
A third component of the HAART, protease inhibitors, have been studied as potential anticancer drugs. According to a recent report, Akt inhibition by nelfinavir, among other protease inhibitors, was the mechanism responsible of growth inhibition of non-small cell lung carcinoma xenografts and decreased viability of a panel of drug-resistant breast cancer cell lines by induction of endoplasmic reticulum stress, autophagy, and apoptosis [29].
Summary
In this mini-review a brief description of the models followed to assess genotoxicity of nucleoside analogs used in the therapy of AIDS was discussed.
The in vitro models have shown to be very useful tools to indicate the direction of the development of human biomarkers of exposure and, potentially, biomarkers of risk. Studies in rodents have been very revealing indicating the potential carcinogenic capacity of the agents under study. Furthermore studies in monkeys, with very similar dosage protocols than the human therapeutic regimens, gave indication of a genotoxic synergism when combination therapy was applied. Finally the possibility of elucidation of potential cancer risk in those infants exposed in utero is illustrated by the use of AZT-DNA incorporation as a biomarker of exposure.
Conclusion
The understanding of the genotoxicity of the drugs used to treat HIV-1 disease is paramount in the future of the AIDS therapy. The balance between the risks and the benefits provided by the drugs should be always taken into account. However, the understanding of the mechanisms by which drugs exert their detrimental effect should be studied in depth and complete knowledge of their pathways at the cellular and sub-cellular level should be characterized in order to improve therapy and drug design.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ayers KM, Clive D, Tucker WE, Hajian G, De Miranda P. Nonclinical toxicology studies with zidovudine: genetic toxicity tests and carcinogenicity bioassays in mice and rats. Fundam. Appl. Toxicol. 1996;32:148–158. doi: 10.1006/faat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 2.NTP. Toxicology and Carcinogenesis Studies of AZT, National Toxicology Program. NC: Research Triangle Park; 1996. [Google Scholar]
- 3.Olivero OA, Anderson LM, Diwan BA, Haines DC, Harbaugh SW, Moskal TJ, Jones AB, Rice JM, Riggs CW, Logsdon D, Yuspa SH, Poirier MC. Transplacental effects of 3'-azido-2',3'-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J. Natl. Cancer Inst. 1997;89:1602–1608. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 4.IARC; World Health Organization. Lyon, France: International Agency for Research on Cancer; Monographs on the Evaluation of Carcinogenic Risks to Humans. Some antiviral and antineoplastic drugs, and other pharmaceutical agents. 2000;Vol 76:73–127.
- 5.Olivero OA, Shearer GM, Chougnet CA, Kovacs AA, Landay AL, Baker R, Stek AM, Khoury MM, Proia LA, Kessler HA, Sha BE, Tarone RE, Poirier MC. Incorporation of zidovudine into leukocyte DNA from HIV-1-positive adults and pregnant women, and cord blood from infants exposed in utero. AIDS. 1999;13:919–925. doi: 10.1097/00002030-199905280-00007. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal RP, Olivero OA. Genotoxicity and mitochondrial damage in human lymphocytic cells chronically exposed to 3'-azido-2',3'-dideoxythymidine (AZT) Mutat. Res. 1997;390:223–231. doi: 10.1016/s1383-5718(97)00014-4. [DOI] [PubMed] [Google Scholar]
- 7.Furman PA, Fyfe JA, St Clair MH, Weinhold K, Rideout JL, Freeman GA, Lehrman SN, Bolognesi DP, Broder S, Mitsuya H, Barry DA. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, Ciraru-Vigneron N, Lacroix C, Rouzioux C, Mandelbrot L, Desguerre I, Rotig A, Mayaux M-J, Delfraissy J-F. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 9.Wutzler P, Thust R. Genetic risks of antiviral nucleoside analogues--a survey. Antiviral Res. 2001;49:55–74. doi: 10.1016/s0166-3542(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 10.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol. Appl. Pharmacol. 2004;199:151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Strahl C, Blackburn EH. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol. Cell Biol. 1996;16:53–65. doi: 10.1128/mcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez DE, Kassim A, Olivero OA. Preferential incorporation of 3'-Azido-2',3'-dideoxythymidine (AZT) in telomeric sequences of CHO cells. Int. J. Oncol. 1995;7:1057–1060. doi: 10.3892/ijo.7.5.1057. [DOI] [PubMed] [Google Scholar]
- 13.Gomez DE, Tejera AM, Olivero OA. Irreversible telomere shortening by 3'-azido-2', 3'-dideoxythymidine (AZT) treatment. Biochem. Biophys. Res. Commun. 1998;246:107–110. doi: 10.1006/bbrc.1998.8555. [DOI] [PubMed] [Google Scholar]
- 14.Olivero OA, Poirier MC. Preferential incorporation of 3'-azido-2',3'-dideoxythymidine (AZT) into telomeric DNA and Z-DNA-containing regions of chinese hamster ovary cells. Mol. Carcinog. 1993;8:81–88. doi: 10.1002/mc.2940080204. [DOI] [PubMed] [Google Scholar]
- 15.Olivero OA, Fernandez JJ, Antiochos BB, Wagner JL, St CM, Poirier MC. Transplacental genotoxicity of combined antiretroviral nucleoside analogue therapy in Erythrocebus patas monkeys. J. Acquir. Immune. Defic. Syndr. 2002;29:323–329. doi: 10.1097/00126334-200204010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Emdad L, Sarkar D, Su ZZ, Boukerche H, Bar-Eli M, Fisher PB. Progression elevated gene-3 (PEG-3) induces pleiotropic effects on tumor progression: modulation of genomic stability and invasion. J. Cell Physiol. 2005;202:135–146. doi: 10.1002/jcp.20097. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Diwan BA, Anderson LM, Logsdon D, Olivero OA, Haines DC, Rice JM, Yuspa SH, Poirier MC. Skin tumorigenesis and Ki-ras and Haras mutations in tumors from adult mice exposed in utero to 3'-azido-2',3'-dideoxythymidine. Mol Carcinog. 1998;23:45–51. [PubMed] [Google Scholar]
- 18.Diwan BA, Riggs CW, Logsdon D, Haines DC, Olivero OA, Rice JM, Yuspa SH, Poirier MC, Anderson LM. Multiorgan transplacental and neonatal carcinogenicity of 3'-azido-3'-deoxythymidine in mice. Toxicol. Appl. Pharmacol. 1999;15:82–99. doi: 10.1006/taap.1999.8782. [DOI] [PubMed] [Google Scholar]
- 19.Walker DM, Malarkey DE, Seilkop SK, Ruecker FA, Funk KA, Wolfe MJ, Treanor CP, Foley JF, Hahn FF, Hardisty JF, Walker VE. Transplacental carcinogenicity of 3'-azido-3'-deoxythymidine in B6C3F1 mice and F344 rats. Environ. Mol. Mutagen. 2007;48:283–298. doi: 10.1002/em.20297. [DOI] [PubMed] [Google Scholar]
- 20.Olivero OA, Parikka R, Poirier MC, Vahakangas K. 3'-azido-3'-deoxythymidine (AZT) transplacental perfusion kinetics and DNA incorporation in normal human placentas perfused with AZT. Mutat. Res-Fund. Mol. M. 1999;428:41–47. doi: 10.1016/s1383-5742(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez IL, Olivero OA, Poirier MC. Altered AZT metabolism may induce cellular drug resistance in human cells. Environmental and Molecular Mutagenesis. 2004;44:234. Abstract # 186. [Google Scholar]
- 22.Antonelli G, Turriziani O, Verri A, Narciso P, Ferri F, D'Offizi G, Dianzani F. Long-term exposure to zidovudine affects in vitro and in vivo the efficiency of phosphorylation of thymidine kinase. AIDS Res. Hum. Retroviruses. 1996;12:223–228. doi: 10.1089/aid.1996.12.223. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsson B, Britton S, He Q, Karlsson A, Eriksson S. Decreased thymidine kinase levels in peripheral blood cells from HIV-seropositive individuals: implications for zidovudine metabolism. AIDS Res. Hum. Retroviruses. 1995;11:805–811. doi: 10.1089/aid.1995.11.805. [DOI] [PubMed] [Google Scholar]
- 24.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL, Jimenez E, O'Neill E, Bazin B, Delfraissy J-F, Culnane M, Coombs R, Elkins M, Moye J, Stratton P, Balsley J. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 25.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, O'Sullivan MJ, Van Dyke RB, Jimenez E, Rouzioux C, Flynn PM, Sullivan JL. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group [see comments] N. Engl. J. Med. 1996;335:1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 26.Capparelli E, Rakhmanina N, Mirochnick M. Pharmacotherapy of perinatal HIV. Semin. Fetal Neonatal Med. 2005;10:161–175. doi: 10.1016/j.siny.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Mofenson LM. Successes and challenges in the perinatal HIV-1 epidemic in the United States as illustrated by the HIV-1 Serosurvey of childbearing women. Arch. Pediatr. Adolesc. Med. 2004;158:422–425. doi: 10.1001/archpedi.158.5.422. [DOI] [PubMed] [Google Scholar]
- 28.Meng Q, Walker DM, Olivero OA, Shi X, Antiochos BB, Poirier MC, Walker VE. Zidovudine-didanosine coexposure potentiates DNA incorporation of zidovudine and mutagenesis in human cells. Proc Natl Acad Sci U. S A. 2000;97:12667–12671. doi: 10.1073/pnas.220203197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, bu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, Hollander MC, Kawabata S, Tsokos M, Figg WD, Steeg PS, Dennis PA. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin. Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 30.Olivero OA, Fernandez JJ, Antiochos BB, Wagner JL, St Claire ME, Poirier MC. Transplacental genotoxicity of combined antiretroviral nucleoside analogue therapy in Erythrocebus patas monkeys. J. Acquir. Immune. Defic. Syndr. 2002;29:323–329. doi: 10.1097/00126334-200204010-00001. [DOI] [PubMed] [Google Scholar]
- 31.Olivero OA, Borojerdi JP, Semino-Mora C, Ward Y, Poirier MC. Genomic instability induced by AZT in cultured normal human mammary epithelial cells (NHMECs) generates aneuploidy. Retrovirology. 2006;3 Suppl 1:48. [Google Scholar]


