Abstract
You have decided to develop a protocol for insulin therapy in your intensive care unit (ICU). You wonder about the merit of using intensive insulin therapy (IIT) to maintain tight blood glucose control in your patients.
Pro: Intensive insulin therapy targeting tight blood glucose control is of benefit in critically ill patients
Hyperglycaemia is a common accompaniment of acute illness. In published trials insulin treatment was required in more than 98% of ICU patients in whom the goal was to maintain normoglycaemia [1-3]. The hyperglycaemia is thought to result from a number of processes; elevated levels of cortisol, epinephrine, norepinephrine and glucagon increase gluconeogenesis [4-8] and glycogenolysis [9] whilst insulin resistance leads to a decrease in insulin-stimulated uptake of glucose in heart and adipose tissue. In addition, exercise induced uptake of glucose in skeletal muscle is absent in immobilized critically ill patients [10,11]. Hyperglycaemia may cause harm by direct toxicity and through increased intracellular oxidative stress due to higher mitochondrial peroxide production [12,13]. The clinical consequence of hyperglycaemia appears to be an increase in morbidity and mortality in a variety of clinical settings, including heterogeneous populations of critically ill patients [14]. In trauma patients hyperglycaemia is associated with higher mortality and an increased rate of infectious complications [15] as well as with worse neurological outcome in the subset of patients with traumatic brain injury [16]. In patients with sepsis and haematological malignancy, hyperglycaemia at hospital admission predicts higher mortality [17,18]. Hyperglycaemia has also been associated with higher mortality and poor functional recovery in non-diabetic stroke patients [19], and with increased risk of in-hospital mortality, congestive heart failure and cardiogenic shock after myocardial infarction [20]. In summary, hyperglycaemia is common in critically ill patients and its occurrence is clearly associated with a worse outcome; thus, it is natural to ask whether hyperglycaemia is simply a marker of illness severity, or is hyperglycaemia itself harmful, in which case does normalizing blood glucose improve patients' outcomes?
The evidence that short-term treatment of hyperglycaemia is beneficial in acute illness is consistent across a number of populations of patients. Insulin treatment targeting a lower blood glucose concentration significantly reduces long term mortality following myocardial infarction [21], and lowers the risk of cardiovascular disease and cardiovascular events in patients with type I diabetes [22]. A meta-analysis evaluating 35 randomized controlled trials (RCTs) of insulin therapy in critically ill hospitalized patients found a beneficial effect of insulin therapy on mortality; the benefit was limited to trials in which insulin was administered with the goal of achieving a particular blood glucose target [23].
Although the majority of studies included in the meta-analysis were conducted in patients with coronary artery disease, the single largest study was the surgical ICU (SICU) study by Van den Berghe and colleagues [1]. In this study, the investigators randomly assigned mechanically ventilated SICU patients to either intensive insulin therapy (IIT; blood glucose target 4.4 to 6.1 mmol/l) or conventional treatment (blood glucose target 10.0 to 11.1 mmol/l). The study was stopped after inclusion of 1,548 patients when a planned interim analysis indicated a significant reduction in mortality in patients assigned to IIT. Patients assigned to IIT had lower ICU mortality (4.6% versus 8.0%, adjusted p < 0.04) and inhospital mortality (7.2% versus 10.9%, p = 0.01). The beneficial effect of IIT occurred in patients who remained in the ICU for more than five days (ICU mortality 10.6% versus 20.2%, p = 0.01); the number of deaths in the first five days of intensive care was similar in both groups. Van den Berghe and colleagues repeated their study in medical ICU (MICU) patients expected to be in the ICU for three days or more [2]. They enrolled 1,200 patients, of whom 767 were treated in the ICU for three days or longer. Overall, in-hospital mortality was lower in patients assigned to IIT (37.3% versus 40.0%), but the difference was not statistically significant (p = 0.33). In patients who were treated in the ICU for 3 days or more, inhospital mortality was reduced in those assigned to IIT (43.0% versus 52.5%, p = 0.009). In addition to its impact on survival, Van den Berghe and colleagues' studies suggest IIT is associated with beneficial effects on a variety of indicators of morbidity; benefits included a decrease in incidence of critical illness polyneuropathy [24,25] and acute renal failure [2], and reduced duration of mechanical ventilation. In Van den Berghe and colleagues' SICU study there was also a reduction in blood stream infections and reduced use of renal replacement therapy and blood transfusion [1].
Following publication of Van den Berghe and colleagues' first study, IIT was adopted in some ICUs and improved outcomes were reported in comparison with historical controls [26,27]. IIT is associated with increased ICU resource use for administration of insulin and close monitoring of blood glucose levels [28]. However, a post hoc analysis of health care resource utilization derived from the data from Van den Berghe and colleagues' SICU study reported an overall reduction in medical costs [29], and similar cost savings compared to historical controls were reported by Krinsley and Jones [30]. In both studies the cost savings occurred due to a shorter ICU stay and reduced expenditure on mechanical ventilation.
Two European multi-centre RCTs of IIT have been initiated but then stopped because of rates of hypoglycaemia considered unacceptable by their data monitoring committees [3,31]. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study [3], which recruited 537 participants in 18 hospitals in Germany, is the only trial report to be published in full to date. Early stopping of the trial resulted in only 247 patients being treated with IIT across 18 centres, an average of less than 14 patients per centre. As IIT is a complex treatment to administer, it is possible that the limited number of patients treated at each site was responsible for, or at least contributed to, the lack of treatment effect; this phenomenon has been observed in at least one prior trial in patients with severe sepsis [32].
In conclusion, the available data from two RCTs and large observational studies suggest that maintaining strict normoglycaemia by means of IIT reduces mortality and morbidity in both MICU and SICU patients. The beneficial effect seems to be even more pronounced in the most severely ill patients requiring prolonged intensive care. The additional costs for the more complex therapy and intensive monitoring are more than offset by the reduced overall resource consumption.
Con: Intensive insulin therapy targeting tight blood glucose control is of benefit in critically ill patients
It seems clear that hyperglycaemia is a common accompaniment of acute illness and that its occurrence and severity are associated with worse outcomes. Maintaining normoglycaemia through IIT significantly reduced mortality in Van den Berghe and colleagues' SICU trial [1] and reduced morbidity in their MICU trial [2], and their results have subsequently been replicated in studies using historical controls [26,27]. Furthermore, control of blood glucose has proven beneficial in other populations of acutely ill patients [23]. Whilst to some such data are sufficient to advocate IIT as a standard of care for critically ill adults [26], it is more prudent to critically evaluate the strength of the evidence before subjecting critically ill patients to such treatment.
The evidence in favour of IIT in ICU patients comes from two RCTs conducted sequentially in the SICUs and MICUs at a University Hospital in Leuven [1,2]. The results, particularly in the SICU population, where the relative risk of in-hospital death was reduced by 33.9%, seem compelling but, as noted by the authors, the patients studied in that trial were ventilated surgical patients admitted to the ICU after predominantly cardiac surgery and these results cannot be extrapolated to other ICU patient groups. Whether the results can be extrapolated to SICU patients worldwide has been questioned by a number of commentators [33,34]; concerns raised include an apparently high mortality rate in the control group, unusual concomitant treatment in the form of a high dose intravenous glucose regimen and that the remarkable reduction in mortality may itself call the trial results into question [35]. The in-hospital mortality of 10.9% in the conventional treatment group seems higher than expected for surgical ICU patients with a median Acute Physiology and Chronic Health Evaluation (APACHE) II score of 9. An ICU mortality rate of 5.1% for cardiac surgery patients also appears high as reported hospital mortality rates range from 0.9% to 3.6% [36-38]. In support of this contention, Egi and colleagues [39] identified patients from the databases of four hospitals in Australia who most closely matched the control group patients in Van den Berghe and colleagues' SICU study; they reported an in-hospital mortality rate of 3.8%, which was substantially lower than both the conventional and IIT groups in Van den Berghe and colleagues' study.
In both Van den Berghe and colleagues' studies, the patients received predominantly parenteral nutrition; the average daily intravenous glucose load was 160 ± 66 g in the conventionally treated patients and 161 ± 64 g in patients treated with IIT [40] – in an inception cohort study in Australia and New Zealand the median daily intravenous glucose load was 12.2 g [41]. In the pooled data analysis of Van den Berghe and colleagues' studies, 86% of all surgical and medical patients received predominately parenteral calories; only 39% received any enteral nutrition during their ICU stay. The percentage of total calories administered by the enteral route was 17.7% for the conventional group and 14.6% for the IIT group [40]. Neither the use of high-dose intravenous glucose nor the early institution of parenteral nutrition is recommended in current practice guidelines [42-44]. The participants in the VISEP trial received over 40% of their kilocalories by the enteral route and, given the potentially complex interplay between glucose administration, glycaemic control and outcome, it may be unsafe to extrapolate Van den Berghe and colleagues' findings to patients receiving more conventional feeding regimens.
Enrolment in Van den Berghe and colleagues' SICU study was stopped for efficacy after 1,548 of the planned 2,500 patients had been recruited. Trials that are stopped early may systematically overestimate treatment effects and where the number of accrued outcome events is small, the overestimation may be very large [45,46]. Where mortality is the primary outcome it may be wise to continue a study until 200 to 400 deaths have occurred and the p-value for the difference in mortality is less than 0.001 [46].
Van den Berghe and colleagues' MICU study [2] included a more heterogeneous population of patients, with a higher severity of illness represented by a mean APACHE II score of 23. Although there was a reduction in mortality with IIT, the difference did not reach the traditionally accepted level of statistical significance in the intention-to-treat analysis. The intention-to-treat analysis is always the most relevant result for any randomized controlled trial as it represents the effect of the treatment in all the patients studied [47], and the likely outcome if others use the treatment in similar populations of patients. Although the authors reported reduced mortality in patients who stayed in the ICU for three days or more, these patients could not be identified at the time treatment was started and so clinicians can not know to which of their patients this result might apply.
In both Van den Berghe and colleagues' studies hypoglycaemia occurred significantly more often in the IIT group (5.1 versus 0.8% in the surgical patients; 18.7 versus 3.1% in medical patients). MICU patients might be at higher risk for occurrence of hypoglycaemia due to the higher incidence of sepsis, and the necessity for renal replacement therapy and inotropic support, all of which have been identified as risk factors in the context of IIT [48].
In contrast to Van den Berghe and colleagues' findings, two multi-centre RCTs have stopped early after interim analyses found that IIT increased the risk of severe hypoglycaemia without any evidence of improved survival [3,31]. The VISEP study was conducted in 18 academic tertiary hospitals in Germany using treatment protocols based on Van den Berghe and colleagues' studies [3]. The study stopped after inclusion of 488 patients because of an increased incidence of severe hypoglycaemia with IIT (17.0 versus 4.1%) and no mortality benefit. The Glucontrol study examined the impact of IIT versus conventional treatment in 21 ICUs in 19 hospitals in 7 European countries [31]. The study was stopped after recruiting 1,101 of the planned 3,500 patients; severe hypoglycaemia occurred in 9.8% of the IIT group compared to 2.7% in the conventional arm, and ICU mortality did not differ significantly between the two groups (16.7% versus 15.2%) [31]. A further RCT, which recruited 523 patients in a mixed MICU and SICU in Saudi Arabia has also reported an increased risk of severe hypoglycaemia but no mortality benefit [49].
Although a number of authors have reported decreased mortality after adopting IIT [26,27], these observations provide, at best, very weak evidence in favour of IIT. As noted by Stewart and colleagues [27], changes in treatment protocols do not occur in isolation; in their surgical trauma ICU, institution of IIT was accompanied by a reduction in mortality, but during the period of study they also implemented a ventilator management protocol, a sedation protocol, and introduced a pneumonia prevention bundle. It is impossible to say which (if any) of these interventions was responsible for reduced mortality and the same criticism applies to all studies using historical controls.
In conclusion, critical appraisal of the high-quality evidence must conclude that IIT is an unproven treatment that should not be adopted widely until benefit is confirmed in large, high-quality, multi-centre RCTs.
Authors' opinion: What is the merit of using intensive insulin therapy to maintain tight blood glucose control in our patients?
Our clinical practice is in a multidisciplinary adult ICU that admits medical patients and patients following general surgery, trauma, burns, spinal injuries, cardiothoracic surgery and neurosurgery; is IIT indicated in any or all of these patient groups? Given the bewildering proliferation of literature on the subject, selective or biased reviews of the evidence can advance convincing arguments both for and against the use of IIT. In our view, the best evidence for or against any treatment comes from large, high-quality RCTs; we agree with Collins and MacMahon [50] that the reliable assessment of effects of treatment on major outcomes requires studies that guarantee strict control of bias through proper randomisation and appropriate analysis and interpretation (with no undue emphasis placed on specific parts of the evidence), and strict control of random error by reporting (in the present context) large numbers of deaths. Additionally, the argument for using any treatment is strengthened if supported by trials that have run to their planned conclusion and if clinical trials report consistent results.
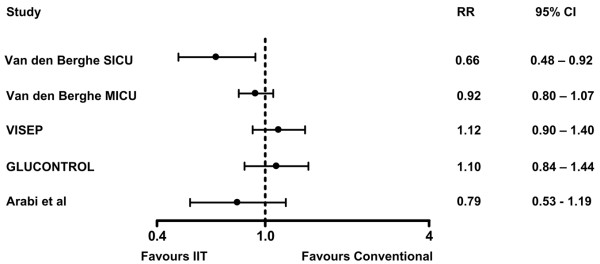
If we apply these principles, should we use IIT? To date, only one high-quality RCT supports the use of IIT (Figure 1); as that trial was stopped early and reported only 140 deaths, we can not exclude the possibility of significant random error [45,50]. The results have not been confirmed by subsequent multi-centre studies, but both multi-centre studies reported to date were stopped early and neither reported more than 200 deaths [3,31]. The one large published study that has run to completion [40], and which reported 442 deaths, did not find a significant treatment effect and so we would consider the evidence in favour of IIT to be equivocal at best. An ongoing international multi-centre study (The NICE-SUGAR study) that plans to recruit 6,100 patients and expects to report between 1,500 and 1,800 deaths may provide strong evidence either for or against the use of IIT [33].
Figure 1.
Relative risk (RR) and 95% confidence interval (CI) for five studies of intensive insulin therapy (IIT). The calculation of RR is based on the latest reported mortality: 90-day mortality for Van den Berghe and colleagues' medical intensive care unit (MICU) study and the Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study; hospital mortality for Van den Berghe and colleagues' surgical ICU (SICU) study; and ICU mortality for the GLUCONTROL study and Arabi and colleagues' study [49].
Even if we conclude that current evidence is not strong enough to advocate targeting normoglycaemia, the concept of IIT remains intuitively appealing and doing nothing is not an option. Glucose control appears beneficial in other populations of patients [21,23], although some of the trials have been small [21]. As in many areas of intensive care practice, the choice of a target range for blood glucose is a matter of balancing potential benefit against potential risk, and considering the resource implications of the treatment against a background of equivocal or incomplete evidence. Use of IIT has consistently resulted in an increased risk of severe hypoglycaemia. Two case-control studies that examined the association between hypoglycaemia and death reached different conclusions; one concluded that hypoglycaemia was independently associated with death [51], whilst the other concluded it was not [52]. Given these conflicting results, and the limited reliability of case-control studies, we conclude that whilst there is no consistent evidence that rapidly corrected hypoglycaemia is harmful, this possibility can not currently be ruled out. The other significant negative factor is that IIT is labour intensive and may require as much as two hours of nursing time per patient per day [28].
The balance between benefit and harm of a specific blood glucose target range depends on a variety of factors, including case mix, baseline morbidity and mortality as well as the characteristics of the individual ICU, such as availability of staff and laboratory equipment [39]. Until the balance of risks and benefits is better defined, we consider universal treatment guidelines or recommendations to target normoglycaemia to be premature. Each ICU should define a blood glucose range that can be achieved without causing a significant increase in severe hypoglycaemia and that fits within the constraints of their nursing and economic resources; for our multi-disciplinary ICU, and pending the results of the NICE-SUGAR study, the upper limit of this range is currently 8 to 10 mmol/l (140 to 180 mg/dl).
Abbreviations
APACHE II = Acute Physiology and Chronic Health Evaluation II score; ICU = intensive care unit; IIT, intensive insulin therapy; MICU = medical intensive care unit; RCT = randomized controlled trial; SICU = surgical intensive care unit; VISEP, Volume Substitution and Insulin Therapy in Severe Sepsis.
Competing interests
The authors declare that they have no competing interests.
References
- Berghe G van den, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Berghe G Van den, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K, German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- Lang CH, Dobrescu C, Bagby GJ. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130:43–52. doi: 10.1210/en.130.1.43. [DOI] [PubMed] [Google Scholar]
- Tappy L, Cayeux MC, Schneiter P, Schindler C, Temler E, Jequier E, Chiolero R. Effects of lactate on glucose metabolism in healthy subjects and in severely injured hyperglycemic patients. Am J Physiol. 1995;268:E630–635. doi: 10.1152/ajpendo.1995.268.4.E630. [DOI] [PubMed] [Google Scholar]
- Tappy L, Chiolero R, Berger M. Autoregulation of glucose production in health and disease. Curr Opin Clin Nutr Metab Care. 1999;2:161–164. doi: 10.1097/00075197-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci (Lond) 2001;101:739–747. doi: 10.1042/cs1010739. [DOI] [PubMed] [Google Scholar]
- McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–124. doi: 10.1016/S0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- Gustavson SM, Chu CA, Nishizawa M, Farmer B, Neal D, Yang Y, Donahue EP, Flakoll P, Cherrington AD. Interaction of glucagon and epinephrine in the control of hepatic glucose production in the conscious dog. Am J Physiol Endocrinol Metab. 2003;284:E695–707. doi: 10.1152/ajpendo.00308.2002. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Piper RC, Slot JW, James DE. Interaction of insulin and exercise on glucose transport in muscle. Diabetes Care. 1992;15:1679–1689. doi: 10.2337/diacare.15.11.1679. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Geer RJ, Rice DE, Bracy D, Flakoll PJ, Brown LL, Hill JO, Abumrad NN. Interaction of exercise and insulin action in humans. Am J Physiol. 1991;260:E37–45. doi: 10.1152/ajpendo.1991.260.1.E37. [DOI] [PubMed] [Google Scholar]
- Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293:H3404–3414. doi: 10.1152/ajpheart.00761.2007. [DOI] [PubMed] [Google Scholar]
- Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Berghe G Van den. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–38. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–342. doi: 10.1097/00006123-200002000-00015. discussion 342–333. [DOI] [PubMed] [Google Scholar]
- Leonidou L, Mouzaki A, Michalaki M, DeLastic AL, Kyriazopoulou V, Bassaris HP, Gogos CA. Cytokine production and hospital mortality in patients with sepsis-induced stress hyperglycemia. J Infect. 2007;55:340–346. doi: 10.1016/j.jinf.2007.05.177. [DOI] [PubMed] [Google Scholar]
- Ali NA, O'Brien JM, Jr, Blum W, Byrd JC, Klisovic RB, Marcucci G, Phillips G, Marsh CB, Lemeshow S, Grever MR. Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer. 2007;110:96–102. doi: 10.1002/cncr.22777. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2004;164:2005–2011. doi: 10.1001/archinte.164.18.2005. [DOI] [PubMed] [Google Scholar]
- Berghe G Van den, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, Bruyninckx F, Berghe G Van den. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175:480–489. doi: 10.1164/rccm.200605-665OC. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- Reed CC, Stewart RM, Sherman M, Myers JG, Corneille MG, Larson N, Gerhardt S, Beadle R, Gamboa C, Dent D. Intensive insulin protocol improves glucose control and is associated with a reduction in intensive care unit mortality. J Am Coll Surg. 2007;204:1048–1054. doi: 10.1016/j.jamcollsurg.2006.12.047. discussion 1054–1045. [DOI] [PubMed] [Google Scholar]
- Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370–377. [PubMed] [Google Scholar]
- Berghe G Van den, Wouters PJ, Kesteloot K, Hilleman DE. Analysis of healthcare resource utilization with intensive insulin therapy in critically ill patients. Crit Care Med. 2006;34:612–616. doi: 10.1097/01.ccm.0000201408.15502.24. [DOI] [PubMed] [Google Scholar]
- Krinsley JS, Jones RL. Cost analysis of intensive glycemic control in critically ill adult patients. Chest. 2006;129:644–650. doi: 10.1378/chest.129.3.644. [DOI] [PubMed] [Google Scholar]
- Devos P, Preiser JC, Mélot C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycaemia: final results of the Glucontrol study. Intensive Care Med. 2007;33(Suppl 2):S189. [Google Scholar]
- Macias WL, Vallet B, Bernard GR, Vincent JL, Laterre PF, Nelson DR, Derchak PA, Dhainaut JF. Sources of variability on the estimate of treatment effect in the PROWESS trial: implications for the design and conduct of future studies in severe sepsis. Crit Care Med. 2004;32:2385–2391. doi: 10.1097/01.CCM.0000147440.71142.AC. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Egi M. Glycemic control in the intensive care unit: why we should wait for NICE-SUGAR. Mayo Clin Proc. 2005;80:1546–1548. doi: 10.4065/80.12.1546. [DOI] [PubMed] [Google Scholar]
- Angus DC, Abraham E. Intensive insulin therapy in critical illness. Am J Respir Crit Care Med. 2005;172:1358–1359. doi: 10.1164/rccm.2508009. [DOI] [PubMed] [Google Scholar]
- Wheatley K, Clayton D. Be skeptical about unexpected large apparent treatment effects: the case of an MRC AML12 randomization. Control Clin Trials. 2003;24:66–70. doi: 10.1016/S0197-2456(02)00273-8. [DOI] [PubMed] [Google Scholar]
- Welke KF, Peterson ED, Vaughan-Sarrazin MS, O'Brien SM, Rosenthal GE, Shook GJ, Dokholyan RS, Haan CK, Ferguson TB., Jr Comparison of cardiac surgery volumes and mortality rates between the Society of Thoracic Surgeons and Medicare databases from 1993 through 2001. Ann Thorac Surg. 2007;84:1538–1546. doi: 10.1016/j.athoracsur.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Kalkat MS, Edwards MB, Taylor KM, Bonser RS. Composite aortic valve graft replacement: mortality outcomes in a national registry. Circulation. 2007;116(11 Suppl):I301–306. doi: 10.1161/CIRCULATIONAHA.106.681437. [DOI] [PubMed] [Google Scholar]
- Humphries KH, Gao M, Pu A, Lichtenstein S, Thompson CR. Significant improvement in short-term mortality in women undergoing coronary artery bypass surgery (1991 to 2004) J Am Coll Cardiol. 2007;49:1552–1558. doi: 10.1016/j.jacc.2006.08.068. [DOI] [PubMed] [Google Scholar]
- Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P, Li W, Bates S. Intensive insulin therapy in postoperative intensive care unit patients: a decision analysis. Am J Respir Crit Care Med. 2006;173:407–413. doi: 10.1164/rccm.200506-961OC. [DOI] [PubMed] [Google Scholar]
- Berghe G Van den, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, Schetz M. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- Mitchell I, Finfer S, Bellomo R, Higlett T. Management of blood glucose in the critically ill in Australia and New Zealand: a practice survey and inception cohort study. Intensive Care Med. 2006;32:867–874. doi: 10.1007/s00134-006-0135-4. [DOI] [PubMed] [Google Scholar]
- Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, Berghe G van den, Wernerman J, DGEM (German Society for Nutritional Medicine) Ebner C, Hartl W, Heymann C, Spies C, ESPEN (European Society for Parenteral and Enteral Nutrition) ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Jolliet P, Pichard C, Biolo G, Chioléro R, Grimble G, Leverve X, Nitenberg G, Novak I, Planas M, Preiser JC, Roth E, Schols AM, Wernerman J. Enteral nutrition in intensive care patients: a practical approach. Working Group on Nutrition and Metabolism, ESICM. European Society of Intensive Care Medicine. Intensive Care Med. 1998;24:848–859. doi: 10.1007/s001340050677. [DOI] [PubMed] [Google Scholar]
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Ann Intern Med. 2007;146:878–881. doi: 10.7326/0003-4819-146-12-200706190-00009. [DOI] [PubMed] [Google Scholar]
- Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M, Lacchetti C, Leung TW, Darling E, Bryant DM, Bucher HC, Schünemann HJ, Meade MO, Cook DJ, Erwin PJ, Sood A, Sood R, Lo B, Thompson CA, Zhou Q, Mills E, Guyatt GH. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294:2203–2209. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- Fontanarosa PB, DeAngelis CD. Publication of clinical trials in JAMA: information for authors. JAMA. 2008;299:95–96. doi: 10.1001/jama.2007.28. [DOI] [Google Scholar]
- Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, Hoekstra JB. Predisposing factors for hypoglycemia in the intensive care unit. Crit Care Med. 2006;34:96–101. doi: 10.1097/01.CCM.0000194536.89694.06. [DOI] [PubMed] [Google Scholar]
- Arabi Y, Tamim H, Alshimemeri A, Memish Z, Junaid S, Rishu A, Daker M, Haddad S, Kahoul S, Britts R, Sakkijha M. Intensive versus standard insulin therapy: a randomized controlled trial in medical surgical critically ill patients. Crit Care Med. 2006;34:A65. doi: 10.1097/00003246-200612002-00227. [DOI] [PubMed] [Google Scholar]
- Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357:373–380. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB, Schultz MJ, Rosendaal FR, Hoekstra JB. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med. 2006;34:2714–2718. doi: 10.1097/01.CCM.0000241155.36689.91. [DOI] [PubMed] [Google Scholar]