Abstract
Introduction
Patients requiring prolonged acute mechanical ventilation (PAMV) represent one-third of those who need mechanical ventilation, but they utilize two-thirds of hospital resources devoted to mechanical ventilation. Measures are needed to optimize the efficiency of care in this population. Both duration of intensive care unit stay and mechanical ventilation are associated with anemia and increased rates of packed red blood cell (pRBC) transfusion. We hypothesized that transfusions among patients receiving PAMV are common and associated with worsened clinical and economic outcomes.
Methods
A retrospective analysis of a large integrated claims database covering a 5-year period (January 2000 to December 2005) was conducted in adult patients receiving PAMV (mechanical ventilation for ≥ 96 hours). The incidence of pRBC transfusions was examined as the main exposure variable, and hospital mortality served as the primary outome, with hospital length of stay and costs being secondary outcomes.
Results
The study cohort included 4,344 hospitalized patients receiving PAMV (55% male, mean age 61.5 ± 16.4 years). Although hemoglobin level upon admission was above 10 g/dl in 75% of patients, 67% (n = 2,912) received at least one transfusion, with a mean of 9.1 ± 12.0 units of pRBCs transfused per patient over the course of hospitalization. In regression models adjusting for confounders, exposure to pRBCs was associated with a 21% increase in the risk for hospital death (95% confidence interval [CI] = 1.00 to 1.48), and marginal increases in length of stay (6.3 days, 95% CI = 5.1 to 7.6) and cost ($48,972, 95% CI = $45,581 to $52,478).
Conclusion
Patients receiving PAMV are at high likelihood of being transfused with multiple units of blood at relatively high hemoglobin levels. Transfusions independently contribute to increased risk for hospital death, length of stay, and costs. Reducing exposure of PAMV patients to blood may represent an attractive target for efforts to improve quality and efficiency of health care delivery in this population.
Introduction
Patients requiring prolonged acute mechanical ventilation (PAMV), defined as 96 hours of mechanical ventilation (MV) or longer, are a group with high hospital utilization intensity [1]. Although constituting roughly one-third of all hospitalized MV patients, they account for about two-thirds of all the hospital resources allocated to the MV group [1]. For example, in the USA in 2003, PAMV patients occupied 6,728,819 hospital days, at an aggregate annualized hospital cost of over $16 billion [1]. At the same time their hospital mortality of 35% is similar to that observed among ventilated patients who require fewer than 96 hours of MV. Based on age-adjusted and disease-specific incidence rates, this population is projected to more than double by the year 2020, thus mandating increased emphasis on efficiency of health care delivery to patients requiring PAMV [2].
Anemia is a frequent complication of critical illness, and its etiology is multifactorial [3-5]. Despite evidence from a large randomized controlled trial suggesting that tolerating a lower hemoglobin among critically ill patients results in unimpaired outcomes, more recent observational data indicate that adherence to this recommendation is poor across the board, and is worst among patients requiring MV [3,5,6]. At the same time, a large body of work specifically addressing the critically ill points to a strong association of exposure to allogeneic blood with such complications as acute lung injury (ALI), ventilator-associated pneumonia, and blood stream infection (BSI) [7-11], and morbidity and attendant hospital resource utilization stemming from such complications may be avoided by more restrictive use of allogeneic blood [12-15].
Because by virtue of having a prolonged critical illness the PAMV population is at greater risk for exposure to packed red blood cell (pRBC) transfusions [16], we hypothesized that in this population more liberal use of allogeneic blood is associated with worse clinical and economic outcomes. Conversely, it would follow that a more restrictive approach to transfusions might result in fewer complications, better outcomes, and thus better quality health care and more efficient health care delivery.
Materials and methods
Human subjects protection
Approval was obtained from the Institutional Review Board of the Henry Ford Health System. No informed consent was required because the study involved the use of de-identified claims data.
Data source
We performed a retrospective cohort study within the Henry Ford Health System (HFHS) database. HFHS is a large, vertically integrated health care system that includes seven hospitals serving the primary and specialty health care needs of residents in the Midwestern USA. The care provided includes more than 2.5 million patient contacts, 20,000 ambulatory surgeries, and 65,000 hospital admissions annually. Most of the care is provided under system-affiliated, salaried physician groups with nearly 900 physicians in more than 40 specialties. Approximately 60% of HFHS members are enrolled in a large nonprofit, mixed-model health maintenance organization. This subset population includes a substantial number of both Medicare (n = 16,000) and Medicaid (n = 22,000) enrollees.
Cohort identification
In the present analysis we used data from all hospital admissions that took place between January 2000 and December 2005. Patients were included if they were 18 years old or older and had charges associated with at least one procedure code for insertion of an endotracheal tube for MV and at least one code for 96 continuous hours of ventilation. The index date was defined as the date of the first hospital admission containing these codes. Patients on dialysis before the index admission and with a diagnosis code for chronic renal failure were not included in the analysis. Additional file 1 presents the inclusion criteria and associated procedure codes for study patients.
Exposure variables
The primary exposure of interest was transfusion of pRBCs during the entire period of hospitalization, based on blood bank data linked to specific patient encounters. A transfusion episode was identified by the receipt of at least one unit of pRBCs on any given day of hospitalization. An additional measure of transfusion exposure was units of RBCs transfused per patient. Transfusion exposure was further stratified based on the baseline, pretransfusion, and nadir hemoglobin. Baseline hemoglobin was calculated using the first laboratory measurement on the date of admission; or, if this was not available, the last measurement on the day before admission; or, if none on that day, the first laboratory measurement on the day after admission. Pretransfusion hemoglobin was defined as the lowest hemoglobin measured on or the day before the day of transfusion. Pretransfusion hemoglobin was divided into the following categories: <7 g/dl, 7 to 8 g/dl, 8 to 9 g/dl, 9 to 10 g/dl, and ≥ 10 g/dl [3]. Nadir hemoglobin was defined as the lowest hemoglobin recorded for a patient during the inpatient stay. PAMV patients who received transfused blood were compared with those who did not, based on demographic and clinical characteristics. The burden of chronic illness was assessed using the modified Charlson Comorbidity Index score [17]. Using International Classification of Diseases, ninth revision (ICD-9) codes, 16 weighted co-morbidity variables were defined: a patient's total score (the sum of the scores from each condition present; maximum 34) indicated his or her chronic disease burden.
Outcome measures
The primary outcome measure was hospital mortality, compared between transfused and nontransfused PAMV patients. Resource utilization (hospital length of stay), hospital costs, discharge hemoglobin, and destination served as the secondary outcomes of interest. Although discharge hemoglobin and destination were examined in a descriptive manner only, hospital mortality, length of stay (LOS) and costs were looked at in multivariable models (see Statistical analyses, below).
Statistical analyses
Values are expressed as percentages and mean ± standard deviation, and P < 0.05 was considered to represent statistical significance.
Frequencies (for categorical variables) and distributions (for continuous variables), from the index admission date to the date of hospital discharge, were compared using χ2 and Student's t-tests, respectively, for all normal data stratified by transfused versus nontransfused patients. Mann-Whitney tests were applied to hospital LOS and cost to assess the significance of differences across exposure to pRBCs. The independent contribution of transfusion exposure to hospital mortality, LOS, and costs was assessed in multivariable models. Although hospital mortality was examined in a logistic regression, the attributable hospital LOS and costs were computed in linear regressions. Because both LOS and costs have highly skewed distributions, the values were log-transformed for the models, and the resultant coefficients were retransformed using the Duan smearing method [18] to yield incremental days and dollars, respectively. The co-variates initially included in the multivariate analyses were patient demographics (age, sex, and race), comorbidities (Charlson Comorbidity Index score), and baseline and nadir hemoglobin levels. Additional variables examined as potential confounders of the relationship between transfusion exposure and outcomes were such complications as hospital-acquired pneumonia (HAP) and BSI (based on the presence of an ICD-9-CM code for pneumonia, bacteremia, or septicemia; see Additional file 1). Finally, potential confounding by such markers of blood loss as gastrointestinal endoscopy, or such surgical procedures as abdominal, cardiac (on-pump and off-pump), and orthopedic surgeries was examined. Those covariates with P > 0.2 were eliminated in a manual backward selection process. Diagnostics were performed to ensure the validity of each final model.
Results
Cohort characteristics
We identified 4,344 hospitalized patients with PAMV during the time frame examined. Most members of the study population were male (54.5%) and African-American (57.6%), with the mean age being 61.5 ± 16.4 years and the mean Charlson Comorbidity Index score being 7.3 ± 3.6.
The transfusion rate in the cohort was 67.0% (n = 2,912). Transfused patients were older (62.0 ± 16.5 years versus 60.4 ± 16.2 years; P = 0.0014), more likely to be female (46.8% versus 42.9%; P = 0.018), had a significantly higher chronic disease burden (Charlson Comorbidity Index scores of 7.8 ± 3.6 versus 6.2 ± 3.3; P < 0.0001), a lower mean baseline hemoglobin (11.1 ± 2.4 g/dl versus 13.0 ± 2.0 g/dl; P < 0.0001), and a lower mean nadir hemoglobin (7.3 ± 1.1 g/dl versus 9.9 ± 1.7 g/dl; P < 0.0001) than did those who did not require a transfusion (Table 1). The distribution of the top 5 admitting diagnoses is shown in Table 2. Acute respiratory failure was the most frequent admitting diagnosis in both patient groups, namely those who required a transfusion and those who did not (5.1% versus 12.4%).
Table 1.
Baseline demographic and clinical characteristics of patients on PAMV by transfusion status
| Characteristic | Transfused (n = 2,912) | Nontransfused (n = 1,432) | P value |
| Sex (% female) | 46.8 | 42.9 | 0.018 |
| Age (years; mean ± SD) | 62.0 ± 16.5 | 60.4 ± 16.2 | 0.0014 |
| Race (%) | |||
| African American | 55.5 | 61.9 | <0.0001 |
| Caucasian | 36.9 | 32.7 | |
| Other | 8.6 | 5.4 | |
| Chronic concomitant comorbidities, % | |||
| Cardiac disease | 88.0 | 79.8 | <0.0001 |
| Hypertension | 69.4 | 65.9 | 0.02 |
| Anemia | 64.7 | 22.8 | <0.0001 |
| Pulmonary disease | 64.6 | 65.9 | 0.43 |
| Gastrointestinal disease | 57.6 | 31.1 | <0.0001 |
| Central nervous system disease | 50.8 | 44.8 | 0.0002 |
| Cancer | 39.1 | 26.1 | <0.0001 |
| Immunologic disease | 38.9 | 22.8 | <0.0001 |
| Thromboembolic disease | 38.5 | 30.0 | <0.0001 |
| Renal disease | 35.3 | 15.8 | <0.0001 |
| Diabetes | 31.6 | 26.1 | 0.0002 |
| Musculoskeletal disease | 31.4 | 14.6 | <0.0001 |
| Peripheral vascular disease | 29.2 | 26.9 | <0.0001 |
| Cerebrovascular disease | 28.7 | 21.7 | 0.11 |
| Primary hematologic disease | 15.2 | 7.5 | <0.0001 |
PAMV, prolonged acute mechanical ventilation; SD, standard deviation.
Table 2.
Distribution of top 5 primary diagnoses among patients on PAMV by transfusion status
| Admitting diagnoses (%) | Transfused (n = 2,912) | Nontransfused (n = 1,432) |
| Acute respiratory failure | 5.1 | 12.4 |
| Heart failure | 4.1 | 6.1 |
| Subendocardial AMI | 4.0 | 3.4 |
| Pneumonia | 3.7 | 5.0 |
| Intracerebral hemorrhage | 2.3 | 3.6 |
AMI, acute myocardial infarction; PAMV, prolonged acute mechanical ventilation.
Hemoglobin and transfusions
The total number of transfusion episodes during the index admission was 7,787 among 2,912 transfused patients. Each patient who was transfused received an average of 3.2 ± 2.8 units/transfusion episode and a total 9.1 ± 12.0 units of blood over the course of the hospitalization. Mean pretransfusion hemoglobin for the 7,787 transfusion episodes was 8.2 ± 1.4 g/dl. The majority of transfusion episodes (69% total) occurred at pretransfusion hemoglobin levels 7 to <8 g/dl (35%; mean units of blood/transfusion episode 3.1 ± 2.9) and 8 to <9 g/dl (34%; mean units of blood/transfusion episode 3.0 ± 3.6; Figure 1). Only 12% of all transfusion episodes in the cohort occurred at the pretransfusion hemoglobin below 7 g/dl, and in this group the mean number of units/transfusion episode was high (5.3 ± 6.5). Conversely, 7% of the episodes occurred in the setting of a pretransfusion hemoglobin above 10 g/dl.
Figure 1.
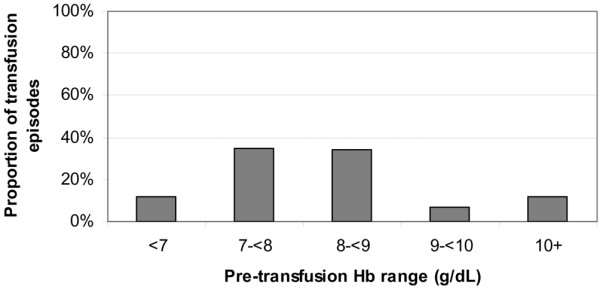
Transfusion episodes occurring at each level of pretransfusion hemoglobin among transfused patients on PAMV. Hb, hemoglobin; PAMV, prolonged acute mechanical ventilation.
Outcomes
Crude hospital mortality was significantly higher among PAMV patients who underwent transfusion as compared with those who did not (odds ratio [OR] = 1.51, 95% confidence interval [CI] = 1.31 to 1.75; P < 0.0001). Compared with nontransfused patients, transfused patients were also more likely to experience HAP (OR = 1.63, 95% CI = 1.38 to 1.93) or BSI (OR = 2.90, 95% CI = 2.53 to 3.34; P < 0.0001 for each) and to have undergone a procedure serving as a marker of high bleeding risk (P < 0.0001 for all five procedures). Similarly, transfused patients had a significantly greater hospital LOS and corresponding total hospital costs than did nontransfused patients (Table 3). Of the patients discharged alive, the odds of being discharged directly home were significantly lower among those transfused (OR = 0.38, 95% CI = 0.32 to 0.44; P < 0.0001) than those who were not transfused. Conversely, a discharge to an intermediate health care facility was 2.64 (95% CI = 2.25 to 3.11; P < 0.0001) times as likely in the transfused group. Finally, despite a high transfusion rate, those transfused were discharged with significantly lower hemoglobin than those who did not receive any transfusions (9.8 ± 1.4 g/dl versus 10.8 ± 1.8 g/dl; P < 0.0001; Table 3).
Table 3.
Hospital events and unadjusted outcomes among patients on PAMV by transfusion status
| Events and outcomes | Transfused (n = 2,912) | Nontransfused (n = 1,432) | P value |
| Complications (%) | |||
| Pneumonia | 86.2 | 79.3 | <0.0001 |
| Blood stream infection | 49.7 | 20.1 | <0.0001 |
| Tracheostomy | 71.9 | 69.8 | 0.16 |
| Processes of care | |||
| Gastrointestinal endoscopy | 31.2 | 12.4 | <0.0001 |
| Abdominal surgery | 47 | 18.4 | <0.0001 |
| Cardiac surgery (on-pump) | 9.2 | 0.7 | <0.0001 |
| Cardiac surgery (off-pump) | 9.9 | 2.7 | <0.0001 |
| Orthopedic surgery | 9.3 | 1.8 | <0.0001 |
| Hospital mortality (%) | 32.2 | 23.9 | <0.0001 |
| Discharge destination (n [%]) | |||
| Home | 433 (22.0) | 465 (42.7) | <0.0001 |
| Skilled nursing facility | 496 (25.1) | 200 (18.4) | |
| Rehabilitation center | 382 (19.4) | 143 (13.1) | |
| Home health service | 275 (13.9) | 147 (13.5) | |
| Rehabilitation with long-term ventilator care | 150 (7.6) | 39 (3.6) | |
| Hospice | 52 (2.6) | 20 (1.8) | |
| Other health facility | 151 (7.7) | 47 (4.3) | |
| Other/unknown | 34 (1.7) | 29 (2.7) | |
| Hemoglobin (mean ± SD) | |||
| Baseline | 11.1 ± 2.4 | 13.0 ± 2.0 | <0.0001 |
| Nadir | 7.3 ± 1.1 | 9.9 ± 1.7 | <0.0001 |
| Discharge | 9.8 ± 1.4 | 10.8 ± 1.8 | <0.0001 |
| LOS | |||
| Mean ± SD | 29.6 ± 22.5 | 15.5 ± 10.9 | <0.0001 |
| 25th percentile | 15 | 9 | |
| 50th percentile | 24 | 13 | |
| 75th percentile | 37 | 19 | |
| Charges | |||
| Mean ± SD | 101,828 ± 72,925 | 239,312 ± 187,882 | <0.0001 |
| 25th percentile | 55,294 | 115,870 | |
| 50th percentile | 83,537 | 185,542 | |
| 75th percentile | 126,623 | 294,389 |
LOS, length of stay; PAMV, prolonged acute mechanical ventilation; SD, standard deviation.
Regression modeling confirmed the independent contribution of pRBC transfusions to hospital mortality, LOS, and aggregate costs of hospitalization. Thus, in a logistic regression, exposure to allogeneic blood was associated with a 21% (95% CI = 1.00 to 1.48) increase in the risk of death, and linear models suggested that transfusions alone were responsible for a marginal 6.3 (95% CI = 5.12 to 7.62) day increase in hospital LOS and $48,972 (95% CI = $45,582 to $52,478) increase in hospital costs (Table 4).
Table 4.
Mortality, hospital LOS, and costs attributable to transfusion exposure among patients on PAMV
| Variable | Adjusted estimate | 95% confidence interval |
| Hospital mortality (adjusted odds ratio) | 1.21 | 1.00 to 1.48 |
| Hospital LOS (days) | 6.33 | 5.12 to 7.62 |
| Hospital costs ($) | $48,973 | $45,582 to $52,478 |
Each estimate adjusted for the following confounding variables: age, sex, race, Charlson Comorbidity Index, baseline and nadir hemoglobin, hospital-acquired pneumonia, blood stream infection, gastrointestinal endoscopy, abdominal surgery, cardiac surgery (on and off bypass), and orthopedic surgery. Mortality outcomes were adjusted additionally for hospital LOS. Hospital LOS and cost outcomes were adjusted for mortality. LOS, length of stay; PAMV, prolonged acute mechanical ventilation.
Discussion
In the present study we have demonstrated that transfusions are commonly used among patients requiring PAMV, occur at relatively liberal hemoglobin triggers, and are associated with worsened hospital outcomes, including mortality, LOS, and costs. We have also shown that PAMV patients undergoing a transfusion are less likely to be discharged home and, despite a high transfusion burden, leave the hospital with a lower hemoglobin level than those not undergoing a transfusion. Specifically, we have documented a 67% transfusion rate among these patients, with the attendant attributable mortality, LOS, and cost increases of 21%, 6.3 days, and $48,972, respectively. Importantly, these numbers reflect the adjustment for potential contributions to these outcomes of such high risk and costly events as nosocomial infections (HAP and BSI) and gastrointestinal bleeding, and cardiac, abdominal and orthopedic surgeries.
Health care costs in the USA have reached an unprecedented $2.1 trillion, representing 16% of the gross domestic product [19]. A large proportion of this price tag, approximately one-third, is attributed to hospital expenses, and these have been rising steadily [20]. The intensive care unit (ICU), in turn, is a high-intensity use area of the hospital, whose utilization by the Medicare population alone has grown by over 12% in a recent 5-year period [21]. MV, whose attributable cost is $1,500/day in 2002 US dollars, is the single greatest driver of the ICU costs [22]. Illustrating this, the 2003 aggregate hospital costs for all patients undergoing any MV were $25 billion, 64% of which (or $16 billion) was consumed by patients requiring PAMV [1]. Furthermore, given the historic approximately 6% crude annual growth in the volume of PAMV in US hospitals, age-specific incidence change in PAMV over time, and the age-adjusted US population growth, PAMV patients are likely to number over 600,000 cases by year 2020, more than doubling their current numbers [2]. Such substantial utilization of health care resources demands closer scrutiny.
Given that hospital survival rate among patients on PAMV is comparable to that among patients requiring shorter term ventilatory support [1], institution of measures that are directed at optimizing efficiency of health care delivery to this population are critical. Allogeneic blood transfusion is one area of health care delivery in which the penetration of evidence-based practices has remained suboptimal [3,5]. Despite the fact that a randomized controlled trial performed a decade ago confirmed safety of reducing hemoglobin transfusion triggers among critically ill from the traditional 10 g/dl to 7 g/dl [23], the mean pretransfusion hemoglobin remains in the region of 8.5 g/dL among all ICU patients, and is even higher among those requiring MV [3,5,6]. Our study demonstrates nonadherence not only to this recommendation but also to the guidance supporting the administration of only 1 unit of pRBCs per transfusion episode, as was done in the TRICC (Transfusion Requirements in Critical Care) trial [23], because we have observed that on average a transfused PAMV patient receives more than 3 units of blood in a single transfusion episode.
Unfortunately, this nonadherence to recommendations based on the best evidence is not without consequences. Evidence points to a strong association between exposure to blood transfusions and such complications as ventilator-associated pneumonia and BSIs [9,10,24], as well as multiple other infectious and immune complications [25-27]. Evidence for the connection between acute respiratory distress syndrome (ARDS) and transfusion exposure is also mounting. For example, not only did the difference in the ARDS incidence nearly reach statistical significance in the TRICC trial (P = 0.06), with the more favorable results in the restrictive arm [23], but also several prospective cohort studies have strengthened this association [7,8,11], particularly because some were able to detect a dose-response relationship between the magnitude of blood exposure and risk for subsequent development of ARDS [7,11]. Giving further credence to this causal relationship is a recent report from the Mayo clinic [13], in which adherence to a restrictive transfusion protocol along with the use of a lung-protective ventilation strategy resulted in a reduction in ALI incidence from 28% to 10%. In general, it is estimated that adopting restrictive triggers more ubiquitously could result in avoidance of nearly 40,000 acute complications associated with transfusions, and, more specifically, approximately 17,000 cases of ARDS alone [14,15]. This may even be an under-estimate, given the results of the nested case-control study conducted by Gajic and coworkers [28], in which ALI was prospectively observed in 8% of all patients exposed to blood products. Our study, by focusing on the population of MV patients at greatest risk for transfusions (namely those receiving PAMV), deepens our understanding not only of transfusion practices in PAMV patients but also of how altering these practices in line with the best evidence might result in lower costs and better outcomes for patients. Given that more than a half of all of the observed transfusion episodes were administered at or above a hemoglobin concentration of 8 g/dl, and given the noted contribution of allogeneic pRBC exposure to mortality, hospital LOS, and costs in the PAMV population, there is a substantial opportunity to improve these outcomes via an evidence-driven reduction in transfusion triggers.
Our study has a number of limitations. First, its retrospective nature lends itself to multiple forms of bias, the most important of which is a selection bias. We have mitigated this possibility by employing uniform inclusion criteria that are based on a strict definition of the population, as well as by following all patients to the hard end-points of hospital discharge or death. Second, having been performed in a single health care system, the underlying population characteristics may limit the generalizability of our findings. Third, the accuracy of the identification of hospital complications (HAP and BSI) and such processes of care as the surgical procedures we adjusted for may be at least somewhat questionable, because we utilized administrative coding, and not clinical data, to define these conditions. Although it is possible that the first two are prone to being over-reported in the hospital's billing system, particularly for the sicker patients, the reporting of surgical procedures is less likely to have an inherent bias. Fourth, because of its observational nature, and although we attempted to adjust for confounders, the possibility of residual confounding remains. Finally, our study was performed before widespread utilization of leukoreduction, and thus it may overestimate the association between transfusions and such adverse outcomes as infectious complications or ALI. However, this potential inflation of the association, if present, is likely to be small, because a recent randomized controlled trial failed to observe a reduction in the incidence of either infection or ALI in the group exposed to leukoreduced blood [29,30].
Conclusion
In summary, we have demonstrated that two-thirds of all patients on PAMV are exposed to allogeneic blood during their hospitalization. Furthermore, the total amount of blood is, on average, a staggering 9 units per transfused patient. Even with a pretransfusion hemoglobin of >7 g/dl, the number of units administered per one episode of transfusion is over 3. This potential overuse of allogeneic blood is associated with a 21% increase in adjusted hospital mortality, and an incremental hospital LOS and costs of 6.3 days and $48,972, respectively. Our data support the idea that evidence-based transfusion practices in this population of critically ill patients may be one of the ways to improve quality and efficiency of health care delivery.
Key messages
• Patients on PAMV (defined as MV for ≥ 96 hours) are at a high risk for transfusion with pRBCs.
• Only 12% of all transfusion episodes took place at a hemoglobinn concentration below 7 g/dL, with an additional 35% of episodes occurring between 7 and <8 g/dl.
• On average, a transfusion episode consists of 3.2 ± 2.8 units of pRBCs.
• Exposure to allogeneic blood is associated with an adjusted increase in hospital mortality of 21%, hospital LOS of 6.3 days, and hospital costs of $48,972.
Abbreviations
ALI = acute lung injury; ARDS = acute respiratory distress syndrome; BSI = blood stream infection; CI = confidence interval; HAP = hospital-acquired pneumonia; HFHS = Henry Ford Health System; ICD-9 = International Classification of Diseases, ninth revision; ICU = intensive care unit; LOS = length of stay; MV = mechanical ventilation; OR = odds ratio; PAMV = prolonged acute mechanical ventilation; pRBC = packed red blood cell.
Competing interests
At the time of this study, MDZ was an employee of Ortho Biotech Clinical Affairs, LLC (Bridgewater, NJ, USA). She currently serves as a consultant to Ortho Biotech Clinical Affairs, LLC, and is a stockholder in Johnson & Johnson (New Brunswick, NJ, USA), its parent company. At the time of this study LSS, DPW, and JJD were employees of Analytica International (New York, NY, USA), which has received research funds from Ortho Biotech Clinical Affairs, LLC. AFS is a consultant to and has received funding from Ortho Biotech Clinical Affairs, LLC.
Authors' contributions
MDZ, DPW, and AFS were responsible for study design, data interpretation, and drafting the manuscript. LSS and JJD were responsible for study design, data analyses, and data interpretation. All authors read and approved the final manuscript.
Supplementary Material
Inclusion criteria and associated procedure codes for study patients. Presented are the inclusion criteria and associated procedure codes for study patients.
Acknowledgments
Acknowledgements
The study and this manuscript were funded by Ortho Biotech Clinical Affairs, LLC. Employees of and consultants to the sponsor were involved in the design of the study. The funding body had no role in data interpretation, manuscript drafting, or the decision to submit the manuscript for publication.
We are indebted to Don Chalfin, MD, MS, an employee of Analytica International, the organization that received funding from Ortho Biotech Clinical Affairs, LLC to conduct this study; and to Monika Raut, PhD and Samir Mody, PharmD, MBA, both of Ortho Biotech Clinical Affairs, LLC, the study sponsor, for their early contributions to the conception and design of the study. No medical writer was involved in the study or its reporting.
Contributor Information
Marya D Zilberberg, Email: mzilberb@schoolph.umass.edu.
Lee S Stern, Email: lstern@analyticaintl.com.
Daniel P Wiederkehr, Email: dwiederkehr@analyticaintl.com.
John J Doyle, Email: jdoyle@analyticaintl.com.
Andrew F Shorr, Email: AFShorr@dnamail.com.
References
- Zilberberg MD, Luippold RS, Sulsky S, Shorr AF. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36:724–730. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- Zilberberg MD, de Wit M, Pirone JR, Shorr AF. Growth in prolonged acute mechanical ventilation: Implications for healthcare delivery. Crit Care Med. 2008;36:1451–1455. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: Anemia and blood transfusion in the critically ill – current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- Rodriguez RM, Corwin HL, Gettinger A, Corwin MJ, Gubler D, Pearl RG. Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness. J Crit Care. 2001;16:36–41. doi: 10.1053/jcrc.2001.21795. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. Anemia and blood transfusion in critically ill patients. Jama. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- Levy MM, Abraham E, Zilberberg M, MacIntyre NR. A descriptive evaluation of transfusion practices in patients receiving mechanical ventilation. Chest. 2005;127:928–935. doi: 10.1378/chest.127.3.928. [DOI] [PubMed] [Google Scholar]
- Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.CCM.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34:196–202. doi: 10.1097/01.CCM.0000194540.44020.8E. [DOI] [PubMed] [Google Scholar]
- Shorr AF, Duh MS, Kelly KM, Kollef MH. Red blood cell transfusion and ventilator-associated pneumonia: a potential link? Crit Care Med. 2004;32:666–674. doi: 10.1097/01.CCM.0000114810.30477.C3. [DOI] [PubMed] [Google Scholar]
- Shorr AF, Jackson WL, Kelly KM, Fu M, Kollef MH. Transfusion practice and blood stream infections in critically ill patients. Chest. 2005;127:1722–1728. doi: 10.1378/chest.127.5.1722. [DOI] [PubMed] [Google Scholar]
- Zilberberg MD, Carter C, Lefebvre P, Raut M, Vekeman F, Duh MS, Shorr AF. Red blood cell transfusions and the risk of acute respiratory distress syndrome among the critically ill: a cohort study. Crit Care. 2007;11:R63. doi: 10.1186/cc5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plurad D, Martin M, Green D, Salim A, Inaba K, Belzberg H, Demetriades D, Rhee P. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: the potential role of lung protective ventilation and conservative transfusion practice. J Trauma. 2007;63:1–7. doi: 10.1097/TA.0b013e318068b1ed. discussion 8. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Keegan MT, Iscimen R, Afessa B, Buck CF, Hubmayr RD, Gajic O. Toward the prevention of acute lung injury: protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35:1660–1666. doi: 10.1097/01.CCM.0000269037.66955.F0. quiz 1667. [DOI] [PubMed] [Google Scholar]
- Zilberberg MD. Transfusion-attributable acute respiratory distress syndrome, hospital utilization and costs in the united states: a model simulation. Transfus Alternatives Transfus Med. 14 Feb 2008; doi: 10.1111/j.1778-428X.2007.00087.x.
- Zilberberg MD, Shorr AF. Effect of a restrictive transfusion strategy on transfusion-attributable severe acute complications and costs in the US ICUs: a model simulation. BMC Health Serv Res. 2007;7:138. doi: 10.1186/1472-6963-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin HL, Parsonnet KC, Gettinger A. RBC transfusion in the ICU. Is there a reason? Chest. 1995;108:767–771. doi: 10.1378/chest.108.3.767. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Duan N. Smearing estimate: a nonparametric retransformation method. J Am Stat Assoc. 1983;78:605–610. doi: 10.2307/2288126. [DOI] [Google Scholar]
- Catlin A, Cowan C, Hartman M, Heffler S, the National Health Expenditure Accounts T National health spending in 2006: a year of change for prescription drugs. Health Aff. 2008;27:14–29. doi: 10.1377/hlthaff.27.1.14. [DOI] [PubMed] [Google Scholar]
- Heffler S, Smith S, Keehan S, Borger C, Clemens MK, Truffer C. U.S. health spending projections for 2004–2014. Health Aff (Millwood) 2005. pp. W5-74–W75-85. [DOI] [PubMed]
- Centers for Medicare & Medicaid Services . Medicare Provider Analysis and Review (MEDPAR) of Short-stay Hospitals. Baltimore, MD: Centers for Medicare & Medicaid; 2004. [Google Scholar]
- Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33:1266–1271. doi: 10.1097/01.CCM.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Earley AS, Gracias VH, Haut E, Sicoutris CP, Wiebe DJ, Reilly PM, Schwab CW. Anemia management program reduces transfusion volumes, incidence of ventilator-associated pneumonia, and cost in trauma patients. J Trauma. 2006;61:1–5. doi: 10.1097/01.ta.0000225925.53583.27. discussion 5–7. [DOI] [PubMed] [Google Scholar]
- Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–572. [PubMed] [Google Scholar]
- Taylor RW, Manganaro L, O'Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249–2254. doi: 10.1097/00003246-200210000-00012. [DOI] [PubMed] [Google Scholar]
- Taylor RW, O'Brien J, Trottier SJ, Manganaro L, Cytron M, Lesko MF, Arnzen K, Cappadoro C, Fu M, Plisco MS, Sadaka FG, Veremakis C. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302–2308. doi: 10.1097/01.CCM.0000234034.51040.7F. quiz 2309. [DOI] [PubMed] [Google Scholar]
- Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–347. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- Watkins TR, Rubenfeld GD, Martin TR, Caldwell E, Nathens AB. Effects of leukoreduced blood transfusion on acute lung injury in trauma patients: a randomized controlled trial [abstract] Proc Am Thoracic Soc. 2006;3:A301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inclusion criteria and associated procedure codes for study patients. Presented are the inclusion criteria and associated procedure codes for study patients.


