Abstract
Physiological mineralization in growth plate cartilage is highly regulated and restricted to terminally differentiated chondrocytes. Because mineralization occurs in the extracellular matrix, we asked whether major extracellular matrix components (collagens) of growth plate cartilage are directly involved in regulating the mineralization process. Our findings show that types II and X collagen interacted with cell surface-expressed annexin V. These interactions led to a stimulation of annexin V-mediated Ca2+ influx resulting in an increased intracellular Ca2+ concentration, [Ca2+]i, and ultimately increased alkaline phosphatase activity and mineralization of growth plate chondrocytes. Consequently, stimulation of these interactions (ascorbate to stimulate collagen synthesis, culturing cells on type II collagen-coated dishes, or overexpression of full-length annexin V) resulted in increase of [Ca2+]i, alkaline phosphatase activity, and mineralization of growth plate chondrocytes, whereas inhibition of these interactions (3,4-dehydro-l-proline to inhibit collagen secretion, K-201, a specific annexin channel blocker, overexpression of N terminus-deleted mutant annexin V that does not bind to type II collagen and shows reduced Ca2+ channel activities) decreased [Ca2+]i, alkaline phosphatase activity, and mineralization. In conclusion, the interactions between collagen and annexin V regulate mineralization of growth plate cartilage. Because annexin V is up-regulated during pathological mineralization events of articular cartilage, it is possible that these interactions also regulate pathological mineralization.
The extracellular matrix not only plays a major role in maintaining the function of a tissue but it also interacts and communicates directly with the cell via cell receptor/matrix interactions. These interactions play crucial roles in cell adhesion, migration, proliferation, and differentiation. Various types of collagens are the main organic components of extracellular matrices of many tissues. For example, in bone the main organic extracellular matrix component is type I collagen, whereas the main collagenous component in growth plate cartilage is type II collagen (1). Type II collagen is highly expressed in the proliferative and prehypertrophic zones of growth plate cartilage. Just before mineralization starts, type II collagen synthesis is reduced and the hypertrophic growth plate chondrocytes produce type X collagen (2, 3).
Several cell surface receptors that mediate cell/matrix interactions have been identified in growth plate chondrocytes. Among these receptors are integrin receptors and non-integrin receptors, like annexin V and CD44. Several integrins have been identified to be expressed by growth plate chondrocytes. Among these integrins are α1, α2, α5, α6, αV, β1, and β5. Most of these integrins are expressed in all growth plate zones, whereas some integrins are restricted to certain zones. For example, β5 integrin is restricted to the hypertrophic zone (1, 4). The main collagen receptors in growth plate cartilage are α1β1 and α2β1 integrins (5). Annexin V was identified as another protein binding to types II and X collagen, and it has been demonstrated that chondrocytes attach to type II collagen using annexin V (6). It was originally isolated from the chondrocyte membrane fractions as a type II collagen-binding protein (7, 8). We and others have shown that annexin V, among other annexins, is highly expressed by hypertrophic and terminally differentiated growth plate chondrocytes (9, 10). Annexins are normally located in the cytoplasm or at the inner plasma membrane surface. However, several annexins have been detected extracellularly or surface-exposed. In addition, several annexins have been shown to act as receptors for a variety of molecules, including extracellular matrix proteins, fetuin A, vitamin D, and other cell surface receptors (11–15). We provided evidence that annexin V mediates Ca2+ influx into growth plate chondrocytes and that this annexin-mediated Ca2+ influx regulates terminal differentiation and mineralization events (16, 17). In addition, Ca2+ influx studies into liposomes have shown that binding of type II or type X collagen to annexin V stimulated its Ca2+ channel activities, resulting in an increased Ca2+ influx into liposomes (18). However, it is not known whether collagen/annexin V interactions occur under physiological conditions and whether these interactions play a role in the differentiation events of growth plate chondrocytes. To determine whether collagen/annexin V interactions occur physiologically, we determined whether annexin V is surface-exposed on growth plate chondrocytes using flow cytometric analysis and whether cell surface-exposed annexin V interacts with type II or X collagen using co-immunoprecipitation analysis. To determine the function of collagen/annexin V interactions in growth plate chondrocytes, we interfered with collagen synthesis and secretion and annexin V expression and function (collagen binding abilities and channel function). We analyzed how these interferences affect cytosolic Ca2+ concentration, [Ca2+]i, as a regulator of mineralization and terminal differentiation events (16), tissue nonspecific alkaline phosphatase (APase)2 activity, an enzyme required for mineralization, and the degree of mineralization of growth plate chondrocytes.
EXPERIMENTAL PROCEDURES
Reagents—The preparation and specificity of rabbit polyclonal antibodies specific for chicken annexin V were described previously (9). Monoclonal antibodies specific for type II and type X collagen were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City) and have been described by Mills and Daniel (19) and Schmid and Linsenmayer (20). Antibodies specific for chicken β1 integrin were purchased from Chemicon International Inc. (Temecula, CA). 3,4-Dehydro-l-proline and ascorbate were purchased from Sigma-Aldrich. Native chicken type II collagen was obtained from Chondrex, Inc. (Redmond, WA). 1,4-Benzothiazepine derivative K-201 is cell-permeable and a specific annexin channel blocker and was obtained from Toshizo Tanaka, Japan Tobacco Inc., Osaka, Japan (21). Phospholipids were obtained from Avanti Polar Lipids (Birmingham, AL). Fura-2 and fura-2AM were obtained from Molecular Probes/Invitrogen.
Chondrocyte Culture—Chondrocytes were isolated from the hypertrophic zone of day 19 embryonic chick tibia growth plate cartilage as described previously (22). Cells were grown in monolayer cultures in Dulbecco's modified Eagle's medium (Invitrogen) containing 5% fetal calf serum (HyClone, Logan, UT), 2 mm l-glutamine (Invitrogen), and 50 units/ml penicillin and streptomycin (Invitrogen) (complete medium) on tissue culture dishes or tissue culture dishes coated with type II collagen. For collagen coating, a 1:100 dilution of chicken type II collagen solution (Chondrex Inc.) was added to each well of 6-well tissue culture plates and incubated at 4 °C. After overnight incubations, excess solution was removed and freshly isolated chondrocytes were plated onto the collagen-coated wells. After cells reached confluency the various treatments were started. Cells were treated with 50 μg/ml ascorbate and 0.5 mm 3,4-dehydro-l-proline or 20 μm K-201 for various time points. The same concentrations of 3,4-dehydro-l-proline and K-201 were used in previous studies to inhibit collagen synthesis in fibroblasts, growth plate chondrocytes, and osteoblasts or annexin V channel activities in growth plate chondrocytes (16, 23–25). These concentrations were shown not to be toxic to growth plate chondrocytes (16, 25). The medium was changed every other day. For annexin overexpression studies, growth plate chondrocytes were incubated after 3 days with high titer retroviral stocks of replication-competent, non-transforming Rous sarcoma virus-based expression vector (RCAS-BP) or RCAS-BP containing full-length annexin V or N terminus-deleted mutant annexin V cDNA in a small volume (5 × 106 colony-forming units/106 cells in less than 1 ml of medium) for 4 h. Thereafter, cells were cultured in complete medium in the presence of 50 μg/ml ascorbate for up to 8 days. The degree of overexpression was detected by immunoblotting using antibodies specific for annexin V or antibodies specific for c-Myc. The construction and production of chicken retrovirus RCAS-BP was performed as described previously (26).
Fluorescence-activated Cell Sorter Analysis—Freshly isolated chondrocytes were cultured for 48 h and then trypsinized. After blocking with 4% heat-inactivated goat serum for 30 min, cells were stained with a rabbit anti-annexin V IgG and mouse anti-β1 integrin for 2 h on ice, followed by incubation with the respective secondary antibodies (Alexa Fluor 633 and Alexa Fluor 488; Molecular Probes/Invitrogen). A fluorescence-activated cell sorter (LSR1; BD Biosciences) was used to quantify bound antibody.
Cytosolic Calcium Concentration [Ca2+]i Measurements—Chondrocytes were trypsinized, and then 2 × 106 cells were incubated with 4 μm fura-2 AM (Molecular Probes/Invitrogen) for 15 min at 37 °C. [Ca2+]i was measured as described previously (27). Briefly, cells were resuspended in a buffer containing 140 mm NaCl, 5 mm KCl, 1 mm CaCl2, 20 mm HEPES, 1 mm NaH2PO4, 5.5 mm glucose, pH 7.4. This suspension was transferred into a cuvette (magnetically stirred and thermostated at 37 °C). Fluorescence was measured in a fluorimeter (Photon Technology; excitation and emission wavelengths were 340 and 505 nm, respectively). The fluorescence maximum (FMax) was determined by addition of ionomycin (2 pmol/liter; Calbiochem), and the fluorescence minimum (FMin) was determined in the presence of 1 mm EGTA/10 mm Tris, pH 7.4. [Ca2+]i was calculated according to the following equation: [Ca2+]i = Kd × [(F – FMin)/(FMax – F)], with Kd = 224 nm (28).
Measurement of APase Activity and Protein Content and Alizarin Red S Staining—APase activity was measured using p-nitrophenyl phosphate (Sigma-Aldrich) as a substrate as described previously (16). Protein content was analyzed by the BCA protein assay from Pierce. To determine the degree of mineralization, chondrocytes were stained with alizarin red S as described previously (16). To quantify the alizarin red S staining, alizarin red S-stained cultures were incubated with 100 mm cetylpyridinium chloride for 1 h to release calcium-bound alizarin red S into solution. The absorbance of the released alizarin red S staining was measured at 570 nm using a spectrophotometer. Data were expressed as units of alizarin red S released/mg of protein in each culture.
Immunoprecipitation—Growth plate chondrocytes were cultured for 48 h in the presence of 50 μg/ml ascorbate. Cells were then extracted with a buffer containing 50 mm Tris/HCl, pH 7.4, 1% Nonidet P-40, 0.1% Triton X-100, 150 mm NaCl, 5 mm EDTA, and proteinase inhibitor mixture. 1 mg of total protein from the cell extract was incubated with 3 μg of monoclonal mouse anti-chicken type II or chicken type X collagen IgG fractions for overnight at 4 °C. After incubation, 60 μl of 50% slurry of protein A beads (Pharmacia) were added. After 1 h of incubation at 4 °C, beads were harvested and washed four times with extraction buffer. 50 μl of SDS loading buffer was added, and the samples were boiled at 95 °C for 5 min. Supernatants were analyzed by SDS-PAGE and immunoblotting.
Measurement of Ca2+ Influx into Fura-2-loaded Liposomes and Annexin V/Collagen Liposome Binding Studies—Large thin-walled liposomes containing phosphatidylserine and phosphatidyl-ethanolamine in a ratio of 9:1 were prepared using a dehydration/hydration method and were loaded with fura-2 as described previously (29). Ca2+ influx into fura-2-loaded liposomes was measured in the absence or presence of recombinant full-length annexin V or N terminus-deleted mutant annexin V (200 nm) as described previously (29). Recombinant full-length and N terminus-deleted mutated annexin V were prepared using the pGEX expression vector (Pharmacia) as described previously (29). To test the binding of type II or type X collagen to liposomes in the absence or presence of full-length annexin V or N terminus-deleted mutant annexin V, 200 nm of these annexins were incubated with liposomes in the presence of 400 μm Ca2+. Liposomes containing annexin or without annexin were then incubated with type II or type X collagen (10 μg) for 1 h at room temperature. Liposomes were quantitatively pelleted by centrifugation at 200,000 × g for 15 min and washed twice, and aliquots of the liposome suspension were dotted onto nitrocellulose membranes and immunostained with antibodies specific for annexin V, type II collagen, or type X collagen as described previously (29). The optical density of the color reaction was determined using a densitometer, and the optical density for staining of liposomes containing full-length annexin V and type II or type X collagen and immunostained with antibodies specific for type II or type X collagen was set as 1.
Cell Viability Assay—Cell viability was determined using the Cell Counting Kit-8 (CCK-8) from Dojindo Molecular Technologies, Inc. (Gaithersburg, MD) following the manufacturer's instructions. Briefly, the CCK-8 solution was added to each culture dish and incubated for 4 h at 37 °C. After incubation the absorption was measured at 450 nm. The absorption value for the untreated cells was set as 100%.
SDS-PAGE and Immunoblotting—To determine the secretion and synthesis of types II and X collagen the cell layer was extracted with 0.1% Triton X-100, 1 m NaCl, and 5 mm EDTA in 50 mm Tris-HCl, pH 7.4, including protease inhibitor mixture from Sigma-Aldrich and 1 mm phenylmethylsulfonyl fluoride. Cell extracts (30 μg of total protein) were subjected to SDS-PAGE and immunoblotted with primary antibodies specific for type II or type X collagen as described previously (16).
Statistical Analysis—Numerical data are presented as means ± S.D. (n ≥ 3), and statistical significance between the groups was identified by using the two-tailed Student t test (p values are reported in the figure legends).
RESULTS
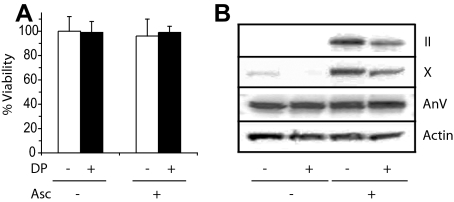
To establish the role of the interactions between collagen and annexin V in terminal differentiation and mineralization events of growth plate chondrocytes, we treated chondrocytes isolated from the hypertrophic zone of day 19 embryonic chick tibia growth plate cartilage with ascorbate in the absence or presence of 3,4-dehydro-l-proline. Ascorbate is required for collagen synthesis, whereas 3,4-dehydro-l-proline reduces the hydroxylation of prolyl residues and the secretion of collagen from the cell (30). We used the same concentration of 3,4-dehydro-l-proline (0.5 mm) as used in previous studies to inhibit collagen synthesis in fibroblasts, sternal growth plate chondrocytes, and osteoblasts (24, 25, 31). Fig. 1A shows that a treatment over 6 days with 3,4-dehydro-l-proline in the absence or presence of ascorbate did not affect cell viability compared with the cell viability of untreated or ascorbate-treated cells. The amounts of type II and X collagen were markedly increased in cell extracts from ascorbate-treated growth plate chondrocytes compared with the amounts of these collagens in extracts from untreated cells (Fig. 1B, II, X). We only determined the amounts of collagens in cell and cell layer extracts and not the collagen amounts released in the medium because only the collagen remaining in the cell layer interacts with cell receptors, including annexin V. The expression of annexin V was not affected by the 72-h ascorbate treatment. 3,4-Dehydro-l-proline treatment did not affect annexin V expression in growth plate chondrocyte cultures in the absence or presence of ascorbate (Fig. 1B, AnV). 3,4-Dehydro-l-proline, however, markedly reduced the amounts of types II and X collagen in total cell extracts from ascorbate-treated growth plate chondrocytes (Fig. 1B, II, X).
FIGURE 1.
A, cell viability of growth plate chondrocytes cultured for 6 days in the absence or presence of 50 μg/ml ascorbate and 0.5 mm 3,4-dehydro-l-proline (white bars: –3,4-dehydro-l-proline; black bars: +3,4-dehydro-l-proline). 0.5 mm 3,4-l-Dehydroproline did not affect cell viability of growth plate chondrocytes cultured in the absence or presence of ascorbate. Data were obtained from three different cultures and expressed as mean ± S.D. B, type II collagen, type X collagen, and annexin V protein synthesis in untreated growth plate chondrocytes or treated with 3,4-dehydro-l-proline (DP), ascorbate (Asc), or 3,4-dehydro-l-proline and ascorbate. Growth plate chondrocytes were cultured in the absence or presence of 50 μg/ml ascorbate and/or 0.5 mm 3,4-dehydro-l-proline for 72 h. Cell and matrix extracts (30 μg of total protein) were analyzed by SDS-PAGE and immunoblotting with antibodies specific for type II collagen, type X collagen, or annexin V. Note the increased levels of type II and type X collagen protein in ascorbate-treated cultures compared with untreated cultures. 3,4-Dehydro-l-proline reduced the levels of type II and type X collagen protein in ascorbate-treated cultures. Annexin V protein levels were not affected by these treatments.
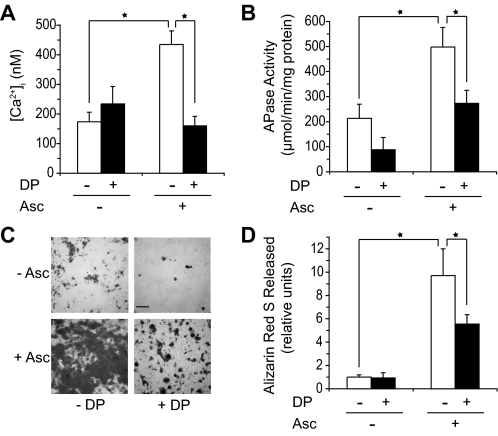
To determine whether types II and X collagen, which bind to annexin V and stimulate its Ca2+ channel activities in an artificial liposome system (18), affect annexin Ca2+ channel activities and ultimately mineralization events in growth plate chondrocytes, we determined first whether the inhibition of collagen secretion by 3,4-dehydro-l-proline in ascorbate-treated growth plate chondrocytes affected their [Ca2+]i and their degree of mineralization. Ascorbate increased [Ca2+]i compared with [Ca2+]i of untreated growth plate chondrocytes (Fig. 2A). 3,4-Dehydro-l-proline (0.5 mm) led to a decrease of [Ca2+]i in ascorbate-treated cultures to levels similar to those of untreated cells (Fig. 2A). 3,4-Dehydro-l-proline led to a slight, but statistically not significant, increase of [Ca2+]i compared with the levels of untreated cells (Fig. 2A). Ascorbate treatment resulted in a marked increase of APase activity and degree of mineralization compared with the levels of APase activity and mineralization in untreated cultures, which showed only very little signs of mineralization (Fig. 2, B–D). APase activity was measured in cell extracts, because most APase activity is retained on either the outer cell surface or in matrix vesicles, which are present in the cell layer (22). APase activity was decreased in ascorbate-treated and untreated growth plate chondrocyte cultures in the presence of 3,4-dehydro-l-proline (Fig. 2B). 3,4-Dehydro-l-proline also resulted in a notable decrease of mineralization of ascorbate-treated growth plate chondrocyte cultures (Fig. 2, C and D). These findings reveal that interfering with collagen secretion by 3,4-dehydro-l-proline affects [Ca2+]i, APase activity, and the degree of mineralization in ascorbate-treated growth plate chondrocytes.
FIGURE 2.
Effect of 3,4-dehydro-l-proline (DP) treatment on [Ca2+]i (A), APase activity (B), and degree of mineralization (C, D). (white bars: –3,4-dehydro-l-proline; black bars: +3,4-dehydro-l-proline). A, [Ca2+]i was measured after 24 h of treatment with ascorbate (Asc) and/or 0.5 mm 3,4-dehydro-l-proline (DP). Ascorbate treatment resulted in ∼3-fold increase of [Ca2+]i compared with [Ca2+]i of untreated cells. 3,4-Dehydro-l-proline resulted in a decrease of [Ca2+]i of ascorbate-treated cultures to levels similar to those of untreated cells. B, APase activity was measured after 6 days of treatment. APase activity was increased in ascorbate-treated cultures compared with APase activity of untreated cells. 3,4-Dehydro-l-proline decreased APase activity of growth plate chondrocytes and growth plate chondrocytes treated with ascorbate. C, alizarin red S staining of growth plate chondrocytes cultured in the absence or presence of ascorbate and 3,4-dehydro-l-proline after 6 days of treatment. Dark areas represent mineralized areas. Bar, 100 μm. D, the density of alizarin red S staining was quantified by determining the release of Ca2+-bound alizarin red S into solution after incubation with 100 mm cethylpyridinium chloride for 1 h. The absorbance of the released alizarin red S staining was measured at 570 nm and normalized to the amount of protein in each culture. Ascorbate treatment markedly increased mineralization of growth plate chondrocytes compared with the degree of untreated cells. 3,4-Dehydro-l-proline notably reduced the degree of mineralization of ascorbate-treated cultures. Data were obtained from three different cultures and expressed as mean ± S.D. *, p ≤ 0.01.
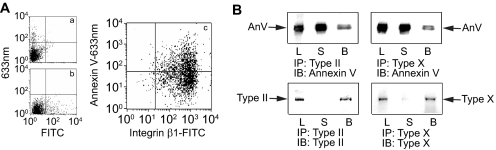
To further determine whether collagen/annexin V interactions mediate these effects, we determined whether annexin V is cell surface-expressed and interacts with types II and X collagen on the cell surface. Annexins are cytosolic proteins, which have no signal peptide for protein secretion. However, several studies have shown the secretion of annexins to the cell surface (14, 32). Flow cytometric analysis of growth plate chondrocytes revealed that the cells express β1 integrin (Fig. 3A, c: lower and upper right quadrants) and annexin V (c: upper left and upper right quadrants) on their surface. Furthermore, we performed co-immunoprecipitation experiments in which total cell extracts from growth plate chondrocytes treated for 48 h with ascorbate were immunoprecipitated with antibodies specific for type II or type X collagen and the immunoprecipitates were immunoblotted with antibodies specific for annexin V. Annexin V co-immunoprecipitated with type II collagen or type X collagen as shown by the immunoblot of type II collagen or type X collagen immunoprecipitates with antibodies specific for annexin V (Fig. 3B). Immunoblotting of the type II collagen immunoprecipitate with antibodies specific for type II collagen revealed a band for type II collagen, whereas immunoblotting of the type X collagen immunoprecipitate with antibodies specific for type X collagen revealed a band for type X collagen (Fig. 3B). These findings reveal that type II and type X collagens interact with cell surface-exposed annexin V.
FIGURE 3.
Flow cytometric analysis of cell surface labeling for annexin V (A) and co-immununoprecipitation of annexin V with antibodies specific for type II or type X collagen (B). A, to determine whether annexin V interacts with type II or type X collagen on the cell surface, we first analyzed cell surface labeling for annexin V by flow cytometry using annexin V-specific primary antibodies followed by Alexa 633-nm-conjugated secondary antibodies. More than 40% of the cells were cell surface-labeled with antibodies specific for annexin V (c, upper right and left quadrants). Cell surfaces were also labeled with antibodies specific for β1 integrin followed by fluorescein isothiocyanate (FITC)-labeled secondary antibodies (c, lower and upper right quadrants). a, control labeled with only the secondary Alexa 633-nm-conjugated antibodies; b, control labeled with only the secondary FITC-conjugated antibodies. B, immunoprecipitation of total cell extracts was performed with antibodies specific for type II or type X collagen. The immunoprecipitates (IP) were then analyzed by immunoblotting (IB) with antibodies specific for annexin V (AnV), antibodies specific for type II collagen (Type II), or antibodies specific for type X collagen (Type X). L, total cell lysate; S, supernatant after immunoprecipitation; B, beads after immunoprecipitation. Note that annexin V co-immunoprecipitated with antibodies specific for type II or type X collagen.
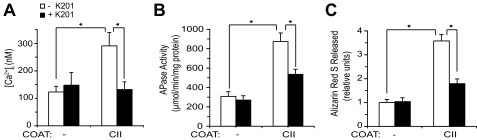
To further establish the role of collagen/annexin V interactions in growth plate chondrocyte mineralization, we cultured growth plate chondrocytes on tissue culture dishes or type II collagen-coated tissue culture dishes in the absence or presence of K-201, a specific annexin V Ca2+ channel blocker (33). K-201 was used in a concentration of 20 μm, which has been previously shown to be an effective concentration to inhibit annexin VCa2+ channel activities without affecting cell viability (16). Cells cultured on type II collagen-coated dishes showed an increase of [Ca2+]i compared with [Ca2+]i of growth plate chondrocytes cultured on tissue culture dishes (Fig. 4A). APase activity and the degree of mineralization were also stimulated by culturing growth plate chondrocytes on type II collagen-coated dishes compared with the APase activity and degree of mineralization of cells cultured on tissue culture dishes (Fig. 4, B and C). The increase of [Ca2+]i, APase activity, and the degree of mineralization of growth plate chondrocytes cultured on type II collagen-coated dishes was inhibited by K-201 (Fig. 4, A–C). K-201 treatment had no effect on [Ca2+]i, APase activity, and mineralization of growth plate chondrocytes cultured on tissue culture dishes (Fig. 4, A–C).
FIGURE 4.
Effect of type II (CII) collagen on [Ca2+]i (A), APase activity (B), and degree of mineralization (C) of growth plate chondrocytes in the absence or presence of K-201, a specific annexin V Ca2+ channel blocker. A, growth plate chondrocytes were cultured on tissue culture dishes (-) or type II collagen-coated tissue culture dishes (CII) in the absence (white bars) or presence (black bars) of K-201, the specific annexin V Ca2+ channel blocker. [Ca2+]i was measured by fura-2AM loading. B, APase activity was measured after cells reached confluency and were cultured for an additional 6 days in complete medium in the absence (white bars) or presence (black bars) of K-201 on tissue culture dishes or type II collagen-coated tissue culture dishes. C, alizarin red S staining was performed at the same time point as APase activity measurement. Quantification of alizarin red S staining was performed as described in Fig. 2. Note that growth plate chondrocytes cultured on type II collagen-coated tissue culture dishes showed increased [Ca2+]i, APase activity, and degree of mineralization compared with the levels of cells cultured on uncoated tissue culture dishes. The increases of [Ca2+]i, APase activity, and degree of mineralization of cells cultured on type II collagen-coated dishes were inhibited by K-201. Data were obtained from three different cultures and expressed as mean ± S.D. *, p ≤ 0.01.
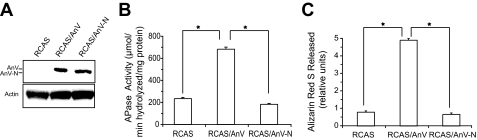
In the final set of experiments we determined whether a N terminus-deleted mutant annexin V, which has been previously shown to have reduced Ca2+ channel activities compared with full-length annexin V (34), had an effect on APase activity and the degree of mineralization of ascorbate-treated growth plate chondrocytes cultures. We prepared full-length annexin V (amino acids 1–321) and N terminus-deleted mutant annexin V (amino acids 13–321) using the pGEX expression vector as described previously (29). We determined whether full-length or N terminus-deleted mutant annexin V mediates Ca2+ influx into fura-2-loaded phosphatidylserine-enriched liposomes. The N terminus-deleted mutant annexin V mediated Ca2+ influx into liposomes to a lesser degree than full-length annexin V (Fig. 5A). Next we tested whether binding of type II or X collagen to liposomes is affected by the N-terminal domain of annexin V. Liposomes were incubated with type II or X collagen in the absence or presence of full-length annexin V or N terminus-deleted mutant annexin V, and liposome-bound type II or X collagen was detected with antibodies specific for type II or type X collagen. In the absence of annexin V, native type II or type X collagen did not bind to phosphatidylserine-enriched liposomes (Fig. 5B). In the presence of full-length annexin V, types II and X collagen bound to the liposomes (Fig. 5B). In the presence of N terminus-deleted mutant annexin V type II collagen did not bind to liposomes, whereas type X collagen bound to the liposomes, suggesting that types II and X collagen use different binding sites in the annexin V protein and that type II collagen binds to the 12-amino acid-long N terminus (Fig. 5B). Finally, we overexpressed full-length annexin V and N terminus-deleted mutant annexin V in growth plate chondrocytes using a retroviral expression vector (RCAS-BP). After infection with viral particles, growth plate chondrocytes were treated with ascorbate to ensure proper collagen synthesis and cultured for up to 8 days. We obtained a 2- to 3-fold increase of annexin V protein expression in cells infected with RCAS-BP containing full-length annexin V or N terminus-deleted mutated annexin V cDNA (data not shown; see also Ref. 17). The virally expressed full-length annexin V or N terminus-deleted annexin V contained a c-Myc tag. Immunoblotting with antibodies specific for c-Myc revealed that the full-length annexin V and the N terminus-deleted mutant annexin V were expressed in equal amounts (Fig. 6A). Infection of growth plate chondrocytes with RCAS-BP containing full-length annexin V cDNA (RCAS/AnV) resulted in an increase of APase activity (Fig. 6B) and degree of mineralization (Fig. 6C) compared with the levels of APase activity and mineralization of growth plate chondrocytes infected with empty RCAS-BP vector (Fig. 6, B and C, RCAS). Infection of growth plate chondrocytes with RCAS-BP containing N terminus-deleted mutant annexin V (RCAS/AnV-N) resulted in decreased APase activity and degree of mineralization compared with the levels of APase activity and mineralization of growth plate chondrocytes infected with RCAS-BP vector containing full-length annexin V (Fig. 6, B and C, RCAS/AnV). The levels of APase activity and degree of mineralization in RCAS-BP vector containing N terminus-deleted mutant annexin V were similar to the levels in RCAS-BP vector-infected cells (Fig. 6, B and C).
FIGURE 5.
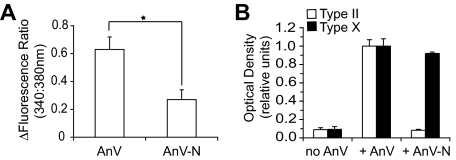
Ca2+ influx into fura-2-loaded liposomes in the presence of recombinant full-length annexin V or recombinant N terminus-deleted mutant annexin V (A) and type II or type X collagen binding to liposomes in the absence or presence of full-length annexin V or N terminus-deleted mutated annexin V (B). A, Ca2+ influx in the presence of full-length annexin V or N terminus-deleted mutated annexin V was measured by the difference in the fluorescence ratio of 340:380 nm (Ca2+-bound to Ca2+-unbound fura-2) before and after adding 200 nm annexin V to a liposome suspension and incubation with annexin V for 30 min. Note that full-length annexin V is more effective in mediating Ca2+ influx into liposomes than N terminus-deleted mutated annexin V. B, liposomes were incubated with native type II or type X collagen (no AnV) or they were first incubated with full-length annexin V (AnV) or N terminus-deleted mutant annexin V (AnV-N) followed by incubation with native type II or type X collagen. Aliquots of the liposome fractions were dotted onto nitrocellulose membranes and immunostained with antibodies specific for type II or type X collagen. The intensities of the dots were analyzed by a densitometer. The optical density obtained for staining of full-length annexin V containing liposomes incubated with type II or type X collagen followed by incubation with type II or type X collagen-specific antibodies was set as 1. Data were obtained from three different experiments and expressed as mean ± S.D. *, p ≤ 0.01.
FIGURE 6.
A, overexpression of full-length annexin V or N terminus-deleted mutant annexin V using a retroviral expression vector (RCAS-BP). The retrovirally expressed proteins were tagged with c-Myc. Immunoblotting with antibodies specific for c-Myc revealed that the retrovirally expressed full-length annexin V (RCAS/AnV) and the N terminus-deleted mutant annexin V (RCAS/AnV-N) were equally expressed in growth plate chondrocytes. Infection with RCAS-BP vector alone (RCAS) revealed no immunostaining with the c-Myc-specific antibodies. The membrane was also stained with antibodies specific for β-actin (Actin) to demonstrate equal loading of the lanes. B and C, APase activity (B) and degree of mineralization (C) of growth plate chondrocytes overexpressing full-length annexin V or N terminus-deleted mutant annexin V. Growth plate chondrocytes were infected with RCAS-BP viral particles (RCAS) or RCAS-BP viral particles containing full-length annexin V (RCAS/AnV) or N terminus-deleted mutant annexin V cDNA (RCAS/AnV-N). After ∼90% of growth plate chondrocytes were infected, cells were treated with ascorbate for 8 days. APase activity (B) and the degree of mineralization (C) were increased in ascorbate-treated cultures overexpressing full-length annexin V (RCAS/AnV) compared with ascorbate-treated cultures infected with RCAS-BP alone (RCAS). Overexpression of N terminus-deleted annexin V(RCAS/AnV-N) resulted in decreased APase activity and degree of mineralization compared with the levels of RCAS/AnV-infected cells. Data were obtained from three different experiments and expressed as mean ± S.D. *, p ≤ 0.01).
DISCUSSION
Mineralization of tissues has to be highly regulated and restricted to certain sites. Many factors, including growth factors and vitamins, have been implicated in the regulation of mineralization. Very little, however, is known about the mechanisms and role of the extracellular matrix and its interactions with cell receptors in the regulation of mineralization events of growth plate chondrocytes. In this study we provide evidence that types II and X collagen via interactions with annexin V regulate mineralization of growth plate chondrocytes. The interactions between annexin V and types II and/or X collagen increased [Ca2+]i, APase activity, and the degree of mineralization of growth plate chondrocytes. Our previous studies have shown that annexin V mediates Ca2+ influx into growth plate chondrocytes and that this annexin-mediated increase of [Ca2+]i results in stimulation of terminal differentiation and mineralization events (16, 17, 26). Therefore, the findings of the present study show a novel mechanism of how growth plate chondrocytes terminally differentiate and mineralize their extracellular matrix. Binding of type II collagen and/or type X collagen to annexin V stimulates its Ca2+ channel activities, leading to an influx of extracellular Ca2+ ions into growth plate chondrocytes. This influx results in constitutively elevated [Ca2+]i levels, which then initiate a terminal differentiation process that leads to increased APase activity and eventual mineralization of the extracellular matrix. Consequently, interfering with collagen secretion or with annexin V channel activities and binding to type II collagen resulted in decreased [Ca2+]i, APase activity, and mineralization. On the other hand, stimulation of collagen and annexin V synthesis and secretion, culturing growth plate chondrocytes on collagen-coated tissue culture dishes, increased [Ca2+]i, APase activity, and mineralization of growth plate chondrocytes.
The increase of [Ca2+]i mediated through annexin V results in a constitutive increase of [Ca2+]i in growth plate chondrocytes. These findings confirm previous findings showing a constitutive increase of [Ca2+]i during hypertrophic and terminal differentiation of growth plate chondrocytes (27, 35, 36). In addition, previous studies have shown that this constitutive increase of [Ca2+]i is involved in the regulation of the entire terminal differentiation program (26, 36). Most of this increase of [Ca2+]i during terminal differentiation is mediated by annexins and results in the regulation of expression of terminal differentiation marker genes, APase activity, mineralization, and ultimately apoptosis (16, 17, 26). Using 1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′,-tetraacetic acid (BAPTA-AM), an intracellular Ca2+ chelator, K-201, the annexin-specific Ca2+ channel blocker, or annexin V-specific small interference RNA to suppress annexin V expression, these terminal differentiation events were inhibited (16, 17, 26). In this study we show that type II and/or type X collagen bind to cell surface-exposed annexin V, resulting in an increase of annexin V-mediated Ca2+ influx into growth plate chondrocytes and ultimately stimulation of mineralization. Using K-201, which has been previously shown to inhibit annexin V-mediated Ca2+ influx into liposomes, matrix vesicles, and growth plate chondrocytes (18, 26), the stimulatory effects of type II collagen on [Ca2+]i and mineralization were inhibited, supporting our hypothesis that collagen regulates terminal differentiation events via binding to annexin V and stimulating its Ca2+ channel activities. This hypothesis is further supported by previous findings showing that interactions of type II or type X collagen with annexin V resulted in an increased influx of Ca2+ into liposomes (18).
Annexin V consists of a small N-terminal domain and a core domain. Based on our findings reported in this study and a previous study, the N-terminal domain is required for optimal Ca2+ channel formation and/or activity (34). The N terminus-deleted annexin V is less effective in mediating Ca2+ influx. Interestingly, the N terminus-deleted mutant annexin V also lost its ability to bind to type II collagen. These findings suggest that the N-terminal domain may play an important role in the regulation of the opening of the channel and the lack of the N-terminal domain results in reduced annexin V-mediated Ca2+ flux. Binding of type II collagen to the N-terminal domain may further open the channel, thereby stimulating its activities. Consequently, overexpression of full-length annexin V in growth plate chondrocytes further increased APase activity and mineralization in ascorbate-treated growth plate chondrocyte cultures, whereas overexpression of the N terminus-deleted mutant annexin V did not increase APase activity and mineralization in ascorbate-treated cultures. These findings suggest that overexpression of full-length annexin V resulted in an increased number of annexin V molecules that form Ca2+ channels and are cell surface-exposed and interact with collagen, whereas overexpression of the N terminus-deleted mutant annexin V does not add additional functional annexin V to the endogenous annexin V pool present in ascorbate-treated growth plate chondrocytes and therefore does not affect APase activity and mineralization in these cells. Currently, the binding region in annexin V for type X collagen is not known. Therefore, it is not clear how type X collagen stimulates annexin V channel activities. It also has to be determined whether both collagens can bind simultaneously to annexin V and/or synergistically activate its channel activities.
Ascorbate is required as a cofactor for prolyl-4-hydroxylase, an enzyme that hydroxylates proline. In the absence of ascorbate the collagen α chains are not being hydroxylated efficiently. Underhydroxylated collagen α chains cannot form triple helices, resulting in impaired collagen secretion. Therefore, connective tissue cells treated in the presence of ascorbate produce more matrix than cells cultured in the absence of ascorbate (37–39). In addition, ascorbate has been shown to stimulate proliferation and APase expression and activity in osteoblastic cells and growth plate chondrocytes (23, 40, 41). It was suggested that the effect of ascorbate on cell proliferation and APase expression resulted from altered matrix protein secretion and consequently altered cell/matrix interactions (23). Our results confirm this suggestion and present a novel mechanism of how collagen regulates the mineralization process of growth plate chondrocytes. Increased types II and X collagen secretion in the presence of ascorbate results in increased interactions with annexin V, stimulation of annexin V-mediated Ca2+ influx, and, ultimately as a consequence of increased [Ca2+]i, stimulation of APase expression and activity and mineralization. Exposure of growth plate chondrocytes to ascorbate and a collagen secretion inhibitor (3.4-dehydro-l-proline) not only resulted in an inhibition of collagen synthesis but also reversed ascorbate-stimulated APase activity and mineralization. In our experiments, 0.5 mm 3,4-dehydro-l-proline reduced collagen synthesis in ascorbate-treated cultures by ∼50–60%. This reduction in collagen synthesis was sufficient to inhibit the increase of [Ca2+]i to levels similar to the levels of untreated cells. However, APase activity and the degree of mineralization were not reduced to the levels of untreated cells. As shown in a previous study, freshly isolated articular chondrocytes attached to type II collagen using annexin V and β1 integrin (6). Therefore, it is possible that the reduction of collagen synthesis by 3,4-dehydro-l-proline was sufficient to completely inhibit the collagen/annexin V interactions but not APase activity and the degree of mineralization because of possible remaining interactions of collagen with other cell surface receptors, including integrins, that may affect cell differentiation and mineralization. Alternatively, ascorbate may act on cells by not only affecting collagen synthesis but also other cellular functions independently of the stimulation of collagen synthesis. For example, a previous study has shown that treatment of cephalic sternal chondrocytes from day 14 embryonic chickens with ascorbate resulted in the up-regulation of APase and type X collagen expression even upon the addition of inhibitors of collagen secretion (24).
Interestingly, annexin V is also expressed in human osteoarthritic cartilage, but not in healthy human articular cartilage (9, 10). Furthermore, type X collagen expression was also detected in human osteoarthritic cartilage, suggesting hypertrophic and terminal differentiation events occur in articular cartilage during osteoarthritis (9). In addition, collagen fragments are generated in the growth plate during terminal differentiation and mineralization and in osteoarthritic cartilage. Most of these fragments are generated by cleavage of matrix metalloproteinases. Interestingly, some of the matrix metalloproteinase cleavages release the telopeptide regions of type II collagen, which have been shown to bind to annexin V. In addition, it has been shown that these cleavage products are often found in the pericellular matrix close to the chondrocytes where they can interact with cell surface receptors (42, 43). Chondrocytes in osteoarthritic cartilage also show increased synthesis of type II collagen in an attempt to repair the extracellular matrix (44). Therefore, it is plausible to speculate that plenty of type II collagen fragments and/or newly produced type II collagen are available in hypertrophic growth plate cartilage and osteoarthritic cartilage to interact with annexin V, resulting in increase of [Ca2+]i and ultimately terminal differentiation and mineralization events. Although these terminal differentiation and mineralization events are required for endochondral bone formation, they will lead to cartilage destruction when happening in articular cartilage.
In conclusion, our study demonstrates that type II and type X collagen/annexin V interactions play a regulatory role in terminal differentiation and mineralization events of growth plate chondrocytes. Both collagens bind to cell surface annexin V and stimulate annexin V-mediated Ca2+ influx into growth plate chondrocytes. The accelerated increase of [Ca2+]i via collagen/annexin V interactions is a key regulator for the initiation of the mineralization events in the growth plate. Interestingly, type II and type X collagen protein concentrations are the highest in the growth plate just before initiation of mineralization, suggesting that at that time plenty of types II and X collagen are available to interact with annexin V and to regulate the initiation of mineralization (45). The accelerated increase of [Ca2+]i mediated by collagen/annexin V interactions may play a key regulatory role not only in the initiation of mineralization events in growth plate cartilage but also in pathological terminal differentiation and mineralization events of osteoarthritic cartilage, where these interactions may also occur. Future experiments using surface-exposed annexin V and annexin V non-surface-exposed chondrocytes have to be performed to determine the effect of collagen/annexin V interactions during cartilage pathology.
Acknowledgments
We thank R. Holmdahl, K. Rubin, J. A. Katzman, and T. F. Linsenmayer for developing the monoclonal antibodies specific for chicken type II and type X collagen, which were obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Biological Sciences, University of Iowa, Iowa City. The Developmental Studies Hybridoma Bank is maintained under contract N01-HD-7-3263 from NICHD, National Institutes of Health.
This work was supported by NIAMS/National Institutes of Health Grants R01AR046245 and R01AR049074 (to T. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviation used is: APase, alkaline phosphatase.
References
- 1.Hausler, G., Helmreich, M., Marlovits, S., and Egerbacher, M. (2002) Calcif. Tiss. Inter. 71 212–218 [DOI] [PubMed] [Google Scholar]
- 2.Mwale, F., Tchetina, E., Wu, C. W., and Poole, A. R. (2002) J. Bone Miner. Res. 17 275–283 [DOI] [PubMed] [Google Scholar]
- 3.Kirsch, T., and von der Mark, K. (1992) Bone Miner. 18 107–117 [DOI] [PubMed] [Google Scholar]
- 4.Wang, W., and Kirsch, T. (2006) J. Biol. Chem. 281 30848–30856 [DOI] [PubMed] [Google Scholar]
- 5.Durr, J., Goodman, S., Potocnik, A., von der Mark, H., and von der Mark, K. (1993) Exp. Cell Res. 207 235–244 [DOI] [PubMed] [Google Scholar]
- 6.Reid, D. L., Aydelotte, M. B., and Mollenhauer, J. (2000) J. Orthop. Res. 18 364–373 [DOI] [PubMed] [Google Scholar]
- 7.Mollenhauer, J., Bee, J. A., Lizarbe, M. A., and von der Mark, K. (1984) J. Cell Biol. 98 1572–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirsch, T., and Pfaeffle, M. (1992) FEBS Lett. 310 143–147 [DOI] [PubMed] [Google Scholar]
- 9.Kirsch, T., Swoboda, B., and Nah, H.-D. (2000) Osteoarthritis Cartilage 8 294–302 [DOI] [PubMed] [Google Scholar]
- 10.Mollenhauer, J., Mok, M. T., King, K. B., Gupta, M., Chubinskaya, S., Koepp, H., and Cole, A. (1999) J. Histochem. Cytochem. 47 209–220 [DOI] [PubMed] [Google Scholar]
- 11.Chung, C. Y., and Erickson, H. P. (1994) J. Cell Biol. 126 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, N. X., O'Neill, K. D., Chen, X., Duan, D., Wang, E., Sturek, M. S., Edwards, J. M., and Moe, S. M. (2007) Amer. J. Physiol. 292 F599–F606 [DOI] [PubMed] [Google Scholar]
- 13.Baran, D. T., Quail, J. M., Ray, R., Leszyk, J., and Honeyman, T. (2000) J. Cell Biochem. 78 34–46 [PubMed] [Google Scholar]
- 14.Siever, D. A., and Erickson, H. P. (1997) Int. J. Biochem. Cell Biol. 29 1219–1223 [DOI] [PubMed] [Google Scholar]
- 15.Takagi, H., Asano, Y., Yamakawa, N., Matsumoto, I., and Kimata, K. (2002) J. Cell Sci. 115 3309–3318 [DOI] [PubMed] [Google Scholar]
- 16.Wang, W., and Kirsch, T. (2002) J. Cell Biol. 157 1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, W., Xu, J., and Kirsch, T. (2005) Exp. Cell Res. 305 156–165 [DOI] [PubMed] [Google Scholar]
- 18.Kirsch, T., Harrison, G., Golub, E. E., and Nah, H.-D. (2000) J. Biol. Chem. 275 35577–35583 [DOI] [PubMed] [Google Scholar]
- 19.Mills, D. K., and Daniel, J. C. (1993) Connect. Tissue Res. 30 37–57 [DOI] [PubMed] [Google Scholar]
- 20.Schmid, T. M., and Linsenmayer, T. F. (1985) J. Cell Biol. 100 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko, N., Ago, H., Matsuda, R., Inagaki, E., and Miyano, M. (1997) J. Mol. Biol. 274 16–20 [DOI] [PubMed] [Google Scholar]
- 22.Kirsch, T., Nah, H. D., Shapiro, I. M., and Pacifici, M. (1997) J. Cell Biol. 137 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschi, R. T., Iyer, B. S., and Cui, Y. (1994) J. Bone Miner. Res. 9 843–854 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan, T. A., Uschmann, B., Hough, R., and Leboy, P. S. (1994) J. Biol. Chem. 269 22500–22506 [PubMed] [Google Scholar]
- 25.Abe, T., Abe, Y., Aida, Y., Hara, Y., and Maeda, K. (2001) J. Cell Physiol. 189 144–151 [DOI] [PubMed] [Google Scholar]
- 26.Wang, W., Xu, J., and Kirsch, T. (2003) J. Biol. Chem. 278 3762–3769 [DOI] [PubMed] [Google Scholar]
- 27.Kirsch, T., Swoboda, B., and von der Mark, K. (1992) Differentiation 52 89–100 [DOI] [PubMed] [Google Scholar]
- 28.Grynkiewicz, G., Poenie, M., and Tsien, R. Y. (1985) J. Biol. Chem. 260 3440–3450 [PubMed] [Google Scholar]
- 29.Kirsch, T., Nah, H.-D., Demuth, D. R., Harrison, G., Golub, E. E., Adams, S. L., and Pacifici, M. (1997) Biochemistry 36 3359–3367 [DOI] [PubMed] [Google Scholar]
- 30.Kerwar, S. S., and Felix, A. M. (1976) J. Biol. Chem. 251 503–509 [PubMed] [Google Scholar]
- 31.Xiao, G., Cui, Y., Ducy, P., Karsenty, G., and Franceschi, R. T. (1997) Mol. Endocrinol. 11 1103–1113 [DOI] [PubMed] [Google Scholar]
- 32.Castro-Caldas, M., Duarte, C. B., Carvalho, A. P., and Lopes, M. C. (2002) Mol. Cell. Biochem. 237 31–38 [DOI] [PubMed] [Google Scholar]
- 33.Kaneko, N., Matsuda, R., Toda, M., and Shimamoto, K. (1997) Biochim. Biophys. Acta 1330 1–7 [DOI] [PubMed] [Google Scholar]
- 34.Berendes, R., Burger, A., Voges, D., Demange, P., and Huber, R. (1993) FEBS Lett. 317 131–134 [DOI] [PubMed] [Google Scholar]
- 35.Iannotti, J. P., and Brighton, C. T. (1989) J. Orthop. Res. 7 511–518 [DOI] [PubMed] [Google Scholar]
- 36.Zuscik, M. J., D'Souza, M., Ionescu, A. M., Gunter, K. K., Gunter, T. E., O'Keefe, R. J., Schwarz, E. M., Puzas, J. E., and Rosier, R. N. (2002) Exp. Cell Res. 276 310–319 [DOI] [PubMed] [Google Scholar]
- 37.Hajek, A. S., and Solursh, M. (1977) J. Exp. Zool. 200 377–388 [DOI] [PubMed] [Google Scholar]
- 38.Meier, S., and Solursh, M. (1978) J. Ultrastruc. Res. 65 48–59 [DOI] [PubMed] [Google Scholar]
- 39.Kivirikko, K. I., and Myllyla, R. (1987) Methods Enzymol. 144 96–114 [DOI] [PubMed] [Google Scholar]
- 40.Harada, S., Matsumoto, T., and Ogata, E. (1991) J. Bone Miner. Res. 6 903–908 [DOI] [PubMed] [Google Scholar]
- 41.Leboy, P. S., Vaias, L., Uschmann, B., Golub, E., Adams, S. L., and Pacifici, M. (1989) J. Biol. Chem. 264 17281–17286 [PubMed] [Google Scholar]
- 42.Lucic, D., Mollenhauer, J., Kilpatrick, K. E., and Cole, A. A. (2003) Connect. Tissue Res. 44 225–239 [PubMed] [Google Scholar]
- 43.Aurich, M., Poole, A. R., Reiner, A., Mollenhauer, C., Margulis, A., Kuettner, K. E., and Cole, A. A. (2002) Arthritis Rheum. 46 2903–2910 [DOI] [PubMed] [Google Scholar]
- 44.Aigner, T., Bertling, W., Stoess, H., Weseloh, G., and von der Mark, K. (1993) J. Clin. Investig. 91 829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alini, M., Matsui, Y., Dodge, G. R., and Poole, A. R. (1992) Calcif. Tissue Int. 50 327–335 [DOI] [PubMed] [Google Scholar]