Abstract
In Alzheimer disease (AD) brain, the level of I PP2A1, a 249-amino acid long endogenous inhibitor of protein phosphatase 2A (PP2A), is increased, the activity of the phosphatase is decreased, and the microtubule-associated protein Tau is abnormally hyperphosphorylated. However, little is known about the detailed regulatory mechanism by which PP2A activity is inhibited by I PP2A1 and the consequent events in mammalian cells. In this study, we found that both I PP2A1 and its N-terminal half I PP2A(1–120)1, but neither I PP2A(1–163)1 nor I PP2A(164–249)1, inhibited PP2A activity in vitro, suggesting an autoinhibition by amino acid residues 121–163 and its neutralization by the C-terminal region. Furthermore, transfection of NIH3T3 cells produced a dose-dependent inhibition of PP2A activity by I PP2A1. I PP2A1 and PP2A were found to colocalize in PC12 cells. I PP2A1 could only interact with the catalytic subunit of PP2A (PP2Ac) and had no interaction with the regulatory subunits of PP2A (PP2A-A or PP2A-B) using a glutathione S-transferase-pulldown assay. The interaction was further confirmed by coimmunoprecipitation of I PP2A1 and PP2Ac from lysates of transiently transfected NIH3T3 cells. The N-terminal isotype specific region of I PP2A1 was required for its association with PP2Ac as well as PP2A inhibition. In addition, the phosphorylation of Tau was significantly increased in PC12/Tau441 cells transiently transfected with full-length I PP2A1 and with PP2Ac-interacting I PP2A1 deletion mutant 1–120 (I PP2A1ΔC2). Double immunofluorescence staining showed that I PP2A1 and I PP2A1ΔC2 increased Tau phosphorylation and impaired the microtubule network and neurite outgrowth in PC12 cells treated with nerve growth factor.
Reversible protein phosphorylation is an essential regulatory mechanism in many cellular processes. Although in the past much attention has been paid to the regulation of protein kinases, it is now apparent that protein phosphatases like the kinases are highly regulated enzymes that play an equally important role in the control of protein phosphorylation. Four main classes, protein phosphatase (PP)2 1, PP2A, PP2B, and PP2C, comprise the majority of cellular serine/threonine phosphatase activity (1). Among them, PP2A has been most implicated in the etiopathogenesis of Alzheimer disease (AD) (2–5).
In AD brain, the axonal microtubule associated protein (MAP) Tau is heavily phosphorylated due in part to decreased Tau phosphatase activity compared with the control brain (6). PP2A, which accounts for ∼70% of the total Tau phosphatase activity in human brain (5), regulates the phosphorylation of Tau (7–10). A decrease in the activity of the enzyme leads to hyperphosphorylation of Tau which, in turn, suppresses its microtubule binding and assembly activities in adult mammalian brain. Inhibition of PP2A also indirectly promotes the activities of several Tau kinases in brain, such as calmodulin kinase II, protein kinase A, MAP kinase kinase (MEK1/2), extracellular regulated kinase (ERK1/2), and P70S6 kinase (7, 11–13). Thus, the abnormal hyperphosphorylation of Tau that results from the inhibition of PP2A activity is probably due to not only a direct decrease in the dephosphorylation by PP2A but also an increase in the phosphorylation of Tau by Tau protein kinases that are regulated by PP2A. However, the mechanisms participating in the regulation of PP2A activity in AD brain are far from understood.
PP2A minimally contains a well conserved catalytic subunit, the activity of which is highly regulated. Regulation is accomplished mainly by members of a family of regulatory subunits, which determine the substrate specificity, subcellular localization, and catalytic activity of the PP2A holoenzymes (1, 14). Li et al. (15) reported the purification of a ∼30-kDa heat-stable protein, I PP2A1, that specifically inhibits PP2A from bovine kidney. Molecular cloning of I PP2A1 revealed it to be a 249-amino acid full-length protein from bovine kidney (16) and human brain (17), which was previously described as the human PHAPI (putative histocompatibility leukocyte antigen class II associated protein-I), mapmodulin, pp32, and LANP (18–23). I PP2A1 inhibits purified preparations of trimeric PP2A, dimeric PP2A, and isolated catalytic C subunit of the phosphatase in a non-competitive manner (15, 17), and PP2Ac has been found in the immunoprecipitates of PHAPI/pp32 from human colon cancer cells (24). I PP2A1 is involved in important physiological events, which include cell proliferation, apoptosis, mRNA transport, and transcription (25). Subcellular localization studies have shown that I PP2A1 is a cytoplasmic/nuclear protein depending on cell type (17, 19). Our previous study showed a significant increase in the neocortical level of I PP2A1 in AD as compared with control cases by in situ hybridization and immunohistochemical colocalization of this inhibitor with PP2A and with abnormally hyperphosphorylated Tau in the neuronal cytoplasm (26). However, the nature of the interaction of I PP2A1 with PP2A and its role in the abnormal hyperphosphorylation of Tau were not understood.
The present study shows that the PP2A activity is dose-dependently inhibited by I PP2A1 and that this decrease is not due to reduced PP2A expression level in NIH3T3 cells. Furthermore, GST-pulldown assay and coimmunoprecipitation from I PP2A1 transiently transfected NIH3T3 cells show an interaction between I PP2A1 and PP2A catalytic subunit, and no interaction between I PP2A1 and PP2A A or B regulatory subunits. The minimal region required for the association with PP2Ac as well as PP2A inhibition is localized at the N-terminal isotype-specific containing region of I PP2A1. In addition, transfection of Tau stably transfected PC12 cells with I PP2A1 increases the phosphorylation of Tau at M4 (Thr-231/Ser-235), 12E8 (Ser-262/356), and Ser-404 sites, and microtubule network and neurite outgrowth are impaired.
EXPERIMENTAL PROCEDURES
Cloning and Generation of Plasmids—The full-length cDNA and deletion mutants of I PP2A1 were designated as I PP2A1FL (aa 1–249), I PP2A1ΔC1 (aa 1–163), I PP2A1ΔC2 (aa 1–120), I PP2A1ΔC3 (aa 1-90), I PP2A1ΔC4 (aa 1–45), I PP2A1F2 (aa 46–90), I PP2A1F2–4 (aa 46–163), and I PP2A1F5 (aa 164–249) and obtained by PCR using pEGFP-N3/I PP2A1 (wt) as a template (17). I PP2A1FL was generated from primer 1 (5′-GATGGATCCATGGAGATGGGCAGA) and primer 2 (5′-GATCTCGAGTTAGTCATCATCTTC), I PP2A1ΔC1 from primer 1 and primer 3 (5′-GATCTCGAGTTAGTAGCCCTCAGC), I PP21ΔC2 from primer 1 and primer 4 (5′-GATCTCGAGTTAAAGGTCTAAGCT), I PP2A1ΔC3 from primer 1 and primer 5 (5′-GATCTCGAGTTAGAGGTTCGGACA), I PP2A1ΔC4 from primer 1 and primer 6 (5′-GATCTCGAGTTATTCCAGTTCTTC), I PP2A1F2 from primer 7 (5′-GATGGATCCATGTTCTTAAGTACA) and primer 5, I PP2A1F2–4 from primer 7 and primer 3, I PP2A1F5 from primer 8 (5′-GATGGATCCATGGTGGAGGGCCTG), and primer 2 by PCR separately. The BamHI site underlined in the forward primer and the XhoI site underlined in the reverse primer were used to clone these fragments into pGEX6P-1 vector (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and pCMV2B vector (Stratagene, La Jolla, CA). For cloning into C-terminal Myc tagged pcDNA3.1 vector (Invitrogen, Carlsbad, CA), the stop codon was removed. PP2A-PR65α was cloned into pGEX6P-1 vector by PCR using PR65α cDNA (a kind gift from Dr. Brian A. Hemmings) as a template and using primer 9 (5′-GATGGATCCATGGCGGCGGCCGAC, BamHI site underlined) and primer 10 (5′-GATCTCGAGTCAGGCGAGAGACAG, XhoI site underlined). The amplified cDNAs including the mutations were subcloned into the corresponding vectors and were verified by DNA sequencing.
Rat Brain Tissue Extract Preparation and GST Pulldown Assay—Rat brain was homogenized in buffer A (50 mm Tris·HCl, pH 7.6, 150 mm NaCl, 10 mm β-mercaptoethanol, 1 mm EDTA, 2 mm benzamidine·HCl, 1 mm phenylmethylsulfonyl fluoride, 100 μm leupeptin, 1 μm pepstatin, 2 μg/ml aprotinin) using a Polytron homogenizer and then centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant was saved as brain extract. GST fusion proteins encoding I PP2A1, its deletion mutants, and PP2A-A were purified according to the manufacturer's instructions (GE Healthcare). Recombinant GST or GST fusion proteins were incubated with glutathione-Sepharose 4B beads overnight at 4 °C and washed three times for 15 min at 4 °C with phosphate-buffered saline. The beads were equilibrated with binding buffer (20 mm Tris·HCl, 8.0, 150 mm NaCl, 1 mm EDTA, 0.5% Nonidet P-40, 2 mm benzamidine·HCl, 0.1 mm 4-(2-aminoethyl)benzenesulfonylfluoride hydrochloride, 100 μm leupeptin, 1 μm pepstatin, 2 μg/ml aprotinin). Rat Brain extract was precleared with glutathione-Sepharose 4B beads for 2 h at 4°C and then incubated with GST or GST fusion proteins bound to glutathione-Sepharose 4B beads. After incubation at 4 °C overnight, the beads were washed five times for 10 min in binding buffer, and proteins bound to the beads were eluted into sample buffer, followed by SDS-PAGE, and Western blots.
Cell Culture, Transfections, and Differentiation—NIH 3T3 cells (obtained from ATCC, Rockville, MD) were grown in 25-cm2 flasks at 37 °C, containing 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum. Cells were plated on the 6-well plates or 100-mm plates and transfected with expression plasmids using FuGENE 6 (Roche Applied Science, Indianapolis, IN).
PC12 cells or Tau 441 stably transfected PC12 cells were grown in 25-cm2 flasks coated with poly-d -lysine at 37 °C, containing 5% CO2 in RPMI medium 1640 (Invitrogen) supplemented with 10% bovine calf serum, 5% horse serum, and 1% penicillin (27). Cells were plated in 6-well plates or 8-well Lab-Tek II Chamber Slides coated with poly-d -lysine (Nalge Nunc International, Naperville, IL) and then transfected with expression plasmids using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). For determining the induction of hyperphosphorylation of Tau by Western blots, cells were grown to 80–90% confluency, and for studying the number and the length of neurites on NGF differentiation, cells were grown to 30–50% confluency. PEGFP-N1 was employed to monitor the transient transfection efficiency, which was ∼30% when using 80–90% confluent cells and ∼2–5% in the case of 30–50% confluent cells. PC12 cells were differentiated into neurons by adding 100 ng/ml NGF to the culture medium for the indicated times.
Coimmunoprecipitation and Western Blots—For coimmunoprecipitation, NIH3T3 cells, transiently transfected with FLAG-tagged I PP2A1 or vector as a control, were grown for 2 days, and lysed in coimmunoprecipitation lysis buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 1 mm EGTA, 10% glycerol, 1.5 mm magnesium chloride, 1% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 50 units/ml aprotinin). After centrifugation at 16,000 × g for 15 min, the lysates were immunoprecipitated with anti-PP2Ac mouse monoclonal 1D6 (Millipore, Billerica, MA) followed by protein G-Sepharose (Pierce). After five final washings with lysis buffer, proteins were resolved by SDS-PAGE on 10% gels and transferred to polyvinylidene difluoride membrane according to the protocol of the manufacturer. Western blots of immunoprecipitates were developed with anti-FLAG (Sigma) or 1D6, followed by True Blue horseradish peroxidase-conjugated secondary antibody (eBioscience, San Diego, CA) detecting only native, undenatured IgG. Western blots of cell lysates were developed with anti-FLAG. Immunoreactive protein bands were visualized with enhanced chemiluminescence (ECL) reagents (Pierce). For detecting the C-terminal Myc-tagged I PP2A1 and its deletion mutants, the samples were resolved by 15% SDS-PAGE and transferred to polyvinylidene difluoride at lower voltage and time. The primary antibodies used were mAb FLAG (Sigma, 1:1000), mAb 1D6 (Millipore, Billerica, MA, to PP2Ac, 1:1000), mAb β-actin (Sigma, 1:2000), pAb PP2A-A (Millipore, 1:1000), mAb 2G9 (Millipore, to PP2A-B, 1:1000), pAb GST (GE Healthcare Bio-Sciences Corp., 1:1000), mAb Myc (Cell Signaling Technology, Inc., Beverly, MA, 1:1000), Tau mAb M4 (to phosphorylated Thr-231/Ser-235, 1:2000 (28)), Tau mAb 12E8 (to phosphorylated Ser-262/356, 1:500 (29)), Tau pAb pS262 (BIOSOURCE, 1:1000), Tau pAb pS404 (BIOSOURCE, 1:1000).
PP2A Activity Assay—PP2A activity in immunoprecipitates of this enzyme was assayed using p-nitrophenyl phosphate as a substrate (phosphatase assay kit, Millipore). Briefly, the cells were lysed in coimmunoprecipitation lysis buffer (see above) at 4 °C. The lysates were centrifuged at 16,000 × g for 15 min at 4 °C, and the supernatants were incubated with 2.5 μg of anti-PP2A for 2 h, followed by incubation with protein G-agarose for 1 h at 4 °C. The immunoprecipitates were washed twice with lysis buffer, once with 50 mm Tris buffer (50 mm Tris, pH 7.5, 0.1 mm CaCl2), resuspended in assay buffer (50 mm Tris, pH 7.5, 0.1 mm CaCl2, 2.5 mm NiCl2, and 1 mg/ml p-nitrophenyl phosphate), and incubated at 37 °C for 30 min. The reaction was stopped by the addition of 13% K2HPO4, and the absorbance was read at 405 nm.
Immunocytochemical Staining—To study colocalization of I PP2A1 and PP2A, and Tau staining in PC12 cells, the cells were fixed with 4% paraformaldehyde in 0.1 m phosphate buffered saline. In the case of tubulin staining, the cells were rinsed once with warm (37 °C) phosphate-buffered saline and once with warm PEM (80 mm PIPES, 5 mm EGTA, 1 mm MgCl2, pH 6.8), and then fixed with 0.3% glutaraldehyde containing 0.5% Non-idet P-40 in PEM for 10 min at 37 °C. After fixation, the cells were treated with 0.5% Triton X in Tris-buffered saline for 10 min, blocked with 4% normal horse serum containing 0.1% Tween 20 in phosphate-buffered saline for 30 min, and immunostained with primary antibodies, followed by incubation with Alexa 488-conjugated goat anti-mouse antibody (1:1000) and Alexa 594-conjugated goat anti-rabbit antibody (1:1000) (Invitrogen). The primary antibodies used were mAb 1D6 (to PP2Ac, 1:100), pAb Myc (1:400), mAb M4 (to phosphorylated Thr-231/Ser-235, 1:1000), mAb DM1A (to alphatubulin, 1:2000) (Sigma), rabbit affinity-purified antibody R-42089 to a synthetic peptide corresponding to amino acid residues 10–23 of I PP2A1 (30). Representative images from several cells were generated using a PCM 2000 Confocal Imaging System (Nikon, Melville, NY) or an Act-2U Digital Imaging System (Nikon, Melville, NY). The length of the neurites borne by randomly selected 30 differentiated PC12 cells were measured by means of an image analysis system (Nikon), using Image J software without knowledge of the transgenes.
RESULTS
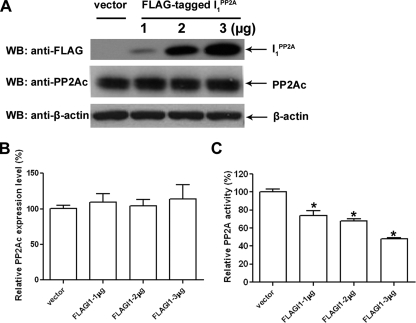
Inhibition of PP2A by I PP2A1 in NIH3T3 Cells—I PP2A1 is known to be a multifunctional protein that, besides inhibiting PP2A, binds to hypoacetylated over hyperacetylated histones and plays a signaling role in integrating histone hypoacetylation to gene inactivation (31–33). We investigated whether I PP2A1 decreased PP2A activity directly or through inhibiting PP2A expression level in NIH3T3 cells transiently transfected with different amounts of FLAG-tagged I PP2A1. We found that in FLAG-tagged I PP2A1-transfected cells, the expression level of I PP2A1 as well as the inhibition of PP2A activity increased in a dose-dependent manner, whereas, PP2A expression level was not significantly altered (Fig. 1). These data suggest that in cells PP2A is inhibited by I PP2A1 in a dose-dependent manner, and that this decrease in the phosphatase activity is not due to the reduction of PP2A expression level.
FIGURE 1.
Inhibition of PP2A activity by I PP2A1 in cultured cells. A, NIH3T3 cells were transiently transfected with different amounts of FLAG-tagged I PP2A1 or mock DNA (vector) as a control. After 48-h transfection, cells were lysed and expression of PP2Ac, I PP2A1, and β-actin were determined by Western blots. B, the relative PP2Ac expression level was normalized by the expression of β-actin. PP2Ac expression level did not show any significant difference among different levels of I PP2A1 transfection in NIH3T3 cells. C, inhibition of PP2A activity by I PP2A1. NIH3T3 cells were transiently transfected with different amounts of FLAG-tagged I PP2A1 or with mock DNA (vector) as a control. After 48-h transfection, cells were lysed and PP2A was immunoprecipitated with mAb 1D6 to PP2A, and the phosphatase activity was assayed colorimetrically using p-nitrophenyl phosphate as a substrate. I PP2A1 inhibited the PP2A activity in a dose-dependent fashion. Error bars indicate means ± S.E.; n = 3. *, p < 0.05 compared with control.
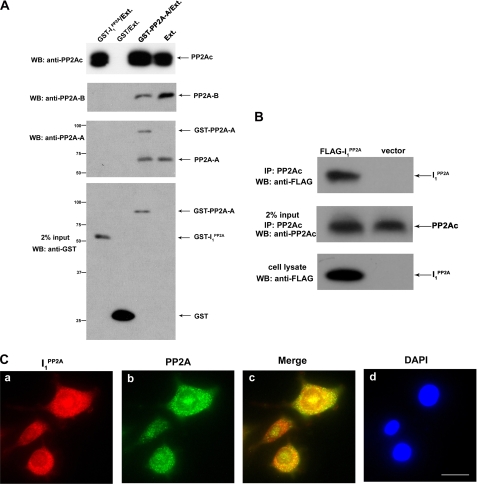
Characterization of Interaction of PP2A with I PP2A1—PP2A consists of a heterotrimer that exists in multiple forms. The core components of all trimeric forms are 36-kDa catalytic subunit (PP2Ac) and the 65-kDa regulatory subunit (A subunit, PR65). This core heterodimer is ubiquitous, and it forms complexes with “variable” B subunits (some of which are expressed in a tissue- and/or in development restricted manner) and confers distinct properties to the enzyme (14). I PP2A1 was shown to inhibit PP2A activity in vitro and could interact with the C subunit of PP2A (15, 24). It was of interest to determine whether I PP2A1 can only bind to PP2A C subunit and the association is sufficient for inhibition of PP2A activity. To study the interaction between PP2A and I PP2A1, in vitro pulldown assays were carried out using bacterially expressed GST alone, GSTPR65α (PP2A-A), or GST-I PP2A1. Rat brain extract, which contains PP2A holoenzyme, was incubated with purified GSTPR65α, GST-I PP2A1, or GST alone on glutathione-Sepharose 4B beads, and subsequently PP2A subunits on beads were detected by Western blots. We found that I PP2A1 interacted with PP2A catalytic subunit (PP2Ac). However, no associations between I PP2A1 and PR65α (PP2A A regulatory subunit) or PR55α (PP2A B regulatory subunit) were observed (Fig. 2A). The interaction between I PP2A1 and PP2Ac was further confirmed by coimmunoprecipitation. NIH3T3 cells were transiently transfected with FLAG-tagged I PP2A1 for 48 h, and then the cell lysate was collected, and endogenous PP2Ac was immunoprecipitated with 1D6 antibody to PP2Ac. The immunoprecipitated PP2Ac complex was resolved by SDS-PAGE, and Western blots were developed with anti-FLAG antibody. We found that the PP2Ac coimmunoprecipitated with FLAG-tagged I PP2A1 but not vector (Fig. 2B). In addition, PP2Ac and I PP2A1 were found to colocalize in PC12 cells double labeled with antibodies to PP2A catalytic subunit and anti-I PP2A1 (Fig. 2C). These findings suggest that I PP2A1 interacts with PP2A catalytic subunit and not with PP2A regulatory subunits PP2A-A or PP2A-B.
FIGURE 2.
Interaction between I PP2A1 and PP2A. A, GST pulldown assay. Rat brain extract (Ext), used as a source of PP2A holoenzyme, was incubated with Sepharose 4B beads bearing GST, GST-I PP2A1, or GST-PP2A-A. After washing, bound PP2Ac, PP2A-B, and PP2A-A were detected by Western blots. The GST, GST-I PP2A1, or GST-PP2A-A used in pulldown assay are shown in the lowest panel. GST-I PP2A1 fusion protein pulled down PP2Ac (PP2A catalytic subunit) but not PR55α (PP2A Bα regulatory subunit) or PR65α (PP2A A regulatory subunit). From left to right, lane 2 (GST/Ext.) is a negative control, and lane 4 (Ext.) is input. Positions of protein size markers are indicated on the left of the panel. B, association of PP2Ac with I PP2A1 was confirmed by in vitro coimmunoprecipitation. Immunoprecipitation was carried out using mAb to PP2Ac in lysates of FLAG-tagged I PP2A1 transiently transfected NIH3T3 cells. I PP2A1 was detected using antibodies against FLAG (shown in the top and bottom panels). The middle panel depicts the amount of immunoprecipitated PP2Ac. C, colocalization of PP2A catalytic subunit and I PP2A1 in PC12 cells. PC12 cells were doubly stained with monoclonal anti-PP2Ac and polyclonal I PP2A1 (R42089), followed by, rhodamine-conjugated anti-rabbit IgG (panel a), fluorescein-labeled anti-mouse IgG (panel b), and additional staining of nuclei with 4′,6-diamidino-2-phenylindole (DAPI, panel d). The colocalization of the two is demonstrated by the yellow color in the merged image (panel c). Scale bar = 10 μm.
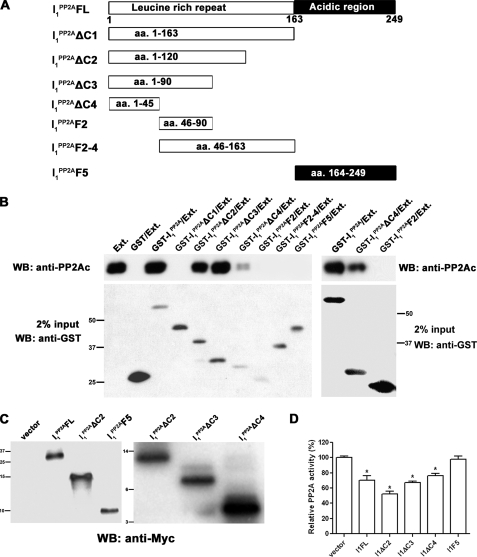
Domains of I PP2A1 Involved in Its Binding to PP2A Catalytic Subunit—The structural feature of I PP2A1 is characterized by four leucine-rich repeats (including the first 25-amino acid subtype-specific N-terminal region), a putative nuclear localization signal, and a long stretch of acidic amino acids at C-terminal ends (18, 22). To define the binding domain as well as PP2A inhibition activity, the full-length I PP2A1 protein and, based on its structure, a series of deletion mutants I PP2A1ΔC1, I PP2A1ΔC2, I PP2A1ΔC3, I PP2A1ΔC4, I PP2A1F2, I1PP2AF2–4, and I1PP2AF5 were generated (Fig. 3A). In vitro pulldown assays were carried out with bacterially expressed GST fusion I PP2A1 full-length protein or its deletion mutants. Rat brain extract as a source of PP-2A holoenzyme was added to purified GST fusion proteins or GST alone bound to glutathione Sepharose 4B beads and then PP2A catalytic subunit that associated with beads was detected by Western blots. We found that the full-length I PP2A1 and I PP2A1 deletion mutants containing the N-terminal isotype-specific region (I PP2AΔC2, I PP2A1ΔC3, and I PP2A1ΔC4) except I PP2A1ΔC1 I PP2A1ΔC1 had the capability to bind with PP2Ac, whereas, the other I PP2A1 mutants without N-terminal isotype-specific region (I PP2A1F2, I PP2A1F2–4, and I PP2A1F5) lost the PP2Ac binding ability (Fig. 3B). Thus, the minimal region required for the binding of PP2Ac is localized at N-terminal region (amino acid 1–45) in I PP2A1.
FIGURE 3.
Interaction between PP2Ac and various domains of I PP2A1. A, schematic diagram of the constructs of functional domains of I PP2A1 employed for the interaction studies. B, rat brain extract as a source of PP-2A holoenzyme was incubated with Sepharose 4B beads bearing GST, GST-I PP2A1, or GST-I PP2A1 deletion mutants. The pulldown assay was carried out using 0.5 μg of GST fusion protein per mg of brain extract, except in cases of GST-I PP2A1F2, and GST alone, double the amount, i.e. 1 μg was employed. After washing, bound PP2Ac was detected by Western blots (upper panel). The GST, GST-I PP2A1, or GSTI PP2A1 deletion mutants used in the pulldown assay are shown in the lower panel. The I PP2A1 constructs, which included the N-terminal (aa 1–45) isotype-specific region, except construct I PP2A1ΔC1, interacted with PP2Ac. The right panel shows that, even in overloaded blots, GST-I PP2A1F2 does not show interaction with PP2Ac. Positions of protein size markers are indicated on the left for the left panel and on the right for the right panel. C, NIH3T3 cells were transiently transfected with C-terminal Myc-tagged I PP2A1, its deletion mutants, or empty vector as a control. After 48-h transfection, transfected Myc-tagged I PP2A1 and its mutants were detected using antibodies against Myc from same amounts of lysates. Positions of protein size markers are indicated on the left. D, relative PP2A activity in transiently transfected NIH3T3 cells with C-terminal Myc-tagged constructs was detected as described in Fig. 1C. I PP2A1FL reduced PP2A activity to ∼70%, whereas I PP2A1ΔC2, I PP2A1ΔC3, and I PP2A1ΔC4 inhibited PP2A activity to ∼50%, 67%, and 76% in NIH3T3 cells, respectively, and I PP2A1F5 had no detectable effect on the activity. *, p < 0.05 compared with the vector transfection control.
To measure the inhibition of PP2A by I PP2A1 mutants, I PP2A1FL, I PP2A1ΔC2, I PP2A1ΔC3, I PP2A1ΔC4, and I PP2A1F5 were chosen to characterize the PP2A activity. NIH3T3 cells were transiently transfected with Myc-tagged-I PP2A1FL, I PP2A1ΔC2, I PP2A1ΔC3, I PP2A1ΔC4, or I PP2A1F5 for 48 h, followed by PP2A activity assays as described above in Fig. 1. We found that compared with vector alone, I PP2A1FL, I PP2A1ΔC2, I PP2A1ΔC3, and I PP2A1ΔC4 reduced PP2A activity to ∼70%, ∼50%, 67, and 76%, respectively. However, I PP2A1F5 did not inhibit PP2A activity in this assay (Fig. 3, C and D). Therefore, in subsequent studies, only I PP2A1FL and I PP2A1ΔC2 were employed.
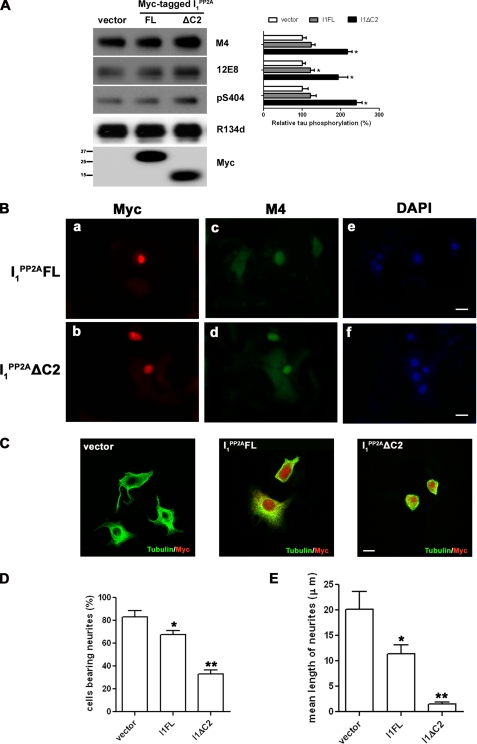
Effect of I PP2A1 on Tau Phosphorylation, Microtubule Assembly, and Neurite Outgrowth—There is accumulating evidence that reduced PP2A activity is sufficient to induce Tau hyperphosphorylation in cultured cells and in brain (3, 4, 10, 34–36). We investigated the functional consequences of the inhibition of PP2A activity by I PP2A1 in cultured cells by Western blots and by immunocytochemistry. To assess whether Tau phosphorylation is altered in cultured cells, the Tau441 stably transfected PC12 cells (PC12/Tau441) were transiently transfected with Myc-tagged I PP2AFL, I PP2A1ΔC2, or I PP2A1 F5, and vector as a control for 48 h, and Tau phosphorylation was studied at several sites by Western blots, and the level of Tau phosphorylation was normalized by total Tau expression. Phosphorylation of Tau at M4 (Thr-231/Ser-235), 12E8 (Ser-262/356), and at Ser-404 sites was significantly increased in cells that overexpressed I PP2A1ΔC2 and in the case of 12E8 site I PP2A1FL but not I PP2A1F5 (data not shown) (Fig. 4A).
FIGURE 4.
The effects of I PP2A1 and its deletion mutants on Tau phosphorylation and neuronal morphology of PC12 cells. A, Tau441 stably transfected PC12 cells were transiently transfected with vector, Myc-tagged I PP2A1FL, or I PP2A1ΔC2. After 48-h transfection, Tau phosphorylation at M4 (pT231/pS235), 12E8, (pS262/356) and pS404, sites, total Tau (R134d), as well as transfected I PP2A1 were detected by Western blots from same amounts of lysates. The level of Tau phosphorylation was quantified by densitometry and normalized to total Tau levels. *, p < 0.05 compared with the vector transfection control. B and C, immunocytochemical staining of PC12 cells transiently transfected with Myc-tagged I PP2A1FL, or I PP2A1ΔC2. At 6 h post-transfection, cells were differentiated with 100 ng/ml NFG for further 90 h and then processed by double immunofluorescence using rabbit polyclonal antibody to Myc and mouse monoclonal antibody M4 to phosphotau (pThr-231/pSer-235) as primary antibodies (B), followed by fluorescein-labeled anti-mouse IgG (panels c and d), or rhodamine-conjugated anti-rabbit IgG (panels a and b) and additional staining of nuclei with 4′,6-diamidino-2-phenylindole (DAPI, panels e and f). M4 signal in cells that overexpressed I PP2A1FL or I PP2A1ΔC2 was increased as compared with non-transfected cells. In C, the cells were processed by double immunofluorescence using rabbit polyclonal antibody to Myc and mouse monoclonal antibody DMIA to tubulin as primary antibodies, followed by rhodamine-conjugated anti-rabbit IgG or fluorescein-labeled anti-mouse IgG, respectively. The morphology of the cells expressing I PP2A1FL or I PP2A1ΔC2 was significantly altered with marked decreases in the number and the length of neurites as compared with mock transfected cells. Scale bar = 10 μm. D, effect of I PP2A1 and its deletion mutant on neurite outgrowth from PC12 cells. The total number of cells expressing Myc-tagged proteins was counted, and cells with processes were regarded as neurite-positive. The number of cells with such outgrowths was then expressed as a percentage of the total number of cells. The percentage of cells bearing neurites in cells transfected with I PP2A1FL and I PP2A1ΔC2 was significantly decreased compared with non-transfected cells used as a control. All cells on any individual dish were counted up to a total of 250 cells, and these results show the average from three independent experiments. E, neurite outgrowth was analyzed quantitatively by measuring the average length of randomly selected 30 neurons in three fields without knowledge of the transgenes under the same conditions described in B and C. The length of neurites in cells transfected with I PP2A1FL and I1PP2AΔC2 was remarkably decreased compared with non-transfected cells used as a control. Error bars indicate means ± S.E.; n = 30. *, p < 0.05; **, p < 0.001.
Besides detecting Tau phosphorylation in PC12/Tau441 cells, wild-type PC12 cells were transiently transfected with Myc tagged I PP2AFL, I PP2A1ΔC2, or I PP2A1F5 as well. At 6 h post-transfection, cells were differentiated with 100 ng/ml NGF for another 90 h to stimulate cell neuronal differentiation as well as increasing endogenous Tau expression level. The cells were then processed to detect endogenous Tau phosphorylation at M4 site and to assess microtubule network by immunohistochemistry using antibody M4 to Tau, and DM1A to tubulin, respectively. Furthermore, the neurite length was measured in randomly selected 30 transfected or non-transfected cells in three fields. I PP2A1FL and I PP2A1ΔC2 but not I PP2A1F5 (data not shown) transfected cells showed Tau phosphorylation at M4 site and as well as a reduction in microtubule network/staining (Fig. 4, B and C). Meanwhile, in 96-h culture of mock transfected cells, ∼80% of PC12 cells possessed neurites, and the mean length of neurites was ∼20 μm (Fig. 4, D and E). For the proportion of PC12 cells expressing I PP2A1FL or I PP2A1ΔC2 the number of neurite bearing cells was reduced to ∼80 and ∼40%, respectively, of the mock transfected cells (Fig. 4D). The mean neurite length of cells overexpressing I PP2A1FL and I PP2A1ΔC2 was reduced significantly from ∼20 μm to ∼11.4 μm and ∼1.5 μm, respectively (Fig. 4E), suggesting that probably the dissociation of Tau from microtubules by I PP2A1FL and I PP2A1ΔC2 impairs neurite outgrowth and neuronal differentiation.
DISCUSSION
Neurofibrillary degeneration of abnormally hyperphosphorylated Tau is a hallmark of AD and a family of related neurodegenerative diseases called tauopathies (37–39). These tauopathies include frontotemporal dementia with Parkinsonism linked to chromosome 17, Pick disease, corticobasal degeneration, progressive supranuclear palsy, Guam Parkinsonism dementia complex, and dementia pugilistica (40). The degree of neurofibrillary degeneration is well known to correlate directly with the degree of dementia in AD patients (41–43). The abnormal hyperphosphorylation of Tau is the key step that, through sequestration of normal MAPs, results in the breakdown of the microtubule network and consequent neurodegeneration (44–50). PP2A, which accounts for >70% of total phosphoseryl/phosphothreonyl protein phosphatase activity in human brain (5), is a major regulator of the phosphorylation of Tau (7–9, 51). The activity of PP2A is compromised in AD brain (4, 34, 52–54). I PP2A1, which is homologous to PHAP-1, pp32, mapmodulin, and LANP (15, 18–20, 22, 23, 55) regulates PP2A activity by functioning as a non-competitive inhibitor of this enzyme (15). In addition, several studies have shown that pp32 is involved in “histone masking,” which can lead to inhibition of histone acetyltransferase-dependent transcriptional activation (19, 33, 56). We have previously shown that the I PP2A1 colocalizes with neurofibrillary pathology and that the transcription of I PP2A1 is up-regulated in the affected areas of AD brain, and I PP2A1 is, thus, a primary suspect in Alzheimer neurofibrillary degeneration (26). However, neither the nature of the interaction between I PP2A1 and PP2A nor the sequence of events by which this interaction could lead to the abnormal hyperphosphorylation of Tau and neurodegeneration were understood.
The present study shows that the PP2A activity is inhibited by I PP2A1 in a dose-dependent manner and that this decrease is not due to any reduction in its expression level of PP2Ac in NIH3T3 cells. Furthermore, I PP2A1 interacts with PP2A catalytic subunit and not with PP2A-A or -B regulatory subunits, and the minimal region required for the association with PP2Ac as well as PP2A inhibition is localized at the N-terminal isotype-specific containing region of the inhibitor. This study also shows that I PP2A1 significantly increases the phosphorylation of Tau at M4 (Thr-231/Ser-135), 12E8 (Ser-262/356) and at Ser-404 sites and impairs microtubule network and neurite outgrowth in PC12 cells during differentiation by NGF.
Unlike the variable B regulatory subunit of the trimeric PP2A holoenzyme, which regulates the phosphatase activity by determining its intracellular localization and substrate specificity, we found that I PP2A1 regulated the PP2A activity by directly interacting with and inhibiting the activity of its catalytic subunit. We found that I PP2A1 interacted with PP2Ac and no such association between the inhibitor and PP2A-A subunit or -B subunit was detected, suggesting that I PP2A1 inhibits PP2A activity by directly interacting with its catalytic subunit. PP2Ac bound to the full-length I PP2A1 and its deletion mutants containing the N-terminal isotype-specific region (I PP2A1ΔC2, I PP2A1ΔC3, and I PP2A1ΔC4) except I PP2A1ΔC1, and not to deletion mutants without the N-terminal isotype-specific region (I PP2A1F2, I PP2A1F2–4, and I PP2A1F5), which suggests that the N-terminal isotype-specific region is required for I PP2A1 binding to PP2Ac as well as the PP2A inhibitor activity. The deletion mutant I PP2A1ΔC1 (aa 1–163) with the N-terminal region but without the C-terminal acidic region and the mutant I PP2A1F5 (aa 164–249) with the C-terminal acidic region alone lost the ability to bind to PP2Ac. These findings suggest 1) that the region between aa 121–163 probably functions as an autoinhibitory domain that negatively affects the interaction of I PP2A1 with PP2Ac and its ability to modulate the PP2A activity, and 2) that the C-terminal acidic region itself cannot bind to PP2Ac but might be involved in neutralizing the aa 121–163 autoinhibitory domain. In cells the inhibitory role of the aa 121–163 domain of I PP2A1 might be regulated through phosphorylation of Ser-158 or both Ser-158 and Ser-204, which have been shown by Hong et al. (57) to be phosphorylated by casein kinase II in the cell.
The present study strongly suggests a link among I PP2A1, PP2A, and abnormally hyperphosphorylated tau. We found that I PP2A1 deletion mutants with the binding capacity to PP2Ac as well as inhibition of PP2A activity, when overexpressed in Tau441 stably transfected PC12 cells, increased the phosphorylation of Tau at M4 (Thr-231/Ser-235), 12E8 (Ser-262/356) and at Ser-404 sites. Phosphorylation of Tau at these sites is critically involved in converting it from microtubule assembly promoting to microtubule assembly inhibitory protein and promoting its self-assembly into paired helical filaments (39, 44, 45, 58–60). This increase in the abnormal hyperphosphorylation of Tau is probably both due to an inhibition of PP2A activity as well as an increase in the activity of one or more Tau kinases. Calmodulin kinase II, protein kinase A, extracellular regulated kinases ERK1 and ERK2, glycogen synthase kinase-3, p70S kinase, and stress-activated kinase p38 are among the Tau kinases activities that are regulated by PP2A (11, 13, 61–63).
In the present study we found that overexpression of I PP2A1 full-length protein and its deletion mutant I PP2A1ΔC2 (aa 1–120) in PC12 cells decreased the microtubule stability as evidenced by a marked reduction in the size of the microtubule network and cell size and reduction in the number and the length of neurites during NGF-induced neuronal differentiation. These deleterious effects of I PP2A1 could occur through the abnormal hyperphosphorylation of Tau, which has been previously demonstrated to disrupt microtubules by sequestering normal MAPs (44, 45, 49, 58, 64). Mapmodulin has been shown to bind to normal MAPs, especially as the free proteins, and in this way destabilize microtubules (21, 55). Thus, I PP2A1 could also contribute to destabilization of microtubules through essentially competing with tubulin.
In short, the direct binding of I PP2A1 to PP2Ac, causing inhibition of the phosphatase activity, provides a new mechanism of the regulation of the cellular PP2A activity. I PP2A1 appears to interact through its N-terminal isotype region (aa 1–45) and have an autoinhibitory domain, which lies within amino acid residues 121–163. This autoinhibitory domain appears to be at least partly neutralized by the C-terminal acidic region (aa 164–249) of I PP2A1, because both the full-length protein and the aa 1–120 N-terminal domains have PP2A inhibitory activities, whereas neither the aa 1–163 nor 46–163 can inhibit PP2A. Inhibition of PP2A by I PP2A1 results in the abnormal hyperphosphorylation of Tau at several sites, which are known to be critically involved in converting normal Tau to an inhibitory and pathological state. Thus, inhibition of I PP2A1 activity offers a very promising therapeutic target for inhibition of neurofibrillary degeneration of the abnormally hyperphosphorylated Tau through the restoration of the PP2A activity.
Acknowledgments
We are grateful to Dr. Brian A. Hemmings for providing the pRC/CMV.HA, PR65α, pCMV5.HA PP2ACatα, and pCMV5.HA PR55; to Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY, for PHF-1 antibody; to Dr. Dale Schenk, Elan Pharmaceuticals, San Francisco, CA, for 12E8 antibody; to Dr. Yasuo Ihara, Tokyo University, Tokyo, Japan, for M4 antibody; and to Dr. Fei Liu from our laboratory for providing Tau441 stably transfected PC12 cells. Janet Murphy provided secretarial assistance, including the preparation of the manuscript.
This work was supported by the New York State Office of Mental Retardation and Developmental Disabilities; by research grants from the Institute for the Study of Aging, New York, and the Alzheimer's Association, Chicago, IL; and by NIA, National Institutes of Health Grant AG-019158. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PP, protein phosphatase; AD, Alzheimer disease; ERK1/2, extracellular regulated kinase; GST, glutathione S-transferase; LANP, leucine-rich acidic nuclear protein; MAP, microtubule-associated protein; MEK1/2, MAP kinase kinase; PHAP, putative histocompatibility leukocyte antigen class II-associated protein; aa, amino acid(s); NGF, nerve growth factor; mAb, monoclonal antibody; CMV, cytomegalovirus; HA, hemagglutinin.
References
- 1.Cohen, P. (1989) Annu. Rev. Biochem. 58 453–508 [DOI] [PubMed] [Google Scholar]
- 2.Iqbal, K., Alonso, A., Gong, C., Khatoon, S., Pei, J. J., Wang, J. Z., and Grundke-Iqbal, I. (1997) in Brain Microtubule Associated Proteins: Modifications and Disease (Kosik, K., and Avila, J., eds) pp. 95–111, Harwood Academic Publishers, New York
- 3.Gong, C. X., Grundke-Iqbal, I., and Iqbal, K. (1994) Neuroscience 61 765–772 [DOI] [PubMed] [Google Scholar]
- 4.Gong, C. X., Singh, T. J., Grundke-Iqbal, I., and Iqbal, K. (1993) J. Neurochem. 61 921–927 [DOI] [PubMed] [Google Scholar]
- 5.Liu, F., Grundke-Iqbal, I., Iqbal, K., and Gong, C. X. (2005) Eur. J. Neurosci. 22 1942–1950 [DOI] [PubMed] [Google Scholar]
- 6.Iqbal, K., Alonso, A. C., Gong, C. X., Khatoon, S., Pei, J. J., Wang, J. Z., and Grundke-Iqbal, I. (1998) J. Neural. Transm. Suppl. 53 169–180 [DOI] [PubMed] [Google Scholar]
- 7.Bennecib, M., Gong, C., Wegiel, J., Lee, M. H., Grundke-Iqbal, I., and Iqbal, K. (2000) Alzheimer's Rep. 3 295–304 [Google Scholar]
- 8.Sontag, E., Nunbhakdi-Craig, V., Lee, G., Bloom, G. S., and Mumby, M. C. (1996) Neuron 17 1201–1207 [DOI] [PubMed] [Google Scholar]
- 9.Sontag, E., Nunbhakdi-Craig, V., Lee, G., Brandt, R., Kamibayashi, C., Kuret, J., White, C. L., 3rd, Mumby, M. C., and Bloom, G. S. (1999) J. Biol. Chem. 274 25490–25498 [DOI] [PubMed] [Google Scholar]
- 10.Gong, C. X., Wegiel, J., Lidsky, T., Zuck, L., Avila, J., Wisniewski, H. M., Grundke-Iqbal, I., and Iqbal, K. (2000) Brain Res. 853 299–309 [DOI] [PubMed] [Google Scholar]
- 11.Li, L., Sengupta, A., Haque, N., Grundke-Iqbal, I., and Iqbal, K. (2004) FEBS Lett. 566 261–269 [DOI] [PubMed] [Google Scholar]
- 12.Pei, J. J., Gong, C. X., An, W. L., Winblad, B., Cowburn, R. F., Grundke-Iqbal, I., and Iqbal, K. (2003) Am. J. Pathol. 163 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kins, S., Kurosinski, P., Nitsch, R. M., and Gotz, J. (2003) Am. J. Pathol. 163 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virshup, D. M. (2000) Curr. Opin Cell Biol. 12 180–185 [DOI] [PubMed] [Google Scholar]
- 15.Li, M., Guo, H., and Damuni, Z. (1995) Biochemistry 34 1988–1996 [DOI] [PubMed] [Google Scholar]
- 16.Li, M., Makkinje, A., and Damuni, Z. (1996) Biochemistry 35 6998–7002 [DOI] [PubMed] [Google Scholar]
- 17.Tsujio, I., Zaidi, T., Xu, J., Kotula, L., Grundke-Iqbal, I., and Iqbal, K. (2005) FEBS Lett. 579 363–372 [DOI] [PubMed] [Google Scholar]
- 18.Chen, T. H., Brody, J. R., Romantsev, F. E., Yu, J. G., Kayler, A. E., Voneiff, E., Kuhajda, F. P., and Pasternack, G. R. (1996) Mol. Biol. Cell 7 2045–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka, K., Taoka, M., Satozawa, N., Nakayama, H., Ichimura, T., Takahashi, N., Yamakuni, T., Song, S. Y., and Isobe, T. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 9670–9674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mencinger, M., Panagopoulos, I., Contreras, J. A., Mitelman, F., and Aman, P. (1998) Biochim. Biophys. Acta 1395 176–180 [DOI] [PubMed] [Google Scholar]
- 21.Ulitzur, N., Humbert, M., and Pfeffer, S. R. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 5084–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaesen, M., Barnikol-Watanabe, S., Gotz, H., Awni, L. A., Cole, T., Zimmermann, B., Kratzin, H. D., and Hilschmann, N. (1994) Biol. Chem. Hoppe. Seyler. 375 113–126 [DOI] [PubMed] [Google Scholar]
- 23.Walensky, L. D., Coffey, D. S., Chen, T. H., Wu, T. C., and Pasternack, G. R. (1993) Cancer Res. 53 4720–4726 [PubMed] [Google Scholar]
- 24.Yu, L. G., Packman, L. C., Weldon, M., Hamlett, J., and Rhodes, J. M. (2004) J. Biol. Chem. 279 41377–41383 [DOI] [PubMed] [Google Scholar]
- 25.Matilla, A., and Radrizzani, M. (2005) Cerebellum 4 7–18 [DOI] [PubMed] [Google Scholar]
- 26.Tanimukai, H., Grundke-Iqbal, I., and Iqbal, K. (2005) Am. J. Pathol. 166 1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G. W., and Gong, C. X. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 10804–10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa, M., Watanabe, A., Takio, K., Suzuki, M., Arai, T., Titani, K., and Ihara, Y. (1993) J. Neurochem. 60 2068–2077 [DOI] [PubMed] [Google Scholar]
- 29.Seubert, P., Mawal-Dewan, M., Barbour, R., Jakes, R., Goedert, M., Johnson, G. V., Litersky, J. M., Schenk, D., Lieberburg, I., Trojanowski, J. Q., and Lee, V. M.-Y. (1995) J. Biol. Chem. 270 18917–18922 [DOI] [PubMed] [Google Scholar]
- 30.Tanimukai, H., Grundke-Iqbal, I., and Iqbal, K. (2004) Brain Res. Mol. Brain Res. 126 146–156 [DOI] [PubMed] [Google Scholar]
- 31.Kutney, S. N., Hong, R., Macfarlan, T., and Chakravarti, D. (2004) J. Biol. Chem. 279 30850–30855 [DOI] [PubMed] [Google Scholar]
- 32.Seo, S. B., Macfarlan, T., McNamara, P., Hong, R., Mukai, Y., Heo, S., and Chakravarti, D. (2002) J. Biol. Chem. 277 14005–14010 [DOI] [PubMed] [Google Scholar]
- 33.Brennan, C. M., Gallouzi, I. E., and Steitz, J. A. (2000) J. Cell Biol. 151 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong, C. X., Shaikh, S., Wang, J. Z., Zaidi, T., Grundke-Iqbal, I., and Iqbal, K. (1995) J. Neurochem. 65 732–738 [DOI] [PubMed] [Google Scholar]
- 35.Goedert, M., Jakes, R., Qi, Z., Wang, J. H., and Cohen, P. (1995) J. Neurochem. 65 2804–2807 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, T., Zhong, J., Iqbal, K., Trenkner, E., and Grundke-Iqbal, I. (1998) FEBS Lett. 426 248–254 [DOI] [PubMed] [Google Scholar]
- 37.Braak, H., Braak, E., Grundke-Iqbal, I., and Iqbal, K. (1986) Neurosci. Lett. 65 351–355 [DOI] [PubMed] [Google Scholar]
- 38.Grundke-Iqbal, I., Iqbal, K., Tung, Y. C., Quinlan, M., Wisniewski, H. M., and Binder, L. I. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iqbal, K., Alonso Adel, C., Chen, S., Chohan, M. O., El-Akkad, E., Gong, C. X., Khatoon, S., Li, B., Liu, F., Rahman, A., Tanimukai, H., and Grundke-Iqbal, I. (2005) Biochim. Biophys. Acta 1739 198–210 [DOI] [PubMed] [Google Scholar]
- 40.Tolnay, M., and Probst, A. (1999) Neuropathol. Appl. Neurobiol. 25 171–187 [DOI] [PubMed] [Google Scholar]
- 41.Alafuzoff, I., Iqbal, K., Friden, H., Adolfsson, R., and Winblad, B. (1987) ActaNeuropathol. (Berl.) 74 209–225 [DOI] [PubMed] [Google Scholar]
- 42.Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T., and Hyman, B. T. (1992) Neurology 42 631–639 [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson, B. E., Blessed, G., and Roth, M. (1970) J. Neurol. Sci. 11 205–242 [DOI] [PubMed] [Google Scholar]
- 44.Alonso, A., Li, B., Grundke-Iqbal, I., and Iqbal, K. (2006) Proc. Natl. Acad. Sci. U. S. A. 23 8864–8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso, A., Zaidi, T., Grundke-Iqbal, I., and Iqbal, K. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 5562–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iqbal, K., Grundke-Iqbal, I., Zaidi, T., Merz, P. A., Wen, G. Y., Shaikh, S. S., Wisniewski, H. M., Alafuzoff, I., and Winblad, B. (1986) Lancet 2 421–426 [DOI] [PubMed] [Google Scholar]
- 47.Liu, S. J., Zhang, J. Y., Li, H. L., Fang, Z. Y., Wang, Q., Deng, H. M., Gong, C. X., Grundke-Iqbal, I., Iqbal, K., and Wang, J. Z. (2004) J. Biol. Chem. 279 50078–50088 [DOI] [PubMed] [Google Scholar]
- 48.Santacruz, K., Lewis, J., Spires, T., Paulson, J., Kotilinek, L., Ingelsson, M., Guimaraes, A., DeTure, M., Ramsden, M., McGowan, E., Forster, C., Yue, M., Orne, J., Janus, C., Mariash, A., Kuskowski, M., Hyman, B., Hutton, M., and Ashe, K. H. (2005) Science 309 476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonso, A., Grundke-Iqbal, I., Barra, H. S., and Iqbal, K. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, B., Chohan, M. O., Grundke-Iqbal, I., and Iqbal, K. (2007) Acta Neuropathol. (Berl.) 113 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong, C. X., Lidsky, T., Wegiel, J., Zuck, L., Grundke-Iqbal, I., and Iqbal, K. (2000) J. Biol. Chem. 275 5535–5544 [DOI] [PubMed] [Google Scholar]
- 52.Vogelsberg-Ragaglia, V., Schuck, T., Trojanowski, J. Q., and Lee, V. M. (2001) Exp. Neurol. 168 402–412 [DOI] [PubMed] [Google Scholar]
- 53.Sontag, E., Luangpirom, A., Hladik, C., Mudrak, I., Ogris, E., Speciale, S., and White, C. L., 3rd. (2004) J. Neuropathol. Exp. Neurol. 63 287–301 [DOI] [PubMed] [Google Scholar]
- 54.Loring, J. F., Wen, X., Lee, J. M., Seilhamer, J., and Somogyi, R. (2001) DNA Cell Biol. 20 683–695 [DOI] [PubMed] [Google Scholar]
- 55.Ulitzur, N., Rancano, C., and Pfeffer, S. R. (1997) J. Biol. Chem. 272 30577–30582 [DOI] [PubMed] [Google Scholar]
- 56.Opal, P., Garcia, J. J., Propst, F., Matilla, A., Orr, H. T., and Zoghbi, H. Y. (2003) J. Biol. Chem. 278 34691–34699 [DOI] [PubMed] [Google Scholar]
- 57.Hong, R., Macfarlan, T., Kutney, S. N., Seo, S. B., Mukai, Y., Yelin, F., Pasternack, G. R., and Chakravarti, D. (2004) Biochemistry 43 10157–10165 [DOI] [PubMed] [Google Scholar]
- 58.Wang, J. Z., Grundke-Iqbal, I., and Iqbal, K. (2007) Eur. J. Neurosci. 25 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alonso, A., Zaidi, T., Novak, M., Grundke-Iqbal, I., and Iqbal, K. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 6923–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, J. Z., Grundke-Iqbal, I., and Iqbal, K. (1996) Brain Res. Mol. Brain Res. 38 200–208 [DOI] [PubMed] [Google Scholar]
- 61.Bennecib, M., Gong, C. X., Grundke-Iqbal, I., and Iqbal, K. (2000) FEBS Lett. 485 87–93 [DOI] [PubMed] [Google Scholar]
- 62.Pei, J. J., Khatoon, S., An, W. L., Nordlinder, M., Tanaka, T., Braak, H., Tsujio, I., Takeda, M., Alafuzoff, I., Winblad, B., Cowburn, R. F., Grundke-Iqbal, I., and Iqbal, K. (2003) Acta Neuropathol. (Berl.) 105 381–392 [DOI] [PubMed] [Google Scholar]
- 63.Sengupta, A., Novak, M., Grundke-Iqbal, I., and Iqbal, K. (2006) FEBS Lett. 580 5925–5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alonso, A., Grundke-Iqbal, I., and Iqbal, K. (1996) Nat. Med. 2 783–787 [DOI] [PubMed] [Google Scholar]