Abstract
Solution structures of complexes between the isolated A (IIAMan) and B (IIBMan) domains of the cytoplasmic component of the mannose transporter of Escherichia coli have been solved by NMR. The complex of wild-type IIAMan and IIBMan is a mixture of two species comprising a productive, phosphoryl transfer competent complex and a non-productive complex with the two active site histidines, His-10 of IIAMan and His-175 of IIBMan, separated by ∼25Å. Mutation of the active site histidine, His-10, of IIAMan to a glutamate, to mimic phosphorylation, results in the formation of a single productive complex. The apparent equilibrium dissociation constants for the binding of both wild-type and H10E IIAMan to IIBMan are approximately the same (KD ∼ 0.5 mm). The productive complex can readily accommodate a transition state involving a pentacoordinate phosphoryl group with trigonal bipyramidal geometry bonded to the Nε2 atom of His-10 of IIAMan and the Nδ1 atom of His-175 of IIBMan with negligible (<0.2Å) local backbone conformational changes in the immediate vicinity of the active site. The non-productive complex is related to the productive one by a ∼90° rotation and ∼37Å translation of IIBMan relative to IIAMan, leaving the active site His-175 of IIBMan fully exposed to solvent in the non-productive complex. The interaction surface on IIAMan for the non-productive complex comprises a subset of residues used in the productive complex and in both cases involves both subunits of IIAMan. The selection of the productive complex by IIAMan(H10E) can be attributed to neutralization of the positively charged Arg-172 of IIBMan at the center of the interface. The non-productive IIAMan-IIBMan complex may possibly be relevant to subsequent phosphoryl transfer from His-175 of IIBMan to the incoming sugar located on the transmembrane IICMan-IIDMan complex.
The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS)5 is a phosphorylation cascade involved in active sugar transport, signaling, and the regulation of carbon catabolite repression as well as an array of other cellular processes (1-3). The initial phosphorylation steps from phosphoenolpyruvate to His-189 (Nε2) of enzyme I and subsequently to His-15 (Nδ1) of HPr are common to all branches of the PTS. Thereafter, the phosphoryl group is transferred to sugar-specific enzymes II, which fall into four major families, glucose (Glc), mannitol (Mtl), mannose (Man), and chitobiose (Chb). The enzymes II are organized into two cytoplasmic domains A and B, and one or two membrane-bound domains, C and D, which may or may not be covalently linked to one another. The A domains from the four major families bear no sequence or structural similarity to one another, but in all cases the active site residue is a histidine (Nε2) that accepts a phosphoryl group from His-15 (Nδ1) of HPr and donates a phosphoryl group to either a cysteine residue in the case of IIBGlc, IIBMtl, and IIBChb or a histidine residue (Nδ2) for IIBMan. As in the case of the A domains, the B domains from the four major families bear no sequence similarity to one another, and with the exception of IIBMtl (4) and IIBChb (5), which have similar topologies, bear no structural similarity either (2, 3).
The various protein-protein complexes of the PTS provide a paradigm to explore the structural basis of protein-protein interactions and the factors that permit the recognition of diverse partners using structurally similar interfaces. Moreover, the complexes of the PTS are generally weak (KD ranging from the micromolar to millimolar range) and to date have proved refractory to crystallization. In a series of reports we have used NMR spectroscopy to solve the structures of complexes of HPr with the N-terminal domain of enzyme I (6), IIAGlc (7), IIAMtl (8), and IIAMan (9), and complexes of IIAGlc and IIAMtl with IIBGlc (10) and IIBMtl (11), respectively. In this report we explore the interaction of the IIAMan dimer (35 kDa) with IIBMan (19 kDa), thereby completing the structure determination of the cytoplasmic complexes of the mannose branch of the E. coli PTS.
The mannose transporter of E. coli comprises four domains, expressed as three proteins: IIABMan, IICMan, and IIDMan (12, 13). The two transmembrane components, IICMan and IIBMan, form a tight complex (13). The cytoplasmic component, IIABMan, is an obligate dimer with all dimerization contacts mediated by the A domain (14-16). The A (residues 1-134) and B (residues 160-323) domains of IIABMan are covalently attached by a flexible 25-residue alanine/proline-rich linker (residues 135-159) and fold independently of one another (15). Phosphoryl transfer occurs between His-10 of IIAMan and His-175 of IIBMan (14, 17). In the homologous sorbose (IISor) permease of Klebsiella pneumoniae (18) and fructose (IILev) permease of Bacillus subtilis (19), the A and B domains are expressed as separate polypeptide chains. To simplify the NMR spectroscopy and facilitate the identification of intermolecular contacts through isotope-filtered/separated nuclear Overhauser enhancement (NOE) experiments we therefore chose to carry out structural work on complexes of isolated IIAMan and IIBMan.
A high (1.7 Å) resolution crystal structure of E. coli IIAMan had previously been solved, but no structure was available for IIBMan. We therefore first solved the solution structure of E. coli IIBMan on the basis of NOE and dipolar coupling data in two alignment media, and then used this structure together with the x-ray structure of IIAMan to solve the structure of the IIAMan-IIBMan complex by conjoined rigid body/torsion angle dynamics on the basis of intermolecular NOE data. The intermolecular data recorded on the wild-type IIAMan-IIBMan complex were not compatible with the existence of a single species. Subsequent mutation of the active site His-10 of IIAMan to Glu to mimic phosphorylation of His-10 resulted in the formation of a single complex that was fully consistent with the stereochemical and geometric requirements for phosphoryl transfer between His-10 of IIAMan and His-175 of IIBMan. Selection of a single complex by the H10E mutation is due to neutralization of the positively charged Arg-172 of IIBMan at the center of the protein-protein interface of the productive complex. With the structure of the productive complex in hand, we were then able to determine the structure of the non-productive complex by accounting for the intermolecular NOE data on the basis of a mixture of productive and non-productive complexes. In the non-productive complex the active site histidines of IIAMan and IIBMan are ∼25 Å apart, and the active site His-175 and associated active site loop of IIBMan are exposed to solvent. We suggest that the non-productive complex may therefore be relevant to subsequent phosphoryl transfer to the incoming sugar located on the cytoplasmic side of the transmembrane IICMan-IIDMan complex. This study illustrates how, for weak protein-protein complexes, a relatively subtle change in a single interfacial residue can have a dramatic impact on the configuration of the resulting complex that, in this instance, may play an important role in both modulating and directing the phosphorylation cascade within the mannose transporter.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of IIAMan and IIBMan—The A domain of wild-type IIABMan (residues 1-136, IIAMan) of E. coli was expressed and purified as described previously (9).
The DNA corresponding to the B domain of IIABMan (residues 157-323, IIBMan) was amplified by PCR using a DNA template derived from E. coli chromosomal DNA provided by A. Peterkofsky. The PCR product contains an NcoI restriction site at the 5′ site and a tandem pair of in-frame termination codons and a BamHI site at the 3′ site introduced during the PCR reaction. The NcoI- and BamHI-cut PCR product was purified and subcloned into the corresponding expression sites of the modified pET32a vector (4) to form a thioredoxin fusion protein with a His6 tag. The selected clone was verified by DNA sequencing.
The following mutations, H10E in IIAMan, and R172Q and H175E in IIBMan were introduced using the QuikChange mutagenesis kit (Stratagene), and the sequences confirmed by DNA sequencing. Expression and purification was carried out using the same protocols as that for the corresponding wild-type proteins.
The plasmids for IIAMan and IIBMan were introduced into E. coli BL21(DE3) (Novagen) cells for protein expression and induced at an A600 ∼ 0.8 with 1 mm isopropyl-β-d-thiogalactopyranoside at 37 °C and 30 °C, respectively. Cells were grown in either Luria-Bertani medium or minimal media (in either H2O or D2O, with 15NH4Cl or 14NH4Cl, and [U-13C/1H]-, [U-12C/1H]-, [U-13C/2H]-, or [U-12C/2H]glucose as the main nitrogen and carbon sources, respectively). Because Leu, Val, Met, and Trp residues are involved in the IIAMan/IIBMan interface, selective labeling was also employed in the preparation of NMR samples. For 15N/13C/2H-(Leu/Val)-methyl-protonated (but otherwise fully deuterated) protein samples, 100 mg of [13C5,3-2H1]α-ketoisovalerate (Cambridge Isotopes) was added to 1 liter of D2O medium 1 h prior to induction (22). The 14N/12C/2H-(Leu/Val)-methyl-protonated (Trp/Met)-protonated (but otherwise fully deuterated) IIAMan samples ([12CH3-LV]/[1H-MW]/[12C/14N/2H]-IIAMan) were prepared by supplementing 1 liter of D2O medium with 100 mg of [12C5,3-2H1]α-ketoisovalerate (Sigma-Aldrich), 150 mg of methionine, and 200 mg of tryptophan 1 h prior to induction.
After induction (3 and 10 h for growths in H2O and D2O, respectively), cells expressing IIBMan protein were harvested, pelleted by centrifugation and resuspended in 50 ml (per liter of culture) of 30 mm Tris, pH 8.0, 10 mm imidazole and 200 mm NaCl. The cell suspension was lysed by three passages through a microfluidizer and centrifuged at 10,000 × g for 20 min. The supernatant was loaded onto a 5-ml nickel-Sepharose column (HisTrap HP, Amersham Biosciences), and the fusion protein was eluted with a 50-ml gradient of imidazole (10-500 mm). The eluted protein was collected and digested with 200 NIH units of thrombin after overnight dialysis against a 4-liter buffer of 25 mm Tris, pH 8.0, and 200 mm NaCl. Thrombin was removed by passage over a benzamidine-Sepharose column (1 ml, Amersham Biosciences), followed by the addition of 1 mm phenylmethylsulfonyl fluoride. The IIBMan protein after cleavage was collected after loading the digested mixture onto a 5-ml nickel-Sepharose column and further purified by gel filtration.
All NMR samples were prepared in a buffer of 20 mm sodium phosphate, pH 6.5, 0.01% sodium azide, and either 90% H2O/10% D2O or 99.996% D2O. IIAMan is a symmetric dimer with two non-overlapping but equivalent binding sites for IIBMan. To achieve optimal line widths we chose to record the majority of spectra on samples comprising a 1:1 mixture of IIAMan dimer to IIBMan monomer (see “Results” and “Discussion”).
NMR Spectroscopy—NMR spectra were recorded at 30 °C on Bruker DMX500, DMX600, DRX600, and DRX800 spectrometers equipped with either x-, y-, and z-shielded gradient triple resonance probes or z-shielded gradient triple resonance cryoprobes. Spectra were processed with the NMRPipe package (23) and analyzed using the programs PIPP, CAPP, and STAPP (24).
Sequential and side-chain assignments of free IIBMan were derived from three-dimensional double and triple resonance through-bond correlation experiments (25, 26): HNCACB, CBCA(CO)NH, HBHA-(CBCACO)NH, C(CCO)NH, H(CCO)NH), HCCH-COSY, and HCCH-TOCSY. Interproton distance restraints were derived from three-dimensional 15N-, 13C-, 13C/15N-, 13C/13C-, and 15N/15N-separated NOE spectra (25, 26). Side-chain rotamers were derived from 3JNCγ (aromatic, methyl, and methylene), 3JC′Cγ (aromatic, methyl, and methylene) and 3JCC scalar couplings measured by quantitative J correlation spectroscopy (27), in combination with data from a short mixing time three-dimensional 13C-separated NOE spectrum recorded in H2O and a three-dimensional 15N-separated ROE spectrum (26). Residual dipolar couplings (RDCs) were measured by taking the difference in J couplings between aligned and isotropic media using well established procedures (28). 1DNH, 1DNC′, and 2DNC′ RDCs were obtained in two alignment media: 10 mg/ml phage pf1 (29) and 5% C12E5 polyethylene glycol/hexanol (30).
Assignments of free wild-type IIAMan were taken from previously published work by Williams et al. (9). Assignments for the H10E mutant of IIAMan were derived from three-dimensional double and triple resonance experiments with reference to the free wild-type IIAMan assignments.
Assignments of 13C, 15N, and 1H chemical shifts in the IIAMan-IIBMan complex were based on the assignments of the free proteins in conjunction with data from titration experiments using constant time 1H-13C HMQC and TROSY 1H-15N spectra, as well as TROSY-based triple resonance through-bond correlation experiments (31).
Intermolecular NOEs were observed on the IIAMan-IIBMan complex in D2O buffer using three-dimensional 12C-filtered(F1)/13C-separated(F2) or 13C-separated(F2)/12C-filtered(F3) NOE experiments and in H2O buffer using two-dimensional 15N separated/13C-edited and two-dimensional 13C-separated/15N-edited NOE experiments (32). The following combinations of isotope-labeled complexes were primarily used for analysis of intermolecular NOEs: [U-1H/13C/15N]-IIBMan with unlabeled IIAMan (wild-type and H10E), [13CH3-LV]/[12C/14N/2H]-IIAMan, [12CH3-LV]/[1H-MW]/[12C/14N/2H]-IIAMan (wild-type and H10E), and [1H-M]/[12C/14N/2H]-IIAMan; unlabeled IIBMan with [U-1H/13C/15N]-IIAMan (wild-type and H10E); [12CH3-LV]/[2H/12C/14N]-IIBMan with [12CH3-LV]/[12C/14N/2H]-IIAMan and [12CH3-LV]/[1H-MW]/[12C/14N/2H]-IIAMan (wild-type and H10E); [12CH3-LV]/[2H/12C/14N]-IIBMan and [13CH3-LV]/[1H/13C-M]/[2H/13C/15N]-IIAMan; [U-1H/13C/15N]-IIBMan(R172Q) with (12CH3-LV]/[1H-MW]/[12C/14N/2H]-IIAMan(H10E); [U-1H/13C/15N]-IIBMan(H175E) and [U-1H/13C/15N]-IIBMan(R172Q) with [U-12C/14N/1H]-IIAMan; and [1HN-15N/12C/2H]-IIBMan with [13CH3-LV]/[1H/13C-M]/[2H/13C/15N]-IIAMan(H10E).
Structure Calculations—Interproton distance restraints were derived from the NOE spectra and classified into generous approximate distance ranges, 1.8-2.7, 1.8-3.5, 1.8-5.0, and 1.8-6.0 Å (with an additional 0.5 Å added to the upper limits for NOEs involving methyl groups), corresponding to strong, medium, weak, and very weak NOE cross-peak intensities, respectively (25, 33). Non-stereospecifically assigned methyl, methylene, and aromatic protons and ambiguous intermolecular NOEs were represented by a (∑r-6)-1/6 sum (26, 34). ϕ/ψ torsion angle restraints for free IIBMan were derived from backbone (N, C′, Cα, Cβ, and Hα) chemical shifts using the program TALOS (35). Side-chain χ torsion angle restraints were derived from 3J heteronuclear couplings and short mixing time NOE and ROE experiments using standard procedures (26). The minimum range for the torsion angle restraints was ±20°.
All structure calculations were carried out using Xplor-NIH (36) and the IVM (21) module for torsion angle and rigid body dynamics. The structure of the free IIBMan was calculated by simulated annealing in torsion angle space (21). The structure determination of the IIAMan-IIBMan complex was carried out using conjoined rigid body/torsion angle dynamics (21, 37). The target function for simulated annealing comprises the following: square well potentials for interproton distance and torsion angle restraints (38); harmonic potentials for 13Cα/13Cβ chemical shift restraints (39), RDC restraints (40), and covalent geometry; and a quartic van der Waals repulsion potential (41), a multidimensional torsion angle data base potential of mean force (42), a backbone hydrogen bonding data base potential of mean force with automatic hydrogen-bond selection (43), and a gyration volume term (44) to represent the non-bonded contacts. The gyration volume term represents a general, weak overall packing potential for any ellipsoidal-shaped molecule based on the observation that proteins pack to a constant density (45). Structures were displayed using VMD-XPLOR (46) and GRASP (47).
RESULTS AND DISCUSSION
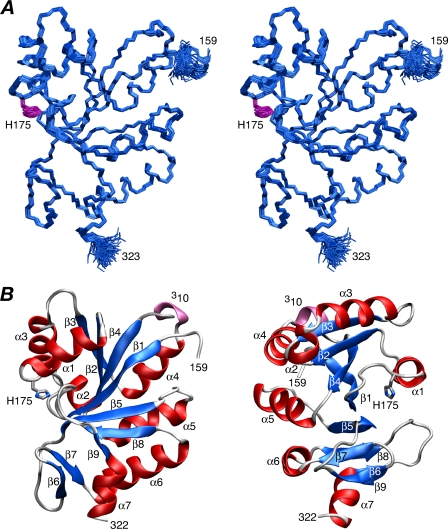
Structure of IIBMan—The solution structure of IIBMan was solved on the basis of 3091 experimental NMR restraints, including 1467 NOE-derived interproton distance restraints and 717 backbone RDCs (1DNH, 1DNC′, and 2DHNC′) in two different alignment media (pf1 phage and polyethylene glycol/hexanol). RDCs provide orientational restraints relative to an external alignment tensor (28), and the two alignment media provide complementary information, because their alignment tensors are significantly different from one another with a normalized scalar product of 0.57. A summary of the structural statistics is provided in Table 1, and a stereoview of a best-fit superposition of the backbone atoms of the 130 final simulated annealing structures is shown in Fig. 1A. Residues 157-159 at the N terminus and 322-323 at the C terminus are disordered. The rest of the structure (residues 160-321) is well defined with a backbone (N, Cα, C′, and O) precision of 0.26 ± 0.04 Å. 93% of the residues occupy the most favorable region of Ramachandran space (48) and the hydrogen bonding data base potential (43) automatically identified 93 backbone hydrogen bonds (of which only 55 were explicitly identified based on the pattern of NOEs).
TABLE 1.
Structural statistics for free IIBMan
<SA> are the final 130 simulated annealing structures. (SA)r is the restrained regularized mean structure derived from the mean coordinates obtained by averaging the coordinates of the 130 simulated annealing structures best-fitted to each other. The number of terms for the various experimental restraints is given in parentheses. None of the structures exhibit interproton distance violations >0.3 A or torsion angle violations 5°.
| <SA> | (SA)r | |
|---|---|---|
| r.m.s. deviation from experimental restraints | ||
| Distances (Å) (1577)a | 0.006 ± 0.001 | 0.008 |
| Torsion angles (°) (478)b | 0.33 ± 0.03 | 0.26 |
| 13Cα shifts (ppm) (161) | 1.26 ± 0.01 | 1.25 |
| 13Cβ shifts (ppm) (158) | 1.24 ± 0.01 | 1.23 |
| RDC R-factors (%)c | ||
| Phage 1DNH (151) | 4.3 ± 0.1 | 4.2 |
| Phage 1DNC′ (113) | 18.7 ± 0.5 | 18.2 |
| Phage 2DHNC′ (113) | 16.9 ± 0.2 | 16.5 |
| PEG/hexanol 1DNH (141) | 5.9 ± 0.1 | 6.0 |
| PEG/hexanol 1DNC′ (96) | 26.5 ± 0.3 | 25.8 |
| PEG/hexanol 2DHNC′ (103) | 24.3 ± 0.3 | 23.5 |
| r.m.s. deviations from idealized covalent geometry | ||
| Bonds (Å) | 0.002 ± 0.0001 | 0.004 |
| Angles (°) | 0.36 ± 0.02 | 0.54 |
| Impropers (°) | 0.53 ± 0.04 | 0.65 |
| Measures of structure qualityd | ||
| % residues in most favored region of Ramachandran plot | 93.1 ± 0.7 | 92.6 |
| Bad contacts per 100 residues | 3.4 ± 1.0 | 1.8 |
| Precision of atomic coordinates (Å)e | ||
| Backbone (N, Cα, C′, and O) | 0.26 ± 0.04 | |
| All heavy atoms | 0.74 ± 0.05 |
There are 1467 interproton distance restraints comprising 451 intra-residue restraints, and 407 |i - j| = 1 sequential, 270 1<|i - j| ≤ 5 medium range and 339 |i - j| > 5 long range inter-residue restraints. In addition there are 110 distance restraints for 55 backbone hydrogen bonds that were added during the final stages of refinement.
The torsion angle restraints comprise 160 ϕ, 157 ψ, 108 X1, 44 X2, and 9 X3 angles.
The RDC R-factor, which scales between 0 and 100%, is defined as the ratio of the r.m.s. deviation between observed and calculated values to the expected r.m.s. deviation if the vectors were randomly distributed, given by [2Da 2(4 + 3η2)/5]½, where Da is the magnitude of the principal component of the alignment tensor, and η is the rhombicity (61). The values of DaNH and η, derived from the distribution of normalized RDCs, are −11.7 Hz and 0.30, respectively, for the data recorded in pf1 phage, and −10.0 Hz and 0.28, respectively, for the data in PEG/hexanol (62).
Calculated with the program PROCHECK (48). The dihedral angle PROCHECK G factors for ϕ/ψ, X1/X2, X1, and X3/X4 are 0.06 ± 0.02, 0.57 ± 0.06, 0.14 ± 0.08, and 0.22 ± 0.13, respectively. The WHATIF first generation packing score is 0.13; a value greater than −0.5 is considered to represent a high quality structure (63).
The precision of the coordinates is defined as the average atomic r.m.s. difference between the individual 130 simulated annealing structures and the corresponding mean coordinates best-fitted to the backbone atoms of residues 160-321. (Residues 156-159 and 322-323 at the N and C termini, respectively, are disordered.)
FIGURE 1.
Solution structure of IIBMan. A, stereoview of a best-fit superposition of 130 simulated annealing structures with the backbone (N, Cα, and C) atoms in blue and the side chain of the active site His-175 in purple. B, two approximately orthogonal views of a ribbon diagram of IIBMan with α-helices in red, sheets in blue, and the single 3-10 helix in lilac.
The structure of IIBMan comprises a central seven-stranded mixed β-sheet (β1, β2, β3, β4, β5, β8, and β9) with a [-2x, -1x, 2x, 2x, 1x, 1] topology, surrounded by seven α-helices and a short anti-parallel β-sheet (β6 and β7). The active site (residues 172-176) immediately precedes helix α1. The side chain of the active site His-175 is in a g+/g+ conformation stabilized by an electrostatic interaction between the carboxylate of Asp-170 and its Nε2-H atom, with the Nδ1 atom exposed to solvent and available for phosphorylation.
Not surprisingly the solution NMR structure of E. coli IIBMan is similar to that of the x-ray structures of K. pneumoniae IIBSor (2.9-Å resolution) (49) and B. subtilis IIBLev (1.75-Å resolution) (50), as expected from their high percentage sequence identity (42 and 49%, respectively). The Cα backbone r.m.s. differences are 1.0 and 1.4 Å, respectively, for the complete polypeptide chain, and 1.1 and 0.7 Å, respectively, for residues 163-186 comprising strand β1 (163-170), the active site loop (residues 171-176), and helix α1 (residues 177-186).
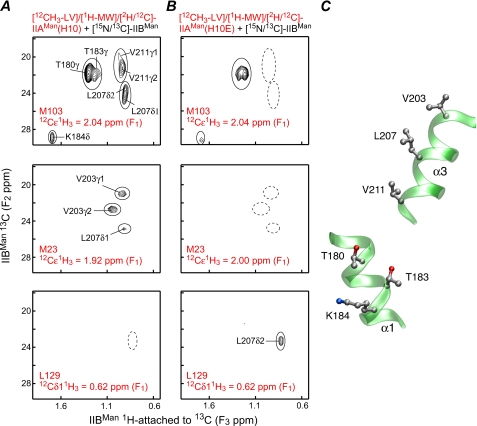
Binding of IIAMan to IIBMan—Three-dimensional 12C-filtered/13C-separated NOE experiments carried out on complexes of wild-type IIAMan with IIBMan revealed a pattern of intermolecular NOEs that was not consistent with a single species and suggested the presence of two co-existing complexes (Fig. 2). This is best illustrated by the top panel in Fig. 2A, which shows intermolecular NOEs from the isolated resonance of the methyl group of Met-103 of [12CH3-LV]/[1H-MW]/[12 C/14N/2H]-IIAMan to the methyl group region of [U-13C/15N]-IIBMan. NOEs are observed to the methyl groups of Leu-207 and Val-211, which form one cluster on the surface of IIBMan, and to the methyl groups of Thr-180 and Thr-183, as well as the δ-methylene group of Lys-184, which form a second cluster. Because the methyl groups of Val-211/Leu-207 are ∼11/18, ∼13/19, and ∼16/22 Å away from the methyl groups of Thr-180 and Thr-183 and the δ-methylene group of Lys-184, respectively, it is evident that the methyl group of Met-103 of IIAMan cannot be close to both clusters of residues simultaneously (Fig. 2C).
FIGURE 2.
Intermolecular NOEs observed in the IIAMan-IIBMan complex. Methyl region of 1H(13C-attached, F3)/13C(F2) planes from a three-dimensional 12C-filtered(F1)/13C-separated(F2) NOE-HSQC spectrum displaying NOEs from the methyl protons of Met-103 (top panels), Met-23 (middle panels), and Leu-129 δ1 (bottom panels) of [12CH3-LV]/[1H-MV]/[2H/12C/14N]-IIAMan to protons attached to 13Cof[U-15N/13C]-IIBMan. A, wild-type IIAMan(H10) and IIBMan. B, IIAMan(H10E) and IIBMan. C, location of the two clusters of residues located on helices α1 and α3 of IIBMan that are far apart in the structure but exhibit NOEs to the methyl group of Met-103 in the wild-type sample. The wild-type sample (A) displays NOEs arising from a mixture of two complexes comprising one that is compatible with phosphoryl transfer (productive) and the other where phosphoryl transfer cannot take place (non-productive); the complex of the phosphomimetic IIAMan(H10E) with IIBMan (B) corresponds to a single productive complex.
Further qualitative interpretation of the intermolecular NOE data suggested that a small number of NOEs were consistent with a productive complex, that is one in which the two active site histidines, His-10 of IIAMan and His-175 of IIBMan, are in close proximity and therefore capable of phosphoryl transfer, whereas the majority of NOEs arose from a non-productive complex. However, in the absence of prior detailed knowledge of one or the other structure, resolving the structures of two complexes simultaneously from the data was not feasible.
We reasoned that a possible explanation for the existence of two complexes could involve the conserved, solvent-exposed Arg-172 in close proximity to the active site His-175 of IIBMan. In the productive complex, Arg-172 would be buried at the interface and had been previously postulated to interact with the negatively charged phosphoryl group on His-10 of IIAMan (50). In the absence of histidine phosphorylation burial of the positively charged guanidinium group of Arg-172 at a protein-protein interface is likely to disfavor the formation of the productive complex. To test this hypothesis we mutated His-10 of IIAMan to Glu to mimic the effect of phosphorylation of His-10 (at its Nε2 position). Analysis of the intermolecular NOE data for the resulting complex of the IIAMan(H10E) mutant with IIBMan was fully consistent with the formation of a single complex corresponding to the productive phosphoryl transfer complex. The change in the pattern of observed intermolecular NOEs can be seen by comparison of Figs. 2A and B.
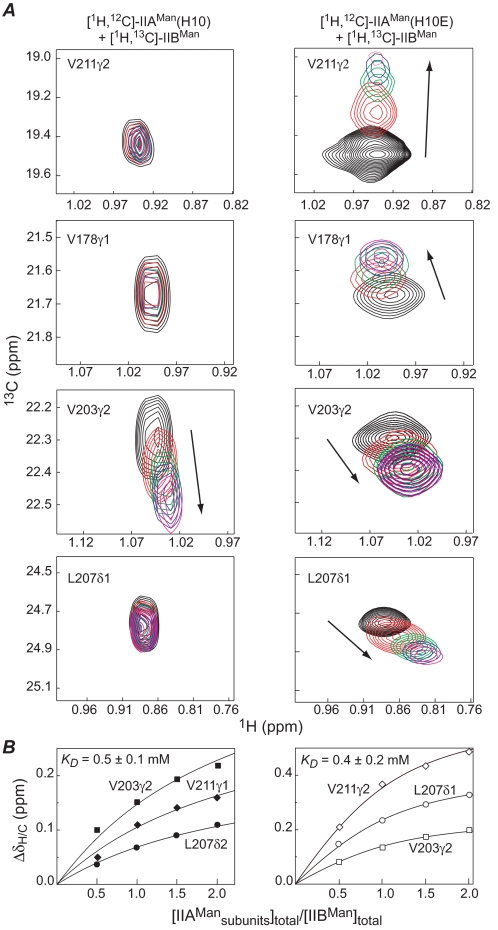
Both wild-type and H10E IIAMan bind weakly to IIBMan, and the complexes are in fast exchange on the chemical shift time scale. The change in pattern of intermolecular NOEs between the complex of wild-type and H10E IIAMan with IIBMan is accompanied by differences in the chemical shift perturbation of methyl groups of IIBMan observed upon titration (Fig. 3A). Interestingly, the apparent affinity of the complex of IIBMan with both wild-type IIAMan and the H10E mutant are very comparable with an equilibrium dissociation constant (KD) of ∼0.5 mm (Fig. 3B). Although binding is weak, in the context of intact IIABMan, where the A and B domains are connected by a flexible 25-residue linker, one can calculate (11, 51), based upon the expected average end-to-end distance of ∼50 Å for the linker (52), that there would be an ∼85% probability of the two domains interacting with one another at any given time.
FIGURE 3.
Binding of IIBMan to wild-type IIAMan (left-hand panels) and the phosphomimetic H10E mutant of IIAMan (right-hand panels). A, contour plots of isolated methyl group resonances of IIBMan obtained from two-dimensional constant-time 1H-13C HMQC spectra recorded at [IIAMan]subunits/[IIBMan]total ratios of 0 (black), 0.5 (red), 1.0 (green), 1.5 (blue), and 2.0 (magenta). B, chemical shift perturbation (ΔδH/C = [25Δδ(1H)2 +Δδ(13C)2]1/2 of selected methyl groups of IIBMan as a function of added IIAMan. The symbols represent the experimental data, and the solid continuous lines are global best-fit theoretical curves for a simple binding isotherm. The concentration of IIAMan is expressed on a subunit basis (i.e. two IIBMan binding sites per IIAMan dimer).
Structure Determination of the Productive and Non-productive IIAMan-IIBMan Complexes—The NMR samples used for structure determination comprised a mixture of 1 equivalent IIAMan dimer to 1 equivalent IIBMan monomer. IIAMan is a symmetric dimer, and the final stoichiometry of both the productive and non-productive complexes is 1 equivalent of IIAMan dimer to 2 equivalents of IIBMan. The use of a 1:1 mixture was chosen to optimize the line widths of both components, IIAMan and IIBMan, simultaneously. Because binding is weak, the samples will always comprise a mixture of free, 1:1 and 1:2 complexes in fast exchange with one another at the concentrations employed in the NMR experiments (0.5-1 mm). The line widths are proportional to the population average molecular weights. Given a KD of ∼0.5 mm, a sample containing 1 mm IIAMan dimer and 1 mm IIBMan (with free molecular masses of 35 and 19 kDa, respectively), will comprise ∼59 and ∼72% complexed IIAMan and IIBMan, respectively, with an apparent molecular mass of ∼49 kDa for both IIAMan and IIBMan. In contrast, a mixture of 1 mm IIAMan and 2 mm IIBMan will yield an effective molecular mass of 58 kDa for IIAMan and 48 kDa for IIBMan.
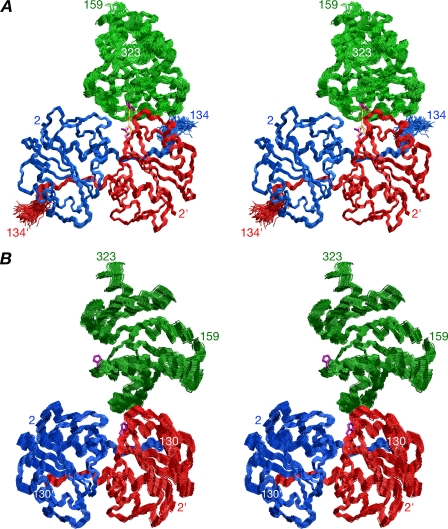
We first solved the structure of the productive complex using data exclusively from the IIAMan(H10E)-IIBMan samples. The changes in 1H/15N shifts upon complexation are very small indicating no significant structural perturbation in the backbone coordinates of either IIAMan or IIBMan occurs upon binding (at the level of detection of the NMR data). We therefore solved the structure of the productive complex using a hybrid approach that employs conjoined rigid body/torsion angle dynamics (20, 21) on the basis of intermolecular NOE data with the coordinates of free IIAMan (x-ray, PDB code 1POD) (16) and IIBMan (the complete ensemble of NMR simulated annealing structures; this report) treated as rigid bodies and the interfacial side chains given torsional degrees of freedom. In addition, the backbone and side chains of residues 130-134 of IIAMan were also given torsional degrees of freedom, because intermolecular NOEs were observed involving residues 129, 133, and 134, although residues 131-133 were not visible in the electron density map of the free crystal structure (16). 37 intermolecular NOEs were identified of which 33 are between unique proton pairs, and the remaining 4 are ambiguous involving potentially alternate partners and were therefore treated as (∑r-6)-1/6 sums. The active site His-10 is located right at the interface of the two identical subunits of IIAMan. Because the Cα atom positions of the two symmetrically related active site histidines, His-10 and His-10′, are separated by 20 Å, there is no issue attributing the intermolecular NOEs to one or other subunit of IIAMan. It should be noted that the use of RDCs to provide orientational information for the structure determination of the complex was precluded owing to uncertainties in the exact proportions of each component in the sample (i.e. free proteins, complex with one IIBMan bound and complex with two IIBMan molecules bound, all of which will have different alignment tensors), and the unfeasibility of deconvoluting the alignment tensors of the 1:1 and 1:2 complexes (because complete occupancy of the 1:2 complex cannot be achieved at concentrations compatible with the alignment media used for RDC measurements). A summary of the structural statistics is given in Table 2, and a best-fit superposition of the final 120 simulated annealing structures is shown in Fig. 4A. The relative orientation of IIBMan relative to the IIAMan dimer is well determined by the intermolecular NOE data with an overall backbone precision for the complex of 0.5 Å.
TABLE 2.
Structural statistics for productive and non-productive IIAMan-IIBMan complexes
The notation is the same as that in Table 1. The final number of simulated annealing structures is 120. The number of experimental restraints for the various terms is given in parentheses, with the first number referring to the data for the productive complex derived from the IIAMan(H10E)-IIBMan complex, and the second to the data from the wild type IIAMan(H10)-IIBMan complex.
|
Productive complex
|
Non-productive complex
|
|||
|---|---|---|---|---|
| <SA> | (SA)r | <SA> | (SA)r | |
| r.m.s. deviations from experimental restraintsa | ||||
| Intermolecular interproton distances (Å) (37/41b) | 0.02 ± 0.01 | 0 | 0.02 ± 0.01 | 0.05 |
| Side-chain torsion angles (°) (47/30)c | 0.60 ± 0.07 | 0.72 | 0.66 ± 0.06 | 1.17 |
| Measures of structure qualityd | ||||
| Intermolecular repulsion energy (kcal.mol−1) | 2.9 + 1.5 | 7.7 | 0.1 ± 0.1 | 0.5 |
| Intermolecular Lennard-Jones energy (kcal.mol−1) | −28.1 ± 4.9 | −30.4 | −10.5 ± 2.4 | −11.1 |
| Coordinate precision of the complex (Å)e | ||||
| Complete backbone (N, Cα, C′, and O) atoms | 0.52 ± 0.15 | 0.76 ± 0.28 | ||
| Interfacial side-chain heavy atoms | 1.30 ± 0.10 | 1.26 ± 0.17 | ||
None of the structures exhibit NOE distance violations >0.3 Å or torsion angle violations >5°.
The data obtained for the wild-type IIAMan(H10E)-IIBMan complex arise from a mixture of productive and unproductive complexes. The NOE data were therefore represented as ambiguous (Σr−6)−⅙ sums. The productive complex was held fixed at the conformation determined from the IIAMan(H10E)-IIBMan data. Of the 41 intermolecular NOEs, only 5 (attributable to the productive complex) are not satisfied (i.e. violations > 0.5 Å) by the non-productive complex alone.
For the productive complex, the side-chain torsion angles comprise 11 χ1 and 5 χ2 for IIAMan, and 14 χ1 and 7 χ2 for IIBMan; in addition, there are 4ϕ and 3ψ backbone torsion angle restraints for residues 130-134 of IIAMan, which were also given torsional degrees of freedom. For the non-productive complex, the side-chain torsion angles comprise 11 χ1 and 5 χ2 for IIAMan, and 5 χ1 and 1 χ2 for IIBMan.
The intermolecular repulsion energy is given by the value of the quartic van der Waals repulsion term calculated with a force constant of 4 kcal.mol−1.Å−4 and a van der Waals radius scale factor of 0.78. The intermolecular Lennard-Jones van der Waals interaction energy is calculated using the CHARMM19/20 parameters and is not included in the target function used to calculate the structures. The percentage of residues present in the most favorable region of the Ramachandran map (48) for the x-ray structure of free IIAMan (PDB code 1 PDO (16)) is 93.3%.
Defined as the average r.m.s. difference between the final 120 conjoined rigid body/torsion angle dynamics simulated annealing structures and the mean coordinate positions. The values quoted for the complete backbone indicate the precision with which the orientation and translation of the IIAMan dimer and IIBMan have been determined relative to one another. Because the backbone of IIAMan is treated as a rigid body, the backbone values do not take into account the error in the x-ray coordinates of IIAMan (estimated at <0.3 Å). For the productive H10E(IIAMan)-IIBMan complex, the values for the backbone do take into account the precision of the free IIBMan coordinates, although the backbone of IIBMan is treated as a rigid body, because the complete ensemble of simulated annealing structures for IIBMan was employed in the calculations. For the non-productive complex the value for the backbone does not take into account the precision of the IIBMan coordinates, because the calculations used the restrained regularized mean coordinates of IIBMan.
FIGURE 4.
Structures of the productive and non-productive IIAMan-IIBMan complexes in solution. Stereoviews showing best-fit superpositions of backbone (N, Cα, and C) atoms for the final 120 simulated annealing structures, with IIBMan in green, and the A and B subunits of IIAMan in blue and red, respectively; the active site side chains of the restrained regularized mean structure are shown in purple. A, structure corresponding to the productive, phosphoryl transfer competent complex, derived from the NOE data obtained with the phosphomimetic H10E mutant of IIAMan. The modeled phosphoryl transition state with a pentacoordinate phosphoryl group and Glu-10 replaced by His is shown in transparent orange. Only negligible changes (<0.2 Å) in local backbone conformation in the immediate vicinity of the active sites (residues 8′-12′ and 173-177) are required to model the phosphoryl transition state. B, structure corresponding to the non-productive, phosphoryl transfer incompetent, complex derived from the NOE data obtained with wild-type IIAMan. The Cα-Cα distance between the active site residues (residue 10′ of the B chain of IIAMan and residue 175 of IIBMan)is11Åforthe productive complex versus 25 Å for the non-productive one.
As noted above, samples of wild-type IIAMan and IIBMan comprise a mixture of productive and non-productive complexes. Of the 41 intermolecular NOEs identified, only 5 satisfied the structure of the productive complex with violations < 0.5 Å, and the remainder are violated by 4-16 Å. To obtain the structure of the non-productive complex, we therefore proceeded as follows. The structure of the productive complex (restrained regularized mean coordinates) was held fixed and a second molecule of IIBMan (restrained regularized mean structure of free IIBMan) was introduced in random starting orientations and its position determined by conjoined rigid body/torsion angle dynamics (21) with the assigned intermolecular NOEs represented as ambiguous restraints, that is (∑r-6)-1/6 sums (34), arising from both the productive and non-productive complexes. (Note that because the intermolecular NOEs are interpreted in terms of loose, conservative distance ranges, and because long distances do not contribute to the (∑r-6)-1/6 sum, it is not necessary to know the proportion of productive and non-productive complexes present in the sample). A table of structural statistics is provided in Table 2, and a best-fit superposition of the ensemble of 120 simulated annealing structures of the non-productive complex is displayed in Fig. 4B. Although the relative orientation of IIBMan relative to IIAMan is not quite as well defined in the non-productive complex relative to the productive one, the backbone precision for the non-productive complex is still rather high (∼0.8 Å). It is worth noting that the same ensemble of structures was obtained for the non-productive complex by simply using the 36 intermolecular NOEs that were violated in the productive complex and not including the structure of the productive complex in the calculations.
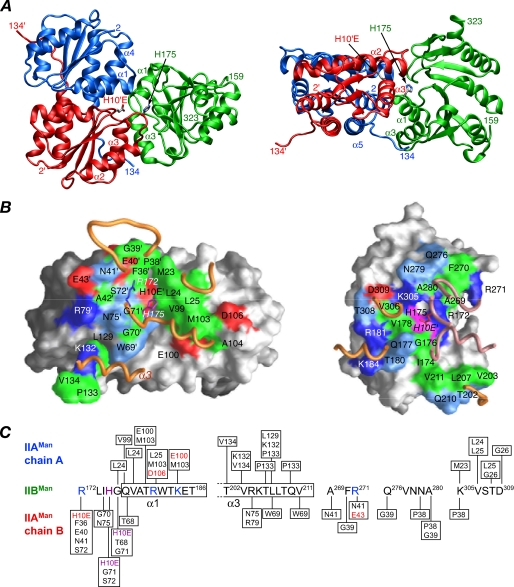
Structure of the Productive IIAMan-IIBMan Complex—A ribbon diagram of the productive complex derived from the IIAMan(H10E)-IIBMan data is shown in Fig. 5A. 1750 Å2 of solvent-accessible surface area is buried at the interface, 870 Å2 originating from IIAMan and 880 Å2 from IIBMan. The dimensions of the interface are ∼40 Å long and 30 Å wide. The gap volume index (ratio of gap volume to buried accessible surface area) is 3.5, which falls in the outer range observed for both hetero (2.4 ± 1.0) and optional (2.7 ± 0.9) protein-protein complexes (53), as expected given the weak binding.
FIGURE 5.
Overall view of the productive, phosphomimetic IIAMan(H10E)-IIBMan complex. A, ribbon diagram with the A and B chains of IIAMan in blue and red, respectively, and IIBMan in green; the active site residues H10E′ of IIAMan (chain B) and H175 of IIBMan are also displayed. B, interaction surfaces for the productive IIAMan(H10E)-IIBMan complex. The left and right panels display the interaction surfaces on IIAMan and IIBMan, respectively. The surfaces are color coded as follows: hydrophobic residues are green, uncharged residues bearing a polar functional group are cyan, negatively charged residues are red, positively charged residues are blue, active site histidines are purple, and non-interfacial residues are gray (with a darker shade for the B subunit of IIAMan). Relevant portions of the backbone of the interacting partner are displayed as tubes (IIBMan in gold, A chain of IIAMan in gold, and B chain of IIAMan in lilac). The side chains of His-175 and Arg-172 of IIBMan are shown in the left-hand panel, and the side chain of H10E′ of the B chain of IIAMan is displayed in the right-hand panel (labeled in italics). C, diagrammatic representation of the intermolecular contacts with the active site histidines colored in purple, and residues involved in potential side chain-side chain intermolecular electrostatic interactions colored in red (acceptor) and blue (donor).
In describing the intermolecular contacts involving IIAMan, the residues of the B-chain (red subunit in Figs. 5, 6, 7 and 8) are denoted by a prime symbol. (Note that the definition of A and B chains of IIAMan is purely arbitrary, and the relationship of the two IIAMan chains to IIBMan at one site is reversed in the symmetry related site.)
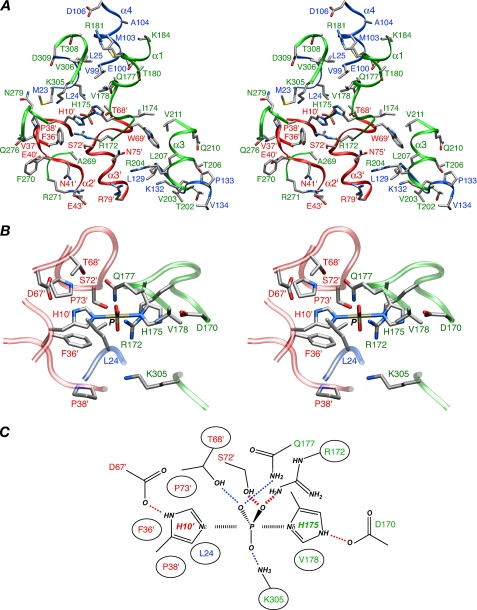
FIGURE 6.
The IIAMan(H10E)-IIBMan interface and the phosphoryl transition state. A, overall stereoview of the interface, including the modeled His-10′—P—His-175 phosphoryl transition state. B, enlarged view depicting the vicinity around the His-10′—P—His-175 transition state. C, schematic diagram of the phosphoryl transition state. In A and B, the backbones are depicted as ribbons (blue, chain A of IIAMan; red, chain B of IIAMan; and green, IIBMan), and the side-chain atoms are colored according to atom type; carbon, gray; nitrogen, blue; oxygen, red; sulfur, yellow, phosphorus, tan. In C, red dashed lines indicate likely hydrogen bonds with donor acceptor distances < 3.5 Å; blue dashed lines represent potential water-bridged interactions with donor-acceptor distances between 5 and 6 Å. The phosphorus and active site histidines are surrounded by a large number of hydrophobic residues (circled), including the long aliphatic side chains of Arg-172 and Lys-305 of IIBMan.
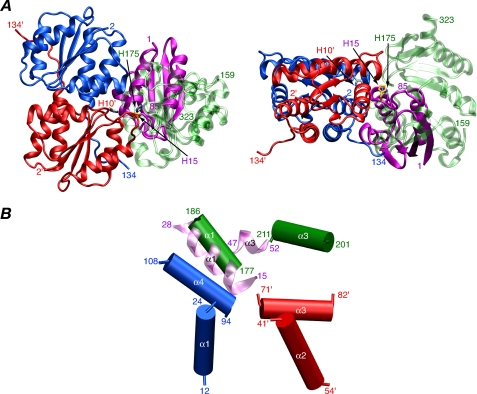
FIGURE 7.
Comparison of the IIAMan-HPr complex with the productive IIAMan-IIBMan complex. A, ribbon diagram showing a comparison of the IIAMan-HPr (PDB code 1VRC (9)) and IIAMan-IIBMan complexes superimposed on the coordinates of IIAMan. The A and B subunits of IIAMan are shown in blue and red, respectively, HPr is shown in purple, and IIBMan is in transparent green. The active site residues are also displayed (His-10′ of the B chain of IIAMan, His-15 of HPr, and His-175 of IIBMan). B, comparison of intermolecular helical-helical interactions in the IIAMan-IIBMan and IIAMan-HPr complexes, helices α1 and α3 of IIBMan in green, helices α1 and α3 of HPr in transparent lilac, helices α1 and α4 of the A chain of IIAMan in blue, and helices α2 and α3 of the B chain of IIAMan in red.
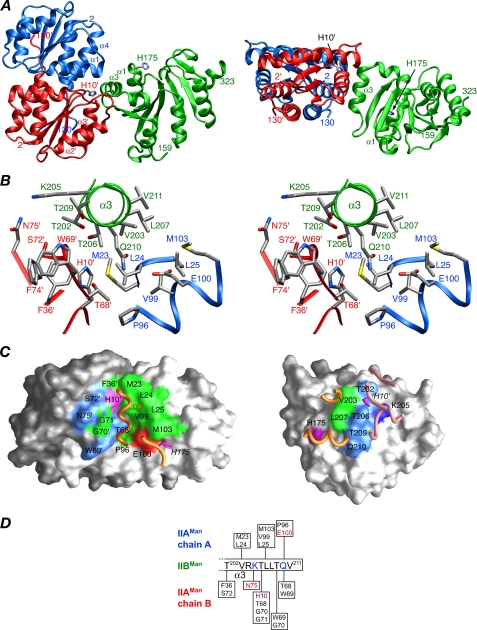
FIGURE 8.
The non-productive, phosphoryl transfer incompetent, IIAMan-IIBMan complex observed with wild-type IIAMan. A, ribbon diagram (blue, chain A of IIAMan; red, chain B or IIAMan; green, IIBMan). The side chains of the active site histidines (His-10′ of IIAMan and His-175 of IIBMan, separated by a Cα-Cα distance of ∼25 Å, are also shown. B, stereoview showing the detailed interactions at the interface. The color coding of the backbone chains (shown as tubes) is as in A, and the side-chain bonds are colored coded according to atom type (see Fig. 6 legend). C, interaction surfaces for the non-productive IIAMan-IIBMan. The left and right panels display the interaction surfaces on IIAMan and IIBMan, respectively, with the color coding as in Fig. 6B. D, diagrammatic representation of the intermolecular contacts with the active site His-10′ colored in purple, and residues involved in potential side chain-side chain intermolecular electrostatic interactions colored in red (acceptor) and blue (donor).
The interface on IIAMan is made up of residues of both subunits and comprises helix α1, helix α4, and the C-terminal five residues of the A-chain: the active site His-10′, the loop between β2′ and α2′, the N-terminal end of helix α2′, and helix α3′ of the B-chain. The interface on IIBMan comprises the active site (residues 172-176, including His-175), helices α1 and α3, and the loops between strands β5 and β6, β6 and β7, and β8 and β9 (Fig. 5, A and C). A summary of the contacts is provided in Fig. 5C, and a stereoview showing the side-chain interactions is shown in Fig. 6A. The A chain of IIAMan primarily interacts with helices α1 and α3, and the loop between strands β8 and β9 of IIBMan, whereas the B-chain primarily contacts the active site loop, helix α3, and the loops between strands β5 and β6 and strands β6 and β7 of IIBMan.
The interface is made up of 57% non-polar atoms and 43% polar ones. The active site histidines, His-10′ and His-175, are located at the center of the interface, as is Arg-172. In the IIAMan(H10E)-IIBMan complex, the positively charged guanidino group of Arg-172 is neutralized by the negative charge on the carboxylate of H10E′, mimicking phosphorylated His-10′. In the absence of neutralization of the guanidino group of Arg-172, the productive complex is destabilized allowing an alternative, non-productive complex to be formed. The majority of intermolecular interactions are hydrophobic in nature, and there are only three additional electrostatic interactions, between Asp-106 and Arg-180, and Glu-100 and Lys-184, which anchor helix α1 of IIBMan, and between Glu-43′ and Arg-271, which anchors the loop between strands β5 and β6 of IIBMan. A ridge of hydrophobic residues comprising Met-23, Leu-24, Leu-25, Val-99, and Met-103 of IIAMan provide non-polar interactions with complementary residues on helix α1 and the loop between strands β8 and β9 of IIBMan. Thr-202, Val-203, Leu-207, and Val-211 of helix α3 of IIBMan are wedged in a hydrophobic groove formed by Leu-129, Lys-132, Pro-133, and Val-134 of the C terminus of the A-chain of IIAMan and Trp-69′ of the B chain of IIAMan.
Phosphoryl transfer between IIAMan and IIBMan, as in other PTS complexes, is known to proceed through a transition state involving a pentacoordinate phosphoryl group in a trigonal bipyramidal geometry with the donor (Nε2 of His-10′ of IIAMan) and acceptor (Nδ1 of His-175 of IIBMan) atoms in apical positions (54, 55). The transition state can readily be modeled on the basis of the structure of the IIAMan(H10E)-IIBMan complex using the procedures described previously (6-9) in which the only portion of the complex allowed to move comprises the backbone and side chains of the active site histidines (His-10′ and His-175), the immediately adjacent residues (residues 8-12′ of IIAMan and 173-177 of IIBMan) and the phosphoryl group (Figs. 4A and 6B). For an N-P distance of 2.5 Å, which corresponds to a mechanism with substantial dissociative character consistent with many phosphoryl transfer reactions (56), the transition state can be accommodated with negligible (<0.2 Å) changes in backbone conformation for residues 9′-11′ and 174-176. Only minor additional backbone changes (∼0.2 Å) for these residues are required for an SN2 mechanism (50% associative) with an N-P distance of 2 Å.
The His-10′-P-His-175 transition state is buried within a largely hydrophobic cavity comprising Leu-24, Phe-36′, Pro-38′, and Pro-73′ of IIAMan, and Val-178 and the aliphatic portions of the long side chains of Arg-172 and Lys-305 of IIBMan. The phosphoryl group is within hydrogen bonding distance of the hydroxyl group of Ser-72′ and the guanidino group of Arg-172, thereby stabilizing the transition state. In addition, there may be water-bridged interactions to the phosphoryl group from the hydroxyl group of Thr-68′, the carboxyamide of Gln-177, and the NH3 group of Lys-305 (Fig. 6, B and C).
The Cα-Cα distances between the N terminus of IIBMan (residue 209) and the C termini (residues 134 and 134′) of the A and B chains of IIAMan are 38 Å and 64 Å, respectively. The expected average end-to-end distance for a random-coil 25-residue linker is ∼50 Å (52). This suggests that, in the intact IIABMan dimer, phosphoryl transfer occurs predominantly in trans, that is between the IIAMan domain of one chain, and the IIBMan domain of the other chain.
The structure of the productive IIAMan-IIBMan complex displays both similarities and differences to the structure of the upstream IIAMan-HPr complex (9). Both IIBMan and HPr have an active site loop followed by an α-helix. The Cα atomic r.m.s. difference between the element of structure form by the active site loop and helix α1 (residues 171-186 of IIBMan and 11-26 of HPr) in the two structures is 1.7 Å. A comparison of the structures of the IIAMan-IIBMan and IIAMan-HPr complexes, superimposed on the coordinates of IIAMan, is shown in Fig. 7. The similar disposition of the active site loop and helix α1 of IIBMan and HPr relative to IIAMan is evident. However, the interface is more extensive in the IIAMan-IIBMan complex than in the IIAMan-HPr complex (1750 Å2 buried versus 1450 Å2). Moreover, HPr has a large contact surface with the A chain of IIAMan, whereas IIBMan has more extensive contacts with the B chain. Despite the reduced contact area, the affinity of HPr for IIAMan (KD ∼ 30 μm 9) is 15- to 20-fold higher than that of IIBMan (KD ∼ 0.5 mm; this report). This may be due to a higher degree of surface complementary, as exemplified, for example, by a 2-fold greater number of electrostatic/hydrogen-bonding interactions between HPr and IIAMan (9).
Structure of the Non-productive IIAMan-IIBMan Complex—A ribbon diagram of the non-productive complex is shown in Fig. 8A. The interaction involves a highly hydrophobic ridge-like protrusion on the surface of IIBMan formed exclusively by helix α3, interacting with a subset of residues on IIAMan that comprise the central portion of the interface involved in the productive complex (Fig. 8C). This subset comprises the predominantly hydrophobic surface formed by helices α1 (Met-23, Leu-24, and Leu-25) and α4 (Pro-96, Val-99, and Met-103) of the A-chain of IIAMan, and the active site His-10′, the loop following strand β2′ (Phe-36′), the loop between strand β3′ and helix α3′ (Thr-68′, Trp-69′, and Gly-70′), and helix α3′ (Ser-72′ and Asn-75′) of the B chain of IIAMan (Fig. 8, B and C). The buried accessible surface area at the interface is 790 Å2, of which 380 Å2 originates from IIAMan and 410 Å2 from IIBMan, approximately half that of the productive complex. The Cα-Cα distance between the two active site histidines, His-10′ and His-175, is 25 Å and therefore incompatible with phosphoryl transfer between IIAMan and IIBMan. The Cα-Cα distances between the N terminus of IIBMan and the C termini of the A and B chains of IIAMan are 60 and 30 Å, respectively (Fig. 8A). Thus, just as in the case of the productive complex, the interaction between the IIBMan domain and IIAMan domain is likely to occur in trans in the intact IIABMan dimer.
The orientation of IIBMan relative to IIAMan in the non-productive complex is related by a ∼90-Å rotation and ∼37-Å translation relative to the productive one. This is readily appreciated from a comparison of the location of helix α3 of IIBMan on the surface of IIAMan in the two complexes provided by Figs. 5B and 8C (left-hand panels).
The active site of IIBMan, including His-175 and Arg-172, is fully exposed to solvent in the non-productive complex, and thus His-175 is potentially available to transfer a phosphoryl group onto the incoming sugar located on the transmembrane IICMan-IIDMan complex. It is interesting to note that His-175 lies close to the upper edge of a deep V-shaped hydrophobic cleft at the bottom of which lies helix α3 of IIBMan, with an outer rim of negatively charged residues (Asp-106, Asp-107, and Asp-108) provided by the A chain of IIAMan and positively charged residues (Arg-172, Arg-181, Arg-204, and Lys-305) by IIBMan. The walls of the cleft are formed by helices α1 and α4 of the A chain of IIAMan, and the active site loop, helix α1, and the loops between strands β5 and β6 and between β6 and β7 of IIBMan.It is tempting to speculate that this cleft comprises part of the binding site for the membrane-bound IICMan-IIDMan complex. In this regard, it is worth noting that IIABMan has been reported to form a stable complex with the transmembrane IICMan-IIDMan component of the mannose transporter with an apparent KD of 5-10 nm (57), and a IIABMan-IICMan-IIDMan complex can be co-purified (58).
Probing the Role of Arg-172 in Complex Formation—The successful elimination of the non-productive complex as a consequence of the introduction of the phosphomimetic H10E mutation providing charge neutralization of Arg-172, strongly suggests a major role for Arg-172 in conjunction with phosphorylation of His-10 in modulating whether a productive or non-productive complex is formed. To probe the role of Arg-172 further, intermolecular NOEs involving the leucine (Leu-24, Leu-25, and Leu129), valine (Val-99 and Val-134), and methionine (Met-23 and Met-103) methyl groups of IIAMan were analyzed for the following samples: IIAMan-IIBMan(H175E), IIAMan-IIBMan (R172Q), and IIAMan(H10E)-IIBMan(R172Q). A qualitative assessment of the proportion of productive to non-productive complexes in these samples relative to the wild-type IIAMan-IIBMan sample can be obtained by examining (a) the fraction of observed intermolecular NOEs attributable to the productive complex and (b) the ratio of the intermolecular NOE cross-peak intensities involving the methyl group of Met-103 of IIAMan attributable to the productive and non-productive complexes (cf. Fig. 2, top panels). The latter has a value of ∼0.8 for the wild-type sample. (Note this value cannot be converted to populations of the two species in the wild-type sample, because the NOE intensities are related not only to population but also to specific intermolecular interproton distances in the two complexes.)
The pattern of intermolecular NOEs observed for the IIAMan-IIBMan(H175E) sample is very similar to that of the wild-type IIAMan-IIBMan sample, with only ∼15% of the intermolecular NOEs attributable to the productive complex compared with ∼10% for the wild-type sample. The ratio of the cross-peak intensities for the productive to non-productive complexes is increased by ∼1.2 relative to wild-type. These data indicate that the proportion of non-productive complex is only slightly decreased relative to wild type and, therefore, suggest that the H175E mutation does not provide adequate intramolecular charge neutralization of Arg-172. This finding may be relevant to the postulated role of the non-productive complex in transferring a phosphoryl group on to the incoming sugar on the transmembrane IICMan-IIDMan complex. In particular, this result may suggest that, once the phosphoryl group is transferred from His-10′ to His-175, the equilibrium between productive and non-productive complex may be shifted toward the non-productive complex if the intramolecular charge neutralization of Arg-172 by phosphorylated His-175 is less effective than the intermolecular charge neutralization by phosphorylated His-10′. This hypothesis may be supported by the observation that the side chain of Arg-172 is disordered in the crystal structure of B. subtilis IIBLev (49).
For the IIAMan-IIBMan(R172Q) sample, intermolecular NOEs corresponding to both productive and non-productive complexes were observed, but the fraction attributable to the productive complex was increased to ∼30%, and the ratio of the cross-peak intensities of productive to non-productive complexes was increased by ∼5-fold relative to wild type. Thus, the population of productive complex in the IIAMan-IIBMan(R172Q) sample was increased substantially relative to the wild-type IIAMan-IIBMan sample. The R172Q mutation removes the positive charge of Arg-172 but still leaves a potentially unfavorable polar residue in the middle of the interface of the productive complex, thereby accounting for the continued presence of non-productive complex.
Finally, ∼90% of intermolecular NOEs observed for the IIAMan(H10E)-IIBMan(R172Q) sample arise from the productive complex, and the few from the non-productive complex were extremely weak relative to their intensities in the wild-type sample. The ratio of NOE cross-peak intensities of productive to non-productive complexes for Met-103 was increased ∼9-fold relative to wild type. Thus, the productive complex constitutes the major species. This result is consistent with the observation that IIBMan(R172Q) can still be phosphorylated by IIAMan, albeit some-what less efficiently than wild-type IIBMan (59).
Concluding Remarks—This report completes the structures of cytoplasmic complexes of the mannose branch of the PTS. The intriguing finding is that the nature of the IIAMan-IIBMan complex can be modulated by the presence or absence of charge neutralization between the active sites, and in particular of the guanidino group of Arg-172. With wild-type IIAMan, two forms of complex with IIBMan were observed: the predominant one arises from a non-productive complex in which the active site histidines (His-10′ and His-175) are separated by ∼25 Å and therefore incompatible with phosphoryl transfer between IIAMan and IIBMan, whereas the minor one arises from a productive complex in which the active site histidines are in close proximity. Mutation of His-10′ of IIAMan to a Glu to mimic histidine phosphorylation results in the exclusive formation (at the level of detection) of a productive complex that is fully consistent with the formation of a pentacoordinate phosphoryl transition state and in which the positive charge on the guanidinium group of Arg-172 is neutralized by interaction with the negative carboxylate group of H10E′. In the non-productive complex, Arg-172 and the active site His-175 of IIBMan are fully exposed to solvent and potentially available to transfer a phosphoryl group to the sugar located on the cytoplasmic side of the IICMan-IIDMan transmembrane complex.
The structural transition between the productive and non-productive states is dramatic and involves a 90° rotation and concomitant 37-Å translation of IIBMan relative to IIAMan. The interaction surface on IIAMan in the non-productive complex comprises a subset of residues located in the central region of the interaction surface employed in the productive complex, including the active site His-10′. Thus, the non-productive complex does not allow for the formation of a ternary HPr-IIAMan-IIBMan complex, because the interaction surface on IIAMan used by IIBMan is also a subset of the interaction surface on IIAMan used by HPr. The interaction surface on IIBMan in the productive and non-productive complexes also partially overlap insofar that the interaction surface in the non-productive complex comprises exclusively helix α3, which is used in a completely different set of interactions with IIAMan in the productive complex.
The existence of the non-productive IIAMan-IIBMan complex may be fortuitous owing to the presence of a highly hydrophobic protrusion on the surface of IIBMan formed by helix α3 that can readily fit in a groove between the two subunits of IIAMan. Nevertheless, it seems likely that weak binding complexes with KD values in the 0.1-2 mm range may be particularly susceptible to multiple alternative configurations arising from rather small changes at the interface. Indeed, two distinct quaternary structures resulting from a relatively small number of changes at an interface have been observed in the much tighter homodimeric complexes of the chemokine family where the CXC and CC chemokines have high sequence identity, the same monomer folds, but entirely different dimeric structures employing completely different interfaces (60).
Acknowledgments
We thank Alan Peterkofsky for providing the DNA template for IIBMan and Dan Garrett for software support.
The atomic coordinates and experimental NMR restraints (codes 2JZH, 2JZN, 2JZO, and 1VSQ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by the intramural program of NIDDK, National Institutes of Health (NIH), and the Intramural AIDS Targeted Antiviral Program of the Office of the Director of the NIH (to G. M. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PTS, phosphoenolpyruvate:sugar phosphotransferase system; HPr, histidine-containing phosphocarrier protein; IIABMan, the cytoplasmic subunit of the mannose transporter;IICMan-IIDMan complex, transmembrane component of the mannose transporter; Man, mannose; Glc, glucose; Mtl, mannitol; Chb, chitobiose; NOE, nuclear Overhauser effect; HMQC, heteronuclear multiple quantum coherence; HSQC, heteronuclear single quantum coherence; RDC, residual dipolar coupling; r.m.s., root mean square.
References
- 1.Meadow, N. D., Fox, D. K., and Roseman, S. (1990) Annu. Rev. Biochem. 59 497-542 [DOI] [PubMed] [Google Scholar]
- 2.Siebold, C., Flükiger, K., Beutler, R., and Erni, B. (2001) FEBS Lett. 504 104-111 [DOI] [PubMed] [Google Scholar]
- 3.Deutscher, J., Francke, C., and Postma, P. W. (2006) Microbiol Mol. Biol. Rev. 70 939-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legler, P. M., Cai, M., Peterkofsky, A., and Clore, G. M. (2004) J. Biol. Chem. 279 39115-39121 [DOI] [PubMed] [Google Scholar]
- 5.Van Montfort, R. L., Pijning, T., Kalk, K. H., Reizer, J., Saier, M. J., Thunnissen, M. M., Robillard, G. T., and Dijkstra, B. W. (1997) Structure 5 217-225 [DOI] [PubMed] [Google Scholar]
- 6.Garrett, D. S., Seok, Y.-J., Peterkofsky, A., Gronenborn, A. M., and Clore, G. M. (1999) Nature Struct. Biol. 6 166-173 [DOI] [PubMed] [Google Scholar]
- 7.Wang, G., Louis, J. M., Sondej, M., Seok, Y.-J., Peterkofsky, A., and Clore, G. M. (2000) EMBO J. 19 5635-5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornilescu, G., Lee, B. R., Cornilescu, C., Wang, G., Peterkofsky, A., and Clore, G. M. (2002) J. Biol. Chem. 277 42289-42298 [DOI] [PubMed] [Google Scholar]
- 9.Williams, D. C., Cai, M., Suh, J.-Y., Peterkofsky, A., and Clore, G. M. (2005) J. Biol. Chem. 280 20775-20784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai, M., Williams, D. C., Wang, G., Lee, B. R., Peterkofsky, A., and Clore, G. M. (2003) J. Biol. Chem. 278 25191-25206 [DOI] [PubMed] [Google Scholar]
- 11.Suh, J.-Y., Cai, M., Williams, D. C., and Clore, G. M. (2006) J. Biol. Chem. 281 8939-8949 [DOI] [PubMed] [Google Scholar]
- 12.Williams, N., Fox, D. K., Shea, C., and Roseman, S. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 3083-3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erni, B., Zanolari, B., and Kocher, H. P. (1987) J. Biol. Chem. 262 5238-5247 [PubMed] [Google Scholar]
- 14.Erni, B., Zanolari, B., Graff, P., and Kocher, H. P. (1989) J. Biol. Chem. 264 18733-18741 [PubMed] [Google Scholar]
- 15.Markovic-Housley, Z., Cooper, A., Lustig, A., Flükiger, K., Stolz, B., and Erni, B. (1994) Biochemistry 33 10977-10984 [DOI] [PubMed] [Google Scholar]
- 16.Nunn, R. S., Markovic-Housley, Z., Genoveso-Taverne, G., Flükiger, K., Rizkallah, P. J., Jansonius, J. N., Schirmer, T., and Erni, B. (1996) J. Mol. Biol. 259 502-511 [DOI] [PubMed] [Google Scholar]
- 17.Gutknecht, R., Flükiger, K., Lanz, R., and Erni, B. (1999) J. Biol. Chem. 274 6091-6096 [DOI] [PubMed] [Google Scholar]
- 18.Wehmeier, U. F., and Lengeler, J. W. (1994) Biochim. Biophys. Acta 1208 348-351 [DOI] [PubMed] [Google Scholar]
- 19.Martin-Verstraete, I., Debarbouille, M., Lier, A., and Rapoport, G. (1990) J. Mol. Biol. 214 657-661 [DOI] [PubMed] [Google Scholar]
- 20.Clore, G. M. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 9021-9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwieters, C. D., and Clore, G. M. (2001) J. Magn. Reson. 152 288-302 [DOI] [PubMed] [Google Scholar]
- 22.Goto, N. K., Gardner, K. H., Mueller, G. A., Willis, R. C., and Kay, L. E. (1999) J. Biomol. NMR 13 369-374 [DOI] [PubMed] [Google Scholar]
- 23.Delaglio, F., Grzesiek, S., Vuiser, G. W., Zhu, G., Pfiefer, J., and Bax, A. (1995) J. Biomol. NMR 6 277-293 [DOI] [PubMed] [Google Scholar]
- 24.Garrett, D. S., Powers, R., Gronenborn, A. M., and Clore, G. M. (1991) J. Magn. Reson. 95 214-220 [DOI] [PubMed] [Google Scholar]
- 25.Clore, G. M., and Gronenborn, A. M. (1991) Ann. Rev. Biophys. Biophys. Chem. 20 29-63 [DOI] [PubMed] [Google Scholar]
- 26.Clore, G. M., and Gronenborn, A. M. (1998) Trends Biotechnol. 16 22-34 [DOI] [PubMed] [Google Scholar]
- 27.Bax, A., Vuister, G. W., Grzesiek, S., Delaglio, F., Wang, A. C., Tschduin, R., and Zhu, G. (1994) Methods Enzymol. 239 79-105 [DOI] [PubMed] [Google Scholar]
- 28.Bax, A., Kontaxis, G., and Tjandra, N. (2001) Methods Enzymol. 339 127-174 [DOI] [PubMed] [Google Scholar]
- 29.Clore, G. M., Starich, M. R., and Gronenborn, A. M. (1998) J. Am. Chem. Soc. 120 10571-10572 [Google Scholar]
- 30.Rückert, M., and Otting, G. (2000) J. Am. Chem. Soc. 122 7793-7797 [Google Scholar]
- 31.Kay, L. E. (2005) J. Magn. Reson. 173 193-2007 [DOI] [PubMed] [Google Scholar]
- 32.Cai, M., Huang, Y., Zheng, R., Wei, S. Q., Ghirlando, R., Lee, M. S., Craigie, R., Gronenborn, A. M., and Clore, G. M. (1998) Nat. Struct. Biol. 5 903-909 [DOI] [PubMed] [Google Scholar]
- 33.Clore, G. M., Sukuraman, D. K., Nilges, M., Zarbock, J., and Gronenborn, A. M. (1987) EMBO J. 6 529-537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilges, M. (1993) Proteins 17 297-309 [DOI] [PubMed] [Google Scholar]
- 35.Cornilescu, G., Delaglio, F., and Bax, A. (1999) J. Biomol. NMR 13 289-302 [DOI] [PubMed] [Google Scholar]
- 36.Schwieters, C. D., Kuszewski, J., and Clore, G. M. (2006) Progr. NMR Spect. 48 47-62 [Google Scholar]
- 37.Clore, G. M., and Bewley, C. A. (2001) J. Magn. Reson. 154 329-335 [DOI] [PubMed] [Google Scholar]
- 38.Clore, G. M., Nilges, M., Sukuraman, D. K., Brünger, A. T., Karplus, M., and Gronenborn, A. M. (1986) EMBO J. 5 2729-2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuszewski, J., Gronenborn, A. M., and Clore, G. M. (1995) J. Magn. Reson. Ser B 106 92-96 [DOI] [PubMed] [Google Scholar]
- 40.Clore, G. M., Gronenborn, A. M., and Tjandra, N. (1998) J. Magn. Reson. 31 159-162 [DOI] [PubMed] [Google Scholar]
- 41.Nilges, M., Gronenborn, A. M., Brünger, A. T., and Clore, G. M. (1988) Protein Eng. 2 27-38 [DOI] [PubMed] [Google Scholar]
- 42.Clore, G. M., and Kuszewski, J. (2002) J. Am. Chem. Soc. 124 2866-2867 [DOI] [PubMed] [Google Scholar]
- 43.Grishaev, A., and Bax, A. (2004) J. Am. Chem. Soc. 126 7281-7292 [DOI] [PubMed] [Google Scholar]
- 44.Schwieters, C. D., and Clore, G. M. (2007) J. Phys. Chem. B. 10.1021/jp076244o [DOI] [PubMed]
- 45.Quilin, M. L., and Matthews, B. W. (2000) Acta Crystallogr. Sect. D Biol. Crystallogr. 56 791-794 [DOI] [PubMed] [Google Scholar]
- 46.Schwieters, C. D., and Clore, G. M. (2001) J. Magn. Reson. 149 239-244 [DOI] [PubMed] [Google Scholar]
- 47.Nicholls, A, Sharp, K. A., and Honig, B. (1991) Proteins 11 281-296 [DOI] [PubMed] [Google Scholar]
- 48.Laskowski, R. A., MacArthur, M. W., Moss, D. S., and Thornton, J. M. (1993) J. Appl. Crystallogr. 26 283-291 [Google Scholar]
- 49.Shauder, S., Nunn, R. S., Lanz, R., Erni, B., and Schirmer, T. (1998) J. Mol. Biol. 276 591-602 [DOI] [PubMed] [Google Scholar]
- 50.Oriss, G. L., Erni, B., and Schirmer, T. (2003) J. Mol. Biol. 327 1111-1119 [DOI] [PubMed] [Google Scholar]
- 51.Suh, J.-Y., Iwahara, J., and Clore, G. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 104 3153-3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantor, C. R., and Schimmel, P. R. (1980) in Biophysical Chemistry Part III: The Behavior of Biological Macromolecules, Chapter 18, pp. 979-1018, W. H. Freeman & Co., San Francisco
- 53.Jones, S., and Thornton, J. M. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begley, G. S., Hansen, D. E., Jacobsen, G. R., and Knowles, J. R. (1982) Biochemistry 21 5552-5556 [DOI] [PubMed] [Google Scholar]
- 55.Mueller, E. G., Khandekar, S. S., Knowles, J. R., and Jacobsen, G. R. (1990) Biochemistry 29 6892-6896 [DOI] [PubMed] [Google Scholar]
- 56.Hollfelder, F., and Herschlag, D. (1995) Biochemistry 34 12255-12264 [DOI] [PubMed] [Google Scholar]
- 57.Mao, Q., Schunk, T., Flükiger, K., and Erni, B. (1995) J. Biol. Chem. 270 5258-5265 [DOI] [PubMed] [Google Scholar]
- 58.Rhiel, E., Flükiger, K., Wehrli, C., and Erni, B. (1994) Biol. Chem. Hoppe-Seyler 375 551-559 [DOI] [PubMed] [Google Scholar]
- 59.Gutknecht, R., Lanz, R., and Erni, B. (1998) J. Biol. Chem. 273 12234-12238 [DOI] [PubMed] [Google Scholar]
- 60.Clore, G. M., and Gronenborn, A. M. (1995) FASEB J. 9 57-62 [DOI] [PubMed] [Google Scholar]
- 61.Clore, G. M., and Garrett, D. S. (1999) J. Am. Chem. Soc. 121 9008-9012 [Google Scholar]
- 62.Clore, G. M., Gronenborn, A. M., and Bax, A. (1998) J. Magn. Reson. 133 216-221 [DOI] [PubMed] [Google Scholar]
- 63.Vriend, G., and Sander, C. (1993) J. Appl. Crystallogr. 26 47-60 [Google Scholar]