Abstract
Proline and hydroxyproline are metabolized by distinct pathways. Proline is important for protein synthesis, as a source of glutamate, arginine, and tricarboxylic acid cycle intermediates, and for participating in a metabolic cycle that shuttles redox equivalents between mitochondria and cytosol. Hydroxyproline, in contrast, is not reutilized for protein synthesis. The first steps in the degradation of proline and hydroxyproline are catalyzed by proline oxidase (POX) and hydroxyproline oxidase (OH-POX), respectively. Because it is well documented that POX is induced by p53 and plays a role in apoptosis, we considered whether OH-POX also participates in the response to cytotoxic stress. In LoVo and RKO cells, which respond to adriamycin with a p53-mediated induction of POX and generation of reactive oxygen species, we found that adriamycin also induced OH-POX gene expression and markedly increased OH-POX catalytic activity, and this increase in activity was not observed in the cell lines HT29 and HCT15, which do not have a functional p53. We also observed an increase in reactive oxygen species generation and activation of caspase-9 with adriamycin in a hydroxyproline-dependent manner. Therefore, we hypothesize that OH-POX plays a role analogous to POX in growth regulation, ROS generation, and activation of the apoptotic cascade.
Several investigators have shown that proline oxidation is a source of reactive oxygen species (ROS)3 in the apoptotic cascade (1–4). On the other hand, little is known concerning the redox contribution of hydroxyproline oxidation. In contrast to proline, hydroxyproline is not utilized for protein synthesis, and the conversion of free hydroxyproline to free proline does not occur. Instead, hydroxyproline is broken down to two- and three-carbon compounds, and the degradation pathway has no known anaplerotic or regulatory function. The first steps of each respective pathway are catalyzed by distinct mitochondrial membrane-bound oxidases with little substrate crossover (5). At the genetic level, POX, also known as proline dehydrogenase 1 (PRODH1, GenBank™ accession number NM_016335), coding for proline oxidase (POX), is located on 22q11.2, and mutations have been associated with susceptibility to schizophrenia (6–8) and type I hyperprolinemia (7). Of importance to cancer biology, POX is a p53-induced gene (PIG6) (3, 9, 10). OH-POX, also known as proline dehydrogenase 2 (PRODH2, GenBank accession number NM_021232), coding for hydroxyproline oxidase (OH-POX), maps to 19q13.1, and little is known about its expression or function (11) except its association with a rarely reported inborn error of metabolism known as hydroxyprolinemia (12).
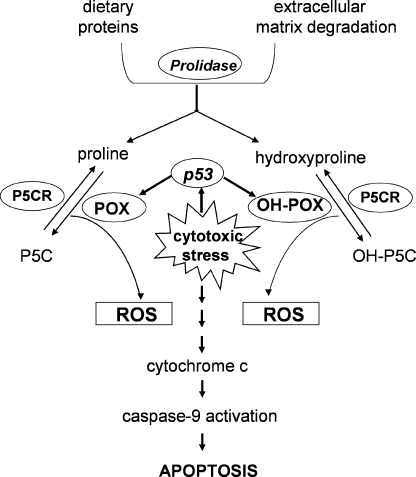
In the mitochondria, proline and hydroxyproline are oxidized to yield Δ1-pyrroline-5-carboxylate (P5C) and Δ1-pyrroline-3-hydroxy-5-carboxylate (OH-P5C), by POX and OH-POX, respectively (Fig. 1). These two intermediates can be converted to glutamate and γ-hydroxy glutamate by a common enzyme, Δ1-pyrroline-5-carboxylate dehydrogenase (5). Work done by Adams and Goldstone (13) showed that Δ1-pyrroline-5-carboxylate reductase can reduce either P5C or OH-P5C to proline or hydroxyproline, respectively.
FIGURE 1.
Hydroxyproline and proline metabolism. Hydroxyproline and proline are metabolized, as shown above, by OH-POX and POX, respectively. P5CR, Δ1-pyrroline-5-carboxylate reductase; P5CD, Δ1-pyrroline-5-carboxylate dehydrogenase; TCA, tricarboxylic acid; KG, ketoglutarate.
Hydroxyproline in protein, predominantly in collagen (30), is formed from proline after peptide linkage. Preformed hydroxyproline, whether derived from dietary protein or released from collagen turnover, is not incorporated into protein. Since the hydroxyproline produced from OH-P5C is not used for protein formation, we considered the possibility that hydroxyproline oxidase participates in redox generation. The role of the proline cycle in redox generation is well documented (3); therefore, the question arose whether the hydroxyproline cycle may play a similar role.
EXPERIMENTAL PROCEDURES
Reagents—Adriamycin, hydroxyproline, and O-aminobenzaldehyde (OAB) were obtained from Sigma, and 2′7′-dichlorofluorescein diacetate was purchased from Molecular Probes (Eugene, OR). The Stealth Select siRNAs were purchased from Invitrogen.
Cell Culture—The RKO and LoVo cell lines were obtained from the American Type Culture Collection. The cell lines, HT29 and HCT15, were obtained from the Division of Cancer Treatment and Diagnosis Tumor Repository at the NCI, National Institutes of Health (Frederick, MD). All cells were cultured in DMEM (Quality Biologicals, Gaithersburg, MD) at 37 °C and 5% CO2, supplemented with 2 mm glutamine, penicillin, and streptomycin and 10% fetal bovine serum (HyClone, Logan, UT). To monitor OH-POX gene expression and OH-POX enzymatic activity, cells were plated into 6-well plates to 60–70% confluence and treated with adriamycin at a concentration of 0.5 μm for various time periods.
Plasmid Constructs—The pCi control vector was obtained from Promega (Madison, WI). The p53 expression construct was made by amplifying p53 cDNA and was subsequently cloned into pTarget (Promega) as described (1).
Transfections and siRNA—The RKO cells were cultured in 6-well plates in growth medium and transfected with pCi control vector or p53 expression vector. Transfections were performed with Lipofectamine 2000 (Invitrogen), according to the manufacturer's directions. Stealth Select RNA interference and siRNA for OH-POX and POX were purchased from Invitrogen and then transfected with Lipofectamine 2000 according to the manufacturer's directions.
RT-PCR—Total RNA was isolated from cell lines by TRIzol (Invitrogen) according to the manufacturer's directions and quantified using a Beckman DU-65 spectrophotometer. A two-step RT-PCR reaction, using 0.5 μg of total RNA, 0.5 μg of random primers, and 0.2 μm OH-POX-specific primers, was performed using RT-PCR beads (Amersham Biosciences). Gene-specific primers for human OH-POX were: forward, 5′-GCGTGGTATGAGGGGAACCTC-3′, and reverse, 5′-AGCCGCTCGAATGTGTCCTTTAGA-3′, which amplify a 475-bp fragment. The PCR conditions were 30 cycles at 94 °C for 45 s, 63 °C for 45 s, and 72 °C for 1 min, with a 7-min final extension at 72 °C. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control primers (Clontech) were amplified with the following changes to the PCR conditions: annealing at 61 °C, and the reactions were removed after 20 cycles. Following amplification, 20 μl of the reactions were run on 2% agarose gels containing ethidium bromide. The products were recorded and quantified using the electrophoresis documentation and analysis system (Kodak Digital Science).
OH-POX Enzyme Assay—RKO cells were cultured, with the appropriate treatments. After the desired time, cells were rinsed and scraped in cold phosphate-buffered saline, pelleted, and then resuspended in cold sucrose extraction buffer (0.25 m sucrose, 3.5 mm Tris-HCl (pH 7.4) and 1 mm EDTA). Cells were then sonicated for 12 s at 25% (Branson Sonifier 450; Branson Ultrasonics Corp., Danbury, CT). Total protein was determined by the Bio-Rad protein assay (Bio-Rad). The formation of OH-P5C when complexed with OAB (14) was detected using a spectrophotometric method. Briefly, a 200-μl reaction mixture (0.1 m KPO4 (pH 7.4), with 0.5 mm hydroxyproline, 0.12 mg/ml OAB, 0.012 mg/ml cytochrome c, and 50 μg of protein) was incubated at 37 °C for 30 min. The reaction was terminated by the addition of 20 μl of OAB (10 mg/ml in 20% ethanol and 80% 6 n HCl). The samples were centrifuged briefly, and the absorbance was measured at 440 nm. Reactions were performed in triplicate with the proper protein blanks for each measurement ± S.D. A calibration curve was generated using P5C and the amount of OH-P5C (nmol/min/μg of protein) produced was determined.
Generation and Measurement of Intracellular ROS—RKO cells were plated in growth medium containing dialyzed serum in the presence or absence of adriamycin and/or hydroxyproline and/or transfection with siRNA for 24 h before analysis of ROS. The dye, 2,7-dichlorofluorescein diacetate, was used to indicate the amount of intracellular ROS. For the measurement, medium was removed and washed with phosphate-buffered saline. Phenol red-free medium, containing 50 μm 2,7-dichlorohydro fluorescein diacetate, was added to the monolayer, and the plates were incubated at 37 °C for 30 min. The plates were read on a CytoFluor 4000 (PerSeptive Biosystems, Framingham, CT) with excitation wavelength of 485 nm and emission of 530 nm. Cells were then washed and collected, and protein content was determined as described above. The samples were assayed in triplicate, and data are shown as arbitrary units of fluorescence ±S.D.
Caspase-9 Activity Assay—Activity of caspase-9 was determined by a colorimetric assay (Chemicon International). The assay is based on the hydrolysis of Ac-LEHD-pNA by caspase-9, resulting in the release of the p-nitroaniline (pNA) moiety with an increase in absorbance at 405 nm. After appropriate treatment of cells, the reaction mixtures were incubated at room temperature for 2 h, and the formation of pNA was measured at 405 nm. The concentration of pNA was calculated from a standard curve, and all values were normalized for protein levels. Experiments were performed in triplicate.
Western Blotting—Cell lysates were prepared and quantified according to established methods. Equal amounts of cell lysates were electrophoresed on SDS-polyacrylamide gels and transferred to nitrocellulose membrane using a semi-dry blotter (Bio-Rad). Membranes were blocked using Tris-buffered saline with 3% nonfat milk (pH 8.0; Sigma). Blots were then probed with the primary anti-caspase-9 (Santa Cruz Biotechnology) or anti-PARP (Cell Signaling Technologies) antibody in blocking buffer, and subsequently, by a secondary antibody conjugated to horseradish peroxidase (1:2000). All blots were washed in Tris-buffered saline with Tween 20 (pH 8.0; Sigma) and developed using the enhanced chemiluminescence (ECL) procedure (Amersham Biosciences). Monoclonal anti-actin antibody (Sigma) (1:2000) was used as loading control. Anti-rabbit antibody (Santa Cruz Biotechnology) was used as secondary antibody.
RESULTS
Adriamycin Induces OH-POX Gene Expression in RKO and LoVo Cells—Earlier work in our laboratory showed that cytotoxic agents can induce POX gene expression in LoVo cells in a p53-dependent manner for generation of proline-dependent ROS (3). Therefore, to determine whether OH-POX gene was also inducible by adriamycin, we used RKO and LoVo colon cancer cells, both of which have a wild type p53. Adriamycin is a powerful quinone-containing anthracycline antibiotic effective against a variety of human neoplasms (15) and is able to induce apoptosis (16), and it has been well documented that adriamycin induces p53 (17, 18). The cells were exposed to 0 or 0.5 μm adriamycin for 24 h. The RNA was isolated and analyzed by RT-PCR for OH-POX expression and normalized to a GAPDH control (Fig. 2, A and B). Our results showed that the addition of adriamycin increased OH-POX expression 2.5-fold over control in the RKO cells. In LoVo cells, untreated controls showed no detectable levels of OH-POX expression, but the addition of adriamycin induced a dramatic increase of OH-POX expression. However, in HCT15 and HT29 cells, induction of OH-POX expression by adriamycin was not observed (data not shown). This result suggested that the increase in expression in LoVo and RKO cells in response to adriamycin may be dependent on p53. Donald et al. (3) reported a 4-fold increase of POX expression, over control, by adriamycin treatment in LoVo cells at 24 h. Thus, adriamycin up-regulates the expression of both POX and OH-POX, indicating that OH-POX may also play a role along with POX in the adriamycin-induced effect.
FIGURE 2.
Adriamycin (Adr) induces OH-POX expression. RKO and LoVo cells were treated with and without adriamycin for 24 h. A, total RNA was prepared, and RT-PCR was performed for OH-POX and GAPDH as a control. The RT-PCR products were then run on an ethidium bromide gel. B, densitometry was performed on the bands from A, and levels of expression were normalized for GAPDH expression. The values represent means ± S.E. of the mean (n = 3). The control levels in LoVo cells were undetectable; however, both cell lines showed a significant increase (**, p < 0.01) in expression of OH-POX after induction with adriamycin.
Adriamycin-induced OH-POX Gene Expression in RKO and LoVo Cells Correlates with OH-POX Catalytic Activity—It has been previously shown that adriamycin treatment increased POX enzymatic activity in LoVo cells (3). To determine whether this occurs for OH-POX enzymatic activity, we treated RKO, LoVo, HT29, and HCT15 cells with or without (0.5 μm) adriamycin for 24 h. Cells were collected as described, and OH-POX enzyme activity was measured (Fig. 3A). Parallel to the increase in expression, OH-POX catalytic activity was found to be 2-fold higher in RKO cells treated with adriamycin as compared with control. A 5-fold increase in OH-POX enzymatic activity was observed in LoVo cells concomitant to the dramatic increase in expression of OH-POX. However, in HT29 and HCT15, which have mutant p53, there was no increase in OH-POX activity following adriamycin treatment, further suggesting that the effect on OH-POX induction may also be due to p53.
FIGURE 3.
Adriamycin increases OH-POX catalytic activity. RKO, LoVo, HT 29, and HCT 15 cells were treated with and without 0.5 μm adriamycin. A, cells were collected at 24 h and lysed, and the activity of hydroxyproline oxidase was determined using an OAB spectrophotometric assay. The values represent means ± S.E. of the mean (n = 3). Adriamycin treatment significantly increased OH-POX enzymatic activity over control in RKO and LoVo cells (**, p < 0.01), which have wild type p53; however, there is no observable difference in OH-POX activity in the adriamycin-treated HT 29 or HCT 15 cells, which have mutant p53. B, RKO cells were treated as above, collected over time, and assayed for OH-POX catalytic activity. The values represent means ± S.E. of the mean (n = 3).
To assess the time course of induction of OH-POX catalytic activity, RKO cells were treated with and without 0.5 μm adriamycin and collected over time. A time-dependent increase in OH-POX enzymatic activity was observed in cells treated with adriamycin as compared with controls (Fig. 3B). A maximum increase in OH-POX catalytic activity up to 4-fold was obtained at 48 h in cells treated with adriamycin as compared with controls.
Induction of OH-POX Is Mediated by p53—The adriamycin-induced OH-POX activity was only observed in cell lines with wild type p53. To confirm the response to be dependent on p53, we overexpressed p53 and monitored the effect on OH-POX enzymatic activity. RKO cells were transfected with either pCi control vector or p53 expression vector. Cells were collected over time, and lysates were assayed for OH-POX enzymatic activity. The results revealed that expression of p53 alone is sufficient to induce OH-POX catalytic activity within 16 h. By 40 h, activity of OH-POX increased almost 3-fold over control (Fig. 4). These data showed that the adriamycin-induced OH-POX enzymatic activity is mediated via p53 similarly to POX.
FIGURE 4.
Expression of p53 increases OH-POX enzymatic activity. RKO cells were transfected with equivalent amounts of pCi control vector (open circles) or p53 cDNA expression vector (closed circles). Cells were collected over time. Lysates were prepared, and protein was quantified and assayed for activity of OH-POX. The values represent means ± S.E. of the mean (n = 3). These data show that p53 alone is sufficient for inducing OH-POX catalytic activity, in the absence of adriamycin, and increases over time.
To further confirm that p53 is involved in the OH-POX response, we also overexpressed p53 in HT29 and HCT15, cells that both have mutant p53 and failed to induce the OH-POX activity by adriamycin. Forced expression of p53 in these cells markedly induced OH-POX activity as shown in Fig. 5. In both the cell lines, the induction of p53 significantly increased OH-POX activity. In HT29 cells, a 5-fold increase in OH-POX activity over control was obtained, and in HCT15 cells, almost a 2-fold increase in OH-POX activity over control was obtained. These results validate the p53-dependent induction of OH-POX.
FIGURE 5.
Overexpression of p53 in cells with mutant p53 increases OH-POX enzymatic activity. The cell lines HT29 and HCT15 with mutant p53 were transfected with equivalent amounts of pCi control vector or p53 cDNA expression vector. After 6 h, the medium was replaced with new medium for 24 h. Cells were harvested, and cell lysates were quantified for protein and assayed for OH-POX activity. The values represent means ± S.E. of the mean (n = 3). By overexpression of p53, OH-POX enzymatic activity is significantly increased in both HT29 and HCT15 cells (**, p < 0.01).
Hydroxyproline-dependent Generation of Intracellular ROS—Earlier work has shown that the p53-induced expression of POX is accompanied by a proline-dependent increase in ROS generation (3). To determine whether this was also true of the p53-induced expression of OH-POX, RKO cells were subjected to the peroxide-sensitive fluorescent probe 2′,7′-dichlorofluorescein in the presence or absence of adriamycin in addition to hydroxyproline. As previously reported (19), adriamycin alone contributes to ROS generation (Fig. 6A), and hydroxyproline alone had no effect on ROS generation. However, hydroxyproline added to cells treated with adriamycin over adriamycin alone demonstrated a significant increase in intracellular ROS. The direct role of OH-POX was further validated by studies using siRNA. RKO cells were treated with and without 0.5 μm adriamycin and were transfected with siRNA for OH-POX. After 24 h in cells transfected with siRNA, the OH-POX catalytic activity was knocked down to the level of the control cells (Fig. 6B). Furthermore, the siRNA abolished the increase in ROS obtained in the presence of added hydroxyproline, thus confirming that the OH-POX catalytic activity is contributing to the generation of ROS (Fig. 6A). Interestingly, the increase in ROS was blocked by the presence of siRNAs for both OH-POX and POX used in combination and also by p53 siRNA alone. In fact, under both these conditions, the ROS levels were no different from controls without adriamycin, indicating that both the enzyme activities are induced by p53 and contribute to the bulk of ROS generated under these conditions.
FIGURE 6.
Hydroxyproline-dependent generation of ROS by adriamycin (Adr). A, RKO cells were grown in 6-well plates to 60–70% confluence and first transfected with siRNAs either for OH-POX or with OH-POX + POX and p53. The transfection medium was changed after 6 h and replaced with medium containing dialyzed serum, which has no hydroxyproline, and either 0 or 0.5 μm adriamycin (Adr) and/or 1 mm hydroxyproline (HYP). After 24 h, the cells were exposed to the fluorescent dye 2′7′-dichlorofluorescein diacetate. Fluorescence was normalized for protein content. The values represent means ± S.E. of the mean (n = 3). There is a significant hydroxyproline-mediated increase in ROS generation in the presence of hydroxyproline (**, p < 0.01) over cells treated with adriamycin alone. This effect is significantly reduced (++, p < 0.01) with the addition of siRNA for OH-POX. B, RKO cells were transfected with siRNA for OH-POX. After 24 h, cells were collected and assayed for OH-POX enzymatic activity. The values represent means ± S.E. of the mean (n = 3). Adriamycin treatment significantly increases (**, p < 0.01) OH-POX activity over control. However, treatment with siRNA for OH-POX significantly (++, p < 0.01) knocks down the increased activity of OH-POX in the presence of adriamycin.
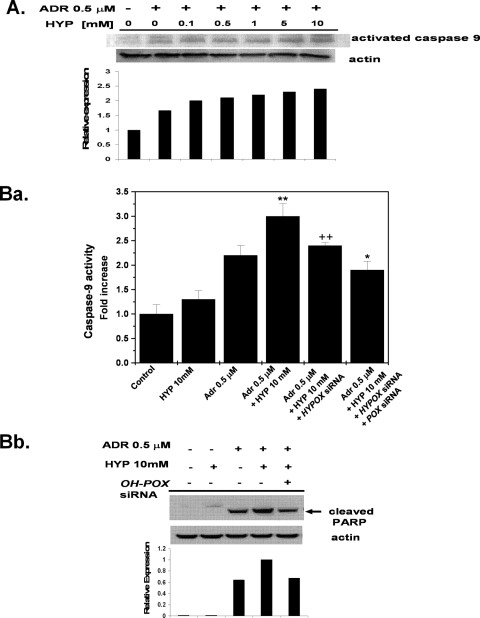
Increase in Caspase-9 Activation in Adriamycin-induced Cells in the Presence of Hydroxyproline—In the mitochondrial-mediated apoptotic pathway, cytochrome c is released from the mitochondria into the cytosol and binds to Apaf-1, inducing the sequential activation of caspase-9 and caspase-3. Caspase-9 activation is an indicator of apoptosis (20). We examined the activation of caspase-9 in RKO cells treated with 0.5 μm adriamycin in the presence of increasing concentrations of hydroxyproline (Fig. 7A). The effect of hydroxyproline on caspase-9 activation was observed at a concentration of hydroxyproline as low as 0.5 mm. As the concentration of hydroxyproline increased, the cleavage of caspase-9 increased, indicating the induction of apoptosis. The activation of caspase-9 was also confirmed by a colorimetric assay in adriamycin-treated RKO cells in the presence or absence of 10 mm hydroxyproline for 24 h. The activity of caspase-9 was significantly higher in cells treated with hydroxyproline and adriamycin than those treated with adriamycin alone (Fig. 7B, panel a). Similar to the ROS studies, the increase in caspase-9 activity was decreased in the presence of OH-POX siRNA, and the activity decreased further in the presence of both OH-POX and POX siRNAs (Fig. 7B, panel a). Furthermore, an increase in levels of cleaved PARP was observed in the presence of added hydroxyproline, and this effect was again suppressed with OH-POX siRNA (Fig. 7B, panel b), thus validating that OH-POX contributes to the apoptotic response.
FIGURE 7.
Hydroxyproline-dependent increase in caspase-9 activation. A, the RKO cells were treated with adriamycin (Adr) in the presence of various concentrations of hydroxyproline (HYP) as indicated for 24 h. Cell lysates were analyzed by Western blot analysis for activation of caspase-9. The graph shows the bands of activated caspase-9 analyzed by densitometry and normalized with actin. A representative result of several independent experiments has been shown. B, RKO cells were first transfected with siRNAs either for OH-POX or for OH-POX + POX. The transfection medium was changed after 6 h and replaced with medium containing dialyzed serum, which has no hydroxyproline, and either 0 or 0.5 μm adriamycin and/or 10 mm hydroxyproline. Cells were collected and lysed, and protein was quantified. Panel a, caspase-9 activity was determined colorimetrically. The results are cumulative of three independent experiments, and the values represent the means ± S.E. of the mean (n = 3). There is a significant hydroxyproline-mediated increase in caspase-9 activity in the presence of hydroxyproline (**, p < 0.01) over cells treated with adriamycin alone. This effect is significantly reduced (++, p < 0.01) with the addition of siRNA for OH-POX. A further decrease in activity was obtained with the addition of both siRNAs for OH-POX + POX as compared with the addition of siRNA for OH-POX (*, p < 0.05). Panel b, cell lysates were analyzed by Western blot analysis for cleavage of PARP. The bands of cleaved PARP were analyzed by densitometry and were normalized with actin. A representative result of several independent experiments has been shown.
DISCUSSION
The role of proline metabolism in metabolic regulation was proposed several decades ago (21), but the importance of this regulation was not fully appreciated until POX was shown to be a downstream mediator of p53-induced apoptosis (3). The mechanism of this effect was shown to be the generation of proline-dependent ROS (3, 22). The association of POX with mitochondrial inner membranes and the transfer of electrons to cytochrome c with an intervening flavoprotein (21) may be the basis for this effect. In addition, the interconversion of proline and P5C provide a metabolic shuttle of redox equivalents in the proline cycle (23). P5C is produced accompanying the generation of ATP or ROS, and it can then be transported into the cytoplasm as a source of oxidizing potential. Since OH-POX, also localized in the inner mitochondrial membrane, catalyzes the degradation of the structurally similar compound hydroxyproline, we considered the possibility of OH-POX playing a similar role to POX in apoptosis.
Little has been published on OH-POX since the studies of Adams and Goldstone (13, 24, 25) in the 1960s. He and others (5) showed that there are two distinct enzymes for the first step in metabolic degradation of proline and hydroxyproline (POX and OH-POX, respectively), and Downing et al. (26) showed that these two enzymes have little or no substrate crossover. Although the first degradative step of proline and hydroxyproline is carried out by distinct enzymes, their respective metabolites are degraded by a series of analogous reactions of biochemical similarity (Fig. 1). Δ1-Pyrroline-5-carboxylate reductase is the enzyme responsible for reducing both Δ1-pyrroline-5-carboxylate and Δ1-pyrroline-3-hydroxy-5-carboxylate (OH-P5C) to yield proline and hydroxyproline, respectively. More importantly, the ability to reduce OH-P5C is a property only of the mammalian enzyme, presumably because hydroxyproline is not produced in lower organisms. Nevertheless, when hydroxyproline arose at the metazoan stage of evolutionary development, it was metabolized by pre-existing mechanisms already in place for synthesizing or degrading the structurally related compound, proline (24). This ability to reduce OH-P5C to hydroxyproline does not contribute to protein synthesis since hydroxyproline is not incorporated into proteins, but in the context of the apoptotic response, the stress-induced cycling of hydroxyproline between the mitochondria and cytoplasm in a manner similar to the cycling of proline can provide substrate for the production of ROS. Our results indeed show that cytotoxic stress resulting from treatment with adriamycin induced the expression of OH-POX gene with a concomitant increase in OH-POX catalytic activity both in RKO and in LoVo cells, and similar to POX, this response may be important in the induction of apoptosis.
POX has been demonstrated to be a downstream mediator in the p53-induced apoptotic response. Cytotoxic stress by adriamycin induces p53 and leads to induction of POX; therefore, we considered whether p53 could also mediate the induction of OH-POX. Increase in OH-POX activity was observed only in the RKO and LoVo cell lines that have wild type p53. However, the HCT15 and HT29 cells with mutant p53 failed to induce OH-POX activity, indicating that similar to POX, the induction of OH-POX with adriamycin also requires p53. Additionally, the involvement of p53 was also observed by the increase in OH-POX activity by forced expression of wild type p53 in cell lines with mutant p53. Thus, in response to cytotoxic stress, the expression of OH-POX is up-regulated specifically by p53 induction. In fact, our study is the first to identify OH-POX as being induced by p53.
ROS usually include hydrogen peroxide, hydroxyl radicals, and superoxide radicals. Although these compounds are capable of reacting with and damaging various molecular targets (27), they are also mediators of biological signals, important for the apoptotic response (28). The mechanism of POX-dependent regulation is due to generation of proline-dependent ROS, specifically in the form of superoxide radicals (3, 22). In this current study, we asked whether OH-POX could generate hydroxyproline-dependent ROS. Our findings show that adriamycin was able to enhance a hydroxyproline-dependent production of intracellular ROS concomitant with increase in OH-POX activity. We found that in the presence of OH-POX siRNA, the additional effect of hydroxyproline on ROS generation was suppressed, demonstrating the direct role of OH-POX in generation of ROS. In the presence of both OH-POX and POX siRNAs, the production of ROS was inhibited, indicating that OH-POX and POX both contribute to ROS in the apoptotic response to adriamycin, which is mediated via p53. Since caspase-9 activation is a critical step in the apoptotic cascade and the most frequently used diagnostic test for apoptosis (29), we determined the effect of OH-POX induction on caspase-9 activation to further demonstrate that ROS generation by OH-POX is contributing to the apoptotic response. The amount of caspase-9 activation and PARP cleavage was increased in the presence of added hydroxyproline in adriamycin-treated cells, and this effect was significantly decreased in the presence of OH-POX siRNA. This result indicated that increased substrate availability superimposed on increased OH-POX activity contributes to the activation of caspases by increasing ROS. Although the significance of the work on OH-POX has yet to be fully determined, it is clear that the oxidation of hydroxyproline can increase the generation of ROS, which we have shown to be important in the apoptotic response. Admittedly, the biochemical mechanisms by which OH-POX contributes to apoptosis and ROS are largely inferred based on its similarities to POX. Both enzymes are up-regulated in cells treated with adriamycin and accompanying expression of p53 by transient transfection of p53 in null cells. OH-POX and POX generate hydroxyproline- and proline-dependent ROS, respectively, and they initiate the apoptotic cascade. We assume that OH-POX can contribute to apoptosis as has been shown for POX. Fig. 8 shows our proposed model for OH-POX in hydroxyproline-mediated apoptosis.
FIGURE 8.
Proposed paradigm through which OH-POX can induce apoptosis. Under conditions of cytotoxic stress p53 mediates the up-regulation of both POX and OH-POX enzyme activities, catalyzing the conversion of proline to P5C and hydroxyproline to OH-3-P5C, respectively. During this process, electrons are generated to reduce oxygen resulting in the production of ROS, which can influence the mitochondrial membrane potential and be one of the mechanisms mediating apoptosis by releasing cytochrome c with the subsequent activation of caspase-9. P5CR, Δ1-pyrroline-5-carboxylate reductase.
The function of POX in modulating carcinogenic pathways was supported by our recent finding that POX expression was activated by PPARγ and its ligands, a signaling pathway shown to stimulate differentiation and apoptosis of cancer cells (1). One might speculate that the apparent link of POX with both p53 and PPARγ would produce greater consequences than those found in patients with Type I hyperprolinemia or in Pro/Re mice. In both conditions in humans and animals, respectively, POX is deficient. Nevertheless, the associated symptoms are mild and confined to the central nervous system. Although this metabolic response system may only be apparent under “stress conditions” and limited to certain organ systems, we considered an alternative possibility, i.e. the lack of a conspicuous phenotype may be due to a redundant metabolic system. We now propose that the metabolism of hydroxyproline by OH-POX may back up that provided by proline and POX. Some 46 years after Adams and Goldstone (13) initially pointed out the unusual aspects of hydroxyproline metabolism, we have discovered functions for hydroxyproline metabolism perhaps supplementing those for proline.
Recognizing that OH-POX may contribute to apoptosis, the source of free hydroxyproline becomes of considerable interest. Of course, diet is a major source of hydroxyproline. Another important source for hydroxyproline comes from the degradation of extracellular matrix (ECM). The ECM is composed predominately of collagen (80%); 25% of collagen is either proline or hydroxyproline (30). The hydroxyproline residues play an important role in stabilizing the collagen fibers (31). Cell growth and migration are dependent on the ECM, which is maintained in a complex balance of synthesis and degradation. Degradation is mediated by enzymes known as matrix metalloproteinases, and their regulation has been implicated in many physiological and pathological processes. Of special relevance to cancer, matrix metallo-proteinase mediates cancer invasion, metastasis, angiogenesis, and apoptosis (30). Activation of matrix metallo-proteinase under conditions of genotoxic, nutrient, or inflammatory stress markedly increases ECM degradation. The accompanying induction of POX or OH-POX utilizes the products of ECM degradation, free proline, or hydroxyproline that can be metabolized for bioenergetics and survival or to initiate the apoptotic cascade.
This work was supported by federal funds from the NCI, National Institutes of Health, under contract N01-CO-12400 and by a grant from the Intramural Research Program of the National Institutes of Health, NCI, Center for Cancer Research. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: ROS, reactive oxygen species; POX, proline oxidase; OH-POX, hydroxyproline oxidase; P5C, Δ1-pyrroline-5-carboxylate; OH-P5C, Δ1-pyrroline-3-hydroxy-5-carboxylate; OAB, o-aminobenzaldehyde; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; pNA, p-nitroaniline; siRNA, small interfering RNA; RT-PCR, reverse transcription-PCR; PARP, poly(ADP-ribose) polymerase-2; ECM, extracellular matrix.
References
- 1.Pandhare, J., Cooper, S. K., and Phang, J. M. (2006) J. Biol. Chem. 281 2044–2052 [DOI] [PubMed] [Google Scholar]
- 2.Maxwell, S. A., and Rivera, A. (2003) J. Biol. Chem. 278 9784–9789 [DOI] [PubMed] [Google Scholar]
- 3.Donald, S. P., Sun, X. Y., Hu, C. A., Yu, J., Mei, J. M., Valle, D., and Phang, J. M. (2001) Cancer Res. 61 1810–1815 [PubMed] [Google Scholar]
- 4.Liu, Y., Borchert, G. L., Surazynski, A., Hu, C. A., and Phang, J. M. (2006) Oncogene 25 5640–5647 [DOI] [PubMed] [Google Scholar]
- 5.Valle, D., Goodman, S. I., Harris, S. C., and Phang, J. M. (1979) J. Clin. Investig. 64 1365–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterlini, M., Zakharenko, S. S., Lai, W. S., Qin, J., Zhang, H., Mukai, J., Westphal, K. G., Olivier, B., Sulzer, D., Pavlidis, P., Siegelbaum, S. A., Karayiorgou, M., and Gogos, J. A. (2005) Nat. Neurosci. 8 1586–1594 [DOI] [PubMed] [Google Scholar]
- 7.Bender, H. U., Almashanu, S., Steel, G., Hu, C. A., Lin, W. W., Willis, A., Pulver, A., and Valle, D. (2005) Am. J. Hum. Genet. 76 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogendoorn, B., Coleman, S. L., Guy, C. A., Smith, S. K., O'Donovan, M. C., and Buckland, P. R. (2004) Hum. Mutat. 24 35–42 [DOI] [PubMed] [Google Scholar]
- 9.Polyak, K., Xia, Y., Zweier, J. L., Kinzler, K. W., and Vogelstein, B. (1997) Nature 389 300–305 [DOI] [PubMed] [Google Scholar]
- 10.Maxwell, S. A., and Davis, G. E. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 13009–13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler, D. L., Barrett, T., Benson, D. A., Bryant, S. H., Canese, K., Chetvernin, V., Church, D. M., DiCuccio, M., Edgar, R., Federhen, S., Geer, L. Y., Helmberg, W., Kapustin, Y., Kenton, D. L., Khovayko, O., Lipman, D. J., Madden, T. L., Maglott, D. R., Ostell, J., Pruitt, K. D., Schuler, G. D., Schriml, L. M., Sequeira, E., Sherry, S. T., Sirotkin, K., Souvorov, A., Starchenko, G., Suzek, T. O., Tatusov, R., Tatusova, T. A., Wagner, L., and Yaschenko, E. (2006) Nucleic Acids Res. 34 D173–D180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, S. Z., Varvogli, L., Waisbren, S. E., and Levy, H. L. (1997) J. Pediatr. 130 437–441 [DOI] [PubMed] [Google Scholar]
- 13.Adams, E., and Goldstone, A. (1960) J. Biol. Chem. 235 3499–3503 [PubMed] [Google Scholar]
- 14.Phang, J. M., Downing, S. J., Valle, D. L., and Kowaloff, E. M. (1975) J. Lab. Clin. Med. 85 312–317 [PubMed] [Google Scholar]
- 15.Green, P. S., and Leeuwenburgh, C. (2002) Biochim Biophys Acta 1588 94–101 [DOI] [PubMed] [Google Scholar]
- 16.Kalyanaraman, B., Joseph, J., Kalivendi, S., Wang, S., Konorev, E., and Kotamraju, S. (2002) Mol. Cell Biochem. 234–235, 119–124 [PubMed] [Google Scholar]
- 17.Gewirtz, D. A. (2000) Breast Cancer Res. Treat. 62 223–235 [DOI] [PubMed] [Google Scholar]
- 18.L'Ecuyer, T., Sanjeev, S., Thomas, R., Novak, R., Das, L., Campbell, W., and Vander Heide, R. S. (2006) Am. J. Physiol. 291 H1273–H1280 [DOI] [PubMed] [Google Scholar]
- 19.Mizutani, H., Tada-Oikawa, S., Hiraku, Y., Kojima, M., and Kawanishi, S. (2005) Life Sci. 76 1439–1453 [DOI] [PubMed] [Google Scholar]
- 20.Cho, S., and Choi, E. (2002) J. Biochem. Mol. Biol. 35 24–27 [DOI] [PubMed] [Google Scholar]
- 21.Phang, J. M. (1985) Curr. Top. Cell. Regul. 25 91–123 [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., Borchert, G. L., Donald, S. P., Surazynski, A., Hu, C. A., Weydert, C. J., Oberley, L. W., and Phang, J. M. (2005) Carcinogenesis 26 1335–1342 [DOI] [PubMed] [Google Scholar]
- 23.Hagedorn, C. H., and Phang, J. M. (1983) Arch. Biochem. Biophys. 225 95–101 [DOI] [PubMed] [Google Scholar]
- 24.Adams, E., and Goldstone, A. (1960) J. Biol. Chem. 235 3504–3512 [PubMed] [Google Scholar]
- 25.Adams, E., and Goldstone, A. (1960) J. Biol. Chem. 235 3492–3498 [PubMed] [Google Scholar]
- 26.Downing, S. J., Phang, J. M., Kowaloff, E. M., Valle, D., and Smith, R. (1977) J. Cell. Physiol. 91 369–376 [DOI] [PubMed] [Google Scholar]
- 27.Shen, H. M., and Liu, Z. G. (2006) Free Radic. Biol. Med. 40 928–939 [DOI] [PubMed] [Google Scholar]
- 28.Skulachev, V. P. (2006) Apoptosis 11 473–485 [DOI] [PubMed] [Google Scholar]
- 29.Koh, D. W., Dawson, T. M., and Dawson, V. L. (2005) Pharmacol. Res. 52 5–14 [DOI] [PubMed] [Google Scholar]
- 30.Ii, M., Yamamoto, H., Adachi, Y., Maruyama, Y., and Shinomura, Y. (2006) Exp. Biol. Med. (Maywood) 231 20–27 [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharjee, A., and Bansal, M. (2005) IUBMB Life 57 161–172 [DOI] [PubMed] [Google Scholar]