Abstract
The nature of the connection between mitochondrial Fe-S cluster synthesis and the iron-sensitive transcription factor Aft1 in regulating the expression of the iron transport system in Saccharomyces cerevisiae is not known. Using a genetic screen, we identified two novel cytosolic proteins, Fra1 and Fra2, that are part of a complex that interprets the signal derived from mitochondrial Fe-S synthesis. We found that mutations in FRA1 (YLL029W) and FRA2 (YGL220W) led to an increase in transcription of the iron regulon. In cells incubated in high iron medium, deletion of either FRA gene results in the translocation of the low iron-sensing transcription factor Aft1 into the nucleus, where it occupies the FET3 promoter. Deletion of either FRA gene has the same effect on transcription as deletion of both genes and is not additive with activation of the iron regulon due to loss of mitochondrial Fe-S cluster synthesis. These observations suggest that the FRA proteins are in the same signal transduction pathway as Fe-S cluster synthesis. We show that Fra1 and Fra2 interact in the cytosol in an iron-independent fashion. The Fra1-Fra2 complex binds to Grx3 and Grx4, two cytosolic monothiol glutaredoxins, in an iron-independent fashion. These results show that the Fra-Grx complex is an intermediate between the production of mitochondrial Fe-S clusters and transcription of the iron regulon.
Iron is an essential element required for all eukaryotes and most prokaryotes. Iron is also potentially dangerous, since it can participate in the generation of toxic oxygen molecules, such as superoxide anion and the hydroxyl radical. Iron transport is highly regulated in all species, and iron transporters are only expressed under conditions of iron need. Transcriptional and post-transcriptional regulation of iron transport systems occurs in all organisms ranging from yeast to humans. Consequently, iron acquisition in all species is tightly controlled and is coordinated with iron use. The budding yeast Saccharomyces cerevisiae expresses two different high affinity iron transport systems. One system is composed of a closely related family of four siderophore transporters. Siderophores are small organic molecules that exhibit an extremely high affinity (Kd = 10–33) for iron (1). Although S. cerevisiae does not synthesize siderophores, it can accumulate siderophores produced by other organisms. The second high affinity iron transport system mediates the acquisition of ionic iron and is composed of a cell surface multicopper oxidase, Fet3, and a transmembrane permease, Ftr1. The multicopper oxidase converts Fe2+ to Fe3+, which is then transported by the transmembrane permease.
The transcriptional activator Aft1 regulates both high affinity iron transport systems (2). Aft1 is cytosolic when cells are iron-replete, but under conditions of iron depletion, Aft1 translocates into the nucleus, where it activates the transcription of ∼20 genes (3). These genes, referred to as the iron regulon, include the siderophore transporters, the high affinity iron transport system, FET3 and FTR1, and genes involved in the assembly of the high affinity iron transport system as well as genes involved in iron-based metabolism and their regulation. There is a second transcription factor, Aft2, which is a paralogue of Aft1. When overexpressed, Aft2 can also affect transcription of the iron regulon, although deletion of AFT2 alone has little effect on iron transport and metabolism (4, 5).
It is clear that translocation of Aft1 into the nucleus and transcription of the iron regulon responds to cellular iron deprivation. We recently suggested that Aft1 does not respond directly to iron but rather to the production of iron-sulfur clusters within mitochondria. This conclusion was based on the observation that defects in mitochondrial Fe-S cluster synthesis induce transcription of the iron regulon even in cells that are iron-replete (6). Aft1, however, does not appear to be an Fe-S cluster-containing protein (6). Deletion of genes required for the assembly of cytosolic Fe-S cluster-containing proteins does not lead to activation of the iron regulon (7). Thus, the mechanism by which mitochondrial Fe-S cluster synthesis affects the iron regulon is unclear. In this paper, we present a genetic test of the hypothesis that mitochondrial Fe-S cluster synthesis is involved in regulating the iron regulon by screening for mutants that would activate the iron regulon even in the presence of high media iron. Analysis of the mutants demonstrated that most were defective in genes required for mitochondrial Fe-S cluster synthesis. Two novel genes were identified that encode nonmitochondrial proteins that are required for regulation of Aft1-mediated expression of the iron regulon.
EXPERIMENTAL PROCEDURES
Yeast Strains and Growth Media—The strains employed in this study are described in Table 1. Yeast were grown in YPD (1% yeast extract, 2% peptone, and 2% dextrose), synthetic complete medium (CM),3 or synthetic defined medium (SD). Cells were iron-loaded by the addition of the indicated amount of iron (50–250 μm FeSO4 in 0.1 m HCl) to the medium and iron-starved by the addition of 40 μm bathophenanthroline disulfonate (BPS) to the medium.
TABLE 1.
Yeast strains used in the study
| Strains | Genotype | Source/Reference |
|---|---|---|
| DY150 | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade2, Mata | Ref. 6 |
| DY1457 | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, Matα | Ref. 6 |
| OCY354 | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade2, ho::FET3LacZ, Mata | This paper |
| OCY355 | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade2, ho::FET3LacZ, Matα | This paper |
| OCY356 | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::FET3LacZ, Mata | This paper |
| OCY357 | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::FET3LacZ, Matα | This paper |
| IF17B (fra1-1) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::FET3LacZ, fra1-1, Mata | This paper |
| OCY456 (Δfra2) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::FET3LacZ, FRA2::KanMX4, Mata | This paper |
| OCY464 (fra1-1Δfra2) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::FET3LacZ, fra1-1, FRA2::KanMX4, Mat nt | This paper |
| OCY516 (Δnfs1, pMET3NFS1) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::Fet3LacZ, NFS1::his3, pMET3NFS1, Mat nt | This paper |
| OCY519 (Δfra1) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::Fet3LacZ, FRA1::KanMX4, Matα | This paper |
| OCY593 (Δnfs1Δfra1, pMET3NFS1) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::Fet3LacZ, NFS1::his3, FRA1::KanMX4, pMET3NFS1, Matα | This paper |
| OCY597 (Δnfs1fra2, pMET3NFS1) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, ho::Fet3LacZ, NFS1::his3, FRA2::KanMX4, pMET3NFS1, Matα | This paper |
| OCY525 (Δfra1) | ura3-52, leu2-3, trp2-1, his3-11, can1-100(oc), ade6, Matα | This paper |
| BY4741 | his3Δ1, leu3Δ0, met15Δ0, ura3Δ0, Mata | Research Genetics |
| AFT1-TAP in BY4741 | his3Δ1, leu3Δ0, met15Δ0, ura3Δ0, AFT1-TAP, Mata | Ref. 8 |
| BY4743 | his3Δ1/his3Δ1, leu3Δ0/leu3Δ0, met15Δ0/+, lys2Δ0/+, ura3Δ0/ura3Δ0, Mata/Matα | Research Genetics |
| Δfra1 in BY4743 | his3Δ1/his3Δ1, leu3Δ0/leu3Δ0, met15Δ0/+, lys2Δ0/+, ura3Δ0/ura3Δ0, FRA1::KanMX4/FRA1::KanMX4 | Research Genetics |
| Δfra2 in BY4743 | his3Δ1/his3Δ1, leu3Δ0/leu3Δ0, met15Δ0/+, lys2Δ0/+, ura3Δ0/ura3Δ0, FRA2::KanMX4/FRA2::KanMX4 | Research Genetics |
Integration of the FET3-lacZ Reporter at the HO Locus in Wild Type Yeast—A 500-bp upstream FET3 promoter was PCR-amplified and cloned into YEp345. The FET3-lacZ reporter was further amplified and cloned into plasmid HO-hisG-URA3-hisG-poly-HO obtained from Dr. David Stillman (University of Utah). The resulting construct was digested, and the fragment containing the FET3-lacZ reporter with the HO sequence on both ends was integrated at the HO locus in wild type yeast DY150 × DY1457. The integrants were further selected by uracil prototrophy. The diploid was sporulated and dissected to create the yeast strains OCY354, OCY355, OCY356, and OCY357.
Genetic Screen and Identification of Mutants—Wild type yeast cells with the FET3-lacZ reporter integrated at the HO locus, OCY356 and OCY357, were plated on YPD supplemented with 50 μm iron (Fe2SO4) and mutagenized by UV to reach a 50% reduction in viability. After 3 days of growth at 30 °C, colonies were replicated to nylon membranes. Cells were disrupted by liquid nitrogen, and X-gal, a substrate for β-galactosidase, was added on top of the membrane. Membranes were incubated at 30 °C for 16 h, and the development of blue color indicated FET3 expression. Mutants that developed blue color were collected. Recessive mutants attributed to a single gene mutation were subjected to a genomic library screen by complementation analysis. A wild type genomic library constructed in YCp50 (ATCC catalog number 37415) was used for all library screens in this study. Complementing library clones were rescued, and library inserts were determined by DNA sequencing. If the insert contained multiple open reading frames, the responsible gene was determined by subcloning each of the open reading frames.
Targeted Gene Deletion—Deletion of FRA1 (YLL029W) or FRA2 (YGL220W) was accomplished, utilizing the homozygous deletion collection (Research Genetics, Inc.) as a PCR template. Specific A and D primers for each gene disruption were obtained from the Saccharyomyces Genome Deletion Project. PCR DNA was transformed into diploid DY150 × DY1457, with or without the FET3-lacZ reporter integrated in the genome, or in the diploid OCY355 × OCY356 containing the integrated FET3-lacZ reporter. Disruption of genes was verified by PCR. Diploids, heterozygous for the disruption, were sporulated and dissected to generate the gene deletion in a haploid. The haploid gene deletion was confirmed by PCR.
Plasmid DNA Construction—FRA1 was subcloned from a library clone pIF17B, composed of 78944–90188 of chromosome 12. BspHI digestion released a 4870-bp fragment containing the FRA1 open reading frame and 0.5 kb of the 5′ and 2 kb of the 3′-flanking region. The fragment was blunt-ended using Escherichia coli DNA polymerase I, large (Klenow) fragment (New England Biolabs), and the 5′-phosphate group was removed by alkaline phosphatase (New England BioLabs). The fragment was cloned into YCp33. To create a gap-repaired plasmid for rescuing the fra1-1 allele, a BstEII/AfeI fragment was removed from the FRA1 subclone described above. The resulting fragment was transformed into the fra1-1 mutant, and the rescued allele was sequenced. N-terminal and C-terminal YFP fragments (a kind gift of Colin Duckett) were subjected to PCR and subcloned into the yeast expression vector YCp pCM189 (URA or LEU) under the control of the doxycycline-regulated tetO7 promoter (8). Grx4 was fused to a linker sequence (RSIAT) and YFPN-(1–155) or YFPN-(1–155) and a linker sequence (GSANSSIDLISVPVDSRAV) via homologous recombination in pCM189 (URA), yielding pGrx4YN and pYNGrx4, respectively. Fra2 was fused to a linker sequence (RPACKIPNDLKQKVMNH) and YFPC-(156–239) or YFPC-(156–239) and a linker sequence (GSANSSIDLISVPVDSRAV) via homologous recombination in pCM189 (LEU), yielding pFra2YC and pYCFra2, respectively. Four additional constructs were generated by exchanging Fra2 for Grx4 and Grx4 for Fra2 in the above constructs (yielding pFra2YN, pYNFra2, pGrx4YC, and pYCGrx4, respectively).
A c-Myc-tagged Grx4-Fis1 fusion protein was prepared as follows. FIS1 was excised as a BamHI/SalI fragment from plasmid B1607 (generously provided by J. M. Shaw) and inserted into pRS416-MET25. A three-c-Myc tag-GRX4 fragment flanked with SpeI-BamHI sites was generated by overlapping PCR technique and then inserted into pRS416-MET25-FIS1. Plasmid CM1089 GRX4-c-MYC has been described previously (9). All plasmid constructs were confirmed by DNA sequencing.
Bacteria and Yeast Transformation—For rescuing plasmid DNA from yeast, total cellular DNA was extracted and transformed into DH10β (Invitrogen)-competent E. coli by electroporation using a Bio-Rad GenePulser Xcell according to the manufacturer's instructions. Yeast transformation of plasmid DNA, gap-repaired DNA, and the gene disruption fragment was performed using standard protocols.
Other Procedures—Cell lysates for immunoblotting were prepared by glass bead beating cells in 10% trichloroacetic acid, 100 mm Tris acetate buffer, pH 8. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with the indicated antibodies and detected using chemiluminescence (Western Lightening; PerkinElmer Life Sciences). Rabbit peroxidase anti-peroxidase (Sigma) or rabbit anti-TAP (made against the carboxyl terminus of the TAP construct after TEV cleavage (Open Biosystems)) was used for TAP detection at a 1:1000 dilution; rabbit polyclonal anti-His6 (1:3000; Abcam), mouse monoclonal anti-c-Myc (1:1000; Covance), or rabbit polyclonal anti-c-Myc (1:2000; Santa Cruz Biotechnology, Inc.) was used to detect tagged proteins on Western blots. Cell lysates for immunoprecipitation analysis were prepared by glass bead beating the cells in 50 mm Tris-Cl, pH 7.5, 150 mm sodium chloride, 0.1% Nonidet P-40, 0.05% sodium deoxycholate, and Complete Protease Inhibitor Mixture tablets (Roche Applied Science). The immunoprecipitated proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5.0% nonfat milk and probed with the indicated antibodies as before. Cell lysates for Ni2+-NTA-agarose (Qiagen) purification of His-tagged proteins were prepared as described above except that the lysis buffer contained 10 mm imidazole, and the His-tagged proteins were eluted from the beads using lysis buffer containing 500 mm imidazole (5 min at room temperature). Chromatin immunoprecipitation was performed as described previously (10). The quantitative β-galactosidase activity assay and the reporter constructs used were described previously (6). The aconitase activity assay was performed as described (11). Intracellular localization of Aft1 was determined by fluorescence microscopy of the green fluorescence protein (GFP)-tagged protein, as described previously (12). The percentage of cells showing nuclear Aft1-GFP was quantified. Two independent experiments were performed, and in each experiment, 10 fields/condition were examined, and the percentage of cells showing nuclear fluorescence was determined. Greater than 300 cells/condition were counted. Subcellular localization of Grx3 was determined by immunoblot using postmitochondrial supernatant, crude mitochondria, and purified mitochondrial extracts prepared as previously described (13).
Polyclonal Antisera—New Zealand White rabbits were immunized with Fra1 protein expressed in E. coli. The FRA1 open reading frame with a carboxyl-terminal His6 tag was cloned into the pET21a vector (Novagen) and expressed in Rosetta DE3 cells (Novagen). Protein production was induced using isopropyl-β-d-thiogalactopyranoside, and the protein was purified using an Ni2+-NTA column (Qiagen). Fra1-specific antibodies were purified from serum using a Fra1-agarose column generated by immobilizing recombinant Fra1 using the AminoLink Plus Kit (Pierce) according to the manufacturer's instructions using the pH 7.2 coupling buffer. Rabbit polyclonal anti-Fra2 was generated commercially by Washington Biotechnology using the sequence RETKRQRHYKMPVTEQ conjugated to KLH; anti-Fra2 was used at a 1:1000 dilution for Western blotting and immunoprecipitation. For Grx3 antibody production, the Grx3 open reading frame lacking the first 35 amino acids (Δ1–35) was cloned into pET21a and expressed in BL21(DE3) cells (Novagen). The protein was precipitated with ammonium sulfate, resuspended, desalted, and then loaded onto a DEAE column followed by a Superdex 75 gel filtration column (GE Healthcare). The purified protein was used to prepare rabbit-generated anti-Grx3 antibodies (Cocalico Biologicals, Reamstown, PA).
RESULTS
We utilized a genetic approach to identify mutant genes that activate the iron regulon in iron-replete cells. Three classes of mutants might be expected to show increased expression of the iron regulon. The first class consists of mutations in genes that encode the high affinity iron transport system (FET3, FTR1) or in genes required for the assembly of the high affinity transport systems. Mutations in these genes lead to reduced cellular iron and increased expression of the iron transport system. The second class includes mutations in genes that encode elements of the mitochondrial Fe-S cluster synthesis and export system. The third class of mutants is expected to interrupt the regulatory signal between mitochondrial Fe-S clusters and Aft1.
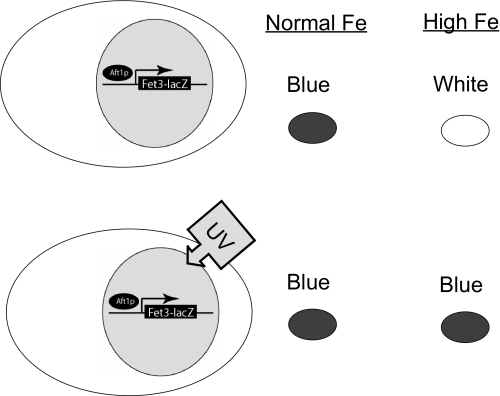
Activation of the iron regulon by the first class of mutations can be suppressed by incubation of cells in high iron medium, since transport of iron by the low affinity iron transport systems would satisfy cellular iron needs and down-regulate high affinity iron transport. The other two classes of mutations would not be suppressed by high external iron. A yeast strain was generated that contained a FET3-lacZ reporter construct integrated at the HO locus. Abundant β-galactosidase activity would be produced when cells are incubated in medium made iron limited through the addition of the cell-impermeable iron chelator BPS (Fig. 1). Little FET3-lacZ activity is seen when wild type cells are incubated in SD. If a deletion in FET3 is introduced into this strain, β-galactosidase activity is activated due to defective iron acquisition. The addition of 50 μm iron to SD, however, suppresses the production of β-galactosidase activity, since cells become iron-replete by the transport of iron through low affinity iron transporters. Based on this result, we mutagenized cells containing an integrated copy of FET3-lacZ by UV light and selected for colonies that showed increased β-galactosidase activity (blue-white assay) when plated on medium containing 50 μm iron. We screened 40,000 colonies to identify those that showed increased β-galactosidase activity in the presence of iron-replete medium. Each mutant was crossed to a wild type strain (also carrying an integrated FET3-lacZ), and all were found to be recessive and, when sporulated, behaved as a single gene defect, showing 2:2 segregation.
FIGURE 1.
Model of the genetic screen designed to identify genes that regulate the iron regulon. Yeast cells containing a FET3-lacZ reporter gene integrated at the HO locus were deleted for the FET3 gene. When grown in standard culture medium, these cells will express FET3-lacZ due to impaired iron transport, thereby turning blue when exposed to X-gal, a substrate for β-galactosidase. The addition of 50 μm iron to the growth medium will completely suppress FET3-lacZ expression, since cells obtain iron through low affinity iron transporters. Based on this observation, wild type cells containing the integrated FET3-lacZ reporter were mutagenized by UV and plated on medium supplemented with 50 μm iron. Colonies that were blue in iron-replete medium were further studied.
Each mutant was transformed with a low copy library, and transformants that regained the ability to regulate the FET3-lacZ reporter in an iron-dependent manner were further analyzed. We identified six genes that affected expression of FET3-lacZ. Two of the genes encoded proteins shown to have a direct role in Fe-S cluster synthesis, NFS1 (14, 15) and ISU1 (16). A third gene, GSH2, encodes an enzyme involved in glutathione synthesis. It is known that deletion of GSH1 decreases Fe-S cluster synthesis (17), and it might be expected that deletion of GSH2 would also affect Fe-S cluster synthesis. The fourth gene, MTM1, is a member of the mitochondrial carrier family. In the absence of MTM1, there is a defect in the activity of Sod2 due to increased mitochondrial iron (18). Deletion of MTM1 was also shown to activate the iron regulon, although the mechanism is not known. These results confirm the hypothesis that iron-sulfur cluster synthesis participates in the regulation of Aft1 activity.
The fifth gene identified encodes Sin4, which is part of the multiprotein Mediator complex (19). This complex interacts with RNA polymerase II to positively or negatively regulate transcription. Mutations in members of the Mediator complex have been shown to alter the basal rate of transcription of Aft1 target genes (20).
Identification of a Novel Gene FRA1 (YLL029W) Involved in the Regulation of the Iron Regulon—The sixth gene identified by the screen was an unknown open reading frame, YLL029W. This gene complemented three independent mutants that were in the same complementation group. Several lines of evidence demonstrate that YLL029W affects activation of the iron regulon. First, we rescued YLL029W from mutant IF17B, sequenced the rescued allele, and identified a missense mutation, which results in an amino acid change from leucine 590 to proline. Second, in haploids obtained from the mating of wild type and mutant strain IF17B, the mutant allele co-segregated with increased expression of FET3-lacZ. Third, deletion of YLL029W in the S288C diploid deletion collection (Research Genetics) also showed increased expression of FET3-lacZ in high iron-containing medium. Fourth, a targeted deletion of YLL029W in W303, the background strain used in this screen, showed increased expression of FET3-lacZ, and a diploid between the deletion strain and mutant IF17B showed increased FET3-lacZ expression. Based on these data, we refer to YLL029W as FRA1 (Fe repressor of activation-1), and we refer to yll029wL590P as fra1-1.
To determine if transcriptional activation of the FET3-lacZ reporter construct is dependent on Aft1, we generated a Δaft1 fra1-1 strain containing an integrated FET3-lacZ reporter construct. Deletion of AFT1 negated the FET3-lacZ expression in fra1-1 cells, since no expression of β-galactosidase was detected by blue-white assay. This result shows that FRA1 participates in the regulation of Aft1-mediated expression of the iron regulon.
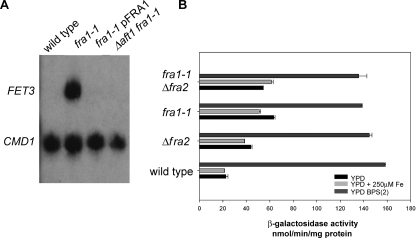
Wild type cells show no FET3 mRNA when cultured in high iron medium (Fig. 2A). Under similar conditions, the fra1-1 strain showed robust expression of FET3 mRNA, which was reduced upon transformation with a FRA1-containing plasmid. Expression of FET3 mRNA in fra1-1 cells was dependent on the transcription factor Aft1, since deletion of AFT1 in fra1-1 cells resulted in the loss of FET3 mRNA. We assayed β-galactosidase activity expressed from a FET3 reporter construct to quantify transcriptional activity. This reporter construct contains the FET3 promoter but lacks the endogenous 5′- and 3′-untranslated regions, precluding post-transcriptional regulation of the reporter construct. Wild type cells show minimal expression of β-galactosidase in cells incubated in either YPD or YPD supplemented with iron (Fig. 2B). In contrast, fra1-1 cells grown in either YPD or iron-supplemented YPD showed increased expression of β-galactosidase. Incubation of fra1-1 mutant cells in low iron medium, however, resulted in a further increase in reporter activity, suggesting that iron depletion affects the regulation of the iron regulon even in the absence of FRA1. Transformation of fra1-1 or Δfra1 cells with plasmids containing reporter constructs for the Mac1-regulated copper regulon (CTR1-lacZ) or the Zap1 regulated zinc regulon (ZRT1-lacZ) showed no increased expression over wild type cells (data not shown). This result shows that deletion of FRA1 is specific for transcriptional activation of FET3.
FIGURE 2.
Deletion or mutation of FRA genes affects transcription of FET3. A, wild type, fra1-1, and fra1-1 cells transformed with a plasmid expressing FRA1 under the control of the endogenous promoter and fra1-1Δaft1 cells were incubated in high iron medium (YPD + 250 μm FeSO4). Cells were grown to midlog phase and harvested, and mRNA levels for FET3 and for calmodulin (CMD1) were analyzed by S1 analysis. B, wild type, fra1-1, Δfra2, and fra1-1Δfra2 cells containing an integrated FET3-lacZ reporter construct were incubated in low iron (dark gray bars; YPD + 40 μm BPS + 2 μm FeSO4), iron-sufficient (black bars; YPD), and high iron (light gray bars; YPD + 250μm FeSO4) medium. The cells were grown for 12 h, and β-galactosidase activity was determined.
Deletion of FRA2 Affects Activation of the Iron Regulon—A large scale interaction study reported that Fra1 physically interacts with Grx3, Grx4, and a protein encoded by an unknown open reading frame, YGL220W (21). We examined the effect of deletion of YGL220W on expression of the FET3-lacZ reporter construct, since two recent studies showed that deletion of GRX3 and GRX4 leads to induction of the iron regulon (9, 22). W303 cells with a deletion in YGL220W are viable and show increased expression of FET3 mRNA as analyzed by S1 analysis (data not shown). To examine the relationship between FRA1 and FRA2, we generated a double mutant strain, fra1-1Δfra2, and measured the activity of the integrated FET3-lacZ reporter gene under various iron conditions. Deletion of FRA2 led to an increase in β-galactosidase expression that was similar to that seen in fra1-1 cells. Deletion of FRA2 in a fra1-1 strain did not lead to any further increase in FET3-lacZ expression (Fig. 2B), suggesting that Fra1 and Fra2 are part of the same pathway. Additionally, incubation of fra1-1Δfra2 cells in low iron medium resulted in a further increase in transcriptional activity. We then tested whether deletion of GRX3 or GRX4 in combination with deletion of FRA1 and FRA2 affected expression of FET3-lacZ. We confirmed the published reports that deletion of GRX3 or GRX4 singly had little effect on FET3-lacZ activity (9, 22). Deletion of either glutaredoxin gene alone in combination with deletions in FRA1 or FRA2 did not have an additive effect in activating the iron regulon (data not shown). Previous work showed that deletion of both GRX3 and GRX4 resulted in maximal induction of the iron regulon that was independent of medium iron (9, 22). In contrast, we observed that iron deprivation increased activation of the iron regulon in fra1-1, Δfra2, and fra1-1Δfra2 cells. There is no measurable growth defect in fra1-1Δfra2 or Δfra1Δfra2 strains; however, there is a severe growth deficit in Δgrx3Δgrx4 cells. It is possible that the increased expression of the iron regulon in Δgrx3Δgrx4 cells is a downstream response to those conditions that lead to poor growth.
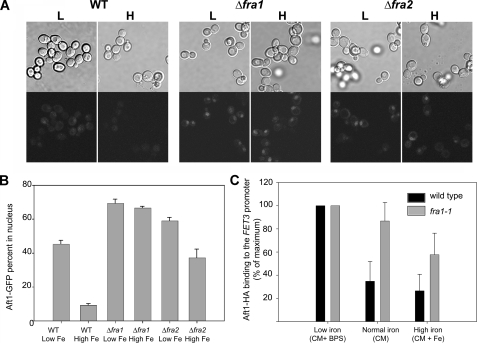
Aft1 Occupies the FET3 Promoter in Δfra1 Cells—There are at least two discrete steps required for Aft1-mediated transcriptional activation; Aft1 must translocate from cytosol to nucleus, and within the nucleus it must occupy the Aft1 binding site and then recruit activators or co-activators. We used a GFP-tagged Aft1 to follow Aft1 movement in wild type, Δfra1, and Δfra2 cells. Under high iron conditions in wild type cells, Aft1-GFP is primarily cytosolic, with less than 10% of cells showing Aft1-GFP localized in the nucleus, whereas under low iron conditions, Aft1-GFP is detected in the nucleus of about 50% of the cells (Fig. 3, A and B). In contrast, Aft1-GFP is localized in the nuclei of Δfra1 and Δfra2 cells under both high and low iron conditions. We note that the accumulation of Aft1-GFP in the nucleus of wild type cells grown in low iron is not as distinct as in Δfra1 or Δfra2 cells, although we cannot explain this observation. We examined whether nuclear Aft1 occupies the FET3 promoter in wild type versus fra1-1 cells by performing chromatin immunoprecipitation experiments using HA-tagged Aft1. Cells expressing Aft1-HA were grown in high (CM + 250 μm FeSO4), medium (CM), and low (CM + 40 μm BPS) iron media, and chromatin immunoprecipitation was performed using an anti-HA antibody. Primers to the FET3 promoter and the control CMD1 (calmodulin) gene were used for PCR amplification. As shown before, the iron regulon is fully induced in wild type and fra1-1 cells grown in low iron medium, and the amount of Aft1 bound to the FET3 promoter was similar in wild type and fra1-1 cells. In moderate and high iron medium, however, more Aft1-HA was bound to the FET3 promoter in fra1-1 cells than in wild type cells (Fig. 3C), demonstrating that induction of the iron regulon in fra1-1 cells is the consequence of Aft1 activating transcription.
FIGURE 3.
Aft1 enters the nucleus and occupies the FET3 promoter in Δfra1 cells. A, wild type (WT), Δfra1, and Δfra2 cells were transformed with a low copy plasmid expressing a GFP-tagged Aft1 under the control of its endogenous promoter. The cells were grown overnight in low (L) and high iron (H) medium, and the subcellular localization of Aft1-GFP was examined by fluorescence microscopy. B, the percentage of cells showing Aft1-GFP in the nucleus was quantified. C, wild type (black bars) and fra1-1 cells (gray bars) were transformed with a low copy plasmid containing AFT1-HA. The cells were grown in iron-limited (CM + 40 μm BPS iron), “normal” iron (CM), or iron-replete (CM + 250 μm Fe2SO4) media. After 12 h of growth, cells were harvested, and chromatin was immunoprecipitated using the HA epitope of Aft1. After cross-linking was reversed, PCR was performed using primers, which flank the Aft1 binding site of the FET3 promoter, with CMD1 used as a positive control. Aft1 binding to the FET3 promoter in normal and high iron conditions is shown as the percentage of the low iron (maximum) values, and the error bars represent the S.E. These results are the average of three independent experiments.
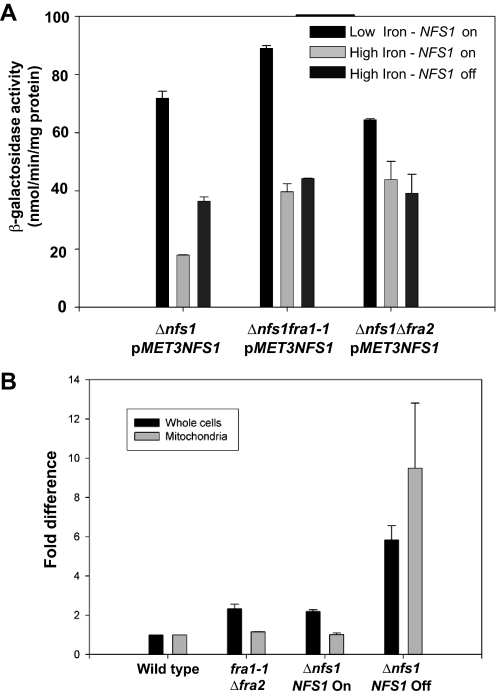
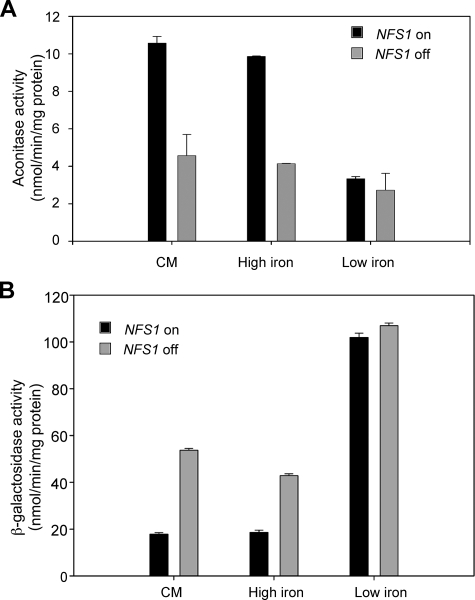
Fra1 and Fra2 Act in the Same Pathway as Fe-S Cluster Synthesis—We have previously shown that decreased mitochondrial Fe-S cluster synthesis activates the iron regulon (6). To determine if Fra1 and Fra2 are part of the Fe-S cluster synthesis-sensing pathway, we examined the effect of inhibiting Fe-S cluster synthesis in fra1-1 or Δfra2 cells. We generated cells with a deletion in NFS1, the cysteine desulfurase, which is essential for Fe-S cluster synthesis. The strains are maintained by expression of NFS1 under the control of a MET3 promoter. Incubation of cells expressing NFS1 in low iron medium led to induction of FET3-lacZ (Fig. 4A). Incubation of Δnfs1 cells expressing NFS1 in high iron medium led to a decrease in induction of FET3-lacZ. In the absence of NFS1 expression, FET3-lacZ is expressed, although the level of expression is less than that seen when cells are grown in low iron medium. When NFS1 was expressed in fra1-1 or Δfra2 cells grown in high iron medium, the level of FET3-lacZ was higher than in Δnfs1 cells expressing NFS1. In the absence of NFS1 expression, there was no further increase in FET3-lacZ expression in Δnfs1 fra1-1 or Δnfs1 Δfra2 cells.
FIGURE 4.
Fra1 and Fra2 are part of the mitochondrial Fe-S cluster synthesis-sensing pathway. A, cells (Δnfs1, Δnfs1 fra1-1, Δnfs1Δfra2) containing an integrated FET3-lacZ reporter construct were transformed with a plasmid containing MET3-regulated NFS1. Cells were grown in medium lacking methionine to induce expression of NFS1. The cells were transferred to medium lacking methionine (on) or medium containing 10 times the normal level of methionine (off) to prevent expression of NFS1. The media were either iron-limited (black bars; low iron CM + 40 μm BPS) or iron-replete (high iron CM + 50 μm FeSO4). The cells were grown in the specified media for 6 h, and then β-galactosidase activity was measured. B, wild type, fra1-1Δfra2, and Δnfs1(pMET3NFS1) cells were grown in CM medium in the presence or absence of methionine for 12 h. Cells were harvested, and the amounts of iron in whole cells and purified mitochondria were determined by inductively coupled plasma mass spectrometry. The data were normalized to cell or mitochondrial protein, and μg of iron/mg of protein is expressed as -fold difference compared with wild type levels. The error bars are the S.D. of three experiments.
Mutation of genes involved in Fe-S cluster synthesis or export leads to increased expression of the iron regulon and high levels of mitochondrial iron. In the absence of NFS1 expression, there is an increase in cellular and mitochondrial iron (Fig. 4B). In contrast, fra1-1Δfra2 cells showed a small increase in cellular iron compared with wild type cells and show no increase in mitochondrial iron. We note that overexpression of NFS1 leads to a small increase in whole cell iron levels relative to wild type cells.
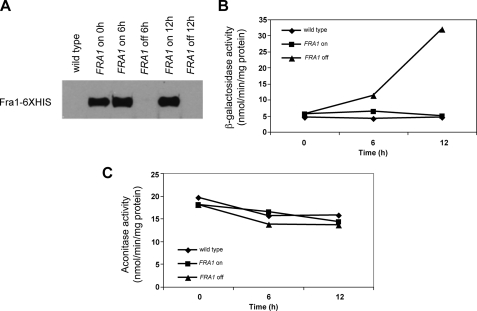
We tested the possibility that the FRA genes affect mitochondrial Fe-S production by measuring the activity of the mitochondrial Fe-S cluster-containing protein aconitase in cells that did not express FRA1. We transformed fra1-1 cells with a plasmid containing a β-estradiol-regulated FRA1-His6. In the absence of β-estradiol, no Fra1-His6 could be detected by Western blot analysis, whereas abundant protein was detected in the presence of β-estradiol (Fig. 5A). If β-estradiol was removed, Fra1-His6 was no longer detected 6 h later. Removal of β-estradiol resulted in a time-dependent increase in FET3-lacZ activity over 12 h (Fig. 5B). Over that same time course, there was no measurable difference in aconitase activity in wild type cells or in fra1-1 cells expressing or not expressing Fra1-His6 (Fig. 5C).
FIGURE 5.
Depletion of Fra1 does not affect mitochondrial aconitase. A, fra1-1 cells containing a FET3-lacZ reporter construct were transformed with a plasmid containing a β-estradiol-inducible FRA1. The cells were grown in CM in the presence of β-estradiol, and then either cells were maintained with β-estradiol, or β-estradiol was removed for the specified times. Samples were taken, and Fra1-His6 protein was determined by Western blot analysis. Aliquots were also taken to assay β-galactosidase (B) and aconitase (C) activity.
It is possible that the turnover rate of aconitase is slow enough that changes in Fe-S cluster synthesis would not be detected in the time frame of the experiment. To test this possibility, we measured the effect of regulating expression of NFS1 on aconitase activity. Reduction of NFS1 expression through the use of the MET3-regulated NFS1 led to a greater than 60% decrease in aconitase activity within 8 h (Fig. 6A). This result shows that the turnover or synthesis rate of aconitase is fast enough to conclude that loss of FRA1 does not affect Fe-S cluster synthesis. When NFS1-deficient cells were placed in low iron medium, there was a small decrease in aconitase activity compared with cells cultured in iron-replete medium. Under the same conditions, iron depletion led to a large increase in expression of FET3-lacZ (Fig. 6B). These results suggest that iron depletion can increase the induction of the iron regulon even in cells with compromised Fe-S cluster synthesis. These data are consistent with results obtained in fra1-1 or Δfra2 cells, which showed that iron depletion increased expression of the iron regulon (Fig. 2). We conclude that Fra1 and Fra2 act in the same pathway as Fe-S cluster synthesis, and iron influences transcription of the iron regulon beyond its affect as a substrate for Fe-S cluster synthesis.
FIGURE 6.
Depletion of Nfs1 affects aconitase and induces expression of FET3-lacZ. A, Δnfs1 cells containing pMET3NFS1 and a FET3-lacZ reporter were grown for 8 h in normal (CM), high iron (50 μm FeSO4), or low iron media (CM + 40 μm BPS + 2 μm FeSO4) either in the presence (black bars) or absence of methionine (gray bar). The cells were harvested, and aconitase (A) and β-galactosidase (B) activity was measured.
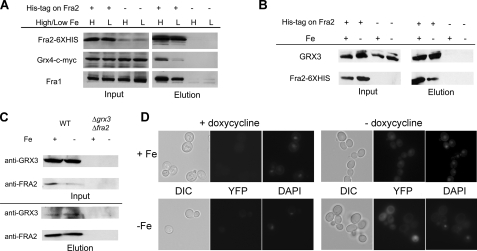
Fra1 and Fra2 Form an Iron-independent Complex with Grx3-Grx4—Published studies (21) have shown a physical interaction between Fra1 and Fra2. We examined the effect of iron status on the interaction of Fra1, Fra2, Grx3, and Grx4. Cells (Δfra2) were transformed with a plasmid containing GRX4-c-MYC construct and either a FRA2-His6 construct or a FRA2 construct lacking the HIS epitope. Extracts were prepared from cells grown in either iron-replete or iron-limited medium. Fra2-His6 was purified using Ni2+-NTA resin, and the eluate was probed by Western blotting. We detected Fra1 and Grx4-c-Myc in the eluate prepared from both high and low iron-grown cells, indicating that the interaction of Fra1, Fra2, and Grx4-c-Myc is iron-independent (Fig. 7A). As a control for affinity purification, no Fra1 or Grx4-c-Myc was detected in extracts from cells transformed with an untagged Fra2. We note that there was an apparent increased association of Grx4 with Fra2-His6 in high iron conditions. This result, however, was not consistent. We also detected endogenous Grx3 associated with affinity-purified Fra2-His6 in both iron-replete and iron-depleted cells (Fig. 7B). We took advantage of our anti-Fra2 antibody to determine if Fra2 and Grx3 would form a complex when expressed at endogenous levels. Immunoprecipitation of Fra2 resulted in the co-immunoprecipitation of Grx3 from both iron-replete and iron-depleted cells (Fig. 7C). The levels of both proteins were low; however, there was clear evidence of a complex. No complex was detected in Δfra2Δgrx3 cells, reflecting the specificity of the antibodies. We confirmed the iron-independent Fra-Grx complex formation using a split YFP tagging (23). Cells were transformed with plasmids containing tetracycline-regulated FRA2-YFP and GRX4-YFP (8). No YFP fluorescence was detected in cells transformed with pFRA2-YFP or pGRX4-YFP alone in the presence or absence of doxycycline (data not shown). Fluorescence was detected in cells transformed with both constructs but only in the absence of doxycycline (Fig. 7D). The fluorescence appeared to be primarily cytosolic, and the distribution and levels of fluorescence were iron-independent. These results suggest that Fra2 and Grx3 form a complex that is not affected by cytosolic iron levels.
FIGURE 7.
Immunoprecipitation of Fra2-His6 protein identifies a protein complex. A, cells (Δfra2) were transformed with a low copy plasmid containing a GRX4-c-Myc construct and a plasmid containing FRA2-His6 or FRA2 without the epitope tag. The cells were grown for 6 h in either low (L; CM + 40 μm BPS) or in high iron (H; CM + 50 μm FeSO4) medium. Extracts were applied to Ni2+-NTA resin, and the eluates were tested for the indicated proteins by Western blotting. Grx4-c-Myc was detected using an antibody specific for c-Myc. Fra2-His6 was detected using an anti-His antibody, and Fra1 was detected using a rabbit anti-Fra1 antibody. B, cells (Δfra2) were transformed with a high copy plasmid containing FRA2-His6 or FRA2 without the epitope tag. Cells were grown as in A and affinity-purified as in A, and eluates were probed for Fra2-His6 and endogenous Grx3 using anti-His6 or rabbit anti-Grx3 antibodies. C, wild type (WT) cells were grown as in A, extracts were obtained, and Fra2 was immunopurified using rabbit anti-Fra2 antibodies bound to Dynabeads (sheep anti-rabbit IgG; Invitrogen). Samples were tested for Grx3 and Fra2 by Western blotting. D, wild type cells were transformed with plasmids containing Grx4-YFP and Fra2-YFP. Cells were grown on CM-Ura-Leu supplemented with either FeS04 (100 μm) or BPS (100 μm) at 30 °C. After 20 h, cells were diluted 100× into fresh media containing doxycycline (1 μg/ml). After 18 h, the cultures were serially diluted (50× and then 20×) into fresh media and grown for an additional 20 h. Aliquots were removed from both dilutions and treatments, stained with 4′,6-diamidino-2-phenylindole (DAPI), and fixed to microscope slides treated with concanavalin A. Cells were stained with 4′,6-diamidino-2-phenylindole, mounted onto a coverslip, and examined for YFP and 4′,6-diamidino-2-phenylindole fluorescence, using an Olympus BX51 microscope with a ×100 1.4A oil immersion lens. Images were captured using PictureFramer software. DIC, differential interference contrast.
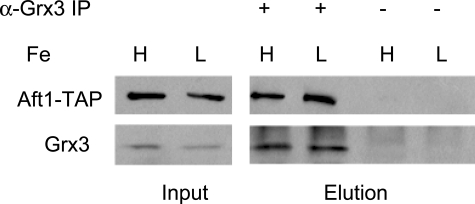
We did not find any evidence that Aft1 bound to the Fra1-Fra2 complex in either high or low iron medium, although an interaction between Aft1 and Grx3 and Grx4 was previously reported. Those studies used overexpressed Aft1 and overexpressed Grx3 and Grx4 (9, 22). To verify that Aft1 and Grx3-Grx4 interact under normal expression conditions, we generated cells containing an AFT1-TAP gene integrated at the chromosomal locus of AFT1. We confirmed that this epitope-tagged Aft1 regulated the expression of FET3 in an iron-dependent manner (data not shown). Cells were incubated in iron-replete or iron-limited medium, and Grx3 was immunoprecipitated using polyclonal antisera. The immunoprecipitated Grx3 was eluted, and the eluate was probed by Western blot analysis. Aft1-TAP co-purified with Grx3 under both low and high iron conditions (Fig. 8). These results extend the published studies by showing that Aft1 and Grx3 form an iron-independent complex even in the absence of overexpression.
FIGURE 8.
Aft1-TAP co-immunoprecipitates with Grx3. Cells containing an AFT1-TAP integrated into the genome were grown for 6 h in high and low iron medium. Cells were lysed and immunoprecipitated as described under “Experimental Procedures” using a Grx3-specific antibody. The immunoprecipitate (IP) was probed for Aft1-TAP using a polyclonal anti-TAP antibody and a polyclonal anti-Grx3 using Western blot analysis.
Fra1, Fra2, and Grx3 Are Cytosolic Mediators of Fe-S Sensing—Fra1 and Fra2 were found to be mainly cytosolic proteins by a genome-wide GFP tagging study (24). We confirmed the observation that Fra2-GFP is cytosolic; however, we observed that the carboxyl-GFP acts as a dominant negative protein. Expression of Fra2-GFP in wild type cells led to activation of the iron regulon even in iron-replete medium. The subcellular localization of Fra1 and Fra2 is hampered by the lack of useful reagents, since none of the tagged versions of low copy expressed Fra1 or Fra2 were detected by immunofluorescence. Antiserum generated against Fra1, which detects the protein on Western blots, did not detect the protein by immunofluorescence. Although both membrane and cytosolic fractions of yeast cells reacted with the anti-Fra1 antibody, purified mitochondrial lysates were negative (data not shown).
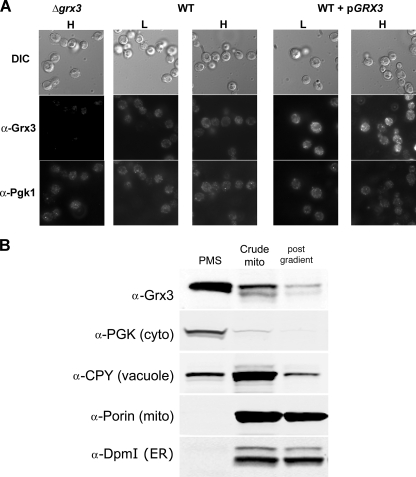
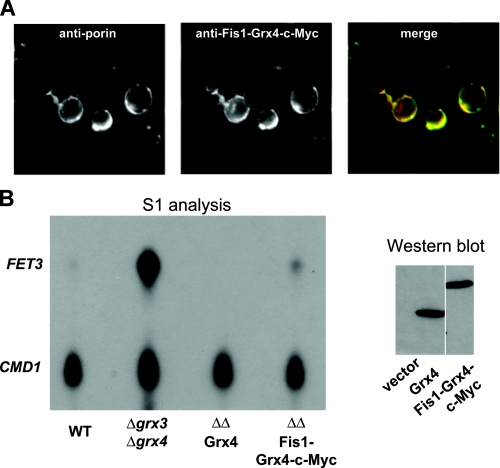
Grx3-GFP was shown to localize to the nucleus; however, the protein was overexpressed (25). We used a polyclonal Grx3 antibody, which detects native Grx3, for immunofluorescence. Immunofluorescence of Grx3 in wild type cells showed homogenous staining of yeast cytosol, no staining was seen in Δgrx3 cells, and there was increased cytosolic staining in cells expressing a high copy GRX3 plasmid. The cytosolic staining seen in Grx3-containing cells was similar to that seen in cells stained for the cytosolic enzyme Pgk1, which is consistent with a cytosolic localization (Fig. 9A). Expression of GRX3 from a plasmid in wild type cells led to increased staining but did not affect localization. The cytosolic staining remained unchanged in cells grown in high and low iron conditions. Subcellular fractionation of cells indicated that most Grx3 is primarily cytosolic. Purified mitochondria show a relative depletion in Grx3, although some protein can be detected associating with mitochondria (Fig. 9B). To determine if Grx4 acts in the cytosol, we generated a Grx4-Fis1 fusion protein, which tethers Grx4 to mitochondrial outer membrane in which the active site of the enzyme is in the cytosol (Fig. 10A). This fusion protein was able to partially complement the Δgrx3Δgrx4 phenotype of activation of the iron regulon, indicating that the cytosol-restricted protein is partially functional (Fig. 10B).
FIGURE 9.
Grx3 localizes to the cytoplasm. A, wild type, Δgrx3, or wild type (WT) cells transformed with a high copy plasmid containing GRX3 under the control of its endogenous promoter, were grown in high (H) and low (L) iron media and processed for immunofluorescence using a polyclonal anti-Grx3 antibody (1:100) and the monoclonal anti-Pgk1 antibody followed by Alexa 594-conjugated goat anti-rabbit IgG and Alexa 488-conjugated goat anti-mouse IgG (1:750; Invitrogen). DIC, differential interference contrast. B, wild type cells were grown to midlog phase and homogenized, and postnuclear supernatant was separated into a postmitochondrial fraction (PMS) and a crude mitochondrial fraction (Crude mito). Mitochondria were further purified by ultracentrifugation in a sucrose gradient (post gradient). Equal amounts of protein are loaded in each lane. The presence of organellar markers was determined by Western blot using anti-Grx3, anti-Pgk1 (cytosolic marker), anti-CPY (vacuolar marker), anti-porin (mitochondrial marker), and anti-Dpm1 (endoplasmic reticulum ER marker) (Invitrogen).
FIGURE 10.
Cytosolic Grx3-Grx4 affects Aft1 function. A, cells expressing Fis1-Grx4-c-Myc fusion protein were grown to midlog and processed for immunofluorescence using rabbit anti-c-Myc (1:100; Covance) and mouse anti-porin (1:100; Invitrogen), followed by Alexa 594-conjugated goat anti-rabbit IgG and Alexa 488-conjugated goat anti-mouse IgG (1:750; Invitrogen). The c-Myc-tagged Fis1-Grx4 co-localized with the mitochondrial outer membrane protein porin, showing that the fusion protein is tethered to the mitochondria, thus restricting Grx4 to the cytosol. B, wild type (WT), Δgrx3Δgrx4, or Δgrx3Δgrx4 transformed with empty vector, pGRX4-c-Myc, or pFIS1-GRX4-c-Myc were grown to midlog, and samples were harvested for FET3 and CMD1 S1 analysis or Western blot using an anti-c-Myc tag antibody. Grx4-c-Myc and Fis1-Grx4-c-Myc are expressed at comparable levels.
DISCUSSION
We have previously shown that the high affinity iron transport system is regulated by the products of the Fe-S cluster pathway. Expression of the iron regulon occurs in the absence of genes that encode components of the Fe-S cluster pathway even when cells are not iron-limited (6). In yeast, Fe-S clusters are exported from the mitochondria and are assembled on cytosolic or nuclear apoproteins by a cytosolic multiprotein complex (26). Deletion of genes that encode proteins that participate in that complex led to defective cytosolic Fe-S protein assembly but did not induce the iron regulon (7). These studies revealed that Aft1 sensed a compound intimately linked to mitochondrial Fe-S cluster synthesis but not to cytosolic Fe-S protein assembly. Thus, the role of Fe-S cluster synthesis in regulation of the iron regulon is unclear. We took a genetic approach to confirm the role of mitochondrial Fe-S cluster synthesis in iron-sensing and to identify additional elements of the iron-sensing machinery. Our screen identified several elements of the Fe-S cluster pathway (NFS1, ISU1, and GSH2) as well as two genes (MTM1 and SIN4) known to affect expression of the iron regulon. The screen also led to the identification of an unknown gene we named FRA1 that when deleted activated the iron regulon. Large scale proteomic studies showed that this protein was part of a complex that consisted of Grx3, Grx4, and another unknown protein we refer to as Fra2. Deletion of any of these genes in a Δgrx4 strain leads to increased expression of the iron regulon. Based on these results and the observation that Fra1 and Fra2 are cytosolic proteins, we speculate that these proteins are involved in the same pathway as mitochondrial Fe-S clusters and that they interpret the Fe-S cluster synthesis signal. In the absence of either Fra1 or Fra2, Aft1 is predominantly nuclear and occupies the FET3 promoter inducing FET3 transcription. FRA1 and FRA2 were identified in a large scale genomic screen as genes that, when deleted, led to increased expression of SIT1 (ARN1), the siderophore transporter for ferroxamine B (27). Since siderophore transporters are regulated by Aft1, these results suggest that Fra1 and Fra2 regulate siderophore transporters as well as the transporters for ionic iron.
Fra1 and Fra2 are ubiquitously expressed in all eukaryotes. Fra2 is a member of a protein family of unknown function, which is named after one of the E. coli members, BolA. BolA-like proteins have been hypothesized to work together with monothiol glutaredoxins based on computational and biochemical analyses (28). Recently, a chloroplast-localized protein homologous to BolA was identified that participates in chloroplast Fe-S synthesis of Arabidopsis thaliana (29). The N-terminal domain shows similarity to SufE proteins, whereas the C-terminal domain is homologous to BolA. Bacterial SufE proteins participate in Fe-S assembly through their ability to transfer sulfur between proteins (30, 31). FRA1 encodes an aminopeptidase P-like protein. Aminopeptidase P-like proteins are manganese-containing enzymes that can hydrolyze peptides with an N-terminal Xaa-Pro motif, but the function of Fra1 in this regard has not yet been tested. The transcription factor Spt16 also has an aminopeptidase-like domain, which is thought to act in protein binding.4 We do not know if this domain is important for binding of Fra1 to Fra2.
Our results suggest that Fra1 and Fra2 are cytosolic proteins that interpret the Fe-S cluster signal, and in their absence, the iron regulon is induced. The Fra proteins are outside of the mitochondria, and we have no evidence that mutation or deletion of either protein affects mitochondrial processes. Furthermore, our data indicate that induction of the iron regulon by deletion of the FRA genes is not additive with induction due to the absence of Fe-S cluster synthesis. Iron limitation leads to a further increase in FET3 transcription in both FRA-deleted cells and in cells with defects in Fe-S cluster synthesis. We interpret these results to indicate that induction of the iron regulon responds both to Fe-S cluster synthesis, of which iron is a required substrate, and to iron limitation beyond its role as a substrate for mitochondrial synthetic activities. The caveat to this interpretation is that we only examined cells that had reduced levels of Nfs1. NFS1 is an essential gene, and a complete loss of Nfs1 compromises cell viability.
Grx3 and Grx4 are monothiol glutaredoxins. Deletion of both genes results in full expression of the iron regulon, and Δgrx3Δgrx4 cells show a significant growth deficit resulting from increased oxidative damage (22). Our results support the hypothesis that the BolA homologue Fra2 is a Grx-binding protein. The levels of Fra1, Fra2, Grx3, and Grx4 are not appreciably affected by changes in cytosolic iron. It is noteworthy that there are reports that monothiol Grxs may dimerize, with a Fe-S cluster holding the two monomers together (32, 33). Preliminary results show that bacterial expressed Grx3 contains a Fe-S complex.5 Glutaredoxins are involved in sulfhydryl chemistry, reducing disulfides, or resolving mixed disulfides. Current studies are directed at determining whether the Fra1-Fra2 complex affects the activity of the bound glutaredoxins or if it targets those enzymes to a specific substrate.
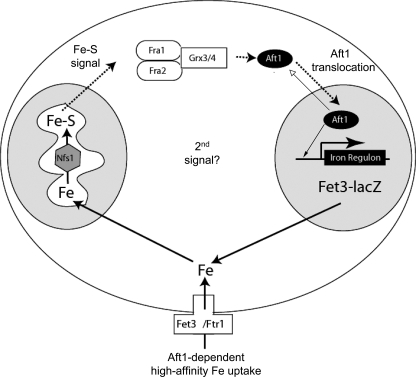
The available data lends itself to a working model (Fig. 11) in which there are two signals regulating Aft1: products of mitochondrial Fe-S cluster synthesis and an unknown signal that might be iron itself. Reduction in cellular iron levels will lead to decreased Fe-S cluster synthesis and decreased signaling through the Fra1-Fra2-Grx3-Grx4 complex. Our data do not provide evidence that the Fra1-Fra2 complex binds to Aft1, although we have confirmed that Aft1 can bind to Grx3-Grx4. The determination of how the Fra1-Fra2-Grx3-Grx4 complex affects Aft1 remains to be determined. In the absence of either mitochondrial Fe-S cluster synthesis or Fra1 or Fra2, Aft1 translocates to the nucleus, where it activates the FET3 promoter. Recently, Ueta et al. (34) published data suggesting that iron affects the nuclear export of Aft1. In the presence of iron, Aft1 multimerizes, and the multimer is recognized by the exportin Msn5, which exports Aft1 to the cytosol. This finding is not discrepant with our model. In our model, we suggest that the Grx3-Grx4-Fra1-Fra2 complex acts in the cytosol. Of particular note is the observation that Grx4 tethered to the mitochondria can prevent activation of the iron regulon. These data are consistent with a model in which Aft1 cycles between cytosol and nucleus, and the nuclear localization of Aft1 reflects a change in the rate constant of translocation. One possibility is that Aft1 is constantly “sampling” the cytosol. This model predicts that Aft1 cycles between nucleus and cytosol even in high iron-grown cells. Our studies also show that once within the nucleus, incubation of cells in iron-depleted media increases the binding of Aft1 to the FET3 promoter without any significant effect on Fe-S cluster synthesis, at least as measured by mitochondrial aconitase activity. These observations lead to an interesting question of whether all nuclear Aft1 is associated with DNA or whether iron levels can affect promoter occupancy. These results suggest that iron affects the transcriptional activity of Aft1 independent of the Fe-S cluster signaling pathway.
FIGURE 11.
Model for the role of Fra1-Fra2 in the regulation of Aft1 transcriptional activity. In the absence of mitochondrial Fe-S cluster synthesis, Aft1 translocates into the nucleus and induces the transcription of its target genes, such as the plasma membrane high affinity iron permease complex Fet3-Ftr1. The signal from the mitochondria is not known, but it is interpreted in the cytosol by a protein complex consisting of the Fra1-Fra2 proteins with Grx3-Grx4. This complex inhibits the translocation of Aft1 into the nucleus. Our data suggest that Aft1 does not bind to the Fra1-Fra2 complex, indicating that there is not a direct physical interaction between the two. The Fra1-Fra2-Grx complex may affect sulfhydryl status on Aft1 or on an Aft1-interacting protein. Low iron conditions reduce the rate of Fe-S cluster synthesis, resulting in a signal through the Fra1-Fra2-Grx complex and in Aft1 translocating to the nucleus, where it occupies the FET3 promoter. Further decreases in cytosolic iron may affect the amount of Aft1 bound to the promoter, leading to an increase in transcriptional activation.
Acknowledgments
We thank members of the Kaplan laboratory for critically reading the manuscript.
This work was supported by National Institutes of Health (NIH) Grants DK30534 and DK52380 (to J. K.), GM083292 (to D. W.), and ES13780 (to C. O.). Support for use of the Core Facilities was provided through NCI, National Institutes of Health, Grant NCI-CCSG P30CA 42014 and NIDDK, NIH, Center of Excellence Award 5P30KD72437. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: CM, complete medium; SD, synthetic defined medium; BPS, bathophenanthroline disulfonate; X-gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; GFP, green fluorescent protein; HA, hemagglutinin; YFP, yellow fluorescent protein.
T. Formosa, personal communication.
C. Outten, unpublished data.
References
- 1.Van Ho, A., Ward, D. M., and Kaplan, J. (2002) Annu. Rev. Microbiol. 56 237–261 [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi-Iwai, Y., Dancis, A., and Klausner, R. D. (1995) EMBO J. 14 1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi-Iwai, Y., Ueta, R., Fukunaka, A., and Sasaki, R. (2002) J. Biol. Chem. 277 18914–18919 [DOI] [PubMed] [Google Scholar]
- 4.Blaiseau, P. L., Lesuisse, E., and Camadro, J. M. (2001) J. Biol. Chem. 276 34221–34226 [DOI] [PubMed] [Google Scholar]
- 5.Rutherford, J. C., Jaron, S., Ray, E., Brown, P. O., and Winge, D. R. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 14322–14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, O. S., Crisp, R. J., Valachovic, M., Bard, M., Winge, D. R., and Kaplan, J. (2004) J. Biol. Chem. 279 29513–29518 [DOI] [PubMed] [Google Scholar]
- 7.Rutherford, J. C., Ojeda, L., Balk, J., Muhlenhoff, U., Lill, R., and Winge, D. R. (2005) J. Biol. Chem. 280 10135–10140 [DOI] [PubMed] [Google Scholar]
- 8.Gari, E., Piedrafita, L., Aldea, M., and Herrero, E. (1997) Yeast 13 837–848 [DOI] [PubMed] [Google Scholar]
- 9.Ojeda, L., Keller, G., Muhlenhoff, U., Rutherford, J. C., Lill, R., and Winge, D. R. (2006) J. Biol. Chem. 281 17661–17669 [DOI] [PubMed] [Google Scholar]
- 10.Crisp, R. C., Adkins, E. M., Kimmel, E., and Kaplan, J. (2006) EMBO J. 25 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, O. S., Hemenway, S., and Kaplan, J. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisp, R. J., Pollington, A., Galea, C., Jaron, S., Yamaguchi-Iwai, Y., and Kaplan, J. (2003) J. Biol. Chem. 278 45499–45506 [DOI] [PubMed] [Google Scholar]
- 13.Meisinger, C., Sommer, T., and Pfanner, N. (2000) Anal. Biochem. 287 339–342 [DOI] [PubMed] [Google Scholar]
- 14.Kispal, G., Csere, P., Prohl, C., and Lill, R. (1999) EMBO J. 18 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, J., Kogan, M., Knight, S. A., Pain, D., and Dancis, A. (1999) J. Biol. Chem. 274 33025–33034 [DOI] [PubMed] [Google Scholar]
- 16.Schilke, B., Voisine, C., Beinert, H., and Craig, E. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 10206–10211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sipos, K., Lange, H., Fekete, Z., Ullmann, P., Lill, R., and Kispal, G. (2002) J. Biol. Chem. 277 26944–26949 [DOI] [PubMed] [Google Scholar]
- 18.Luk, E., Carroll, M., Baker, M., and Culotta, V. C. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10353–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y., Bjorklund, S., Jiang, Y. W., Kim, Y. J., Lane, W. S., Stillman, D. J., and Kornberg, R. D. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 10864–10868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Peppel, J., Kettelarij, N., van Bakel, H., Kockelkorn, T. T., van Leenen, D., and Holstege, F. C. (2005) Mol. Cell 19 511–522 [DOI] [PubMed] [Google Scholar]
- 21.Ho, Y., Gruhler, A., Heilbut, A., Bader, G. D., Moore, L., Adams, S. L., Millar, A., Taylor, P., Bennett, K., Boutilier, K., Yang, L., Wolting, C., Donaldson, I., Schandorff, S., Shewnarane, J., Vo, M., Taggart, J., Goudreault, M., Muskat, B., Alfarano, C., Dewar, D., Lin, Z., Michalickova, K., Willems, A. R., Sassi, H., Nielsen, P. A., Rasmussen, K. J., Andersen, J. R., Johansen, L. E., Hansen, L. H., Jespersen, H., Podtelejnikov, A., Nielsen, E., Crawford, J., Poulsen, V., Sorensen, B. D., Matthiesen, J., Hendrickson, R. C., Gleeson, F., Pawson, T., Moran, M. F., Durocher, D., Mann, M., Hogue, C. W., Figeys, D., and Tyers, M. (2002) Nature 415 180–183 [DOI] [PubMed] [Google Scholar]
- 22.Pujol-Carrion, N., Belli, G., Herrero, E., Nogues, A., and de la Torre-Ruiz, M. A. (2006) J. Cell Sci. 119 4554–4564 [DOI] [PubMed] [Google Scholar]
- 23.Hu, C. D., Chinenov, Y., and Kerppola, T. K. (2002) Mol. Cell 9 789–798 [DOI] [PubMed] [Google Scholar]
- 24.Huh, W. K., Falvo, J. V., Gerke, L. C., Carroll, A. S., Howson, R. W., Weissman, J. S., and O'Shea, E. K. (2003) Nature 425 686–691 [DOI] [PubMed] [Google Scholar]
- 25.Molina, M. M., Belli, G., de la Torre, M. A., Rodriguez-Manzaneque, M. T., and Herrero, E. (2004) J. Biol. Chem. 279 51923–51930 [DOI] [PubMed] [Google Scholar]
- 26.Lill, R., and Muhlenhoff, U. (2006) Annu. Rev. Cell Dev. Biol. 22 457–486 [DOI] [PubMed] [Google Scholar]
- 27.Lesuisse, E., Knight, S. A., Courel, M., Santos, R., Camadro, J. M., and Dancis, A. (2005) Genetics 169 107–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huynen, M. A., Spronk, C. A., Gabaldon, T., and Snel, B. (2005) FEBS Lett. 579 591–596 [DOI] [PubMed] [Google Scholar]
- 29.Ye, H., Abdel-Ghany, S. E., Anderson, T. D., Pilon-Smits, E. A., and Pilon, M. (2006) J. Biol. Chem. 281 8958–8969 [DOI] [PubMed] [Google Scholar]
- 30.Layer, G., Gaddam, S. A., Ayala-Castro, C. N., Ollagnier-de Choudens, S., Lascoux, D., Fontecave, M., and Outten, F. W. (2007) J. Biol. Chem. 282 13342–13350 [DOI] [PubMed] [Google Scholar]
- 31.Outten, F. W., Wood, M. J., Munoz, F. M., and Storz, G. (2003) J. Biol. Chem. 278 45713–45719 [DOI] [PubMed] [Google Scholar]
- 32.Feng, Y., Zhong, N., Rouhier, N., Hase, T., Kusunoki, M., Jacquot, J. P., Jin, C., and Xia, B. (2006) Biochemistry 45 7998–8008 [DOI] [PubMed] [Google Scholar]
- 33.Johansson, C., Kavanagh, K. L., Gileadi, O., and Oppermann, U. (2007) J. Biol. Chem. 282 3077–3082 [DOI] [PubMed] [Google Scholar]
- 34.Ueta, R., Fujiwara, N., Iwai, K., and Yamaguchi-Iwai, Y. (2007) Mol. Biol. Cell 18 2980–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]