Abstract
Seven transmembrane receptors (7TMRs) exert strong regulatory influences on virtually all physiological processes. Although it is historically assumed that heterotrimeric G proteins mediate these actions, there is a newer appreciation that β-arrestins, originally thought only to desensitize G protein signaling, also serve as independent receptor signal transducers. Recently, we found that activation of ERK1/2 by the angiotensin receptor occurs via both of these distinct pathways. In this work, we explore the physiological consequences of β-arrestin ERK1/2 signaling and delineate a pathway that regulates mRNA translation and protein synthesis via Mnk1, a protein that both physically interacts with and is activated by β-arrestins. We show that β-arrestin-dependent activation of ERK1/2, Mnk1, and eIF4E are responsible for increasing translation rates in both human embryonic kidney 293 and rat vascular smooth muscle cells. This novel demonstration that β-arrestins regulate protein synthesis reveals that the spectrum of β-arrestin-mediated signaling events is broader than previously imagined.
Seven transmembrane-spanning receptors (7TMRs3/G protein-coupled receptors), the largest class of cell surface receptors, regulate nearly every known physiologic process in mammals. Canonical signaling by 7TMRs involves an agonist-induced change in receptor conformation that causes the activation of heterotrimeric G proteins, leading to second messenger generation and downstream signaling. The termination of this cascade, a process known as desensitization, occurs when G protein-coupled receptor kinases phosphorylate the activated receptor and promote the subsequent binding of β-arrestins, which act sterically to block further G protein activation. However, in recent years a previously unappreciated role of β-arrestins has become evident as distinct transducers of 7TMR signals independent of G proteins. By serving as multiprotein binding scaffolds, β-arrestins facilitate the activation of numerous signaling pathways, including the mitogen-activated protein kinases (MAPKs), c-Src, and Akt (1–3). The diverse array of cellular outcomes that involve β-arrestin-mediated signaling are just beginning to be appreciated and thus far include chemotaxis (4), cardiomyocyte contractility (5), and the prevention of apoptosis (6), among others (2).
One of the most well studied targets of β-arrestin-mediated signaling from 7TMRs is the MAPK extracellular-regulated kinase 1/2 (ERK1/2) (7, 8). Classically, G protein stimulation via second messenger effectors like protein kinase C (PKC) and protein kinase A promotes ERK1/2 phosphorylation and nuclear translocation to modulate transcription. However, our laboratory and others have found that after β-arrestin-dependent ERK1/2 activation, the kinase remains solely in the cytoplasm (9, 10), where it presumably phosphorylates a distinct subset of targets. The elucidation of these cytosolic β-arrestin-ERK1/2 targets has become a focus of recent study. In reviewing the literature on such cytosolic substrates of ERK1/2, we found a number of proteins involved in the modulation of protein synthesis, including MAP kinase-interacting kinase 1 (Mnk1). Because Mnk1 can be activated by a 7TMR (11), the angiotensin II receptor, we reasoned it might well be a β-arrestin ERK1/2 substrate and that β-arrestins might be involved in signaling to protein synthesis.
Protein synthetic regulation is critical for cellular growth and development, and its dysregulation is implicated in numerous diseases such as Alzheimer disease, tumorigenesis, and various proliferative conditions such as neointimal hyperplasia (12). Multiple cell signaling molecules, including ERK1/2, can elicit changes in the rate of mRNA translation. These situations arise when environmental conditions demand heightened protein synthesis, and cells can respond either by increasing the rate of general translation or that of only a specific subset of mRNAs, the latter typically occurring through sequence-specific factors in the 5′-non-translated region of the message. On the other hand, because nearly all mammalian mRNAs contain a 5′-methylguanosine cap, increasing the assembly of the multiprotein cap binding complex is one way that cells can elicit more general increases in message translation.
The major rate-limiting factors in cap-dependent translation are the availability of and mRNA affinity for the cap binding complex member protein eukaryotic translation initiation factor 4E (eIF4E). The amount of free eIF4E available for cap binding is determined by 4E-binding proteins (4EBPs). Only when the 4EBPs are dephosphorylated are they capable of binding eIF4E (12). The 4EBP kinase that allows for eIF4E release is the mammalian target of rapamycin (mTOR), a protein also implicated in other mRNA translation steps (13). The affinity of eIF4E for mRNA is determined by its phosphorylation state: when phosphorylated at serine 209, affinity decreases (14), a process carried out by Mnk1 (15, 16). This decreased affinity for mRNA cap structure seems counterintuitive for a pathway that seeks to increase mRNA translation. However, the link between eIF4E phosphorylation and protein synthesis is well established, leading to the hypothesis that cap affinity needs to be reduced to allow the ribosome to move along the untranslated mRNA (17). Thus, Mnk1, a serine/threonine kinase and substrate of ERK1/2 and p38 (15, 16), serves as a key point of convergence for signal transduction pathways regulating protein synthesis. Recently, the potential targeting of Mnk1 for proliferative diseases was described, based on the observation that HER2-positive breast cancer lines show heightened Mnk1 activity (18).
Using rat vascular smooth muscle cells, Ishida et al. (19) measured the protein synthetic response to angiotensin II (AngII) (11). By using a chemical inhibitor of Mnk1 kinase activity, the authors found that Mnk1 activity was absolutely required for AngII stimulation of protein synthesis. Also, Mnk1 phosphorylation in response to AngII in these cells was entirely mediated by ERK1/2, and not by p38 (11). The upstream mediators of this signaling, however, remain unknown.
Based on the observations that Mnk1 is a target of cytosolic ERK1/2 (15, 16) and can be activated by the AT1AR (11), we hypothesized that Mnk1 might be a potential downstream target of β-arrestin-mediated ERK1/2 signaling. Thus, we sought to investigate the potential role of β-arrestins in 7TMR signaling to Mnk1 activation and the resulting increase in protein synthesis.
EXPERIMENTAL PROCEDURES
Plasmids and Cloning—Mnk1 cDNA was amplified from pEBG3X-Mnk1 (gift from Jonathan Cooper, Fred Hutchinson Cancer Center) with an N-terminal FLAG epitope and cloned into pcDNA3.1. For bacterial expression, pGEX-Mnk1 was a gift from Tony Hunter (Salk Institute). The plasmid encoding HA-AT1AR has been previously described (20); AT1ARDRY-AAY mutant was made by site-directed mutagenesis (QuikChange; Stratagene) of the wild type plasmid. For the COS7 studies in supplemental Fig. S2, β-arrestin overexpression plasmids were described previously (21).
Reagents—(Sar1, Ile4, Ile8)-AngII was synthesized as described (20, 22) and used at 10 μm in all figures, in accordance with its KD being ∼200-fold less than AngII (22). AngII (Sigma A9525) was used at 100 nm in all figures. Ro31–8425 (1 μm), U0126 (5 μm), and SB203580 (20 μm) were purchased from Calbiochem. Phospho-Mnk1 and phospho-eIF4E antibodies were purchased from Cell Signaling (2111 and 9741). Total Mnk1 and eIF4E antibodies were purchased from Santa Cruz Biotechnology (SC-6962 and SC-6968). CGP57380 was a gift from Hermann Gram, Novartis (Basel, Switzerland) and was used at 30 μm.
Cell Culture—Vascular smooth muscle cells (VSMCs) were prepared from 10–12-week-old male Sprague-Dawley rats (∼250 g) as previously described (11). VSMCs were cultured in M199 medium with 10% fetal bovine serum and penicillin/streptomycin. rVSMCs were used for signaling and translation assays prior to four passages, due to loss of angiotensin receptor expression and responsiveness over time. Rat vascular smooth muscle cells were used because, unlike mouse VSMCs, they express a tractable amount of AT1AR. HEK-293 and COS7 cells were cultured in minimal essential medium with 10% fetal bovine serum and penicillin/streptomycin as described previously (23). Transfection of siRNAs in HEK-293s was carried out using Gene Silencer (Gene Therapy Systems) as described previously (24). Silencing achieved for β-arrestins was 70–80%, consistent with previous reports (24, 25). Sequences of human siRNAs used were the following: β-arrestin 1 (5′-AAAGCCUUCUGCGCGGAGAAU-3′), β-arrestin2 (5′-AAGGACCGCAAAGUGUUUGUG-3′), and for supplemental Fig. S2 the second β-arrestin2 siRNA was (5′-CCAACCUCAUUGAAUUUGA-3′). Pooled human Mnk1 siRNA was purchased from Santa Cruz Biotechnology (sc-39106). Transfection of plasmids for all other experiments in HEK-293 cells was done using FuGENE 6 (Roche Applied Science) according to the manufacturer's protocol. For siRNA experiments in rat cells, siRNAs were the following: β-arrestin2-1 (5′-AAGGACCGGAAAGTGTTTGTG-3′) and β-arrestin2-2 (5′-ACCAACCTCATTGAATTCGA-3′). Pooled murine Mnk1 siRNA was purchased from Santa Cruz Biotechnology (sc-39107). Transfection was achieved using 60 μl of Lipofectamine/10-cm dish of rVSMCs at 90% confluence according to the manufacturer's protocol.
Immunoprecipitations and Western Blots—For IPs, HeLa cells (500 μg of total protein/sample) or mouse tissue homogenate (1 mg/sample) in radioimmune precipitation lysis buffer was pre-cleared with protein A beads and then incubated with A1CT antibody (26) or an equivalent volume of preimmune serum from the same rabbit. 24 h later, bound proteins were precipitated with protein A-agarose beads and washed several times with radioimmune precipitation buffer. Western blots were carried out according to standard protocols. β-Arrestins were detected with a monoclonal antibody (BD 610550). For the antigen-blocking experiment, A1CT-coated beads were preincubated with 100 ng of recombinant ratβ-arr1 (or equivalent volume of buffer) and washed thoroughly before proceeding with IP. For epitope-tagged proteins, HA antibody was Covance 12CA5 and FLAG antibody was M2 (Sigma F9291). For agonist-stimulated IPs the time point was 30 min. Detection of β-arr2 in rVSMCs was performed with A2CT antibody (26).
Protein Synthesis Measurements—Rat VSMCs or HEK-293 cells were seeded in 12-well plates. After 24 h the medium was replaced with serum-free medium. 24 h later, 2 uCi/ml of [3H]leucine (NET460005MC; Amersham Biosciences) and either phosphate-buffered saline (for nonstimulated conditions), 100 nm AngII, or 10 μm SII were added to the medium. 24 h later, medium was removed and newly generated protein was quantitated as previously described (11).
RESULTS
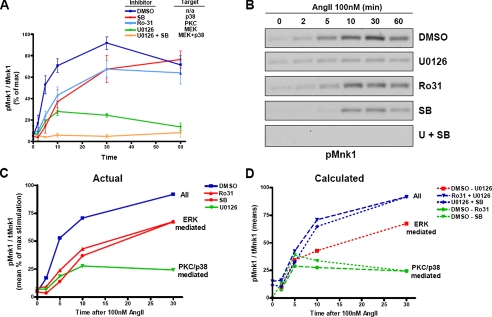
Mnk1 Activation Occurs in an ERK-dependent but PKC-independent Manner—In HEK-293 cells, AT1AR activation leads to ERK1/2 phosphorylation by both G protein/PKC and β-arrestin-dependent mechanisms (9, 20, 27). In the presence of a PKC inhibitor, all AngII-induced ERK1/2 phosphorylation is carried out by the β-arrestin2-specific signaling arm (27). Thus, if AngII stimulation leads to phosphorylation of a protein that is resistant to PKC inhibition, but is sensitive to ERK inhibition, it is a candidate β-arrestin signaling target. We used this rationale as a screening strategy to identify potential β-arrestin-ERK1/2 signaling targets among a panel of known cytosolic ERK1/2 substrates. One protein, Mnk1, showed the expected pattern of a β-arrestin target but was very difficult to detect at endogenous levels in HEK-293 cells. Thus, we generated stable cells that express both the AT1AR and Mnk1 to further investigate this protein. These cells were pretreated with vehicle (Me2SO) or chemical inhibitors of PKC (Ro31–8425), MEK (U0126), p38 (SB203580), or a combination of inhibitors and then stimulated with AngII as shown in Fig. 1. AngII-induced Mnk1 phosphorylation was primarily dependent on ERK1/2 rather than p38, especially at later time points, consistent with previously published data for Mnk1 activation in rat vascular smooth muscle cells (11). In the early portion of the curve, there was a slight effect of p38 inhibition, PKC inhibition, and MEK inhibition, suggesting that all three effectors can contribute to Mnk1 phosphorylation at these shorter time points. The virtual superimposition of the p38 and PKC inhibition curves suggests that p38 and PKC may lie in the same pathway, which is parallel to and independent of the ERK1/2 pathway. Further support for this idea is provided by the additive nature of these curves. As can be seen in Fig. 1, C and D, the Mnk1 phosphorylation that is resistant to MEK inhibition (U0126) added to either the PKC-inhibited curve (Ro31) or p38-inhibited curve (SB) accounts for the total Mnk1 phosphorylation activity, drawn in blue lines. Similarly, the subtraction of the MEK-inhibited curve from the total activity curve results in a curve that perfectly overlies either the PKC- or p38-inhibited curve (Fig. 1D). At later times, Mnk1 phosphorylation is largely resistant to p38 and PKC inhibition, which strongly suggests G protein independence. These kinase inhibitor studies indicate that in HEK-293 cells activation of Mnk1 occurs through two pathways, one of which relies upon G protein activation, PKC, and p38 and the other which is likely mediated by β-arrestin2-dependent ERK1/2 signaling.
FIGURE 1.
Sensitivity of AngII-stimulated pMnk1 activation to kinase inhibitors. A, HEK-293 cells stably expressing HA-AT1AR and Mnk1 were serum-starved for 16 h, pretreated with vehicle Me2SO (DMSO) or the indicated kinase inhibitor for 30 min, and then stimulated with 100 nm AngII for the indicated times. Western blots for phospho-Mnk1 and tMnk1 were performed. The percentage of pMnk1/tMnk1 was calculated for each data point and plotted as a percentage of maximal activity (Me2SO-treated cells at 30 min) ± S.E. These data represent the average ± S.E. of seven independent experiments. B, representative pMnk1 Western blots. C, means of individual data points (as a percent of maximal stimulation) up to 30 min from experiments performed in A. D, calculations based on addition or subtraction of means from A as shown in the legend. Solid lines in C represent actual data; dashed lines in D represent mathematically calculated values.
Mnk1 and eIF4E Activation Depends upon Expression of β-Arrestin2—We used siRNA to directly test the involvement of β-arrestins in the AngII-induced Mnk1 activation in the same cell system used for Fig. 1. β-arrestin1 silencing had a small effect at early time points compared with control siRNA but had no effect at 30 and 60 min, times when Mnk1 was fully activated (Fig. 2A). However, β-arrestin2 siRNA inhibited Mnk1 phosphorylation at all time points tested by ∼50%. The pMnk1 signal that persisted after β-arrestin2 siRNA was largely the result of PKC-dependent, MEK-independent signaling, as predicted from the kinase inhibitor studies in Fig. 1 (data not shown). Western blotting for β-arrestins demonstrates that siRNA silencing was >70%, consistent with the amount of silencing previously reported (24). A second siRNA sequence targeting a different portion of the β-arrestin2 mRNA was also tested and a similar result was obtained (supplemental Fig. S1A), thus confirming the necessity of β-arrestin2 expression for full Mnk1 activation. Similarly, overexpression of β-arrestin2, but not β-arrestin1, in Cos7 cells, which have low levels of endogenous β-arrestins, resulted in an increase in Mnk1 phosphorylation (supplemental Fig. S1B).
FIGURE 2.
Effect of β-arrestin siRNAs on AngII-stimulated pMnk1 and peIF4E activation. A, HEK-293 cells stably expressing HA-AT1AR and Mnk1 were transfected with either control siRNA (CTL) or β-arrestin1-specific or β-arrestin-2-specific siRNAs (β-arr1, β-arr2). 72 h post-transfection, cells were serum-starved for 16 h and stimulated with 100 nm AngII for the indicated times. Western blots for phospho-Mnk1 and total Mnk1 were performed. The percentage of pMnk1/tMnk1 was calculated for each data point and plotted as a percentage of maximal activity (CTL-transfected cells at 30 min). These data represent the average of six independent experiments ± S.E. Representative pMnk1- and β-arrestin-silencing Western blots are shown. B, cells were treated as in A and stimulated with 100 nm AngII for 30 min, and Western blots were performed for phospho-eIF4E and total eIF4E. Data in graph represent the means ± S.E. of five experiments; statistical significance was calculated by one way ANOVA with post-test comparison of AngII-stimulated populations (*, p < 0.05). Representative Western blot for p-eIF4E is shown.
To confirm that β-arrestin-mediated Mnk1 activation indeed continues down the pathway to eIF4E, we used a similar approach (Fig. 2B). We found that eIF4E phosphorylation in HEK-293 cells stimulated by AngII was significantly inhibited by β-arrestin2 siRNA (Fig. 2B), indicating that β-arrestins are capable of signaling to eIF4E, the rate-limiting step in cap-dependent translation.
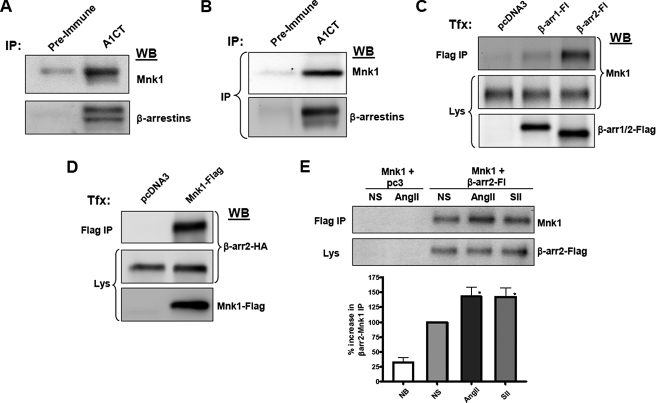
β-Arrestins Physically Interact with Mnk1—β-Arrestins are known to serve as scaffolds for the activation of numerous signaling molecules (1), including ERK in complex with Raf-1 and Mek-1. Based on the above observations, we speculated that similar to other MAPK pathway members, Mnk1 might be found in a complex with β-arrestins. Thus, we performed immunoprecipitations using an antibody that recognizes both β-arrestin1 and -2 from either HeLa cells (Fig. 3A) or mouse spleen (Fig. 3B) and immunoblotted for Mnk1. We found that Mnk1 was ∼10-fold more abundant in samples immunoprecipitated with the β-arrestin antibody versus an equivalent amount of pre-immune serum from the same rabbit. These results were consistent across all mouse tissues tested, including skeletal muscle, heart, kidney, and liver (data not shown). Further, when the β-arrestin antibody-coated beads were preincubated with the immunizing antigen, the Mnk1-β-arrestin co-immunoprecipitation was blocked (data not shown). These results confirm a specific physical interaction of these proteins at endogenous expression levels in cultured cells as well as in vivo.
FIGURE 3.
β-Arrestins physically interact with Mnk1 in vivo and in vitro. A, HeLa cells were lysed and immunoprecipitated with aβ-arrestin-specific antibody (A1CT) or pre-immune serum from the same rabbit. Western blots were performed with antibodies to detect human Mnk1 (upper panel) or a monoclonal antibody to detect immunoprecipitated β-arrestins (lower panel). B, spleens were harvested from C57Black6 mice and immunoprecipitated with A1CT or pre-immune serum. Western blots were performed with an antibody for murine Mnk1 or with a monoclonal β-arrestin antibody. C, Mnk1 and β-arrestins interact in transfected cells. HEK-293 cells stably expressing Mnk1 were transfected with empty vector or plasmids expressing β-arrestin1-FLAG or β-arrestin2-FLAG and immunoprecipitated with a FLAG-M2 antibody. Western blots were performed for Mnk1. D, reciprocal IP. Empty vector or Mnk1-FLAG expression plasmid was cotransfected with a β-arrestin2-HA expression plasmid. Lysates were immunoprecipitated with a FLAG-M2 antibody, and HA Western blots were performed to detect β-arrestin2. All results shown are representative of three similar experiments. E, agonist effect on IP. Similar methods as in C, except cells were serum-starved and stimulated for 30 min with AngII or SII. Results of six experiments ± S.E. are shown in the graph. NB, no bait control, NS, nonstimulated. NB versus NS (p < 0.05), NS versus AngII (*, p < 0.05) or SII (*, p < 0.05).
To determine whether there was β-arrestin isoform specificity for the physical interaction with Mnk1, we transfected HEK-293 cells with Mnk1 and epitope-tagged β-arrestin1 or -2 (Fig. 3C). We then immunoprecipitated the β-arrestins with epitope antibody and compared the levels of Mnk1. Although both β-arrestins bound more Mnk1 than the control condition, β-arrestin2 bound much more robustly than β-arrestin1. A reciprocal immunoprecipitation of Mnk1 followed by β-arrestin Western blotting was also performed and confirmed the interaction (Fig. 3D).
In other systems, such as ERK1/2 and JNK3 (8, 21), the functional significance of β-arrestin scaffolding is to activate the target kinase by giving it access to its upstream activator. Thus, we explored whether agonist stimulation could modulate the interaction of βarrestin-2 and Mnk1 using a co-immunoprecipitation assay. We observed a moderate, yet significant, increase in the amount of Mnk1 immunoprecipitated by β-arrestin2 after stimulation of the AT1AR with either AngII or SII for 30 min (Fig. 3E). Also, the cellular stores of Mnk1 bound by β-arrestin2 were phosphorylated upon receptor stimulation, as confirmed by immunoblotting for phospho-Mnk1 in the β-arrestin2 immunoprecipitation (supplemental Fig. S3). These data suggest a dynamic interaction of β-arrestin2 and Mnk1 that can be regulated by the receptor and that may facilitate Mnk1 phosphorylation. Alternatively, the basal interaction of these two proteins alone may be important for facilitating Mnk1 phosphorylation.
β-Arrestin-mediated Signaling to Mnk1—As mentioned above, when AngII binds the extracellular surface of the AT1AR it stimulates the independent coupling of receptor to both G protein- and β-arrestin-mediated signaling pathways (20). To circumvent the issues of discerning which pathway is eliciting a particular response, we utilized two reagents that specifically activate only the β-arrestin signaling arm. S1I4I8-AngII (SII) is a mutated peptide analog of the natural ligand that induces a receptor conformation that is unable to activate Gαq yet maintains the capacity to activate ERK1/2 in a β-arrestin-dependent manner (20, 22). Similarly, the AT1AR-DRY/AAY mutated receptor, when treated with AngII, only activates β-arrestin signaling and does not couple to G proteins (20, 28). Hence, these reagents allow for clearer investigation of the role of β-arrestin in Mnk1 activation. In HEK-293 cells, SII stimulated significant Mnk1 phosphorylation, ∼22% as well as AngII. We believe that this lower response, though statistically significant, is likely because the SII peptide leads to exclusive, albeit less efficient, coupling of the receptor to the β-arrestin signaling pathway. The SII mechanism for Mnk1 activation was dependent upon ERK1/2, but not PKC, activity (Fig. 4A). Similarly, the AT1AR-DRY/AAY, which is uncoupled from G proteins yet still recruits β-arrestins and leads to ERK1/2 activation, also stimulated Mnk1 activation (Fig. 4B). We also observed that eIF4E phosphorylation could be stimulated via SII-AngII treatment of the wild type AT1AR or by AngII stimulation of the AT1AR-DRY/AAY mutated receptor (data not shown).
FIGURE 4.
Selective β-arrestin pathway stimulation via SI4I8 AngII or AT1ARDRY/AAY results in Mnk1 activation. A, HEK-293 cells stably expressing HA-AT1AR and Mnk1 were serum-starved and pretreated with Me2SO (DMSO), U0126, or Ro31–8425 for 30 min, followed by stimulation with either 100 nm AngII or 10 μm SI4I8 AngII for 30 min. Western blots for phospho-Mnk1 and total Mnk1 were performed. The percentage of pMnk1/tMnk1 was calculated and plotted as a percentage of maximal AngII-mediated activity. Results are representative of six independent experiments ± S.E. Post test comparisons: NS versus AngII (**, p < 0.001), NS versus SII (*, p < 0.01), Ro31 NS versus Ro31 SII (**, p < 0.01), SII versus U0126 SII (p < 0.01), U0126 NS versus U0126 SII (not significant). NS, nonstimulated. B, HEK-293 cells were transiently transfected with a plasmid encoding the HA-AT1ARDRY/AAY mutant (which is uncoupled from G protein signaling) and Mnk1. Cells were serum-starved and stimulated with 100 nm AngII for the indicated times. Western blots for phospho-Mnk1 were performed and quantitated as in A. Results are representative of the mean of three independent experiments ± S.E.
β-Arrestin2 and Mnk1 Dependence of AngII-induced Protein Synthesis—The terminal event in the Mnk1 signaling pathway is the stimulation of protein synthesis. As has been observed in other systems, AngII treatment led to an increase in protein synthesis by ∼50% in AT1AR-stable HEK-293 cells. Interestingly, SII stimulation also resulted in significant increases in protein synthesis over basal rates, with a magnitude of about half that observed with AngII (Fig. 5A). We confirmed that this pathway required β-arrestin2 expression by using siRNA transfection and found that AngII-induced translation was reduced and SII-induced translation was completely inhibited in the presence of a β-arrestin2-silencing RNA. Importantly, the basal rate of protein synthesis did not change upon transfection of β-arrestin2 siRNA. We also performed this assay in the presence of Mnk1 siRNAs and found that although the basal rate was decreased there was no significant increase imparted by SII stimulation of the receptor, indicating that the β-arrestin-mediated pathway requires Mnk1 activity. We further used a chemical inhibitor of Mnk1, CGP57380, to demonstrate the necessity of Mnk1 in β-arrestin-mediated protein synthesis. As shown in Fig. 5B, pretreatment of AT1AR-expressing cells with the Mnk1 inhibitor CGP57380 had very little effect on AngII-stimulated protein synthesis from the wild type receptor in these cells. However, in AT1AR-DRY/AAY-expressing cells, inhibition by the Mnk1 inhibitor was all but complete, indicating total reliance upon the β-arrestin-Mnk1 pathway for this receptor. These data suggest that in HEK-293 cells, protein synthesis can be stimulated by the AT1AR in at least two ways, one of which requires β-arrestin2 and Mnk1 activity and another that requires G protein activity and is not as sensitive to Mnk1 kinase inhibition.
FIGURE 5.
Angiotensin-induced protein synthesis is dependent upon β-arrestin2 expression and Mnk1 activity. A, HEK-293 cells stably expressing the AT1AR were transfected with either control siRNA (CTL), β-arrestin2 siRNA (β-arr2), or Mnk1 siRNA (Mnk1) for 48 h. Cells were serum-starved for an additional 24 h to arrest cycling. Phosphate-buffered saline (nonstimulated), AngII (100 nm), or SII-AngII (10 μm) along with 3H-labeled leucine was added to the medium; 24 h later, cells were harvested as described under “Experimental Procedures” and radiolabeled proteins were precipitated and quantitated. Data have been normalized such that the control siRNA, nonstimulated is set to 100%. Results are representative of nine independent experiments each performed, at a minimum, in duplicate. Statistical significance was calculated using one-way ANOVA with post test CTL NS versus CTL AngII (***, p < 0.001) or CTL SII (*, p < 0.05) and CTL AngII versus β-arr2 (**, p < 0.01) AngII and Mnk1 AngII (**, p < 0.01). B, HEK-293 cells either stably expressing HA-AT1AR or transiently expressing HA-AT1ARDRY/AAY (as indicated) were serum-starved for 24 h and then pretreated for 1 h with either Me2SO (DMSO) or 30 μm CGP57380, the Mnk1 inhibitor (reported IC50 2.2 μm). Cells were then stimulated with AngII in the presence of [3H] leucine for 24 h, and protein synthesis was calculated as described in A. Results depicted represent the mean of four independent experiments ± S.E. Using a one-way ANOVA with post test, the Me2SO AngII-stimulated condition is significantly greater (*, p < 0.05) than the CGP AngII condition for each receptor.
β-Arrestin-dependent Mnk1 Phosphorylation and Protein Synthesis in rVSMCs—Rat VSMCs serve as an excellent model for endogenous AngII receptor biology and have been previously reported to require Mnk1 activity for AngII-induced protein synthesis (11). To investigate whether β-arrestins are capable of linking 7TMR signaling to Mnk1 activity and translation in these cells, we used a variety of approaches, including both AngII and SII stimulation of endogenously expressed AngII receptors, kinase inhibitors, and siRNAs targeting rat Mnk1 and rat β-arrestin2. In Fig. 6A, we observe that AngII and SII stimulation for 30 min results in dramatic increases in Mnk1 phosphorylation, which are not reduced by PKC inhibition but almost completely abolished by ERK1/2 inhibition. Surprisingly, PKC inhibition serves to increase signaling from both AngII and SII. This may be the result of suppressing PKC-mediated inhibition of the β-arrestin arm of signaling or releasing PKC-mediated inhibition of Mnk1 phosphorylation itself. Using a fluorescent calcium-sensing dye, we further confirmed that SII was unable to stimulate any G protein coupling in rVSMCs (data not shown). Unlike the case with HEK-293 cells, which derive ∼25% of their total Mnk1 activation through a pathway involving PKC, rVSMCs do not seem to rely upon this pathway at all, suggesting a nearly complete independence from G protein activity for Mnk1 phosphorylation in these cells. We further investigated the potential role of β-arrestins in Mnk1 phosphorylation in rVSMCs by transfecting siRNA for β-arrestin2. In the presence of either of two different siR-NAs for rat β-arrestin2, AngII-induced pMnk1 was greatly reduced and SII-induced pMnk1 was abolished (Fig. 6B). These studies conclusively link β-arrestin signaling to Mnk1 phosphorylation in this physiologically relevant system.
FIGURE 6.
β-Arrestin2 signaling stimulates Mnk1 activation and protein synthesis in vascular smooth muscle cells. A, rVSMCs were serum-starved and pretreated with vehicle Me2SO (DMSO), the PKC inhibitor Ro31–8425, or the MEK inhibitor U0126 for 30 min. Cells were stimulated with either 100 nm AngII (A) or 10 μm SII-AngII (S) for an additional 30 min. Western blots for pMnk1 were performed and quantitated. Results depicted are representative of four experiments ± S.E. Statistical significance was calculated using a one-way ANOVA with post test. Me2SO-nonstimulated was compared with the following, Me2SO AngII (**, p < 0.01), Me2SO SII (*, p < 0.05), Ro31 AngII (***, p < 0.001), and Ro31 SII (**, p < 0.01). No other conditions are significant with respect to the Me2SO-nonstimulated group. B, rVSMCs were transfected with siRNA for β-arr2 and subjected to pMnk1 analysis as in A at 96 h post-transfection. Graph depicts results from six experiments ± S.E. Statistical significance was calculated using a one-way ANOVA with post test. CTL-nonstimulated was compared with CTL AngII (***, p < 0.001) and CTL SII (**, p < 0.01). CTL AngII was compared with βarr2 AngII (**, p < 0.01). No other comparisons are significant. C, rat vascular smooth muscle cells (rVSMC) were serum-starved and stimulated with AngII or SII in the presence of [3H]leucine for 24 h. Radiolabeled proteins were quantitated as described under “Experimental Procedures.” Data are normalized such that nonstimulated cells are set to 100%. Results depicted are representative of four experiments (means ± S.E.). Statistical significance was calculated using a one-way ANOVA with post test: N.S. versus AngII was *, p < 0.01 and N.S. versus SII was **, p < 0.05. D, rVSMC were transfected with siRNAs for β-arrestin2 or Mnk1 and subjected to protein synthesis analysis as in C at 96 h post-transfection. Results depicted are representative of six experiments (means ± S.E.). Statistical significance was calculated using a one-way ANOVA with post test. CTL N.S. compared with CTL AngII was *, p < 0.05. CTL AngII compared with βarr2 AngII and Mnk1 AngII were both **, p < 0.01.
When we examined AngII-induced increases in protein synthesis in rVSMCs, we observed an ∼50% increase over basal (Fig. 6C), similar to previously published results (11). We then reasoned that if SII could reproduce the effects of the natural ligand AngII, then β-arrestins are likely to be involved in this signal transduction process. We found that AngII treatment leads to an ∼50% increase in translation rate but, strikingly, SII also led to significant increases in translation over basal rates, with the magnitude being nearly half that produced by AngII (Fig. 6C). When we directly investigated the effect of β-arrestin2 and Mnk1 siRNAs on the rate of translation, we found that both proteins were absolutely required for AngII-induced increases (Fig. 6D). These findings clearly show that in rVSMCs SII and the natural ligand AngII are activating a signaling pathway to Mnk1 and protein synthesis. Further, our siRNA data conclusively demonstrate that this pathway is comprised of both β-arrestin-mediated signaling and Mnk1 activation in rVSMCs.
DISCUSSION
Here we describe the novel finding that β-arrestin is necessary for full stimulation of protein synthesis by agonism of a model 7TMR. Although it has been known for several years that β-arrestins transduce receptor signals to multiple biochemical pathways, the cellular outcomes of this signaling have not been clear. The results presented here demonstrate the first example of a β-arrestin-dependent signaling target downstream of ERK1/2 and introduce the regulation of translation as one of many cellular outcomes of this new signaling paradigm, thus solidifying the appreciation of β-arrestins as positive regulators of receptor signaling.
Cellular regulation of protein synthetic rates is complex and as yet incompletely understood. In particular, the exact roles of Mnk1 and eIF4E in the regulation of this process have been controversial. From their discovery (15, 16), these two proteins were believed to lie in a stimulatory pathway of sequential phosphorylation that results in a change in mRNA cap binding and translation. However, subsequent reports have disputed the necessity of these proteins for this signaling cascade. For example, it has been reported that siRNA targeting Mnk1, which eliminated eIF4E phosphorylation, had no effect on protein synthesis in HEK-293 cells (29). Similarly, the Mnk1/Mnk2 knock-out mouse is viable and fertile, with no obvious phenotype despite a complete lack of phosphorylated eIF4E (30). This may reflect a conditional specificity, where Mnk1 and eIF4E increase translation only in response to certain upstream stimuli, as we have reported here for AngII. Numerous others have reported the necessity for Mnk1 or eIF4E in translation, particularly in cardiac cells (11, 31, 32), supporting the observations made in this report.
In HEK-293 cells, our current understanding of the signaling pathway originating from the AT1AR and culminating in increased protein synthesis involves many signaling intermediates (supplemental Fig. S1). These pathways, which include elements such as mTOR, P70-S6 kinase, and eIF4B, can act in parallel (12). One way in which this is accomplished is through eIF4E, which must be freed from 4EBP1 before it can be phosphorylated by Mnk1. When phosphorylated by mTOR, 4EBP1 is unable to trap eIF4E. The AT1 angiotensin receptor activates mTOR via a calcium-dependent pathway, reflective of Gαq activation (33). Thus, it appears that the receptor can stimulate protein synthesis by two concerted pathways, G protein-mediated signaling accounting for mTOR activation and β-arrestin signaling resulting in phosphorylation and activation of Mnk1 and eIF4E. In this model, stimulation of the Mnk1 pathway alone might result in increased protein synthesis to varying degrees, dependent on the basal activation and other eIF4F components, such as eIF4B. This feature of the model explains why SII stimulation of the AngII receptor results in protein synthesis increases that are significant, yet blunted by comparison to AngII. Similarly, the basal level of phosphorylated eIF4E in a given cell system could dictate its ability to stimulate mRNA translation independent of Mnk1 activation. However, maximal activation only occurs when both pathways are stimulated.
We document here that the main upstream contributor to Mnk1 phosphorylation in HEK-293 cells is ERK1/2, and not p38. These data are similar to what has been reported using rVSMCs, where ERK was the singular contributor to Mnk1 phosphorylation in these cells (11). However, we also found that AngII-stimulated HEK-293 cells can increase protein synthesis independent of Mnk1, unlike the situation in rVSMCs, which cannot carry out AngII-stimulated protein synthesis in the presence of a Mnk1 inhibitor (11) or Mnk1 siRNA (Fig. 6D). Although the data from rVSMCs is clearly the more physiologically relevant result, especially with regard to AngII-responsive tissues, the HEK-293 cell data displaying translational independence from Mnk1 might be reflective of the biology of other cell types in the body.
The experiments performed here used the AT1AR as a model receptor; however, the central finding that β-arrestin-mediated ERK1/2 signaling leads to increased protein synthesis might well be reflective of the biology of many 7TMRs. For example, the prostaglandin2 receptor EP4, which is Gαs-coupled, was shown to stimulate cardiac myocyte hypertrophy via a PKA-independent mechanism that required ERK1/2 activation (34). Also, the Gαi-coupled lysophosphatidic acid receptor, which we have previously shown signals via β-arrestins in cultured cells (35), is also capable of stimulating protein synthesis through eIF4E phosphorylation (36). These results may well be explained by β-arrestin-dependent ERK1/2 signaling that activates the downstream target Mnk1.
The findings that β-arrestins are necessary for activation of a signaling pathway, such as the one described here, would have been quite surprising only a few years ago. However, the preponderance of recent data, in agreement with what we have found here, supports the idea that β-arrestins can act as transducers of receptor signals. For signaling pathways such as JNK3 (21, 37) and ERK1/2 (8), the mechanism of β-arrestin-mediated activation involves a scaffolding of upstream kinases. In the case of the Raf-1, Mek1, and ERK1/2 β-arrestin scaffold, the amount of ERK1/2 bound by β-arrestin increases after agonist stimulation and phosphorylated ERK remains associated with β-arrestin after activation (8, 38). Mnk1 is a known binding partner of ERK1/2, and the complex dissociates upon ERK1/2 activation (16). This suggests a direct interaction of β-arrestin2 and Mnk1; however, we have been unable to detect such an interaction using purified recombinant proteins in vitro (data not shown). Nonetheless, we feel that these data do not definitively rule out the possibility that such an interaction exists in vivo. Our data suggest that by tethering activated ERK1/2, β-arrestin2 may allow Mnk1 preferential access to its upstream kinase for activation, evidenced by the presence of phosphorylated Mnk1 in the β-arrestin2-bound fraction (supplemental Fig. S3).
Although the SII ligand and the AT1ARDRY/AAY receptor can both activate Mnk1 and eIF4E phosphorylation, as well as stimulate protein synthesis, we do not endorse a direct comparison of the magnitude of these responses with that of the natural ligand or the wild type receptor. We believe these signaling events are driven by alterations in receptor conformation rather than simple inhibition or promotion of one arm of signaling over another. For example, it is possible that SII is capable of constraining the receptor in a conformation that is exclusive to β-arrestin recruitment and signaling, yet is less efficient at doing so. Indeed, there exists mutational evidence for a conformation of the receptor induced by SII that is distinct from that induced by AngII (39). As such, for the purposes of this report, the fact that the β-arrestin-specific mutant ligand and receptor can signal to Mnk1 activation is more important than the magnitude of the response.
With regard to AT1AR and β-arrestin signaling to ERK1/2, there exists a phenomenon referred to as reciprocal regulation (27) whereby one isoform of β-arrestin facilitates the signal while the other inhibits it. For the AT1AR, only β-arrestin2 siRNA lowers ERK1/2 phosphorylation, while β-arrestin1 siRNA increases it (27). The exact mechanism for this is not known, although β-arrestin dimerization and competition for receptors among β-arrestin isoforms have been offered as explanations (1). For Mnk1 phosphorylation, we did not detect a reciprocal regulation. Only β-arrestin2 siRNA affected Mnk1 activity, whereas β-arrestin1 had little to no effect (Fig. 2). These data agree with the immunoprecipitation data, which show a preferential binding of Mnk1 and β-arrestin2 over β-arrestin1. This lack of reciprocal regulation could be due to the fact that while ERK1/2 is downstream of both G protein and β-arrestin pathways, Mnk1 is primarily a β-arrestin signaling component in these cells. Thus, the cellular pools of ERK1/2 that are affected by β-arrestin1 siRNA may not have access to Mnk1.
The finding that there are two pathways that lead from the AT1AR to Mnk1 activation in HEK-293 cells parallels what has been observed for other β-arrestin-mediated signaling events. For example, whereas AngII-stimulated ERK phosphorylation is primarily G protein- and PKC-mediated at early time points, late ERK activity depends upon β-arrestins (9). Here, we describe an early component of Mnk1 signaling that is both p38- and PKC-mediated, but also a later component that is ERK-mediated and PKC-independent (Fig. 1C). The reason for having two, seemingly redundant, pathways remains unclear. One possible explanation is that, as in the ERK situation, the spatial and temporal separation may be important for determining the downstream outcomes. For example, Mnk1 was shown to be important for mRNA stability independent of eIF4E, suggesting that Mnk1 may act as a kinase for other substrates besides eIF4E (9, 40). Thus, subcellular localization of these potential Mnk1 targets may be important in determining which activated pool of Mnk1 has access to various substrates. As other targets become identified, the involvement of β-arrestin in their activation can be assessed.
In summary, the work described here conclusively shows that β-arrestin2 expression is necessary for activation of a signaling pathway that begins with ERK1/2 phosphorylation, continues to Mnk1 and eIF4E activation, and results in increased protein synthesis. These findings further expand the signaling functions of β-arrestins to regulation of the critical cellular process of mRNA translation and protein synthesis.
Acknowledgments
We thank Donna Addison and Elizabeth Hall for excellent secretarial assistance.
This work was supported in part by National Institutes of Health Grants HL16037 and HL70631. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
Footnotes
The abbreviations used are: 7TMR, seven transmembrane receptor; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; PKC, protein kinase C; AngII, angiotensin II; VSMC, vascular smooth muscle cell; rVSMC, rat VSMC; HEK, human embryonic kidney; siRNA, small interfering RNA; IP, immunoprecipitation; HA, hemagglutinin; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; ANOVA, analysis of variance; JNK, c-Jun N-terminal kinase.
References
- 1.DeWire, S. M., Ahn, S., Lefkowitz, R. J., and Shenoy, S. K. (2007) Annu. Rev. Physiol. 69 483-510 [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz, R. J., Rajagopal, K., and Whalen, E. J. (2006) Mol. Cell 24 643-652 [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz, R. J., and Shenoy, S. K. (2005) Science 308 512-517 [DOI] [PubMed] [Google Scholar]
- 4.DeFea, K. A. (2007) Annu. Rev. Physiol. 69 535-560 [DOI] [PubMed] [Google Scholar]
- 5.Rajagopal, K., Whalen, E. J., Violin, J. D., Stiber, J. A., Rosenberg, P. B., Premont, R. T., Coffman, T. M., Rockman, H. A., and Lefkowitz, R. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16284-16289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Povsic, T. J., Kohout, T. A., and Lefkowitz, R. J. (2003) J. Biol. Chem. 278 51334-51339 [DOI] [PubMed] [Google Scholar]
- 7.DeFea, K. A., Zalevsky, J., Thoma, M. S., Dery, O., Mullins, R. D., and Bunnett, N. W. (2000) J. Cell Biol. 148 1267-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luttrell, L. M., Roudabush, F. L., Choy, E. W., Miller, W. E., Field, M. E., Pierce, K. L., and Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 2449-2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn, S., Shenoy, S. K., Wei, H., and Lefkowitz, R. J. (2004) J. Biol. Chem. 279 35518-35525 [DOI] [PubMed] [Google Scholar]
- 10.Tohgo, A., Choy, E. W., Gesty-Palmer, D., Pierce, K. L., Laporte, S., Oakley, R. H., Caron, M. G., Lefkowitz, R. J., and Luttrell, L. M. (2003) J. Biol. Chem. 278 6258-6267 [DOI] [PubMed] [Google Scholar]
- 11.Ishida, M., Ishida, T., Nakashima, H., Miho, N., Miyagawa, K., Chayama, K., Oshima, T., Kambe, M., and Yoshizumi, M. (2003) Circ. Res. 93 1218-1224 [DOI] [PubMed] [Google Scholar]
- 12.Proud, C. G. (2007) Biochem. J. 403 217-234 [DOI] [PubMed] [Google Scholar]
- 13.Wang, X., and Proud, C. G. (2006) Physiol. (Bethesda) 21 362-369 [DOI] [PubMed] [Google Scholar]
- 14.Scheper, G. C., van Kollenburg, B., Hu, J., Luo, Y., Goss, D. J., and Proud, C. G. (2002) J. Biol. Chem. 277 3303-3309 [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga, R., and Hunter, T. (1997) EMBO J. 16 1921-1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waskiewicz, A. J., Flynn, A., Proud, C. G., and Cooper, J. A. (1997) EMBO J. 16 1909-1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheper, G. C., and Proud, C. G. (2002) Eur. J. Biochem. 269 5350-5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chrestensen, C. A., Shuman, J. K., Eschenroeder, A., Worthington, M., Gram, H., and Sturgill, T. W. (2007) J. Biol. Chem. 282 4243-4252 [DOI] [PubMed] [Google Scholar]
- 19.Knauf, U., Tschopp, C., and Gram, H. (2001) Mol. Cell Biol. 21 5500-5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei, H., Ahn, S., Shenoy, S. K., Karnik, S. S., Hunyady, L., Luttrell, L. M., and Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 10782-10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J., and Lefkowitz, R. J. (2000) Science 290 1574-1577 [DOI] [PubMed] [Google Scholar]
- 22.Miura, S., Zhang, J., Matsuo, Y., Saku, K., and Karnik, S. S. (2004) Hypertens. Res. 27 765-770 [DOI] [PubMed] [Google Scholar]
- 23.Violin, J. D., Dewire, S. M., Barnes, W. G., and Lefkowitz, R. J. (2006) J. Biol. Chem. 281 36411-36419 [DOI] [PubMed] [Google Scholar]
- 24.Ahn, S., Nelson, C. D., Garrison, T. R., Miller, W. E., and Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 1740-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisler, J. W., Dewire, S. M., Whalen, E. J., Violin, J. D., Drake, M. T., Ahn, S., Shenoy, S. K., and Lefkowitz, R. J. (2007) Proc. Natl. Acad. Sci. U. S. A. [DOI] [PMC free article] [PubMed]
- 26.Attramadal, H., Arriza, J. L., Aoki, C., Dawson, T. M., Codina, J., Kwatra, M. M., Snyder, S. H., Caron, M. G., and Lefkowitz, R. J. (1992) J. Biol. Chem. 267 17882-17890 [PubMed] [Google Scholar]
- 27.Ahn, S., Wei, H., Garrison, T. R., and Lefkowitz, R. J. (2004) J. Biol. Chem. 279 7807-7811 [DOI] [PubMed] [Google Scholar]
- 28.Gaborik, Z., Jagadeesh, G., Zhang, M., Spat, A., Catt, K. J., and Hunyady, L. (2003) Endocrinology 144 2220-2228 [DOI] [PubMed] [Google Scholar]
- 29.O'Loghlen, A., Gonzalez, V. M., Salinas, M., and Martin, M. E. (2004) FEBS Lett. 578 31-35 [DOI] [PubMed] [Google Scholar]
- 30.Ueda, T., Watanabe-Fukunaga, R., Fukuyama, H., Nagata, S., and Fukunaga, R. (2004) Mol. Cell Biol. 24 6539-6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuxworth, W. J., Jr., Saghir, A. N., Spruill, L. S., Menick, D. R., and McDermott, P. J. (2004) Biochem. J. 378 Pt. 1, 73-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wada, H., Ivester, C. T., Carabello, B. A., Cooper, G. T., and McDermott, P. J. (1996) J. Biol. Chem. 271 8359-8364 [DOI] [PubMed] [Google Scholar]
- 33.Yamakawa, T., Tanaka, S., Kamei, J., Kadonosono, K., and Okuda, K. (2003) Eur. J. Pharmacol. 478 39-46 [DOI] [PubMed] [Google Scholar]
- 34.Mendez, M., and LaPointe, M. C. (2005) Am. J. Physiol. 288 H2111-H2117 [DOI] [PubMed] [Google Scholar]
- 35.Gesty-Palmer, D., El Shewy, H., Kohout, T. A., and Luttrell, L. M. (2005) J. Biol. Chem. 280 32157-32167 [DOI] [PubMed] [Google Scholar]
- 36.Voisin, L., Foisy, S., Giasson, E., Lambert, C., Moreau, P., and Meloche, S. (2002) Am. J. Physiol. 283 C446-C455 [DOI] [PubMed] [Google Scholar]
- 37.Miller, W. E., McDonald, P. H., Cai, S. F., Field, M. E., Davis, R. J., and Lefkowitz, R. J. (2001) J. Biol. Chem. 276 27770-27777 [DOI] [PubMed] [Google Scholar]
- 38.Tohgo, A., Pierce, K. L., Choy, E. W., Lefkowitz, R. J., and Luttrell, L. M. (2002) J. Biol. Chem. 277 9429-9436 [DOI] [PubMed] [Google Scholar]
- 39.Yee, D. K., Suzuki, A., Luo, L., and Fluharty, S. J. (2006) Mol. Endocrinol. 20 1924-1934 [DOI] [PubMed] [Google Scholar]
- 40.Spruill, L. S., and McDermott, P. J. (2006) FASEB J. 20 2133-2135 [DOI] [PubMed] [Google Scholar]