Abstract
Ikaros and Notch1, two regulators of gene transcription, are critically important at many stages of T cell development. Deregulation of Ikaros and Notch activities cooperate to promote T cell leukemogenesis, providing evidence that they function in converging pathways in developing T cells. In this report, a mechanism for Ikaros:Notch cooperativity is described, revealing a non-redundant role for Ikaros in regulating expression of the Notch target gene Hes1 in a leukemia T cell line. We provide evidence that Ikaros directly represses Hes1 in concert with the transcriptional repressor, RBP-Jκ, allowing for cross-talk between Notch and Ikaros that impacts regulation of CD4 expression. Taken together, these data describe a potential mechanism for Ikaros' function during T cell development and define Ikaros as an obligate repressor of Hes1.
Ikaros is a nuclear protein whose function is essential for normal T cell development (1–3). Ikaros also functions as a tumor suppressor in the T cell lineage as evidenced by the 100% penetrance of T cell leukemogenesis observed in genetically engineered Ikaros-deficient mice (4–6). The Notch receptor is also essential for T cell development and its constitutive activation also leads to T leukemogenesis in mice (7–9). Interestingly, deregulation of Ikaros and Notch pathways cooperate in leukemogenesis in both mouse and humans (10–13). However, the mechanism of cooperative function of Ikaros and Notch, which likely also contributes to the normal developmental process, is unknown.
The Notch receptor is a transmembrane protein that undergoes two proteolytic cleavage events upon recognition of its extracellular ligand (members of the Delta or Serrate/Jagged family) (14). These cleavages free the intracellular domain, which travels to the nucleus. In the nucleus, intracellular Notch regulates transcription of Notch target genes through its binding to and activation of the transcriptional repressor CSL (designated RBP-Jκ/CBF-1 in mammals). Ikaros and RBP-Jκ (RBP-J) are able to bind to the same DNA sequences in electrophoretic mobility shift assays, and it has been suggested that they may compete for binding (10).
The mammalian Notch family consists of four receptors, Notch1, -2, -3, and -4 (15). Although thymocytes express Notch1, -2, and -3 (16), a non-redundant essential role in T cell development has only been established for Notch1 (7). However, constitutively active forms of Notch1, Notch2, or Notch3 expressed as a transgene in mouse bone marrow can lead to T cell leukemogenesis (9). This suggests that whereas Notch1 may be the critical Notch receptor for T cell development, deregulation of any Notch is sufficient to induce leukemogenesis. Leukemogenesis induced by constitutively active Notch is similar in phenotype to that induced by Ikaros deficiency, suggesting that Ikaros and Notch lie in a linear pathway during T cell development. In both cases, leukemia is specific for the T cell lineage, arises within thymocyte population, and requires pre-TCR or TCR signaling (2, 5, 17).
Despite the importance of Notch1 in leukemogenesis and T cell development, only a handful of direct Notch target genes have been identified in developing T cells (18, 19). One of the first described target genes for Notch-induced transcriptional activation was the gene encoding the transcriptional repressor, Hairy Enhancer of Split-1 (Hes1) (20). Hes1, like Notch1, is essential for T cell development (21). Although most Hes1 gene targets that are important in regulation of T cell development are unknown, two potential targets have been defined. CD4 was the first identified target for Hes1 repressive activity in T cells (22, 23). The cell cycle-dependent kinase inhibitor gene p27kip1 also has been identified as a potential Hes1 target (24). Interestingly, CD4 and p27kip1 are also potential Ikaros target genes because their expression is up-regulated upon re-introduction of Ikaros into the JE131 Ikaros null T leukemia cell line (6). Because of this potential connection, we began to investigate the role of Ikaros in regulation of Hes1.
In this report, we demonstrate that Ikaros is an obligate repressor of Hes1. We show that Ikaros-mediated repression is accomplished through direct binding within regulatory elements of Hes1 and provide evidence that Ikaros binds these sequences in a cooperative fashion with RBP-J. Therefore, we propose a novel mechanism of Notch target gene repression whereby RBP-J is an inefficient repressor in the absence of Ikaros. In addition, we show that the role of Ikaros in Hes1 repression contributes to the mechanism underlying Ikaros-induced expression of CD4 in JE131 cells.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture—JE131 cells were derived from the thymus of an Ikaros null mouse with spontaneous T cell leukemia as previously described (6). Cells were maintained in RPMI medium (Invitrogen) supplemented with 10% bovine growth serum, 50 μm β-mercaptoethanol, and 100 units/ml of penicillin-streptomycin (RPMI complete). For inhibitor assays, equal volumes of the γ-secretase inhibitor N,N-(3,5-difluorophenacetyl)-l -alanyl-(S)-phenylglycine t-butyl ester (DAPT)3 (Calbiochem) resuspended in Me2SO or the carrier (Me2SO) were added to cultures.
Protein Preparation and Immunoblotting—Protein extracts were prepared by cell lysis with 420 mm NaCl Lysis Buffer (20 mm Tris pH 7.5, 0.1% bovine serum albumin, 1 mm EDTA, 1% Nonidet P-40) supplemented with leupeptin (4 μg/ml), aprotonin (2 μg/ml), and phenylmethylsulfonyl fluoride (5 μg/ml). Cells were lysed for 30 min on ice followed by centrifugation. 30 μg/lane of protein extracts were electrophoresed on a SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane overnight at 4 °C. Membranes were blocked for 1 h in Tris-buffered saline, 5% milk. Antibodies against cleaved Notch1-Val-1744 (Cell Signaling Technology) were diluted 1:500 in Tris-buffered saline, 5% bovine serum albumin and incubated with the membrane overnight at 4 °C. Blots were washed with Tris-buffered saline 3 times for 5 min and incubated with horseradish peroxidase-conjugated antibody for 1 h at room temperature. Proteins were visualized by incubation with enhanced chemiluminescence reagent and exposure to film.
Retroviral Constructs—MSCV IRES H-2Kk and MSCV IRES Ik-1 H-2Kk constructs were described previously (6). Ikaros mutants were generated by site-directed PCR mutagenesis (25). 5′ and 3′ end primers used to insert restriction enzyme sites for cloning were (5′ to 3′): 5′ extension (BglII), GCCGACCGTCAGATCTATGGACTACAAGGACGACGATGACAAG; 3′ extension (XhoI), GCTGTAGGAATTCGCTGTAGCTCGAGTTAGCTCAGGTGGTAACGATGCTCC. Mutation introducing primers: F2 DBD forward, GCCTCCTTTACCCGGAAAGGCAACCTCC; F2 DBD reverse, GGAGGTTGCCTTTCCGGGTAAAGGAGGC; F3 DBD forward, TGCCTGCCGCCAGAGGGACGCCCT; and F3 DBD reverse, AGGGCGTCCCTCTGGCGGCAGGCA. Following BglII-XhoI restriction enzyme digest of PCR products, mutant Ik-1 sequences were subcloned into MSCV IRES H-2Kk. All constructs were sequenced at the University of Chicago Cancer Research DNA Sequencing Facility. MSCV IRES Hes1 GFP was a gift of Dr. Warren Pear (University of Pennsylvania, Philadelphia, PA).
Retroviral Transduction and Magnetic Cell Sorting—Retroviral plasmid constructs were transfected into Phoenix ecotropic packaging cells using Lipofectamine reagent (Invitrogen). Viral supernatants were harvested at 48 and 72 h postinfection, passed through a 0.22-μm filter, and stored at –80 °C. JE131 cells were infected using 1 ml of supernatant per 2 × 106 cells supplemented with 8 μg/ml of Polybrene in a 24-well tissue culture plate. Plates were centrifuged at 500 × g for 2 h at 32 °C. Supernatants were removed and cells were cultured with RPMI complete medium. Successfully transduced cells were sorted with the MiniMacs system (Miltenyi) using the H-2Kk expression marker as previously described (6). Purity was assessed using the MACSelect control fluorescein isothiocyanate antibody and was consistently >90%.
Flow Cytometry—For flow cytometric analyses, the following antibodies were used: anti-CD4 (GK1.5, eBiosciences), anti-CD8 (53-6.7, eBiosciences), MACSelect control fluorescein isothiocyanate antibody (Miltenyi Biotech), and anti-H-2Kk (H100-27.R55, Miltenyi Biotech). Antibodies were allophycocyanin, fluorescein isothiocyanate, or phycoerythrin conjugates. For staining, cells were plated in microwell staining plates at 5 × 105 to 1 × 106 cells per well. Fluorochrome-conjugated antibodies were added to cells and incubated on ice for 15 min. Stained cells were analyzed by flow cytometry on a FACScalibur (BD Biosciences) flow cytometer using CellQuest Pro software.
RT-PCR—mRNA was prepared with TRIzol reagent (Invitrogen) from H-2Kk positive retrovirally transduced JE131 cells sorted 24 h postinfection or JE131 cells treated with DAPT for 36 h. cDNA was generated using a Superscript II kit (Invitrogen). Quantitative real-time RT-PCR (qRT-PCR) was performed using a Bio-Rad iQ5 Real Time PCR machine and iQ SYBR Green Supermix (Bio-Rad). Analyses of qRT-PCR were performed using the Pfaffl method. Primers were designed using Beacon Design software and synthesized by IDT DNA Technologies. Primer sequences are available upon request.
Chromatin Immunoprecipitation—Chromatin immunoprecipitation (ChIP) was performed using chromatin prepared from H-2Kk positive JE131 cells infected with MSCV IRES H-2Kk or MSCV IRES Ik-1 H-2Kk retroviruses at 24 h postinfection using the ChIP Assay Kit (Upstate). Briefly, 106 cells were used per sample. Proteins bound to DNA were cross-linked by treating cells with 1% formaldehyde for 10 min at room temperature. Cells were washed, lysed, and sonicated (Fisher Scientific Sonic Dismembrator Model 100) to shear DNA. Samples were precleared with Protein G or Protein A-agarose/salmon sperm beads (Upstate). Protein-DNA complexes were immunoprecipitated using the ChIP Assay Kit (Millipore; catalog number 17-295) and antibodies against Ikaros, RBP-J (Santa Cruz and Chemicon), anti-acetylated histone H3 (Upstate), or a control IgG (Santa Cruz). Complexes were collected with Protein G or Protein A-agarose/salmon sperm beads and washed. Protein-DNA complexes were eluted off the beads and cross-links were reversed by heating at 65 °C overnight. DNA was recovered by phenol:chloroform extraction and precipitated by ethanol. qPCR analyses were performed on immunoprecipitated DNA and normalized to total chromatin input using the Pfaffl method. Primers were used with iQ SYBR Green Supermix (Bio-Rad) and were as follows (5′ to 3′): Hes1 forward, CTGTGGGAAAGAAAGTTTGGGAAG; Hes1 reverse, GCTCCAGATCCTGTGTGATCC; upstream 1 kb forward, CTCCCTTGTCCGCCGTCTATCC; upstream 1 kb reverse, CGCTCGTTCCTCCGCCACTCTC; upstream 7 kb forward, GAGAGGCAACCACGGACTTG; upstream 7 kb reverse, ACAGGCTCCAGGCACCAC; downstream 1 kb forward, GCGTGCGTCCCCTCTCTG; downstream 1 kb reverse, GCTGAATGCCTCTCACAACCG; downstream 2.5 kb forward, GCGGCTCCCAACTCACTCC; downstream 2.5 kb reverse, ACAGACAAATGAAGGTCCCAATGC; Deltex 1 forward, TCAGCCAGATTACATCCATTAGTC; Deltex1 reverse, CAGAGAGGTTACTCAGTTTGTCC.
For ChIP-Western blot, ChIP was performed with anti-Ikaros monoclonal antibodies or control IgG. Chromatin utilized was derived from 5 × 106 magnetically sorted H-2Kk positive JE131 cells infected with MSCV IRES H-2Kk or MSCV IRES Ik-1 H-2Kk retroviruses. Immunoprecipitated complexes were eluted from beads by incubation in Laemmli buffer at 95 °C for 10 min and subjected to Western blot analyses using antibodies against RBP-J (Chemicon) or Ikaros.
RESULTS
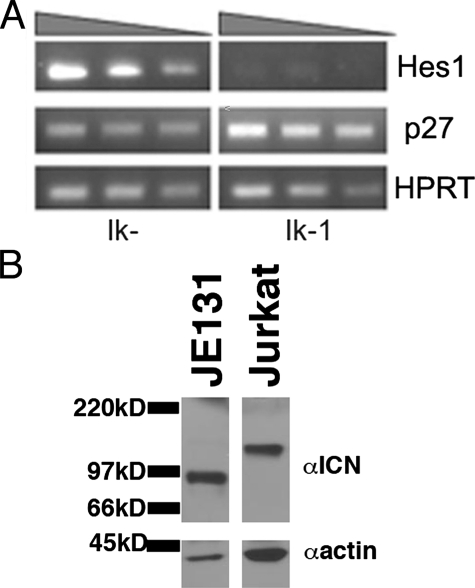
Restoration of Ikaros Results in Down-regulation of Hes1 Expression in an Ikaros Null T Leukemia Cell Line with Activated Notch—The basic helix-loop-helix transcription factor, Hes1, has previously been shown to repress expression of the T cell differentiation marker, CD4, and the cell cycle regulator, p27kip1 (22–24). Interestingly, reintroduction of Ikaros to the JE131 Ikaros null T leukemia cell line has been shown to up-regulate both CD4 and p27kip1 (6). Therefore, we postulated that up-regulation of CD4 and p27kip1 in JE131 cells transduced with Ikaros may be the result of reduced expression of Hes1. This reduction would relieve Hes1-induced repression of CD4 and p27kip1. To begin to address this possibility, Hes1 expression was analyzed in JE131 cells transduced with the Ikaros isoform, Ik-1 (Ik-1) or the negative control retrovirus (Ik–). Semi-quantitative RT-PCR of cDNA prepared from JE131 cells without Ikaros revealed high levels of Hes1 expression. JE131 cells transduced with Ik-1, however, displayed a dramatic down-regulation of Hes1 expression (Fig. 1A).
FIGURE 1.
Expression of the Notch target gene, Hes1, is repressed upon reintroduction of Ikaros to an Ikaros null T leukemia cell line that contains ICN. A, 24 h post-infection, JE131 cells successfully infected with MSCV IRES H-2Kk (Ik–) or MSCV IRES Ik-1 H-2Kk (Ik-1) retroviruses were purified using H-2Kk as a marker of successful transduction. cDNA was prepared and analyzed by semi-quantitative RT-PCR. Expression of Hes1, p27kip1, and the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase in each population is shown. The shaded triangle above the blots indicates decreasing amounts of cDNA. B, Western blot analysis of JE131 and Jurkat whole cell extracts. Blots were probed with antibodies directed against intracellular Notch1 (ICN) that has been properly cleaved at Va1–744 (αICN). Blots were also probed with anti-actin antibodies as a loading control (αactin). Jurkat cells served as a positive control because they constitutively express ICN (46). Differences in mobility of ICN in Jurkat and JE131 are likely due to mutations that arise during leukemogenesis as previously described (27).
Notch signaling up-regulates Hes1 expression, suggesting that high Hes1 expression in JE131 cells may result from activated Notch in these cells. In support of this, it was reported that T leukemia cell lines arising from mice with greatly diminished Ikaros expression (Ik L/L mice) (10) constitutively express intracellular Notch (ICN), the active form of Notch. Therefore, JE131 cells were examined for the presence of ICN. Whole cell extracts from JE131 cells prepared and analyzed by Western blot with an anti-ICN specific antibody showed that ICN was constitutively generated in JE131 cells (Fig. 1B). It is important to note that although the generation of ICN in JE131 cells is likely to contribute to deregulation of Hes1 expression in JE131 cells, reintroduction of Ikaros alone is sufficient to down-regulate expression of Hes1.
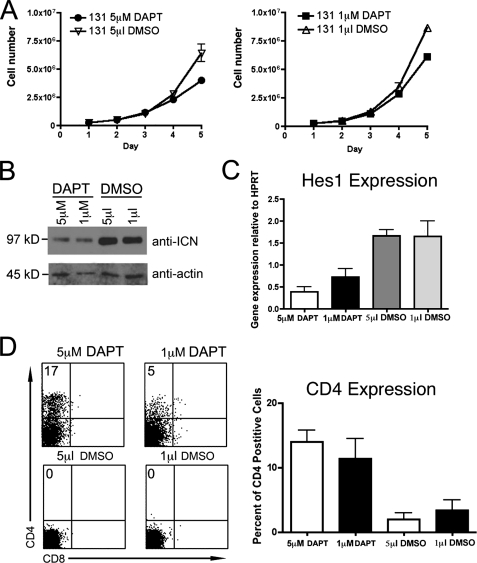
Treatment with γ-Secretase Inhibitor Has Minimal Effect on Proliferation of JE131 Cells—Notch signaling is required to maintain proliferation and viability of many murine and human T leukemia cell lines (26, 27). The presence of ICN and a high level of Hes1 expression in JE131 cells suggests that ICN may function to promote the leukemic phenotype of these cells through the constitutive activation of Notch signaling. Given these data, we next determined if generation of ICN was essential for viability and/or proliferation of JE131 cells. For these studies, the pharmacological γ-secretase inhibitor (GSI), DAPT was utilized. DAPT blocks cleavage of Notch, preventing formation of ICN and its translocation to the nucleus. Previous data have shown that addition of GSI to rapidly growing mouse leukemia cell lines that express ICN frequently results in G0/G1 arrest and apoptosis (10, 28). In particular, 5 μm DAPT has been shown to be sufficient to induce cell cycle arrest and apoptosis in murine T-acute lymphoblastic leukemia cell lines with activating Notch1 mutations (28). However, addition of 5 or 1 μm DAPT to JE131 cells had a minimal effect on their ability to proliferate (Fig. 2A).
FIGURE 2.
The γ-secretase inhibitor, DAPT, has minimal effects on growth of an Ikaros null leukemia cell line, but leads to up-regulation of CD4. A, 2.5 × 105JE131 cells were cultured with 5 μm DAPT, 1 μm DAPT, 5 μl of Me2SO (DMSO), or 1 μm Me2SO in a 24-well plate. Counts of viable cells were performed every 24 h. Graphs depict results of two experiments with error bars (±S.D.). B, Western blot analysis of 30 μg/lane of protein extracts prepared from JE131 cells treated with 5 μm DAPT, 1 μm DAPT, 5 μl of Me2SO, or 1 μm Me2SO for 4 days. Blots were probed with antibodies directed against ICN that has been properly cleaved at Val-1744. Blots were also probed with anti-actin antibodies as a loading control. C, qRT-PCR analyses of Hes1 expression were performed using cDNA prepared from JE131 cells treated with 5 μm DAPT, 1 μm DAPT, 5 μl of Me2SO, or 1 μl of Me2SO. y axis shows gene expression normalized to expression of the housekeeping gene, HPRT (hypoxanthine-guanine phosphoribosyltransferase). Values were determined by the Pfaffl method and are shown as ratios (2–ΔCT target:2–ΔCT reference). Target = gene of interest; reference = HPRT. Bar graph depicts results of two experiments performed in duplicate with error bars (±S.D.). D, JE131 cells treated with 5 μm DAPT, 1 μm DAPT, 5 μl of Me2SO, or 1 μl of Me2SO for 4 days were stained with fluorochrome-conjugated antibodies against CD4 and CD8. Representative flow cytometric dot plots are shown. Bar graph depicts results of three experiments with error bars (±S.D.).
To confirm the efficiency of DAPT treatment, ICN levels were measured by Western blot analyses using protein extracts prepared after 5 days of culture. In DAPT-treated cells, a decrease in levels of ICN was observed compared with Me2SO-treated controls (Fig. 2B). Therefore, high levels of ICN are not required for viability and proliferation of JE131 cells. This is in contrast to JE131 cells transduced with Ikaros, which undergo rapid cell cycle arrest (6), suggesting that the proliferative capacity of JE131 cells is primarily dependent upon lack of Ikaros rather than generation of ICN.
Reduction of ICN Levels Leads to Reduced Expression of Hes1 and Up-regulation of the T Cell Differentiation Marker, CD4—To verify that the reduction observed in levels of ICN was sufficient to effect Notch target gene expression, Hes1 expression was analyzed. qRT-PCR was performed on cDNA generated from JE131 cells treated with DAPT or Me2SO (negative control). Treatment of JE131 cells with DAPT led to a decrease in Hes1 expression compared with that observed in cells treated with Me2SO (Fig. 2C). Therefore, the reduction in ICN levels that occurred as a result of DAPT treatment was sufficient to reduce Hes1 expression in JE131 cells, even though proliferation was unaffected.
The phenotype of the JE131 cells, CD4-CD8-TCR-CD44-CD25+, places them at the DN3 stage of T cell development. Reintroduction of Ikaros in these cells induces a T cell-specific program of gene expression in which expression of T cell differentiation markers such as CD4 and CD8 are up-regulated (6). Because Hes1 has been implicated in repression of CD4 (22, 23) and DAPT reduces Hes1 expression levels, we hypothesized that JE131 cells grown in the presence of DAPT may up-regulate CD4 as do those transduced with Ikaros. Indeed, JE131 cells treated with DAPT up-regulate CD4 (Fig. 2D). These data suggest that inactivation of Notch signaling is sufficient to induce CD4 expression in JE131 cells, even in the absence of Ikaros. Note that this is not the case for CD8 (Fig. 2D).
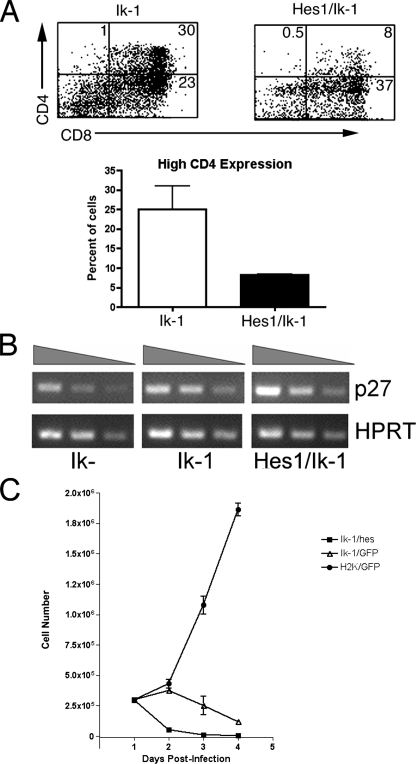
Forced Expression of Hes1 in the Presence of Ikaros Interferes with High Level CD4 Expression—In JE131 cells, reintroduction of Ikaros and treatment with DAPT both result in up-regulation of CD4 and down-regulation of Hes1, indicating that in Ikaros-transduced JE131 cells, CD4 up-regulation may result from repression of Hes1 by Ikaros. To test this, JE131 cells were co-transduced with Hes1 and Ikaros (Ik-1) to force continued high-level expression of Hes1 in the presence of Ikaros, where it would normally be down-regulated. In co-transduced JE131 cells, expression levels of CD4 differed from those observed in JE131 cells transduced with Ikaros alone. More specifically, co-transduction with Hes1 decreased Ikaros' ability to induce high-level expression of CD4 (Fig. 3A). These data provide evidence that up-regulation of CD4 observed in Ikaros-transduced JE131 cells is partially induced by Ikaros-driven repression of Hes1.
FIGURE 3.
Preventing down-regulation of Hes1 decreases Ikaros' ability to induce high-level expression of CD4. A, JE131 cells were infected with MSCV IRES GFP and MSCV IRES Ik-1 H-2Kk (Ik-1 alone) or MSCV IRES Hes-1 GFP and MSCV IRES Ik-1 H-2Kk (Hes1/Ik-1) retroviruses, and sorted for H-2Kk expression using magnetic beads. Four days post-infection, cells were stained with fluorochrome-conjugated antibodies against CD4 and CD8. Flow cytometric dot plots depict CD4 and CD8 staining for GFP positive cells in each culture. “High” CD4 expression appears in the upper right and upper left quadrants of each plot. Percentages of cells that fall into each quadrant are shown. Bar graph depicts results of two experiments with error bars (±S.D.). B, semiquantitative RT-PCR analysis of p27kip1 expression was performed using cDNA from JE131 transduced with Ik–, Ik-1, or Ik-1 and Hes1 retroviruses. Twenty-four hours post-infection, JE131 cells were purified using H-2Kk and GFP as markers of successful transduction. cDNA was prepared and analyzed. Expression of p27kip1 and the housekeeping gene, HPRT (hypoxanthine-guanine phosphoribosyltransferase), are shown for each population. The shaded triangle above the blots indicates decreasing amounts of cDNA. C, 24 h postinfection, 3.0 × 105 sorted JE131 cells successfully transduced with Ik–, Ik-1, or Ik-1 and Hes retroviruses were cultured in RPMI complete media in a 48-well plate. Counts of viable cells were performed every 24 h. Graph represents results of two experiments performed in quadruplicate with error bars (±S.D.). On day 4 post-infection, average cell counts/ml were 4.25 × 103 (Ik-1/Hes), 1.17 × 105 (Ik-1/GFP), and 1.86 × 106 (H-2K/GFP).
Hes1 has been implicated as a repressor of p27kip1, and p27kip1 levels increase upon expression of Ikaros in the Ikaros null JE131 cells (6). Therefore, Ikaros-induced down-regulation of Hes1 may be the mechanism behind up-regulation of p27kip1 in Ikaros-transduced JE131 cells. To examine this possibility, expression levels of p27kip1 were analyzed in JE131 cells transduced with Ikaros and Hes1 retroviruses by semi-quantitative RT-PCR. p27kip1 expression levels were unaffected in the presence of Ikaros and Hes1 compared with Ikaros alone (Fig. 3B). Thus, Hes1 repression by Ikaros does not contribute to the up-regulation of p27kip1 in JE131 cells transduced with Ikaros.
A role for Hes1 in regulating cell survival and proliferation has been documented (21, 29, 30). Therefore, we next wanted to test if down-regulation of Hes1 by Ikaros underlies the reduction in proliferative capacity observed in Ikaros-transduced JE131 cells (6). However, this was not the case because Ikaros/Hes1-expressing JE131 cells exhibited dramatically slowed growth, similar to that observed in cells transduced with Ikaros alone (Fig. 3C). Interestingly, this correlates with the inability of forced Hes1 expression to block Ikaros-induced up-regulation of p27kip1 expression (Fig. 3B).
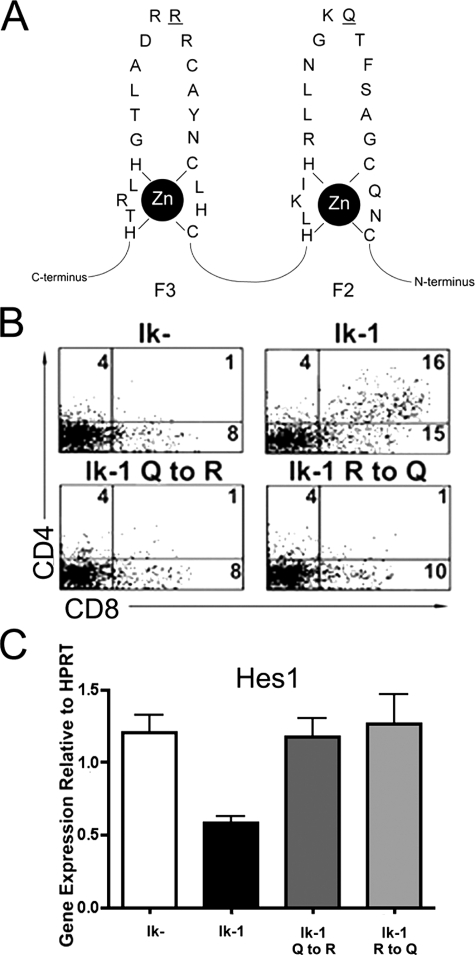
Repression of Hes1 Expression Requires Ikaros' Sequence-specific DNA Binding Ability—Two mechanisms by which Ikaros could repress Hes1 are: 1) through direct binding to Hes1 regulatory regions, or 2) through interaction with and sequestration of factors required for Hes1 expression. To begin to distinguish between these mechanisms, point mutations were introduced separately into two of Ikaros' DNA binding zinc fingers (F2 and F3) that would not disrupt zinc finger formation, but would abrogate Ikaros' ability to bind at its sequence-specific DNA binding site (31). These subtle mutations preserve Ikaros' tertiary structure and, therefore, would be highly unlikely to affect its ability to interact with other proteins (31) (Fig. 4A). Both mutations prevented Ikaros-induced up-regulation of CD4 and CD8 in JE131 cells (Fig. 4B). Furthermore, both rendered Ikaros completely unable to repress Hes1 expression, as shown by qRT-PCR (Fig. 4C). These data indicate that down-regulation of Hes1 expression in JE131 cells transduced with Ikaros occurs through sequence-specific DNA binding of Ikaros.
FIGURE 4.
DNA binding specificity is required for Ikaros' ability to repression Hes1. A, point mutations that abrogate Ikaros' ability to bind to its consensus DNA binding site, GGGAA, were generated by site-directed PCR mutagenesis. The mutated DNA bases within the second and third N-terminal zinc fingers (F2 and F3) are underlined. B, JE131 cells were transduced with Ikaros DNA binding-deficient mutants (Ik-1 Q to R and Ik-1 R to Q) and stained with fluorochrome-conjugated antibodies against H-2Kk, CD4, and CD8 4 days post-infection. C, Hes1 expression was analyzed in JE131 cells transduced with Ikaros DNA-binding deficient mutants by qRT-PCR. Twenty-four hours post-infection, H-2Kk positive JE131 cells were purified and cDNA prepared. Expression level for each population is normalized to HPRT expression. Bar graph depicts results of two experiments with error bars (±S.D.).
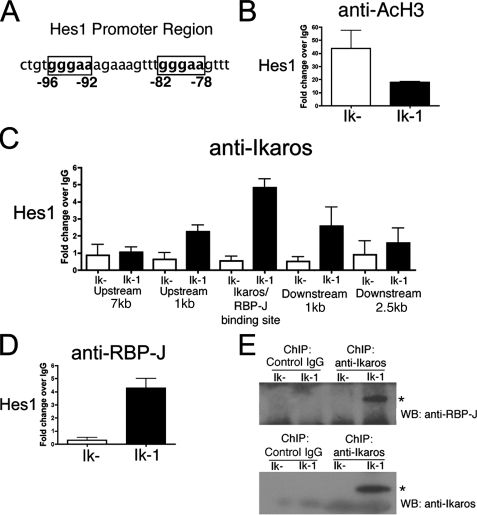
Hes1 Promoter Region Is Deacetylated in the Presence of Ikaros—Ikaros participates in chromatin remodeling as a component of both activating and repressing chromatin remodeling complexes. These complexes facilitate chromatin modification, thereby altering accessibility of DNA to transcription factors. Some of these complexes are associated with enzymes, histone acetyltransferases and histone deacetyltransferases, which alter chromatin formation by addition or removal of acetyl groups, respectively. Ikaros is believed to target these complexes to specific gene loci. Repression of Hes1 expression in JE131 cells transduced with Ikaros may occur though Ikaros' ability to target chromatin remodeling complexes to the Hes1 promoter. To determine the chromatin state of the Hes1 promoter, ChIP assays were performed on chromatin prepared from JE131 cells transduced with the control retrovirus (Ik–) or Ikaros (Ik-1) using an antibody against acetylated histone H3. Acetylated histone H3 was chosen because Ikaros has previously been shown to cause a widespread change in histone H3 acetylation in JE131 cells (6). Using real-time qPCR analysis of ChIP DNA, a decrease in acetylation of histone H3 at the Hes1 promoter was observed in Ikaros-transduced JE131 cells as compared with those not expressing Ikaros (Fig. 5B). These data suggest that the presence of Ikaros in JE131 cells represses Hes1 expression by facilitating changes in histone acetylation at the Hes1 locus.
FIGURE 5.
Ikaros and RBP-Jκ bind to the regulatory region of Hes1. A, Hes1 promoter region with RBP-Jκ/Ikaros binding sites are shown in boxes. B–D, ChIP with antibodies against acetylated histone H3 (anti-AcH3)(B), Ikaros (anti-Ikaros)(C), or RBP-Jκ (anti-RBP-J)(D) were performed on chromatin prepared from populations of JE131 cells successfully transduced with the MSCV IRES H-2Kk retrovirus (Ik–) or the MSCV IRES Ik-1 H-2Kk (Ik-1) retrovirus 24 h post-infection. Analyses of ChIP DNA were performed using qPCR with primers surrounding Ikaros and/or RBP-Jk (RBP-J) binding sites in the upstream regulatory region of Hes1. All ChIP analyses were performed with two independent chromatin preparations with at least two immunoprecipitations per chromatin preparation. Bar graphs depict results of these two experiments with error bars (±S.D.). E, ChIP was performed using chromatin prepared from H-2Kk positive JE131 cells that had been infected with MSCV IRES H-2Kk (Ik–) or MSCV IRES Ik-1 H-2Kk (Ik-1) retroviruses. Control IgG or anti-Ikaros monoclonal antibodies were utilized for immunoprecipitation of the chromatin. Complexes were eluted from beads using Laemmli buffer and Western blot analyses performed using anti-RBP-J (top blot) or anti-Ikaros (bottom blot) antibodies. Stars indicate specific bands. Experiment was performed twice and a representative experiment is shown.
Ikaros Binds to Hes1 Regulatory Regions in Vivo—To demonstrate direct Ikaros regulation of Hes1, Ikaros ChIP experiments were performed. Because it has been shown that Ikaros and RBP-J, the Notch inducible transcriptional activator, can bind the same DNA sequences in electrophoretic mobility shift assays (10), primers were generated to the Hes1 regulatory region containing the identified RBP-J binding site (Fig. 5A). Interestingly, this region contains two consensus Ikaros/RBP-J binding sites (GGGAA) in a head-to-tail configuration. Chromatin was prepared from JE131 cells that had been transduced with Ikaros (Ik-1) as well as those transduced with the control retrovirus (Ik–), and ChIP was performed with anti-Ikaros antibodies. In this way, it was determined that Ikaros binds directly within the Hes1 regulatory region that has been shown to bind RBP-J (Fig. 5C, Ikaros/RBP-J binding site).
Although the binding site for RBP-J within Hes1 has been firmly established by electrophoretic mobility shift assay (20), ChIP (32), and reporter assays (33), this is not the case for Ikaros. Therefore, we tested two additional primer pairs on each side of the Ikaros binding region within Hes1. A peak of Ikaros binding was observed within the regulatory region that also contains the RBP-J binding site (Fig. 5C).
Ikaros and RBP-J Bind to DNA Simultaneously, Not Antagonistically in a Leukemia Cell Line—It has been hypothesized that because Ikaros and RBP-J share the same DNA binding consensus sequence, they bind competitively (11). If RBP-J and Ikaros compete for binding within Hes1 regulatory sequences, then RBP-J binding within these sequences should be reduced in the presence of Ikaros. To test if this was the case, RBP-J binding was examined by ChIP analyses with anti-RBP-J antibodies using chromatin prepared from JE131 cells transduced with Ikaros (Ik-1) or the control retrovirus (Ik–). We surveyed the same consensus Ikaros/RBP-J sites in the regulatory region of Hes1 as described above to determine whether the presence of Ikaros negatively affected levels of RBP-J binding. RBP-J and Ikaros were both detected bound to the Hes1 regulatory region in JE131 cells transduced with Ik-1 (Fig. 5, C and D). Surprisingly, RBP-J binding levels did not decrease, but actually increased in the presence of Ikaros. In fact, RBP-J binding was undetectable unless Ikaros was also expressed.
As a further test to determine whether Ikaros and RBP-J bind DNA concomitantly, ChIP was performed followed by Western blot analyses. Chromatin prepared from Ikaros-transduced JE131 cells was immunoprecipitated with anti-Ikaros antibodies, followed by Western blot analyses of the protein component of the immunoprecipitate using anti-RBP-J and anti-Ikaros antibodies. Anti-Ikaros antibodies precipitated chromatin fragments that contained both Ikaros and RBP-J (Fig. 5E). Thus, these data confirm that Ikaros and RBP-J can bind together within small regions of chromatin, supporting our model of concurrent and not antagonistic binding.
Ikaros' Role as a Direct Repressor of Notch Target Genes Extends to Deltex1—To determine whether this mode of regulation extends to other Notch target genes, we investigated whether Ikaros could bind within potential regulatory sequences of the Notch target gene, Deltex1. Deltex1 encodes a putative E3-ubiquitin ligase whose expression is highly induced by Notch signals in thymocytes (34).
Levels of Deltex1 expression were compared by qRT-PCR in JE131 cells transduced with Ikaros (Ik-1) or control retroviruses (Ik–). Deltex1 expression decreased upon reintroduction of Ikaros to JE131 cells (Fig. 6A). Although Deltex1 expression is frequently used as a marker for Notch activation, its regulatory elements have not been well defined. Therefore, we scanned sequences upstream of the Deltex1 transcriptional start site for potential RBP-J/Ikaros binding sites using the UCSC Genome Browser. Two such sites were defined within region –1424 to –1133 relative to the start site of transcription (Fig. 6B).
FIGURE 6.
Ikaros and RBP-Jκ bind to the regulatory regions of Deltex1. A, qRT-PCR analysis of Deltex1 expression in sorted JE131 cells transduced with MSCV IRES H-2Kk (Ik–) or MSCV IRES Ik-1 H-2Kk (Ik-1). Bar graph depicts results of two experiments performed in duplicate with error bars (±S.D.). B, Deltex1 sequences with RBP-J/Ikaros binding sites shown in boxes. C, ChIP were performed on H-2Kk positive JE131 cells 24 h post-infection with the MSCV IRES H-2Kk retrovirus (Ik–) or the MSCV IRES Ik-1 H-2Kk (Ik-1) retrovirus using antibodies against Ikaros (anti-Ikaros) or RBP-Jκ (anti-RBP-J). qPCR analyses of ChIP DNA were performed with primers surrounding Ikaros and/or RBP-Jκ (RBP-J) binding sites in upstream regulatory regions of Deltex1. ChIP analyses were performed with two independent chromatin preparations with at least two immunoprecipitations per chromatin preparation. Bar graph depicts results of these two experiments with error bars (±S.D.).
ChIP was performed with anti-Ikaros antibodies and chromatin prepared from JE131 cells transduced with Ikaros (Ik-1) or the control retrovirus (Ik–). Binding of Ikaros to Deltex1 was observed suggesting that Ikaros directly represses Deltex1 expression by binding to its regulatory regions (Fig. 6C). ChIP was also performed with anti-RBP-J antibodies and chromatin from JE131 cells transduced with Ik-1 or Ik–. As with the Hes1 promoter region, RBP-J was detected within the Deltex1 regulatory region only in the presence of Ikaros (Fig. 6C).
DISCUSSION
Cooperative mutations in protooncogenes and tumor suppressor genes occur in many forms of cancer (e.g. colon cancer, pancreatic cancer, melanoma, acute leukemia) (35–38). Strong evidence indicates that loss of Ikaros, a tumor suppressor gene, and constitutive activation of Notch1, a proto-oncogene, fit this paradigm in the genesis of T-acute lymphoblastic leukemia (10–13). It is likely, therefore, that Ikaros and Notch signaling also intersect in regulation of normal T cell development. Here, we provide evidence for a unique role for Ikaros in direct repression of Notch target genes, by focusing our studies on the role of Ikaros in repression of the canonical Notch target gene, Hes1.
In this report, we have demonstrated that, in the JE131 Ikaros null leukemia T cell line, Ikaros is required for repression of Hes1. Three explanations for these observations were evaluated. The first possibility was that Ikaros might interact with a factor necessary for induction of Notch target gene expression (i.e. Mastermind-like), thereby sequestering it from interacting with RBP-J until Notch/Notch-ligand interactions occur. To test this possibility, point mutations were introduced that would abrogate Ikaros' sequence-specific DNA binding ability without affecting its tertiary structure. These point mutations abrogated Ikaros' ability to down-regulate Hes1, making this possibility unlikely. Second, Ikaros may compete for binding with RBP-J and function as a more efficient repressor of Notch target genes. However, evidence was provided that Ikaros and RBP-J can both be chromatin immunoprecipitated with regulatory elements of Hes1 and Deltex1 when these genes are repressed, suggesting that this mechanism is also incorrect. The third, and we suggest the correct mechanism is that Ikaros and RBP-J bind simultaneously to the regulatory regions of Hes1 and Deltex1 (Fig. 7). These data provide evidence, for the first time, that RBP-J may require the presence of another DNA binding factor, in this case Ikaros, to efficiently repress gene expression. A precedent exists for functional interaction of RBP-J and zinc finger DNA-binding proteins in Notch gene regulation, albeit in gene activation, not repression as we show here. It has been suggested that the Notch protein, Notch 3, requires binding of a zinc finger DNA-binding protein in conjunction with RBP-J for Notch gene activation (39), although it is unknown, to date, how these two proteins function together.
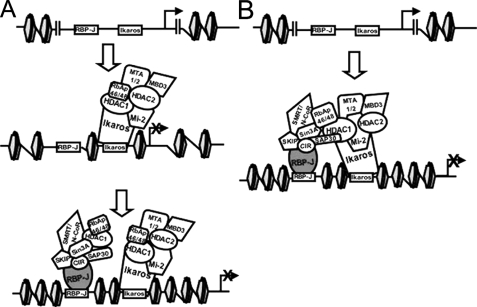
FIGURE 7.
Two hypotheses for cooperative DNA binding of Ikaros and RBP-Jκ during gene repression. A, Ikaros binding is required to alter chromatin structure, thereby facilitating RBP-Jκ (RBP-J) binding to DNA. B, Ikaros (or its interacting proteins) interacts directly with RBP-Jκ (or its interacting proteins) on the DNA, thereby stabilizing interactions of RBP-Jκ with the DNA.
We hypothesize that the role of Ikaros in Notch target gene repression may be to stabilize interaction of components of the co-repressor complex (i.e. histone deacetylase activity) to regulatory elements bound by RBP-J or to facilitate changes in DNA structure that allow more efficient RBP-J DNA binding (Fig. 7). Within the Hes1 promoter, there are two RBP-J/Ikaros sites in close proximity. Therefore, Ikaros and RBP-J may each bind to one of these sites. We hypothesize that this is also the case for the two consensus Ikaros/RBP-J binding sites in Deltex1. In the case of Deltex1, however, the two sites are farther apart than those observed in Hes1. Interestingly, the distance separating them, 291 bp, corresponds almost exactly with two turns of the DNA around the nucleosome (146 bp/turn × 2 = 292 bp), which places them in closer vicinity in three-dimensional space than would be expected from the linear DNA sequence.
It cannot yet be explained why RBP-J binding to Hes1 and Deltex1 regulatory regions was only robustly observed when Ikaros was present. One possibility is that when RBP-J is in the context of a co-activator complex in T lymphocytes, the epitopes for the immunoprecipitating antibodies are not accessible. Nevertheless, it is evident from the data presented here that Ikaros and RBP-J bind together to facilitate repression of Hes1. This cooperation extends to another Notch target gene, Deltex1, suggesting it may be a more universal mechanism for Notch target gene repression in developing T cells. Experiments are underway to address this possibility.
We contend that lack of Ikaros results in de-repression, as opposed to activation, of Notch target genes. Therefore, constitutive generation of ICN in Ikaros-deficient cells may further increase expression of de-repressed Notch target genes. This is likely the mechanism behind cooperative Ikaros loss-of-function mutations that were identified in ICN-induced leukemia in mice, which significantly accelerated the disease course (11). Interestingly, both activating mutations in Notch1 and aberrant Ikaros expression have been described in human T-acute lymphoblastic leukemia (27, 40, 41). The importance of high-level ICN expression to viability of human leukemia cells has been demonstrated through the use of GSIs. Some, but not all, T-acute lymphoblastic leukemia with activating Notch mutations are sensitive to treatment with GSIs, displaying apoptosis and/or growth arrest (26). A recent report has demonstrated that GSI decreases growth of a leukemia cell line arising in a mouse with a hypomorphic Ikaros allele (10). However, we demonstrate that the JE131 cell line is refractory to treatment with GSI, showing that it is not dependent on ICN for viability and proliferation. It could be suggested that this is the result of cumulative mutations that have arisen in these cells over time in culture. However, it must be emphasized that growth of JE131 cells can still be dramatically and consistently slowed through restoration of Ikaros by retroviral transduction (6). These data suggest that viability and proliferative capacity of JE131 cells is driven predominantly by their lack of Ikaros rather than constitutive generation of ICN. It is likely that the relative contribution of lack of Ikaros and constitutive ICN generation to viability will differ from leukemia to leukemia.
These studies began with the observation that re-introduction of Ikaros into JE131 Ikaros null leukemia cells promotes up-regulation of the T cell differentiation marker, CD4, and the cell cycle regulator, p27kip1. We initially hypothesized that down-regulation of Hes1 expression may underlie up-regulation of CD4 and p27kip1 in Ikaros-transduced JE131 cells, because Hes1 is a common factor reported to be involved in their regulation (22–24). In this report, we demonstrate that forced expression of Hes1 in Ikaros-transduced cells can interfere with Ikaros' ability to induce high-level expression of CD4, suggesting that Hes1 down-regulation by Ikaros does indeed contribute to Ikaros' ability to initiate high-level CD4 expression in these cells. These data also provide evidence for a role for Hes1 in fine-tuning levels of CD4 gene expression in developing thymocytes. The Runx1 and Runx3 transcriptional repressors, known to be critically important for CD4 silencing (42–44), are also Notch target genes (45). Because co-transduction of Hes1 does not affect Ikaros' ability to induce low levels of CD4 expression, studies are underway to investigate if Ikaros-induced down-regulation of Runx proteins could also contribute to the mechanism behind Ikaros' positive role in regulation of CD4 expression.
Acknowledgments
We thank Dr. Warren Pear for the Hes1 expressing retroviral construct and encouragement, Dr. Greg Gregory for initial assistance with the chromatin immunoprecipitation experiments, Dr. Melissa Brown for use of the iCycler as well as valuable advice, and Dr. Katia Georgopoulos for anti-Ikaros monoclonal antibodies.
This work was supported in part American Cancer Society Grant RSG-04-054-01-GMC and National Institutes of Health Grant R01 CA104962-01A1 (to S. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: DAPT, N,N-(3,5-difluorophenacetyl)-l-alanyl-(S)-phenylglycine t-butyl ester; ICN, intracellular Notch1; GFP, green fluorescent protein; RT, reverse transcriptase; ChIP, chromatin immunoprecipitation; qRT-PCR, quantitative real-time PCR; IRES, internal ribosomal entry site; GSI, γ-secretase inhibitor.
References
- 1.Georgopoulos, K., Winandy, S., and Avitahl, N. (1997) Annu. Rev. Immunol. 15 155–176 [DOI] [PubMed] [Google Scholar]
- 2.Winandy, S., Wu, L., Wang, J. H., and Georgopoulos, K. (1999) J. Exp. Med. 190 1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban, J. A., and Winandy, S. (2004) J. Immunol. 173 4470–4478 [DOI] [PubMed] [Google Scholar]
- 4.Winandy, S., Wu, P., and Georgopoulos, K. (1995) Cell 83 289–299 [DOI] [PubMed] [Google Scholar]
- 5.Wang, J. H., Nichogiannopoulou, A., Wu, L., Sun, L., Sharpe, A. H., Bigby, M., and Georgopoulos, K. (1996) Immunity 5 537–549 [DOI] [PubMed] [Google Scholar]
- 6.Kathrein, K. L., Lorenz, R., Minniti Innes, A., Griffiths, E., and Winandy, S. (2005) Mol. Cell. Biol. 25 1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke, F., Wilson, A., Stark, G., Bauer, M., van Meerwijk, J., MacDonald, H. R., and Aguet, M. (1999) Immunity 10 547–558 [DOI] [PubMed] [Google Scholar]
- 8.Pui, J. C., Allman, D., Xu, L., DeRocco, S., Karnell, F. G., Bakkour, S., Lee, J. Y., Kadesch, T., Hardy, R. R., Aster, J. C., and Pear, W. S. (1999) Immunity 11 299–308 [DOI] [PubMed] [Google Scholar]
- 9.Aster, J. C., and Pear, W. S. (2001) Curr. Opin. Hematol. 8 237–244 [DOI] [PubMed] [Google Scholar]
- 10.Dumortier, A., Jeannet, R., Kirstetter, P., Kleinmann, E., Sellars, M., dos Santos, N. R., Thibault, C., Barths, J., Ghysdael, J., Punt, J. A., Kastner, P., and Chan, S. (2006) Mol. Cell. Biol. 26 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beverly, L. J., and Capobianco, A. J. (2003) Cancer Cell 3 551–564 [DOI] [PubMed] [Google Scholar]
- 12.Beverly, L. J., and Capobianco, A. J. (2004) Trends Mol. Med. 10 591–598 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Nieva, P., Santos, J., and Fernandez-Piqueras, J. (2004) Carcinogenesis 25 1299–1304 [DOI] [PubMed] [Google Scholar]
- 14.Schweisguth, F. (2004) Curr. Biol. 14 R129–R138 [PubMed] [Google Scholar]
- 15.Baron, M. (2003) Semin. Cell Dev. Biol. 14 113–119 [DOI] [PubMed] [Google Scholar]
- 16.Felli, M. P., Maroder, M., Mitsiadis, T. A., Campese, A. F., Bellavia, D., Vacca, A., Mann, R. S., Frati, L., Lendahl, U., Gulino, A., and Screpanti, I. (1999) Int. Immunol. 11 1017–1025 [DOI] [PubMed] [Google Scholar]
- 17.Zweidler-McKay, P. A., and Pear, W. S. (2004) Semin. Cancer Biol. 14 329–340 [DOI] [PubMed] [Google Scholar]
- 18.Reizis, B., and Leder, P. (2002) Genes Dev. 16 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson, A. C., Robey, E. A., and Huang, Y. H. (2001) Curr. Opin. Genet. Dev. 11 554–560 [DOI] [PubMed] [Google Scholar]
- 20.Jarriault, S., Brou, C., Logeat, F., Schroeter, E. H., Kopan, R., and Israel, A. (1995) Nature 377 355–358 [DOI] [PubMed] [Google Scholar]
- 21.Tomita, K., Hattori, M., Nakamura, E., Nakanishi, S., Minato, N., and Kageyama, R. (1999) Genes Dev. 13 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, H. K., and Siu, G. (1998) Mol. Cell. Biol. 18 7166–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen, R. D., III, Kim, H. K., Sarafova, S. D., and Siu, G. (2001) Mol. Cell. Biol. 21 3071–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murata, K., Hattori, M., Hirai, N., Shinozuka, Y., Hirata, H., Kageyama, R., Sakai, T., and Minato, N. (2005) Mol. Cell. Biol. 25 4262–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton, R. M., Cai, Z. L., Ho, S. N., and Pease, L. R. (1990) BioTechniques 8 528–5352357375 [Google Scholar]
- 26.Weng, A. P., Ferrando, A. A., Lee, W., Morris, J. P., Silverman, L. B., Sanchez-Irizarry, C., Blacklow, S. C., Look, A. T., and Aster, J. C. (2004) Science 306 269–271 [DOI] [PubMed] [Google Scholar]
- 27.Pear, W. S., and Aster, J. C. (2004) Curr. Opin. Hematol. 16 426–433 [DOI] [PubMed] [Google Scholar]
- 28.O'Neil, J., Calvo, J., McKenna, K., Krishnamoorthy, V., Aster, J. C., Bassing, C. H., Alt, F. W., Kelliher, M., and Look, A. T. (2006) Blood 107 781–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomita, K., Ishibashi, M., Nakahara, K., Ang, S. L., Nakanishi, S., Guillemot, F., and Kageyama, R. (1996) Neuron 16 723–734 [DOI] [PubMed] [Google Scholar]
- 30.Ishibashi, M., Moriyoshi, K., Sasai, Y., Shiota, K., Nakanishi, S., and Kageyama, R. (1994) EMBO J. 13 1799–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koipally, J., Heller, E. J., Seavitt, J. R., and Georgopoulos, K. (2002) J. Biol. Chem. 277 13007–13015 [DOI] [PubMed] [Google Scholar]
- 32.Fryer, C. J., White, J. B., and Jones, K. A. (2004) Mol. Cell 16 509–520 [DOI] [PubMed] [Google Scholar]
- 33.Kageyama, R., and Ohtsuka, T. (1999) Cell Res. 9 179–188 [DOI] [PubMed] [Google Scholar]
- 34.Deftos, M. L., He, Y. W., Ojala, E. W., and Bevan, M. J. (1998) Immunity 9 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pons, M., and Quintanilla, M. (2006) Clin. Transl. Oncol. 8 466–474 [DOI] [PubMed] [Google Scholar]
- 36.Camos, M., and Colomer, D. (2006) Clin. Transl. Oncol. 8 550–559 [DOI] [PubMed] [Google Scholar]
- 37.Hruban, R. H., Goggins, M., and Kern, S. E. (1999) Curr. Opin. Gastroenterol. 15 404. [DOI] [PubMed] [Google Scholar]
- 38.Shattuck-Brandt, R. L., and Dubois, R. N. (1999) Curr. Opin. Gastroenterol. 15 3. [DOI] [PubMed] [Google Scholar]
- 39.Ong, C. T., Cheng, H. T., Chang, L. W., Ohtsuka, T., Kageyama, R., Stormo, G. D., and Kopan, R. (2006) J. Biol. Chem. 281 5106–5119 [DOI] [PubMed] [Google Scholar]
- 40.Sun, L., Heerema, N., Crotty, L., Wu, X., Navara, C., Vassilev, A., Sensel, M., Reaman, G. H., and Uckun, F. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, L., Goodman, P. A., Wood, C. M., Crotty, M. L., Sensel, M., Sather, H., Navara, C., Nachman, J., Steinherz, P. G., Gaynon, P. S., Seibel, N., Vassilev, A., Juran, B. D., Reaman, G. H., and Uckun, F. M. (1999) J. Clin. Oncol. 17 3753–3766 [DOI] [PubMed] [Google Scholar]
- 42.Taniuchi, I., Sunshine, M. J., Festenstein, R., and Littman, D. R. (2002) Mol. Cell 10 1083–1096 [DOI] [PubMed] [Google Scholar]
- 43.Taniuchi, I., Osato, M., Egawa, T., Sunshine, M. J., Bae, S. C., Komori, T., Ito, Y., and Littman, D. R. (2002) Cell 111 621–633 [DOI] [PubMed] [Google Scholar]
- 44.Taniuchi, I., and Littman, D. R. (2004) Oncogene 23 4341–4345 [DOI] [PubMed] [Google Scholar]
- 45.Burns, C. E., Traver, D., Mayhall, E., Shepard, J. L., and Zon, L. I. (2005) Genes Dev. 19 2331–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Neil, J., Grim, J., Strack, P., Rao, S., Tibbitts, D., Winter, C., Hardwick, J., Welcker, M., Meijerink, J. P., Pieters, R., Draetta, G., Sears, R., Clurman, B. E., and Look, A. T. (2007) J. Exp. Med. 204 1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]