Abstract
The mammalian amino acid response (AAR) pathway is up-regulated by protein or amino acid depletion. This pathway involves detection of uncharged tRNA by the GCN2 kinase, phosphorylation of the translation initiation factor eIF2α (eukaryotic initiation factor 2α), and, through subsequent translational control, enhanced de novo synthesis of the transcription factor ATF4. The present studies demonstrate that inhibition of MEK activation in HepG2 human hepatoma cells by PD98059 or U0126 blocked the increased phosphorylation of eIF2α and ATF4 synthesis triggered by amino acid limitation, showing that the AAR requires activation of the MEK-ERK pathway. Inhibitors of the JNK or p38 MAPK pathways were ineffective. Consequently, inhibition of MEK activation blocked transcriptional induction of ATF4 target genes, but the induction was rescued by overexpression of ATF4 protein. Furthermore, the enhanced ERK phosphorylation following amino acid deprivation required GCN2 kinase activity and eIF2α phosphorylation. Inhibition of protein phosphatase 1 action on phospho-eIF2α by knockdown of GADD34 did not block the sensitivity to PD98059, suggesting that MEK functions to enhance GCN2-dependent eIF2α phosphorylation rather than suppressing dephosphorylation. Collectively, these results document a critical interdependence between the MEK-ERK MAPK signaling pathway and the amino acid stress-activated pathway.
Activation of the mammalian amino acid response (AAR)4 pathway occurs in response to protein and/or amino acid deprivation in vivo (1-4) as well as to amino acid limitation in vitro (reviewed in Ref. 5). Without an adequate supply of essential amino acids, the binding of uncharged tRNA activates the GCN2 (general control nondeprepressible-2) kinase, which in turn, phosphorylates serine 51 of the α subunit of the translation initiation factor eIF2 (eukaryotic initiation factor 2) (6). The Ser-51-phosphorylated eIF2α (p-eIF2α) functions as an inhibitor of eIF2B, which is responsible for the GDP-GTP exchange that regulates eIF2 activity; therefore, the resulting elevated level of p-eIF2α suppresses global translation initiation (6). Dephosphorylation of Ser-51 p-eIF2α is mediated by protein phosphatase 1 (PP1), which is targeted to p-eIF2α by interaction with the growth arrest and DNA damage gene GADD34 (7, 8). The PP1-GADD34 action on p-eIF2α is proposed to be a component of the program of recovery from the translational inhibition to allow for synthesis of stress-responsive proteins (9). Although eIF2α phosphorylation leads to a suppression of general protein synthesis, paradoxically, there is an increase in the synthesis of ATF4 (activating transcription factor 4) from preexisting mRNA through a mechanism based on ribosome scanning and reinitiation efficiency involving upstream open reading frames (uORFs) within the ATF4 mRNA (10, 11). Elevated ATF4 protein results in the activation of a wide array of stress-induced genes (12), including those that contain an amino acid response element (AARE) (5). Among the ATF4-responsive, AARE-containing genes is SNAT2 (System A neutral amino acid transporter) (13-15).
Cells also sense and respond to their environment via the activation of other protein kinase cascades, such as the mitogen-activated protein kinase (MAPK) pathways. In mammalian cells, there are three major MAPK cascades, the extracellular signal-regulated kinase (ERK), the c-Jun N-terminal kinase (JNK), and the p38 MAPK. The ERK pathway involves the sequential activation of rapidly accelerated fibrosarcoma protein (Raf), MAPK/ERK kinase (MEK), and ERK kinases (16). Amino acid depletion has been shown to activate Raf-1 activity by causing hypophosphorylation at Ser-259 by protein phosphatase 2A (17, 18). The enhanced Raf-1 kinase activity triggers the MEK/ERK signaling cascade, which in turn stimulates autophagy in an amino acid-dependent manner via a Gα-interacting protein that regulates the rate of GTP hydrolysis by Gαi3 protein (17, 19). A potential direct link between the MEK/ERK pathway and the eIF2α-ATF4 transcription signaling cascade has not been well documented, and the purpose of the present work was to investigate this possibility. Only one previous report has provided evidence for a relationship between the ERK and eIF2α phosphorylation. Monick et al. (20) showed that alveolar macrophages are different from most cells in that they exhibit a constitutively high ERK activity, which promotes hypophosphorylation of eIF2α by enhancing the action of PP1 and, in this way, promoting general protein synthesis. The authors of that study propose that this unique elevation of translation accounts for the prolonged life span of alveolar macrophages despite being exposed to a wide range of environmental stresses.
Depending on the tissue or cell type, the enhanced expression of particular cellular functions following amino acid deprivation has been shown to be dependent on one or more of the MAPK pathways. For example, Franchi-Gazzola et al. (21) showed that after complete amino acid starvation, the adaptive increase in System A transport activity was blocked by the MEK activation inhibitor PD98059. At the time of those studies, the three SNAT genes that encode System A transport activity were not yet identified, so the molecular basis for MEK action in the induction of System A transport activity was not known. It was later shown that the SNAT2 gene encoded the amino acid-regulated System A activity (22, 23), and our laboratory has documented that the SNAT2 gene contains an ATF4-responsive AARE within the first intron (13, 14). Consequently, induction of SNAT2 expression in response to amino acid limitation is dependent on eIF2α phosphorylation and the subsequent synthesis of ATF4 (15).
Our present data, collected in HepG2 human hepatoma cells, demonstrate an interdependent relationship between activation of the MEK/ERK pathway and eIF2α phosphorylation during nutrient stress. The results document that, following amino acid deprivation, MEK and ERK are activated, as revealed by increased ERK phosphorylation, and this activation required GCN2 kinase activity. Using agents that prevent MEK activation, the phosphorylation of eIF2α on Ser-51 by GCN2 and the subsequent production of the transcription factor ATF4 was observed to be dependent on MEK/ERK activation. Inhibition of eIF2α phosphatase activity by siRNA against GADD34 (growth arrest and DNA damage-inducible gene 34) did not alter the sensitivity to MEK inhibition, indicating that MEK functions to enhance GCN2-dependent eIF2α phosphorylation rather than to suppress the dephosphorylation step. Consistent with a requirement for enhanced MEK activity within the AAR pathway, the loss of transcriptional signaling due to MEK blockade could be rescued by expression of exogenous ATF4. Therefore, the data show that MEK is activated by a GCN2-dependent process and that there is an absolute requirement for MEK/ERK signaling upstream of eIF2α-mediated translational control in the mammalian AAR pathway.
MATERIALS AND METHODS
Inhibitors and Antibodies—The β-actin antibody (catalog number A2066) and the following inhibitors were purchased from Sigma: MEK inhibitor U0126, p38 inhibitor SB203580, MEK inhibitor PD98059, and JNK inhibitor SP600125.
Antibodies to ATF4 (catalog number sc-200), total ERK (catalog number sc-94), and p-ERK (catalog number sc-7383) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies to eIF2α (catalog number 9722) and p-eIF2α at Ser-51 (catalog number 9721) were purchased from Cell Signaling (Danvers, MA).
Cell Culture—HepG2 human hepatoma cells were cultured in modified Eagle's minimal essential medium (MEM), pH 7.4 (Mediatech, Herndon, VA), supplemented with 1× nonessential amino acids, 2 mm glutamine, 100 μg/ml streptomycin sulfate, 100 units/ml penicillin G, 0.25 μg/ml amphotericin B, and 10% (v/v) fetal bovine serum (FBS) and maintained at 37 °C in an atmosphere of 5% CO2 and 95% air. Mouse embryonic fibroblasts (MEFs) from wild type and GCN2 knock-out mice that had been immortalized by SV40 were generously supplied by Drs. Heather Harding and David Ron (24). MEF cells isolated from C57B16 mice expressing the wild type eIF2α (S/S) or a knock-in cell line expressing the S51A mutation (eIF2α-A/A) that had been immortalized spontaneously (25). The MEF cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with glutamine (Mediatech), supplemented with 1× nonessential amino acids, 10% fetal bovine serum, 100 μg/ml streptomycin sulfate, 100 units/ml penicillin G, and 0.25 μg/ml amphotericin B. At 12 h prior to initiating all treatments, cells were given fresh medium and serum to ensure that no nutrient deprivation took place prior to the start of experimental incubations. For amino acid deprivation in the presence of kinase inhibitors (50 μm PD98059 (MEK), 10 μm U0126 (MEK), 10 μm SB203580 (P38), or 20 μm SB600125 (JNK)), cells were pretreated with the specified inhibitor in complete MEM conditions for 1 h and then incubated with the inhibitor in complete MEM or MEM minus histidine, each containing 10% dialyzed fetal bovine serum.
Steady State mRNA Analysis—Total mRNA was isolated using the Qiagen RNeasy kit with on-column DNase treatment to eliminate any trace amounts of DNA (Qiagen, Valencia, CA). To measure the steady state ATF4, SNAT2, or GADD34 mRNA, quantitative real time PCR was performed using the DNA Engine Opticon 2 system (Bio-Rad) and SYBR Green chemistry. RNA reaction mixtures were incubated at 48 °C for 30 min followed by 95 °C for 15 min and amplification of 35 cycles at 95 °C for 15 s and then 60 °C for 60 s. To establish that a single product was amplified during the reaction, melting curves were generated for each reaction by a stepwise increase of the temperature from 55 to 95 °C, and measurements were taken at every degree change. Reactions were also run without reverse transcriptase to ensure that there was no DNA amplification. The primers used for ATF4 mRNA amplification were as follows: sense primer, 5′-TGAAGGAGTTCGACTTGGATGCC-3′; antisense primer, 5′-CAGAAGGTCATCTGGCATGGTTTC-3′. The primers used for GADD34 mRNA amplification were as follows: sense primer, 5′-CACTCCTGCTACAGGTGTCT-3′; antisense primer, 5′-TCGGCTGATCCTGTATCA-3′. The primers used to assay steady state SNAT2 mRNA or to measure transcription activity from the SNAT2 gene by assaying hnRNA amplification were reported previously (14). The primers used to measure ERK1 steady state mRNA were as follows: sense primer, 5′-GCGCTACACGCAGTTGCAGTACA-3′; antisense primer, 5′-TGATGGCCACGCGAGTCTTG-3′. For ERK2, the primers were as follows: sense primer, 5′-CGCCGAAGCACCATTCAAGTT-3′ and antisense primer, 5′-TCCTGGCTGGAATCTAGCAGTCTCT-3′.
Protein Isolation and Immunoblotting—Whole cell protein extracts were isolated in sample dilution buffer (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromphenol blue, 5% 2-mercaptoethanol), and protein concentration was determined using a modified Lowry method (26). For immunoblotting, 30 μg of protein/lane was loaded onto Bio-Rad Precast Tris-HCl polyacrylamide gels (Bio-Rad) and then electrotransferred onto polyvinylidene difluoride membranes. Membranes were stained with Fast Green to ensure equal loading and then blocked with 5% blocking solution (5% (w/v) Carnation nonfat dry milk, 30 mm Tris-base, pH 7.5, 0.1% (v/v) Tween 20, and 200 mm NaCl) for 1 h at room temperature. Each primary antibody was used at a dilution between 1:500 and 1:1000 (v/v) in 5% blocking solution and incubated with membranes overnight at 4 °C or for 4 h at room temperature. The blots were washed five times for 5 min each in 1% blocking solution and then incubated with the appropriate secondary antibody at a 1:5,000 to 1:20,000 dilution (v/v) for 1 h at room temperature. The blots were then washed for 5 × 5 min in 1% blocking solution and 2 × 5 min in freshly made TBS/Tween (30 mm Tris-base, 0.1% Tween 20, and 200 mm NaCl, pH 7.5). The bound secondary antibody was detected using an enhanced chemiluminescence kit (Amersham Biosciences) and exposing the blot to Biomax MR film (Eastman Kodak Co.).
DNA Affinity Purification Assay—HepG2 nuclear extracts were isolated as described previously (27). Annealing complementary 5′-biotinylated DNA strands at a concentration of 1 μg/μl generated a 29-bp double-stranded DNA probe (5′-GACGATCGATATTGCATCAGTTTTCTTTC-3′) containing an AARE (underlined). PBS was used to wash a 4% slurry of streptavidin-agarose beads (Sigma), and then 500 μg of HepG2 nuclear extract plus 5 μg of biotinylated probe was added to the bead slurry. The total volume was adjusted to 500 μl with PBS containing protease and phosphatase inhibitors (27) and incubated for 1 h at room temperature. The beads were then washed four times for 5 min each with cold PBS, resuspended in 100 μl of sample buffer, and then boiled for 5 min to elute the bound protein. A 25-50-μl aliquot was loaded onto Tris-HCl polyacrylamide gels (Bio-Rad) and then immunoblotted according to the protocol above.
Transient Transfection and Luciferase Assays—HepG2 cells were seeded in 24-well plates at a density of 1.5 × 105 cells/well and grown for 24 h prior to transfection. As described previously (14), Superfect (Qiagen) was used to transfect cells with either 1 μg of pGL3 Firefly luciferase reporter plasmid encoding the AARE-containing SNAT2 genomic region (nucleotides -512 to +770) alone or in combination with 100 ng of pcDNA3.1 plasmid containing the cDNA for ATF4 driven by the cytomegalovirus promoter. The ATF4 cDNA lacks the upstream open reading frame that renders the native mRNA subject to translational control. Consequently, the ATF4 protein is produced constitutively from this construct. For all wells, total DNA concentration was kept constant at 1.1 μg by adding empty pcDNA3.1 plasmid to the transfection complex. Cells were transfected for 3 h, given fresh MEM for ∼18 h, and incubated for 8 h in MEM, MEM plus PD98059, MEM minus histidine, or MEM minus histidine plus PD98059. Firefly luciferase activities were measured using the luciferase reporter assay system (Promega, Madison, WI). Total protein, measured for each well, was used to normalize data between replicate wells. The data are presented as the averages ± S.D. of at least four determinations.
To investigate the role of MEK activity on ATF4 translation per se, HepG2 cells were transiently transfected, using the Superfect protocol described above, with 1 μg of either a wild type ATF4-luciferase fusion construct or a uORF1 and uORF2 doubly mutated ATF4-luciferase fusion construct, both created by the laboratory of Dr. Ron Wek (10). Cells were also transfected with 0.5 ng of an SV40 promoter-driven Renilla construct as a transfection control. Cells were incubated in either control or histidine-depleted medium with or without 50 μm PD98059 for 4 h, and then Firefly and Renilla luciferase activities were measured using the dual luciferase reporter assay system (Promega, Madison, WI).
To test whether or not MEK or ERK modulated eIF2α phosphorylation on Ser-51, human embryonic kidney 293T cells were transiently transfected, using calcium phosphate (14), with cDNA constructs containing the coding regions for a constitutively active MEK, wild type eIF2α, or a mutant eIF2α lacking the serine 51 phosphorylation site (S51A). At 36 h after transfection, whole cell extracts were immunoblotted for total eIF2α protein, p-eIF2α, or actin.
Short Interfering RNA (siRNA) Transfection—The human PPP1R15A (GADD34) siRNA (catalog number D-004442-02), siControl nontargeting siRNA (catalog number D-001210-02), and DharmaFECT 4 transfection reagent were purchased from Dharmacon, Inc. (Lafayette, CO). HepG2 cells were seeded in 12-well plates at a density of 2.5 × 105 cells/well in MEM and grown for 16 h. Transfection was performed according to the instructions of Dharmacon using 3 μl of DharmaFECT-4 and a 100 nm per well final siRNA concentration. HepG2 cells were treated with transfection reagent for 24 h and then rinsed with PBS, given fresh MEM, and cultured for another 24 h. The medium was then removed and replaced with control MEM or MEM lacking histidine, with or without 50 μm PD98059. Total RNA and protein extracts were isolated at specific times and analyzed by reverse transcription-PCR or immunoblotting, respectively.
RESULTS
Inhibition of MEK Activation Blocks the Induction of an AARE-containing Gene and the in Vitro Binding of ATF4 to an AARE—Franchi-Gazzola et al. (21) showed that the increased System A amino acid transport activity that occurs following amino acid deprivation is blocked by inhibition of MEK activation using the inhibitor PD98059. Subsequently, amino acid-regulated System A activity was established to result from increased transcription from the SNAT2 gene (22, 23). Our laboratory has documented previously that histidine deprivation of HepG2 cells causes increased ERK1/2 phosphorylation, which is completely blocked by PD98059 (28). Consistent with these observations, when HepG2 cells were deprived for histidine in the presence of PD98059 (labeled -MEK), the increase in SNAT2 mRNA content was completely blocked (Fig. 1A). An inhibitory effect of PD98059 was also observed for transcriptional induction of another amino acid-regulated gene, asparagine synthetase (data not shown).
FIGURE 1.
Inhibition of MEK blocks the induction of an AARE-containing gene and the in vitro binding of ATF4 to an AARE. HepG2 cells were incubated for 8 h in either complete MEM or MEM lacking histidine (MEM - His) in the presence (-MEK) or absence (+MEK) of the MEK inhibitor PD98059 (50 μm). A, total RNA was used to measure SNAT2 transcription activity (heterogeneous nuclear RNA) and steady-state mRNA content. The results represent three independent experiments, each measured in duplicate, and are depicted as the means ± S.E. B, nuclear extracts were isolated from HepG2 cells incubated with or without PD98059 and histidine as described above. DAPA analysis was used to monitor ATF4 binding to an AARE consensus sequence as described under “Materials and Methods.” The autoradiographic film shown is representative of several separate experiments using independently prepared nuclear extracts.
The induction of transcription from these AARE-containing genes following amino acid limitation coincides with the increase in ATF4 protein production (29). Furthermore, overexpression of ATF4 increases transcription from a SNAT2-driven reporter gene, and by both in vivo and in vitro techniques, it has been documented that ATF4 binds to the AARE within intron I of the SNAT2 gene (14). Consequently, the effect of MEK inhibition on nuclear ATF4 binding activity was monitored (Fig. 1B). A DNA affinity purification assay was performed using an AARE-containing DNA probe that was incubated with nuclear extracts from HepG2 cells that had been incubated for 8 h in either complete MEM or MEM lacking histidine. The amount of ATF4 binding activity was considerably greater in the extracts from amino acid-depleted cells compared with those from MEM-incubated control cells (Fig. 1B). However, when the histidine-deprived cells were simultaneously treated with the MEK inhibitor, the increase in nuclear ATF4 binding activity was completely blocked.
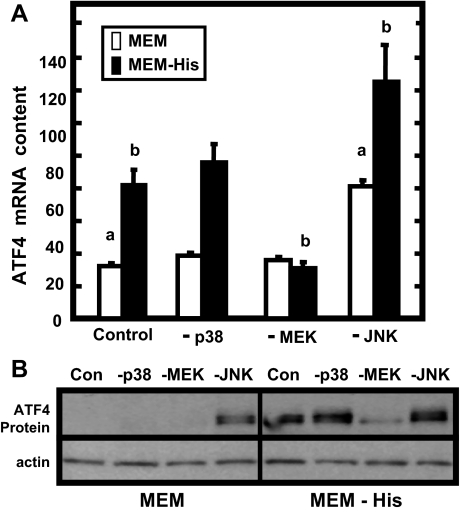
The Effect of MAP Kinase Inhibitors on ATF4 mRNA and Protein Levels during Amino Acid Limitation—In addition to enhanced ATF4 translation, there is also an increase in ATF4 mRNA content following amino acid deprivation (30). Therefore, to investigate the effect of MEK activity on ATF4 mRNA and protein expression during histidine deprivation, HepG2 cells were treated with PD98059 (-MEK), as well as with selective inhibitors for either p38 (-p38) or JNK (-JNK) (Fig. 2). The p38 and JNK inhibitors did not prevent the induction of ATF4 mRNA levels, although blocking JNK activity actually elevated the basal levels of ATF4 mRNA (Fig. 2A). In contrast, the inhibitor of MEK/ERK signaling, PD98059, completely prevented the increase in ATF4 mRNA. As discussed above, although transcription from the ATF4 gene is increased, the primary mode of increasing ATF4 protein expression following amino acid deprivation is enhanced translation of preexisting ATF4 mRNA (10, 11), so the increase in ATF4 protein content was also measured in the presence of the MAPK inhibitors (Fig. 2B). The p38 inhibitor had no effect on the amino acid deprivation-induced increase in ATF4 protein levels, but once again, inhibition of MEK blocked the enhancement. Interestingly, inhibition of JNK caused an enhanced ATF4 level, even in the basal (complete MEM) condition.
FIGURE 2.
Effect of MAPK inhibitors on ATF4 mRNA and protein levels during amino acid limitation. HepG2 cells were incubated for 8 h in either amino acid complete MEM or MEM lacking histidine (MEM - His) in the absence (Control) or the presence of a MAPK inhibitor (-p38, SB203580; -MEK, PD98059; -JNK, SP600125). Total RNA or whole cell extracts were isolated and probed for ATF4 mRNA (A) or protein (B) content, respectively. The quantitative PCR ATF4 mRNA data represent three independent experiments, each measured in duplicate, from which the results are depicted as the means ± S.E. Those values marked with a letter (a, MEM; b, MEM - His) are significantly different (p < 0.05) from the corresponding medium lacking inhibitor (Control). The immunoblot shown in B is representative of multiple experiments.
De novo synthesis results in a detectable increase in HepG2 ATF4 protein levels after 1-2 h of histidine deprivation and a peak of accumulation around 4 h (29). Given that the initial MAPK pathway screening shown in Fig. 2 was conducted at a single treatment time of 8 h, a time course of ATF4 synthesis with or without PD98059 treatment was investigated (Fig. 3A). As expected, the ATF4 content of control cells increased at 2 h after histidine withdrawal, with a maximum level attained by 4 h. However, the cells treated with PD98059 (-MEK) showed a complete block of ATF4 protein accumulation at all time points. As mentioned above, the translation of preexisting ATF4 mRNA is enhanced following amino acid limitation, because increased phosphorylation of eIF2α favors translation initiation at the ATF4 coding sequence rather than at an out-of-frame uORF (10, 11). To determine if the translation of ATF4 was directly blocked by inhibition of MEK activity, cells were transfected with an ATF4-Firefly luciferase construct containing the wild type ATF4 uORFs or mutations within the uORFs so as to prevent the regulated translation (10). As expected, the translation of the ATF4-luciferase fusion was enhanced after histidine deprivation, and this synthesis was blocked by PD98059 (Fig. 3B). Mutation of the uORF sequences such that their translation was prevented resulted in elevated, but unregulated, basal ATF4 synthesis and loss of an effect by PD98059. The lack of a negative effect on Renilla activity (data not shown) and on the unregulated ATF4 synthesis argues that PD98059 does not inhibit global translation. Thus, MEK kinase activity is required for both the transcriptional and the eIF2α-regulated translational mechanisms that contribute to increased ATF4 production following amino acid limitation.
FIGURE 3.
Inhibition of MEK blocks the time-dependent induction of ATF4 protein synthesis following amino acid deprivation. For A, HepG2 cells were transferred to either amino acid complete MEM or MEM lacking histidine (MEM - His) for 2, 4, or 8 h. For each condition, the cells were incubated with (-MEK) or without (Control) 50 μm PD98059, which inhibits MEK activation. Whole cell protein extracts were collected and subjected to immunoblotting with antibodies specific for ATF4. Although the top panel shows a single experiment, densitometric quantification of the ATF4 protein bands from at least three independent immunoblot experiments was performed to generate the bar graph. In B, HepG2 cells were co-transfected with a Renilla reporter gene driven by SV40 and a 5′ portion of the ATF4 mRNA sequence containing either wild type or mutated uORFs fused to the Firefly luciferase reporter gene (10). At 18 h after transfection, the cells were incubated for 4 h in complete MEM or MEM lacking histidine (-His) in the presence (-MEK) or absence (Control) of 50 μm PD98059. Cell lysates were collected, and luciferase activity (ATF4-Luciferase Expression) was measured. Data are expressed as the ratio of Firefly to Renilla luciferase activity.
ATF4 Overexpression Rescues the Inhibition of AARE-driven Transcription by MEK Blockade—It is possible that inhibition of MEK is blocking SNAT2 transcription (Fig. 1) independently of its negative effect on ATF4 protein production. If neither MEK inhibition nor PD98059 have secondary effects, inhibition of SNAT2 transcription should be rescued by expression of exogenous ATF4 protein. To investigate this possibility, a Firefly luciferase reporter gene driven by an AARE-containing SNAT2 genomic fragment was co-transfected into HepG2 cells along with an unregulated ATF4 expression plasmid. The ATF4 construct lacked the upstream regulatory open reading frame within the native mRNA such that constitutively high levels of ATF4 are produced. Following transfection, the cells were incubated in amino acid complete or histidine-deficient medium, with or without the presence of the MEK activation inhibitor (Fig. 4). In the absence of exogenous ATF4, the SNAT2 AARE mediated an approximately 7-fold increase in luciferase activity under conditions of amino acid limitation, and the majority of this increase was blocked when cells were treated with PD98059 (Fig. 4, left, Vector only), similar to the data for the endogenous SNAT2 gene shown in Fig. 1. In contrast, when the SNAT2-driven reporter was co-expressed with an expression plasmid for ATF4 (Fig. 4, right, ATF4 transfected), transcription was induced 80-fold (complete MEM) to 100-fold (MEM - His), but the presence of the MEK inhibitor had little or no effect. These data demonstrate that the loss of transcriptional activation following inhibition of MEK activity can be rescued by supplying exogenous ATF4.
FIGURE 4.
ATF4 overexpression rescues the inhibition of AARE-driven transcription by MEK blockade. HepG2 cells were transfected with a Firefly luciferase reporter gene driven by a SNAT2 genomic fragment containing the promoter and the intronic AARE (nucleotides -512/+770). One-half of the cells were co-transfected with an expression plasmid for ATF4 (ATF4 transfected; right), and the remainder received empty pcDNA3.1 plasmid (Vector only; left). At 18 h after transfection, the cells were incubated for 12 h in complete MEM or MEM lacking histidine (-His) in the presence (-MEK) or absence (Control) of 50 μm PD98059. Cell lysates were collected, and SNAT2 AARE-driven luciferase activity was measured. The data shown in both panels are depicted as the -fold change relative to the value (marked 1.0 in the left panel) obtained for the control cells (i.e. no PD98059) that were incubated in complete MEM and that were transfected with empty pcDNA3.1 plasmid (Vector only). Note the difference in the relative units of luciferase activity for those cells co-transfected with the ATF4 expression plasmid (right panel).
Effect of MEK Inhibition on Amino Acid-regulated Phosphorylation of eIF2α—Given that PD98059 blocked production of ATF4 protein, it was plausible that MEK activity is also required for the AAR pathway upstream step of eIF2α phosphorylation. Cell extracts from HepG2 cells incubated for 0-8 h in either complete MEM or MEM minus histidine, with (-MEK) or without (+MEK) PD98059, were immunoblotted for total eIF2α or p-eIF2α (Fig. 5A). As expected, during amino acid limitation, there was an increase in the phosphorylation of eIF2α at 2 h that reached a level of 3-4-fold between 4 and 8 h. However, when the MEK activation was blocked, phosphorylation of eIF2α was inhibited (Fig. 5A). To determine if these effects were specific for MEK, similar studies were performed in the presence of a second inhibitor of MEK activation, U0126, which also resulted in a decrease in the stress-induced phosphorylation of eIF2α and the subsequent synthesis of ATF4 (Fig. 5B). These data document that regulated ATF4 mRNA translation requires the action of MEK at a step upstream of eIF2α phosphorylation.
FIGURE 5.
Effect of MEK inhibition on amino acid-regulated phosphorylation of eIF2α. In A, HepG2 cells were incubated in amino acid complete MEM or MEM lacking histidine (MEM - His) for 2, 4, or 8 h, in the presence (-MEK) or absence (+MEK) of 50 μm PD98059. At the times indicated, whole cell protein extracts were collected and subjected to immunoblotting with antibodies specific for p-eIF2α, total eIF2α, p-ERK, or total ERK. The p-eIF2α data from three independent experiments were quantified by densitometry to generate the bar graph. For B, HepG2 cells were incubated in MEM or MEM - His for 4 h in the presence or absence of 10 μm U0126. Whole cell protein extracts were collected and subjected to immunoblotting with antibodies specific for either p-eIF2α, total eIF2α, ATF4, p-ERK, or total ERK.
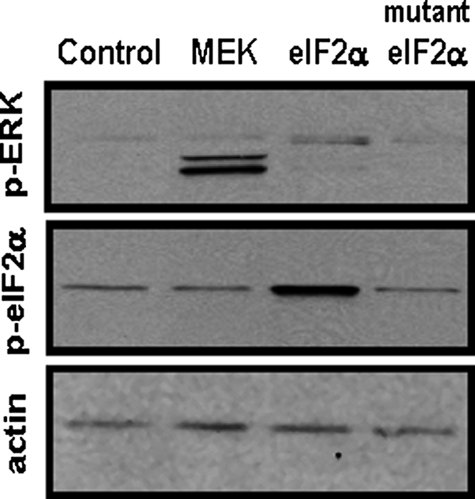
Neither MEK nor ERK Acts Directly to Phosphorylate eIF2α on Ser-51—To determine if activated MEK or its downstream target ERK contribute to the enhancement of phosphorylation of eIF2α on Ser-51, a constitutively active form of MEK was overexpressed in 293T cells (Fig. 6). As a control, eIF2α wild type or a mutated eIF2α form lacking serine 51 (S51A) was also expressed. Immunoblotting whole cell extracts for p-ERK1/2 documented that overexpression of active MEK caused activation of ERK1/2. When the same extracts were tested for the phosphorylation status of eIF2α, the expression of MEK had no stimulatory effect (Fig. 6). These data suggest but do not prove that, independent of GCN2 activation, neither MEK nor ERK directly phosphorylates eIF2α on Ser-51. The data do not rule out the possibility that MEK, ERK, or a downstream effector of ERK phosphorylates eIF2α at a site other than Ser-51. Consistent with these results, computer analysis of the eIF2α protein sequence does not reveal any MAPK kinase consensus sites.
FIGURE 6.
Neither MEK2 nor ERK1/2 act directly to phosphorylate eIF2α on Ser-51. Human embryonic kidney 293T cells were transiently transfected with expression plasmids encoding constitutively active MEK2, wild-type eIF2α, or mutant eIF2α lacking the Ser-51 phosphorylation site (S51A). Whole-cell extracts were prepared 36 h after transfection and subjected to immunoblotting for p-ERK, p-eIF2α, or actin.
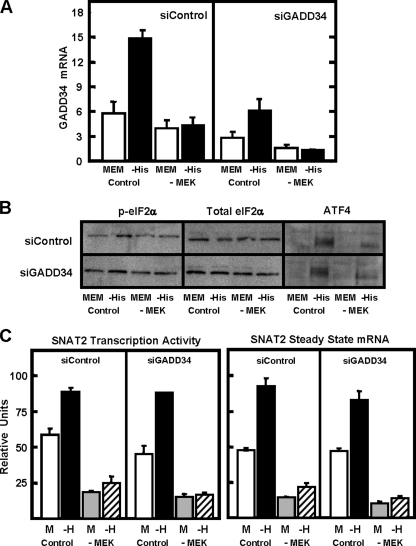
Does Activated MEK/ERK Promote eIF2α Phosphorylation or Inhibit the eIF2α Phosphatase?—Amino acid limitation activates the eIF2α kinase GCN2, but as part of a negative feedback loop, a phosphatase activity, mediated by a complex composed of protein phosphatase 1 and GADD34 (PP1-GADD34), is induced as the result of de novo GADD34 synthesis (7, 9). The PP1 phosphatase is targeted to p-eIF2α because of the association with GADD34 (8). It was possible that the dependence of enhanced eIF2α phosphorylation on MEK/ERK was due to MEK-dependent inhibition of the PP1-GADD34 activity, either by blocking GADD34 transcription or by inhibiting the phosphatase complex. To test the first possibility, the effect of blockade of MEK activation on GADD34 mRNA was analyzed to determine if it caused an increase in GADD34 mRNA content (Fig. 7A, siControl panel). As expected, histidine deprivation caused an increase in GADD34 mRNA, but this induction was prevented when MEK activation was blocked. These data are consistent with the requirement of MEK for ATF4 synthesis, given that ATF4 is probably responsible for the induction of the GADD34 gene. However, the results indicate that MEK/ERK do not act to enhance eIF2α phosphorylation by decreasing GADD34 synthesis; otherwise, an increase in GADD34 mRNA would have been observed after PD98059 treatment. To test whether or not MEK promotes p-eIF2α levels by inhibiting the activity of the PP1-GADD34 complex, HepG2 cells were treated with an siRNA against GADD34, and the AAR pathway was monitored at several levels, including phosphorylation of eIF2α (Fig. 7B), ATF4 production (Fig. 7B), and SNAT2 transcription (Fig. 7C). As a positive control, these experiments included parallel incubations with PD98059. The siRNA blockade of the increase in steady state GADD34 mRNA following amino acid limitation was confirmed, and the lack of MEK activation further reduced GADD34 expression (Fig. 7A, siGADD34 panel). Knockdown of GADD34 slightly raised the basal level of p-eIF2α content, but the increased eIF2α phosphorylation (Fig. 7B), ATF4 synthesis (Fig. 7B), and SNAT2 transcription (Fig. 7C) were still evident after activation of the AAR pathway, despite the lack of GADD34 expression (compare Control MEM versus -His in each figure). Furthermore, even in the absence of GADD34, inhibition of MEK largely prevented the AAR pathway.
FIGURE 7.
Knockdown of the GADD34 protein by siRNA does not restore eIF2α phosphorylation or SNAT2 induction in the absence of active MEK. HepG2 cells were treated with either a control siRNA (siControl) or a siRNA specific for GADD34 (siGADD34) for 24 h followed by incubation in complete MEM for 24 h. The cells were then incubated for 4 h in complete MEM (MEM) or MEM lacking histidine (-His) in the presence (-MEK) or absence (Control) of 50 μm PD98059. For A, total RNA was isolated and probed for steady state GADD34 mRNA. For B, whole cell protein extracts were collected and subjected to immunoblotting with antibodies specific for either p-eIF2α, total eIF2α, or ATF4. For C, the total RNA was used to measure SNAT2 transcription activity and steady state mRNA. All of the quantitative reverse transcription-PCR mRNA data represent three independent experiments, each measured in duplicate, from which the results are depicted as the means ± S.E.
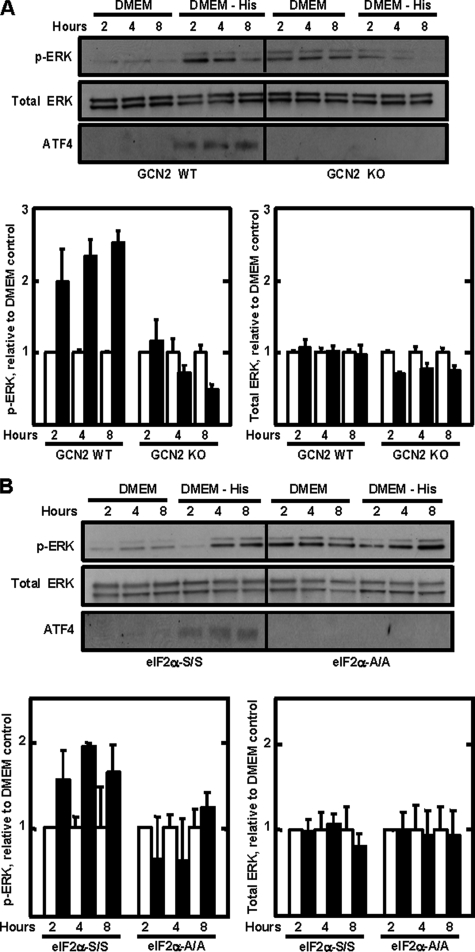
Activation of the MEK/ERK Pathway Is Dependent on GCN2 and p-eIF2α—Collectively, the data indicate that the function of MEK/ERK in promoting eIF2α phosphorylation is to permit or even enhance the GCN2-mediated phosphorylation rather than through inhibition of PP1-GADD34-dependent dephosphorylation. However, whether or not the AAR pathway is the mechanism by which MEK/ERK signaling is activated is unknown. To investigate whether or not either ERK1 or ERK2 was an amino acid-responsive gene, steady state mRNA expression was measured after histidine deprivation, but neither gene's mRNA content was increased (data not shown). These data, consistent with the lack of change in total ERK protein (Fig. 5B), suggest that the increased p-ERK following amino acid deprivation was upstream of ATF4 action. Consequently, MEF cells were used to determine if the activation of ERK was dependent on GCN2 function or eIF2α phosphorylation. Wild type MEFs were compared with MEFs from GCN2 knock-out mice or mice containing an eIF2α knock-in that expresses alanine instead of serine at position 51 (eIF2α-A/A). The cells were subjected to histidine deprivation for 2-8 h, and then ERK phosphorylation and ATF4 production were measured (Fig. 8). As expected, increased ATF4 synthesis did not occur in cells either lacking GCN2 expression (Fig. 8A) or containing the eIF2-A/A mutation (Fig. 8B). Histidine deprivation resulted in the expected increase in p-ERK levels in the GCN2 wild type and eIF2α-S/S MEF cells. However, increased phosphorylation of ERK was not observed in the cells deficient for GCN2 (Fig. 8A). Although the basal level of p-ERK was higher in the cells expressing the eIF2α-A/A mutant, after quantification of three independent experiments and normalization to total ERK levels, there was no increase in the level of phosphorylation after amino acid depletion (Fig. 8B).
FIGURE 8.
ERK phosphorylation by amino acid starvation requires GCN2 and serine 51 phospho-eIF2α. A, MEF cells from gcn2+/+ (GCN2 WT) and gcn2-/- (GCN2 KO) mice were incubated in amino acid complete DMEM (DMEM) or DMEM lacking histidine (DMEM - His) for 2, 4, or 8 h. For the data in B, the same incubations were performed for eif2α-S/S (wild type) or eif2α-A/A MEF cells. Total protein was isolated and immunoblotted for p-ERK, total ERK, or ATF4 protein. The immunoblots shown are a single experiment, whereas the quantification below represents the average ± S.D. for three independent experiments using different batches of cells.
DISCUSSION
The results from this study are the first to demonstrate that phosphorylation of eIF2α by the amino acid-sensing kinase GCN2 requires MEK activity. As a consequence, the de novo synthesis of ATF4 and its subsequent transcription program is also dependent on activation of the MEK/ERK signaling MAPK pathway. It was also confirmed that amino acid deprivation activates MEK activity, as judged by ERK phosphorylation. The results suggest that the site of MEK action is to permit and/or enhance GCN2-mediated eIF2α phosphorylation, not PP1-GADD34-mediated dephosphorylation. Thus, as depicted in Fig. 9, the data illustrate an interdependence between two major stress-activated pathways within the cell. Specifically, the following novel observations are reported. 1) The activation of MEK/ERK signaling by amino acid limitation was dependent on GCN2 and phosphorylation of eIF2α. 2) Of the three major MAPK pathways tested, only the MEK/ERK pathway was essential for activation of eIF2α-ATF4 signaling in HepG2 cells. 3) Following activation of the AAR by amino acid limitation, enhanced phosphorylation of eIF2α was dependent on MEK activation. 4) Consistent with the link between MEK activity and eIF2α phosphorylation, the stress-induced increase in ATF4 protein content and nuclear DNA binding activity was dependent on increased MEK activity. 5) Reporter assays illustrated that AARE-driven transcription was also dependent on MEK activity and that PD98059-induced inhibition of transcription could be rescued by expression of exogenous ATF4. 6) Knockdown of GADD34 expression did not prevent the dependence of eIF2α phosphorylation on MEK, arguing against a MEK/ERK action on the PP1-GADD34 phosphatase.
FIGURE 9.
There is an interdependence between the MEK-ERK signaling pathway and the amino acid response pathway. The diagram depicts activation of the MEK-ERK pathway by amino acid limitation and the function of the MEK/ERK pathway to permit or enhance the GCN2-dependent phosphorylation of eIF2α.
Although it remains possible that sensors other than GCN2 exist to detect amino acid limitation, the present studies involving GCN2-deficient MEF cells show that the activation of MEK/ERK occurs downstream of and is completely dependent on GCN2. How GCN2 triggers MAPK signaling remains to be established. A number of previous studies have shown that amino acid limitation causes increased MEK-dependent ERK phosphorylation. Franchi-Gazzola et al. (21) demonstrated that medium completely devoid of amino acids caused an increase in ERK phosphorylation. Codogno and co-workers (18, 19) have documented that incubating cells in amino acid-free medium induces autophagy by activating ERK1/2 via a Raf-1-dependent mechanism. Sharp et al. (31) showed that elevated levels of ERK and eIF2α are co-localized in a group of specific neurons, which were thought to be involved in sensing dietary amino acids. Depletion of the culture medium for the single amino acid histidine, as in the present studies, causes an increase in ERK phosphorylation, which was necessary for a parallel increase in p21 expression (28). However, there is only one previous report showing a functional dependence between the MEK/ERK pathway and eIF2α phosphorylation, but in that instance, described in alveolar macrophages, the relationship was opposite to that observed in the present studies. Alveolar macrophages exhibit an unusually high level of basal ERK activity, and inhibition of that activity by the MEK inhibitor U0126 caused an increase in the phosphorylation state of eIF2α via a cascade involving suppression of JNK-mediated inhibition of PP1 activity (20). In the HepG2 hepatoma cells studied here, inhibition of the MEK/ERK pathway blocked the AAR-induced eIF2α phosphorylation, suggesting that the relationship between the MEK and eIF2α pathways may be cell-specific. However, Monick et al. (20) also showed that JNK inhibits PP1 activity, which in turn, leads to an increase in phosphorylated eIF2α, because PP1 mediates eIF2α dephosphorylation (7, 9). That observation is consistent with the present data showing that inhibition of JNK caused an increase in ATF4 production, even in amino acid-replete cells. This result suggests that in both the HepG2 hepatoma cells and the alveolar macrophages, JNK can modulate the degree of eIF2α phosphorylation by inhibition of PP1 activity.
Increased phosphorylation of eIF2α is the result of a balance between one or more of the stress-induced eIF2 kinases, GCN2 included, and the rate of dephosphorylation. It is known that these stress pathways induce the synthesis of GADD34 (7, 9) and that GADD34 is responsible for targeting the PP1 to the endoplasmic reticulum and eIF2α (8). The present observations document that the increase in eIF2α phosphorylation after amino acid deprivation is still dependent on MEK activation in cells with reduced GADD34 expression. This result argues that the MEK/ERK pathway acts to enhance the phosphorylation of eIF2α by GCN2 rather than suppressing the dephosphorylation. Despite repeated attempts, the phosphorylation of GCN2 with and without PD98059 treatment could not be documented by immunoblotting or immunoprecipitation because of the poor quality of the commercially available phospho-GCN2 antibody. Therefore, we were unable to establish whether the action of MEK is upstream or downstream of GCN2.
Deprivation of eukaryotic cells for a single amino acid triggers a complex response that extends beyond the pathways immediately associated with that particular amino acid. For example, deprivation of Saccharomyces cerevisiae for histidine results in altered expression of hundreds of genes encompassing not only amino acid metabolism but also genes involved in vitamin metabolism, glycogen homeostasis, peroxisomes, and mitochondrial transport (32). Microarray analysis of ATF4-deficient mouse embryonic fibroblasts has also revealed a wide range of affected genes (12). In mammalian cells, several independent eIF2α kinases have been identified (33), and this and other observations have led Ron and co-workers (12, 34) to coin the term “integrated stress response,” which underscores the convergence of several stress-activated kinases on p-eIF2α/ATF4 signaling. Although the present data document that the MAPK pathway containing MEK is only necessary for the GCN2 eIF2α kinase, it is possible that one or more of the MAPK pathways may also be required for action by other eIF2α kinases. Consistent with this hypothesis, while this report was in preparation, Zykova et al. (35) published data showing that UV irradiation leads to PKR-mediated phosphorylation of eIF2α in an ERK-dependent mechanism. Liang et al. (36) reported that thapsigargin, but not tunicamycin, initiated a PERK-dependent activation of the JNK and p38 MAPKs. Future studies will provide further evidence to determine which array of cellular stress signals, ultimately transduced through eIF2α phosphorylation, are also dependent on co-activation of the MAPK pathways.
Acknowledgments
We thank other members of the Kilberg laboratory for technical advice, reagents, and helpful discussion. We appreciate the ATF4-luciferase expression plasmids from Dr. Ron Wek (Indiana University), the MEK expression plasmid provided by Dr. Xingming Deng (University of Florida), the eIF2α wild type and S51A expression plasmids provided by Dr. Maria Hatzoglou (Case Western Reserve University), and the GCN2 wild type and deficient cells from Drs. Heather Harding and David Ron (New York University).
This work was supported by National Institutes of Health Grants DK-52064 and DK70647 (to M. S. K.) and DK-42394 and HL-52173 (to R. J. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: AAR, amino acid response; AARE, amino acid response element; p-eIF2α, eIF2α phosphorylated at Ser-51; ERK, extracellular signal-regulated kinase; p-ERK, phosphorylated ERK; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; PP1, protein phosphatase 1; uORF, upstream open reading frame; MEM, minimal essential medium; DMEM, Dulbecco's modified Eagle's medium; MEF, mouse embryo fibroblast; siRNA, short interfering RNA.
References
- 1.Straus, D. S., Burke, E. J., and Marten, N. W. (1993) Endocrinology 132 1090-1100 [DOI] [PubMed] [Google Scholar]
- 2.Endo, Y., Fu, Z., Abe, K., Arai, S., and Kato, H. (2002) J. Nutr. 132 3632-3637 [DOI] [PubMed] [Google Scholar]
- 3.Hao, S., Sharp, J. W., Ross-Inta, C. M., McDaniel, B. J., Anthony, T. G., Wek, R. C., Cavener, D. R., McGrath, B. C., Rudell, J. B., Koehnle, T. J., and Gietzen, D. W. (2005) Science 307 1776-1778 [DOI] [PubMed] [Google Scholar]
- 4.Maurin, A. C., Jousse, C., Averous, J., Parry, L., Bruhat, A., Cherasse, Y., Zeng, H., Zhang, Y., Harding, H. P., Ron, D., and Fafournoux, P. (2005) Cell Metab. 1 273-277 [DOI] [PubMed] [Google Scholar]
- 5.Kilberg, M. S., Pan, Y. X., Chen, H., and Leung-Pineda, V. (2005) Annu. Rev. Nutr. 25 59-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinnebusch, A. G. (1997) J. Biol. Chem. 272 21661-21664 [DOI] [PubMed] [Google Scholar]
- 7.Novoa, I., Zeng, H., Harding, H. P., and Ron, D. (2001) J. Cell Biol. 153 1011-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brush, M. H., Weiser, D. C., and Shenolikar, S. (2003) Mol. Cell. Biol. 23 1292-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novoa, I., Zhang, Y., Zeng, H., Jungreis, R., Harding, H. P., and Ron, D. (2003) EMBO J. 22 1180-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vattem, K. M., and Wek, R. C. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 11269-11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, P. D., Harding, H. P., and Ron, D. (2004) J. Cell Biol. 167 27-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding, H. P., Zhang, Y., Zeng, H., Novoa, I., Lu, P. D., Calfon, M., Sadri, N., Yun, C., Popko, B., Paules, R., Stojdl, D. F., Bell, J. C., Hettmann, T., Leiden, J. M., and Ron, D. (2003) Mol. Cell 11 619-633 [DOI] [PubMed] [Google Scholar]
- 13.Palii, S. S., Chen, H., and Kilberg, M. S. (2004) J. Biol. Chem. 279 3463-3471 [DOI] [PubMed] [Google Scholar]
- 14.Palii, S. S., Thiaville, M. M., Pan, Y. X., Zhong, C., and Kilberg, M. S. (2006) Biochem. J. 395 517-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaccioli, F., Huang, C. C., Wang, C., Bevilacqua, E., Franchi-Gazzola, R., Gazzola, G. C., Bussolati, O., Snider, M. D., and Hatzoglou, M. (2006) J. Biol. Chem. 281 17929-17940 [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer, H. J., and Weber, M. J. (1999) Mol. Cell. Biol. 19 2435-2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham, D., Podar, K., Pacher, M., Kubicek, M., Welzel, N., Hemmings, B. A., Dilworth, S. M., Mischak, H., Kolch, W., and Baccarini, M. (2000) J. Biol. Chem. 275 22300-22304 [DOI] [PubMed] [Google Scholar]
- 18.Pattingre, S., Bauvy, C., and Codogno, P. (2003) J. Biol. Chem. 278 16667-16674 [DOI] [PubMed] [Google Scholar]
- 19.Ogier-Denis, E., Pattingre, S., El Benna, J., and Codogno, P. (2000) J. Biol. Chem. 275 39090-39095 [DOI] [PubMed] [Google Scholar]
- 20.Monick, M. M., Powers, L. S., Gross, T. J., Flaherty, D. M., Barrett, C. W., and Hunninghake, G. W. (2006) J. Immunol. 177 1636-1645 [DOI] [PubMed] [Google Scholar]
- 21.Franchi-Gazzola, R., Visigalli, R., Bussolati, O., Dall'Asta, V., and Gazzola, G. C. (1999) J. Biol. Chem. 274 28922-28928 [DOI] [PubMed] [Google Scholar]
- 22.Gazzola, R. F., Sala, R., Bussolati, O., Visigalli, R., Dall'Asta, V., Ganapathy, V., and Gazzola, G. C. (2001) FEBS Lett. 490 11-14 [DOI] [PubMed] [Google Scholar]
- 23.Bain, P. J., LeBlanc-Chaffin, R., Chen, H., Palii, S. S., Leach, K. M., and Kilberg, M. S. (2002) J. Nutr. 132 3023-3029 [DOI] [PubMed] [Google Scholar]
- 24.Harding, H. P., Novoa, I., Zhang, Y., Zeng, H., Wek, R., Schapira, M., and Ron, D. (2000) Mol. Cell 6 1099-1108 [DOI] [PubMed] [Google Scholar]
- 25.Scheuner, D., Song, B., McEwen, E., Liu, C., Laybutt, R., Gillespie, P., Saunders, T., Bonner-Weir, S., and Kaufman, R. J. (2001) Mol. Cell 7 1165-1176 [DOI] [PubMed] [Google Scholar]
- 26.Kilberg, M. S. (1989) Methods Enzymol. 173 564-575 [DOI] [PubMed] [Google Scholar]
- 27.Leung-Pineda, V., and Kilberg, M. S. (2002) J. Biol. Chem. 277 16585-16591 [DOI] [PubMed] [Google Scholar]
- 28.Leung-Pineda, V., Pan, Y., Chen, H., and Kilberg, M. S. (2004) Biochem. J. 379 79-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, H., Pan, Y. X., Dudenhausen, E. E., and Kilberg, M. S. (2004) J. Biol. Chem. 279 50829-50839 [DOI] [PubMed] [Google Scholar]
- 30.Siu, F., Bain, P. J., LeBlanc-Chaffin, R., Chen, H., and Kilberg, M. S. (2002) J. Biol. Chem. 277 24120-24127 [DOI] [PubMed] [Google Scholar]
- 31.Sharp, J. W., Ross-Inta, C. M., Hao, S., Rudell, J. B., and Gietzen, D. W. (2006) J. Comp. Neurol. 494 485-494 [DOI] [PubMed] [Google Scholar]
- 32.Natarajan, K., Meyer, M. R., Jackson, B. M., Slade, D., Roberts, C., Hinnebusch, A. G., and Marton, M. J. (2001) Mol. Cell. Biol. 21 4347-4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroder, M., and Kaufman, R. J. (2005) Annu. Rev. Biochem. 74 739-789 [DOI] [PubMed] [Google Scholar]
- 34.Harding, H. P., Calfon, M., Urano, F., Novoa, I., and Ron, D. (2002) Annu. Rev. Cell Dev. Biol. 18 575-599 [DOI] [PubMed] [Google Scholar]
- 35.Zykova, T., Zhu, F., Zhang, Y., Bode, A. M., and Dong, Z. (2007) Carcinogenesis 28 543-1551 [DOI] [PubMed] [Google Scholar]
- 36.Liang, S. H., Zhang, W., McGrath, B. C., Zhang, P., and Cavener, D. R. (2006) Biochem. J. 393 201-209 [DOI] [PMC free article] [PubMed] [Google Scholar]