Abstract
Sugars evoke a distinctive perceptual quality (“sweetness” in humans) and are generally highly preferred. The neural basis for these phenomena is reviewed for rodents, in which detailed electrophysiological measurements have been made. A receptor has been identified that binds sweeteners and activates G-protein-mediated signaling in taste receptor cells, which leads to changes in neural firing rates in the brain, where perceptions of taste quality, intensity, and palatability are generated. Most cells in gustatory nuclei are broadly-tuned, so quality perception presumably arises from patterns of activity across neural populations. However, some manipulations affect only the most sugar-oriented cells, making it useful to consider them as a distinct neural subtype. Quality perception may also arise partly due to temporal patterns of activity to sugars, especially within sugar-oriented cells that give large but delayed responses. Non-specific gustatory neurons that are excited by both sugars and unpalatable stimuli project to ventral forebrain areas, where neural responses provide a closer match with behavioral preferences. This transition likely involves opposing excitatory and inhibitory influences by different subgroups of gustatory cells. Sweeteners are generally preferred over water, but the strength of this preference can vary across time or between individuals, and higher preferences for sugars are often associated with larger taste-evoked responses.
Keywords: sweet, rat, mouse, gustatory coding, hedonics
Nearly all mammals can respond to sugars by taste. This is not surprising, given that glucose is an essential source of energy, and survival depends on blood glucose concentrations being maintained within narrow limits. Other sugars, such as sucrose and fructose, are useful to animals because they can be converted to glucose, to which these compounds taste similar. Short-chain polysaccharides and starches can also be converted, but a larger amount of energy is required; although the former can induce taste responses directly, they do so using different mechanisms than those activated by sugars (Sclafani, 1991; Sako et al., 1994).
Ingestion of sugars immediately stimulates neural and behavioral responses that are distinct from those evoked by compounds with salty, sour, bitter, and umami tastes. In humans, sugars generate the distinctive taste quality of sweetness. There is no way for rodents to verbalize such perceptions, but the unique reactions that they demonstrate to sugars confirm that these compounds can be considered to have a unique taste quality for them. For example, rats trained to bar-press for sugars do not generalize the behavior to compounds known to taste salty, sour, or bitter to humans (Morrison, 1969), and rodents that are made ill after ingesting sugars avoid a variety of compounds that humans label “sweet”, but not non-sweet compounds (Nowlis et al., 1980; Ninomiya et al., 1984a). Throughout this review, compounds will be considered to taste “sweet” to rodents if they are treated similarly to sucrose in such behavioral tests, with the caveat that such taste quality perceptions must be inferred. The distinctive taste of sugars is useful, in that it provides an immediate signal that a source of readily available calories has been sampled.
After sugars stimulate gustatory transduction mechanisms, the neurons that receive the resulting signals serve several important roles. A specific taste quality perception is generated, which allows sugars to be differentiated from other compounds. Perceptions of taste intensity also occur and allow animals to react appropriately to different concentrations of sugars. In addition, perceptions of palatability and reward help to guide consumption of sugars based on a dynamic process than can accommodate short-term changes in physiological state and long-term changes due to learning or development. For example, the significance of sugar consumption for an animal varies depending on whether or not it has eaten recently, and gustatory cells alter their responses accordingly in a way that helps to maintain glucose homeostasis. This review describes the different gustatory aspects of sugars in rodents, for which there is detailed information about taste-evoked neural activity at all levels of the gustatory system. Sugars are given the most emphasis, since they are the most biologically relevant sweeteners, but other compounds that taste similar to sugars are also considered.
I. Transduction mechanisms and central projections
When sugars are ingested by a rodent, they come in contact with taste buds in the tongue and other parts of the oral cavity, such as the soft palate and nasoincisor ducts. In the tongue, taste buds can be found within protuberances called papillae. Fungiform papillae are found on the anterior two-thirds of the tongue, whereas circumvallate and foliate papillae are located on the posterior one-third. Each of these buds forms a complex, interactive unit with approximately 50–150 taste receptor cells, some of which project into the taste pore at their apical ends to allow binding of compounds, and some of which contact peripheral gustatory nerves to allow transmission of action potentials to the brain (Fish et al., 1944; Miller, 1995; Herness and Gilbertson, 1999).
The first step in gustatory transduction of sugars is thought to be binding to a receptor that is apically expressed in taste receptor cells and consists of a dimer of the seven-transmembrane-spanning domain proteins T1R2 and T1R3, which are coded for by the genes Tas1r2 and Tas1r3, respectively. The dimer is then coupled to G-proteins for intracellular signaling. The Tas1r3 gene corresponds to the Sac locus (Bachmanov et al., 2001a; Kitagawa et al., 2001; Max et al., 2001; Montmayeur et al., 2001; Nelson et al., 2001; Sainz et al., 2001), which had been proposed to be important in sweet taste based on inherited differences in preferences for saccharin in mice (Fuller, 1974). There is also evidence that T1R3 alone, possibly acting as a homodimer, can bind sugars at high concentrations (Zhao et al., 2003).
In vitro work has shown that expression of the rat forms of T1R2 and T1R3 results in binding of compounds that appear to taste sweet to rodents (Nelson et al., 2001; Li et al., 2002). These include the sugars sucrose and fructose; artificial sweeteners such as saccharin, dulcin, sucralose, and acesulfame-K; the amino acids glycine and D-tryptophan; and other compounds, such as the sugar alcohol D-sorbitol. Binding of the sugars glucose, maltose, lactose, and galactose was found in one study (Li et al., 2002), but not in another that used similar concentrations (Nelson et al., 2001). The diversity of chemical structures for the compounds listed above raises the possibility that there are multiple binding sites on the T1R2/T1R3 receptor, and work with the human sequences of these proteins supports this view (Xu et al., 2004). Specific binding sites have not yet been identified for the rodent forms, though there is evidence that the N-terminal domains of mouse T1R2 and T1R3 are both involved in binding, but to different degrees for different sugars (Nie et al., 2005). The rat form of the receptor differs from the human form, in that it is unable to bind aspartame, which explains why rats do not show strong preferences for this compound (Sclafani and Abrams, 1986). Mice are normally insensitive to aspartame (Bachmanov et al., 2001b), but transgenic animals that express the human form of T1R2 consume it avidly, which is consistent with it tasting sweet to them (Zhao et al., 2003).
Sequences of the Tas1r3 gene differ between inbred mouse strains that vary in their preferences for sweeteners in two-bottle tests (Bachmanov et al., 2001b; Kitagawa et al., 2001; Montmayeur et al., 2001; Sainz et al., 2001; Reed et al., 2004). The inbred strains used in these studies differ on many genes, but work with transgenic and congenic mouse strains has directly implicated variation in Tas1r3 as the primary cause of the behavioral differences (Bachmanov et al., 2001a; Li et al., 2001; Nelson et al., 2001; Inoue et al., 2007). In these studies, the Tas1r3 allele from a strain with high sweetener preferences was expressed on the background of a strain with low preferences, and the resulting animals had high preferences for sweet compounds. Insight into differences between mouse strains has also been provided by work using a binding assay; T1R3 with an amino acid sequence variant found in the high-preferring strains exhibited more effective binding of sugars than did T1R3 with a sequence variant found in the strains with low preferences (Nie et al., 2005). This suggests that the first stage of gustatory transduction has a major impact on the palatability of sweeteners, though it is likely not the sole determinant (see section IV.B for a more thorough consideration of this issue).
Studies with knock-out mice have provided additional evidence that T1R2/T1R3 is the primary taste receptor for sweeteners. Targeted deletion of Tas1r2 and/or Tas1r3 results in dramatic reductions in preferences for sugars and evoked responses in the chorda tympani nerve (Damak et al., 2003; Zhao et al., 2003). Nonetheless, Tas1r3 knock-out mice prefer high concentrations of sucrose and glucose (Damak et al., 2003). One explanation is that there are other, less sensitive sugar receptors that remain to be determined. For example, the dpa locus is known to influence neural and behavioral sensitivity to sucrose in mice (Shigemura et al., 2005), though the mechanism by which it does so has not been determined yet. It is also possible that sugars have non-gustatory sensory characteristics that allow knockout animals to detect them, and sugar intake is then driven by associating the other sensations with reinforcement from calories (see section IV. A, Sclafani and Glendinning, 2005, or Scalafani, 2007 for more consideration of how post-ingestive effects can affect subsequent sugar intake).
After binding to T1R2/T1R3, there follows a series of events that eventually results in changes in neural firing in the brain. Activation of heterotrimeric G-proteins is the first step. In particular, T1R2 is thought to be the site of this interaction, based on in vitro work with chimeric receptors containing both human and rat sequences (Xu et al., 2004). One of the important G-protein subunits is α-gustducin, which is co-expressed extensively with T1R2 and T1R3 in the palate, and co-expressed to a lesser extent in the tongue (Montmayeur et al., 2001; Stone et al., 2007). Mice with a targeted deletion of α-gustducin have reduced neural and behavioral responses to sweeteners, though they retain some sensitivity to high concentrations (Wong et al., 1996) and the reduction in intake depends in part on the exact testing methods that are used (Glendinning et al., 2005a). Thus, there must be an additional G-protein alpha subunit, whose identity still needs to be determined, that can couple to T1R2/T1R3. Gαi and Gαs have been suggested as possibilities (Stone et al., 2007), though direct evidence has not been obtained.
G-protein activation initiates a cascade of events that causes the depolarization of a taste receptor cell that expresses T1R2/T1R3 (figure 1). One important pathway involves stimulation of adenylate cyclase activity to effect an increase in cyclic AMP, which then inhibits K+ channels via protein kinase A, causing a depolarization of the cell (Tonosaki and Funakoshi, 1988; Striem et al., 1989; Bernhardt et al., 1996). Another proposed pathway involves activation of phospholipase C B2 (PLCB2) which then stimulates the second messengers inositol triphosphate (IP3) and diacylglycerol. There is evidence that this second pathway is activated only weakly by sugars and plays a larger role in transduction of artificial sweeteners (Bernhardt et al., 1996; Gilbertson et al., 2000). However, knockout of PLCB2 nearly abolishes neural and behavioral responses to sucrose and glucose in mice, suggesting a major role for the second pathway in sugar transduction (Zhang et al., 2003; Dotson et al., 2005). IP3 then causes intracellular stores to release Ca++, which opens TRPM5 channels to allow an influx of positive cations, which depolarize the cell (Zhang et al., 2003). Targeted deletion of TRPM5 severely impairs neural and behavioral responses to sugars, though reports vary as to whether TRPM5-knockout mice retain some residual sensitivity (Zhang et al., 2003; Damak et al., 2006). In a recent study, deletion of TRPM5 in mice did not completely eliminate cellular depolarization by intracellular Ca++ in dissociated taste receptor cells, suggesting that there is likely to be another cation channel whose identity has not yet been determined (Zhang et al., 2007).
Figure 1.
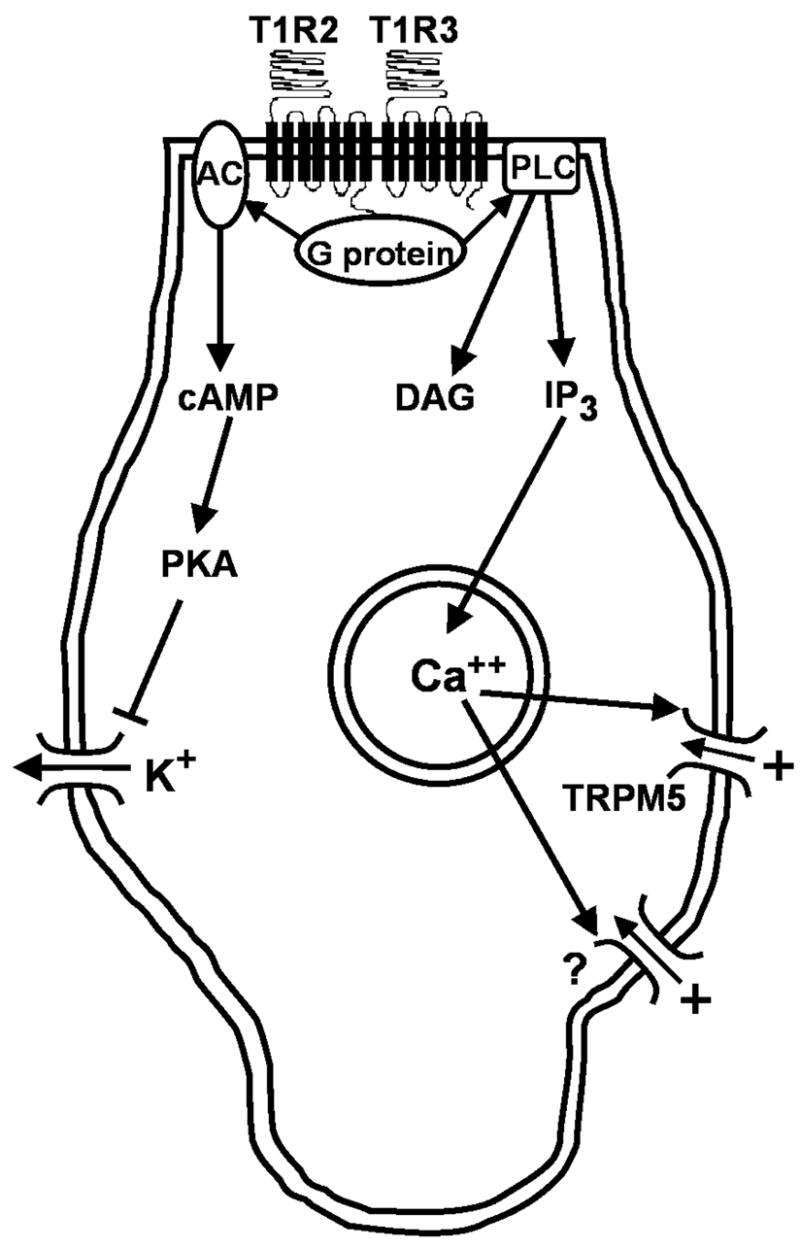
Intracellular cascades that follow binding of sugars to a putative taste receptor and that result in depolarization of a taste receptor cell. Sugars are thought to bind to a dimer of the proteins T1R2 and T1R3. T1R2 then activates a complex of G proteins which contains alpha, beta, and gamma subunits, including α-gustducin in some cases. One transduction pathway involves G-protein-mediated activation of adenylate cyclase (AC), which then increases production of cyclic AMP (cAMP), which causes inhibition of basolateral K+ channels through PKA. Depolarization of the cell results from decreased K+ efflux. An alternate pathway may also be involved, though evidence is stronger for artificial sweeteners than for sugars. This pathway involves activation of phospholipase C B2 (PLC) by the T1R2-coupled G-protein. PLC then causes inositol triphosphate (IP3) to release Ca++ from intracellular stores. Calcium opens non-selective TRPM5 channels, which allow cations to enter the cell, causing depolarization. There is also evidence for an unidentified cation channel (?) that is activated by Ca++ and can induce depolarization of the cell.
Gustatory signals are transmitted from the taste bud to peripheral nerves, but the exact process remains unclear. It has been proposed that sugar-responsive taste cells may communicate directly with gustatory nerve terminals via ATP (Finger et al., 2006; Romanov et al., 2007). However, there is evidence that receptor cells that respond to sweeteners do not have the cellular machinery for making synapses onto peripheral nerve fibers (DeFazio et al., 2006), and that they release ATP onto an adjacent cell, which then communicates with the peripheral nerve via serotonin (Huang et al., 2007). Consistent with this, recent evidence suggests that sucrose first activates narrowly-tuned type II receptor cells, which then influence less specific “presynaptic cells” that are also responsive to non-sweet compounds (Tomchik et al., 2007). In fact, the events involved could be even more complicated, as electrical and chemical coupling is common between taste bud cells (Lindemann, 1996; Herness et al., 2005), and so there may be substantial processing of a response to sugars within a taste bud before it is transmitted to a nerve.
Figure 2 illustrates the primary central gustatory projections. Taste information is carried to the brain via the chorda tympani (CT) and greater superficial petrosal (GSP) branches of the facial nerve, the glossopharyngeal nerve, and the superior laryngeal branch of the vagus. All peripheral gustatory information synapses first in the nucleus of the solitary tract (NST), followed by projections to the parabrachial nucleus (PBN) in the pons, the thalamus (the ventral posterior medial subnucleus), and then agranular insular cortex (Norgren and Leonard, 1971; Norgren, 1977; Saper, 1982). These four areas will be considered as the “gustatory” or “taste-responsive” brain regions, as neural responses across populations of cells within each nucleus provide a close match with presumed taste quality. This label is partly a matter of convenience, though. It will be used with the understanding that these nuclei also receive somatosensory and visceral input (Contreras et al., 1982; Yamamoto, 1984; Baird et al., 2001; Karimnamazi et al., 2002; Verhagen et al., 2003) and that other brain areas, such as the amygdala, lateral hypothalamus, and orbitofrontal cortex, contain cells that change their firing rates after stimulation of taste buds (Norgren, 1970; Azuma et al., 1984; Gutierrez et al., 2006). Moreover, there is extensive feedback from ventral forebrain areas onto the NST, and so the “gustatory” pathway described above does not operate independently of brain areas related to visceral sensation, homeostasis, and palatability (Van der Kooy et al., 1984).
Figure 2.
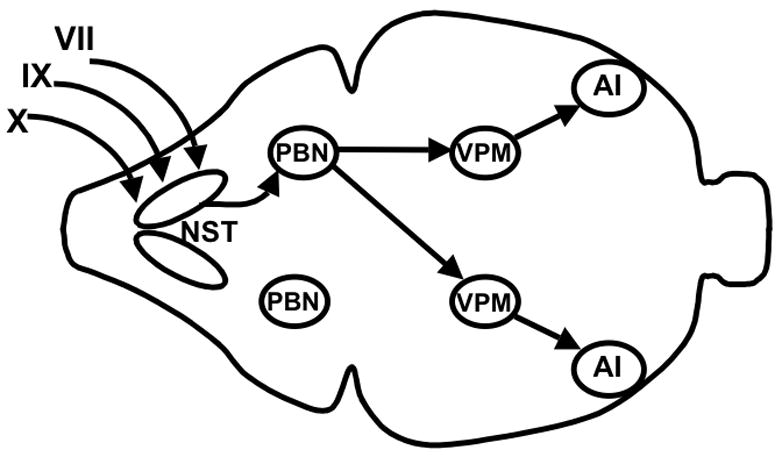
Schematic overhead view of the main central gustatory pathways in the rat. Projections originating from only one side of the oral cavity are shown for the sake of simplicity. Branches of the facial (VII), glossopharyngeal (IX), and vagus (X) nerves innervate taste buds in the tongue and other parts of the oral cavity. These peripheral taste-responsive fibers synapse in the nucleus of the solitary tract (NST) in the caudal hindbrain. Projections are then sent rostrally to the ipsilateral parabrachial nucleus (PBN), which projects bilaterally to the ventral posterior medial subnucleus of the thalamus (VPM). The VPM then projects ipsilaterally to the cortical gustatory area in agranular insular cortex (AI).
II. Perceived intensity
Perceptions of taste intensity and quality are generated in the brain. Intensity perceptions allow an animal to estimate the concentration of a sugar and thus the amount of calories it provides. Rats cannot rate the intensity of sugars verbally, but several methods have provided converging evidence that the perceived intensity rises monotonically as concentration is increased, up to the point of saturation. These include measurements of short-term intake, sham-drinking, initial lick rate, the size of licking bursts, and amount of bar-pressing (Young and Greene, 1953; Guttman, 1954; Davis, 1973; Cagan and Maller, 1974; Weingarten and Watson, 1982; Spector et al., 1998). Naturally, the question arises as to whether these measures provide direct insight into intensity perceptions in animals, given that the values obtained may be influenced by perceptions of taste quality or reward value. However, validation that they can be used in such a manner has been provided by close matches between their outcomes in rats and psychophysical ratings in human subjects (Guttman, 1954), and by similar findings when generalizations of conditioned aversions are used to estimate perceived glucose intensity in rats (Scott and Giza, 1987).
The neural basis for generating intensity perceptions appears straightforward. For concentrations above threshold, mean neural responses to sucrose rise monotonically with increases in concentration in the CT (Ganchrow and Erickson, 1970), NST (Ganchrow and Erickson, 1970), and PBN (Scott and Perrotto, 1980) of the rat. This is consistent with a simple rate code for signaling the perceived intensity of sugars. Further support is provided by studies in rats involving intravenous infusions of glucose, which reduces the multiunit NST response to oral glucose by 43% (Giza and Scott, 1983); behavioral tests measuring generalization of a conditioned taste aversion to 1 M glucose indicated that glucose infusion reduced the perceived intensity of the sugar by a similar amount (47%; Giza and Scott, 1987a). The monotonic relationship between sugar concentration and net response does not hold for the mean response averaged across all thalamic taste cells. However, there are individual thalamic neurons whose firing rates are related to sucrose concentration, which allows for a rate code carried by a subset of neurons (Scott and Yalowitz, 1978).
A rate code for perceived intensity is partially supported by comparisons that have been made between sugars. The most consistent finding in such comparisons has been that sucrose is the most effective sugar, both in driving peripheral and central taste-evoked responses (Hagstrom and Pfaffmann, 1959; Nejad, 1986; Travers and Norgren, 1991; Nakamura and Norgren, 1993; Harada et al., 1997) and in evoking behaviors such as short-term licking and bar-pressing that have minimal post-ingestive contributions (Guttman, 1954; Shuford, 1959; Hammer, 1968; Cagan and Maller, 1974). Among other sugars, the order of effectiveness has generally been fructose > glucose > maltose in electrophysiological recordings made from rats and hamsters, especially when only sucrose-oriented neurons are considered (Hagstrom and Pfaffmann, 1959; Smith et al., 1983b; Chang and Scott, 1984; Beidler and Tonosaki, 1985; Nejad, 1986; Giza et al., 1991; McCaughey et al., 1997; Verhagen et al., 2003; but see Harada et al., 1997 and Nakamura and Norgren, 1993 for exceptions). Short-term intake tests have also supported a more intense taste for fructose compared to glucose in rats (Davis, 1973; Cagan and Maller, 1974), but maltose has often been consumed more avidly than other sugars when matched for concentration, despite its ineffectiveness in driving taste-evoked neural responses (Davis et al., 1976; Sclafani and Mann, 1987).
It should also be noted that the order of effectiveness of sugars can depend on concentration (Cagan and Maller, 1974). Rats prefer 30 mM maltose over 30 mM sucrose in 3-min tests, but switch their preference to sucrose if the concentration of both is raised to 500 or 1000 mM (Sclafani and Mann, 1987). Prior experience and test duration are other factors that could contribute to the complicated pattern of results when comparing effectiveness of sugars between experiments (Hammer, 1968; Ramirez, 1996). In addition, different sugars may vary not only in perceived intensity, but also in perceived taste quality, as it is possible that sugars have non-sweet side tastes in rodents (see section III). For example, the preferences for low concentrations of maltose over sucrose have been proposed to be related to starch taste, not sweetness perception (Sclafani and Mann, 1987).
There are several known ways of reducing taste-evoked neural responses to sugars in rodents, including lingual application of gurmarin (Imoto et al., 1991; Lemon et al., 2003); extracts from the Zizyphus jujube plant (Yamada and Imoto, 1987); CuCl2 or ZnCl2 (Iwasaki and Sato, 1986); alloxan (Zawalich, 1973); methyl 4,6-dichloro-4,6-dideoxy-gamma-D-galactopyranoside (Jakinovich, 1983); and proteolytic enzymes (Hiji and Ito, 1977). In all cases, reductions were limited to stimuli thought to taste sweet to the animals. For gurmarin, behavioral tests indicate that the perceived intensity of sugars is decreased by the treatment (Murata et al., 2003).
At present, there is not definitive evidence to explain the underlying mechanisms for these rodent sweet taste blockers, as there is for lactisol, which reduces sweet taste in humans and whose binding site on the human T1R2/T1R3 receptor has been identified (Xu et al., 2004). However, comparisons of gurmarin and proteolytic enzyme effects between mouse strains have suggested that the blockers act on the product of the dpa locus, but not on T1R3 (Sanematsu et al., 2005; Ninomiya et al., 1991). In addition, gurmarin application has resulted in suppression of sucrose responses in the whole GSP (Harada and Kasahara, 2000) and in a subset of CT fibers (Ninomiya et al., 1999), but not in the glossopharyngeal nerve (Ninomiya et al., 1997); these data are difficult to reconcile with action of gurmarin on T1R2/T1R3, which is widely expressed in taste buds throughout the tongue and palate (Nelson et al., 2001). The possibility remains that the selective block of sweetness perception results from interference with the general functioning of receptor cells that express sweetener-binding receptors, especially given the slow time course (more than 30 min) of recovery from zizyphus extract (Yamada and Imoto, 1987) and gurmarin (Ninomiya et al., 1999) treatments.
III. Perceived taste quality
Sugars presumably evoke a distinctive taste quality in rodents, based on the fact that they are treated differently from compounds thought to possess salty, sour, bitter, or umami tastes (Morrison, 1969; Nowlis et al., 1980; Ninomiya et al., 1984a; Dotson and Spector, 2007). There are a variety of other compounds that can be labeled “sweeteners”, based on behavioral tests such as generalization of conditioned taste aversions (Nowlis et al., 1980; Danilova et al., 1998; MacKinnon et al., 1999; Ninomiya et al., 1984a). These non-sugar sweeteners are also thought to possess non-sweet side tastes to varying degrees, with concentration an important factor. For example, sodium saccharin is thought to taste primarily sweet to rats at 1 mM, but acquires a substantial salty component as the concentration is raised to 20 mM or higher (Ogawa et al., 1969; Ganchrow and Erickson, 1970; Chang and Scott, 1984; Giza et al., 1996). Saccharin may also taste bitter to rats at high concentrations, as it does to humans (Bartoshuk, 1979), but the paper cited most often in support of this view does not include statistics for the comparison that is relevant to this issue (Morrison and Jessup, 1977). In fact, Nowlis and colleagues (1980) found that taste aversions to saccharin at three concentrations (1, 40, and 200 mM) did not generalize to quinine in hamsters, suggesting a lack of bitter taste for the sweetener in this species. Some amino acids, such as D-phenylalanine and glycine, appear to taste similar to sugars, but are also treated differently from them in some cases (Nowlis et al., 1980; Ninomiya et al., 1984a; Eylam and Spector, 2004; Shigemura et al., 2005; Manita et al., 2006; Dotson and Spector, 2007).
Sugars may also evoke non-sweet side tastes in rodents, as they do in humans (Richter and Campbell, 1940a), but resolving this issue is complicated by the fact that rodents cannot report such perceptions directly. Instead, the animals’ perceptions must be inferred based on generalizations of their reactions to a reference stimulus, which is usually a sugar that is assumed to taste primarily sweet. This approach does not resolve what the reference stimulus tastes like to the animal. Rats have been able to discriminate different sugars from each other in some studies (Berridge et al., 1981; Ramirez, 1994; Spector et al., 1997), and there are instances in which conditioned taste aversions have not generalized between different sugars (Dugas du Villard et al., 1981; MacKinnon et al., 1999). One explanation for these results is that sugars have non-sweet side tastes, rather than tasting purely sweet. However, mice were unable to discriminate some concentrations of glucose or fructose from sucrose in recent work by Dotson and Spector (2007). This suggests that the sweetness evoked by all three sugars is similar in nature, and it raises the possibility that sugars were discriminated from each other in prior experiments based on intensity cues, rather than differences in taste quality. Still, these new data do not directly address the full range of perceptions evoked by the sugars (i.e., all three may have similar sweet and non-sweet components to their taste). In recordings from the NST and PBN, the across-neuron profile of activity evoked by sucrose has often been poorly correlated with the profiles of NaCl, HCl, and quinine, relative to the correlations found when other sugars are compared with these non-sweet stimuli (Perrotto and Scott, 1976; Van Buskirk and Smith, 1981; Smith et al., 1983b; McCaughey, 2007). This suggests that sucrose tastes more purely sweet than other sugars, which could contribute to it being the most highly preferred sugar in short-term tests (Cagan and Maller, 1974).
In addition, it is not clear what is perceived by rodents at the lowest concentrations at which sugars can be tasted. Detection thresholds, defined as the lowest concentration that can be discriminated from water, have varied widely between studies for sucrose (Table 1). No doubt, this variation is due in part to differences in the kinds of animals used, though methodological differences are likely to also be important. For example, the ability of rats to discriminate sucrose or maltose from water depends not only on concentration, but also on the volume of solution provided (Brosvic et al., 1991). Typical detection thresholds for sucrose, though, are about 2 mM. Recognition thresholds, defined as the lowest concentration that is treated similarly as a reference stimulus that is clearly suprathreshold and presumed to taste sweet, are generally higher than detection thresholds, as are preference thresholds, at which a sweetener is first preferred over water in two-bottle tests (Table 1). The comparatively low thresholds for detection could occur because sugars do not evoke perceptions of sweetness in rodents at the lowest concentrations that can be differentiated from water. Such results have been reported for human subjects (Richter and Campbell, 1940a), but resolving this issue is more difficult for non-human species, and an alternate explanation is that successful performance in the different threshold tasks depends on different levels of perceived intensity. Mice with a targeted deletion of the protein T1R3, which makes up half of the putative receptor for sweeteners, do not differ from wild-type controls in their detection threshold for sucrose, though this does not resolve whether the detection occurs due to a T1R3-independent mechanism for sweetness or to perception of non-sweet taste (Delay et al., 2006).
Table 1.
Sucrose thresholds in rats and mice. Detection thresholds (D) refer to a minimum concentration that could be discriminated from water behaviorally. Recognition thresholds (R) refer to a minimum concentration at which there was generalization of a conditioned taste aversion to a higher concentration of sucrose, except where noted. Preference thresholds (P) refer to the lowest concentration that was preferred significantly to water in a two-bottle test.
| Study | Threshold type | Species | Sex | Strain | Threshold (mM) |
|---|---|---|---|---|---|
| Koh and Teitelbaum, 1961 | D | rat | F | albino | 14* |
| Morrison and Norrison, 1966 | D | rat | – | – | 2 |
| Brosvic and Slotnick, 1986 | D | rat | M | albino and hooded | 1 |
| Willner et al., 1990 | D | rat | M | hooded | 0.07** |
| Thaw and Smith, 1992 | D | rat | M | Sprague-Dawley | 2 |
| Delay et al., 2006 | D | mouse | – | C57BL/6J | 2 |
| Tas1R3 KO | 3† | ||||
| Dugas du Villard et al., 1981 | R | rat | M | Wistar | 40 |
| Lal et al., 1982 | R | rat | M | Long-Evans | 13‡ |
| Ramirez, 1991 | R | rat | F | CD | 1.5 |
| Curtis et al., 2005 | R | rat | F | Sprague-Dawley | 75+ |
| 25++ | |||||
| Richter and Campbell, 1940b | P | rat | M | – | 17 |
| Lasiter et al., 1985 | P | rat | M | Sprague-Dawley | 15 |
| Bachmanov et al., 2001b | P | mouse | M | C57BL/6J | 58 |
| 129P3/J | 234 | ||||
| Lewis et al., 2005 | P | mouse | M | C57BL/6J | 29 |
| CD-1 | 0.003# | ||||
| DBA/2J | 292 | ||||
| 129P3/J | 73 |
–, information not provided in the original paper;
, value given is for non-food-deprived rats that were tested using a shock avoidance paradigm, whereas deprived animals tested with a food reinforcement paradigm could detect sucrose at 6 mM;
, the testing procedure involved extensive experience with sucrose intake compared to the other studies listed, which may be responsible for the unusually low threshold that was found;
, the threshold for knock-outs (KO) did not differ from the threshold for C57BL/6ByJ mice with a functional Tas1r3 allele;
, threshold was defined as the lowest concentration that was treated similarly to 100 mM sucrose in a bar-pressing task;
, in ovariectomized rats with estrogen replacement;
, in ovariectomized rats without estrogen replacement; concentrations lower than 25 mM were not tested, so the actual recognition threshold may be lower;
, animals preferred the lowest concentration tested, so the true threshold may be even lower.
At the initial stage of taste transduction, the binding to receptors expressed in taste receptor cells, there is clear specificity for sweeteners that distinguishes them from other molecules. The T1R2/T1R3 dimer is not activated by non-sweet compounds (Li et al., 2002), and taste receptor cells in which the dimer is found also do not express proteins involved in sour, bitter, or umami transduction (Nelson et al, 2001; Huang et al., 2006). This specificity may extend to the cellular level, as there is a report that most type II cells in taste buds do not respond to compounds with different taste qualities (Tomchik et al., 2007). In addition, a neural tracing study has reported segregated anatomical pathways for sweet and bitter taste that stretch from receptor cells to gustatory cortex (Sugita and Shiba, 2005). However, perception arises from neural firing in the brain, not directly from anatomical connections, and there is little specificity for sugars in central gustatory responses. That is, those neurons in the brain that respond to sugars almost always respond significantly to moderate to high concentrations of acids, NaCl, or mineral salts.
What is the neural basis for the perception of sweetness? The coding of taste quality has generally been described in terms of opposing “labeled-line” and “across-neuron pattern” theories (Erickson, 2000; Spector and Travers, 2005). Typically, the former proposes that the activity of a subclass of highly sugar-responsive neurons is necessary and sufficient for the perception of sweetness. The across-neuron pattern theory, as it is usually defined, proposes that a unique spatial pattern of activity, distributed across all cells within a brain area and perhaps also between nuclei (Jones et al., 2006), is necessary and sufficient for the perception of sweetness.
The available data disprove labeled-line coding as it is phrased above. The change in firing rate of a particular class of neurons (e.g., sugar-oriented) cannot be sufficient to signal sweet taste, since the cells give identical responses to some non-sweet stimuli. Recent analyses conducted on rat NST responses confirmed that neural subclasses and individual cells do not provide unambiguous information about the taste quality of a stimulus (Lemon and Smith, 2006). Although reports of highly sweetener-specific neurons have been common for the periphery (Ninomiya et al., 1984b; Danilova et al., 1998; Sollars and Hill, 2005), such data have been rare for central regions that are more directly relevant to perception.
The apparent solution to this problem is to assume that taste quality is determined only by populations of cells, not by individual ones or by narrowly defined neural subclasses. However, it is also true that the strict definition of across-neuron patterning described above does not lead itself easily to explicit testing, and evidence against labeled-line coding does not directly address the necessity and sufficiency of neuronal patterns. Moreover, some investigators have pointed out that labeled-line and across-neuron coding are not mutually exclusive (Pfaffmann, 1985; Smith et al., 2000; Scott and Giza, 2000), and it may be counter-productive to consider across-cell patterning in the extreme form described earlier. For instance, is the most relevant comparison across unclassified neurons, each of which is considered separately, or should cells be placed into subgroups, which are then compared with each other? Furthermore, should all cells within a given brain area be treated as necessary and equal contributors to the across-neuron pattern? To answer this, Smith and colleagues (1981, 1983a) subtracted different subgroups of hamster PBN neurons and looked at the effects on analyses of across-neuron response profiles. They showed that a sucrose-oriented subgroup of neurons is essential for creating a unique across-neuron profile of responding to sugars, but salt- or acid-oriented neurons are not necessary. This work reinforces that while it is possible to propose strict versions of the across-pattern and labeled-line theories, it may be more reasonable to consider a compromise between them, in which populations of cells with different sensitivities play a role, but it is also useful to group cells into classes, with some classes being more important for a particular taste quality than others.
Furthermore, a specific outcome can generally be viewed equally effectively from labeled-line and across-neuron perspectives (Erickson, 2000; Scott and Giza, 2000). For example, an experiment involved creation of transgenic mice, in which expression of an opioid receptor was driven in taste receptor cells that would normally express T1R2 (Zhao et al., 2003). The ligand for the receptor was highly preferred in the transgenic animals, but not in wild-type mice, as if it tasted sweet to only the former group. If the opioid antagonist activated the same taste receptor cells that are normally activated by sugars in the transgenic mice, then it would have generated a sugar-like across-neuron profile in the brain. Naturally, it would have also stimulated large responses in the neural subgroup with the most sugar-oriented cells, but not in subgroups that respond poorly to sugars. Thus, this experiment does not provide evidence that favors a particular theory of gustatory coding. The data are useful, though, in that they reinforce that central neurons do not have direct access to stimuli applied to the oral cavity, and so they are completely dependent on their input from the periphery, with gustatory transduction the crucial first step.
Separate considerations are given below to across-neuron patterns evoked by sugars and to responses within sugar-oriented neural subgroups, with the understanding that neither may provide a full explanation for the neural basis of sweetness. Sugars also evoke unique temporal patterns of activity, which may contribute to taste quality perception, and these patterns are considered in a separate section.
A. Across-neuron patterns evoked by sugars
Sensitivity to sugars is not distributed evenly throughout the oral cavity, though papillae in all areas show some degree of responsiveness. In rats, stimulation of the nasoincisor ducts with sucrose is more effective at driving NST responses than is stimulation of the anterior tongue (Travers et al., 1986). In addition, the GSP, which innervates the palate and nasoincisor ducts, gives larger sugar responses than does the CT, which innervates the anterior two-thirds of the tongue (Nejad, 1986; Harada et al., 1997; Sollars and Hill, 2005). Furthermore, transection of the GSP has a larger effect on behavioral sensitivity to sucrose than does CT transection (Krimm et al., 1987).
It is also possible to identify subregions within central gustatory areas that vary in their responsiveness to sugars, but there are no regions that respond exclusively to sweet stimuli (Halpern, 1967; Yamamoto et al., 1989; DiNardo and Travers, 1997; Accolla et al., 2007). Sugars can be differentiated from non-sweet stimuli, though, by considering patterns of responding across broad populations of cells. The across-neuron patterns of activity evoked by different stimuli can be correlated with each other, and multidimensional scaling, cluster analysis, and similar methods can then be performed on the resulting correlation matrix. An example is shown in figure 3, which shows a two-dimensional space that was generated based on across-neuron patterns of NST responses in C57BL/6ByJ mice (McCaughey, 2007). Non-sugar sweeteners generally have similar across-neuron patterns as sugars, suggesting a shared taste quality. However, there are also instances in which artificial sweeteners and supposedly sweet amino acids have not been grouped with sugars (Giza et al., 1996; Verhagen et al., 2003; McCaughey, 2007), presumably due to non-sweet side-tastes (see section IV.B for more detail). Interestingly, ethanol evokes an across-neuron pattern similar to that of sugars in the NST of rats (Lemon et al., 2004). This suggests that it tastes sweet to them, a hypothesis that is supported by CTA generalization tests (Di Lorenzo et al., 1986).
Figure 3.
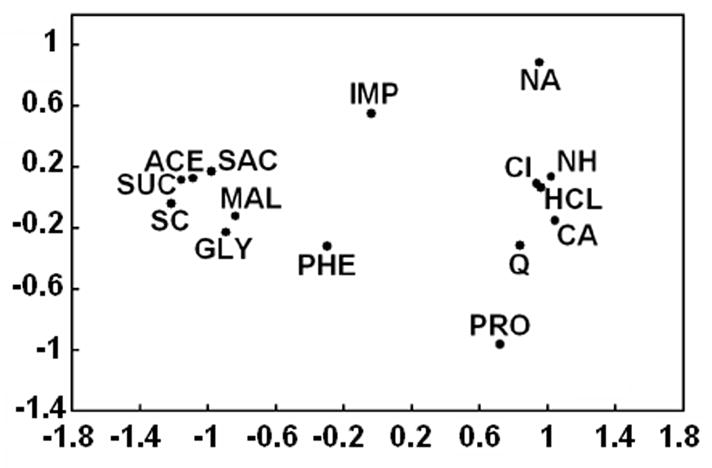
Comparison of gustatory stimuli based on activity in the nucleus of the solitary tract of C57BL/6ByJ mice. Across-neuron patterns of activity evoked by stimuli were correlated with each other, and multidimensional scaling was used to generate a two-dimensional space in which stimuli with similar patterns were located close to each other. The sugars sucrose and maltose are grouped with other compounds that are thought to taste sweet to mice and in a distinct location from compounds thought to taste salty, sour, or bitter. ACE, 20 mM acesulfame-K; CA, 100 mM CaCl2; CI, 10 mM citric acid; GLY, 100 mM glycine; HCL, 10 mM HCl; IMP, 10 mM disodium inosine 5′ monophosphate; MAL, 500 mM maltose; NA, 100 mM NaCl; NH, 100 mM NH4Cl; PHE, 100 mM D-phenylalanine; PRO, 100 mM L-proline; Q, 20 mM quinine HCl; SAC, 10 mM NaSaccharin; SC, 1 mM SC-45647; SUC, 500 mM sucrose. Reprinted from McCaughey, 2007 with permission.
Although across-neuron patterns correlate well with presumed taste quality in rodents, they are not necessarily the immediate cause of sweetness perception. One issue is whether taste quality arises due to the activity of neurons in a particular brain area. Pfaffmann and colleagues (1977) proposed that projections to gustatory thalamus and cortex are especially important for allowing discriminations between compounds by rats, and cortex is thought to be important for conscious perception in general (Verhagen, 2007). However, thorough tests of the necessity of cortex for sweetness perception have not been conducted, and gustatory cortex feeds back onto the NST, rather than acting as a terminus for taste information (Van der Kooy et al., 2004). This does not necessarily preclude an important role for gustatory cortex in causing perceptions of taste quality, but it means that it may do so as part of a distributed, multi-area circuit, rather than by itself (Jones et al., 2006; Verhagen, 2006).
Sweeteners elicit not only perceptions, but also more tangible consequences that can be measured directly in rodents, such as cephalic phase insulin release and facial reactions that promote ingestion. Furthermore, sweetness perception can stimulate specific behaviors, such as lever pressing, that are not observed for non-sweet compounds (Morrison, 1969). These behaviors involve certain alpha motor neurons, each of which can be considered a final common path. This raises the question of exactly how such sugar-specific responses are generated from the output of broadly-tuned taste responsive cells. In the usual application of across-neuron patterning to address this issue, all neurons that have been measured in a particular gustatory nucleus are included in mathematical calculations, and each cell is given equal weight. However, such calculations are unlikely to reflect the in vivo anatomical projections that are relevant to this issue. When a particular motor neuron changes its firing rate following ingestion of sugar, but not of non-sweet compounds, the pre-synaptic input responsible is unlikely to involve an equal contribution from every neuron in every gustatory area. Naturally, only some taste-responsive neurons would have an influence, and those that do would vary in how much influence they have, based on factors such as synaptic strengths.
Thus, the proposal for a comparison across neurons can be described abstractly, but it also requires a specific physical basis. A finite number of broadly-tuned taste-responsive neurons must converge onto a common post-synaptic neuron, which summates their combined excitatory and inhibitory influences to generate action potentials. This same process is then continued with a new group of post-synaptic cells, and at some point these events can result in sweetener-specific neural responses. Can we say anything specific about how this is accomplished? Although this process must be complex, some relevant data were published recently. Lemon and Smith (2006) demonstrated that the responses of individual neurons in the rat NST do not contain sufficient information to identify the kind of gustatory stimulus that was applied to the oral cavity. However, they estimated that patterns of activity across small groups of cells (as few as six) would be sufficient. Thus, one possibility is that the NST and other gustatory areas consist of networks of neurons, with each network containing a small number of cells that are capable of generating sweetener-specific post-synaptic events, but with different networks being processed in parallel and projecting to different neurons. This mechanism offers a realistic explanation for how sugar intake could stimulate not only a particular taste quality, but specific behavioral and physiological reactions. The question would remain, though, of how to consider such networks collectively and in relation to each other.
B. Sugar-oriented neurons
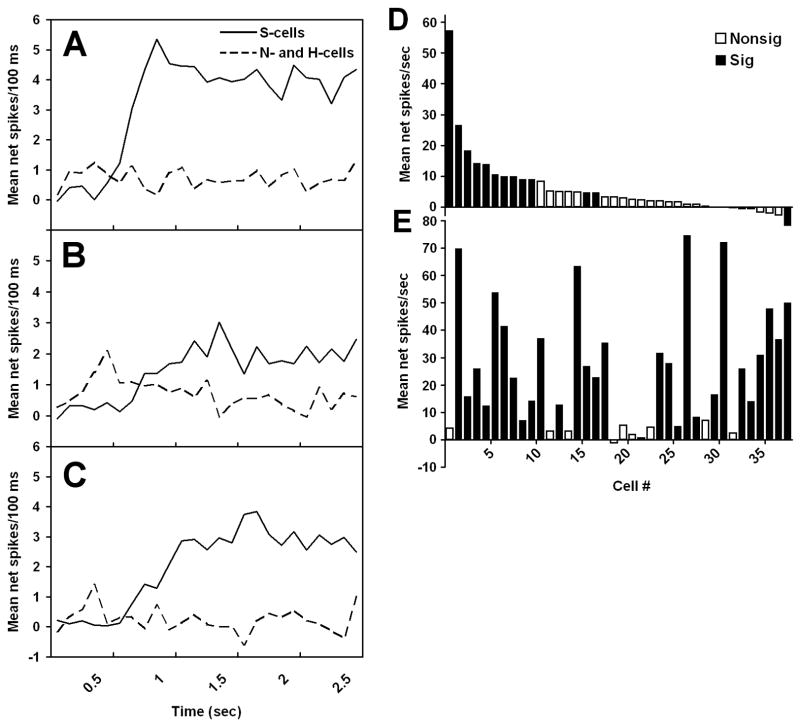
Gustatory neurons have been classified using several criteria, the most common being their response profile across prototypical sweet, salty, sour, and bitter stimuli. This method of categorization has generally resulted in a clear distinction between sugar-oriented neurons (i.e., the subset of cells with the largest responses to sugars relative to those of non-sweet stimuli, regardless of the identity of each cell’s most effective stimulus), often called S-cells, and other neurons. This is especially true in the rat GSP, whose large multiunit response to sugars derives from pronounced sugar sensitivity in a small subset of cells (Sollars and Hill, 2005), but it also applies to central areas (Smith et al. 1983a; Verhagen et al., 2003). Even within S-cells, though, it is rare to find a neuron that responds to only sweet stimuli, and the breadth of tuning metric (i.e., entropy) of sucrose-oriented neurons increases going from the CT to NST to PBN (Smith, 1985). In a recent study in which NST responses were measured in C57BL/6ByJ mice, all of the cells that responded significantly to 500 mM sucrose also responded significantly to 100 mM NaCl, 10 mM HCl, or 20 mM quinine HCl (McCaughey, 2007).
The mere fact that cells can be classified as especially sugar-oriented is uninteresting. The relevant question is whether knowing that a cell is sugar-oriented provides other useful information. There are several indications that it does. First, sugar-oriented neurons in the CT and PBN show response suppression to mixtures of sucrose with HCl or quinine, whereas other subgroups of cells do not show this effect (Vogt and Smith, 1993; Formaker et al., 1997). In addition, sucrose-oriented neurons in the CT, NST, and PBN can also be distinguished from other neural subgroups by their large responses to umami stimuli (Nishijo et al., 1991; Formaker et al., 2004; Geran and Travers, 2006, McCaughey, 2007). Furthermore, S-cells differ from other cells not only in the overall size of their sugar response, but also in its time course. The post-stimulus time histograms (PSTHs) of sugar responses are distinctively shaped for S-cells in the NST of rats and mice, due to a response latency of more than 600 ms (see section III.C for more detail). There are also several manipulations that have altered gustatory responses of S-cells, but not of other neurons, in the NST and PBN of rats. These include intraduodenal infusion of lipids, formation of a conditioned taste aversion to saccharin, and deprivation of sodium or calcium (see sections IV.D, V.A and V.C for more details).
Together, these data reinforce the usefulness of knowing that a gustatory neuron has a sugar-oriented response profile. Such knowledge provides an ability to predict other characteristics of the cell, at least relative to chance levels, and these relationships provide insight into the organization of the gustatory system. Moreover, the coincidence of multiple features in the same neurons provides evidence that S-cells may constitute a discrete “type” of cell, rather than belonging on a continuum with other neurons.
Another factor to consider, though, is the stability of neural classifications over time. This has been examined thoroughly in only a small number of studies (Ogawa et al., 1973; DiLorenzo and Victor, 2003; Chen and DiLorenzo, 2008). Most rat NST neurons consistently maintain the same best stimulus among the four prototypical taste qualities if stimuli representing these tastes are applied multiple (8–27) times, but some cells do not (DiLorenzo and Victor, 2003). Naturally, this raises the issue of whether response profile should be considered a stable characteristic of a cell, or whether it would be more appropriate to describe a “sugar-oriented state” that central gustatory neurons can enter and leave. One relevant factor is that taste-responsive cells are known to change their evoked activity to sugars based on changes in blood glucose levels and other variables (see section V). Thus, it may be that response profile serves as a useful criterion for categorizing cells, but only if an animal is maintained in a specific physiological state, and fluctuations from that state can affect sugar responses to the degree that cells become reclassified. One way of answering this question will be to determine additional characteristics that tend to define S-cells, apart from giving large responses to sugars, and to find properties that are especially stable over time. In addition, it may be preferable to define S-cells based on a constellation of features, such as the ones described above that tend to differ between S-cells and other neurons, rather than using a single feature.
C. Temporal characteristics of sugar responses
Sugars evoke peripheral and central neural responses with slow onsets relative to responses to non-sweet compounds (Beidler, 1953; Perrotto and Scott, 1976; Yamamoto et al., 1984; Pfaffmann, 1985; Verhagen et al., 2003). The mean response to sucrose in the NST of rats and mice generally peaks at more than 800 msec after it is applied to the oral cavity, whereas responses to salty and sour compounds peak within less than 600 msec (McCaughey et al., 1997; McCaughey, 2007). Moreover, sucrose responses in the rat NST peak even later when it is applied to only the anterior tongue and not to the nasoincisor ducts (Travers and Norgren, 1989).
There is also evidence that sugar recognition has a relatively long latency compared to non –sweet compounds in rats, as it does in humans (Yamamoto and Kawamura, 1981). Behavioral tests conducted by Halpern (1985) resulted in durations of 229 and 854 ms for rats to behaviorally identify 500 mM NaCl and 500 mM sucrose, respectively. Presumably, then, the neural basis for sweetness perception in rats must be found within the action potentials occurring less than 900 ms after sucrose contacts the oral cavity. This topic is relatively unexplored, though, and further work needs to be conducted on the time course of sugar perception in rodents.
Why do sugar responses have a long latency? The phenomenon appears to originate within taste buds. For example, rat CT fibers that respond to both NaCl and sucrose give a rapid response to the former and a delayed response to the latter, and so the difference is not due to properties of the nerve fibers themselves (Pfaffmann, 1985). To some extent, the slow response to sucrose may be due to the dependence of sugar transduction on G-protein-mediated cascades, rather than on direct passage of ions through channels and into taste receptor cells, as is thought to occur for sodium ions and protons (Heck et al., 1984; Lindemann, 1996). However, this mechanism is unlikely to provide a full explanation, given that bitter transduction is also thought to be G-protein mediated (Wong et al., 1996; Zhang et al., 2003), but responses to quinine and other bitter stimuli typically peak within 600 msec in the NST of rodents (Giza et al., 1991; McCaughey, 2007). Another explanation for slow responses to sugars is high viscosity, since the compounds are often used at concentrations of 500 mM or greater. Data from the NST of mice contradict this possibility, though, given that similar time courses of responding are observed for 500 mM sucrose and the high potency sweetener SC-45647 at 1 mM (Figure 4A and C; McCaughey, 2007). One process that would explain large but delayed responses to sugars is a positive feedback loop, possibly involving reciprocal interactions between adjacent taste receptor cells, but this would be difficult to verify in vivo.
Figure 4.
Sweeteners evoke independent phasic and tonic responses in the nucleus of the solitary tract (NST) of C57BL/6ByJ (B6) mice. A–C. Post-stimulus time histograms showing mean net responses to three sweeteners in different subgroups of NST cells during the first 2.5 sec after stimulus onset in 100-ms bins. Sucrose at 500 mM (A), 500 mM maltose (B), and the artificial sweetener SC-45647 at 1 mM (C) evoked small responses with a rapid onset in NST cells that had NaCl- or HCl-oriented profiles based on evoked responding across a 5-sec evoked period (n = 17; dashed lines). In contrast, these compounds evoked responses that were larger, but more delayed, in cells with sucrose-oriented response profiles (n = 21; solid lines). D–E. Mean net responses to 500 mM sucrose in the NST of B6 mice during phasic (D) and tonic (E) response periods. Responses that deviated significantly from baseline firing are indicated by solid bars, whereas ones that did not are indicated by open bars. Data are shown for 38 individual neurons, placed in descending order of their phasic response to sucrose for both graphs. The phasic period was defined as the first 600 ms after stimulus onset, and the tonic period lasted from 600–5000 ms after onset. Both periods allowed adequate time for cells to show a significant change in firing rate compared to baseline. However, the Pearson product moment correlation between cells’ phasic and tonic sucrose responses was −0.06, indicating that the two periods are independent. This figure is previously unpublished, but data are taken from the experiment for McCaughey, 2007.
A recent study suggests that sweeteners evoke two independent response periods in mice: a phasic period with short latency and brief time course, and a tonic period that is more sustained and has a longer latency (McCaughey, 2007). Salt- and acid-oriented NST neurons in C57BL/6ByJ (B6) mice tended to give small, transient responses to sweeteners that had peaked by 600 ms after stimulus onset; the cells that were the most sucrose-oriented across 5 sec, in contrast, evoked little response to sucrose until 600–900 ms after onset (figure 4A–C). Based on this, net responses of NST neurons were calculated based on either the first 600 ms after onset or on 600–5000 ms after onset in order to define phasic and tonic responses, respectively. The across-neuron profiles of phasic and tonic responses tended to be poorly correlated with each other for sweeteners, but not for other compounds. The correlation for sucrose was only −0.06 (figure 4D–E). In other words, the phasic and tonic neural activity generated by sucrose in the NST were generally found in different neurons.
The independence of phasic and tonic sweetener responses was also supported by comparing data between B6 and 129P3/J (129) mice, which differ in sweetener preferences and have different forms of the T1R2/T1R3 receptor. Sucrose responses of the B6 strain were significantly larger, but only when 1200 ms or more of response time was considered. Thus, there was no strain difference in the phasic response to sucrose, despite the differences between B6 and 129 mice in sequence of the primary sweet taste receptor. In addition, phasic responses tended to be especially large for the sweeteners thought to have the largest non-sweet side-tastes in both strains. Taken as a whole, these data suggest that sweeteners activate an initial phasic response that is neither mediated by T1R2/T1R3 nor sent preferentially to a certain neural subgroup, as well as a longer latency tonic response that begins with binding to T1R2/T1R3 and is directed primarily to S-cells in the NST. The tonic response appears to be associated with perceptions of sweetness, whereas the phasic response may be more closely related to non-sweet side-tastes.
Work by DiLorenzo and colleagues (1993, 2003) also reinforces the importance of temporal patterns of activity in signaling taste quality. Rats received electrical stimulation of the NST using artificial spike trains, whose timing was based on the responses to sucrose recorded from individual NST cells in different animals. When these patterns of activity were “played back”, the animals reacted as if they were experiencing perceptions of sweet taste, but the effect disappeared if the temporal pattern of the spike trains was shuffled. Thus, the temporal characteristics of an individual cell’s sucrose response must have been sufficient to signal sweetness, though these results do not resolve what aspect(s) of the time course were important. A sugar response can be considered in terms of the general course of its development over a period of several hundred milliseconds to seconds, as described earlier in this section. Responses can also be described in terms of their rhythms or patterns. For example, it has been common to observe rhythmic “swells” or “bursts” of firing in response to sucrose and other sweeteners in a subset of neurons in the CT (Ogawa et al., 1969; Sato et al., 1969; Mistretta, 1972; Ogawa et al., 1973; Ogawa et al., 1974; Nagai and Ueda, 1981; Frank et al., 2005) and, to a lesser extent, in the PBN (Perrotto and Scott, 1976) and gustatory thalamus (Scott and Erickson, 1971). In contrast, reports of these patterns are rare for non-sweet stimuli. The direct impact of such bursting on perception is not clear. Nonetheless, there is evidence that NST responses in rats contain quality-specific information related to the precise timing of spikes, particularly during the first 2 sec of the response (DiLorenzo and Victor, 2003).
IV. Preference
Rodents normally consume sugars avidly and prefer them in two-bottle tests versus water, though there appear to be two partially independent aspects to this behavior. In addition to the palatability or hedonic aspect of a stimulus (i.e., the extent to which an animal “likes” it), it is also possible to describe its incentive properties based on its effectiveness as a reward or reinforcer (i.e., the extent to which the animal “wants” it). These two processes have been described thoroughly in several excellent reviews (see Hoebel, 1985; Yamamoto, 2003; Norgren et al., 2006; Peciña et al., 2006). Thus, they will be summarized here only briefly, with an emphasis on how these processes are influenced by the output of gustatory nuclei after sugars are tasted.
There are indications that sugars cause sensations of pleasure in rodents, although the animals cannot report this verbally. In rats, sugars evoke facial reactions, such as lateral tongue protrusions (Grill and Norgren, 1978a), that are thought to be related more strongly to liking than to wanting (Peciña et al., 2006). For example, these facial reactions are observed when sugars are infused intraorally in decerebrate animals, which do not seek out sugars on their own (Grill and Norgren, 1978b). The resulting feelings of pleasure are thought to arise partly due to the action of endogenous opioid peptides acting in several brain areas, including a restricted portion of the outer shell of the nucleus accumbens (see Peciña et al., 2006 for a review). Furthermore, ingestion of sucrose or saccharin increases levels of the endogenous opiate β-endorphin in plasma and CSF of rats (Yamamoto et al., 2000), and sucrose intake can have analgesic effects (Blass et al., 1987; Segato et al., 2005).
Sugars are also reinforcing, indicating a high incentive salience. Rats will run towards a known source of sugars rapidly (Young and Shufford, 1955) and will work to obtain them (Guttman, 1954). Access to sugar can condition a place preference (Agmo and Marroquin, 1997), and rats will also forego mild brain stimulation of reward pathways in order to obtain access to sucrose (Conover and Shizgal, 1994). Many experimenters, in fact, have measured sucrose or glucose intake without an obvious interest in sugars per se. Rather, they have used the compounds as prototypical natural reinforcers that can be used to gain insight into pathways and neurochemicals that control motivation. This work suggests that sugar consumption stimulates dopaminergic fibers that originate in the ventral tegmental area and project widely throughout the nucleus accumbens shell, where the amount of dopamine released is well-correlated with behavioral measures of reward (Hoebel, 1985; Norgren et al., 2006; Peciña et al., 2006; see below for more details).
Of course, sugars are not the only stimuli that can serve as rewards and stimulate feelings of pleasure, and there is evidence that different reinforcers act on similar neural mechanisms. There is a high correlation between preferences for sweeteners and ethanol (see Kampov-Polevoy et al., 1999 for a review). This may derive in part from ethanol tasting sweet (Lemon et al., 2004; Di Lorenzo et al., 1986), but likely also involves common neural substrates in non-gustatory areas related to reward (Phillips et al., 1994). In addition, the amount of self-administration of addictive drugs such as cocaine or morphine serves as a predictor of sweetener intake in rats (see Levine et al., 2003 for a review), which suggests that high preferences for sucrose do not derive exclusively from gustatory factors. Furthermore, when sucrose is delivered to rats on a restricted schedule, it can become addictive to them, generating changes in the brain that are similar to those observed with drugs of abuse (Colantuoni et al., 2001; Bello et al., 2003; Spangler et al., 2004), as well as withdrawal-like reactions when not delivered (Colantuoni et al., 2002).
A. Role of learning
Sugars provide one of the clearest examples of an appetite with an unlearned component, and intake of them can be driven solely by immediate sensory factors. Sucrose is consumed avidly by rat pups that have had no prior experience with it (Hall and Bryan, 1981; Vigorito and Sclafani, 1988; Ackerman et al., 1992). Adult rodents will lick rapidly for sugars as soon as they are presented, prior to any significant post-ingestive consequences (Davis, 1973). In addition, rats ingest large volumes of sucrose and glucose under sham-drinking conditions, which prevents them from retaining the liquid and deriving calories from it (Mook, 1963; Weingarten and Watson, 1982). Moreover, there is evidence that short-term licking or sham-drinking of sweeteners is sufficient to activate dopaminergic reward pathways (Xenakis and Sclafani, 1981; Schneider et al., 1986). These results reflect the strong stimulatory impact of sucrose’s oral sensory qualities (e.g., sweetness) on intake, whereas the compound’s inhibitory qualities during a meal derive primarily from satiety.
Although the avid ingestion of sugars is innate, experience can also play a role. Rats and mice increase their sucrose intake with exposure (Perez and Sclafani, 1990; Amico et al., 2005; Sclafani, 2006b). A possible contributor to this change is association of the taste of sucrose with the calories that it provides. For example, intragastric infusion of carbohydrates is effective at conditioning preferences for formerly neutral tastes (Scalfani, 2004; Sclafani and Glendinning, 2005), and saccharin intake is enhanced following infusion of carbohydrate, with evidence that an increase in palatability is partly responsible (Ramirez, 1994; Ramirez, 1997). Presumably, a similar process may act to increase preferences for sugars as an animal experiences their sweet taste followed by calories. However, the fact that fructose is only weakly effective at causing post-ingestive reinforcement (Sclafani et al., 1993) suggests that factors other than caloric value also need to be considered.
B. Individual differences
Sugars are rarely avoided by rodents, but there can be large differences between individuals in their preferences for sweeteners. For instance, inbred mice from the 129P3/J (129) strain prefer sucrose to water only at concentrations of 234 mM or greater, whereas C57BL/6ByJ mice prefer 58 mM sucrose (Bachmanov et al., 2001b). In general, variability in sucrose preferences across mouse strains is closely related to sequences of the Tas1r3 gene (Bachmanov et al., 2001b; Kitagawa et al., 2001; Montmayeur et al., 2001; Sainz et al., 2001; Reed et al., 2004), though the strength of the relationship can depend on the sucrose concentration used (Lewis et al., 2005). Binding assays suggest that the Tas1r3 sequence variant found in the mouse strains with the lowest preferences results in poor binding of sweeteners to the T1R2/T1R3 receptor (Nie et al., 2005). Electrophysiological recordings indicate that the ineffective activation of this receptor in 129 mice, relative to that found in B6 mice, then results in small sugar responses in the whole CT nerve and averaged across all NST neurons (Bachmanov et al., 1997; Inoue et al., 2001; Reed et al., 2004; McCaughey, 2007). In addition, whole-nerve CT responses to sucrose and saccharin are smaller in DBA/2J mice, which have a 129-like sequence of Tas1r3, than in B6 mice, and the B6 strain also has a higher preference for these compounds in one- and two-bottle tests (Frank and Blizard, 1999; Bachamanov et al., 2001a). Thus, a simple difference in effectiveness of receptor binding, under the control of Tas1r3 and maintained across at least two synapses, presumably leads to differences in perceived intensity that can explain the strain variation in preferences for sugars.
However, some recent data are inconsistent with this mechanism and reinforce that numerous stages intervene between taste transduction and preference behavior. For example, 129 mice consume more sucrose than do B6 mice in short-term lick tests when high concentrations are used (Dotson and Spector, 2004; Glendinning et al., 2005b). Furthermore, 129 mice increase their preference for sweeteners upon repeated exposure to them, so that they end up preferring them to the same extent as B6 mice that receive identical exposure (Sclafani, 2006b). One study suggested that an important factor may be the effectiveness of sugar intake in stimulating reward pathways, which is high in 129 mice that have experience with the compound (Sclafani, 2006a). In addition, most NST cells give small responses to sugars in 129 mice that have had no exposure to sugars, but there is a small percentage of sucrose-oriented cells whose sweetener responses are comparable to those of S-cells in C57BL/6ByJ mice (McCaughey, 2007). Thus, there appears to be some process by which ineffective sugar transduction at the start of taste processing can be counteracted at later stages. Altogether, these data reinforce that sugar preference is determined by an interaction of genetics and environment, and inheritance of a T1R2/T1R3 receptor that binds sweeteners poorly can be overcome by other factors.
Preferences for saccharin also vary widely, and this variation has a genetic basis in rats and mice (Nachman, 1959; Fuller, 1974), though different loci may be involved for the two species. In mice, the genes Tas1r3 and dpa (Capeless and Whitney, 1995; Bachmanov et al., 1997; Reed et al., 2004) are known to play a role, but a recent study ruled out an involvement of Tas1r3 for rats (Lu et al., 2005). A possible explanation for the latter result is that variation in saccharin preferences in rats is due to variation in non-sweet transduction mechanisms. Supporting evidence is provided by NST recordings made in rats that preferred or avoided 30 mM NaSaccharin in two-bottle tests with water (Giza et al., 1996). The mean NST response to saccharin was the same in the two groups, but it evoked an across-neuron profile that was more similar to that of NaCl and less similar to that of sucrose in the saccharin-avoiding animals. This suggests that the differences in saccharin preference were due to differences in perceived taste quality, not intensity. In saccharin-avoiding rats, the 30 mM saccharin presumably tasted primarily salty, rather than sweet, as is the case for higher concentrations of saccharin that are avoided by all rats. The same kind of perceptual change may explain why ovariectomy reduces preferences for saccharin in female rats. DiLorenzo and Monroe (1990) found that saccharin evoked a similar mean response across all cells in the PBN of intact and ovariectomized females, but the compound’s across-neuron profile was more similar to that of NaCl and HCl in the ovariectomized group. Furthermore, similar results were found in C57BL/6ByJ (B6) and 129 mice (McCaughey, 2007), which differ in their saccharin preferences, with higher scores in the former (Bachmanov et al., 2001b). The two strains did not differ in their mean saccharin response across all NST cells, but saccharin’s across-neuron profile was more highly correlated with that of NaCl and less highly correlated with that of sucrose in 129 relative to B6 mice (figure 5).
Figure 5.
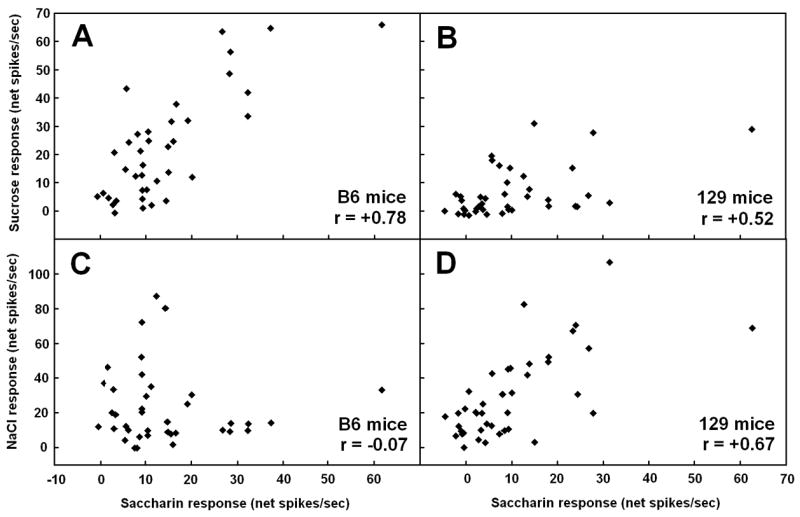
Scatterplots showing the relationship between responses evoked by 10 mM NaSaccharin and those evoked by 500 mM sucrose (A,B) or 100 mM NaCl (C,D) in cells from the nucleus of the solitary tract of C57BL/6ByJ (B6; A,C) or 129P3/J (129; B,D) mice. The Pearson product moment correlation is shown in the bottom right of each panel. Responses to saccharin were more highly correlated with responses to sucrose in B6 mice than in 129 mice, whereas saccharin responses were more highly correlated with NaCl responses in 129 animals than in the B6 strain. These results suggests that saccharin tastes more sweet and less salty to B6 mice than to 129 mice, given that the correlation between across-neuron profiles provides a good match with similarity of perceived taste quality. Such a perceptual difference between strains likely contributes to the higher preferences for saccharin in B6 mice compared to 129 mice. This figure is previously unpublished, but data are taken from the experiment for McCaughey, 2007.
C. Anatomy
There is a clear anatomical basis for the taste of sugars affecting neurons related to behavioral preferences in rodents. Two main pathways, one originating in the PBN and the other in gustatory cortex, allow taste-responsive cells to immediately alter the firing rates of cells thought to be responsible for perceptions related to palatability and reward. In particular, neural tracing in rats has shown dense projections from the gustatory PBN to areas in the ventral forebrain such as the amygdala and hypothalamus (Norgren, 1976), which contain neurons that respond to oral sugar stimulation, albeit in ways that are more closely related to the palatability of stimuli than to their taste quality (Norgen, 1970; Nishijo et al., 1998; de Araujo et al., 2006). The amygdala and hypothalamus then project to the ventral tegmental area (VTA), which sends fibers to the nucleus accumbens, where the release of dopamine is closely related to the reward value of stimuli (Yamamoto, 2003; Norgren et al., 2006).
These same areas may also be influenced by primary gustatory cortex, which sends projections to brain regions, including prefrontal cortex and amygdala, that are reciprocally connected to the hypothalamus and other parts of the ventral forebrain (Yamamoto, 2003; Norgren et al., 2006; Yamamoto, 2006). Simultaneous recordings from gustatory cortex, oribitofrontal cortex, amygdala, and the lateral hypothalamus indicate that neurons in the different areas compose a neural circuit in which changes in firing are closely related to sucrose licking as a rat cycles through satiety and hunger (de Araujo et al., 2006). Thus, there appear to be two main anatomical pathways through which gustatory responses can influence preference behavior. There is evidence, based on lesions of the gustatory thalamus in rats, that the projections from the PBN are more important in influencing sugar intake in rats than are the projections from cortex (Hajnal and Norgren, 2005). However, the distinction between the pathways is blurred by extensive interconnections between them, which create an elaborate neural circuit.
Naturally, the expression of sugar preferences also requires a motor output. One important pathway is thought to involve projections from the nucleus accumbens to the basal ganglia, so that the reward value of sugars can be translated into licking behavior (Mogenson et al., 1980). The nucleus accumbens may also influence motor areas via the ventral pallidum, which has also been implicated as an important area for creating sensations of pleasure in response to sugars (Kalivas et al., 1999; Pecina et al., 2006). However, these are not the only important pathways. Decerebrate rats make ingestive facial reactions upon intraoral infusion of sugars and consume more of them as concentration increases (Flynn and Grill, 1988), and these behaviors must be due to projections caudal to the midbrain. One of the important parts of this circuit appears to be GABA-ergic cells within the parabrachial nucleus (Higgs and Cooper, 1996; Söderpalm and Berridge, 2000).
D. Neural coding
Anatomical connections provide a route by which tasting sugars can lead to intake of them, as described above. Furthermore, the neural coding of sugar preference appears to be straightforward when the responses of cells in areas such as the nucleus accumbens and ventral pallidum are examined. For example, of 52 neurons in the nucleus accumbens that responded significantly to intraoral sucrose infusion, 47 either gave no response to quinine or changed their firing rate in the opposite direction relative to their sucrose response (Roitman et al., 2005). In the ventral pallidum, there are cells that increase their firing rate in response to sugar infusion; these neurons show only moderate increases in firing upon infusion of 1.5 M NaCl in replete rats that find the solution unpleasant, but large increases in firing after the animals are sodium-deprived and develop an appetite for NaCl (Tindell et al, 2006). Thus, there are cells in the ventral forebrain whose firing rates closely track preference behavior. This allows for the possibility of a strict labeled-line, rather than across-neuron, strategy, in contrast to the neural coding of taste quality (see section III).
However, these data raise a separate issue, which is how neurons in ventral forebrain areas are able to give different responses to sugars and non-preferred taste stimuli. In other words, what is unique about the gustatory responses generated by sugars that causes them, but not avoided taste stimuli, to generate specific outcomes such as release of dopamine in the nucleus accumbens? As described earlier (section III), neurons in gustatory nuclei are broadly tuned when using an array of taste stimuli with sugars, acids, NaCl, and presumed bitter compounds. Although there are reports of individual gustatory neurons that are excited by sugars and inhibited by unpalatable stimuli, or vice-versa (Travers and Smith, 1979; Yamamoto et al., 1989), there are few such cells in gustatory nuclei that could provide unambiguous information about palatability to post-synaptic neurons. This suggests a need for a comparison across cells, as is the case for the generation of taste quality perceptions, which raises the issues of exactly what is compared in the nervous system and how. Another question is whether separate populations of cells within a given gustatory nucleus contribute to perceptions of taste quality and control taste preferences. In general, there is not clear evidence that a certain subpopulation of taste-responsive cells provides quality information exclusively. This suggests that taste is similar to vision, with individual neurons encoding multiple stimulus parameters simultaneously (Erickson 1974), but proving this is challenging.
One way to facilitate consideration of these complicated issues is to look at the mean responses of neural subgroups, defined based on their profiles of responding to sweet, salty, sour, and bitter compounds. For example, there is evidence that excitation of sugar-oriented neurons (S-cells) in the NST and PBN tends to stimulate ingestion. After rats are deprived of sodium or calcium, compounds with the needed mineral become more preferred and palatable (Berridge et al., 1984; McCaughey et al., 2005), and there are also increases in taste-evoked excitation by CaCl2 or NaCl in S-cells in the NST, but not in other neural subgroups (Jacobs et al., 1988; McCaughey and Tordoff, 2001; but see Nakamura and Norgren, 1995 and McCaughey and Scott, 2000 for failures to replicate the data by Jacobs and colleagues). In addition, S-cells in the CT, NST, and PBN of rats give larger responses to umami stimuli, which tend to be highly preferred, than do other subgroups of cells (Nishijo et al., 1991; Formaker et al., 2004; Geran and Travers, 2006). Furthermore, decreases in S-cell responses to sucrose have been observed in the rat PBN following intraduodenal infusion of intralipid (Hajnal et al., 1999), which also suppresses sucrose intake (Foster et al., 1998).
At the same time, the broad tuning of S-cells in gustatory nuclei prevents an exact correspondence between their firing rates and behavioral preference. That is, if S-cells in the NST and PBN are excited by unpalatable stimuli, such as quinine or hypertonic NaCl, then why don’t these other responses also drive neural firing that stimulates intake? The likely answer is that they do, but they are balanced out by inhibitory influences from other subgroups of gustatory cells, which give robust responses to the unpalatable stimuli, but weak responses to sugars. Support for this idea is provided by studies in which an appetite for NaCl was created in rats. The most common effect of sodium appetite on taste-evoked neural activity has been reduced neural responding to NaCl, and this effect has typically been most pronounced in N-cells, the neurons that are tuned most narrowly to NaCl (Contreras, 1977; Contreras and Frank, 1979; Contreras et al., 1984; Jacobs et al., 1988; McCaughey and Scott, 2000; Tamura and Norgren, 2003). These reduced neural responses are not associated with a decrease in perceived intensity or ability to detect NaCl (Fregley, 1958; Carr, 1952; Breslin et al., 1993). Rather, they can be viewed in terms of a shift in the relative amounts of activity carried by different neural subtypes (McCaughey and Scott, 1998). A reduction in responding to NaCl in N-cells would result in a greater proportion of the total NaCl response carried in S-cells, and these data help to explain sodium appetite if S- and N-cells exert excitatory and inhibitory influences, respectively, on ventral forebrain neurons that stimulate feelings of pleasure and/or reward. Further support is provided by the effects of creating a conditioned taste aversion to NaCl in rats, which include both reduced NaCl intake and increased taste-evoked responses in the PBN that are limited to cells that are amiloride-sensitive and narrowly-tuned to NaCl (Tokita et al., 2004).
Figure 6 illustrates how a summation of opposing effects by sucrose- and NaCl-oriented gustatory cells could result in post-synaptic responses that are proportional to preference for a taste stimulus. The need to include acid/bitter-oriented gustatory neurons (H-cells) in this model is not clear. They, too, may cause inhibition of neurons important for pleasure and reward, in which case their responses would compensate for the small but significant excitatory responses to acids and bitter stimuli found in sugar-responsive NST and PBN neurons. There are few studies, though, that provide direct support, such as changes in responses of H-cells following manipulations that affect intake. There is a report of unusually small H-cell responses to MgCl2 in the NST of rats that had been conditioned to prefer the compound. However, conditioning a preference to citric acid in another group of animals did not result in decreased responses to this compound in H-cells (Giza et al., 1997).
Figure 6.
Model to explain how the firing of broadly-tuned gustatory cells could result in post-synaptic responses that closely track the palatability of a taste stimulus. The gustatory signal is sent to a sucrose-oriented cell in the PBN (S), which then excites a hypothetical hedonically-oriented cell in the ventral forebrain (HC), whose responses are related more closely to stimulus palatability than taste quality. Taste stimuli can also drive firing in a NaCl-oriented PBN cell (N), which inhibits the HC. Line thicknesses are proportional to evoked firing rates, and neural excitation is indicated by arrows and inhibition by dashed lines. A) Oral sucrose causes robust responses in PBN S-cells, but weak responses in N-cells, resulting in a large response by the HC. B) Infusion of lipids is known to reduce sucrose intake, as well as the size of sucrose responses in S-cells in the PBN, possibly due to smaller input from the NST, though interactions between taste and visceral inputs in the PBN are another potential source of the effect. PBN N-cell responses are unaffected by lipid infusion. The net result in the HC would be smaller excitation by sucrose. C) Umami stimuli, such as a mixture of MSG and GMP, are known to evoke larger responses in S- than N-cells in the NST and PBN. The summation of PBN outputs would cause excitation of the HC by umami compounds, which tend to be highly preferred. D) PBN S-cells respond not only to sucrose, but also are strongly excited by moderately preferred or avoided concentrations of NaCl. However, NaCl also effectively drives firing of N-cells in the PBN, with the net result that the HC would not give large response to NaCl under normal conditions. E) When rats are sodium-deprived, or when an appetite for NaCl is created by other means, there is a reduction in the size of the response evoked by N-cells in the NST, which provide input to N-cells in the PBN. There is also a report of elevated S-cell responses to NaCl in the NST of sodium-deprived rats. Thus, sodium appetite would be accompanied by robust HC responses when NaCl is tasted. F) Creation of a conditioned taste aversion to NaCl causes a decrease in NaCl intake and palatability in rats. There is also an increase in the taste-evoked response to NaCl in the PBN, which is limited to amiloride-sensitive cells that are narrowly tuned to NaCl. Stronger excitatory input to these PBN N-cells is depicted as a possible cause of this elevated responding to NaCl, though this has not been tested directly.
Assuming such a model is correct, it still leaves open the question of exactly where responses of different taste-responsive neurons are compared in the brain. That is, in what nucleus does the output of broadly-tuned gustatory cells get converted into a labeled-line code more appropriate for guiding behavior? The available evidence indicates that there is no single location, but rather there is a gradual convergence of information related to preference, and the process has been proposed to occur across at least two synapses (Norgren et al., 2006). Taste-responsive neurons in the hypothalamus and amygdala are thought to respond based on the palatability of stimuli, rather than their taste quality, in part due to shifts in firing rates following manipulations that affect food intake. However, it is still possible to find neurons that respond to both sucrose and quinine in these areas (Norgren, 1970; Azuma et al., 1984). This suggests that the hypothalamus and amygdala act as transitional areas, in which inputs from the PBN may be processed to result in a closer relationship between neural firing rates and palatability, but still not to the extent observed in the nucleus accumbens or ventral pallidum. The hypothalamus and amygdala then project to the nucleus accumbens directly or via the ventral tegmental area, and another comparison could be made across neurons, resulting in post-synaptic firing rates that closely track the palatability of gustatory stimuli.
Figure 6 is meant to provide some general principles for how the output of gustatory nuclei could lead to preference-oriented responses to sugars and other stimuli, but it is not intended to capture the full complexity of neural responding in vivo. For instance, it is unlikely that mean firing rates of N-cells in the PBN could simply be subtracted from those of S-cells to determine how activity of the subgroups is summated by post-synaptic neurons. Such summation would depend not only on mean firing rates, but also on the time course of firing. This includes the latency of responses, which varies across neural subgroups with different response profiles (see section III.C), but another relevant factor would be firing rhythms. In the NST of C57BL/6ByJ mice, 70% of N-cells fire often with interspike intervals that are very short (< 10 ms), whereas only 19% of S-cells do (McCaughey, unpublished observations); in the visual system, short-interval firing has been shown to be especially effective at driving post-synaptic action potentials compared to firing rhythms that have longer intervals (Usrey et al., 1998), and so N- and S-cells in the taste system likely differ in their effectiveness at influencing other cells. Temporal factors would also play a large role in how the activity of gustatory cortex influences palatability-related areas such as the amygdala and orbitofrontal cortex, since taste-evoked cortical responses are known to have complex temporal patterns that often include periods of inhibition (Katz et al., 2001).
V. Changes in preferences
Sugars are rarely harmful to rodents, especially given that excess glucose can be stored as glycogen and then reconverted later to provide energy. Still, sugars are more useful at some moments than others, so it is appropriate for animals to alter sugar preference based on need. At the same time, it should be important for sweeteners to maintain a unique taste quality as any changes in palatability or reward value occur. Thus, one might expect sweetener responses in gustatory nuclei to remain unchanged over time and provide stable input to neurons in hedonic areas, which then alter their firing rates based on visceral and hormonal factors. However, this is not the case. A number of variables that affect preferences for sweeteners also affect taste-evoked responses to them, though not necessarily to such a degree that sugars no longer generate distinctive across-neuron patterns (Chang and Scott, 1984). This is not surprising, though, given that labeling areas as “gustatory” and “hedonic” is partly a matter of convenience and ignores the extensive interconnections between them.
A. Satiety
Sugars become less palatable as a rodent consumes them. For example, the rate at which rats lick for sucrose declines across a test session, (Davis and Perez, 1993). Furthermore, ingestive facial reactions to oral sucrose infusion occur less frequently if a rat has just eaten for an hour or received an intragastric infusion (Cabanac and Lafrance, 1990; Berridge, 1991). These satiety effects include both sensory-specific and non-specific components (Mook et al., 1983) and are appropriate, given the elevation of blood glucose and decreased usefulness of sugars for the animal after it has fed.
Food intake causes a wide array of physiological changes that could contribute to decreases in sugar preference. Many of these satiety-related responses have been examined individually and found to have effects on taste-responsive neurons in rats. Stomach distension (Glenn and Erickson, 1976) and increases in plasma levels of glucose (Giza and Scott, 1983), insulin (Giza and Scott, 1987b), and glucagon (Giza et al., 1993) reduce the multiunit NST response to orally applied sugars. However, neural responses in this area are unaffected by changes in cholecystokinin levels (Giza et al., 1990), despite the peptide’s effectiveness in causing a reduction in intake of sugars (Grill and Smith, 1988). The effects of gastric distension have been examined in the rat PBN, where taste-evoked responses to sucrose were reduced, but only in a subset of the cells (Baird et al., 2001). Sucrose responses also decline in the PBN following intraduodenal administration of lipids (Hajnal et al., 1999). In addition, CT and glossopharyngeal nerve responses to sucrose are reduced in mice by administration of leptin (Kawai et al., 2000), which can inhibit long-term food intake, though it is not considered a satiety factor that causes meal termination (Friedman and Halaas, 1998).
Satiety is also associated with decreased neural responses to sugars in the amygdala, lateral hypothalamus, and orbitofrontal cortex. Recent data indicate that cells in these areas change their firing rates as a rat licks sucrose to satiety, with the mean firing rate across neuronal populations decreasing as the duration of intervals between sucrose licking bouts increases (de Araujo et al., 2006). Thus, one component of decreased sugar preference in satiated animals appears to be smaller activation of gustatory nuclei by sugars, which causes them to be less effective at driving cells in hedonic areas. Of course, describing mean responses across large populations is not productive in explaining the specific motor responses associated with ingestion, as described earlier (see section III.A). Thus, an area for future research is determining exactly how comparisons are made across cells to reduce licking rates for sugars towards the end of a meal.
B. Hunger
Food-deprived rats show even larger intake of sugars than do those that are non-deprived (Smith and Duffy, 1957), and this is true even for 3-day-old pups (Hall and Bryan, 1980). However, the full neural basis for this behavioral shift remains less clear than is the case for satiety-induced decreases in sugar consumption. There are mixed reports as to whether sugars are more palatable to rats when they are food-deprived, based on measurements of ingestive facial reactions and the size of licking clusters, with concentration, experimental design, and measurement technique as apparent contributors to the variability (Berridge, 1991; Davis and Perez, 1993; Grill et al., 1996; Spector et al., 1998).
Decerebrate rats show enhanced intake of intraorally-infused sucrose when food-deprived, indicating that caudal brainstem areas are sufficient for the effect (Grill and Norgren, 1978c). Can this result be explained by food deprivation producing elevated taste-evoked responses to sugars, in a complimentary fashion to the decreases in responding that accompany satiety? Recordings from the NST of rats indicated that there was no increase in responsiveness to oral sugar application after 72-h of deprivation, although there were other changes, including a decrease in the mean spontaneous firing rate of the cells (Nolan and Scott, 1994). The cause of elevated sugar intake in food-deprived decerebrates could be changes in gustatory responses in the PBN, where taste and visceral signals overlap to a greater extent than in the NST, but this has not been tested.
Food deprivation has been reported to alter taste-evoked responses to sucrose in the hypothalamus, where the percentage of cells that responded to sucrose but not quinine was larger after 48-h deprivation than during baseline conditions (Norgren, 1970). This effect would not need to depend on altered input from taste-responsive nuclei such as the PBN, though, since hypothalamic nuclei show a variety of deprivation-induced changes, including elevation of levels of neuropeptide Y (NPY), that are mediated by changes in blood glucose or hormones related to energy balance (Kalra et al., 1999; Williams et al., 2001). NPY can also be administered exogenously to stimulate ingestion, especially of sugars (Lynch et al., 1993; Kalra et al., 1999). There is some evidence that NPY increases sucrose intake by affecting orosensory factors (Torregrossa et al., 2006), but other data contradict this possibility and suggest that the enhanced ingestion is due primarily to changes in post-ingestive mechanisms (Baird et al., 2006). Measurement of the effects of NPY infusion on taste-evoked neural responding could help to resolve this controversy, but such work has not been performed.
C. Conditioned taste aversions
Sweeteners can be made less preferred by pairing intake of them with illness, which results in a conditioned taste aversion (CTA; Garcia et al., 1955). Such conditioning has widespread effects on the consequences of sugar ingestion. After a CTA to a sweetener is created, its taste no longer generates cephalic-phase release of insulin (Berridge et al., 1981), release of dopamine in the nucleus accumbens (Mark et al., 1991), and increases in B-endorphin levels in CSF and plasma (Yamamoto et al., 2000). Sweeteners not only lose some of their normal properties, they can also acquire characteristics of naturally aversive compounds, such as quinine. Rats increase the number of aversive facial reactions in response to intraoral sugar infusion after pairing the compound with a manipulation that induces nausea (Berridge et al., 1981; Pelchat et al., 1983). This does not necessarily mean that sweeteners become bitter or change their taste quality after a CTA is formed. The fact that aversions to sugars and saccharin show little generalization to stimuli such as quinine argues against this possibility (Nowlis et al., 1980; Ninomiya et al., 1984a). In recordings from the NST of rats, conditioning a CTA to saccharin did result in the compound generating an across-neuron profile of responding that was more similar to that of quinine than was the case for unconditioned animals, which suggests an increase in bitter taste for saccharin (Chang and Scott, 1984). However, saccharin’s profile was still more similar to those of sugars than that of quinine within the conditioned animals, and so it is likely that saccharin still tasted predominantly sweet after conditioning.
In the same study, there was also an increase in saccharin responses in conditioned animals that was limited to S-cells and to a well-defined peak of activity that occurred about 900 ms after stimulus onset (Chang and Scott, 1984). This result is unusual, given the evidence that increases in S-cell responses are often associated with increases in preference (see section IV.D). One explanation is that only delayed portions of the NST response are important in causing perceptions of sweetness (see section III.C), and therefore an increase that has only a small effect on tonic responding would have little consequence for the animal perceptually. In addition, an increased NST S-cell response to saccharin has also been found in rats that formerly had a conditioned taste aversion to this compound, but that then underwent thorough behavioral extinction and no longer avoided it (McCaughey et al., 1997). Thus, the effect appears to be associated with the prior pairing of saccharin and illness, rather than with behavioral rejection of saccharin per se. Enhanced responses to saccharin have also been observed in a subset of neurons in gustatory cortex after creation of a CTA (Bures et al., 1998).
Creation of a CTA to a sweetener can also cause ingestion of the compound to generate responses similar to those induced by nausea in regions that receive visceral information, such as the intermediate NST and lateral PBN, and these areas may then have an influence on taste-evoked responses due to convergence of gustatory and visceral signals in other parts of the PBN (Swank and Bernstein, 1994; Yamamoto et al., 1994). Nonetheless, saccharin consumption retains the ability to increase expression of c-fos in the central medial nucleus of the PBN, a response typically observed for intake of sucrose but not non-sweet compounds, even after aversive conditioning (Yamamoto et al., 1994). A change in taste-evoked PBN responses to a sweetener upon formation of a CTA would have widespread effects on ventral forebrain areas related to palatability and food intake. In fact, effects of aversive conditioning have been observed in the hypothalamus of rats during measurement of taste-evoked responses to saccharin, with conditioning causing a shift in the relative reactivity of the lateral and ventromedial nuclei (Aleksanyan et al., 1976). The percentage of lateral versus ventromedial cells that responded to saccharin was smaller after the CTA was created, and the lateral and ventromedial nuclei are thought to exert excitatory and inhibitory influences, respectively, on feeding, so these data provide a likely neural contributor to rejection of saccharin after conditioning. Similar support has been provided by measurements of taste-evoked activity to saccharin in the amygdala before and after formation of a CTA. Increases in excitatory responses were observed often in the basolateral amygdala after conditioning, whereas larger inhibitory responses were observed most frequently in the central subnucleus, and these effects are consistent with the presumed roles of these nuclei in feeding behavior (Yasoshima et al., 1995).
D. Gender differences
When sweetener consumption was initially compared between male and female rats, daily intake of glucose and saccharin was higher in females, and the difference was attributed to effects of ovarian hormones, but not testosterone (Valenstein et al., 1967; Zucker, 1969; Gabri and Soljaci, 1975). However, since then a more complicated picture has emerged. There have been observations of larger sweetener intake in males or ovariectomized females than in intact females, with indications that the outcome is affected by testing procedure, concentration, and type of sweetener (Marks, 1974; Kenney and Redick, 1980; Perez and Sclafani et al., 1990; Hrupka et al., 1997; Curtis et al., 2004).
Measurement of licking microstructure and sham-drinking of sucrose suggests that estrogen reduces the sucrose intake of rats through enhancement of negative post-ingestive signals, without an effect on the compound’s sensory properties (Geary et al., 1995; Hrupka et al., 1997). In another study, though, short-term licking of low concentrations (25–50 mM) of sucrose was inversely related to estrogen levels (Curtis et al., 2004), and estrogen treatment has been found to increase the recognition threshold for sucrose (see table 1; Curtis et al., 2005). Work involving taste reactivity and electrophysiology also supports an influence of ovarian hormones on the sensory properties of sucrose, but contradicts the two studies just mentioned in the direction of the effect. Female rats that received intraoral sucrose infusions during diestrus or proestrus, when estrogen levels are especially high, showed a larger number of tongue protrusions than did male rats or females tested during estrus or metestrus (Clarke and Ossenkopp, 1998). Furthermore, taste-evoked responses to sucrose and saccharin in the PBN were larger in intact and ovariectomized females than in male rats, suggesting that there is increase in perceived intensity of sweeteners mediated by an organizational effect of ovarian hormones (Di Lorernzo and Monroe, 1989; Di Lorernzo and Monroe, 1990).
VI. Summary and perspectives
Sugars evoke distinctive gustatory responses when considered from several perspectives. However, there is no simple rule that explains the neural basis for all consequences of sugar ingestion. Sweeteners evoke unique patterns of activity across large populations of cells, and these patterns provide a useful tool for inferring perceived sweetness in animals. At the same time, consideration of smaller cell populations, possibly organized as parallel networks, will be necessary in explaining how sugar intake causes specific behavioral and physiological effects. It is also appropriate to focus on the responses of a sugar-oriented neural subgroup in some situations, such as when examining the effects of manipulations related to sugar taste and metabolism. Thus, current attempts to prove “across-fiber” or “labeled-line” theories are counter-productive, especially to the extent that the theories are defined in their extreme forms and treated as mutually exclusive. Instead, across-fiber patterns and responses within neural subgroups should be treated as alternate analyses that provide different perspectives on a given set of data. Temporal patterns of activity provide still another way of describing sugar responses, and recent data indicate that these patterns help rodents to identify and react to sugars. In the past, spatial (i.e., across-neuron) and temporal patterns of gustatory activity have been considered separately. However, in future work it will be important to consider them together, as has been done for olfaction (Moulton, 1976; Spors et al., 2006) and somatosensation (Ghazanfar and Nicolelis, 1999), given that both kinds of patterns occur simultaneously as an animal ingests sugars.
The responses to sugars in gustatory nuclei, whose cells are broadly-tuned and whose across-neuron patterns of activity correlate well with presumed taste quality, get converted into more narrowly-tuned responses in ventral forebrain areas, allowing for control of sugar ingestion. One way of accomplishing this is for subgroups of PBN neurons with different response profiles to exert opposite effects on post-synaptic cells in the ventral forebrain. Specifically, there is evidence that excitation of sugar-oriented PBN cells tends to stimulate intake, whereas excitation of salt-oriented cells tends to inhibit intake; at present, there is not strong evidence suggesting that acid- or bitter-sensitive cells are important in this process. Further work will be needed to determine exactly how the responses of different neural subtypes should be compared in order to match their combined activity with intake. It will also be important to re-evaluate this model as new experiments are conducted, with manipulations that alter sugar preferences being especially useful. Such manipulations could include food deprivation or creation of a CTA to sweeteners, as they have well-characterized behavioral effects, but their influence on neural responses in the PBN has not been examined.
The preference for sweeteners is not static, but can vary within an animal over time, as well as between individuals, and in many cases these outcomes are related to neural responses in gustatory brain areas. For sugars, intake and preference differences are often proportional to differences in the mean response across a population of taste-responsive cells, which implicates variation in perceived intensity as the underlying cause. In contrast, differences in saccharin preference have been related more closely to variation in its perceived quality, with a more sugar-like across-neuron pattern of responding in taste responsive nuclei being associated with greater preference. Sugar consumption can be dramatically reduced by food intake and individual satiety factors, as well as by aversive conditioning, and there are changes in taste-evoked responses in brainstem gustatory nuclei and ventral forebrain areas that provide a neural correlate to these behavioral shifts. It is also possible to increase sugar intake beyond baseline levels through manipulations such as food deprivation, but it is not clear to what extent this is due to changes in the taste of sugars, and post-ingestive factors influencing feeding may play a primary role.
Acknowledgments
The author thanks Drs. Danielle Reed and Alexander Bachmanov for their comments on an earlier draft of this manuscript. This work was supported by NIH grant R03 DC005929.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2-3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci. 2007;27(6):1396–404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman SH, Albert M, Shindledecker RD, Gayle C, Smith GP. Intake of different concentrations of sucrose and corn oil in preweanling rats. Am J Physiol. 1992;262:R624–7. doi: 10.1152/ajpregu.1992.262.4.R624. [DOI] [PubMed] [Google Scholar]
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Agmo A, Marroquin E. Role of gustatory and postingestive actions of sweeteners in the generation of positive affect as evaluated by place preference conditioning. Appetite. 1997;29(3):269–89. doi: 10.1006/appe.1997.0101. [DOI] [PubMed] [Google Scholar]
- Aleksanyan ZA, Buresova O, Bures J. Modification of unit responses to gustatory stimuli by conditioned taste aversion in rats. Physiol Behav. 1976;17(2):173–9. doi: 10.1016/0031-9384(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol. 2005;289(6):R1798–806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- Azuma S, Yamamoto T, Kawamura Y. Studies on gustatory responses of amygdaloid neurons in rats. Exp Brain Res. 1984;56(1):12–22. doi: 10.1007/BF00237437. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm Genome. 1997;8(8):545–8. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001a;26(7):925–33. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001b;26(7):905–13. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol. 2001;281(5):R1581–93. doi: 10.1152/ajpregu.2001.281.5.R1581. [DOI] [PubMed] [Google Scholar]
- Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci. 2006;120(4):937–51. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 1979;205(4409):934–5. doi: 10.1126/science.472717. [DOI] [PubMed] [Google Scholar]
- Beidler LM. Properties of chemoreceptors of tongue of rat. J Neurophysiol. 1953;16(6):595–607. doi: 10.1152/jn.1953.16.6.595. [DOI] [PubMed] [Google Scholar]
- Beidler LM, Tonosaki K. Multiple sweet receptor sites and taste theory. In: Pfaff DW, editor. Taste, Olfaction, and the Central Nervous System. Rockefeller University Press; New York: 1985. pp. 47–64. [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol. 2003;284(5):R1260–8. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490(2):325–36. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16(2):103–20. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. J Comp Physiol Psychol. 1981;95(3):363–82. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- Blass EM, Fitzgerald E, Kehoe P. Interactions between sucrose, pain and isolation distress. Pharmacol Biochem Behav. 1987;26:483–489. doi: 10.1016/0091-3057(87)90153-5. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Kaplan JM, Spector AC, Zambito CM, Grill HJ. Lick rate analysis of sodium taste-state combinations. Am J Physiol. 1993;264(2 Pt 2):R312–R318. doi: 10.1152/ajpregu.1993.264.2.R312. [DOI] [PubMed] [Google Scholar]
- Brosvic GM, Slotnick BM. Absolute and intensity-difference taste thresholds in the rat: evaluation of an automated multi-channel gustometer. Physiol Behav. 1986;38(5):711–7. doi: 10.1016/0031-9384(86)90268-4. [DOI] [PubMed] [Google Scholar]
- Brosvic GM, Hecht GS, LaHaye S, Rowe MM, Risser JM, Clementson E, Dihoff RE. Quality-specific differences in rat taste detection performance as a function of stimulus volume. Physiol Behav. 50(4):711–6. doi: 10.1016/0031-9384(91)90007-b. [DOI] [PubMed] [Google Scholar]
- Bures J, Bermudez-Rattoni F, Yamamoto T. Conditioned taste aversion: memory of a special kind. Oxford University Press; Oxford: 1998. [Google Scholar]
- Cabanac M, Lafrance L. Postingestive alliesthesia: the rat tells the same story. Physiol Behav. 1990;47(3):539–43. doi: 10.1016/0031-9384(90)90123-l. [DOI] [PubMed] [Google Scholar]
- Cagen RH, Maller O. Taste of sugars: brief exposure single-stimulus behavioral method. J Comp Physiol Psych. 1974;87:47–55. doi: 10.1037/h0036585. [DOI] [PubMed] [Google Scholar]
- Capeless CG, Whitney G. The genetic basis of preference for sweet substances among inbred strains of mice: preference ratio phenotypes and the alleles of the Sac and dpa loci. Chem Senses. 1995;20(3):291–8. doi: 10.1093/chemse/20.3.291. [DOI] [PubMed] [Google Scholar]
- Carr JM. The effect of adrenalectomy upon the NaCl taste threshold in rat. J Comp Physiol Psych. 1952;45:377–380. doi: 10.1037/h0056378. [DOI] [PubMed] [Google Scholar]
- Chang FC, Scott TR. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci. 1984;4(7):1850–62. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Di Lorenzo PM. Responses to binary taste mixtures in the nucleus of the solitary tract: neural coding with firing rate. J Neurophysiol. 2008 doi: 10.1152/jn.01020.2007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Clarke SN, Ossenkopp KP. Taste reactivity responses in rats: influence of sex and the estrous cycle. Am J Physiol. 274(3):R718–24. doi: 10.1152/ajpregu.1998.274.3.R718. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12(16):3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 10(6):478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Conover KL, Shizgal P. Competition and summation between rewarding effects of sucrose and lateral hypothalamic stimulation in the rat. Behav Neurosci. 1994;108:537–548. doi: 10.1037//0735-7044.108.3.537. [DOI] [PubMed] [Google Scholar]
- Contreras RJ. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 1977;121:373–378. doi: 10.1016/0006-8993(77)90162-7. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979;73:569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst. 1982;6(3):303–22. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Kosten T, Frank ME. Activity in salt taste fibers: peripheral mechanism for mediating changes in salt intake. Chem Senses. 1984;8:275–288. [Google Scholar]
- Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav. 2004;80(5):657–64. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Stratford JM, Contreras RJ. Estrogen increases the taste threshold for sucrose in rats. Physiol Behav. 2005;86(3):281–6. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301(5634):850–3. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31(3):253–64. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G, Tinti JM, Nofre C. Gustatory responses of the hamster Mesocricetus auratus to various compounds considered sweet by humans. J Neurophysiol. 1998;80(4):2102–12. doi: 10.1152/jn.1998.80.4.2102. [DOI] [PubMed] [Google Scholar]
- Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav. 1973;11(1):39–45. doi: 10.1016/0031-9384(73)90120-0. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Collins BK, Levine MW. Peripheral control of meal size: interaction of gustatory stimulation and postingestional feedback. In: Novin D, Wyrwicka W, Bray G, editors. Hunger: Basic mechanisms and clinical implications. Raven Press; New York: 1976. pp. 395–408. [Google Scholar]
- de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr, Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51(4):483–94. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 26(15):3971–80. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 2006;31(4):351–7. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Hecht GS. Perceptual consequences of electrical stimulation in the gustatory system. Behav Neurosci. 1993;107(1):130–8. doi: 10.1037/0735-7044.107.1.130. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of male, female and pregnant rats. Brain Res Bull. 1989;23(3):219–27. doi: 10.1016/0361-9230(89)90151-2. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of ovariectomized rats. Brain Res Bull. 1990;25(5):741–8. doi: 10.1016/0361-9230(90)90052-2. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol. 2003;90(3):1418–31. doi: 10.1152/jn.00177.2003. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol. 1986;3:55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Hallock RM, Kennedy DP. Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci. 2003;117(6):1423–33. doi: 10.1037/0735-7044.117.6.1423. [DOI] [PubMed] [Google Scholar]
- DiNardo LA, Travers JB. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. J Neurosci. 17(10):3826–39. doi: 10.1523/JNEUROSCI.17-10-03826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Spector AC. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem Senses. 2004;29:489–498. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC. PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses. 2005;30(7):593–600. doi: 10.1093/chemse/bji053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas du Villard X, Her C, MacLeod P. Qualitative discrimination of sweet stimuli: behavioural study on rats. Chem Senses. 1981;6:143–148. [Google Scholar]
- Erickson RP. Parallel “population” neural coding in feature extraction. In: Schmitt FO, Worden FG, editors. The Neurosciences: Third study program. MIT Press; Cambridge: 1974. pp. 155–170. [Google Scholar]
- Erickson RP. The evolution of neural coding ideas in the chemical senses. Physiol Behav. 2000;69:3–13. doi: 10.1016/s0031-9384(00)00193-1. [DOI] [PubMed] [Google Scholar]
- Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in Sac ‘taster’ and ‘non-taster’ mice. Chem Senses. 2004;29(7):639–49. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–9. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Fish HS, Malone PD, Richter CP. The antomy of the tongue of the domestic Norway rat. Anat Rec. 1944;89:429–440. [Google Scholar]
- Flynn FW, Grill HJ. Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci. 1988;102(6):934–41. doi: 10.1037//0735-7044.102.6.934. [DOI] [PubMed] [Google Scholar]
- Formaker BK, MacKinnon BI, Hettinger TP, Frank ME. Opponent effects of quinine and sucrose on single fiber taste responses of the chorda tympani nerve. Brain Res. 1997;772:239–42. doi: 10.1016/s0006-8993(97)00845-7. [DOI] [PubMed] [Google Scholar]
- Formaker BK, Stapleton JR, Roper SD, Frank ME. Responses of the rat chorda tympani nerve to glutamate-sucrose mixtures. Chem Senses. 2004;29(6):473–82. doi: 10.1093/chemse/bjh049. [DOI] [PubMed] [Google Scholar]
- Foster LA, Boeshore K, Norgren R. Intestinal fat suppressed intake of fat longer than intestinal sucrose. Physiol Behav. 1998;64(4):451–5. doi: 10.1016/s0031-9384(98)00053-5. [DOI] [PubMed] [Google Scholar]
- Frank ME, Blizard DA. Chorda tympani responses in two inbred strains of mice with different taste preferences. Physiol Behav. 1999;67(2):287–97. doi: 10.1016/s0031-9384(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Frank ME, Formaker BK, Hettinger TP. Peripheral gustatory processing of sweet stimuli by golden hamsters. Brain Res Bull. 2005;66(1):70–84. doi: 10.1016/j.brainresbull.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Fregley MJ. Specificity of the sodium chloride appetite of adrenalectomized rats: Substitution of lithium chloride for sodium chloride. Am J Physiol. 1958;195:645–653. doi: 10.1152/ajplegacy.1958.195.3.645. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Single-locus control of saccharin preference in mice. J Hered 1974. 1974;65(1):33–6. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- Gabri D, Soljaci M. Effect of gonadectomy on taste preference for glucose solutions in rats. Physiol Behav. 1975;15(2):145–8. doi: 10.1016/0031-9384(75)90227-9. [DOI] [PubMed] [Google Scholar]
- Ganchrow JR, Erickson RP. Neural correlates of gustatory intensity and quality. J Neurophysiol. 1970;33(6):768–83. doi: 10.1152/jn.1970.33.6.768. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122(3160):157–8. [PubMed] [Google Scholar]
- Geary N, Trace D, Smith GP. Estradiol interacts with gastric or postgastric food stimuli to decrease sucrose ingestion in ovariectomized rats. Physiol Behav. 1995;57(1):155–8. doi: 10.1016/0031-9384(94)00271-6. [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol. 2006;96(5):2513–27. doi: 10.1152/jn.00607.2006. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Nicolelis MA. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex. 1999;9(4):348–61. doi: 10.1093/cercor/9.4.348. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol. 2000;10(4):519–27. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR. Blood glucose selectively affects taste-evoked activity in rat nucleus tractus solitarius. Physiol Behav. 1983;31(5):643–50. [PubMed] [Google Scholar]
- Giza BK, Scott TR. Blood glucose level affects perceived sweetness intensity in rats. Physiol Behav. 1987a;41(5):459–64. doi: 10.1016/0031-9384(87)90081-3. [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR. Intravenous insulin infusions in rats decrease gustatory-evoked responses to sugars. Am J Physiol. 1987b;252:R994–1002. doi: 10.1152/ajpregu.1987.252.5.R994. [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR, Antonucci RF. Effect of cholecystokinin on taste responsiveness in rats. Am J Physiol. 1990;258:R1371–9. doi: 10.1152/ajpregu.1990.258.6.R1371. [DOI] [PubMed] [Google Scholar]
- Giza BK, Scott TR, Sclafani A, Antonucci RF. Polysaccharides as taste stimuli: their effect in the nucleus tractus solitarius of the rat. Brain Res. 1991;555(1):1–9. doi: 10.1016/0006-8993(91)90852-m. [DOI] [PubMed] [Google Scholar]
- Giza BK, Deems RO, Vanderweele DA, Scott TR. Pancreatic glucagon suppresses gustatory responsiveness to glucose. Am J Physiol. 1993;265:R1231–7. doi: 10.1152/ajpregu.1993.265.6.R1231. [DOI] [PubMed] [Google Scholar]
- Giza BK, McCaughey SA, Zhang L, Scott TR. Taste responses in the nucleus of the solitary tract in saccharin-preferring and saccharin-averse rats. Chem Senses. 1996;21(2):147–57. doi: 10.1093/chemse/21.2.147. [DOI] [PubMed] [Google Scholar]
- Giza BK, Ackroff K, McCaughey SA, Sclafani A, Scott TR. Preference conditioning alters taste responses in the nucleus of the solitary tract of the rat. Am J Physiol. 1997;273:R1230–40. doi: 10.1152/ajpregu.1997.273.4.R1230. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005a;30(4):299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of Tas1r3 polymorphisms. Chem Senses. 2005b;30:601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- Glenn JF, Erickson RP. Gastric modulation of gustatory afferent activity. Physiol Behav. 1976;16:561–568. doi: 10.1016/0031-9384(76)90216-x. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978a;143(2):263–79. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978b;143(2):281–97. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978c;201(4352):267–9. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol. 1988;254:R853–6. doi: 10.1152/ajpregu.1988.254.6.R853. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Roitman MF, Kaplan JM. A new taste reactivity analysis of the integration of taste and physiological state information. Am J Physiol. 1996;271:R677–687. doi: 10.1152/ajpregu.1996.271.3.R677. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Carmena JM, Nicolelis MA, Simon SA. Orbitofrontal ensemble activity monitors licking and distinguishes among natural rewards. J Neurophysiol. 2006;95(1):119–33. doi: 10.1152/jn.00467.2005. [DOI] [PubMed] [Google Scholar]
- Guttman N. Equal-reinforcement values for sucrose and glucose solutions compared with equal-sweetness values. J Comp Physiol Psychol. 1954;47(5):358–61. doi: 10.1037/h0062710. [DOI] [PubMed] [Google Scholar]
- Hagstrom EC, Pfaffmann C. The relative taste effectiveness of different sugars for the rat. J Comp Physiol Psychol. 1959;52(3):259–62. doi: 10.1037/h0040519. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84(3):363–9. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci. 1999;19(16):7182–90. doi: 10.1523/JNEUROSCI.19-16-07182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WG, Bryan TE. The ontogeny of feeding in rats: II. Independent ingestive behavior. J Comp Physiol Psych. 1980;94:746–756. [Google Scholar]
- Hall WG, Bryan TE. The ontogeny of feeding in rats: IV. Taste development as measured by intake and behavioral responses to oral infusions of sucrose and quinine. J Comp Physiol Psych. 1981;95:240–251. doi: 10.1037/h0077771. [DOI] [PubMed] [Google Scholar]
- Halpern BP. Chemotopic coding for sucrose and quinine hydrochloride in the nucleus of the fasciculus solitarius. In: Hayashi T, editor. Olfaction and Taste II. Pergamon; New York: 1967. pp. 549–562. [Google Scholar]
- Halpern BP. Time as a factor in gustation: temporal patterns of taste stimulation and response. In: Pfaff DW, editor. Taste, olfaction, and the central nervous system. Rockefeller University Press; New York: 1985. pp. 181–209. [Google Scholar]
- Hammer LR. Relationship of reinforcement value to consummatory behavior. J Comp Physiol Psych. 1968;66:667–672. doi: 10.1037/h0026554. [DOI] [PubMed] [Google Scholar]
- Harada S, Kasahara Y. Inhibitory effect of gurmarin on palatal taste responses to amino acids in the rat. Am J Physiol. 2000;278(6):R1513–7. doi: 10.1152/ajpregu.2000.278.6.R1513. [DOI] [PubMed] [Google Scholar]
- Harada S, Yamamoto T, Yamaguchi K, Kasahara Y. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses. 1997;22(2):133–40. doi: 10.1093/chemse/22.2.133. [DOI] [PubMed] [Google Scholar]
- Heck GI, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- Herness MS, Gilbertson TA. Cellular mechanisms of taste transduction. Annu Rev Physiol. 1999;61:873–900. doi: 10.1146/annurev.physiol.61.1.873. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Shen T, Lu SG, Cao Y. Communication routes within the taste bud by neurotransmitters and neuropeptides. Chem Senses. 2005;30:i37–i38. doi: 10.1093/chemse/bjh101. [DOI] [PubMed] [Google Scholar]
- Higgs S, Cooper SJ. Hyperphagia induced by direct administration of midazolam into the parabrachial nucleus of the rat. Eur J Pharmacol. 1996;313:1–9. doi: 10.1016/0014-2999(96)00446-3. [DOI] [PubMed] [Google Scholar]
- Hiji Y, Ito H. Removal of “sweetness” by proteases and its recovery mechanism in rat taste cells. Comp Biochem Physiol. 1977;58:109–113. [Google Scholar]
- Hoebel BG. Brain neurotransmitters in food and drug reward. Am J Clin Nutr. 1985;42:1133–50. doi: 10.1093/ajcn/42.5.1133. [DOI] [PubMed] [Google Scholar]
- Hrupka BJ, Smith GP, Geary N. Ovariectomy and estradiol affect postingestive controls of sucrose licking. Physiol Behav. 1997;61(2):243–7. doi: 10.1016/s0031-9384(96)00360-5. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–8. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104(15):6436–41. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto T, Miyasaka A, Ishima R, Akasaka K. A novel peptide isolated from the leaves of Gymnema sylvestre: I. Characterization and its suppressive effect on the neural responses to sweet taste stimuli in the rat. Comp Biochem Physiol A. 1991;100(2):309–14. doi: 10.1016/0300-9629(91)90475-r. [DOI] [PubMed] [Google Scholar]
- Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole-nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129. B6-Tas1r3 congenic mice. Physiol Genomics. 2007;32(1):82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Sato M. Inhibition of taste nerve responses to sugars and amino acids by cupric and zinc ions in mice. Chem Senses. 1986;1:79–88. [Google Scholar]
- Jacobs KM, Mark GP, Scott TR. Taste responses in the nucleus tractus solitarius of sodium-deprived rats. J Physiol. 1988;406:393–410. doi: 10.1113/jphysiol.1988.sp017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakinovich W., Jr Methyl 4,6-dichloro-4,6-dideoxy-gamma-D-galactopyranoside: an inhibitor of sweet taste responses in gerbils. Science. 1983;219:408–410. doi: 10.1126/science.6849141. [DOI] [PubMed] [Google Scholar]
- Jones LM, Fontanini A, Katz DB. Gustatory processing: a dynamic systems approach. Curr Opin Neurobiol. 2006;16(4):420–8. doi: 10.1016/j.conb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann NY Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34(3):386–95. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2003;957(2):193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21(12):4478–89. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney NJ, Redick JH. Effects of ovariectomy and subsequent estradiol replacement on intake of sweet solutions. Physiol Behav. 1980;24(4):807–9. doi: 10.1016/0031-9384(80)90418-7. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283(1):236–42. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- Koh SD, Teitelbaum P. Absolute behavioral taste thresholds in the rat. J Comp Physiol Psychol. 1961;54:223–9. doi: 10.1037/h0048474. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Nejad MS, Smith JC, Miller IJ, Jr, Beidler LM. The effect of bilateral sectioning of the chorda tympani and the greater superficial petrosal nerves on the sweet taste in the rat. Physiol Behav. 1987;41(5):495–501. doi: 10.1016/0031-9384(87)90086-2. [DOI] [PubMed] [Google Scholar]
- Lal H, Shearman GT, Cafaro MP. Discrimination of sucrose-taste by the rat as a potential bioassay for sweeteners. Drug Nutr Interact. 1982;1(2):119–24. [PubMed] [Google Scholar]
- Lemon CH, Smith DV. Influence of response variability on the coding performance of central gustatory neurons. J Neurosci. 2006;26(28):7433–43. doi: 10.1523/JNEUROSCI.0106-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Imoto T, Smith DV. Differential gurmarin suppression of sweet taste responses in rat solitary nucleus neurons. J Neurophysiol. 2003;90(2):911–23. doi: 10.1152/jn.00215.2003. [DOI] [PubMed] [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol. 2004;92(1):536–44. doi: 10.1152/jn.00097.2004. [DOI] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78(4):834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85(5):546–56. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mamm Genome. 2001;12(1):13–6. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99(7):4692–6. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Taste reception. Physiol Rev. 1996;76(3):718–66. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- Lu K, McDaniel AH, Tordoff MG, Li X, Beauchamp GK, Bachmanov AA, VanderWeele DA, Chapman CD, Dess NK, Huang L, Wang H, Reed DR. No relationship between sequence variation in protein coding regions of the Tas1r3 gene and saccharin preference in rats. Chem Senses. 2005;30(3):231–40. doi: 10.1093/chemse/bji019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WC, Grace M, Billington CJ, Levine AS. Effects of neuropeptide Y on ingestion of flavored solutions in nondeprived rats. Physiol Behav. 1993;54(5):877–80. doi: 10.1016/0031-9384(93)90295-q. [DOI] [PubMed] [Google Scholar]
- MacKinnon BI, Frank ME, Hettinger TP, Rehnberg BG. Taste qualities of solutions preferred by hamsters. Chem Senses. 1999;24(1):23–35. doi: 10.1093/chemse/24.1.23. [DOI] [PubMed] [Google Scholar]
- Manita S, Bachmanov AA, Li X, Beauchamp GK, Inoue M. Is glycine “sweet” to mice? Mouse strain differences in perception of glycine taste. Chem Senses. 2006;31(9):785–93. doi: 10.1093/chemse/bjl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551(1–2):308–10. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- Marks HE. Reactivity to different saccharin concentrations as a function of testing procedure and alterations in body weight of intact and oophorectomized female rats. Physiol Behav. 1974;12(1):29–38. doi: 10.1016/0031-9384(74)90064-x. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28(1):58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- McCaughey SA. Taste-evoked responses to sweeteners in the nucleus of the solitary tract differ between C57BL/6ByJ and 129P3/J mice. J Neurosci. 2007;27:35–45. doi: 10.1523/JNEUROSCI.3672-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey SA, Scott TR. The taste of sodium. Neurosci Biobehav Rev. 1998;22(5):663–76. doi: 10.1016/s0149-7634(97)00067-5. [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Scott TR. Rapid induction of sodium appetite modifies taste-evoked activity in the rat nucleus of the solitary tract. Am J Physiol. 2000;279:R1121–R1131. doi: 10.1152/ajpregu.2000.279.3.R1121. [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Tordoff MG. Calcium deprivation alters gustatory-evoked activity in the rat nucleus of the solitary tract. Am J Physiol. 2001;281(3):R971–8. doi: 10.1152/ajpregu.2001.281.3.R971. [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Giza BK, Nolan LJ, Scott TR. Extinction of a conditioned taste aversion in rats: II. Neural effects in the nucleus of the solitary tract. Physiol Behav. 1997;61(3):373–9. doi: 10.1016/s0031-9384(96)00412-x. [DOI] [PubMed] [Google Scholar]
- McCaughey SA, Forestell CA, Tordoff MG. Calcium deprivation increases the palatability of calcium solutions in rats. Physiol Behav. 2005;84:335–342. doi: 10.1016/j.physbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Miller IJ. Anatomy of the peripheral taste system. In: Doty RL, editor. Handbook of Olfaction and Gustation. Marcel Dekker, Inc; New York: 1995. pp. 521–57. [Google Scholar]
- Mistretta CM. A quantitative analysis of rat chorda tympani fiber discharge patterns. In: Schneider D, editor. Olfaction and Taste IV. Wissenschaftliche; Stuttgart: 1972. pp. 294–300. [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4(5):492–8. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Mook DG. Oral and postingestional determinants of the intake of various solutions in rats with esophageal fistulas. J Comp Physiol Psychol. 1963;56:645–659. [Google Scholar]
- Mook DG, Brane JA, Kushner LR, Whitt JA. Glucose solution intake in the rat: the specificity of postingestive satiety. Appetite. 1983;4(1):1–9. doi: 10.1016/s0195-6663(83)80039-7. [DOI] [PubMed] [Google Scholar]
- Morrison GR. Taste psychophysics in animals. In: Pfaffmann C, editor. Olfaction and Taste III. Rockefeller University Press; New York: 1969. pp. 512–516. [Google Scholar]
- Morrison GR, Jessup A. A dual taste for saccharin in the rat. II. Taste changes following alloxan injection. Chem Senses Flavor. 1977;2:395–400. [Google Scholar]
- Morrison GR, Norrison W. Taste detection in the rat. Can J Psychol. 1966;20(2):208–17. doi: 10.1037/h0082938. [DOI] [PubMed] [Google Scholar]
- Moulton DG. Spatial patterning of response to odors in the peripheral olfactory system. Physiol Rev. 1976;56(3):578–93. doi: 10.1152/physrev.1976.56.3.578. [DOI] [PubMed] [Google Scholar]
- Murata Y, Nakashima K, Yamada A, Shigemura N, Sasamoto K, Ninomiya Y. Gurmarin suppression of licking responses to sweetener-quinine mixtures in C57BL mice. Chem Senses. 2003;28(3):237–43. doi: 10.1093/chemse/28.3.237. [DOI] [PubMed] [Google Scholar]
- Nachman M. The inheritance of saccharin preference. J Comp Physiol Psychol. 1959;52:451–457. doi: 10.1037/h0048853. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ueda K. Stochastic properties of gustatory impulse discharges in rat chorda tympani fibers. J Neurophysiol. 1981;45(3):574–92. doi: 10.1152/jn.1981.45.3.574. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: an extended stimulus array. J Neurophysiol. 1993;70(3):879–91. doi: 10.1152/jn.1993.70.3.879. [DOI] [PubMed] [Google Scholar]
- Nejad MS. The neural activities of the greater superficial petrosal nerve of the rats in response to chemical stimulation of the palate. Chem Senses. 1986;11:283–293. [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106(3):381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 2005;15(21):1948–52. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res. 1984a;322(1):83–92. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Mizukoshi T, Higashi T, Katsukawa H, Funakoshi M. Gustatory neural responses in three different strains of mice. Brain Res. 1984b;302(2):305–14. doi: 10.1016/0006-8993(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Katsukawa H, Funakoshi M. Taste receptor mechanisms influenced by a gene on chromosome 4 in mice. In: Wysocki CJ, Kare MR, editors. Genetics of Perception and Communication. Marcel Dekker; New York: 1991. pp. 267–278. [Google Scholar]
- Ninomiya Y, Inoue M, Imoto T, Nakashima K. Lack of gurmarin sensitivity of sweet taste receptors innervated by the glossopharyngeal nerve in C57BL mice. Am J Physiol. 1997;272:R1002–6. doi: 10.1152/ajpregu.1997.272.3.R1002. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Imoto T, Sugimura T. Sweet taste responses of mouse chorda tympani neurons: existence of gurmarin-sensitive and -insensitive receptor components. J Neurophysiol. 1999;81(6):3087–91. doi: 10.1152/jn.1999.81.6.3087. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Norgren R. Parabrachial gustatory neural responses to monosodium glutamate ingested by awake rats. Physiol Behav. 1991;49(5):965–71. doi: 10.1016/0031-9384(91)90209-7. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Uwano T, Tamura R, Ono T. Gustatory and multimodal neuronal responses in the amygdala during licking and discrimination of sensory stimuli in awake rats. J Neurophysiol. 1998;79(1):21–36. doi: 10.1152/jn.1998.79.1.21. [DOI] [PubMed] [Google Scholar]
- Nolan LJ, Scott TR. The effect of food deprivation on gustatory-evoked activity in the nucleus tractus solitarius of the rat. Soc Neurosci Abstracts. 1994;20:1224. [Google Scholar]
- Norgren R. Gustatory responses in the hypothalamus. Brain Res. 1970;21(1):63–77. doi: 10.1016/0006-8993(70)90021-1. [DOI] [PubMed] [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Norgren R. A synopsis of gustatory neuroanatomy. In: Le Magnen J, MacLeod P, editors. Olfaction and Taste VI. Information Retrieval; London: 1977. pp. 225–232. [Google Scholar]
- Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173:1136–1139. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89(4):531–5. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlis GH, Frank ME, Pfaffmann C. Specificity of acquired aversions to taste qualities in hamsters and rats. J Comp Physiol Psych. 1980;94:932–942. doi: 10.1037/h0077809. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Gustatory impulse discharges in response to saccharin in rats and hamsters. J Physiol. 1969;204(2):311–29. doi: 10.1113/jphysiol.1969.sp008915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Sato M, Yamashita S. Variability in impulse discharges in rat chorda tympani fibers in response to repeated gustatory stimulation. Physiol Behav. 1973;11:469–479. doi: 10.1016/0031-9384(73)90033-4. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Yamashita S, Sato M. Variation in gustatory nerve fiber discharge pattern with change in stimulus concentration and quality. J Neurophysiol. 1974;37(3):443–57. doi: 10.1152/jn.1974.37.3.443. [DOI] [PubMed] [Google Scholar]
- Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–11. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Grill HJ, Rozin P, Jacobs J. Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. J Comp Psychol. 1983;97(2):140–53. [PubMed] [Google Scholar]
- Perez C, Sclafani A. Developmental changes in sugar and starch taste preferences in young rats. Physiol Behav. 1990;48(1):7–12. doi: 10.1016/0031-9384(90)90252-y. [DOI] [PubMed] [Google Scholar]
- Perrotto RS, Scott TR. Gustatory neural coding in the pons. Brain Res. 1976;110(2):283–300. doi: 10.1016/0006-8993(76)90403-0. [DOI] [PubMed] [Google Scholar]
- Pfaffmann C. De Gustibus: Praeteriti, Praesentis, Futuri. In: Pfaff DW, editor. Taste, Olfaction, and the Central Nervous System, New York. Rockefeller University Press; New York: 1985. pp. 19–44. [Google Scholar]
- Pfaffmann C, Norgren R, Grill HJ. Sensory affect and motivation. Ann NY Acad Sci. 1977;290:18–34. doi: 10.1111/j.1749-6632.1977.tb39713.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18(4):931–41. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Thresholds for starch and Polycose are lower than for sucrose in rats. Physiol Behav. 1991;50(4):699–703. doi: 10.1016/0031-9384(91)90005-9. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Sucrose and fructose have qualitatively different flavors to rats. Physiol Behav. 1994;56:549–554. doi: 10.1016/0031-9384(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Ramirez I. Is fructose sweeter than glucose for rats? Physiol Behav. 1996;60(5):1299–306. doi: 10.1016/s0031-9384(96)00259-4. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24(4):938–46. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Campbell KH. Sucrose taste thresholds of rats and humans. Am J Physiol. 1940a;128:291–297. [Google Scholar]
- Richter CP, Campbell KH. Taste thresholds and taste preferences of rats for five common sugars. J Nutr. 1940b;20:31–46. [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45(4):587–97. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26(3):657–67. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77(3):896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- Sako N, Shimura T, Komure M, Mochizuki R, Marsuo R, Yamamoto Y. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav. 1994;56:741–745. doi: 10.1016/0031-9384(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Sanematsu K, Yasumatsu K, Yoshida R, Shigemura N, Ninomiya Y. Mouse strain differences in Gurmarin-sensitivity of sweet taste responses are not associated with polymorphisms of the sweet receptor gene, Tas1r3. Chem Senses. 2005;30(6):491–6. doi: 10.1093/chemse/bji041. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Sato M, Yamashita S, Ogawa H. Afferent specificity in taste. In: Pfaffmann C, editor. Olfaction and Taste III. The Rockefeller University Press; New York: 1969. pp. 470–487. [Google Scholar]
- Schneider LH, Gibbs J, Smith GP. D-2 selective receptor antagonists suppress sucrose sham feeding in the rat. Brain Res Bull. 1986;17(4):605–11. doi: 10.1016/0361-9230(86)90231-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Starch and sugar tastes in rodents: an update. Brain Res Bull. 1991;27:383–6. doi: 10.1016/0361-9230(91)90129-8. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81(5):773–9. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav. 2006a;87:734–744. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav. 2006b;87:745–756. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiol Behav. 2007;90(4):602–11. doi: 10.1016/j.physbeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Abrams M. Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav. 1986;37(2):253–6. doi: 10.1016/0031-9384(86)90228-3. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol. 2005;289(3):R712–20. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Mann S. Carbohydrate taste preferences in rats: glucose, sucrose, maltose, fructose, and polycose compared. Physiol Behav. 1987;40:563–568. doi: 10.1016/0031-9384(87)90097-7. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 1993;265(2 Pt 2):R320–5. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- Scott TR, Erickson RP. Synaptic processing of taste-quality information in thalamus of the rat. J Neurophysiol. 1971;34(5):868–83. doi: 10.1152/jn.1971.34.5.868. [DOI] [PubMed] [Google Scholar]
- Scott TR, Giza BK. A measure of taste intensity discrimination in the rat through conditioned taste aversions. Physiol Behav. 1987;41(4):315–20. doi: 10.1016/0031-9384(87)90394-5. [DOI] [PubMed] [Google Scholar]
- Scott TR, Giza BK. Issues of gustatory neural coding: where they stand today. Physiol Behav. 2000;69:65–76. doi: 10.1016/s0031-9384(00)00189-x. [DOI] [PubMed] [Google Scholar]
- Scott TR, Perrotto RS. Intensity coding in pontine taste area: gustatory information is processed similarly throughout rat’s brain stem. J Neurophysiol. 1980;44(4):739–50. doi: 10.1152/jn.1980.44.4.739. [DOI] [PubMed] [Google Scholar]
- Scott TR, Yalowitz MS. Thalamic taste responses to changing stimulus concentration. Chem Senses. 1978;3:167–175. [Google Scholar]
- Segato EN, Reboucas EC, Freitas RL, Caires MP, Cardoso AV, Resende GC, Shimizu-Bassi G, Elias-Filho DH, Coimbra NC. Effect of chronic intake of sweet substance on nociceptive thresholds and feeding behavior of Rattus norvegicus (Rodentia, Muridae) Nutr Neurosci. 2005;8(2):129–40. doi: 10.1080/10284150500069413. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Yasumatsu K, Yoshida R, Sako N, Katsukawa H, Nakashima K, Imoto T, Ninomiya Y. The role of the dpa locus in mice. Chem Senses. 2005;30:i84–i85. doi: 10.1093/chemse/bjh125. [DOI] [PubMed] [Google Scholar]
- Shuford EH., Jr Palatability and osmotic pressure of glucose and sucrose solutions as determinants of intake. J Comp Physiol Psychol. 1959;52(2):150–3. doi: 10.1037/h0044382. [DOI] [PubMed] [Google Scholar]
- Smith DV. Brainstem processing of gustatory information. In: Pfaff DW, editor. Taste, Olfaction, and the Central Nervous System. Rockefeller University Press; New York: 1985. pp. 151–177. [Google Scholar]
- Smith DV, Van Buskirk RL, Travers JB, Bieber SL. Gustatory neuron types in hamster brain stem. J Neurophysiol. 1983a;50(2):522–40. doi: 10.1152/jn.1983.50.2.522. [DOI] [PubMed] [Google Scholar]
- Smith DV, Van Buskirk RL, Travers JB, Bieber SL. Coding of taste stimuli by hamster brain stem neurons. J Neurophysiol. 1983b;50(2):541–58. doi: 10.1152/jn.1983.50.2.541. [DOI] [PubMed] [Google Scholar]
- Smith DV, St John SJ, Boughter JD. Neuronal cell types and taste quality coding. Physiol Behav. 2000;69(1–2):77–85. doi: 10.1016/s0031-9384(00)00190-6. [DOI] [PubMed] [Google Scholar]
- Smith M, Duffy M. Consumption of sucrose and saccharine by hungry and satiated rats. J Comp Physiol Psychol. 1957;50(1):65–9. doi: 10.1037/h0041824. [DOI] [PubMed] [Google Scholar]
- Söderpalm AH, Berridge KC. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 2000;877(2):288–97. doi: 10.1016/s0006-8993(00)02691-3. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. In vivo recordings from rat geniculate ganglia: taste response properties of individual greater superficial petrosal and chorda tympani neurones. J Physiol. 2005;564:877–93. doi: 10.1113/jphysiol.2005.083741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124(2):134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4(3):143–91. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 1997;272:R1210–8. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112(3):678–94. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26(4):1247–59. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and gustducin in palatal taste buds of mice. Chem Senses. 2007;32(3):255–62. doi: 10.1093/chemse/bjl053. [DOI] [PubMed] [Google Scholar]
- Striem BJ, Pace U, Zehavi U, Naim M, Lancet D. Sweet tastants stimulate adenylate cyclase coupled to GTP-binding protein in rat tongue membranes. Biochem J. 1989;260(1):121–6. doi: 10.1042/bj2600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M, Shiba Y. Genetic tracing shows segregation of taste neuronal circuitries for bitter and sweet. Science. 2005;309(5735):781–5. doi: 10.1126/science.1110787. [DOI] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636(2):202–8. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Tamura R, Norgren R. Intracranial renin alters gustatory neural responses in the nucleus of the solitary tract of rats. Am J Physiol. 2003;284(4):R1108–18. doi: 10.1152/ajpregu.00574.2002. [DOI] [PubMed] [Google Scholar]
- Thaw AK, Smith JC. Conditioned suppression as a method of detecting taste thresholds in the rat. Chem Senses. 1992;17:211–223. [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96(5):2399–409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Tokita K, Karádi Z, Shimura T, Yamamoto T. Centrifugal inputs modulate taste aversion learning associated parabrachial neuronal activities. J Neurophysiol. 2004;92(1):265–79. doi: 10.1152/jn.01090.2003. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 2007;27(40):10840–8. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonosaki K, Funakoshi M. Cyclic nucleotides may mediate taste transduction. Nature. 1988;331(6154):354–6. doi: 10.1038/331354a0. [DOI] [PubMed] [Google Scholar]
- Torregrossa AM, Davis JD, Smith GP. Orosensory stimulation is sufficient and postingestive negative feedback is not necessary for neuropeptide Y to increase sucrose intake. Physiol Behav. 2006;87(4):773–80. doi: 10.1016/j.physbeh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Travers JB, Smith DV. Gustatory sensitivities in neurons of the hamster nucleus tractus solitarius. Sens Processes. 1979;3(1):1–26. [PubMed] [Google Scholar]
- Travers SP, Norgren R. The time course of solitary nucleus gustatory responses: influence of stimulus and site of application. Chem Senses. 1989;14:55–74. [Google Scholar]
- Travers SP, Norgren R. Coding the sweet taste in the nucleus of the solitary tract: differential roles for anterior tongue and nasoincisor duct gustatory receptors in the rat. J Neurophysiol. 1991;65(6):1372–80. doi: 10.1152/jn.1991.65.6.1372. [DOI] [PubMed] [Google Scholar]
- Travers SP, Pfaffmann C, Norgren R. Convergence of lingual and palatal gustatory neural activity in the nucleus of the solitary tract. Brain Res. 1986;365(2):305–20. doi: 10.1016/0006-8993(86)91642-2. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395(6700):384–7. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156(3777):942–3. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Van Buskirk RL, Smith DV. Taste sensitivity of hamster parabrachial pontine neurons. J Neurophysiol. 1981;45(1):144–71. doi: 10.1152/jn.1981.45.1.144. [DOI] [PubMed] [Google Scholar]
- Verhagen JV. The neurocognitive bases of human multimodal food perception: consciousness. Brain Res Rev. 2007;53(2):271–86. doi: 10.1016/j.brainresrev.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Giza BK, Scott TR. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol. 2003;89(1):265–75. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- Vigorito M, Sclafani A. Ontogeny of polycose and sucrose appetite in neonatal rats. Dev Psychobiol. 1988;21(5):457–65. doi: 10.1002/dev.420210505. [DOI] [PubMed] [Google Scholar]
- Vogt MB, Smith DV. Responses of single hamster parabrachial neurons to binary taste mixtures: mutual suppression between sucrose and QHCl. J Neurophysiol. 1993;69(3):658–68. doi: 10.1152/jn.1993.69.3.658. [DOI] [PubMed] [Google Scholar]
- Weingarten HP, Watson SD. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav. 1982;28:401–407. doi: 10.1016/0031-9384(82)90131-7. [DOI] [PubMed] [Google Scholar]
- Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- Willner P, Papp M, Phillips G, Maleeh M, Muscat R. Pimozide does not impair sweetness discrimination. Psychopharmacology (Berl) 1990;102(2):278–82. doi: 10.1007/BF02245934. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Xenakis S, Sclafani A. The effects of pimozide on the consumption of a palatable saccharin-glucose solution in the rat. Pharmacol Biochem Behav. 1981;15(3):435–42. doi: 10.1016/0091-3057(81)90274-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101(39):14258–63. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Imoto T. Inhibitory effect of the extract from Zizyphus jujuba leaves on sweet taste responses of the chorda tympani in the rat and hamster. Comp Biochem Physiol A. 1987;88(2):355–60. doi: 10.1016/0300-9629(87)90497-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Taste responses of cortical neurons. Prog Neurobiol. 1984;23(4):273–315. doi: 10.1016/0301-0082(84)90007-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Brain mechanisms of sweetness and palatability of sugars. Nutr Rev. 2003;61:S5–9. doi: 10.1301/nr.2003.may.S5-S9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–255. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kawamura Y. Gustatory reaction time in human adults. Physiol Behav. 1981;26(4):715–9. doi: 10.1016/0031-9384(81)90149-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I Response characteristics. J Neurophysiol. 1984;51(4):616–35. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste responses of cortical neurons in freely ingesting rats. J Neurophysiol. 1989;61:1244–1258. doi: 10.1152/jn.1989.61.6.1244. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65(2):123–37. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Maeda S. Effects of taste stimulation on beta-endorphin levels in rat cerebrospinal fluid and plasma. Physiol Behav. 2000;69(3):345–50. doi: 10.1016/s0031-9384(99)00252-8. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Shimura T, Yamamoto T. Single unit responses of the amygdala after conditioned taste aversion in conscious rats. Neuroreport. 1995;6(17):2424–8. doi: 10.1097/00001756-199511270-00034. [DOI] [PubMed] [Google Scholar]
- Young PT, Greene JT. Relative acceptability of saccharine solutions as revealed by different methods. J Comp Physiol Psychol. 1953;46(4):295–8. doi: 10.1037/h0056212. [DOI] [PubMed] [Google Scholar]
- Young PT, Shuford EH., Jr Quantitative control of motivation through sucrose solutions of different concentrations. J Comp Physiol Psychol. 1955;48(2):114–8. doi: 10.1037/h0045576. [DOI] [PubMed] [Google Scholar]
- Zawalich WS. Depression of gustatory sweet response by alloxan. Comp Biochem Physiol A. 1973;44(3):903–9. doi: 10.1016/0300-9629(73)90154-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27(21):5777–86. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115(3):255–66. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zucker I. Hormonal determinants of sex differences in saccharin preference, food intake and body weight. Physiol Behav. 1969;4:595–602. [Google Scholar]