Abstract
Evidence indicates that dopamine receptors regulate processes of procedural learning in the sensorimotor striatum. Our previous studies revealed that the indirect dopamine receptor agonist cocaine alters motor-skill learning-associated gene regulation in the sensorimotor striatum. Cocaine-induced gene regulation in the striatum is principally mediated by D1 dopamine receptors. We investigated the effects of cocaine and striatal D1 receptor antagonism on motor-skill learning. Rats were trained on a running wheel (40–60 min, 2–5 days) to learn a new motor skill, that is, the ability to control the movement of the wheel. Immediately before each training session, the animals received an injection of vehicle or cocaine (25 mg/kg, i.p.), and/or the D1 receptor antagonist SCH-23390 (0, 3, 10 μg/kg, i.p., or 0, 0.3, 1 μg, intrastriatal via chronically implanted cannula). The animal’s ability to control/balance the moving wheel (wheel skill) was tested before and repeatedly after the training. Normal wheel-skill memory lasted for at least 4 weeks. Cocaine administered before the training tended to attenuate skill learning. Systemic administration of SCH-23390 alone also impaired skill learning. However, cocaine given in conjunction with the lower SCH-23390 dose (3 μg/kg) reversed the inhibition of skill learning produced by the D1 receptor antagonist, enabling intact skill performance during the whole post-training period. In contrast, when cocaine was administered with the higher SCH-23390 dose (10 μg/kg), skill performance was normalized 1–6 days after the training, but these rats lost their improved wheel skill by day 18 after the training. Similar effects were produced by SCH-23390 (0.3–1 μg) infused into the striatum. Our results indicate that cocaine interferes with normal motor-skill learning, which seems to be dependent on optimal D1 receptor signaling. Furthermore, our findings demonstrate that D1 receptors in the striatum are critical for consolidation of long-term skill memory.
Keywords: cocaine, D1 receptor, dopamine, running wheel, skill learning, striatum
Introduction
Previous research showed that the dorsal striatum mediates forms of procedural learning, including habit (S-R) learning and motor-skill learning (e.g., Graybiel, 1995; Knowlton et al., 1996; Packard and Knowlton, 2002). The cellular and molecular processes underlying this type of learning, however, remain poorly understood. Evidence indicates that striatal dopamine regulates such processes. For example, studies showed that dopamine receptor agonists infused into the striatum modified procedural maze learning (Packard and White, 1991; Packard et al., 1994). Conversely, skill learning in a rotarod task was impaired by moderate striatal dopamine depletion (Ogura et al., 2005).
It has been proposed that exposure to psychostimulants such as cocaine and amphetamine can lead to abnormal habit learning as a consequence of excessive stimulation of striatal dopamine receptors and ensuing molecular adaptions in striatal neurons (White, 1996; Berke and Hyman, 2000; Everitt et al., 2001). This notion is based in part on findings showing that psychostimulants often affect the same molecular mechanisms that mediate learning and memory in various brain systems (Berke and Hyman, 2000; Nestler, 2001; Kelley, 2004). For example, psychostimulant-induced molecular changes include altered expression of genes that encode transcription factors such as c-fos and zif 268 (e.g., Harlan and Garcia, 1998; Steiner and Gerfen, 1998), and synaptic scaffolding proteins such as homer (e.g., Swanson et al., 2001; Yano and Steiner, 2005). Whereas the former are implicated in various forms of neuroplasticity, including memory consolidation (Stork and Welzl, 1999; Davis et al., 2003), the latter are thought to directly regulate synaptic efficacy (Xiao et al., 2000; Thomas, 2002), presumably a mechanism of long-term memory formation.
We have recently employed c-fos and homer 1a as markers to map the distribution of motor-skill learning-associated molecular changes in the striatum and determine their modification by cocaine (Willuhn et al., 2003; Willuhn and Steiner, 2005; Willuhn and Steiner, 2006). In these studies, we used a novel motor-skill learning paradigm in which rats learn to run on a running wheel, a motor skill that rats acquire within 1–2 trials (Willuhn and Steiner, 2006). Our results showed that this wheel-skill learning is associated with transiently enhanced c-fos and homer 1a inducibility in specific parts of the sensorimotor striatum (Willuhn and Steiner, 2005; Willuhn and Steiner, 2006). These learning-related molecular changes were abnormally enhanced when the wheel-skill training occurred under the influence of cocaine (Willuhn et al., 2003; Willuhn and Steiner, 2005; Willuhn and Steiner, 2006).
In the present behavioral study, we further characterized the previously used wheel-skill learning task and developed and validated a novel test to measure long-term memory for this wheel skill. We also investigated how the same cocaine treatment that altered learning-associated gene regulation in the striatum in our previous studies (see above) affected this wheel-skill learning. Psychostimulant effects on gene regulation in the striatum are primarily mediated by D1 dopamine receptors (e.g., Graybiel et al., 1990; Steiner and Gerfen, 1995; Drago et al., 1996; Moratalla et al., 1996). Moreover, recent findings indicate that D1 receptors are involved in procedural learning (e.g., Eyny and Horvitz, 2003; Hale and Crowe, 2003). In the present study, we thus also investigated the role of striatal D1-type receptors in wheel-skill memory formation, by using systemic and intrastriatal administration of the D1 receptor antagonist SCH-23390 in doses that did not attenuate wheel running.
Experimental procedures
Subjects
Male Sprague–Dawley rats (180–220 g at the beginning of the experiments; Harlan, Madison, WI, USA) were housed in pairs under standard laboratory conditions on a 12:12 h light/dark cycle (lights on at 0700 h). They had free access to food (standard rodent chow) and water. Experiments 1 to 3 were conducted between 1700 and 1900 h, experiment 4 between 1300 and 1600 h. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
Running-wheel training
Training and testing were performed in the same room. In order to minimize the effects of surrounding noise, constant “white noise” was provided during both training and testing. Rats were trained on a running wheel once a day (40- or 60-min session) on 2 or 5 consecutive days. During the training session the animal was free to run, but could not leave the wheel. The running wheels (Wahmann Company, Baltimore, MD, USA) consisted of a rotating metal chamber with a wire mesh floor (diameter, 35 cm; width, 11 cm), attached to a stationary metal wall with an access opening that could be closed. A mechanical counter recorded full wheel revolutions.
Wheel-skill testing
The wheel-skill test is based on our previous findings showing that, in the beginning of the running-wheel training, rats are unable to run with an appropriate speed to remain at the bottom of the wheel (Willuhn and Steiner, 2006). The rat often moves too fast or too slow relative to the speed of the wheel. Consequently, because of the momentum of the wheel, the rat/wheel “swings”. During continued wheel training, the rat learns to minimize wheel swinging by adjusting its movements to control/balance the wheel (“wheel skill”; Willuhn and Steiner, 2006). The wheel-skill test assesses this skill by measuring the rat’s ability to stop the wheel from swinging.
The wheel skill was tested on the day before the first training session (“pre”) and repeatedly after the last training session (“post”). In experiments with only systemic drugs during the training, the animals were tested on post-days 1, 6, 18, 26, and in experiments with intrastriatal drug infusions, on post-days 1, 13, 27. One day before the pre-test the animal was habituated to test/training room and wheel by placing it on a locked wheel for 1 h. On the test day, the rat was again first placed inside the locked wheel for a 2-min habituation period. The wheel with the rat was then gently rotated by 90 degrees (rat’s head up) (Fig. 1a). Upon release, the wheel swung back and forth until the rat stopped the swinging by counterbalancing. Behavior on the wheel was videotaped for analysis. Wheel-skill performance was measured by counting the number of interruptions by the rat’s body (minus tail) of a horizontal line placed at one third of the wheel diameter above the wheel bottom (marked on the video monitor; see Fig. 1a), until the animal failed to interrupt for 3 sec. The reliability of this video analysis was confirmed by a high correlation (r=0.96–0.99) between 2 different raters, as determined in pilot studies. In all studies, two trials per test (inter-trial interval 1 min) were performed, and the scores were averaged for a measure of performance error. The treatment groups were balanced with respect to pre-test scores.
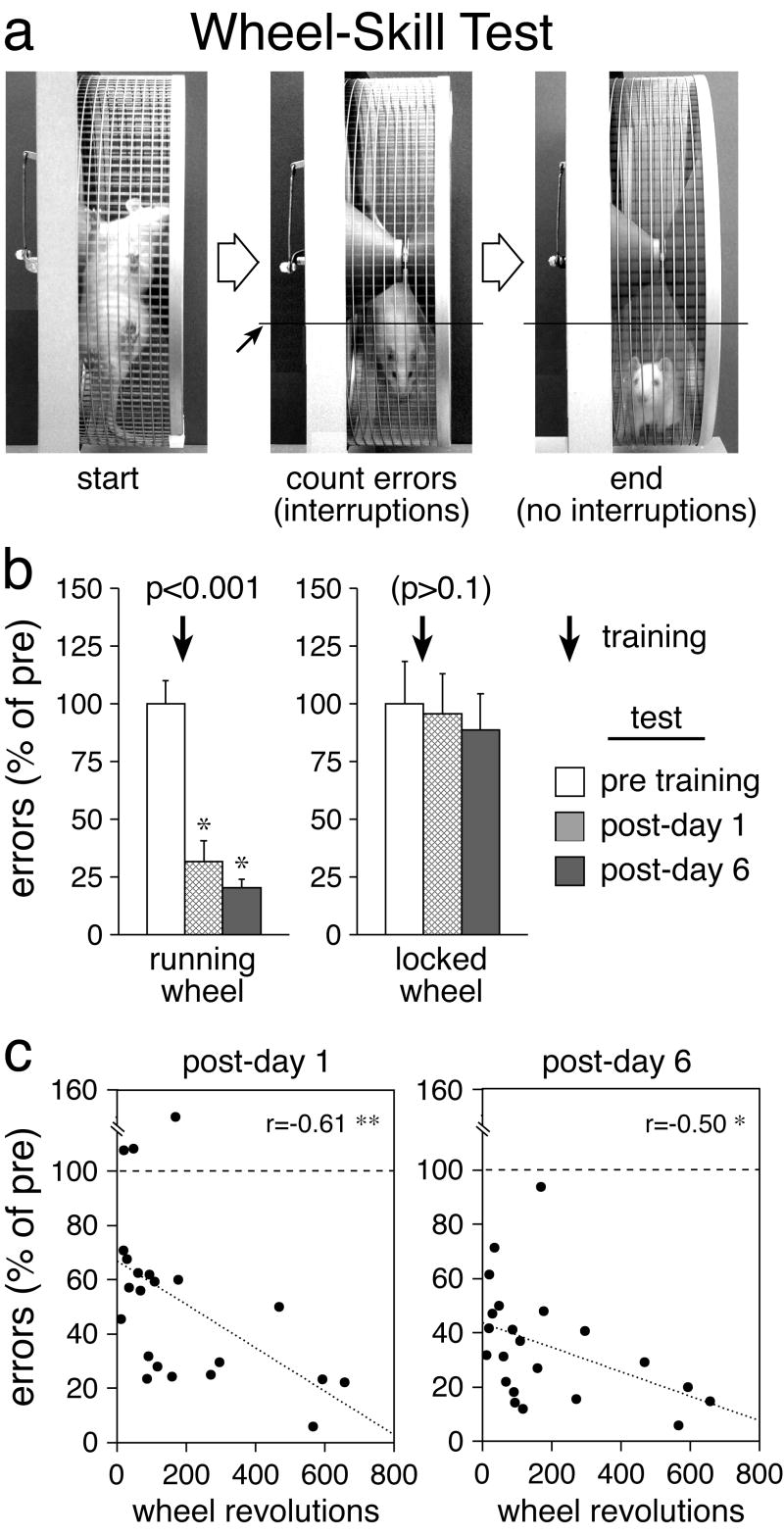
Fig. 1.
Motor-skill learning on the running wheel. a Video stills depict a rat during the wheel-skill test. To start, the wheel with the rat is rotated by 90 degrees (left) and released. The number of interruptions of a line marked on the video monitor (small arrow) by the rat’s body minus tail (middle) is counted until the rat fails to interrupt for 3 sec (right) (see Methods). During the running-wheel training, the rat learns to control/balance the wheel in order to reduce such swinging. These swings (interruptions) serve as an index of performance error. b The number (mean±SEM) of swings (errors) committed during the wheel-skill test before (pre) and 1 and 6 days after (post) a 2-day wheel training (60-min sessions, arrow), expressed as percentage of mean pre-test values, are shown for rats trained on a running wheel or on a locked wheel. The p values for the overall training effect (Friedman test) are also shown. * p<0.05, vs. pre (Wilcoxon test). c Scatter plots depict, for individual animals, the relationship between the total number of wheel revolutions during a 2-day training and the number of performance errors committed during skill tests 1 and 6 days after this training (expressed as percentage of pre-test values). ** p<0.01; * p<0.05.
Implantation of guide cannulas (experiment 4)
Rats were anesthetized with ketamine/xylazine (60/20 mg/kg) and given a dose of the analgesic Banamine (1.5 mg/kg, s.c.). A guide cannula (26 gauge; Plastics One, Roanoke, VA, USA), occluded by a “dummy cannula”, was implanted into the right striatum (A, −0.1 mm; L, 3.0 mm; V, −4.0 mm; Paxinos and Watson, 1998), aiming at the striatal sectors that previously showed wheel learning-associated changes in gene regulation (Willuhn and Steiner, 2006). One week later, one day before the first infusion, the dummy cannula was replaced with a longer one that protruded 2.5 mm beyond the tip of the guide cannula to reduce the probability of acute damage caused by the infusion cannula (33 gauge, 1 mm longer than the guide cannula). The infusion site in the striatum was confirmed after completion of the experiment (4 weeks after the infusions). To mark the track, the long dummy cannula was reinserted into the guide cannula, and the animal was then killed. Cannula placement was verified in Nissl-stained coronal sections (see Fig. 4d).
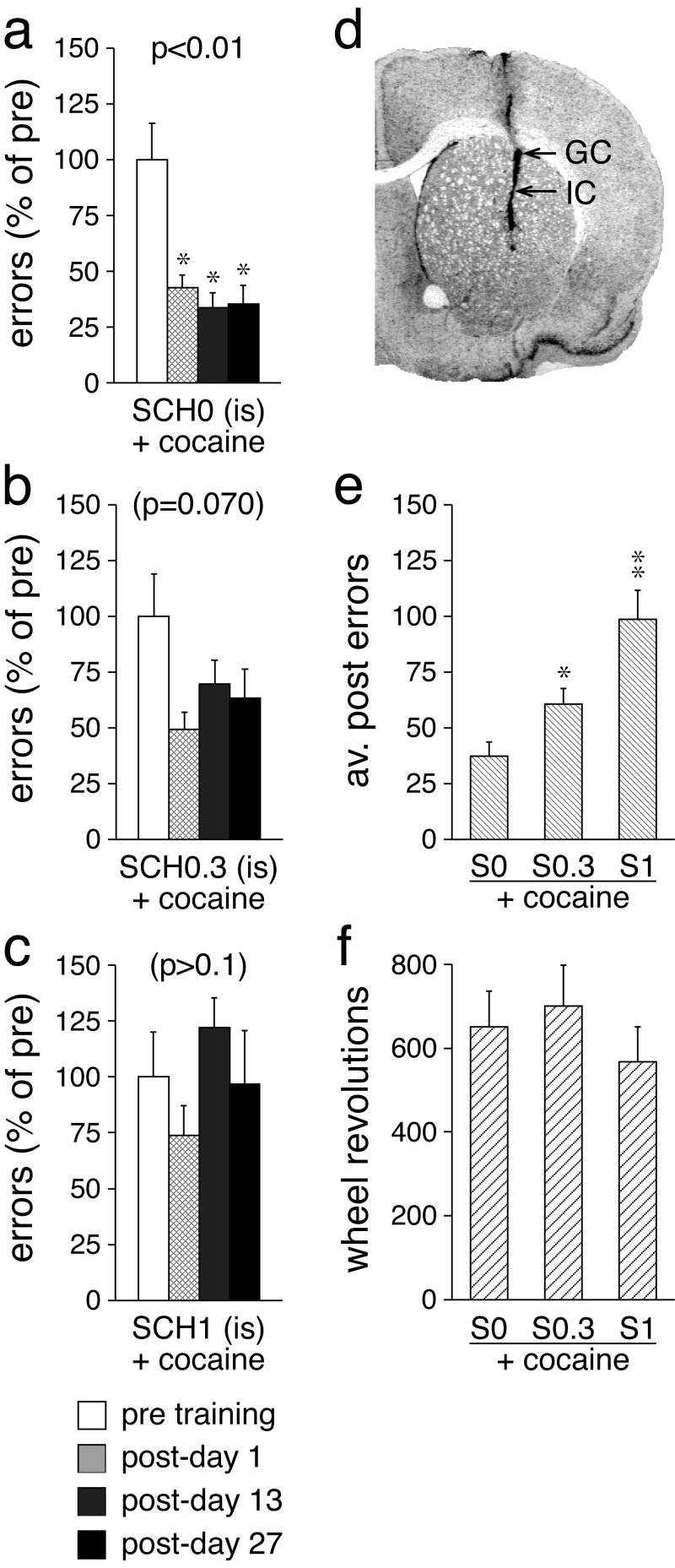
Fig. 4.
Effects of D1 receptor blockade in the striatum on motor-skill learning. a, b, c The number (mean±SEM) of performance errors (in percent of pre-test values) before (pre) and 1, 13 and 27 days after (post) a 2-day training (40-min sessions) are shown for groups of rats that received an intrastriatal (is) infusion of (a) vehicle (SCH0), (b) 0.3 yg (SCH0.3), or (c) 1 yg (SCH1) of SCH-23390 followed by an injection of cocaine (25 mg/kg), before each training session. d Photomicrograph of a Nissl-stained coronal section shows the track produced by the dummy cannula (see Methods). Arrows indicate the approximate position of the tip of the chronically implanted guide cannula (GC) and of the infusion cannula (IC). e The number (mean±SEM) of averaged post-training performance errors is given for the groups that received an intrastriatal infusion of vehicle (S0), 0.3 yg (S0.3), or 1 yg (S1) of SCH-23390 followed by the cocaine injection. f The total number (mean±SEM) of wheel revolutions during the 2-day training is shown for the above treatment groups. ** p<0.01; * p<0.05., vs. pre or S0.
Experimental designs and drug administration
Experiment 1 assessed the effects of practicing running on wheel-skill learning. Rats received an injection of vehicle (0.02% ascorbic acid, 1 ml/kg, i.p.) immediately before they were placed on a running wheel, on 2 days (60 min/day; n=8). Controls were confined to an identical, but immobilized (“locked”) wheel (n=8). Thus, the controls could freely move about the wheel, but could not run (turn the wheel). Experiment 2 investigated the effects of the training duration on wheel learning, with and without cocaine during the training. Animals trained on a running wheel on 2 or 5 days (60 min/day; n=7–8), after an injection of vehicle or cocaine (cocaine hydrochloride; Sigma, St. Louis, MO, USA; 25 mg/kg, in 0.02% ascorbic acid, i.p.). Experiment 3 assessed the effects of cocaine and/or systemic D1 receptor blockade during the training. Rats trained on a running wheel on 2 days (40 min/day), after receiving an injection of the D1 receptor antagonist SCH-23390 (R(+)-SCH-23390 hydrochloride; Sigma; 0, 3 or 10 μg/kg, in 0.02% ascorbic acid, i.p.; n=7–9), followed 10 min later by an injection of cocaine (25 mg/kg) or no injection. Experiment 4 investigated the effects of intrastriatal D1 receptor blockade plus systemic cocaine during the training. Rats received an intrastriatal infusion of SCH-23390 (0, 0.3 or 1 μg in 1 μl of saline; 0.2 μl/min; n=8–9), followed 10 min later by a cocaine injection (25 mg/kg) and the training, on 2 days (40 min/day). In both experiments, SCH-23390 doses were used that did not inhibit wheel running, as determined in pilot studies.
Statistical analysis
Effects of drug treatments and training conditions on skill-test performance after the training (averaged post-training test scores) were determined by nonparametric one-factor analysis of variance (Kruskal-Wallis test) or Mann-Whitney U test (between-group comparisons). Training effects over time within a group were assessed by nonparametric analysis of variance for repeated measures (Friedman test), and post hoc Wilcoxon paired-sample tests were used to describe differences between pre- and post-training test scores (Statistica, Statsoft, Tulsa, OK, USA). Treatment effects on wheel revolutions during the training were determined by Kruskal-Wallis and Mann-Whitney U tests. P values are used as a measure of confidence (Greenwald et al., 1996) to describe the effects; p values for pair-wise comparisons are two-tailed. In order to facilitate comparisons between experiments, the test scores are expressed as percentage of mean pre-test scores.
Results
Wheel-skill learning is dependent on practicing during the training
Experiment 1 assessed the effects of running- vs. locked-wheel training (2 days) on wheel-skill performance on days 1 and 6 after the training (Fig. 1b). In the pre-test, the “running wheel” group showed an average of 15.4±1.5 (mean±SEM) errors (swings) per trial, the “locked wheel” group 15.5±2.9 errors. Similar pre-test error counts were obtained in experiments 2–4 (not shown). Mann-Whitney U test comparing the averaged post-training error scores of running vs. locked groups revealed a significant effect of training condition (Z=3.16, p<0.01). Furthermore, the Friedman test comparing error scores over time revealed that the training on the running wheel significantly improved the subsequent performance in the wheel-skill test (χ2=14.0, p<0.001), as these rats committed significantly fewer performance errors than before the training on both post-days 1 and 6 (p<0.05, Wilcoxon test) (Fig. 1b). In contrast, animals that had no opportunity to practice their wheel skill (i.e., that were on the locked wheel) showed no improvement in their test performance (χ2=0.3, p>0.1) (Fig. 1b). These findings demonstrate that running (practicing) during the training is critical for this motor-skill learning.
To further clarify the relationship between practicing during the running-wheel training and wheel-skill learning, we performed a correlation analysis using the data of vehicle-treated rats from experiments 1, 2 and 3 (2-day training) (Fig. 1c). Our results show that, on both post-days 1 and 6, the number of errors committed during the skill test was negatively correlated with the amount of running during the training (total wheel revolutions in both sessions) (post-day 1, r=−0.61, p<0.01; post-day 6, r=−0.50, p<0.05; Spearman rank correlation; Fig. 1c). These findings demonstrate that wheel-skill improvement is directly related to the amount of practicing during the training.
Effects of cocaine treatment and training duration on wheel-skill learning
In experiment 2, the effects of varying the training duration (2 vs. 5 days), with and without cocaine, on wheel-skill learning were assessed on days 1, 6, 18, and 26 after the last training session (Fig. 2). Analysis of averaged post-training test scores showed that cocaine tended to impair skill learning, while longer training facilitated learning, but these effects did not reach statistical significance (p>0.05, Kruskal-Wallis test). Analysis of skill performance over time for individual groups confirmed these tendencies. Thus, vehicle-injected animals that trained on a running wheel for 2 days showed a significantly improved skill performance after the training compared to pre-training values (χ2=12.9, p<0.05, Friedman test), and they retained this skill for up to 26 days after the training (p<0.05) (Fig. 2a). In contrast, in cocaine-treated rats, the effect of the 2-day training on skill performance did not reach statistical significance (χ2=9.1, p=0.059), although a trend for fewer errors after the training was seen (Fig. 2a). After the 5-day training (Fig. 2b), rats treated with vehicle also displayed an improved wheel skill (χ2=14.1, p<0.01). Moreover, the post hoc analysis showed that these rats reached maximal skill performance already on post-day 1 (p<0.05), whereas vehicle-treated animals that trained for only 2 days continued to improve after the first post-test (Fig. 2a). In contrast, rats that trained under the influence of cocaine for 5 days again failed to show a significantly improved wheel skill after the training (χ2=9.0, p=0.061), but displayed a clear tendency for improvement (Fig. 2b).
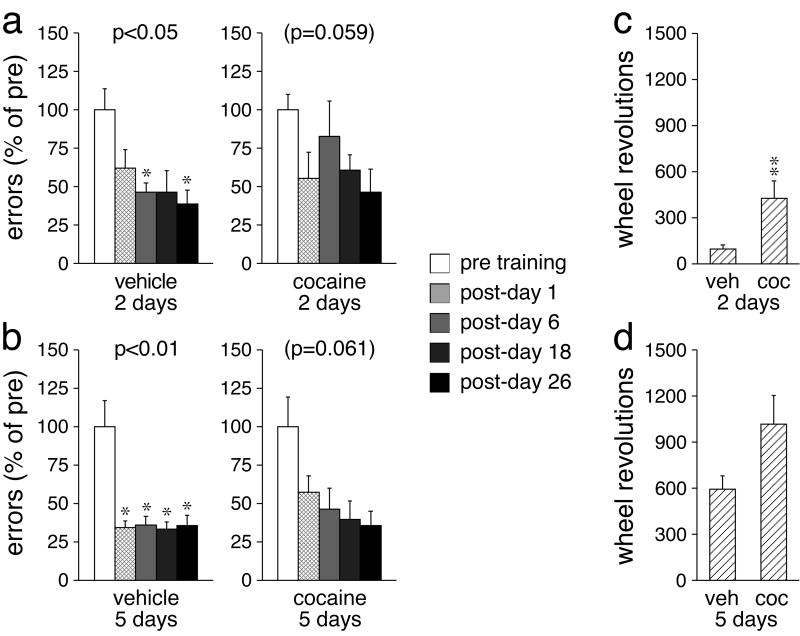
Fig. 2.
Effects of training duration and cocaine on motor-skill learning. a, b The number (mean±SEM) of errors (in percent of pre-test values) before (pre) and 1, 6, 18 and 26 days after (post) a 2-day (a) or 5-day (b) training (60-min sessions) are given for rats that received an injection of vehicle (left) or cocaine (25 mg/kg) (right) before each training session. c, d The total number (mean±SEM) of wheel revolutions during the 2-day (c) or 5-day training (d) is given for vehicle (veh)- and cocaine (coc)-treated groups. ** p<0.01, * p<0.05, vs. pre or vehicle.
Considering the importance of practicing (running) for skill learning (see above), we assessed and compared the total number of wheel revolutions during the training between the vehicle- and cocaine-treated groups. Mann-Whitney U tests showed that cocaine-treated animals tended to run more than vehicle-treated animals during both the 2-day (Z=2.62, p<0.01; Fig. 2c) and the 5-day training (Z=1.72, p=0.085; Fig. 2d). Thus, cocaine appeared to attenuate wheel-skill learning despite enhancing the amount of running (practice) during the training.
Effects of systemic blockade of D1 receptors during the training on wheel-skill learning
Experiment 3 investigated the effects of systemic D1 receptor antagonism, with and without cocaine, on wheel-skill learning (2-day training) (Fig. 3). The D1 receptor antagonist SCH-23390 (0, 3, 10 μg/kg) and cocaine were administered before each training session, and the wheel skill was evaluated on days 1, 6, 18 and 26 after the training. For averaged post-training test scores, statistical analysis revealed no significant overall effects of cocaine (p>0.05, Mann-Whitney U test) or SCH-23390 (p>0.05, Kruskal-Wallis test; data not shown). However, statistical analysis of test scores over time in individual groups showed that training effects varied depending on the drug treatment. Thus, vehicle only-treated rats (SCH0) displayed a highly significant training effect (χ2=19.3, p<0.001), with an improved wheel skill present at all time points up to 26 days after the training (p<0.05) (Fig. 3a). On the other hand, rats that received cocaine alone before each training session (SCH0+cocaine) only showed a marginal training effect (χ2=10.3, p<0.05), with wheel-skill improvement present until day 6 after the training (p<0.05).
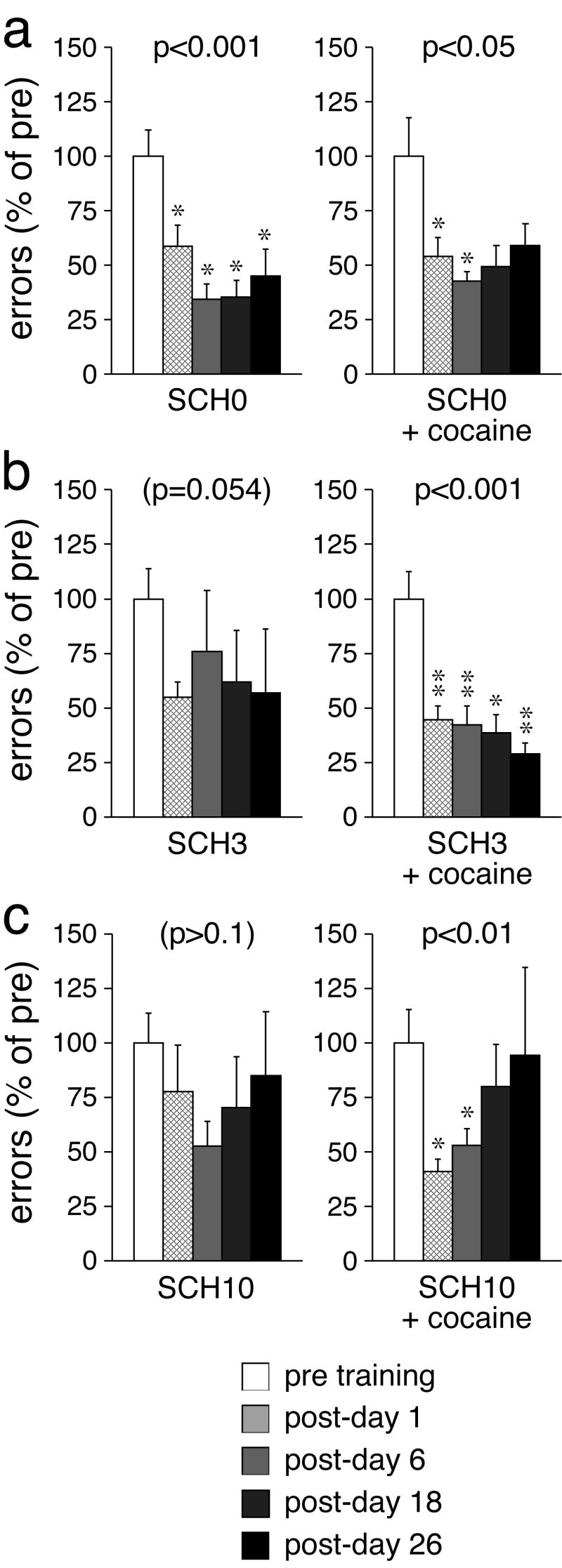
Fig. 3.
Effects of systemic D1 receptor blockade during running-wheel training on motor-skill learning. a, b, c The number (mean±SEM) of performance errors (in percent of pre-test values) before (pre) and 1, 6, 18 and 26 days after (post) a 2-day running-wheel training (40-min sessions) are given for groups of rats that received an injection of (a) vehicle (SCH0), (b) 3 yg/kg (SCH3), or (c) 10 yg/kg (SCH10) of the D1 receptor antagonist SCH-23390, either alone (left) or followed by an injection of cocaine (25 mg/kg) (right), before each training session. ** p<0.01, * p<0.05, vs. pre.
The effects of SCH-23390 were dose-dependent and, when administered in conjunction with cocaine, biphasic. Given alone the D1 receptor antagonist attenuated wheel-skill learning. Thus, no significant effect of the training on skill performance was found in rats that received 3 μg/kg (SCH3; χ2=9.3, p=0.054; Fig. 3b) or 10 μg/kg of SCH-23390 (SCH10; χ2=3.8, p>0.05; Fig. 3c) before each training session, although the group receiving the lower dose did show a trend for improvement (Fig. 3b). In contrast, animals treated with the lower dose of SCH-23390 (3 μg/kg) plus cocaine (SCH3+cocaine) displayed a highly significant training effect on wheel-skill performance (χ2=20.8, p<0.001), with an improved skill that was stable throughout the post-training period (post-days 1–26, p<0.05; Fig. 3b). Conversely, rats treated with the higher dose (10 μg/kg) plus cocaine (SCH10+cocaine) showed a significant training effect (χ2=13.9, p<0.01), but this effect did not last. While these rats did improve to a similar degree on days 1 and 6 after the training (p<0.05), they then lost their improved skill over time (post-days 18 and 26, p>0.05; Fig. 3c). Thus, when given with the lower dose of SCH-23390, cocaine improved both early (post-days 1–6) and late long-term skill memory (post-days 18–26), whereas when given with the higher dose of SCH-23390, this psychostimulant treatment only reversed the inhibition of early, but not late, long-term memory formation (Figs. 3b, c). Together, these results suggest that motor-skill learning is dependent on optimal D1 receptor stimulation. Moreover, D1 receptors appear to be critical for enduring long-term skill-memory consolidation/retrieval.
The number of wheel revolutions during the training was monitored to assess possible effects of the D1 receptor antagonist on running activity. No significant differences between SCH-23390- and vehicle-treated groups were found [SCH0 vs. SCH3 vs. SCH10, 66.3±15.3 (mean±SEM) vs. 112.0±34.8 vs. 100.1±39.3; p>0.05; SCH0+cocaine vs. SCH3+cocaine vs. SCH10+cocaine, 581.7±86.1 vs. 621.8±100.2 vs. 442.4±82.8; p>0.05]. Furthermore, administration of the D1 antagonist did not change the distribution of running activity within the training sessions (data not shown).
Blockade of D1 receptors in the striatum is sufficient to inhibit skill-memory formation
In experiment 4, we investigated whether D1 receptor stimulation in the striatum is necessary for wheel-skill learning (2-day training) (Fig. 4). The D1 receptor antagonist SCH-23390 (0, 0.3 and 1 μg) was infused into the dorsolateral striatum (Fig. 4d) in conjunction with systemic cocaine administration. SCH-23390 had dose-dependent effects on wheel-skill learning. Statistical analysis of the averaged post-training test scores revealed a significant effect of the D1 receptor antagonist treatment (H=12.0, p<0.01, Kruskal-Wallis test) (Fig. 4e). Thus, both rats infused with the lower dose (S0.3+cocaine; Z=2.0, p<0.05, Mann-Whitney U test) and the higher dose of SCH-23390 (S1+cocaine; Z=3.1, p<0.01) showed more performance errors after the training than the controls that received a saline infusion into the striatum (S0+cocaine). S1+cocaine animals also displayed more errors than S0.3+cocaine animals (Z=2.2, p<0.05). Statistical analysis of the test scores over time revealed that the saline controls displayed a significant training effect (χ2=12.0, p<0.01), with reduced error scores present on days 1, 13 and 27 after the training (p<0.05) (Fig. 4a). In contrast, the group that received the lower dose of SCH-23390 (0.3 μg) showed only a trend for improved skill performance (χ2=7.1, p=0.070), with near-normal improvement on post-day 1 (Fig. 4b). The higher dose of SCH-23390 (1 μg) completely prevented this motor learning (χ2=4.2, p>0.1; Fig. 4c). Again, no effect of the D1 receptor antagonist on the amount of running during the training was observed (p>0.05; Fig. 4f).
Discussion
In the present study, we developed and validated a novel test to measure striatum-based procedural learning in a running-wheel skill-learning paradigm. We also investigated the effects of cocaine and D1 receptor antagonism on this form of motor learning. Our findings indicate that both decreasing dopamine tone at the D1 receptor (by the D1 receptor antagonist SCH-23390) and abnormally increasing dopamine tone (by the indirect dopamine receptor agonist cocaine) during the training impaired wheel-skill learning. Combining a low dose of the D1 receptor antagonist with cocaine restored skill learning. These findings suggest that optimal D1 receptor stimulation is necessary for this motor-skill learning. Moreover, our results with intrastriatal D1 receptor antagonism indicate that D1 receptors in the striatum are critical for long-term skill-memory consolidation.
Procedural learning in a novel running-wheel paradigm
In our previous (Willuhn et al., 2003; Willuhn and Steiner, 2005; Willuhn and Steiner, 2006) and present studies, we have used a novel running-wheel paradigm to investigate the neuronal mechanisms of procedural learning. In this paradigm, rats learn to perform a motor skill while running on a wheel. Our findings show that in the beginning of the training rats are not able to run on the wheel without causing the wheel to swing (Willuhn and Steiner, 2006). During continued training, they learn to control the wheel by adjusting their movements in order to stabilize the wheel and avoid swinging (wheel skill). As is typical for motor-skill learning (cf. Costa et al., 2004), rats learn fast within and between the first training sessions (Willuhn and Steiner, 2006; present results).
In this task, wheel swings serve as a measure of performance error for the assessment of skill learning. In our previous study (Willuhn and Steiner, 2006), we counted wheel swings during the training sessions to determine learning. However, because the number of swings during the training is directly related to the amount of running, differences in overall activity levels between experimental groups (for example, as a result of a drug treatment; Willuhn and Steiner, 2006) complicate interpretation of differences in such swing counts. In the present study, we therefore developed a wheel-skill test that is administered before and after the training, thus allowing assessment of skill learning independent of the training situation. As a further advantage, this test can be performed repeatedly after the training to determine the duration of this motor memory. Our results obtained with this test demonstrate that, as expected, wheel-skill learning is dependent on factors such as the amount of running (practicing) during the training and the training duration (number of sessions). These tests also showed that this wheel-skill memory lasted for at least 4 weeks after the training (long-term memory).
Studying motor-skill learning in this running-wheel paradigm has a number of advantages over other learning paradigms used to investigate processes of procedural learning. For example, in contrast to other learning paradigms, wheel-skill learning is not dependent on motivational manipulations such as food deprivation (e.g., used for instrumental learning), electric shocks (e.g., in avoidance learning), or forced locomotion (e.g., skill learning on rotarod), because rats voluntarily run on wheels (Sherwin, 1998). Also, our results demonstrate that, unlike in many other procedural learning paradigms, long training periods are not necessary for robust motor memory formation; even a short training (one to two daily sessions) was sufficient to produce stable long-term memory. Finally, the separation of training and testing permits the assessment of drug effects on learning in a drug-free state, thus avoiding confounds by drug effects on the tested motor response. In summary, our findings demonstrate that this running-wheel paradigm is an efficient and simple tool to investigate procedural learning and memory formation.
Neuronal correlates of procedural learning in the striatum
Previous work implicated the sensorimotor striatum in several forms of procedural learning (White, 1989; Packard and Knowlton, 2002; Yin and Knowlton, 2006). For example, early studies demonstrated a role of the striatum in the acquisition of avoidance learning (e.g., Winocur and Mills, 1969; Neill and Grossman, 1970). More recently, lesions or inactivation of the striatum were shown to disrupt habit/S-R learning in maze, lever-pressing, and discrimination tasks (e.g., Packard and McGaugh, 1992; Packard and McGaugh, 1996; Yin et al., 2004; Featherstone and McDonald, 2005). Conversely, other studies found that neuronal activity patterns in corticostriatal circuits are modified during the acquisition of a procedural T-maze task (Jog et al., 1999; Barnes et al., 2005), conditional visuomotor learning (Brasted and Wise, 2004), or head movement habit formation (Tang et al., 2007).
Motor-skill learning is another form of procedural learning (Squire, 1987) that is also mediated by the striatum (Packard and Knowlton, 2002). In skill learning the animal learns to produce a skilled motor response in order to achieve a specific outcome, and this response eventually reaches automaticity (Poldrack et al., 2005). This process is associated with neuronal changes in the striatum (e.g., Lehericy et al., 2005; Poldrack et al., 2005). For example, a recent study showed changes in striatal ensemble activity during motor-skill learning on a rotarod (Costa et al., 2004). Moreover, we and others have found molecular changes in the striatum associated with skill learning in two different running-wheel paradigms. Kitsukawa and colleagues (Kitsukawa et al., 2002) reported enhanced c-fos induction in striatal neurons related in time to the acquisition of new stepping patterns on a running wheel. Our previous work demonstrated that learning of the wheel skill described above is associated with changes in the expression of several genes in the sensorimotor striatum (Willuhn et al., 2003; Willuhn and Steiner, 2005; Willuhn and Steiner, 2006). Thus, the inducibility of c-fos, homer 1a and substance P in striatal projection neurons was enhanced for at least 24 h after running-wheel training sessions. Importantly, these molecular changes were limited to the first few sessions (peak after a 2-day training) and were no longer present after extended wheel training (8-day training; Willuhn and Steiner, 2006), suggesting that this effect was a correlate of the learning process. Moreover, while these skill learning-associated changes in gene expression were modest when rats trained after a vehicle injection (Willuhn and Steiner, 2005), they were very robust when the wheel training occurred under the influence of cocaine (Willuhn et al., 2003; Willuhn and Steiner, 2005; Willuhn and Steiner, 2006). This latter finding indicated that learning-associated gene regulation was abnormally induced or magnified by the action of this psychostimulant. The present studies further investigated the mechanisms underlying such skill learning.
Effects of cocaine on procedural learning in the running wheel
Considering the effects of cocaine on molecular correlates of wheel-skill learning (see above), we investigated whether this psychostimulant modified skill learning in the present task. Our findings indicate that this is the case. Skill learning tended to be impaired when rats received an injection of cocaine immediately before each training session. While this effect was not very pronounced (and did not reach statistical significance in the direct comparisons), the performance of cocaine-treated animals was consistently inferior to that of vehicle-treated controls, with both 2-day and 5-day training regimens. This cocaine-induced impairment was seen despite the fact that cocaine-treated rats ran more (and thus practiced more) during the training than vehicle-treated rats.
At first glance, this finding is in apparent contrast with our previous findings that showed facilitated wheel-skill learning during training under the influence of cocaine (Willuhn and Steiner, 2006). However, these two studies differed in important aspects. For example, in the previous study, performance errors (swing counts) were measured during the training sessions (i.e., during running), whereas in the present study, skill performance was assessed at different time points after the training in a controlled test setting. Thus, the outcome measure likely assessed different processes, that is, in the previous study, a combination of working/short-term memory and long-term memory, whereas in the present study, only long-term memory (days to weeks after the training). These findings may thus indicate that cocaine exerts differential effects on these different stages of wheel-skill memory formation, a possibility that needs to be clarified in future studies.
It could also be argued that these differences may reflect state-dependency of wheel-skill memory. In both the previous and the present study, animals learned the wheel skill under the influence of cocaine. In contrast to the previous study, the present study assessed recall of skill memory in the drug-free state. There is evidence indicating that recall of memory in a different state can be compromised (e.g., Overton, 1991). Such an effect may have contributed to the tendency for impaired test performance in animals trained but not tested under the influence of cocaine in the present study. However, the time-dependent and biphasic effects of cocaine plus SCH-23390 on skill performance (see below) would appear to argue against state-dependency as a simple explanation for the observed effects of cocaine (and/or SCH-23390). In summary, it is currently unclear whether the findings of our two studies reflect differential effects of cocaine on different skill-memory processes, or were due to differences in the experimental approach.
The present (and previous) behavioral findings were obtained with a relatively high dose of cocaine (25 mg/kg), chosen because this dose produced abnormal gene regulation in our previous molecular studies (see above). In order to fully characterize cocaine effects on wheel-skill learning, the dose-response relationship for a range of cocaine doses will have to be established. It can be speculated that, at a higher dose, cocaine may have more detrimental effects (Quirk et al., 2001); conversely, in the light of the results produced by the D1 receptor blocker (see below), a lower dose of cocaine may have facilitatory rather than attenuating effects also with the present approach. Regardless, our findings indicate that cocaine, presumably by producing excessive stimulation of dopamine (and other) receptors (Ritz et al., 1990), interferes with normal motor-skill learning.
Role of striatal dopamine receptors in wheel-skill learning
Our previous studies revealed effects of cocaine on skill learning-associated gene regulation in the striatum (see above). Such cocaine-induced gene regulation is mediated by dopamine receptors in interaction with other neurotransmitter systems (e.g., glutamate; Hyman et al., 1996; Wang and McGinty, 1996). While D2-type dopamine receptors play a modulatory role (e.g., Gerfen et al., 1995; Zhang et al., 2004), the D1 receptor is critical for psychostimulant-induced gene regulation; these effects are prevented by targeted elimination the D1 receptor (Drago et al., 1996; Moratalla et al., 1996; Zhang et al., 2004), or by the selective D1 receptor antagonist SCH-23390 (e.g., Graybiel et al., 1990; Young et al., 1991; Steiner and Gerfen, 1995).
To assess the role of D1 receptors in wheel-skill learning, we first investigated the effects of systemic D1 receptor antagonism. Injection of SCH-23390 alone before each training session (in doses that did not inhibit running) attenuated wheel-skill learning in a dose-dependent manner. However, when given in conjunction with cocaine, SCH-23390 had biphasic effects on wheel-skill learning and these effects were time-dependent. The lower dose (3 μg/kg) given with cocaine tended to improve skill performance over cocaine-only or SCH-23390-only controls, especially at late time points (days 18–26 after the training). In contrast, rats that were treated with the higher dose of SCH-23390 (10 μg/kg) plus cocaine showed significant skill improvement 1–6 days after the training (i.e., an improvement over rats treated with 10 μg/kg SCH-23390 only), but this motor memory did not last. By post-training day 18 this motor skill was lost. Together, these results demonstrate a critical role for dopamine and D1 receptors in this type of skill learning. Moreover, our results suggest that, similar to the well-known D1 receptor regulation of working memory in the prefrontal cortex (e.g., Goldman-Rakic et al., 2000; Seamans and Yang, 2004), motor-skill learning requires optimal stimulation of D1 receptors.
The above drugs were given systemically, thus disallowing conclusions regarding the location of the relevant D1 receptors. We further assessed the role of striatal D1 receptors by intrastriatal administration of the D1 receptor blocker (in conjunction with cocaine). SCH-23390 was infused into parts of the sensorimotor striatum that displayed robust skill learning-associated changes in gene regulation in our previous studies (see above). Intrastriatal infusion of the D1 receptor antagonist also produced dose-dependent attenuation of wheel-skill learning, which overall showed a similar pattern as systemic D1 receptor antagonism. Rats treated with the lower dose (0.3 μg) plus cocaine displayed a tendency for skill learning. The higher dose (1 μg) completely blocked wheel-skill learning. These findings indicate that D1 receptors in the striatum are critical for the acquisition of this wheel skill (although at higher SCH-23390 doses other receptors may also have been affected; Bourne, 2001). Our most recent studies (Willuhn and Steiner, in preparation) show that such striatal D1 receptor antagonism during the training also prevented the previously discovered learning-associated changes in gene regulation (see above). Together, our findings indicate that striatal D1 receptors mediate molecular changes in the striatum that underlie wheel-skill memory consolidation (see Willuhn and Steiner, 2006, for further discussion).
Overall, our findings are consistent with previous studies showing effects of dopamine receptor stimulation on mechanisms of procedural learning in the striatum (Beninger and Miller, 1998). For example, partial striatal dopamine depletion by 6-OHDA was associated with impaired acquisition of skilled behavior in a rotarod test (Ogura et al., 2005). Moreover, systemic administration of cocaine enhanced avoidance learning (Introini-Collison and McGaugh, 1989; Janak et al., 1992), whereas post-trial intrastriatal infusion of dopamine receptor agonists facilitated memory formation in procedural maze tasks (Packard and White, 1991; Packard et al., 1994). Regarding the specific dopamine receptor subtypes involved, evidence indicates that both D1- and D2-type receptors participate, although D1 receptors appear to play a more prominent role in striatum-based learning (Packard and White, 1991; Packard et al., 1994; Kabai et al., 2004; Dalley et al., 2005; Domenger and Schwarting, 2006; see Beninger and Miller, 1998, for review). Blocking D1 receptors with SCH-23390 has been shown to disrupt, for example, avoidance learning (Hale and Crowe, 2003; Kabai et al., 2004) and habit/S-R learning in a conditioned approach paradigm (Eyny and Horvitz, 2003). Our present findings demonstrate that D1 receptors in the striatum are also critically important for motor-skill learning.
Differential roles for D1 receptors in early vs. late long-term memory consolidation
An important finding of the present study is the dissociation of early- (1–6 days) vs. late-stage (>18 days) long-term skill memory with respect to their D1 receptor dependence. Short-term memory is thought to last ~3–6 h (Izquierdo et al., 2006); memory 1–6 days after the training is thus considered early long-term memory. Our results indicate that the formation of early- and late-stage (18–26 days) long-term memory is mediated by differential processes. Late-stage, but not the early, wheel-skill memory was attenuated when animals were treated with a high enough dose of SCH-23390 (10 μg/kg) before the training, irrespective of whether they also received cocaine. In contrast, while such D1 receptor antagonism alone (SCH-23390 only) also attenuated early wheel-skill memory (1–6 days), this effect was reversed by co-administration of cocaine. Thus, these rats demonstrated apparently normal wheel-skill improvement on post-training days 1–6, but then lost their wheel skill by day 18 after the training. A similar effect was seen with intrastriatal administration of 0.3 μg of SCH-23390. These findings indicate that while consolidation of late-stage long-term skill memory is critically dependent on striatal D1 receptor signaling, early long-term skill memory formation is less so. It is presently unclear whether increased dopamine tone at D1 (or other) dopamine receptors or other neurochemical effects of cocaine mediated this rescue of early long-term memory by cocaine. However, these findings demonstrate that apparently intact early long-term memory formation is not sufficient to guarantee normal consolidation of late long-term memory for this motor skill, if D1 receptor signaling is impaired during the training. Future studies will have to elucidate the differential mechanisms that mediate early- vs. late-stage long-term skill memory formation.
Acknowledgments
This work was supported by USPHS grant DA011261. We would like to thank Joel Beverley for excellent technical assistance.
References
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev. 1998;22:335–345. doi: 10.1016/s0149-7634(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bourne JA. SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev. 2001;7:399–414. doi: 10.1111/j.1527-3458.2001.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Domenger D, Schwarting RKW. The serial reaction time task in the rat: effects of D1 and D2 dopamine-receptor antagonists. Behav Brain Res. 2006;175:212–222. doi: 10.1016/j.bbr.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: Cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Eyny YS, Horvitz JC. Opposing roles of D1 and D2 receptors in appetitive conditioning. J Neurosci. 2003;23:1584–1587. doi: 10.1523/JNEUROSCI.23-05-01584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Lesions of the dorsolateral striatum impair the acquisition of a simplified stimulus-response dependent conditional discrimination task. Neuroscience. 2005;136:387–395. doi: 10.1016/j.neuroscience.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Gauda EB. D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate-early gene response in D1-containing neurons. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, Gonzalez R, Harris RJ, Guthrie D. Effect sizes and p values: what should be reported and what should be replicated? Psychophysiology. 1996;33:175–183. doi: 10.1111/j.1469-8986.1996.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Hale MW, Crowe SF. Facilitation and disruption of memory for the passive avoidance task in the day-old chick using dopamine D1 receptor compounds. Behav Pharmacol. 2003;14:525–532. doi: 10.1097/00008877-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Cole RL, Schwarzschild M, Cole D, Hope B, Konradi C. Molecular mechanisms of striatal gene regulation: a critical role for glutamate in dopamine-mediated gene induction. In: Merchant KM, editor. Pharmacological Regulation of Gene Expression in the CNS. Boca Raton: CRC; 1996. pp. 115–131. [Google Scholar]
- Introini-Collison IB, McGaugh JL. Cocaine enhances memory storage in mice. Psychopharmacology. 1989;99:537–541. doi: 10.1007/BF00589905. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Janak PH, Keppel G, Martinez JL. Cocaine enhances retention of avoidance conditioning in rats. Psychopharmacology. 1992;106:383–387. doi: 10.1007/BF02245422. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kabai P, Stewart MG, Tarcali J, Csillag A. Inhibiting effect of D1, but not D2 antagonist administered to the striatum on retention of passive avoidance in the chick. Neurobiol Learn Mem. 2004;81:155–158. doi: 10.1016/j.nlm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Tomioka R, Utsumi H, Yanagihara D, Yamamori T. Histochemical identification of neuronal processing involved in change of running pattern in mice. Soc Neurosci Abstr. 2002;28:264–268. [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DB, Grossman SP. Behavioral effects of lesions or cholinergic blockade of the dorsal and ventral caudate of rats. J Comp Physiol Psychol. 1970;71:311–317. doi: 10.1037/h0029110. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Ogura T, Ogata M, Akita H, Jitsuki S, Akiba L, Noda K, Hoka S, Saji M. Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci Res. 2005;51:299–308. doi: 10.1016/j.neures.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Overton DA. Historical context of state-dependent learning and discriminative drug effects. Behav Pharmacol. 1991;2:253–264. [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk PL, Richards RW, Avery DD. Subchronic cocaine produces training paradigm-dependent learning deficits in laboratory rats. Pharmacol Biochem Behav. 2001;68:545–553. doi: 10.1016/s0091-3057(01)00462-2. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and Brain. Oxford: Oxford University Press; 1987. [Google Scholar]
- Steiner H, Gerfen CR. Dynorphin opioid inhibition of cocaine-induced, D1 dopamine receptor-mediated immediate-early gene expression in the striatum. J Comp Neurol. 1995;353:200–212. doi: 10.1002/cne.903530204. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H. Memory formation and the regulation of gene expression. Cell Mol Life Sci. 1999;55:575–592. doi: 10.1007/s000180050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Pawlak AP, Prokopenko V, West MO. Changes in activity of the striatum during formation of a motor habit. Eur J Neurosci. 2007;25:1212–1227. doi: 10.1111/j.1460-9568.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Glutamatergic and cholinergic regulation of immediate-early gene and neuropeptide gene expression in the striatum. In: Merchant KM, editor. Pharmacological Regulation of Gene Expression in the CNS. Boca Raton; CRC: 1996. pp. 81–113. [Google Scholar]
- White NM. Reward or reinforcement: what’s the difference? Neurosci Biobehav Rev. 1989;13:181–186. doi: 10.1016/s0149-7634(89)80028-4. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Willuhn I, Steiner H. Motor learning-related gene regulation in the striatum: Effects of cocaine. In: Bolam JP, Ingham CA, Magill PJ, editors. The Basal Ganglia VIII. New York: Plenum Press; 2005. pp. 197–207. [Google Scholar]
- Willuhn I, Steiner H. Motor-skill learning-associated gene regulation in the striatum: Effects of cocaine. Neuropsychopharmacology. 2006;31:2669–2682. doi: 10.1038/sj.npp.1300995. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Mills JA. Effects of caudate lesions on avoidance behavior in rats. J Comp Physiol Psychol. 1969;68:552–557. doi: 10.1037/h0027645. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-Fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]