Abstract
Dopamine D1-like partial receptor agonists such as SKF 83959 have been proposed as potential candidates for the treatment of cocaine addiction. The present studies were conducted to further characterize SKF 83959 by pharmacologically evaluating effects of its R-(+)- and S-(−) enantiomers, MCL 202 and MCL 201, respectively, on overt behavior (eye blinking) and schedule-controlled performance in squirrel monkeys. MCL 202, like the D1 full receptor agonist SKF 82958, produced dose related increases in eye blinking and decreases in rates of fixed-ratio responding. However, the magnitude of effects of MCL 202 on eye blinking was less than observed with SKF 82958. In contrast to the effects of its R-(+) enantiomer, MCL 201 was relatively devoid of behavioral activity up to doses that were approximately 10-fold greater than MCL 202. Pretreatment with the selective D1-like receptor antagonist SCH 39166 dose-dependently antagonized increases in eye blinking produced by MCL 202, confirming the involvement of D1 mechanisms in its effects. A dose-ratio analysis of the antagonism of effects of MCL 202 by SCH 39166 revealed an apparent pA2 value of 7.675 with a slope of −0.78 ± 0.04. In further studies, pretreatment with MCL 202 antagonized the effects of SKF 82958 on eye blinking and, like SCH 39166, schedule-controlled behavior in a dose-related manner. A dose-ratio analysis of the antagonist effects of MCL 202 on the SKF 82958-induced increases in eye blinking revealed ratios of 2.7, 4.8 and 31.1 for 0.1, 0.3 and 1.0 mg/kg dose of the antagonist, respectively, indicative of a significant change in the potency of SKF 82958. These results suggest that MCL 202, like its parent compound SKF 83959, has both D1 receptor-mediated agonist and antagonist properties, consistent with its characterization as a partial agonist at the D1-like receptor. In addition, the inactivity of MCL 201, the S-(−)-enantiomer, suggests that the behavioral effects of SKF 83959 can be attributed primarily to the activity of its R-(+)-enantiomer.
Keywords: Dopamine D1-like receptors, Enantiomers of SKF 83959, Partial agonist, Eye blinking, Schedule-controlled behavior, SKF 82958, Squirrel monkeys
1. Introduction
A considerable body of pharmacological evidence suggests that dopamine D1-like mechanisms may be involved in the reinforcing and other abuse-related behavioral effects of cocaine and other monoaminergic psychomotor stimulants (e.g. Bergman et al., 1990; Corrigall and Coen, 1991; Self and Stein, 1992; Morelli et al., 1993; Weed and Woolverton, 1995; Spealman et al., 1997; Weed et al., 1997; Singh et al., 1997; Tidey and Bergman, 1998; Abrahams et al., 1998a, 1998b; Henry et al., 1998). This evidence has led to the suggestion that D1-like receptor ligands may be candidate medications for the treatment of cocaine dependence (Mendelson and Mello, 1996; Platt et al., 2002). In this regard, D1 ligands with partial agonist effects have been of particular interest for several reasons. First, D1 ligands that are presumed to be partial agonists (e.g., SKF 38393, SKF 77434, SKF 83959) do not appear to have reinforcing effects associated with D1 full receptor agonists such as SKF 82958 or R-6-BrAPB and may produce a less pronounced disruption of motor behavior than observed with D1 receptor blockers such as SCH 39166 (Weed and Woolverton 1996; Platt et al., 2000, 2001; Mutschler and Bergman, 2002). Second, these drugs also appear to attenuate the actions of cocaine or other psychomotor stimulants in studies of their discriminative stimulus and behavioral stimulant effects in rodents and monkeys (Spealman et al., 1997; Katz et al., 1999; Platt et al., 2001). As well, acute treatment with a range of D1 partial receptor agonists, including SKF 38393, SKF 75670, SKF 83959 and SKF 77434 has been reported to reduce or antagonize i.v. self-administration of cocaine in rats and/or monkeys (Bergman and Rosenzweig-Lipson, 1992; Katz and Witkin, 1992; Caine et al., 1999; Mutschler and Bergman, 2002). Third, D1 ligands such as SKF 38393 and SKF 83959 can inhibit the reinstatement of extinguished cocaine-seeking behavior, raising the possibility that these compounds may be effective in attenuating clinical relapse (Spealman et al., 1999).
The relative efficacy of D1-like receptor agonists generally has been determined using both in vitro and in vivo studies (for review see Jutkiewicz and Bergman, 2004). In vitro studies have characterized dopamine D1-like receptors by their positive coupling to adenylyl cyclase (AC) stimulation (Kebabian and Calne, 1979), and more recently to phosphoinositide (PI) hydrolysis (Mahan et al., 1990; Undie and Friedman, 1990; Undie, 1999; Undie et al., 1994, 2000). In these types of studies, efficacy can be estimated by comparing the maximal effects of a D1 ligand to the effects of dopamine itself (e.g. Anderson and Jansen, 1990). Unfortunately, a robust correspondence has not been obtained between the behavioral effects of D1-like receptor agonists and their efficacy in stimulating AC and/or PI activity (e.g. Daly and Waddington, 1992; Gnanalingham et al., 1995a, 1995b; Fornaguera et al., 1999; Katz et al., 1999; Platt et al., 2001; Sinnott and Nader, 2001; Desai et al., 2003a, 2005). This lack of association between in vitro and in vivo efficacy estimates for D1-like receptor agonists is exemplified by the comparison of biochemical and behavioral data obtained for the D1 ligand SKF 83959. In in vitro studies, this compound serves as a potent antagonist of AC-coupled dopamine D1-like receptors in both rats (Arnt et al., 1992; Gnanalingham et al., 1995c) and primates (Andringa et al., 1999). In behavioral studies, however, the effects of SKF 83959 in both rats and primates are more comparable to those of dopamine D1-like full receptor agonists. Thus, SKF 83959, like a range of D1 receptor agonists, induces vacuous chewing and enhanced grooming in rats (Downes and Waddington, 1993) and dose-related increases in eye-blinking in monkeys (Jutkiewicz and Bergman, 2004). Further, SKF 83959, like other D1 receptor agonists, can reduce motor deficits in MPTP-lesioned rats and monkeys (Gnanalingham et al., 1995b, 1995c; Andringa et al., 1999b; Cools et al., 2002) and may induce dyskinesias in haloperidol-sensitized monkeys (Peacock and Gerlach, 2001). Finally, the profile of behavioral effects of SKF 83959, like those of the dopamine D1-like receptor agonist A 68930, which stimulates AC with dopamine-like efficacy, are significantly retained in D1-like receptor knockout mice (Clifford et al., 1999). Taken together, these findings suggest that our current understanding of the cellular mechanisms that mediate D1 efficacy in vivo is incomplete, and that behavioral procedures currently provide the most useful means for evaluating the efficacy of D1-like receptor ligands.
Recent regulatory guidelines in the USA and Europe have recommended the development of single enantiomers of compounds that have clinical applications. This recommendation is based on the idea that single enantiomers may offer clinical advantages over the racemate in terms of potency, efficacy and/or tolerability (reviewed by Caldwell, 2001). With regard to dopamine D1 receptor agonists, Neumeyer et al. (1992, 2003) have synthesized and developed a number of novel substituted phenylbenzazepine congeners of the dopamine D1-like receptor partial agonist SKF 83959 including the R(+)- and S(−)-enantiomers, MCL 202 and MCL 201, respectively. Receptor binding studies have revealed that, in vitro, both MCL 202 and MCL 201 are highly selective for D1-like receptors relative to D2-like receptors (Neumeyer et al., 2003). Further, MCL 202 is approximately 2-fold more potent than its racemic mixture SKF 83959 at the D1 receptor, whereas MCL 201 is 18-fold less potent than SKF 83959 (Neumeyer et al., 2003). The present study was designed to extend these observations with the R(+)- and S(−)-enantiomers of SKF 83959 by characterizing their effects on eye blinking and scheduled controlled behavior in squirrel monkeys. The induction of eye blinking is a robust behavioral measure of D1-like receptor agonist activity and appears to be useful for distinguishing efficacy-related differences in the effects of dopamine D1-like receptor agonists (e.g. Bergman et al., 1995; Elsworth et al., 1991; Kleven and Koek, 1996; Jutkiewicz and Bergman, 2004). The effects of D1 receptor agonists on schedule-controlled responding also have been extensively documented in monkeys, and serves as a second means for evaluating the efficacy-related effects of D1 receptor agonists (Bergman et al. 1991; 1995; Katz et al., 1995).
In the present studies, the agonist effects of MCL 202 and MCL 201 on eye blink rate and rates of responding maintained under stimulus-shock termination first were compared with the effects of the well-established dopamine D1-like full receptor agonist SKF 82958. The agonist effects of MCL 202 on eye-blinking also were studied after treatment with different doses of the selective dopamine D1-like receptor antagonist, SCH 39166 to quantitatively examine the involvement of D1 receptor-mediated mechanisms in these effects. Finally, the antagonist effects of MCL 202 were studied by determining how it modified the effects of SKF 82958 on eye blink rate and scheduled controlled responding. Overall, results of these studies indicate that D1 activity of SKF 83959 resides in its R-(+)-enantiomer (MCL 202) and that its behavioral effects are consistent with its characterization as a D1 partial agonist.
2. Materials and Methods
2.1. Subjects
Eight experimentally naïve male squirrel monkeys (Saimiri sciureus), weighing 700 to 950 g were used as subjects. All monkeys were individually housed in a temperature- and humidity-controlled vivarium. Monkeys had unrestricted access to water and were fed a daily allotment of high protein monkey chow (Purina Monkey Chow, St. Louis, MO), supplemented with fruit and multivitamins. The monkeys were randomly divided into two groups. The subjects, s-2, s-3, s-7 and s-9 were used in observational studies that measured eye blinking and the remaining four monkeys, s-4, s-13, s-8, and s-11, were studied in experiments involving schedule-controlled behavior. All experiments were conducted between 08:00 AM and 06:00 PM under protocols that were approved by the Institutional Animal Care and Use Committee at McLean Hospital. Monkeys were maintained in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, National Institutes of Health. The facility in which subjects were housed and studies were conducted is licensed by the U.S. Department of Agriculture.
2.2. Eye blinking: apparatus and experimental methods
All testing was conducted in a specifically constructed chamber (20.2″ × 10″ × 10″). Monkeys sat in a customized Lexan chair with blackened side and back walls to provide color contrast. The front wall of the chair was removed in order to facilitate videotaping with a compact video camera (JVC, model GR-AX10), equipped with a telephoto lens located at a distance of approximately 2 feet in front of the seated monkey. Observation of eye blink responses was facilitated by a neck plate that was attached to the chair and maintained a relatively constant orientation of the monkeys head. In order to view eye blink responses when the monkey turned its head, a curved piece of mirror Plexiglas was placed behind the monkeys head. Optimum illumination was provided by positioning two vertical fluorescent lights on the side walls of the chamber. A computer-driven program (ATI Multimedia Software, ATI Player) captured the camera image which was viewed on a 20″ monitor and recorded on 6 hour videotapes using a VHS VCR (JVC, model HR-VP69U).
During test sessions, the effects of various does of drugs or vehicle were examined in each monkey for four or five successive 15-min components. Each component comprised a 10-min habituation period followed by a 5-min period during which eye blinking was videotaped. During the entire videotaping session the image of the monkey head was transmitted by the video camera and displayed on the computer screen; this allowed for continuous observation of the subject from an adjacent room. The videotape of each 5-min session component was scored by observers blind to the treatment conditions. An eye blink was defined as a visible closure and opening of the eyelid. Each component was scored by at least one trained observer. To evaluate scoring reliability, test components were periodically rescored by a second trained observer for whom inter-observer reliability criteria (>90% concordance in blink rate during preceding scoring sessions) had been met.
2.3 Schedule-controlled behavior: apparatus and experimental methods
During experimental sessions, subjects sat in Plexiglas chairs (Kelleher and Morse, 1968) in ventilated, sound-attenuating chambers that delivered white noise to the chambers at all times to mask extraneous sounds. While seated, monkeys faced a panel containing colored stimulus lights serving as a visual stimuli and a lever set at 4 inches above the waist plate. Each press of the lever with a force greater than 0.2 N produced an audible click and was recorded as a response. Cumulative recorders were used to track and record patterns of responding over the session. Prior to each session a shaved portion of the monkey’s tail was coated with electrode paste and placed under brass electrodes through which a brief, low-intensity shock stimulus (3 mA for 200 ms) could be delivered.
All subjects were trained to respond under a 30-response fixed ratio schedule of stimulus-termination. Under this schedule, the illumination of stimulus lights on the front panel started a program under which shock stimuli were delivered every 30-s. The completion of 30 consecutive responses turned off the stimulus lights and the associated program of shock delivery, and initiated a timeout (TO) period of 10-s during which all stimulus lights were off and responding had no scheduled consequences. Sessions were terminated after delivery of five shock stimuli in any one component. Subjects were considered to be trained under terminal schedule contingencies when responding reliably terminated visual stimuli within 30-s following their illumination. Daily sessions were comprised of five sequential components. Each component comprised a 9-min period during which no stimuli were presented and responding had no programmed consequences (TO 9′) followed by a 3-min period during which the FR 30, TO 10″ schedule of stimulus–termination was in effect.
2.4 Eye Blinking: drug testing
Cumulative dosing procedures were used to determine the effects of drugs on eye blinking no more than once or twice weekly. In these experiments, incremental doses of a drug were administered at the beginning of the 15-min components of the test session, permitting the evaluation of up to 5 cumulative doses during a single session. In some instances, a complete dose-effect function could be determined in a single session. In other instances, the complete dose-effect was determined by testing two or three overlapping dose ranges over two or three test sessions. Using these procedures, the effects of SKF 82958 (0.03–1.0 mg/kg), MCL 201 (0.003–10.0 mg/kg), and MCL 202 (0.0003–1.0 mg/kg) on eye blinking were determined in four monkeys; the order of drug testing varied irregularly among individual subjects. In separate experiments, the time course of MCL 202-induced changes in eye blinking was determined by videotaping 5-min periods at 5, 10, 15, 30, 45, 60, and 120 min following a single i.m. injection of 0.1 mg/kg MCL 202. For drug combination studies, each of several doses of SCH 39166 (0.1 – 1 mg/kg) was administered i.m. 5 min before test sessions in which the effects of MCL 202 (0.0003 – 1 mg/kg) was re-determined. The effects of SKF 82958 (0.03 – 1 mg/kg) in the presence of MCL 202 (0.1–1.0 mg/kg) were similarly studied.
2.5 Schedule-controlled behavior: drug testing
Cumulative dosing procedures described above also were used to determine the effects of SKF 82958 (0.03–1.0 mg/kg), MCL 201 (0.1–3.0 mg/kg), MCL 202 (0.01–3.0 mg/kg) on responding maintained under the schedule of stimulus-termination. In these experiments, incremental doses of a drug were administered at the beginning of sequential 9-min TO periods, and up to five cumulative doses could be studied in a single session. The effects of pretreatment with SCH 39166 (0.3–1.0 mg/kg) or MCL 202 (0.03–1.0 mg/kg) were studied by administering each pretreatment dose at the start of the session and administering cumulative doses of SKF 82958 in successive components beginning with the second component (i.e. 12 min after the initial treatment).
2.6 Drugs
SKF 82958 ((±)-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine HBr) was purchased from Research Biochemicals International Sigma/RBI, (Natick, MA). SCH 39166 was generously supplied by Schering Plough (Kenilworth, NJ). The synthesis of enantiomers of SKF 83959, MCL 201 [S(−)–enantiomer] and MCL 202 [R(+)–enantiomer] has been described previously (Neumeyer et al., 1992, 2003). The basic chemical structures of dopamine D1 receptor-selective phenylbenzazepines are shown in Fig. 1.
Fig. 1.

Chemical structures of dopamine D1-like receptor-selective phenyl-benzazepines.
2.7 Data analysis
Eye blink rates are expressed as blinks per minute and were computed as total number of eye blinks divided by the duration of time that the subject’s eyes were visible to the observers during the 5-min test component. Response rates under the schedule of stimulus-termination are expressed as responses per second and were calculated for each session component by dividing the total number of lever press responses by the duration of the component minus the time the chamber was darkened (timeout periods). In both observational procedures and experiments involving schedule-controlled responding, saline injections did not appreciably alter, respectively, rates of eye blinking or lever press responding across session components. Therefore, mean control values for rates of eye blinking and rates of responding (mean ± S.E.M) for each subject were determined by averaging rates across components of sessions during which the effects of saline were determined. For individual monkeys, the effects of each cumulative dose in both procedures were calculated as response rate after treatment and as a percentage of mean control rate. Data for saline and all drugs are presented in this report as mean values for the group of monkeys. Mean values (± S.E.M) are expressed as rates of eye blinking (blinks per minute) or lever-press responding (responses per second) or, alternatively, as percentages of control rates of eye blinking or lever-press responding. The effects of a given dose of drug on eye blinking or lever-press responding were considered significant when the mean values for that dose lay outside the 99% confidence interval for mean control values for the given measure.
Each dose-response curve was analyzed using standard ANOVA and linear regression techniques. ED50 values and their 95% confidence limits were determined from data using the linear portions of the dose-effect curves (Snedecor and Cochran, 1967). One-way analyses of variance (ANOVAs) and Dunnett’s t-tests were conducted where appropriate. Relative potency estimates were obtained by parallel-line bioassay techniques; if 1.0 was not included in the 95% confidence limits, then a significant difference in potency was assumed (Finney, 1964). A significance level of P < 0.05 was assumed throughout.
The apparent pA2 value was calculated as described by Dykstra et al. (1988). Dose-ratios were determined by dividing each of the ED50 values of MCL 202 in combination with SCH 39166 by the ED50 value of MCL 202 alone. A Schild plot representing each dose ratio on the ordinate as log (dose ratio − 1) and expressed as a function of the negative logarithm of the dose of antagonist which produced that ratio is generated. The point at which the regression line intercepts the x-axis represents the apparent pA2 value. A competitive antagonism is indicated when the 95% confidence limits for the slope of the Schild plot included the value −1.
3. Results
3.1 Eye Blinking
The effects of control rates of eye blinking are shown in Table 1. The control values for eye blink rates remained consistent throughout the four session components for all monkeys, except monkey Ss 7. Monkey Ss 7 displayed greater variability in control blink rates, with eye blinking decreasing from 8.01 (± 1.30) blinks per min during the first component to 3.93 (± 0.62) blinks per min in the fourth component. The control values for eye blink rates failed to exceed 12 blinks/min across components for any individual monkey, and the eye blink rates for the group of monkeys averaged 6.07 (± 0.5 S.E.M) across the four components.
Table 1.
Control values of eye blinking across each session component for individual subjects. Data are expressed in blinks per minute (± S.E.M).
| Monkey | Session Component Blinks/min (± S.E.M)
|
Mean Blinks/min (± S.E.M) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Ss 2 | 7.22 (1.68) | 8.41 (0.72) | 8.00 (0.78) | 8.61 (0.41) | 8.06 (0.31) |
| Ss 3 | 7.24 (0.61) | 7.13 (0.94) | 6.41 (0.43) | 6.79 (0.48) | 6.89 (0.19) |
| Ss 7 | 8.01 (1.30) | 6.58 (0.71) | 5.13 (0.91) | 3.93 (0.62) | 5.91 (0.88) |
| Ss 9 | 3.90 (0.30) | 3.53 (0.15) | 3.33 (0.36) | 2.82 (0.63) | 3.40 (0.23) |
As previously reported, the dopamine D1-like receptor agonist SKF 82958 produced dose-dependent increases in rates of eye blinking (F4,15 = 9.97; p < 0.05; Fig. 2, open circles). Further analysis revealed that the highest doses of SKF 82958 tested, 0.3 and 1 mg/kg, significantly increased eye blink rates to approximately 10- and 16-fold above control values, respectively. As with SKF 82958, cumulative doses of the R[+] enantiomer of SKF 83959, MCL 202 (0.003 – 1 mg/kg), produced dose-related increases in rates of eye blinking (F8,27 = 18.84; p < 0.05). However, the maximal effects of MCL 202 were less than those achieved with SKF 82958 (Fig. 2, downward triangles). Thus, rates of eye blinking were increased to approximately 4-fold above control values following the cumulative dose of 0.03 mg/kg of MCL 202, and this effect plateaued as the cumulative dose of MCL 202 increased to as much as 1.0 mg/kg. Post-hoc Dunnett’s test revealed that the increase in eye blink rates produced by 0.01 – 1 mg/kg MCL 202 was significantly above control values (ps < 0.05). In contrast to MCL 202, the same range of doses of the S[−] enantiomer of SKF 83959, MCL 201, failed to increase eye blink rates above control values (ps > 0.05). ANOVA revealed a significant effect of dose (F5,18 = 4.55; p < 0.05) that was due to the high cumulative dose of 10 mg/kg MCL 201 which increased rates of eye blinking only to approximately 2-fold of control values (Fig. 2, upward triangles).
Fig. 2.
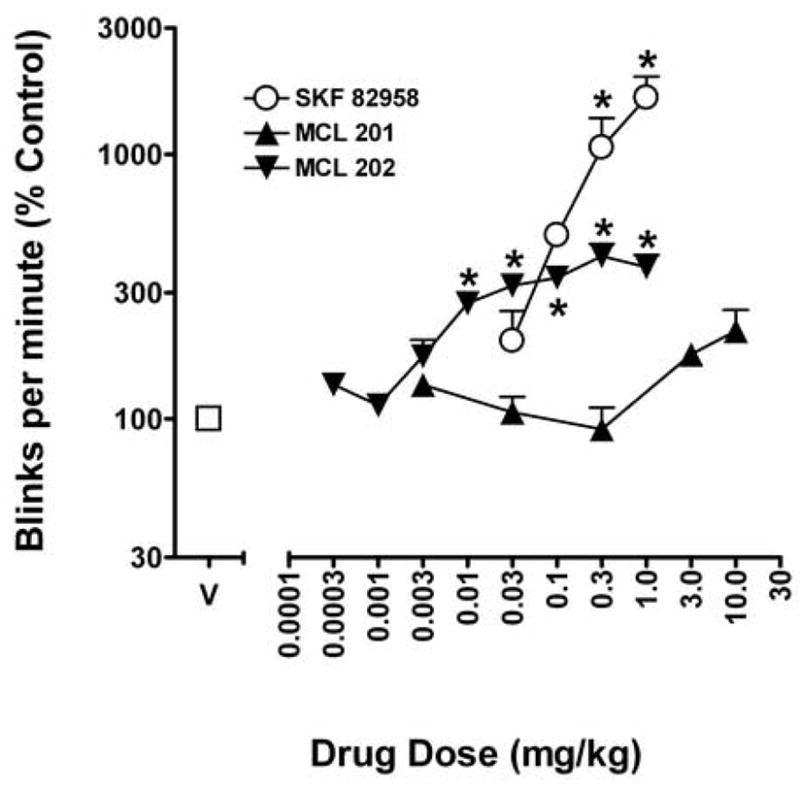
The effects of dopamine D1-like receptor agonists, SKF 82958, MCL 201, and MCL 202 on eye blinking in squirrel monkeys. Ordinates, eye blink rates as a percentage of that obtained after vehicle injections. Abscissa, cumulative dose of drugs in mg/kg. Each point represents the average effect determined in all four monkeys. Results are presented as mean % control (+ S.E.M). Note that the R[+] enantiomer of SKF 83959, MCL 202 significantly increased rates of eye blinking, whereas the S[−] enantiomer of SKF 83959, MCL 201 failed to induced eye blinking in squirrel monkeys, and that both these compounds were less effective than SKF 82958. For all drugs, when apparently absent, error bars are within the symbol. * indicates significantly different from vehicle group (Dunnetts t-test, P < 0.05, on means derived from main effect of dose).
Fig. 3 shows the effects of MCL 202 on eye blinking when administered alone and in combination with several doses of the dopamine D1-like antagonist SCH 39166 (0.1 – 1 mg/kg). SCH 39166 dose-dependently antagonized the effects of MCL 202 on eye blinking, as indicated by a parallel rightward shift in the dose-effect function (Fig. 3, open symbols). The 0.1 mg/kg dose of SCH 39166 shifted the SKF 82958 dose-effects function to the right by a factor of about 11, whereas 0.3 and 1.0 mg/kg SCH 39166 shifted the SKF 82958 dose-response curve approximately 25- and 57-fold to the right, respectively (Fig. 3; Table 2). Relative potency analysis indicated that all three doses of SCH 39166 significantly shifted the dose-effect curve for SKF 82958-induced eye blinking to the right in a dose-related manner (Table 2). A Schild plot analysis of the antagonist effects of SCH 39166 generated an apparent pA2 value of 7.675 (slope: −0.78 ± 0.04).
Fig. 3.
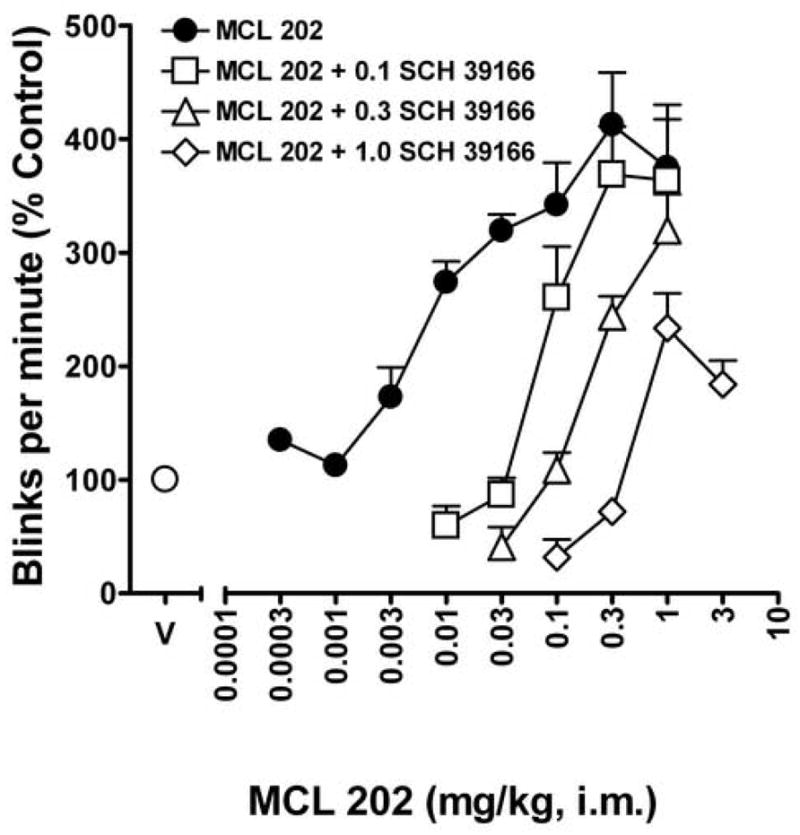
Changes in the MCL 202 dose-effect curve for rates of eye blinking produced by pretreatments with several doses of the dopamine D1-like receptor antagonist SCH 39166. Ordinates, eye blink rates as a percentage of that obtained after vehicle injections. Abscissa, cumulative dose of MCL 202 in mg/kg. Data are presented at as mean % control (+ S.E.M) of n = 4 animals. Schild plot analysis of the results reveals a slope of -0.96 (± 0.08) and a pA2 value of 8.01. Note that pretreatment with SCH 39166 produced a dose-related rightward shift in the MCL 202-induced dose-effect curve.
Table 2.
Effects of interactions of MCL 202 and SKF 82958 with SCH 39166 and MCL 202, respectively, on eye blinking.
| Drug Treatment | Eye Blinking | |
|---|---|---|
| ED50 value (mg/kg) (95% CL) | Relative Potency | |
| MCL 202 alone | 0.01 (0.007 – 0.02) | |
| MCL 202 with 0.1 mg/kg SCH 39166 | 0.11 (0.06 – 0.19) | 0.07 (0.03 – 0.18)a |
| MCL 202 with 0.3 mg/kg SCH 39166 | 0.25 (0.19 – 0.35) | 0.01 (0.006 – 0.03)a |
| MCL 202 with 1.0 mg/kg SCH 39166 | 0.57 (0.42 – 0.92) | 0.003 (0.001 – 0.006)a |
| SKF 82958 alone | 0.20 (0.11 – 0.42) | |
| SKF 82958 with 0.1 mg/kg MCL 202 | 0.54 (0.37 – 0.72) | 0.43 (0.19 – 0.95) |
| SKF 82958 with 0.3 mg/kg MCL 202 | 0.97 (0.30 – 48.82) | 0.21 (0.09 – 0.45) |
| SKF 82958 with 1.0 mg/kg MCL 202 | 6.29 (2.36 – 416.64) | 0.07 (0.02 – 0.20) |
Value is only an estimate because there was a significant effect of preparations.
The activity of MCL 202 was further characterized by examining its ability to antagonize the effects of SKF 82958 on eye blinking. Similar to SCH 39166, pretreatment with several doses of MCL 202 produced a rightward shift in the dose-effect curve for SKF 82958-induced increases in rates of eye blinking (Fig. 4, open symbols). A dose-ratio analysis of the antagonist effects of MCL 202 on the SKF 82958-induced increases in eye blinking revealed ratios of 2.7, 4.8 and 31.1 for 0.1, 0.3 and 1.0 mg/kg dose of the antagonist, respectively (Table 2). A relative potency analysis indicates that all three doses of MCL 202 significantly shifted the dose-effect curve for SKF 82958-induced eye blinking to the right in a dose-related manner (Table 2). Schild plot analysis of the antagonist effects of MCL 202 revealed a slope of −1.26 ± 0.28 for the regression of dose ratio values against antagonist concentration, precluding the determination of an apparent pA2 value.
Fig. 4.
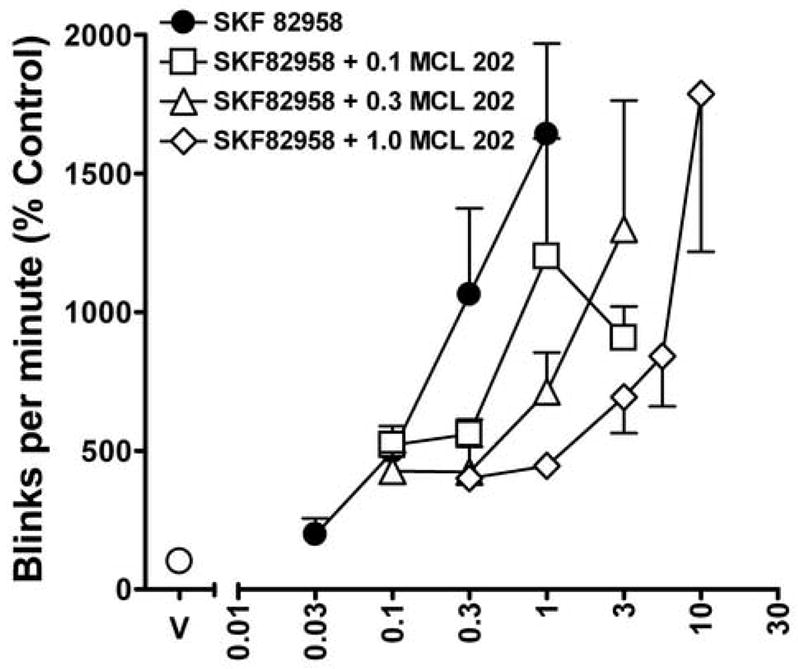
Changes in the SKF 82958 dose-effect curve for rates of eye blinking produced by pretreatments with several doses of the R[+] enantiomer of SKF 83959, MCL 202. Ordinates, eye blink rates as a percentage of that obtained after vehicle injections. Abscissa, cumulative dose of SKF 82958 in mg/kg. Schild plot analysis of the data shows a slope of −0.79 (± 0.11) and a pA2 value of 7.07. Note that pretreatment with MCL 202 produced a dose-related rightward shift in the SKF 82958-induced dose-effect curve. Other details as in Fig. 2.
3.2 Scheduled Controlled Behavior
Table 3 shows control rates of responding maintained under a schedule of stimulus-termination in individual monkeys. All subjects performed consistently throughout the session components with a group mean of 3.10 (± 0.07 S.E.M) responses/s.
Table 3.
Control values of scheduled-controlled behavior across each session component for individual subjects. Data are expressed as responses per second (± S.E.M).
| Monkey | Session Component Responses/s (± S.E.M)
|
Mean Responses/s (± S.E.M) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Ss 4 | 3.72 (0.18) | 3.82 (0.08) | 3.71 (0.13) | 3.66 (0.11) | 3.73 (0.12) | 3.73 (0.03) |
| Ss 13 | 2.38 (0.07) | 2.26 (0.07) | 2.14 (0.03) | 2.17 (0.04) | 2.08 (0.05) | 2.20 (0.05) |
| Ss 11 | 2.70 (0.13) | 2.64 (0.07) | 2.45 (0.03) | 2.46 (0.07) | 2.49 (0.05) | 2.55 (0.05) |
| Ss 8 | 4.42 (1.19) | 4.19 (0.93) | 3.93 (1.38) | 3.36 (0.88) | 3.73 (1.09) | 3.93 (0.18) |
Cumulative doses of SKF 82958 (0.03 – 1.0 mg/kg) produced dose-dependent and significant (F4,10 = 31.74; p < 0.05) decreases in lever-press responding under the schedule of stimulus-termination (Fig. 5, filled circles). The highest cumulative dose of 1.0 mg/kg SKF 82958, which had maximally increased rates of eye blinking, virtually eliminated responding. The R[+] enantiomer of SKF 83959, MCL 202, also produced a dose-related decrease in rates of responding (F6,18 = 8.70; p < 0.05; Fig. 5, open triangles). Maximal decrease in response rates, to 27% of control rates, was achieved after administration of the highest dose of MCL 202 (3 mg/kg). In contrast, cumulative doses of the S[−] enantiomer of SKF 83959, MCL 201, failed to appreciably alter rates of responding (F4,14 = 2.74; p > 0.05; Fig. 4, open squares) and, at the highest dose (3 mg/kg), decreased response rates only to approximately 68% of control values.
Fig. 5.
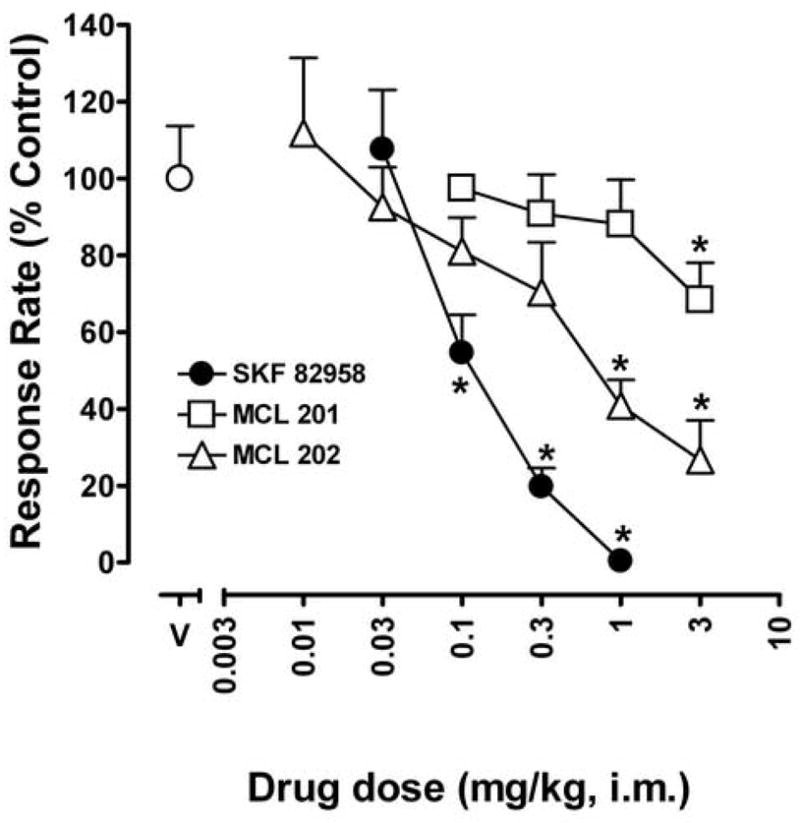
The effects of dopamine D1-like receptor agonists, SKF 82958, MCL 201, and MCL 202 on scheduled controlled behavior. Ordinates, response rates expressed as a percentage of that obtained after vehicle injections. Abscissa, cumulative dose of drugs in mg/kg. Each point represents the average effect determined in three to four monkeys. Results are presented as mean % control (+ S.E.M). Note that the R[+] enantiomer of SKF 83959, MCL 202 significantly decreased response rates, whereas the S[−] enantiomer of SKF 83959, MCL 201 failed to alter rates of responding in squirrel monkeys, and that both these compounds were less effective than SKF 82958. * indicates significantly different from vehicle group (Dunnetts t-test, P < 0.05, on means derived from main effect of dose).
Additional experiments were conducted to compare antagonism of the behavioral effects of SKF 82958 by the D1 receptor blocker SCH 39166 and the D1 agonist MCL 202. As shown in Fig. 6, doses of the D1 receptor blocker SCH 39166 that decreased rates of responding (0.03 and 0.1 mg/kg) also shifted the SKF 82958 dose-effect function to the right in a dose-dependent manner (Fig. 6, open squares and open triangles, respectively). The highest dose of SCH 39166 (0.1 mg/kg) markedly reduced response rates to 16.4 (± 8.40) % of control values (Fig. 6, open triangle) and produced a 3-fold shift to the right in the dose-effect curve, indicative of surmountable antagonism (Fig. 6, open triangles). However, it should be noted that SKF 82958 (0.03 – 0.3 mg/kg) may be antagonizing the rate decreasing effects of SCH 39166.
Fig. 6.

Changes in the SKF 82958 dose-effect curve for response rates of stimulus shock control produced by pretreatments with several doses of the dopamine D1-like receptor antagonist SCH 39166. Ordinates, response rates expressed as a percentage of that obtained after vehicle injections. Abscissa, cumulative dose of SKF 82958 in mg/kg. Other details as in Fig. 4. Note that pretreatment with SCH 39166 produced a dose-related rightward shift in the SKF 82958-induced dose-effect curve.
Like SCH 39166, MCL 202 (0.3 – 1.0 mg/kg) antagonized the effects of SKF 82958 on response rate by dose-dependently shifting the dose effect curve to the right (Fig. 7, open squares and open triangles). The magnitude of antagonism produced by MCL 202 was greater than observed with the highest dose of SCH 39166, perhaps reflecting the more disruptive direct effects of SCH 39166 than of MCL 202 on schedule-controlled responding (Fig. 6, closed symbols above MCL 202). Thus, the higher dose of MCL 202 (1.0 mg/kg) produced an approximately 10-fold shift to the right in SKF 82958’s dose-effect function (Fig. 7, open triangles).
Fig. 7.
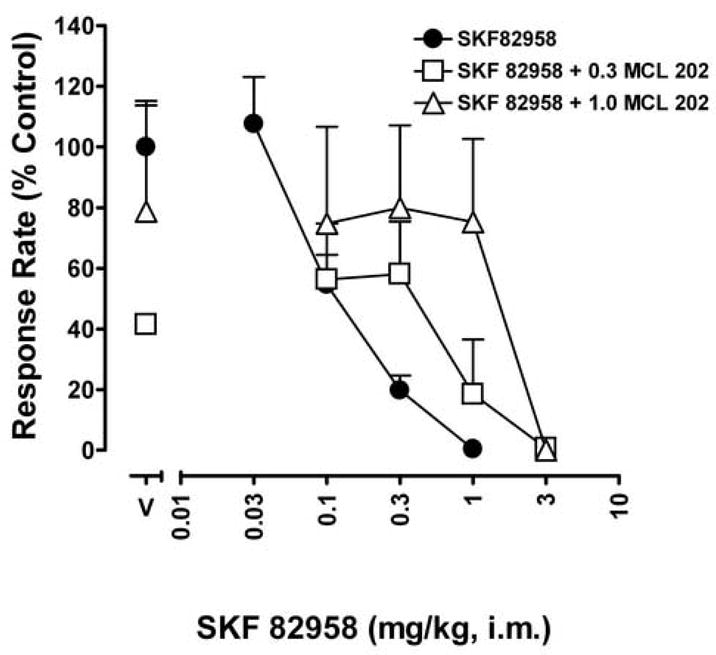
Changes in the SKF 82958 dose-effect curve for response rates of stimulus shock control produced by pretreatments with several doses of the R[+] enantiomer of SKF 83959, MCL 202. Ordinates, response rates expressed as a percentage of that obtained after vehicle injections. Abscissa, cumulative dose of SKF 82958 in mg/kg. Other details as in Fig. 4. Note that pretreatment with MCL 202 produced a dose-related rightward shift in the SKF 82958-induced dose-effect curve.
4. Discussion
The present results in squirrel monkeys reveal interesting similarities and differences in the pharmacology of SKF 82958 and MCL 202, the R-(+)-enantiomer of SKF 83959. Consistent with previous reports, the dopamine D1-like full receptor agonist SKF 82958 produced dose-related increases in eye blinking and decreases in rates of responding (Elsworth et al., 1991, Bergman et al., 1995; Kleven and Koek, 1996, Jutkiewicz and Bergman, 2004). MCL 202 also produced increases in eye blink rate and decreases in response rates. Pretreatment with the selective dopamine D1-like receptor antagonist, SCH 39166, surmountably antagonized the effects of MCL 202 on eye blinking and the effects of SKF 82958 on response rates. Further, pA2 analysis of the shift in the MCL 202 dose-effect curve revealed a slope of −0.78 (± 0.04) and a pA2 value of 7.675, suggestive of competitive antagonism at the D1-like receptor. These results are consistent with the report that SCH 39166 competitively antagonized the effects of R-(+)-6-BrAPB (Jutkiewicz and Bergman, 2004). Together these findings support the view that the behavioral effects of both drugs are mediated by D1 receptor mechanisms, and are highly consistent with the results of earlier in vivo studies with other dopamine D1-like receptor agonists (Bergman et al., 1995; Jutkiewicz and Bergman, 2004) and with those of in in vitro binding studies. In the latter studies, both drugs have been shown to competitively bind to D1-like receptors with high affinity and selectivity (approximately 800-fold for the D1-like over the D2-like receptors (Arnt et al., 1992; Andersen and Jansen, 1990; Neumeyer et al., 2003).
In contrast to SKF 82958 and MCL 202, the S-(−)-enantiomer of SKF 83959, MCL 201, failed to alter measures of either eye blinking or scheduled-controlled behavior in the present study. In previous in vitro binding studies, Neumeyer et al. (2003) have reported a 20-fold difference in the potencies with which MCL 201 and its parent compound, SKF 83959, bind to D1-like receptors, and only a 2- to 3-fold difference in their potency for binding to D2-like receptors (Neumeyer et al., 2003). Taken together, these findings indicate that the behavioral effects of SKF 83959 reside in the R-(+) enantiomer. Based on the limited effects of MCL 201 in the present studies, the S-(−) enantiomer of SKF 83959 appears to be relatively inactive at dopamine D1-like receptors.
Although the effects of both SKF 82958 and MCL 202 appear to be mediated by D1-like receptor mechanisms, the effects of the two drugs differed in the two behavioral assays with respect both to potency and efficacy. For example, based on ED50 values, MCL 202 was approximately 87-fold more potent than SKF 82958 in increasing rates of eye blinking but approximately 3-fold less potent than SKF 82958 in decreasing rates of responding. Moreover, in both types of study the effects of MCL 202 were at levels below those attained with SKF 82958. In conjunction with previous findings in studies of eye blinking, these results are consistent with differences in pharmacological efficacy for the two drugs (Jutkiewicz and Bergman 2004).
Previous studies have demonstrated that SKF 83959, the parent compound of MCL 202, can produce both agonist and antagonist like effects (Gnanalingham et al., 1995a, 1995b, 1995c; Waddington et al., 1995). The agonist effects of SKF 83959 are readily observed in rats and monkeys, both in studies of overt behavior and in studies of anti-Parkinsonian effects (Downes and Waddington 1993; Deveney and Waddington, 1995; Gnanalingham et al., 1995a, b or c; Waddington et al., 1995; Jutkiewicz and Bergman 2004; Desai et al., unpublished data). Moreover, agonist effects of SKF 83959 appear to be significantly conserved in D1-like receptor knockout mice (Clifford et al., 1999). However, recent studies of D1-mediated eye blinking in monkeys showed that SKF 83959 also could partially antagonize the effects of R-(+)-6-Br-APB, a D1-like receptor agonist with higher efficacy (Jutkiewicz and Bergman, 2004). These findings, indicating that SKF 83959 has both agonist and antagonist effects, are consistent with its characterization as a partial agonist at D1-like receptors.
In light of the present findings, it is likely that MCL 202, like its parent compound SKF 83959, also may function as a D1-like partial agonist. In this regard, the effects of MCL 202 on eye blink rates were comparable to those of SKF 83959 (Jukiewicz and Bergman, 2004). Both drugs produced dose-related increases in eye blinking that plateaued at approximately 400% of control values (Jukiewicz and Bergman, 2004). Both drugs antagonized the effects of D1-like receptor agonists with presumably higher efficacy (Jukiewicz and Bergman, 2004). In the present study, for example, MCL 202 dose-dependently antagonized the increased eye blinking and the decrease in response rates produced by SKF 82958, which is fully efficacious in stimulating AC activity. Together, these results suggest that MCL 202, like its parent SKF 83959, has both agonist and antagonist like properties, and therefore can function as a partial agonist at the D1-like receptor.
Acknowledgments
The authors gratefully acknowledge the assistance of Susan Trofimow and Heather Millette. This work was supported by USPHS grants DA 13311 (CAP), DA 145251 (JLN), and DA 03774 (JB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ. Place conditioning with the dopamine D1-like receptor agonist SKF 82958 but not SKF 81297 or SKF 77434. Eur J Pharmacol. 1998;343:111–118. doi: 10.1016/s0014-2999(97)01531-8. [DOI] [PubMed] [Google Scholar]
- Abrahams BS, Rutherford JD, Mallet PE, Beninger RJ. Place conditioning with dopamine D1 and D2 agonists injected peripherally or into nucleus accumbens. Psychopharmacology. 1998;103:271–276. doi: 10.1007/BF02244216. [DOI] [PubMed] [Google Scholar]
- Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Andringa G, Stoof JC, Cools AR. Sub-chronic administration of the dopamine D1 antagonist SKF 83959 in bilaterally MPTP-treated rhesus monkeys: stable therapeutic effects and wearing-off dyskinesia. Psychopharmacology. 1999;146:328–334. doi: 10.1007/s002130051124. [DOI] [PubMed] [Google Scholar]
- Andringa G, Drukarch B, Leysen JE, Cools AR, Stoof JC. The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur J Pharmacol. 1999;364:33–41. doi: 10.1016/s0014-2999(98)00825-5. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Sanchez C. Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol. 1992;213:259–267. doi: 10.1016/0014-2999(92)90690-6. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Spealman RD. Behavioral effects of D1 and D2 dopamine receptor antagonists in squirrel monkeys. J Pharmacol Exp Ther. 1991;258:910–917. [PubMed] [Google Scholar]
- Bergman J, Rosenzweig-Lipson S. Problems of Drug Dependence 1991 NIDA Research Monographs. Vol. 119. US Government Printing Office; Washington, DC: 1992. Cocaine antagonist effects of limited-efficacy D1 agonists; pp. 185–189. [PubMed] [Google Scholar]
- Bergman J, Rosenzweig-Lipson S, Spealman RD. Differential effects of dopamine D1 and D2 receptor agonists on schedule-controlled behavior in squirrel monkeys. J Pharmacol Exp Ther. 1995;273:40–48. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D1-like and D2-like agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- Caldwell J. Do single enantiomers have something special to offer? Hum Psychopharmacol. 2001;16:S67–S71. doi: 10.1002/hup.339. [DOI] [PubMed] [Google Scholar]
- Clifford JJ, Tighe O, Croke DT, Kinsella A, Sibley DR, Drago J, Waddington JL. Conservation of behavioural topography to dopamine D1-like receptor agonists in mutant mice lacking the D1A receptor implicates a D1-like receptor not coupled to adenylyl cyclase. Neuroscience. 1999;93:1483–1489. doi: 10.1016/s0306-4522(99)00297-3. [DOI] [PubMed] [Google Scholar]
- Cools AR, Lubbers L, van Oosten RV, Andringa G. SKF 83959 is an antagonist of dopamine D1-like receptors in the prefrontal cortex and nucleus accumbens: a key to its antiparkinsonian effect in animals? Neuropharmacology. 2002;42:237–245. doi: 10.1016/s0028-3908(01)00169-1. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Cocaine self-administration is increased by both D1 and D2 dopamine antagonists. Pharmacol Biochem Behav. 1991;39:799–802. doi: 10.1016/0091-3057(91)90168-2. [DOI] [PubMed] [Google Scholar]
- Daly SA, Waddington JL. D1 dopamine receptors and the topography of unconditioned motor behavior: studies with the selective ‘full efficacy’ benzazepine D1 agonist SKF 83189. J Psychopharmacology. 1992;6:50–60. doi: 10.1177/026988119200600111. [DOI] [PubMed] [Google Scholar]
- Desai RI, Terry P, Katz JL. Comparison of the discriminative-stimulus effects of SKF 38393 with those of other dopamine receptor agonists. Behav Pharmacol. 2003;14:223–228. doi: 10.1097/00008877-200305000-00006. [DOI] [PubMed] [Google Scholar]
- Desai RI, Terry P, Katz JL. A comparison of the locomotor stimulant effects of D1-like receptor agonists in mice. Pharmacol Biochem Behav. 2005;81:843–848. doi: 10.1016/j.pbb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Deveney AM, Waddington JL. Pharmacological characterization of behavioural responses to Sk&F 83959 in relation to ‘D1-like’ dopamine receptors not linked to adenylyl cyclase. Br J Pharmacol. 1995;116:2120–2126. doi: 10.1111/j.1476-5381.1995.tb16420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes RP, Waddington JL. Grooming and vacuous chewing induced by SK&F 83959, and agonist of dopamine ‘D1-like’ receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur J Pharmacol. 1993;234:135–136. doi: 10.1016/0014-2999(93)90718-w. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Bertalmio AJ, Woods JH. Discriminative and analgesic effects of mu and kappa opioids: In vivo pA2 analysis. Psychopharmacology. 1988;4:107–121. doi: 10.1007/978-3-642-73223-2_9. [DOI] [PubMed] [Google Scholar]
- Ellsworth JD, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Lewis MH, Redmond DE., Jr D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J Pharmacol Exp Ther. 1992;259:595–600. [PubMed] [Google Scholar]
- Finney DJ. Statistical methods in biological assay. 2. Hafner; New York: 1964. [Google Scholar]
- Fornaguera J, Huston JP, Carey RJ, Schwarting RK. Stimulation of D1- or D2-receptors in drug-naive rats with different degrees of unilateral nigro-striatal dopamine lesions. Psychopharmacology. 1995;119:145–154. doi: 10.1007/BF02246155. [DOI] [PubMed] [Google Scholar]
- Gnanalingham K, Erol D, Hunter A, Smith L, Jenner P, Marsden CA. Differential anti-parkinsonian effects of benzazepine D1 dompamine agonists with varying efficacies in the MPTP-treated common marmoset. Psychopharmacology. 1995;117:275–286. doi: 10.1007/BF02246102. [DOI] [PubMed] [Google Scholar]
- Gnanalingham K, Hunter AJ, Jenner P, Mardens CD. Stimulation of adenylate cyclase activity by benzazepine D-1 dopamine agonists with varying efficacies in the 6-hydroxydopamine lesioned rat--relationship to circling behavior. Biochem Pharm. 1995;49:1185–1193. doi: 10.1016/0006-2952(95)00035-x. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Hunter AJ, Jenner P, Marsden CD. The differential behavioural effects of benzazepine D1 dopamine agonists with varying efficacies, co-administered with quinpirole in primate and rodent models of Parkinson’s disease. Psychopharmacology. 1995;117:287–297. doi: 10.1007/BF02246103. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Hu XT, White FJ. Adaptations in the mesoaccumbens dopamine system resulting from repeated administration of dopamine D1 and D2 receptor-selective agonists: relevance to cocaine sensitization. Psychopharmacology. 1998;140:233–242. doi: 10.1007/s002130050762. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Bergman J. Effects of dopamine D1 ligands on eye blinking in monkeys: efficacy, antagonism, and D1/D2 interactions. J Pharmacol Exp Ther. 2004;311:1008–1015. doi: 10.1124/jpet.104.071092. [DOI] [PubMed] [Google Scholar]
- Katz JL, Witkin JM. Selective effects of the D1 dopamine receptor agonist, SKF 38393, on behavior maintained by cocaine injection in squirrel monkeys. Psychopharmacology. 1992;109:241–244. doi: 10.1007/BF02245508. [DOI] [PubMed] [Google Scholar]
- Katz JL, Shores AE, Witkin JM. Effects of D1 dompamine agonists on schedule-controlled behavior in the squirrel monkey. Behav Pharm. 1995;6:143–148. [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Myers KA, Mitkus RJ, Chider M. Behavioral effects of cocaine: Interactions with D1 dopaminergic antagonists and agonists in mice and squirrel monkeys. J Pharmacol Exp Ther. 1999;291:265–279. [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Differential effects of direct and indirect dopamine agonists on eye blink rate in cynomolgus monkeys. J Pharmacol Exp Ther. 1996;279:1211–1219. [PubMed] [Google Scholar]
- Mahan LC, Burch RM, Monsma FJ, Jr, Sibley DR. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc Natl Acad Sci USA. 1990;87:2196–2200. doi: 10.1073/pnas.87.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. New Engl J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Morelli M, Fenu S, Pinna A, Cozzolino A, Carta A, Di Chiara G. ‘Priming’ to dopamine agonist-induced contralateral turning as a model of non-associative sensitization to the expression of the post-synaptic dopamine message. Behav Pharmacol. 1993;4:389–397. [PubMed] [Google Scholar]
- Mutschler NH, Bergman J. Effects of chronic administration of the D1 receptor partial agonist SKF 77434 on cocaine self-administration in rhesus monkeys. Psychopharmacology. 2002;160:362–370. doi: 10.1007/s00213-001-0976-z. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Kula NS, Baldessarini RJ, Baindur N. Stereoisomeric probes for the D1 dopamine receptor: synthesis and characterization of R(+) and S(−) enantiomers of 3-allyl-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine and its 6-bromo analogue. J Med Chem. 1992;35:1466–1471. doi: 10.1021/jm00086a016. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazpines. Eur J Pharmacol. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- Peacock L, Gerlach J. Aberrant behavioral effects of a dopamine D1 receptor antagonist and agonist in monkeys: evidence of uncharted dopamine D1 receptor actions. Biol Psychiatry. 2001;50:501–509. doi: 10.1016/s0006-3223(01)01189-1. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Dissociation of cocaine-antagonist properties and motoric effects of the D1 receptor partial agonists SKF 83959 and SKF 77434. J Pharmacol Exp Ther. 2000;293:1017–1026. [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Modulation of cocaine and food self-administration by low- and high-efficacy D1 agonists in squirrel monkeys. Psychopharmacology. 2001;157:208–216. doi: 10.1007/s002130100779. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology. 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. The D1 agonists SKF 82958 and SKF 77434 are self-administered by rats. Brain Res. 1992;582:349–352. doi: 10.1016/0006-8993(92)90155-3. [DOI] [PubMed] [Google Scholar]
- Singh J, Desiraju T, Raju TR. Dopamine receptor sub-types involvement in nucleus accumbens and ventral tegmentum but not in medial prefrontal cortex: on self-stimulation of lateral hypothalamus and ventral mesencephalon. Behav Brain Res. 1997;86:171–179. doi: 10.1016/s0166-4328(96)02263-2. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Nader MA. Modulation of cocaine’s discriminative stimulus effects by dopamine D(1) agonists in rhesus monkeys. Pharmacol Biochem Behav. 2001;68:301–309. doi: 10.1016/s0091-3057(00)00462-7. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical methods. 6. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- Spealman RD, Bergman J, Rosenzweig-Lipson S. Differential modulation of behavioral effects of cocaine by low- and high-efficacy D1 agonists. Psychopharmacology. 1997;133:283–292. doi: 10.1007/s002130050403. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1–12. [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;622:2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- Undie AS. Relationship between dopamine agonist stimulation of inositol phosphate formation and cytidine diphosphate-diacylglycerol accumulation in brain slices. Brain Res. 1999;816:286–294. doi: 10.1016/s0006-8993(98)01076-2. [DOI] [PubMed] [Google Scholar]
- Undie AS, Berki AC, Beardsley K. Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology. 2000;39:75–87. doi: 10.1016/s0028-3908(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Daly SA, Downes RP, Deveney AM, McCauley PG, O’Boyle KM. Behavioural pharmacology of ‘D-1-like’ dopamine receptors: further subtyping, new pharmacological probes and interactions with ‘D-2-like’ receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:811–831. doi: 10.1016/0278-5846(95)00130-n. [DOI] [PubMed] [Google Scholar]
- Weed MR, Woolverton WL. The reinforcing effects of dopamine D1 receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 1995;275:1367–1374. [PubMed] [Google Scholar]
- Weed MR, Paul IA, Dwoskin LP, Moore SE, Woolverton WL. The relationship between reinforcing effects and in vitro effects of D1 agonists in monkeys. J Pharmacol Exp Ther. 1997;283:29–38. [PubMed] [Google Scholar]


