Abstract
Replication forks stall at DNA lesions or as a result of an unfavorable replicative environment. These fork stalling events have been associated with recombination and gross chromosomal rearrangements. Recombination and fork bypass pathways are the mechanisms accountable for restart of stalled forks. An important lesion bypass mechanism is the highly conserved post-replication repair (PRR) pathway that is composed of error-prone translesion and error-free bypass branches. EXO1 codes for a Rad2p family member nuclease that has been implicated in a multitude of eukaryotic DNA metabolic pathways that include DNA repair, recombination, replication, and telomere integrity. In this report, we show EXO1 functions in the MMS2 error-free branch of the PRR pathway independent of the role of EXO1 in DNA mismatch repair (MMR). Consistent with the idea that EXO1 functions independently in two separate pathways, we defined a domain of Exo1p required for PRR distinct from those required for interaction with MMR proteins. We then generated a point mutant exo1 allele that was defective for the function of Exo1p in MMR due to disrupted interaction with Mlh1p, but still functional for PRR. Lastly, by using a compound exo1 mutant that was defective for interaction with Mlh1p and deficient for nuclease activity, we provide further evidence that Exo1p plays both structural and catalytic roles during MMR.
Keywords: DNA mismatch repair, EXO1, DNA nuclease, DNA damage and post-replication repair
1. Introduction
Cells expend a great deal of energy and enlist a large number of gene products to faithfully duplicate their genomes [1,2]. S-phase replication checkpoints are cellular failsafe mechanisms that arrest cells or slow S-phase progression allowing for the repair, recovery and if these are not possible, the bypass of lesions that prevent duplication of the genome. Replication forks stall because of encounters with upstream lesions or low dNTP pools. Pathways to overcome fork stalling are highly conserved across species. When checkpoints, fork repair or fork bypass processes are defective, stalled replication forks can generate anomalous structures [1]. These recombinogenic structures have been proposed to account for genomic rearrangements at fragile sites and for genomic instability in cancer cells [3]. In fact, human cancers have been shown to have defects in genes for checkpoints, fork repair or fork bypass proteins [3]. Moreover, recent studies have suggested that these replication response pathways serve as the earliest barrier to tumorigenesis [4,5].
Post-replication repair (PRR) is a eukaryotic pathway that facilitates the bypass or tolerance of fork stalling events but does not “repair” these lesions [6-8]. The pathway is defined by the RAD6-RAD18 heterodimer, which encodes a ubiquitin-conjugating and ubiquitin-ligase enzyme complex. This pathway has been shown to consist of minimally three genetic branches: a checkpoint sub-pathway [9]; an error-free sub-pathway; and an error-prone translesion synthesis sub-pathway [10]. Genetic defects in these sub-pathways can result in increased sensitivity to DNA damaging agents, growth deficiencies and effects on spontaneous and induced mutagenesis [11].
Exo1p is a member of the Rad2 family of structure-specific nucleases which based on in vitro studies possess 5′->3′ exonuclease and 5′-flap endonuclease activities [12,13]. First isolated as a nuclease activity induced during meiosis in fission yeast [14], Exo1p has since been implicated in multiple DNA metabolic pathways that include DNA repair, recombination, replication, and telomere integrity [12,13]. EXO1 involvement in these multiple pathways makes it a logical target for mutation during oncogenesis; supported by the tumor prone phenotype of a mouse model [15]. Exo1p has been best characterized by its structural and catalytic role during DNA mismatch repair (MMR) mutation avoidance [16-19]. However, based on the exo1Δ mutational spectra and genetic interaction with REV3, we previously hypothesized that EXO1 participates in at least one MMR-independent mutation avoidance pathway [12]. In agreement with our supposition, recent studies have identified a potential role for EXO1 in the maintenance and repair of stalled replication forks [20-22].
Here, we report on the role of EXO1 in the tolerance to the fork stalling lesion(s) produced from low doses of methylmethane sulfonate (MMS) and the MMR dependency of this function. Based on our findings, we propose that EXO1 functions in the MMS2 error-free branch of the PRR pathway, independent of the role of EXO1 in MMR. Consistent with the idea that EXO1 functions in two separate genome stability pathways, we defined a domain of Exo1p required for PRR distinct from those required for interaction with MMR proteins. Finally, we generated an exo1 missense mutant that was functional for PRR, but defective for MMR.
2. Materials and methods
2.1. Strains and media
E. coli strain DH-10B was used for plasmid construction and amplification. S. cerevisiae strains used in this study are described in Table 1. Bacterial and yeast strains were grown under conditions described previously [19]. Yeast transformations were performed by the polyethylene glycol-lithium acetate method [23].
The genomic exo1 point mutant strains [19] (Table 1) were created with a two-step recombination procedure as described previously (for exo1-D173A) and as below (for exo1-FF447AA). Targeting construct YIp-exo1-FF477AA was digested with XmaI and transformed into appropriate strains as described previously [19]. We screened for the mutant alleles using the PCR oligos: EXO1-999.S, 5′-CGACGACGATATAGATCACCAC-3′; and EXO1-1501.AS, 5′-CACTCAGGTTGTCGTCATCCTC-3′. The exo1-FF4477AA mutation creates a second AluI site in this interval. All point mutants were also confirmed by sequencing using oligo EXO1-1230.S, 5′-CATCCATAGTCTAAGACAAGCGG-3′.
All other yeast deletion mutants were created using PCR generated disruption amplicons from strains provided by the Saccharomyces Genome Deletion Project.
2.2. Plasmid construction
All DNA manipulations were performed using standard molecular biology procedures [24]. DNA sequencing was performed at OHSU Microbiology and Immunology core sequencing facility with an ABI automated sequencer.
(i) Targeting vectors
YIp-exo1-FF447AA was created as follows. A BamHI-XmaI fragment of EXO1 containing the FF447AA mutation taken from pJAS-exo1-FF447AA was cloned into YIp-EXO1 and replaced the wildtype BamHI-XmaI segment. The correct clone was confirmed by sequencing.
(ii) Expression plasmids
The construction of pJAS-EXO1-FLAG and nuclease deficient variants and pJAS-RAD27 were described previously [19]. The pJAS-exo1-FF447AA and pJAS-exo1-D173A,-FF447AA constructs were made as follows.
The exo1-FF4477AA allele was created using the Quikchange™ Site-Directed Mutagenesis Kit (Stratagene) on pJAS-EXO1-FLAG using the following oligos: exo1-FF447AA.S, 5′-GGATACAAGAAGCAAAGCTGCTAATAAACCCTCCATG-3′; and exo1-FF447AA.AS, 5′-CATGGAGGGTTTATTAGCAGCTTTGCTTCTTGTATCC-3′. The region between unique internal BamHI and XmaI sites was sequenced for the FF447AA allele and to exclude any second site mutations. The desired BamHI- XmaI fragment was cloned back into the parent pJAS-EXO1-FLAG and pJAS-exo1-D173A-FLAG to produce pJAS-exo1-FF447AA-FLAG and pJAS-exo1-D173A,-FF447AA-FLAG, respectively.
2.3. Mutation rates assays
Measurement of mutation rates were performed as stated previously [19]. Briefly, strains were streak purified, individual colonies grown to saturation in YPD or -Trp, then various dilutions plated onto complete synthetic media (CSM), -Thr and + Canavanine (+CAN) [60 μg/ml] plates and colonies counted after 2-3 days growth at 30°C. Rates were determined as previously described. Statistical analysis was performed using Prism version 2a software (GraphPad Software Inc.).
Patch assays were performed by streak purifying strains, individual colonies patched onto YPD or –Trp plates, grown to confluence at 30°C, then replica plated onto -Thr or -Thr -Trp plates and colonies counted after 2-3 days growth at 30°C.
2.4. Two-hybrid Analysis
Protein-protein interactions were assessed using the two-hybrid technique as described previously [18]. Four independently generated pGAD-exo1-FF447AA “prey” clones were tested. “Bait” and “prey” plasmids were transformed into L40, growth on –TRP –URA plates served as a control, while growth on –TRP –URA –HIS plates indicated “bait”-“prey” interaction. L40 has a second chromosomal lexA-GAL4A reporter system, URA3::(lexAop) 8-lacZ. β-galactosidase assays were performed on –TRP –URA –HIS plates as described [25]. Reactions were placed at 30°C until desired blue color development was achieved.
2.5. Methylmethane Sulfonate Epistasis Analysis
Designated single and double mutant combinations were grown overnight to saturation in YPD, serially diluted (1:5), dilutions spotted onto YPD and YPD containing 0.0005-0.0175% methylmethane sulfonate (MMS) plates with a 48-prong replicator and allowed to grow at 30°C for 3-4 days.
2.6. Plasmid Loss Assay
To assess synthetic lethality of exo1 mutations in combination with rad27Δ, we performed the assay as described previously 2-3 times per experiment [24].
3. Results
3.1. Epistasis analysis defines a role for EXO1 in the MMS2 error-free branch of PRR that is MMR-independent
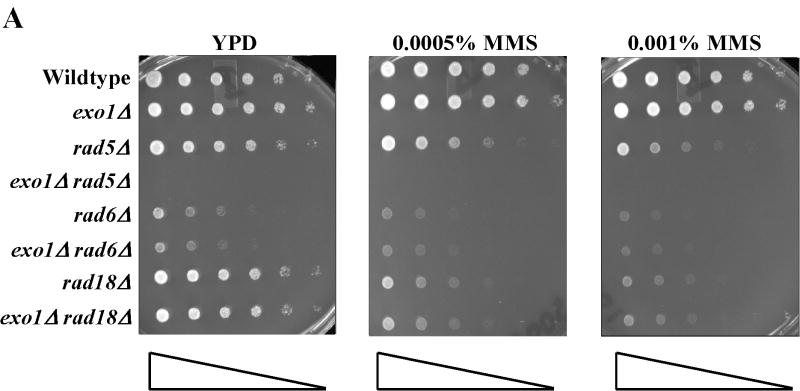
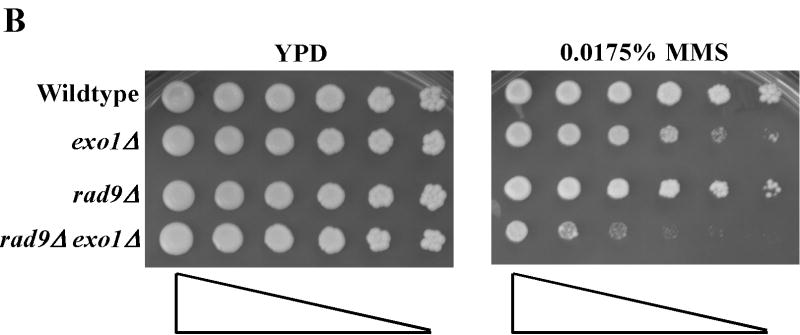
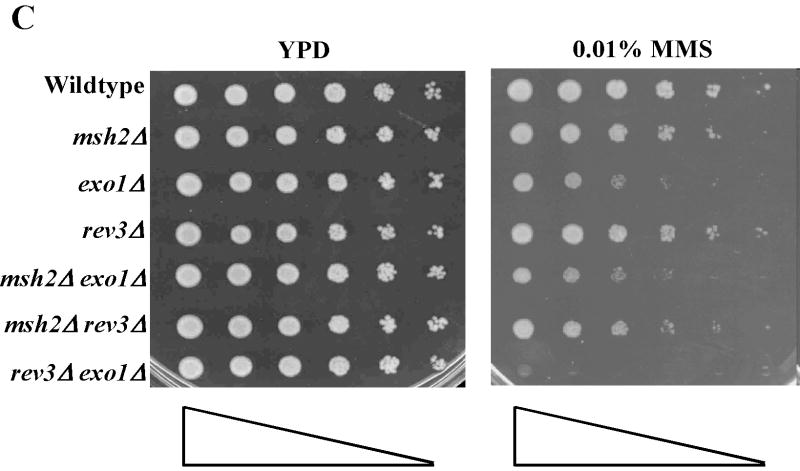
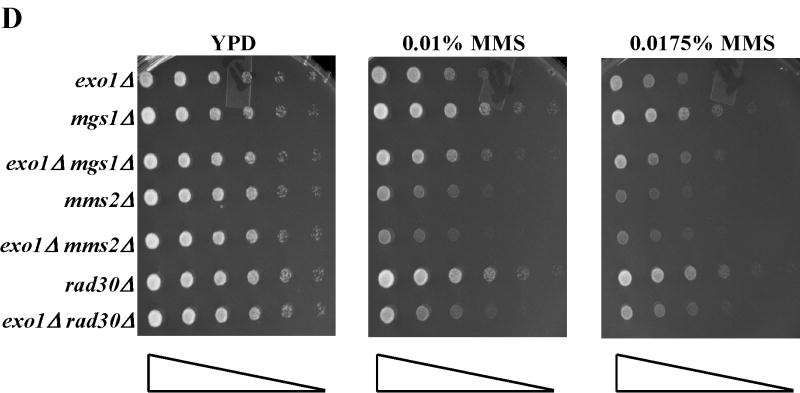
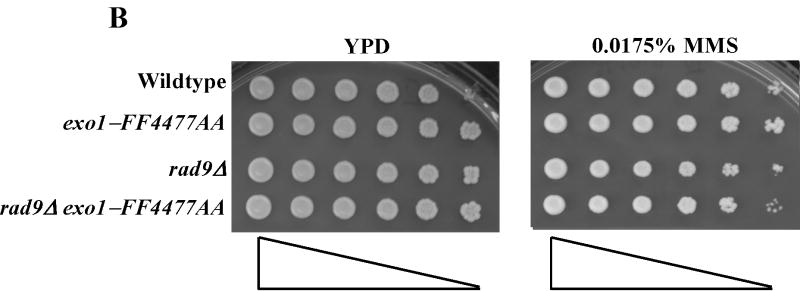
Treatment of S. cerevisiae with low doses of MMS has been used to assess the function of the intra-S-phase checkpoint and replication fork restart/bypass processes [26-29]. We performed epistasis analysis for sensitivity to low doses of MMS between EXO1 and genes known to be involved in PRR or replication fork bypass. The rad6Δ and rad18Δ mutations, the founding genes of the PRR pathway, displayed an epistatic relationship to exo1Δ (Figure 1A) [11], suggesting that EXO1 functioned in PRR for MMS tolerance. As mentioned previously, PRR is composed of at least three genetically distinct sub-pathways: a checkpoint pathway; an error-free pathway; and an error-prone pathway, defined by the genes RAD9 [9], MMS2 [30] and REV3 [10,31,32], respectively. We found that mms2Δ was epistatic to exo1Δ, but that both rad9Δ and rev3Δ were synergistic with exo1Δ (see Figure 1B-D) by epistasis analysis for MMS sensitivity. These data suggested that EXO1 functions in the MMS2 error-free sub-pathway of PRR and is redundant to the RAD9 checkpoint and REV3 error-prone sub-pathways. RAD5 has been mapped to the MMS2-error free branch by both genetic and protein interaction data [33-35]. Interestingly, we were unable to generate an exo1Δ rad5Δ double mutant suggestive of a synthetically lethal interaction (see Figure 1A).
Figure 1.
EXO1 acts in the MMS2 error-free branch of PRR in response to MMS independent of MMR. Overnight saturated cultures were serially diluted (1:5), spotted on YPD plates and YPD plates with increasing concentrations of MMS using a 48-prong replicator and then incubated at 30 °C for 2-4 days. The exo1Δ mutation defines an MMS tolerance pathway that functions in the (A) RAD6/RAD18 PRR pathway, but is redundant with the REV3 error-prone branch and the RAD9 checkpoint branch as the double (B) exo1Δ rad9Δ and (C) exo1Δ rev3Δ mutants are synergistically more sensitive than the single mutants alone. In contrast, (D) EXO1 is hypostatic to MMS2 and other components of the error-free branch of the PRR, but EXO1 did not interact with pathways competing with PRR as defined by MGS1 and RAD30. This EXO1 DNA damage tolerance pathway is independent of MMR as there was no genetic interaction when (C) msh2Δ or (Figure S1B) pms1Δ mutants were examined.
EXO1 was first isolated in a screen for recombination gene products. Recombination plays a role in replication restart of stalled forks independent of the PRR pathway and possibly some facet of the MMS2 error-free bypass pathway of PRR [36]. Consistent with a role of EXO1 in recombinational bypass of stalled replication forks, the recombination mutants rad52Δ and rad51Δ demonstrated a epistatic relationship to exo1Δ for MMS sensitivity (see Supplemental Figure S1A).
MGS1 encodes for a DNA-dependent ATPase with ssDNA annealing activities that has been suggested to compete with the PRR pathway for resolution of fork stalling lesions [6,37,38]. RAD30 has been implicated in an error-free form of translesion synthesis [39,40] that some have placed in the PRR pathway [41]. Neither of these genes demonstrated any sensitivity to low dose MMS as single mutants. Nor did MGS1 or RAD30 have any additive or synergist genetic interaction with EXO1 (see Figure 1D), consistent with our data that EXO1 functions in the MMS2 error-free sub-pathway of PRR.
Finally, this role of EXO1 in MMS tolerance was independent of the role of EXO1 in MMR. Figure 1C and supplemental Figure S1B showed that canonical MMR genes msh2Δ and pms1Δ did not display any sensitivity to low dose MMS consistent with a lack of role in PRR. Similarly, neither msh2Δ or pms1Δ interacted with exo1Δ as double mutants. Consistent with published reports for mutation avoidance [18] and PRR [36], MSH2 or PMS1 did not interact with REV3 in our MMS sensitivity assays. Taken together, our epistasis analyses suggested EXO1 functions in the MMS2 error-free branch of the PRR pathway in response to low dose MMS and that this role is independent of MMR.
3.2. Mapping separate Exo1p “PRR” and “MMR” domains
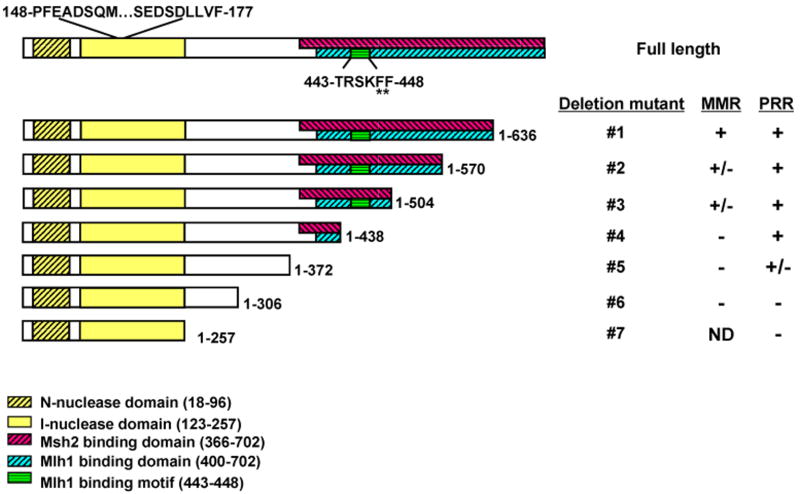
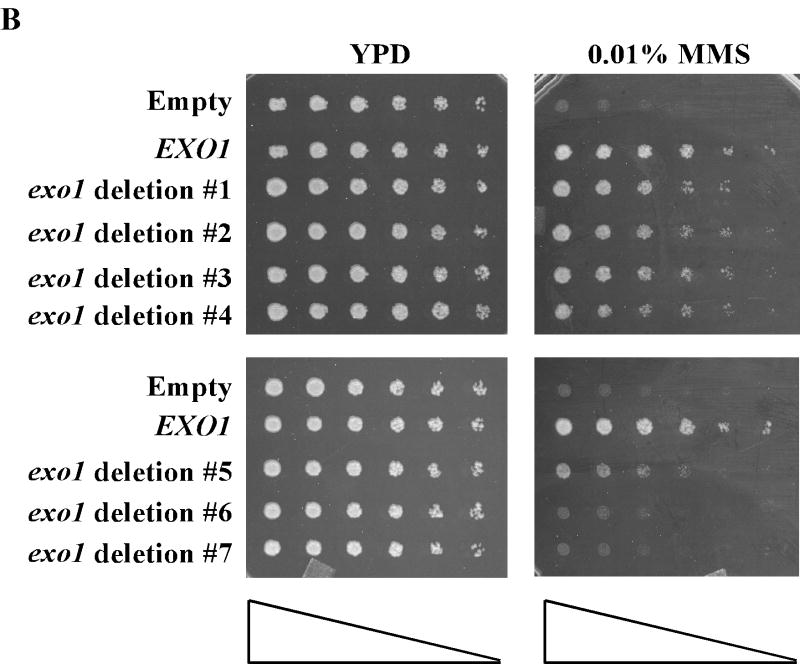
Structure-function studies have delineated modular domains of Exo1p (see Figure 2A) [12,19,42-45]. Functionally, Exo1p can be split into NH2- and COOH-terminal halves, required for nuclease and protein-protein interaction activities [19,42,45], respectively. To identify an Exo1p domain necessary for MMS tolerance we performed complementation assays with progressively larger COOH –terminal deletion exo1 mutants (see Figure 2A-B). To increase the sensitivity of the assay we performed complementation in the exo1Δ rev3Δ double mutant which demonstrates synergistic MMS sensitivity. We have previously shown that many of the functions of Exo1p require active nuclease activity [19], therefore deletions of the NH2-terminal half were not likely to be fruitful. Figure 2B shows that residues 1-438 of Exo1p are required for full complementation of the MMS sensitive exo1Δ phenotype or full activity in PRR (see Figure 2A for summary).
Figure 2.
Defining separate Exo1p PRR and MMR domains. (A) Schematic and summary of phenotypes for Exo1p deletion mutants tested by MMS sensitivity (PRR) and for MMR mutator as shown in Figure 2B and Table 2, respectively. Exo1p with functional domains and motifs as indicated by respective hatched boxes. Site-specific mutations experimentally examined are highlighted by asterisks as detailed in the text. This analysis defines Exo1p deletion mutant #4 (1-438 aa) as a separation of function mutant. (B) Complementation of MMS tolerance in an exo1Δ rev3Δ strain with the listed Exo1p deletion mutant constructs suggests that residues 1-438 are necessary for functional PRR. MMS sensitivity was performed as described previously in Figure 1.
In conjunction with examining the Exo1p domains required for PRR in response to MMS, we also performed complementation studies with these exo1 deletion mutants for MMR mutator phenotype. The mutation reporter hom3-10 reports reversions that occur primarily from a single nucleotide deletion in a homonucleotide run of seven T-A base pairs, and has been shown to be a sensitive marker of MMR activity in vivo [18,46]. Similar to our MMS complementation experiments, we used the double mutant pms1-61 exo1Δ for complementation to improve the sensitivity of our assay. Our complementation data suggested that the COOH-terminal MMR interaction domains of Exo1p are critical for full MMR activity (see Table 2 and Figure 2A for summary). As summarized in Figure 2A, the domains required for PRR (MMS tolerance) and MMR were distinct from one another. Consistent with this idea, we defined an exo1 deletion (Table 2) that was functional for PRR, but not MMR (Exo1p deletion mutant #4).
3.3. The exo1-FF447AA allele is defective for MMR-dependent mutation avoidance
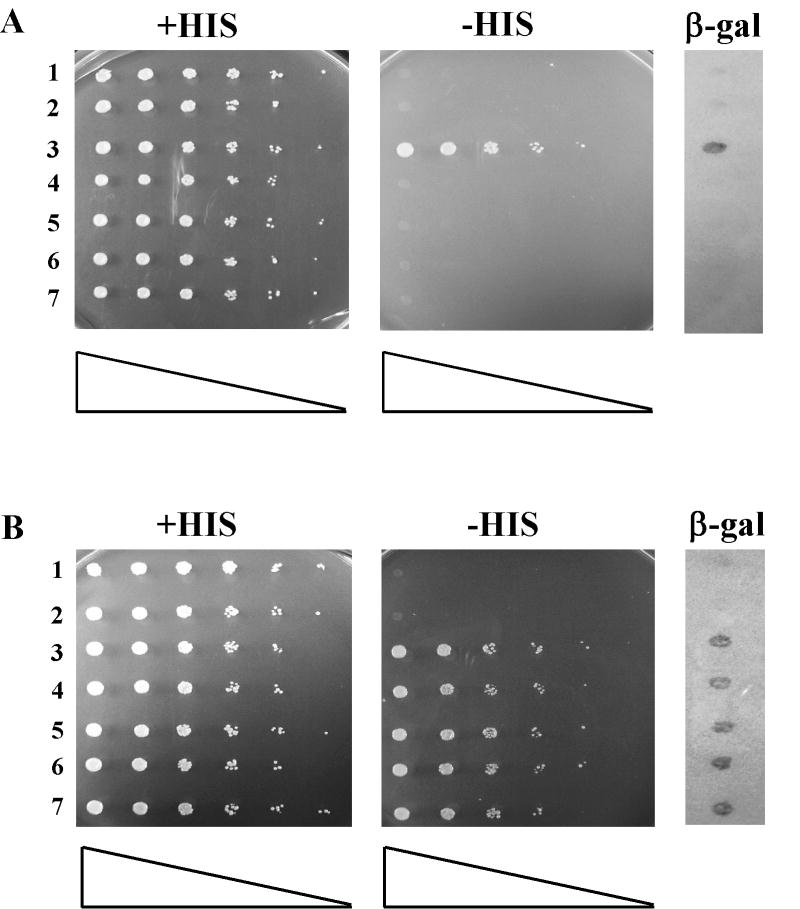
As suggested by the MMR complementation data for EXO1 above, interactions between Exo1p and MMR components are important for full MMR activity, but not PRR. The MutLα heterodimer composed of Mlh1p and Pms1p is a required component in MMR that interacts with Exo1p via the Mlh1p protomer [18,43]. We hypothesized that we could create a more subtle allele of EXO1 that would be defective in MMR, but not PRR by mutating the Mlh1p minimal binding motif in Exo1p [47]: 445-RSKFF-448 to 445-RSKAA-448 (designated as exo1-FF447AA for the remainder of this manuscript). We validated that exo1p-FF447AA no longer interacted with Mlh1p by yeast two-hybrid (see Figure 3). However, this exo1 mutant was still capable of interaction with Msh2p, another required component of MMR, by yeast two-hybrid (Figure 3). Retention of Msh2p interaction would seem to rule out that gross disruption of the Exo1p COOH-terminal tertiary structure or increased turnover was the reason for the lack of an exo1p-FF447AA-Mlh1p interaction (Figure 3).
Figure 3.
The FF->AA mutation prevents Exo1p from interacting with Mlh1p, but not Msh2p by yeast two-hybrid. Strains with designated bait-prey sets were grown in nonselective media (-TRP -URA) to saturation, serially diluted (1:5), spotted on the indicated plates using a 48-prong replicator and then incubated at 30 °C for 3 days. Strains were assayed for β-gal activity by lift assays as described in the Materials and Methods. Growth on –HIS plates and blue color development on the β -gal assay indicates interaction between the bait and prey fusion proteins. (A) Mlh1p-LexAp bait and Exo1p-Gad4p prey combinations demonstrate that the exo1-FF447AA mutation prevents Exo1p-Gad4p interaction with Mlh1p-LexAp. Lanes: 1, pBTM (empty bait control) + pGAD (empty prey control); 2, pBTM-MLH1 + pGAD; 3, pBTM-MLH1 + pGAD-EXO1; 4, pBTM-MLH1 + pGAD-exo1-FF447AA # 1; 5, pBTM-MLH1 + pGAD-exo1-FF447AA # 5; 6, pBTM-MLH1 + pGAD-exo1-FF447AA # 6; and 7, pBTM-MLH1 + pGAD-exo1-FF447AA # 10. (B) Msh2p-LexAp bait and Exo1p-Gad4p prey combinations demonstrate that the exo1-FF447AA mutation does not prevent Exo1p-Gad4p interaction with Msh2p-LexAp. Lanes: 1, pBTM (control) + pGAD (control); 2, pBTM-MSH2 + pGAD; 3, pBTM- MSH2 + pGAD-EXO1; 4, pBTM- MSH2 + pGAD-exo1-FF447AA # 1; 5, pBTM- MSH2 + pGAD-exo1-FF447AA # 5; 6, pBTM- MSH2 + pGAD-exo1-FF447AA # 6; and 7, pBTM- MSH2 + pGAD-exo1-FF447AA # 10. Clones pGAD-exo1-FF447AA #1, 5, 6 & 10 represent four independently generated “prey” constructs.
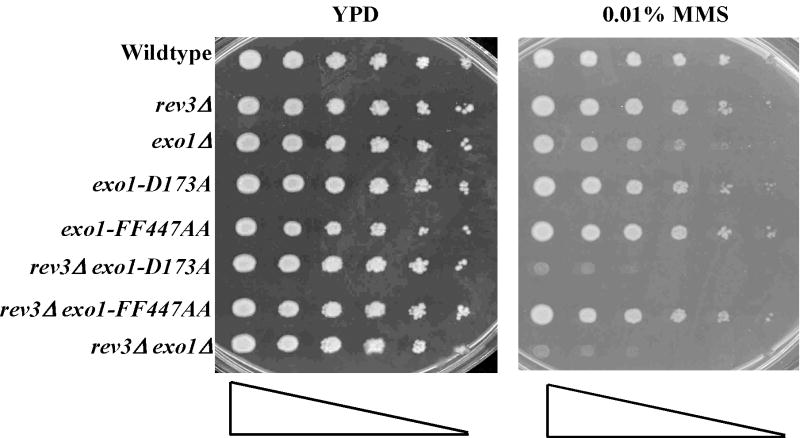
To validate the failure of exo1p-FF447AA to interact with Mlh1p in vivo, we made use of the dominant negative allele, exo1-D173A, which when overexpressed in a hypomorphic MMR defective background resulted in a synergistic MMR defect using the hom3-10 reporter (Table 3) [19]. We chose to use the pms1-E61A allele because the role of EXO1 in MMR can be more readily appreciated with the use of hypomorphic alleles of mlh1 or pms1, which demonstrate synergistic defects in MMR when combined with exo1 mutants [18,19]. As demonstrated by Table 3, the exo1-FF447AA allele was able to complement the defect of exo1Δ using the “general” mutation reporter CAN1, which reports a wide variety of mutational events including small frameshift insertion/deletions, nucleotide substitutions and large deletions. However, the compound exo1-D173A, -FF447AA mutant was no longer able to exert a dominant negative effect, per the MMR-specific hom3-10 reporter, as compared to the exo1-D173A allele when expressed in the background of a weak pms1 defective allele. Taken together these data suggest that the protein encoded by exo1-FF447AA is stable and functional for MMR-independent mutation avoidance, but is not capable of interaction with the MutLα heterodimer, consistent with our two-hybrid data above.
We next examined the effect of the exo1-FF447AA allele when expressed from the native EXO1 chromosomal locus. Previously we ascribed much of the CAN1 mutator phenotype of an exo1Δ to a defect in a MMR-independent mutation avoidance pathway based both on mutational spectra data and suppression by deletion of REV3 [12,19]. In agreement with the argument that the exo1-FF447AA mutant is functional for PRR or fork bypass, we did not observe an increased mutation rate at the CAN1 mutation reporter (Table 4, strain PTY1300). We next analyzed the effect of the exo1-FF447AA mutant on the MMR mutator phenotype using the hom3-10 reporter. As described previously, a singular defect in EXO1 has little or no effect on MMR-dependent mutation avoidance as compared to the primary components MLH1 or PMS1 (Table 4). The lack of a strong MMR mutator phenotype is likely due to EXO1 redundant activities [12]. As stated previously, we used hypomorphic alleles of mlh1 or pms1 to better evaluate the effect of exo1-FF447AA on MMR, because mlh1-E31A and pms1-E61A alleles demonstrate synergistic defects in MMR when combined with exo1 mutants [18,19]. As shown in Table 4, exo1-FF447AA demonstrated synergistic defects in MMR with either mlh1 (strain PTY223) or pms1 (strain PTY224) hypomorph alleles. Taken together, these data suggest that the exo1-FF447AA mutant is defective for MMR function most likely due to loss of interaction with the MutLα heterodimer.
3.4. The exo1-FF447AA allele is proficient for PRR
We next examined the function of the exo1-FF447AA mutant for PRR, or fork bypass, by sensitivity to low dose MMS. As shown in Figure 4A-B, the exo1-FF447AA mutant was no more sensitive to low dose MMS than wildtype. In addition, the exo1-FF447AA mutant displayed no synergistic defects with either rev3Δ or rad9Δ mutations (see Figure 4A-B). As stated previously, RAD5 has been mapped to the MMS2-error free branch [33-35] and we demonstrated above a synthetic interaction between exo1Δ and rad5Δ (Figure 1A). Consistent with our hypothesis that the exo1-FF447AA allele is only dysfunctional for MRR, we were able to generate a double exo1-FF447AA rad5Δ mutant (see Figure S2C). In summary, these data suggest that the exo1-FF4477A allele defines a mutation that separates the PRR and MMR functions of EXO1.
Figure 4.
The exo1-FF447AA mutant is functional for PRR. Overnight saturated cultures were serially diluted (1:5), spotted on YPD plates and YPD plates with increasing concentrations of MMS using a 48-prong replicator and then incubated at 30 °C for 2 days. The exo1-FF447AA mutation shows no defect in the PRR pathway as this mutant is as resistant to MMS as wildtype and does not show any genetic interaction with the redundant (A) REV3- or (B) RAD9-dependent branches of PRR.
4. Discussion
In this study, we report genetic data suggesting that EXO1 functions in the MMS2 error-free branch of PRR in response to MMS. This novel function of EXO1 in PRR was independent of MMR. By performing structure-function studies on Exo1p, we defined an NH2-terminal fragment of Exo1p that was required for PRR. This fragment included regions of Exo1p not attributed previously to any known activities. Finally, using an allele of EXO1 that encodes a protein incapable of binding to Mlh1p, we were able to genetically separate the function of EXO1 in MMR from that in PRR.
Although the mechanism of fork bypass by the MMS2 error-free branch of PRR is poorly defined, selection of which PRR branch is utilized during a fork stalling event is thought to occur via ubiquitin states of PCNA [48-50]. Briefly, upon S-phase DNA damage Rad6p-Rad18p mono-ubiquitinates PCNA on K164 promoting translesion synthesis or mutagenic fork bypass. Alternatively, Mms2-Ubc13p-Rad5p poly-ubiquitinates PCNA on K164 through further chain assembly on K63 of ubiquitin, which directs the stalled fork to error-free bypass. Recent data suggest that the MMS2 error-free branch likely facilitates fork bypass using a recombinational mechanism via copy choice or template switching. This same study also suggested that a smaller fraction of error-free fork bypass events was RAD52-dependent [36]. Our results (Figure 1A-D & Figure 4) showing that mms2Δ was epistatic to exo1Δ suggest that EXO1 plays a role in the MMS2-dependent error-free bypass pathway. Given EXO1's role in other recombinational pathways and its biochemical activity on recombination substrates, we hypothesize that the Exo1p nuclease functions are utilized to generate or resolve intermediates for copy choice or template switching bypass in the MMS2-sub-branch of PRR.
In addition to the role of EXO1 in PRR proposed here, EXO1 has been implicated to function in cell cycle checkpoint, fork maintenance and fork repair pathways [20-22,51,52]. EXO1 has been shown to play subtle roles in end-processing for mitotic recombination and function redundantly with at least one other unidentified nuclease. This “other” redundant nuclease may be regulated by the Mre11p- Rad50p-Xrs2p (MRX) complex for recombination [51]. However, more recently the MRX complex and Exo1p have been implicated in the activation of DSB- and UV-induced Mec1p-dependent checkpoints [52]. The MRX complex and Exo1p were shown to collaborate in producing long ssDNA tails at DSB ends and promote Mec1p association with the DSBs. The long ssDNA tails produced by Exo1p can also revoke fork reversal by resecting newly synthesized chains and resolving the sister chromatid junctions that cause regression of collapsed replication forks in a rad53-deficient background [22]. Consistent with a role in replication fork maintenance, EXO1 has been demonstrated to have a synthetic interaction with SGS1 mutations [20]. The Sgs1p helicase is required for genome stability and is thought to be important for maintenance of stalled forks [20]. Thus, the requirement for Exo1p in the absence of Sgs1p suggests an important role for Exo1p in the maintenance, repair or restart of stalled replication forks. Finally, EXO1 and PSO2 appear to have overlapping roles in the processing of collapsed replication forks due to some forms of endogenous DNA damage and from nitrogen mustard induced interstrand cross-links [21].
Our inability to generate an exo1Δ rad5Δ double mutant seems at odds with a role of EXO1 in the MMS2-sub-pathway of PRR. This can be explained by several studies suggesting a function of yeast RAD5 beyond its activity in PRR. RAD5 deficiency results in higher sensitivity to various types of DNA damage than deletion of either MMS2 or UBC13 [33]. Furthermore, rad5Δ mutants, relative to other PRR mutants, are highly sensitive to ionizing radiation, have elevated rates of spontaneous mitotic recombination, higher rates of gross chromosomal rearrangements, paradoxically increased stability of simple repetitive sequences and higher end-joining activity in a plasmid gap repair [53-56]. Similar to our findings, Chen et. al. recently isolated a separation of function mutant of RAD5 for PRR and MRE11-XRS2-RAD50-dependent double strand break repair [57]. Given the data supporting multiple PRR-independent functions of RAD5, we believe the synthetic lethality between exo1Δ and rad5Δ uncovers a redundancy for an essential PRR-independent function or possibly a strain-dependent phenomenon.
Studies have suggested that Exo1p plays both catalytic and structural roles during MMR-mediated mutation avoidance [16,17,19]. Our results using nuclease-deficient, exo1-D173A, and Mlh1p-binding defective, exo1- FF447AA, exo1 alleles further support both catalytic and structural roles, respectively, for Exo1p in MMR. Interestingly, the “structural-catalytic” double exo1 mutant (exo1-D173A, -FF447AA) behaves like exo1Δ, as determined by synergistic interaction with weak pms1 alleles (see Table 4; compare strain PTY204 to PTY225). Another interesting MMR-related finding is that the Mlh1-binding mutant exo1p-FF447AA (Figure 3) although still capable of interaction with Msh2p, was nevertheless defective in its MMR spellchecker function. These data suggest that a complex interplay between Exo1p and MutLα and MutSα is necessary for optimum MMR activity.
Exo1p is an interesting nuclease that appears to have multiple independent roles in several DNA metabolic processes important for genome duplication and stability (see Figure S3 and Table 5). In this report, we have uncovered an additional function of EXO1 as a component in the MMS2-error free branch of PRR independent of its role in MMR. The findings reported here and those of others strengthen the assertion that EXO1 is truly a “multi-tasking” nuclease [12]. However, similar to the function of EXO1 in MMR [12], the role of EXO1 in PRR will be somewhat difficult to assess because a singular defect in EXO1 is apparently masked by redundant gene functions within the PRR pathway. Further studies of EXO1 in both the MMR and PRR pathways will likely require the use of specific exo1 alleles such as those described here.
Supplementary Material
Supplemental Figure 1 – EXO1 acts in the MMS2 error-free branch of PRR in response to MMS. Overnight saturated cultures were serially diluted (1:5), spotted on YPD plates and YPD plates with increasing concentrations of MMS using a 48-prong replicator and then incubated at 30 °C for 2-4 days. EXO1 is hypostatic to (Figure 1D) MMS2 and (A) recombination genes implicated in PRR, RAD51 and RAD52. (B) EXO1 deletion did not interact with pms1Δ for low dose MMS treatment.
Supplemental Figure 2 – The exo1-FF447AA mutant is functional for PRR. Overnight saturated cultures were serially diluted (1:5), spotted on YPD plates and YPD plates with increasing concentrations of MMS using a 48-prong replicator and then incubated at 30 °C for 2 days. The exo1-FF447AA mutation shows no defect in the PRR pathway as this mutant is as resistant to MMS as wildtype and does not show any genetic interaction with (A) MMS2, MGS1 or RAD30, (B) recombination genes RAD51 and RAD52, or (C) other PRR genes.
Supplemental Figure 3 – Exo1p does not require interaction with Mlh1p to back-up Rad27p. The specified strains were grown to saturation in nonselective media at room temperature, serially diluted (1:5), then spotted onto nonselective YPD plates and media containing 5FAA and incubated at room temperature for 5 days. The 5FAA compound counterselects for TRP prototrophy or pJAS-RAD27 (2μ, TRP1, RAD27) plasmid retention in this case (see section 2.6).
Acknowledgments
We would like to thank members of the Liskay lab for helpful suggestions. PTT is a recipient of a Radiological Society of North America (RSNA) Resident Research Grant (RR0601). This work was supported by the Association pour la Recherche sur le Cancer (ARC-3480 to SB and LG) and the National Institutes of Health (GM45413 to RML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Branzei D, Foiani M. The DNA damage response during DNA replication. Curr Opin Cell Biol. 2005;17:568–575. doi: 10.1016/j.ceb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 3.Kolodner RD, Putnam CD, Myung K. Maintenance of genome stability in Saccharomyces cerevisiae. Science. 2002;297:552–557. doi: 10.1126/science.1075277. [DOI] [PubMed] [Google Scholar]
- 4.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 5.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 6.Barbour L, Xiao W. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat Res. 2003;532:137–155. doi: 10.1016/j.mrfmmm.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence CW. Following the RAD6 pathway. DNA Repair (Amst) 2007 doi: 10.1016/j.dnarep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence C. The RAD6 DNA repair pathway in Saccharomyces cerevisiae: what does it do, and how does it do it? Bioessays. 1994;16:253–258. doi: 10.1002/bies.950160408. [DOI] [PubMed] [Google Scholar]
- 9.Barbour L, Ball LG, Zhang K, Xiao W. DNA Damage Checkpoints Are Involved in Postreplication Repair. Genetics. 2006;174:1789–1800. doi: 10.1534/genetics.106.056283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao W, Chow BL, Broomfield S, Hanna M. The Saccharomyces cerevisiae RAD6 group is composed of an error-prone and two error-free postreplication repair pathways. Genetics. 2000;155:1633–1641. doi: 10.1093/genetics/155.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T, editors. DNA Repair and Mutagenesis. ASM Press; Washington, DC: 2006. [Google Scholar]
- 12.Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 2004;3:1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Liberti SE, Rasmussen LJ. Is hEXO1 a cancer predisposing gene? Mol Cancer Res. 2004;2:427–432. [PubMed] [Google Scholar]
- 14.Szankasi P, Smith GR. A DNA exonuclease induced during meiosis of Schizosaccharomyces pombe. J Biol Chem. 1992;267:3014–3023. [PubMed] [Google Scholar]
- 15.Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T, Kolas NK, Russell R, Hou H, Jr, Kneitz B, Yang G, Kunkel TA, Kolodner RD, Cohen PE, Edelmann W. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17:603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokolsky T, Alani E. EXO1 and MSH6 are high-copy suppressors of conditional mutations in the MSH2 mismatch repair gene of Saccharomyces cerevisiae. Genetics. 2000;155:589–599. doi: 10.1093/genetics/155.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin NS, Nguyen MN, Oh S, Kolodner RD. exo1-Dependent mutator mutations: model system for studying functional interactions in mismatch repair. Mol Cell Biol. 2001;21:5142–5155. doi: 10.1128/MCB.21.15.5142-5155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran PT, Simon JA, Liskay RM. Interactions of Exo1p with components of MutLalpha in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2001;98:9760–9765. doi: 10.1073/pnas.161175998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran PT, Erdeniz N, Dudley S, Liskay RM. Characterization of nuclease-dependent functions of Exo1p in Saccharomyces cerevisiae. DNA Repair (Amst) 2002;1:895–912. doi: 10.1016/s1568-7864(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 20.Ooi SL, Shoemaker DD, Boeke JD. DNA helicase gene interaction network defined using synthetic lethality analyzed by microarray. Nat Genet. 2003;35:277–286. doi: 10.1038/ng1258. [DOI] [PubMed] [Google Scholar]
- 21.Barber LJ, Ward TA, Hartley JA, McHugh PJ. DNA interstrand cross-link repair in the Saccharomyces cerevisiae cell cycle: overlapping roles for PSO2 (SNM1) with MutS factors and EXO1 during S phase. Mol Cell Biol. 2005;25:2297–2309. doi: 10.1128/MCB.25.6.2297-2309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotta-Ramusino C, Fachinetti D, Lucca C, Doksani Y, Lopes M, Sogo J, Foiani M. Exo1 processes stalled replication forks and counteracts fork reversal in checkpoint-defective cells. Mol Cell. 2005;17:153–159. doi: 10.1016/j.molcel.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 23.Gietz RD, Schiestl RH. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast. 1991;7:253–263. doi: 10.1002/yea.320070307. [DOI] [PubMed] [Google Scholar]
- 24.Fritsch TMEF, Sambrook J, editors. Molecular Cloning. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1982. [Google Scholar]
- 25.Welz-Voegele C, Stone JE, Tran PT, Kearney HM, Liskay RM, Petes TD, Jinks-Robertson S. Alleles of the yeast Pms1 mismatch-repair gene that differentially affect recombination- and replication-related processes. Genetics. 2002;162:1131–1145. doi: 10.1093/genetics/162.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myung K, Kolodner RD. Suppression of genome instability by redundant S-phase checkpoint pathways in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2002;99:4500–4507. doi: 10.1073/pnas.062702199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frei C, Gasser SM. The yeast Sgs1p helicase acts upstream of Rad53p in the DNA replication checkpoint and colocalizes with Rad53p in S-phase-specific foci. Genes Dev. 2000;14:81–96. [PMC free article] [PubMed] [Google Scholar]
- 28.Sidorova JM, Breeden LL. Rad53-dependent phosphorylation of Swi6 and down-regulation of CLN1 and CLN2 transcription occur in response to DNA damage in Saccharomyces cerevisiae. Genes Dev. 1997;11:3032–3045. doi: 10.1101/gad.11.22.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulovich AG, Hartwell LH. A checkpoint regulates the rate of progression through S phase in S. cerevisiae in response to DNA damage. Cell. 1995;82:841–847. doi: 10.1016/0092-8674(95)90481-6. [DOI] [PubMed] [Google Scholar]
- 30.Broomfield S, Chow BL, Xiao W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proc Natl Acad Sci U S A. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison A, Christensen RB, Alley J, Beck AK, Bernstine EG, Lemontt JF, Lawrence CW. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989;171:5659–5667. doi: 10.1128/jb.171.10.5659-5667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson JR, Lawrence CW, Hinkle DC. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich HD, Jentsch S. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. Embo J. 2000;19:3388–3397. doi: 10.1093/emboj/19.13.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich HD. The srs2 suppressor of UV sensitivity acts specifically on the RAD5- and MMS2-dependent branch of the RAD6 pathway. Nucleic Acids Res. 2001;29:3487–3494. doi: 10.1093/nar/29.17.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broomfield S, Xiao W. Suppression of genetic defects within the RAD6 pathway by srs2 is specific for error-free post-replication repair but not for damage-induced mutagenesis. Nucleic Acids Res. 2002;30:732–739. doi: 10.1093/nar/30.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Lawrence CW. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc Natl Acad Sci U S A. 2005;102:15954–15959. doi: 10.1073/pnas.0504586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hishida T, Ohno T, Iwasaki H, Shinagawa H. Saccharomyces cerevisiae MGS1 is essential in strains deficient in the RAD6-dependent DNA damage tolerance pathway. Embo J. 2002;21:2019–2029. doi: 10.1093/emboj/21.8.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawabe Y, Branzei D, Hayashi T, Suzuki H, Masuko T, Onoda F, Heo SJ, Ikeda H, Shimamoto A, Furuichi Y, Seki M, Enomoto T. A novel protein interacts with the Werner's syndrome gene product physically and functionally. J Biol Chem. 2001;276:20364–20369. doi: 10.1074/jbc.C100035200. [DOI] [PubMed] [Google Scholar]
- 39.Johnson RE, Prakash S, Prakash L. Requirement of DNA polymerase activity of yeast Rad30 protein for its biological function. J Biol Chem. 1999;274:15975–15977. doi: 10.1074/jbc.274.23.15975. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 41.McDonald JP, Levine AS, Woodgate R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics. 1997;147:1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmutte C, Sadoff MM, Shim KS, Acharya S, Fishel R. The interaction of DNA mismatch repair proteins with human exonuclease I. J Biol Chem. 2001;276:33011–33018. doi: 10.1074/jbc.M102670200. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen FC, Jager AC, Lutzen A, Bundgaard JR, Rasmussen LJ. Characterization of human exonuclease 1 in complex with mismatch repair proteins, subcellular localization and association with PCNA. Oncogene. 2004;23:1457–1468. doi: 10.1038/sj.onc.1207265. [DOI] [PubMed] [Google Scholar]
- 44.Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF, Kolodner RD. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci U S A. 1997;94:7487–7492. doi: 10.1073/pnas.94.14.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee BI, Wilson DM., 3rd The RAD2 domain of human exonuclease 1 exhibits 5′ to 3′ exonuclease and flap structure-specific endonuclease activities. J Biol Chem. 1999;274:37763–37769. doi: 10.1074/jbc.274.53.37763. [DOI] [PubMed] [Google Scholar]
- 46.Chen C, Merrill BJ, Lau PJ, Holm C, Kolodner RD. Saccharomyces cerevisiae pol30 (proliferating cell nuclear antigen) mutations impair replication fidelity and mismatch repair. Mol Cell Biol. 1999;19:7801–7815. doi: 10.1128/mcb.19.11.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gellon L, Werner M, Boiteux S. Ntg2p, a Saccharomyces cerevisiae DNA N-glycosylase/apurinic or apyrimidinic lyase involved in base excision repair of oxidative DNA damage, interacts with the DNA mismatch repair protein Mlh1p. Identification of a Mlh1p binding motif. J Biol Chem. 2002;277:29963–29972. doi: 10.1074/jbc.M202963200. [DOI] [PubMed] [Google Scholar]
- 48.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 49.Ulrich HD. The RAD6 pathway: control of DNA damage bypass and mutagenesis by ubiquitin and SUMO. Chembiochem. 2005;6:1735–1743. doi: 10.1002/cbic.200500139. [DOI] [PubMed] [Google Scholar]
- 50.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 51.Moreau S, Morgan EA, Symington LS. Overlapping functions of the Saccharomyces cerevisiae Mre11, Exo1 and Rad27 nucleases in DNA metabolism. Genetics. 2001;159:1423–1433. doi: 10.1093/genetics/159.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakada D, Hirano Y, Sugimoto K. Requirement of the Mre11 complex and exonuclease 1 for activation of the Mec1 signaling pathway. Mol Cell Biol. 2004;24:10016–10025. doi: 10.1128/MCB.24.22.10016-10025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson RE, Henderson ST, Petes TD, Prakash S, Bankmann M, Prakash L. Saccharomyces cerevisiae RAD5-encoded DNA repair protein contains DNA helicase and zinc-binding sequence motifs and affects the stability of simple repetitive sequences in the genome. Mol Cell Biol. 1992;12:3807–3818. doi: 10.1128/mcb.12.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liefshitz B, Steinlauf R, Friedl A, Eckardt-Schupp F, Kupiec M. Genetic interactions between mutants of the ‘error-prone’ repair group of Saccharomyces cerevisiae and their effect on recombination and mutagenesis. Mutat Res. 1998;407:135–145. doi: 10.1016/s0921-8777(97)00070-0. [DOI] [PubMed] [Google Scholar]
- 55.Friedl AA, Liefshitz B, Steinlauf R, Kupiec M. Deletion of the SRS2 gene suppresses elevated recombination and DNA damage sensitivity in rad5 and rad18 mutants of Saccharomyces cerevisiae. Mutat Res. 2001;486:137–146. doi: 10.1016/s0921-8777(01)00086-6. [DOI] [PubMed] [Google Scholar]
- 56.Smith S, Hwang JY, Banerjee S, Majeed A, Gupta A, Myung K. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2004;101:9039–9044. doi: 10.1073/pnas.0403093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen S, Davies AA, Sagan D, Ulrich HD. The RING finger ATPase Rad5p of Saccharomyces cerevisiae contributes to DNA double-strand break repair in a ubiquitin-independent manner. Nucleic Acids Res. 2005;33:5878–5886. doi: 10.1093/nar/gki902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – EXO1 acts in the MMS2 error-free branch of PRR in response to MMS. Overnight saturated cultures were serially diluted (1:5), spotted on YPD plates and YPD plates with increasing concentrations of MMS using a 48-prong replicator and then incubated at 30 °C for 2-4 days. EXO1 is hypostatic to (Figure 1D) MMS2 and (A) recombination genes implicated in PRR, RAD51 and RAD52. (B) EXO1 deletion did not interact with pms1Δ for low dose MMS treatment.
Supplemental Figure 2 – The exo1-FF447AA mutant is functional for PRR. Overnight saturated cultures were serially diluted (1:5), spotted on YPD plates and YPD plates with increasing concentrations of MMS using a 48-prong replicator and then incubated at 30 °C for 2 days. The exo1-FF447AA mutation shows no defect in the PRR pathway as this mutant is as resistant to MMS as wildtype and does not show any genetic interaction with (A) MMS2, MGS1 or RAD30, (B) recombination genes RAD51 and RAD52, or (C) other PRR genes.
Supplemental Figure 3 – Exo1p does not require interaction with Mlh1p to back-up Rad27p. The specified strains were grown to saturation in nonselective media at room temperature, serially diluted (1:5), then spotted onto nonselective YPD plates and media containing 5FAA and incubated at room temperature for 5 days. The 5FAA compound counterselects for TRP prototrophy or pJAS-RAD27 (2μ, TRP1, RAD27) plasmid retention in this case (see section 2.6).