Abstract
The authors used the meta-analytic approach to examine the effects of aerobic exercise on lipids and lipoproteins in adults 50 years of age and older. Twenty-eight outcomes representing 1427 subjects (806 exercise, 621 control) were available for pooling. Random-effects modeling yielded statistically significant improvements of 1.1%, 5.6%, 2.5%, and 7.1%, respectively, for total cholesterol (mean ± SEM in mg/dL, −3.3±1.7; 95% confidence interval [CI], −6.5 to −0.02; p=0.05), high-density lipoprotein cholesterol (2.5±1.0; 95% CI, 0.7–4.4; p=0.01), low-density lipoprotein cholesterol (−3.9±1.9; 95% CI, −7.7 to −0.08; p=0.05), ratio of total cholesterol to high-density lipoprotein cholesterol (−0.8±0.2; 95% CI, −1.2 to −0.4; p<0.001), but not triglycerides (−7.0±3.6; 95% CI, −14.0 to 0.1; p=0.06). After conducting sensitivity analyses, only the improvements in high-density lipoprotein cholesterol and the ratio of total cholesterol to high-density lipoprotein cholesterol remained statistically significant (p<0.05 for both). It was concluded that aerobic exercise increases high-density lipoprotein cholesterol and decreases the ratio of total cholesterol to high-density lipoprotein cholesterol in older adults.
Less than optimal lipid and lipoprotein levels, a major risk factor for coronary heart disease (CHD), increase with advancing age.1 One potential approach for obtaining and/or maintaining optimal lipid and lipoprotein levels in older adults is aerobic exercise, a low-cost, nonpharmacologic intervention that is available and feasible for most individuals. However, randomized controlled trials dealing with the effects of aerobic exercise on lipids and lipoproteins in adults 50 years of age and older have led to conflicting results.2–23 While previous systematic reviews dealing with the effects of aerobic exercise in adults have been conducted,24–30 none have specifically focused on older adults. Therefore, given: 1) the prevalence of less than optimal lipid and lipoprotein levels in older adults; 2) the conflicting results of previous studies dealing with the effects of aerobic exercise on lipids and lipoproteins in older adults; and 3) the absence of any meta-analytic work that has specifically focused on the effects of aerobic exercise on lipids and lipoproteins in older adults, the purpose of this study was to use the meta-analytic approach to examine the effects of aerobic exercise on lipids and lipoproteins in adults aged 50 years and older and to provide suggestions for future research on this topic.
Methods
Data Sources
Studies were retrieved via: 1) computerized literature searches (MEDLINE, EMBASE, SportDiscus, Current Contents, Dissertation Abstracts International); 2) cross-referencing from retrieved articles; 3) hand searching select journals; and 4) expert review of our reference list (W. Haskell, personal communication, 2003). Search terms used included cholesterol, exercise, physical activity, aerobic, fitness, lipids, lipoproteins, adults, humans, and cardiovascular disease.
Study Selection
The inclusion criteria for this study were as follows: 1) randomized controlled trials; 2) nonexercise control group; 3) prescribed aerobic exercise for 8 weeks or more; 4) adult humans 50 years of age or older; 5) studies published in journal, dissertation, or master's thesis format; 6) studies published in the English language; 7) studies published between January 1, 1955 and January 1, 2003; and 8) assessment of one or more of the following lipid and/or lipoprotein variables in the apparently fasting state: total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and the ratio of TC to HDL-C (TC/HDL-C). The inclusion of foreign-language articles was beyond the scope of this project. We limited our primary outcomes to TC, HDL-C, LDL-C, TG, and TC/HDL-C because these are the variables that are most commonly assessed in the clinical setting. All included studies were prospective.
Data Abstraction
All studies were coded by the first two authors, independent of each other. The major categories of variables that were coded included: 1) study characteristics; 2) subject characteristics; 3) lipid assessment characteristics; 4) training program characteristics; 5) primary outcomes; and 6) secondary outcomes (body weight, body mass index [BMI] in kg/m2, percent body fat, and maximum oxygen consumption [Vo2max] in mL/kg/min).
Statistical Analyses
Primary outcomes were calculated as the difference (exercise–control) of the changes (initial–final) in the mean values from each study. Pooled outcomes were calculated by assigning weights equal to the inverse of the variance for net changes in all lipid and lipoprotein outcomes. Confidence intervals (CIs) (95%) were used to establish the statistical significance of our estimates. If the CI did not cross zero (0.00), our results were considered to be statistically significant. A random-effects model was used for all analyses.31 Secondary outcomes were analyzed using the same approach as for primary outcomes.
To examine the effects of each study on the overall results, analyses were conducted with each study deleted from the model once. Heterogeneity was examined using the Q statistic as well as an extension of the Q statistic (I2).32 Publication bias, i.e., the selective publication of research that is dependent on the nature and direction of results, was assessed using a rank-based, data-augmentation, trim-and-fill procedure.33
Study quality was assessed using a previously validated and reliable quality index.34 The number of points ranged from 0–5, with higher numbers representing greater study quality.
Subgroup analyses for changes in lipids and lipoproteins were accomplished using meta-analytic analysis of variance models35 partitioned according to study source, country, gender, health status, and whether the exercise program was supervised or unsupervised. We did not conduct any type of subgroup comparisons for cigarette smoking, alcohol consumption, or medications that could affect lipid and/or lipoprotein metabolism because none of the studies appeared to limit their inclusion of subjects to those who smoked cigarettes, consumed alcohol, or were taking antihyperlipidemic medications. We also did not conduct any type of subgroup comparisons for diet, because only one study reported changes in diet that might have affected lipids and lipoproteins.23 In addition, we did not conduct any type of subgroup analysis between studies in which subjects were physically active vs. inactive because only three studies reported that some, but not all, of the subjects were physically active before taking part in the study.5,6,16 Metaregression, which is analogous to simple regression for original data,35 was performed for changes in lipids and lipoproteins and their relationship to study quality; year of publication; percent dropout; initial lipid and lipoprotein levels; age; height; initial body weight, changes in body weight; BMI; percent body fat; Vo2max; number of hours fasted; number of hours that exercise was avoided before lipid assessment; length, frequency, intensity, and duration of training; total minutes of training; and compliance. We did not conduct any type of multiple regression analysis because of missing data for different variables and our desire to include the maximum amount of data possible for each analysis. CIs (95%) were used to establish the statistical significance of our estimates. If the CI did not cross zero (0.00), our results were considered to be statistically significant.
Descriptive statistics are reported as mean ± SD, while primary and secondary outcomes are reported as mean ± SEM. The two-tailed α level for statistical significance was set at p≤0.05. While our primary measure of statistical significance for primary, secondary, and metaregression analyses was CI, we also included α values to enhance understanding. The term “sensitivity analysis” is used throughout the manuscript to denote analyses for publication bias as well as analyses conducted with each study deleted from the model once.
Results
Study Characteristics
Twenty-eight outcomes from 22 studies representing 1427 subjects (806 exercise, 621 control) were available for pooling.2–23 Loss to follow-up ranged from approximately 5.0%–45.0% in the exercise groups (mean ± SD, 18.3%±12.7%) and 0.0%–64.0% in the control groups (mean ± SD, 14.9%±16.7%). Study quality ranged from 1–4 (median = 2). A general description of each study is given in Table I.
Table I.
Study Characteristics
| Study | Subjects | Aerobic Exercise Program | Lipids Assessed | Assessment Methods |
|---|---|---|---|---|
| Baker and Al-Najadah2 | 30 Women assigned to either an exercise (n=20) or control (n=10) group | 12 wk of aerobic exercise 2 d/wk, 17 min/session, 65%–80% of MHR | TC, HDL-C, LDL-C, TG | Morning after a 12-h overnight fast |
| Baker et al.3 | 34 Sedentary men, 20 exercise (57.5±5.81 yr of age), 14 controls (59.3±5.61 yr of age) | 20 wk of walking/running, 3 d/w, 48 min/session, 65%–85% of MHRR | TC, HDL-C, LDL-C, TG | Morning after a 14-h overnight fast and 51 h after the last exercise session |
| Blumenthal et al.4 | 63 Sedentary men and women >60 yr of age assigned to either an exercise (n=31) or control (n=32) group | 16 wk of walking, jogging, cycling, and arm ergometry, 3 d/wk, 45 min/session, 60%–70% of MHRR | TC, HDL-C, LDL-C, TG | Morning after a 14-h overnight fast |
| Cauley et al.5 | 204 Women assigned to either an exercise (n=100; 57.95±4.01 yr of age) or control (n=104; 57.39±4.17 yr of age) group | 104 wk of walking, 3 d/wk | TC, HDL-C, TG | Morning after fasting for > 10 h |
| Cunningham et al.6 | 202 Men, 55–65 yr of age, assigned to either an exercise (n=101) or control (n=101) group | 52 wk of walking or jogging, 2.5 d/wk, 30 min/session, at a heart rate of 131 bpm | TC, HDL-C | Morning after an overnight fast of 12–16 h and avoiding exercise |
| Eriksson et al.7 | 14 Sedentary men and women (mean age, 60±18.7 yr) assigned to either an exercise (n=7) or control (n=7) group | 24 wk of walking, jogging, cycling, and swimming, 3 d/wk, 30–60 min/session, 60% of MHR | TC, HDL-C, TG | Morning after a 12-h overnight fast and avoiding exercise for 5–7 d |
| Fahlman et al.8 | 30 Sedentary women, assigned to an aerobic exercise (n=15; 76±5 yr of age) or control (n=15; 74±5 yr of age) group | 10 wk of walking, 3 d/wk, 20–50 min/session, 70% of MHRR | TC, HDL-C, LDL-C, TG, TC/HDL-C | Morning after a 12–14-h overnight fast and avoiding exercise for 48–96 h |
| Ferrier et al.9 | 10 Sedentary men (n=5) and women (n=5), mean age, 64±7 yr, crossed over to an exercise and control condition | 8 wk of cycling, 3 d/wk, 30 min/session, 65% of MHRR | HDL-C, LDL-C, TG | After avoiding exercise for 48 h |
| Hersey et al.10 | 25 Sedentary men and women ages 70–79 assigned to an aerobic exercise (n=16) or control (n=9) group | 24 wk of walking/jogging, 3 d/wk, 20–45 min/session, 50%–85% of MHRR | TC, TG | Morning after a 12-h overnight fast and avoiding exercise for 16–24 h |
| King et al.11 | 300 Sedentary men and women, 50–65 years of age, assigned to high-intensity group-based exercise (n=74), high-intensity home-based exercise (n=77), lower-intensity home-based exercise (n=74), or control (n=75) group | 52 wk of walking/jogging, cycling, 3 d/wk, 40 min/session, 73%–88% of MHR (higher intensity exercise groups) or 5 d/wk, 30 min/session, 60%–73% of MHR (lower intensity exercise groups) | HDL-C, LDL-C, TG | Morning after an overnight fast of ≥ 12 h |
| Ligtenberg et al.12 | 51 Men and women assigned to either an exercise (n=25) or control (n=26) group | 26 wk of walking/running, cycling, swimming, rowing, 3 d/wk, 50 min/session, 60%–80% of Vo2max | TC, HDL-C, LDL-C, TG | Morning after a 12-h overnight fast and within 72 h of the last exercise session |
| Motoyama et al.13 | 30 Men and women, 15 exercise (75.5±5.6 yr of age), and 15 controls (73.7±4.4 yr of age) | 36 wk of treadmill exercise, 5 d/wk, 30 min/session, 50% of Vo2max | TC, HDL-C, LDL-C, TG, TC/HDL-C | Morning after a 12-h overnight fast |
| Nieman et al.14 | 30 Sedentary women, 67–85 yr of age, assigned to an exercise (n=l4) or sham (n=16) group | 12 wk of walking, 5 d/wk, 30–40 min/session, 60% of MHRR | TC, HDL-C, LDL-C, TG, TC/HDL-C | NA |
| Rauramaa et al.15 | 125 Men, 50–60 yr of age, assigned to either an exercise (n=62) or control (n=63) group | 156 wk of walking, jogging, swimming, cycling, and cross-country skiing, 3–5 d/wk, 30–60 min/session, 40%–60% of Vo2max | TC | Morning after a 12-h overnight fast and avoiding exercise for 24 h |
| Raz et al.16 | 38 Men and women, assigned to either an exercise (n=19; 56.7±6.2 yr of age) or control (n=19; 56.5±6.7 yr of age) group | 12 wk of walking, jogging, swimming, cycling, 3 d/wk, 45–50 min/session, 65%–70% of MHRR | TC, HDL-C, TG | Morning after a 12-h overnight fast |
| Ready et al.17 | 25 Postmenopausal women, 15 exercise and 10 control, 62±5.7 yr of age | 24 wk of walking, 5 d/wk, 54 min/session, 54% of MHRR | TC, HDL-C, LDL-C, TG, TC/HDL-C | Morning after a 12-h overnight fast and 12 h after the last exercise session |
| Ready et al.18 | 53 Sedentary women (mean age, 61.3±5.8 yr) assigned to either 3 d of exercise (n=18), 5 d of exercise (n=17), or control (n=18) group | 24 wk of walking, 3 d/wk, 59 min/session, 60% of Vo2max, or 5 d/wk, 56 min/session, 60% of Vo2max | TC, HDL-C, LDL-C, TG | Morning after a 12-h overnight fast and avoiding exercise |
| Ruoti19 | 20 Men and women, 50–68 yr of age, assigned to either an exercise (n=12; 65.16±5.29 yr of age) or control (n=8; 56.00±6.78 yr of age) group | 12 wk of aqua dynamic exercise, 3 d/wk, 60 min/session, 70%–80% of MHR | TC, HDL-C, LDL-C, TG, TC/HDL-C | Morning after an overnight fast |
| Seals et al.20 | 35 Sedentary women assigned to an exercise (n=18; 62±9 yr of age) or no exercise and sodium restriction (n=17; 65±10 yr of age) group | 13 wk of walking, 5.8 d/wk, 40 min/session, 70% of MHR | TC, HDL-C, LDL-C, TG | Morning after an overnight fast |
| Sunami et al.21 | 40 Men and women, 60–77 yr of age (mean age, 67±4 yr), assigned to an exercise (n=20) or control (n=20) group | 20 wk of cycling, 2–4 d/wk, 50% of Vo2max | TC, HDL-C, LDL-C, TG, TC/HDL-C | Morning after a 12-h overnight fast |
| Verissimo et al.22 | 63 Men and women, ages 65–94, assigned to an exercise (n=31; 77.9±7.4 yr of age) or control (n=32; 77.8±7.5 yr of age) group | 32 wk of walking/jogging, cycling, rowing, arm cranking, 3 d/wk, 50 min/session, 60%–80% of MHRR | TC, HDL-C, LDL-C, TG, TC/HDL-C | Morning after a 12-h overnight fast and avoiding exercise for 36 h |
| Verity and Ismail23 | 10 Sedentary women, 50–70 yr of age, assigned to either an exercise (n=5; 61.20±9.17 yr of age) or control (n=5; 57.20±8.27 yr of age) group | 16 weeks of walking, 3 d/wk, 60–90 min/session, 65%–80% of MHRR | TC, HDL-C, TC/HDL-C | Morning after a 10–12-h overnight fast |
Description of studies limited to those subjects and variables that met our inclusion criteria. Number of subjects limited to those in which pre- and post-assessment of lipids took place. Data reported as mean ± SD. Lipid variables listed limited to those that met our inclusion criteria, including availability. MHR=maximum heart rate; TC=total cholesterol; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; TG=triglycerides; MHRR=maximum heart rate reserve; Vo2max=maximum oxygen consumption; NA=not available
Subject, Lipid Assessment, and Training Program Characteristics
Baseline characteristics of the subjects are shown in Table II. Of the 1427 subjects included in the study, 690 were men and 737 were women. One study reported that some of the subjects changed their diet,23 while the remaining studies that provided information reported that no changes in diet that could affect lipids and lipoproteins occurred.3,5,7,8,11,12,14,16–18,20,21 Thirteen studies reported that none of the subjects were taking any type of medication(s) that could affect lipids and lipoproteins,2,3,5,10,11,13,14,17,18,20–23 one reported that none of the subjects were taking any type of medication(s) other than hormone replacement therapy that could affect lipids and lipoproteins,8 while another reported that some subjects were taking medications that could affect lipids and lipoproteins.12 Subjects fasted from 10–14 hours (mean ± SD, 12.2±1.0 h) and refrained from exercise for 12–144 hours (mean ± SD, 45.9±39.5 h) before lipid assessment. Characteristics of the aerobic exercise programs are shown in Table III. For those studies that provided such information, 11 studies had subjects perform supervised exercise,2–4,10,12–14,19,21–23 two involved unsupervised exercise,15,20 and six others involved a combination of both supervised and unsupervised exercise.5–7,16–18 One other study included subjects assigned to either supervised or unsupervised exercise.11
Table II.
Baseline Characteristics of Subjects
| Variable | n | Exercise Mean ± SD | Range* | n | Control Mean ± SD | Range* |
|---|---|---|---|---|---|---|
| Age (yr) | 28 | 62.9±6.6 | 55.0–77.9 | 23 | 63.2±6.9 | 55.0–77.8 |
| Height (cm) | 9 | 160.0±3.2 | 154.0–165.0 | 8 | 160.0±3.3 | 154.0–164.0 |
| Body weight (kg) | 16 | 69.8±7.9 | 53.3–82.4 | 15 | 70.5±9.1 | 55.1–83.8 |
| BMI (kg/m2) | 24 | 27.0±2.0 | 22.0–31.8 | 19 | 27.4±2.8 | 22.7–33.4 |
| Body fat (%) | 10 | 33.5±6.6 | 20.7–41.8 | 9 | 33.7±7.0 | 21.4–42.1 |
| Vo2max (mL/kg/min) | 22 | 24.4±4.8 | 17.3–31.5 | 17 | 23.8±5.0 | 17.8–31.3 |
| TC (mg/dL) | 21 | 223.8±19.7 | 178.9–257.9 | 20 | 223.5±18.1 | 184.5–264 |
| HDL-C (mg/dL) | 26 | 53.6±6.9 | 36.0–70.0 | 21 | 51.3±8.6 | 33.6–63.0 |
| LDL-C (mg/dL) | 21 | 148.8±21.5 | 104.4–196.8 | 16 | 145.9±18.4 | 116.0–187.5 |
| TG (mg/dL) | 25 | 131.0±30.5 | 84.8–210.5 | 20 | 138.2±32.2 | 87.7–219.3 |
| TC/HDL-C | 8 | 4.6±0.6 | 4.2–5.8 | 8 | 4.8±0.6 | 4.2–5.9 |
n=number of groups reporting data; BMI=body mass index; Vo2rnax = maximum oxygen consumption; TC=total cholesterol; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; TG=triglycerides; TC/HDL-C=ratio of total cholesterol to HDL-C;
range represents the mean for each group from each study
Table III.
Training Program Characteristics
| Variable | n | Mean ± SD | Range |
|---|---|---|---|
| Length (wk) | 28 | 35.3±31.8 | 8–156 |
| Frequency (times/wk) | 28 | 3.5±1.0 | 2–6 |
| Intensity (%Vo2max) | 27 | 67.8±9.8 | 50–82 |
| Duration (min/session) | 27 | 42.4±12.1 | 17–75 |
| Compliance* (%) | 16 | 81.8±16.8 | 5–100 |
n=number of groups reporting data; Vo2max=maximum oxygen consumption;
percentage of exercise sessions attended
Primary Outcomes
As can be seen in Table IV, statistically significant improvements were found for TC (p=0.05), HDL-C (p=0.01), LDL-C (p=0.05), and TC/HDL-C (p<0.001) but not for TG (p=0.06). While no publication bias was observed for TC, HDL-C, or TC/HDL-C, adjustment for publication bias for LDL-C resulted in nonsignificant decreases of −1.3±1.8 mg/dL (95% CI, −4.7 to 2.1; p=0.47). With each study deleted from the model once, changes in TC ranged from a statistically significant value of −3.84±1.64 mg/dL (95% CI, −7.0 to −0.6 mg/dL; p=0.02) to a nonsignificant value of −2.43±1.5 (95% CI, −5.3 to 0.4 mg/dL; p=0.10) while increases in HDL-C remained statistically significant throughout the range of both high (3.3±1.2 mg/dL; 95% CI, 0.9–5.7 mg/dL; p=0.01) and low (2.1±0.8; 95% CI, 0.4–3.8 mg/dL; p=0.01) values (Figure 1). Similar to TC, changes in LDL-C with each study deleted from the model ranged from a statistically significant reduction of −5.8±2.5 mg/dL (95% CI, −10.7 to −0.8 mg/dL; p=0.02) to a nonsignificant reduction of −2.9±1.8 (95% CI, −6.4 to 0.6 mg/dL; p=0.10). While only eight outcomes were included because of missing variance data for TC/HDL-C, reductions remained statistically significant with each study deleted from the model once (high, −1.0±0.2; 95% CI, −1.4 to −0.6; p<0.001; low, −0.8.±0.3; 95% CI, −1.4 to −0.3; p=0.001) (Figure 2).
Table IV.
Primary and Secondary Outcomes
| Variable | n | Mean ± SEM | 95% CI | Change (%) |
|---|---|---|---|---|
| Primary outcomes | ||||
| TC (mg/dL) | 21 | −3.3±1.7 | −6.5 to −0.02* | −1.1 |
| HDL-C (mg/dL) | 26 | 2.5±1.0 | 0.7 to 4.4* | 5.6 |
| LDL-C (mg/dL) | 21 | −3.9±1.9 | −7.7 to −0.08* | −2.5 |
| TG (mg/dL) | 25 | −7.0±3.6 | −14.0 to 0.1 | −5.9 |
| TC/HDL-C | 8 | −0.8±0.2 | −1.2 to −0.4* | −7.1 |
| Secondary outcomes | ||||
| Body weight (kg) | 14 | −0.7±0.4 | −1.5 to 0.05 | −1.1 |
| BMI (kg/m2) | 16 | −0.2±0.1 | −0.3 to −0.1* | −1.3 |
| Body fat (%) | 10 | −1.0±0.4 | −1.9 to −0.3* | −3.4 |
| Vo2max (mL/kg/min) | 20 | 2.8±0.2 | 2.1 to 3.6* | 13.0 |
n=number of groups reporting data in which a treatment effect could be calculated; CI=confidence interval; TC=total cholesterol; HDL-C=high-density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; TG=triglycerides; TC/HDL-C=ratio of TC to HDL-C; BMI=body mass index; Vo2max=maximum oxygen consumption;
significantly different from zero
Figure 1.
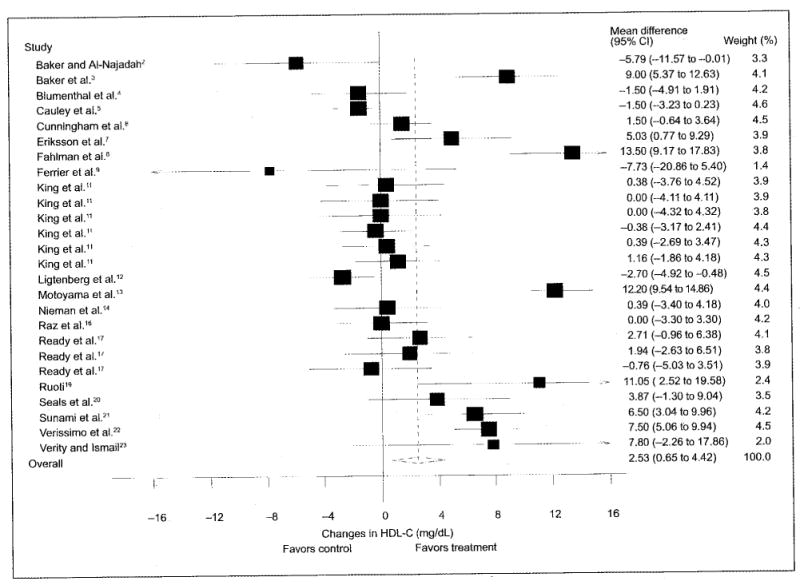
Changes (n=26) in high-density lipoprotein cholesterol (HDL-C) and 95% confidence intervals (CIs) for each outcome, as well as the overall weighted mean difference and 95% CI. The size of the black boxes for each outcome represents the weight given to that outcome. The overall mean difference is shown by the middle of the diamond, while the left and right extremes of the diamond represent the corresponding 95% CI. The vertical dashed line represents the overall mean.
Figure 2.
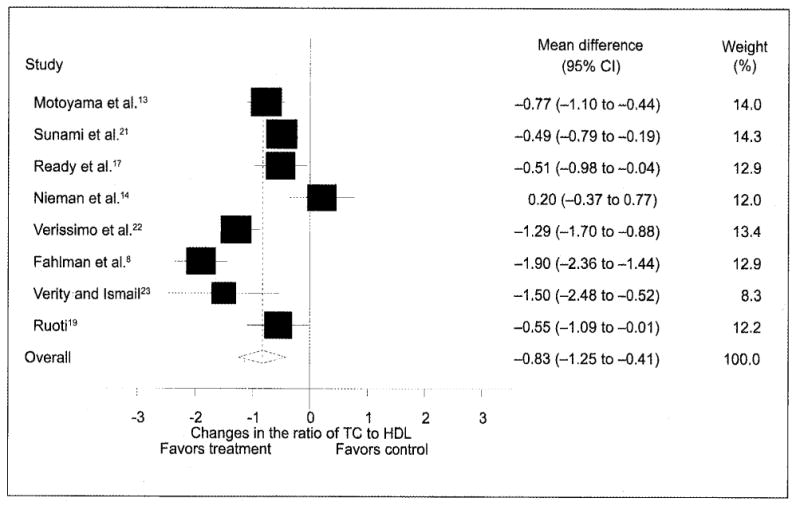
Changes (n=8) in the ratio of total cholesterol (TC) to high-density lipoprotein (HDL) cholesterol and 95% confidence intervals (CIs) for each outcome, as well as the overall weighted mean difference and 95% CI. The size of the black boxes for each outcome represents the weight given to that outcome. The overall mean difference is shown by the middle of the diamond, while the left and right extremes of the diamond represent the corresponding 95% CI. The vertical dashed line represents the overall mean.
Subgroup and Regression Analyses
Decreases in TC were associated with greater reductions in body weight (r=0.66; 95% CI, 0.22–0.88; p=0.001) and BMI (r=0.60; 95% CI, 0.11–0.86; p=0.01) while increases in HDL-C were associated with greater increases in Vo2max (r=0.71; 95% CI, 0.39–0.87; p<0.001) and older age (r=0.58; 95% CI, 0.25–0.79; p<0.001). Post hoc simple regression analysis resulted in a statistically significant relationship between increases in Vo2max and increasing age (r=0.45; 95% CI, 0.01–0.73; p=0.02). Decreases in LDL-C were associated with decreases in body weight (r=0.74; 95% CI, 0.25–0.93; p=0.001), decreases in BMI (r=0.66; 95% CI, 0.26–0.87; p<0.001), and increases in Vo2max (r=0.66; 95% CI, 0.28–0.86; p<0.001) while decreases in TG were associated with decreases in BMI (r=0.75; 95% CI, 0.45–0.89; p<0.001) and increases in Vo2max (r=0.54; 95% CI, 0.11–0.80; p=0.006). In addition, decreases in TC/HDL-C were significantly correlated with increases in Vo2max (r=0.91; 95% CI, 0.33–0.98; p<0.001). No other statistically significant (p>0.05 for all) or clinically relevant differences or relationships were observed for lipids and lipoproteins including, but not limited to, percent dropout, gender, baseline levels, and all aspects of the training programs (length, frequency, intensity, duration, total minutes, and type of supervision).
Secondary Outcomes
There were small, but statistically significant, exercise–control decreases in BMI (p<0.001) and percent body fat (p=0.004), but not body weight (p=0.07) (Table III). In addition, there was a large and statistically significant increase in Vo2max for the exercise groups (p<0.001).
Discussion
While changes in lipids and lipoproteins were all in the direction suggestive of benefit, only HDL-C and TC/HDL-C continued to be statistically significant after conducting sensitivity analyses. Inspection of Figure 1 might lead one to question this since only 7 of 26 outcomes (26.9%) favored treatment. However, this would be relying on a “vote-counting” approach that has been shown to be less valid than the more quantitative meta-analytic approach.36 One of the very reasons for conducting a meta-analysis is to increase the power and precision of primary end points to reach general conclusions about a body of research.37
The increases in HDL-C observed in this study occurred despite the fact that overall baseline levels were well within the normal range.1 While increases in HDL-C were independent of changes in body composition, there was a statistically significant association between increases in HDL-C with both increasing age as well as increases in Vo2max. Since we conducted post hoc analysis and also found a statistically significant and positive association between increasing age and increases in Vo2max, it may be that these associations are the result of older people being sedentary for a longer number of years and, thus, having more to gain from an aerobic exercise program in terms of increasing both HDL-C and Vo2max.
The decreases in TC/HDL-C observed in this study also occurred despite the fact that overall baseline levels were well within the normal range.1 Similar to HDL-C, improvements in TC/HDL-C were independent of changes in body composition and significantly associated with increases in Vo2max. This association is most likely due to the changes observed for HDL-C.
While our overall findings resulted in statistically significant reductions in TC and LDL-C as well as a trend for reduction in TG, the results for TC and LDL-C were no longer significant when sensitivity analyses were performed. Thus, while we do not want to rule out the potential for improvements in TC, LDL-C, and TG as a result of aerobic exercise in older adults, we are not comfortable with reaching any definitive conclusions regarding the benefits of aerobic exercise on these variables. This may be especially true for TG, given the fact that overall baseline levels were well below the normal cutpoint of <150 mg/dL.1 The fact that decreases in BMI were associated with decreases in TC, LDL-C, and TG, while decreases in body weight were associated with decreases in TC and LDL, raises the possibility that improvements in these lipid variables may not be the direct result of the aerobic exercise program itself, but rather a consequence of the decreases in BMI and body weight that occur with an aerobic exercise program. The statistically significant association between increases in Vo2max and decreases in LDL-C and TG is similar to that found for HDL-C. This suggests that greater decreases in LDL-C and TG may occur with greater increases in Vo2max.
While the primary target of therapy is currently focused on lowering LDL-C in those subjects with elevated levels, raising HDL-C is also clinically important.1 For example, since as little as a 1% decrease in HDL-C has been associated with a 2%–3% increase in CHD risk,1 increases in HDL-C of as little as 1% should decrease the risk for CHD. Since we found an approximate 6% vs. 1% increase in HDL-C, greater reductions in CHD risk might be realized in older adults by participating in a regular program of aerobic exercise.
Based on our findings, it would appear plausible to suggest that participation in an aerobic exercise program would be appropriate for increasing HDL-C and decreasing the ratio of TC/HDL-C. This is especially true given the low-cost, minimal side effects, and numerous other benefits that can be derived from participating in an aerobic exercise program.38 While we found no relationship between training program characteristics and changes in HDL-C as well as TC/HDL-C, it is important to realize that the average length of training in our studies was approximately 35 weeks, and the vast majority of trials included in our study adhered to the general guidelines for aerobic exercise as recommended by the American College of Sports Medicine.39 Therefore, it may be appropriate to suggest that training for approximately 35 weeks and adhering to these general guidelines should improve HDL-C and subsequent TC/HDL-C levels to an extent similar to those observed in our study. Briefly, this includes aerobic exercise (e.g., walking, bicycling, swimming) performed for 20–60 minutes, 3–5 days per week, at an intensity ranging from 55% to 90% of maximum heart rate. However, because of adherence problems and other potential hazards of higher intensity training (e.g., musculoskeletal injury), lower intensity sessions of at least 30 minutes or longer are probably more appropriate, especially for older adults. For those who are quite unfit, intensity levels of 55%–64% of maximum heart rate are recommended. In order for these changes to be maintained, it is probably important to continue training so as not to lose these benefits.39
While not the primary focus of this meta-analysis, we found statistically significant decreases in BMI and percent body fat; however, these decreases are probably not important from a clinical perspective. In contrast, the approximate 13.0% increase in Vo2max is clinically important, given that the relative risk of mortality from any cause in individuals with TC values greater than 220 mg/dL is lower in individuals with higher exercise capacities.40 Thus, older adults may reduce their risk of all-cause mortality irrespective of reductions in TC.
One of the reasons for conducting a meta-analysis is to identify areas of weakness and provide direction for future research. With the former in mind, we offer the following general observations. First, since only two studies appeared to use the intention-to-treat approach in the analysis of their data,4,6 we would suggest that future researchers report, and editors publish, results using both an analysis-by-protocol and intention-to-treat approach. The inclusion of intention-to-treat analyses will provide the reader with a better understanding of the effectiveness of aerobic exercise on lipids and lipoproteins in the “real world.” This may be especially appropriate given the dropout rate of exercising subjects in the studies that we included in our analysis. Second, because only one study17 reported that all the subjects were hyperlipidemic, it would appear plausible to suggest that future aerobic exercise training studies limit subjects to those with elevated lipid and lipoprotein levels. The inclusion of those with elevated levels is important because these are the individuals that are usually at the greatest risk for morbidity and mortality from cardiovascular disease.1 Furthermore, none of the studies limited their inclusion criteria to those subjects who smoked cigarettes and/or consumed alcohol. Since cigarette smoking and/or alcohol may have a negative effect on lipid and lipoprotein levels,1 it would be interesting to examine the effects of aerobic exercise on lipids and lipoproteins in these populations. However, we understand the difficulties (e.g., patient recruitment) in conducting studies of this nature. Finally, it would be interesting to examine the interaction, if any, between aerobic exercise and antihyperlipidemic medications (e.g., statins) on lipids and lipoproteins in older adults. This would lead to a more definitive understanding and, thus, more specific recommendations, regarding the use of aerobic exercise and pharmacologic intervention on lipids and lipoproteins in older adults. In conclusion, the results of this study suggest that aerobic exercise increases HDL-C and reduces TC/HDL-C in adults 50 years of age and older.
Acknowledgments
Acknowledgment and disclosure: The authors would like to thank William Haskell, PhD, Stanford University, for reviewing our reference list and providing suggestions for the coding of studies. This study was supported by a grant from the National Institutes of Health—National Heart, Lung, and Blood Institute, Award #R01-HL069802 (G.A. Kelley, Principal Investigator).
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Baker SJ, Al-Najadah R. Effect of ingesting fish oil on serum lipid and lipoprotein concentration in exercising and non-exercising women. Sports Med Train Rehabil. 1946;6:287–297. [Google Scholar]
- 3.Baker TT, Allen D, Lei KY, et al. Alterations in lipid and protein profiles of plasma lipoproteins in middle-aged men consequent to an aerobic exercise program. Metabolism. 1986;35:1037–1043. doi: 10.1016/0026-0495(86)90040-5. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal JA, Emery CF, Madden DJ, et al. Effects of exercise training on cardiorespiratory function in men and women >60 years of age. Am J Cardiol. 1991;67:633–639. doi: 10.1016/0002-9149(91)90904-y. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Kriska AM, LaPorte RE, et al. A two year randomized exercise trial in older women: effects on HDL-cholesterol. Atherosclerosis. 1987;66:247–258. doi: 10.1016/0021-9150(87)90068-2. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham DA, Rechnitzer PA, Howard JH, et al. Exercise training of men at retirement: a clinical trial. J Gerontol. 1987;42:17–23. doi: 10.1093/geronj/42.1.17. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson J, Tuominen J, Valle T, et al. Aerobic endurance exercise or circuit-type resistance training for individuals with impaired glucose tolerance? Horm Metab Res. 1998;30:37–41. doi: 10.1055/s-2007-978828. [DOI] [PubMed] [Google Scholar]
- 8.Fahlman MM, Boardley D, Lambert CP, et al. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J Gerontol A Biol Sci Med Sci. 2002;57:B54–B60. doi: 10.1093/gerona/57.2.b54. [DOI] [PubMed] [Google Scholar]
- 9.Ferrier KE, Waddell TK, Gatzka CD, et al. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38:222–226. doi: 10.1161/01.hyp.38.2.222. [DOI] [PubMed] [Google Scholar]
- 10.Hersey WC, Graves JE, Pollock ML, et al. Endurance exercise training improves body composition and plasma insulin responses in 70- to 79-year-old men and women. Metabolism. 1994;43:847–854. doi: 10.1016/0026-0495(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 11.King AC, Haskell WL, Taylor CB, et al. Group- vs home-based exercise training in healthy older men and women: a community-based clinical trial. JAMA. 1991;266:1535–1542. [PubMed] [Google Scholar]
- 12.Ligtenberg PC, Hoekstra JB, Bol E, et al. Effects of physical training on metabolic control in elderly type 2 diabetes mellitus patients. Clin Sci (Lond) 1997;93:127–135. doi: 10.1042/cs0930127. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama M, Sunami Y, Kinoshita F, et al. The effects of long-term low intensity aerobic training and detraining on serum lipid and lipoprotein concentrations in elderly men and women. Eur J Appl Physiol. 1995;70:126–131. doi: 10.1007/BF00361539. [DOI] [PubMed] [Google Scholar]
- 14.Nieman DC, Warren BJ, O'Donnell KA, et al. Physical activity and serum lipids and lipoproteins in elderly women. J Am Geriatr Soc. 1993;41:1339–1344. doi: 10.1111/j.1532-5415.1993.tb06485.x. [DOI] [PubMed] [Google Scholar]
- 15.Rauramaa R, Vaisanen SB, Kuhanen R, et al. The Rsal polymorphism in the alpha-fibrinogen gene and response of plasma fibrinogen to physical training—a controlled randomised clinical trial in men. Thromb Haemost. 2000;83:803–806. [PubMed] [Google Scholar]
- 16.Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non-insulin-dependent diabetes mellitus. Isr J Med Sci. 1994;30:766–770. [PubMed] [Google Scholar]
- 17.Ready EA, Drinkwater DT, Ducas J, et al. Walking program reduces elevated cholesterol in women postmenopause. Can J Cardiol. 1995;11:905–912. [PubMed] [Google Scholar]
- 18.Ready EA, Naimark B, Ducas J, et al. Influence of walking volume on health benefits in women post-menopause. Med Sci Sports Exerc. 1996;28:1097–1105. doi: 10.1097/00005768-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ruoti RG. The Effects of Calisthenic Water Exercise on Selected Work, Physiologic, and Blood Parameters of Older Adults. Philadelphia, PA: Temple University; 1989. [master's thesis] [Google Scholar]
- 20.Seals DR, Tanaka H, Clevenger CM, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 21.Sunami Y, Motoyama M, Kinoshita F, et al. Effects of low-intensity aerobic training on the high-density lipoprotein cholesterol concentration in healthy elderly subjects. Metabolism. 1999;48:984–988. doi: 10.1016/s0026-0495(99)90194-4. [DOI] [PubMed] [Google Scholar]
- 22.Verissimo MT, Aragao A, Sousa A, et al. Effect of physical exercise on lipid metabolism in the elderly. Rev Port Cardiol. 2002;21:1099–1112. [PubMed] [Google Scholar]
- 23.Verity LS, Ismail AH. Effects of exercise on cardiovascular disease risk in women with NIDDM. Diabetes Res Clin Pract. 1989;6:27–35. doi: 10.1016/0168-8227(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 24.Durstine JL, Grandjean PW, Davis PG, et al. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med. 2001;31:1033–1062. doi: 10.2165/00007256-200131150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Halbert JA, Silagy CA, Finucane P, et al. Exercise training and blood lipids in hyperlipidemic and normolipidemic adults: a meta-analysis of randomized, controlled trials. Eur J Clin Nutr. 1999;53:514–522. doi: 10.1038/sj.ejcn.1600784. [DOI] [PubMed] [Google Scholar]
- 26.Kelley GA, Kelley KS, Tran ZV. Walking, lipids, and lipoproteins: a meta-analysis of randomized controlled trials. Prev Med. 2004;38:651–661. doi: 10.1016/j.ypmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33:S502–S515. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- 28.Lokey EA, Tran ZV. Effects of exercise training on serum lipid and lipoprotein concentration in women: a meta-analysis. Int J Sports Med. 1989;10:424–429. doi: 10.1055/s-2007-1024937. [DOI] [PubMed] [Google Scholar]
- 29.Tran ZV, Weltman A. Differential effects of exercise on serum lipid and lipoprotein levels seen with changes in body weight: a meta-analysis. JAMA. 1985;254:919–924. [PubMed] [Google Scholar]
- 30.Tran ZV, Weltman AW, Glass GV, et al. The effects of exercise on blood lipids and lipoproteins: a meta-analysis of studies. Med Sci Sports Exerc. 1983;15:393–402. [PubMed] [Google Scholar]
- 31.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 34.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 35.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 36.Hedges LV, Olkin I. Vote-counting methods in research synthesis. Psychol Bull. 1980;88:359–369. [Google Scholar]
- 37.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 38.Booth FW, Chakravarthy MV, Gordon SE, et al. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 39.American College of Sports Medicine. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30:992–1008. [PubMed] [Google Scholar]
- 40.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]


