Abstract
Purpose
Use the meta-analytic approach to examine the effects of aerobic exercise on lipids and lipoproteins in adults with cardiovascular disease (CVD).
Methods
Studies were retrieved via electronic databases, review of reference lists from retrieved articles, including reviews, and hand searching. Inclusion criteria were: (1) randomized controlled trials, (2) aerobic exercise ≥4 weeks as an intervention, (3) studies published in English language only between January 1, 1955 and January 1, 2005, (4) studies published in journals or as dissertations or master's theses, (5) human subjects ≥18 years, (6) all subjects diagnosed with some type of CVD, and (7) pre and post data available for total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and/or triglycerides (TG). Random-effects models were used for data analysis.
Results
Of the more than 3,000 studies reviewed, a total of 10 representing 1,260 subjects (580 exercise, 680 control) were included in our analysis. There was a statistically significant increase of 9% in HDL-C (mean ± SEM, 3.7 ± 1.3 mg/dL; 95% CI, 1.2 to 6.1 mg/dL) and a statistically significant decrease of 11% in TG (−19.3 ± 5.4 mg/dL; 95% CI, −30.1 to −8.5 mg/dL), but no statistically significant decreases in TC or LDL-C (TC, −8.8 ± 6.8 mg/dL; 95% CI, −22.3 to 4.7 mg/dL; LDL-C, −7.7 ± 6.0 mg/dL; 95% CI, −19.5 to 4.2 mg/dL).
Conclusions
The present findings suggest that chronic aerobic exercise increases HDL-C and decreases TG in adults, especially men, with CVD.
Keywords: aerobic, cardiovascular disease, exercise, lipids, meta-analysis
Introduction
Suboptimal levels of lipids and lipoproteins represent a major risk factor for cardiovascular disease (CVD), the number 1 cause of mortality in the United States.1 Recent estimates indicate that more than 100 million US adults have a suboptimal lipid and lipoprotein profile.1 Aerobic exercise is a therapeutic lifestyle approach that is recommended for improving lipid and lipoprotein levels for all individuals, including those with CVD.2 However, randomized controlled trials of the effects of aerobic exercise on lipids and lipoproteins in adults with CVD have yielded conflicting results, possibly due to differences in initial lipid and lipoprotein levels, exercise program characteristics, duration of the intervention, or other confounding variables (eg, concomitant dietary and/or medication-induced changes).3–12 For example, of the previously cited studies, only 10% of total cholesterol (TC), 50% of high-density lipoprotein cholesterol (HDL-C), 60% of low-density lipoprotein cholesterol (LDL-C), and 44% of triglyceride (TG) outcomes were reported as statistically significant. However, the vote-counting approach (number and/or percentage of statistically significant results) has been shown to be less valid and reliable than the more quantitative meta-analytic approach.13 Meta-analysis is an approach in which individual studies are combined and analyzed in a quantitative fashion. It is especially appropriate when the number of studies is small, the number of subjects that can be enrolled in any given study is limited, or both.14 In the present study, we used the meta-analytic approach to examine the effects of aerobic exercise on lipids and lipoproteins in adults with CVD.
Methods
Data Sources
For this project, studies were retrieved via (1) electronic databases (MEDLINE, EMBASE, Current Contents, Dissertation Abstracts International), (2) review of reference lists from retrieved articles, and (3) hand searching.
Study Selection
Inclusion criteria for this study were: (1) randomized controlled trials, (2) aerobic exercise of 4 weeks or longer as an intervention, (3) studies published in the English language between January 1, 1955 and January 1, 2005, (4) studies published in journals or as dissertations or master's theses, (5) human subjects 18 years or older, (6) only subjects with known (or documented) CVD, and (7) pre and post data available for TC, HDL-C, LDL-C, and TG. Studies with multiple interventions met our inclusion criteria if the only difference was that the control group did not receive the aerobic exercise intervention. We chose 1955 as the starting date to search because it appears that this was the first time that an aerobic exercise training study on lipids and lipoproteins had been reported.15 If multiple publication bias (same data reported in more than 1 publication) was identified, only data from 1 study was included. Study selection was conducted by the first 2 authors, independent of each other. In addition, the third author examined our reference list for thoroughness and completeness. Discrepancies were resolved by consensus. All studies were entered into a reference database (ProCite, version 5.0.3).16
Data Abstraction
Coding sheets were developed that could hold more than 200 items. The major categories of items that were coded included study, subject, lipid assessment, and training program characteristics, as well as primary (TC, HDL-C, LDL-C, TG) and secondary (body weight, body mass index [BMI] in kg/m2, and maximum oxygen consumption [Vo2max], expressed as mL·kg−1·min−1) outcomes. All studies were coded in Microsoft Excel version 2002 spreadsheets17 by the first 2 authors, independent of each other. The authors then reviewed every entry for accuracy and consistency. Discrepancies were resolved by consensus. Interrater reliability prior to correcting discrepant items was 0.94 (Cohen kappa).18
Statistical Analysis
Before the start of this study, we identified the number of outcomes and subjects that would be available for our primary outcomes analysis and then conducted power analyses designed specifically for meta-analytic datasets.19 Using a small to medium effect size of 0.35,20 a random-effects variance component of 1.0, a 2-tailed alpha level of .05, and a desired power of 0.80, our power to identify a statistically significant difference was estimated to be 0.98, 0.88, 0.83, and 0.96, respectively, for TC, HDL-C, LDL-C, and TG.
Primary outcomes (TC, HDL-C, LDL-C, and TG) were calculated by taking the change score difference between the exercise and control groups. Because most studies provided dispersion statistics for pre and post means but not change outcomes, these were estimated according to procedures developed by Follmann et al.21 All studies were weighted by the inverse of the variance and results were pooled using a random-effects model.22,23 Ninety-five percent confidence intervals were used to establish the precision of our results. Secondary outcomes (changes in body weight, BMI, Vo2max) were analyzed using the same procedures as those for primary outcomes.
To examine for publication bias, we used the regression approach of Egger et al.24 The sensitivity of our results was examined by running all of our primary outcomes analyses with each study deleted from the model once. Study quality was examined using a previously validated and reliable questionnaire that ranged from 0 to 5 points, with higher scores representing greater study quality.25 Analysis of variance–like models for meta-analysis were used to compare differences in lipids and lipoproteins according to whether the intention-to-treat or analysis-by-protocol approach was used.26 We were unable to examine gender differences because none of the studies provided data for women only. Because of missing data for different variables from different studies, a common occurrence with meta-analytic datasets, simple meta-regression was used to examine the relationship between changes in TC, HDL-C, LDL-C, and TG with study quality, initial lipid levels, age, changes in body weight, BMI, Vo2max, length, frequency and duration of training, as well as compliance to the exercise protocol. We were unable to examine the relationship between changes in lipids and lipoproteins and intensity of training because of a lack of reported data. The 2-tailed alpha level for statistical significance was set at P < .05. All data were analyzed using Stata SE (version 8.2)27 and SPSS (version 13.0).28
Results
Study Characteristics
Of more than 3,000 studies reviewed, 11 studies published in journals met our inclusion criteria.3–12,29 However, we were unable to include one study because of a lack of reported data on lipids and lipoproteins.29 Thus, our percent loss that met our inclusion criteria was approximately 9%. A general description of the 10 included studies is shown in Table 1. A total of 1,260 subjects (580 exercise, 680 control) were included in our analysis. The number of subjects in which lipid and lipoprotein data were available ranged from 8 to 110 in the exercise groups (mean ± SD, 58 ± 34) and 18 to 142 in the control groups (mean ± SD, 68 ± 47). Two studies were conducted in the United States,6,8 Scotland,3,11 and Sweden,7,10 whereas 1 each were conducted in China,12 Italy,4 the United Kingdom,5 or Yugoslavia.9 Six studies used the analysis-by-protocol approach to analyze their data,3,5,8–10,12 whereas 4 used intention-to-treat analysis.4,6,7,11 Median study quality was 2.0. Initial characteristics of the subjects are shown in Table 2.
Table 1.
Characteristics of Included Studies
| Study | Subjects | Exercise Program | Lipids |
|---|---|---|---|
| Ballantyne et at (1982)3 | 42 men, 19 exercise (age = 51.0 ± 5.2 years) and 23 control (age = 54.3 ± 6.2 years). All subjects had suffered a myocardial infarction. | 24 weeks of training following the Canadian Air Force 5BX plan | TC, HDL-C, LDL-C, TG |
| Belardinelli et al (2001)4 | 118 men and women (age = 57 ± 10) assigned to either an exercise (n = 59) or control (n = 59) group. Subjects were patients who had undergone coronary angioplasty. | 24 weeks of cycle ergometry performed 3 d/wk | TC, HDL-C, LDL-C, TG |
| Carson et al (1982)5 | 239 men, 104 exercise (age = 50.3 years) and 135 controls (age = 52.8 years). All subjects had suffered a myocardial infarction. | 12 weeks of aerobic circuit exercise performed 2 d/wk | TC, TG |
| Fletcher et al (1994)6 | 88 men, 47 exercise (age = 62.0 ± 8 years) and 41 controls (age = 63.0 ± 7 years). All subjects presented with evidence of coronary artery disease and had at least 1 physical disability. | 24 weeks of stationary wheelchair ergometry performed 5 d/wk, 20 minutes/session | TC, HDL-C, TG |
| Gelin et al (2001)7 | 149 men and women, 45–81 years, assigned to either an exercise (n = 73, age = 67 years) or control (n = 76, age = 67 years) group. All subjects presented with intermittent claudication. | 52 weeks of walking, 2–3 d/wk, 30 minutes/session | TC, TG |
| LaRosa et al (1982)8 | 223 men, 30–64 years, assigned to an exercise (n = 110, age = 51.6 ± 7.3 years) or control (n = 113, age = 52.7 ± 6.4 years) group. All subjects had suffered a myocardial infarction. | 52 weeks of aerobic exercise, 3 d/wk, 30–40 minutes/session, 70–85% of MHR | TC, HDL-C, LDL-C, TG |
| Plavsic et al (1976)9 | 26 men up to 59 years, 8 in the exercise group and 18 in the control group. All subjects had suffered a myocardial infarction. | 72 weeks of aerobic exercise, 2 d/wk | TC, TG |
| Wilhelmsen et al (1975)10 | 209 men and women, 67 in the exercise group and 142 in the control group. All subjects had suffered a myocardial infarction. | 27 weeks of aerobic exercise, 3 d/wk, 30 minutes/session, 80% of MHRR | TC, TG |
| Wosornu et al (1996)11 | 54 men assigned to either an exercise (n = 27, age = 56.5 ± 8.9 years) or control (n = 27, age = 56.6 ± 7 years) group. All subjects had undergone coronary artery bypass surgery. | 24 weeks of aerobic exercise based on the Canadian Air Force 5BX plan, 3 d/wk, 12–40 minutes/session | TC, HDL-C, LDL-C, TG |
| Yu et al (2003)12 | 112 men and women assigned to either an exercise (n = 72, age = 62.3 ± 11.2 years) or control (n = 40, age = 61.2 ± 10.2 years) group. Subjects were overweight/obese with coronary heart disease that recently had an acute myocardial infarction or percutaneous coronary intervention. | 8 weeks of aerobic exercise, 2 d/wk. 60 minutes/session, 65% to 85% of Vo2max | TC, HDL-C, LDL-C, TG |
MHRR indicates maximum heart rate reserve; Vo2max, maximum oxygen consumption; MHR, maximal heart rate; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
Description of studies was limited to those subjects and variables that met our inclusion criteria. The number of subjects was limited to those in which pre and post assessment of lipids took place. Lipid variables listed were limited to those that met our inclusion criteria, including availability. Data were reported as mean ± SD.
Table 2.
Initial Characteristics of Subjects
| Variable | n | Exercise (mean ± SD) | Range | n | Control (mean ± SD) | Range |
|---|---|---|---|---|---|---|
| Age (y) | 8 | 56.7 ± 6.3 | 50–67 | 8 | 58.3 ± 5.2 | 53–67 |
| Body weight (kg) | 6 | 74.4 ± 3.6 | 70–79 | 6 | 75.2 ± 3.3 | 71–81 |
| BMI (kg/m2) | 6 | 25.4 ± 1.6 | 23–28 | 6 | 25.6 ± 1.5 | 24–28 |
| Vo2max (mL·kg−1·min−l) | 4 | 22.5 ± 5.5 | 18–29 | 4 | 23.7 ± 5.5 | 18–30 |
| TC (mg/dL) | 9 | 237.6 ± 27.1 | 192–276 | 9 | 230.6 ± 24.3 | 184–262 |
| HDL-C (mg/dL) | 6 | 40.3 ± 6.9 | 34–49 | 6 | 41.4 ± 6.8 | 35–51 |
| LDL-C (mg/dL) | 5 | 151.1 ± 19.2 | 123–176 | 5 | 152.4 ± 20.6 | 131–178 |
| TG (mg/dL) | 9 | 179.8 ± 32.9 | 143–255 | 9 | 171.4 ± 12.4 | 146–184 |
n indicates number of studies reporting data; mean ± SD, mean ± standard deviation; range represents the means for each group from each study; BMI, body mass index; Vo2max, maximum oxygen consumption; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
Subject Characteristics
Gender
Six studies were limited to men,3,5,6,8,9,11 and 4 others included both men and women.4,7,10,12 Although we were unable to determine the number of men and women for one study,10 the remaining 9 studies included 858 men and 109 women.
Race/Ethnicity
For the one study that provided specific information on race/ethnicity, all subjects were reported to be Chinese.12
Medications and Lipid Metabolism
One study reported that none of the subjects were taking any type of medication(s) that could affect lipid metabolism,4 whereas 4 others had some subjects that were taking some type of medication(s).3,5,11,12 Four studies reported that medication usage did not change during the study,3,4,11,12 whereas another stated that an additional 8 and 5 subjects in the exercise and control groups started taking beta-blockers.5
Cigarette Smoking/Alcohol Consumption
One study reported that none of the subjects smoked cigarettes,6 whereas 8 others stated that some subjects smoked cigarettes.3–5,7,8,10–12 For alcohol consumption, 2 studies reported that some of the subjects consumed alcohol.3,8
Physical Activity/Diet
Three studies stated that some of the subjects in both the exercise and control groups were physically active before taking part in the study.9,10,12 Five studies reported that they provided dietary advice to both the exercise and control groups6,8–10,12 with one reporting concomitant changes in diet that may have effected the alterations in lipids and lipoproteins in the exercise group.6
Comorbidities
Six studies stated that some of the subjects had Type 1 and/or Type 2 diabetes,4,5,7,10–12 while another 2 reported that none of the subjects had diabetes.3,8 For overweight/obesity, one study reported that all of the subjects were overweight or obese,12 while 2 studies stated that some of the subjects were overweight and/or obese.4,9 A description of the subjects in relation to CVD can be found in Table 1.
Lipid Assessment Characteristics
Blood sampling for the assessment of lipids and lipoproteins generally took place in the morning after an overnight fast.
Training Program Characteristics
A description of the individual and group training program characteristics are provided in Tables 1 and 3, respectively. As shown in Table 3, only 20% of the studies reported the average intensity of training.
Table 3.
Training Program Characteristics
| Variable | n | Mean ± SD | Range |
|---|---|---|---|
| Length (weeks) | 10 | 32.8 ± 20.1 | 8–72 |
| Frequency (times/wk) | 8 | 2.9 ± 1.0 | 2–5 |
| Intensity (%Vo2max) | 2 | 70.0 ± 14.1 | 60–80 |
| Duration (minutes/session) | 6 | 35.8 ± 14.3 | 20–60 |
| Compliance (%) | 6 | 71.0 ± 12.8 | 58–94 |
n indicates number of studies reporting data; mean ± SD, mean ± standard deviation; compliance, percentage of exercise sessions attended.
Primary Outcomes
Total Cholesterol
The results for TC are shown in Table 4 and Figure 1. Across all studies, a nonsignificant reduction of approximately 4% was found for TC. With each study deleted from the model once, results remained nonsignificant. Greater decreases in TC were associated with decreases in BMI (r = 0.90, P = .0002) and compliance to the exercise protocol (r = 0.88, P = .0004). No other statistically significant differences or relationships were observed (P > .05 for all).
Table 4.
Primary and Secondary Outcomes
| Variable | Studies (n) | Subjects (n) | Mean ± SEM | P | 95% CI |
|---|---|---|---|---|---|
| Primary Outcomes (mg/dL) | |||||
| TC | 9 | 1,021 | −8.8 ± 6.8 | .20 | −22.3 to 4.7 |
| HDL-C | 6 | 637 | 3.7 ± 1.3 | .004* | 1.2 to 6.1** |
| LDL-C | 5 | 549 | −7.7 ± 6.0 | .20 | −19.5 to 4.2 |
| TG | 9 | 1,172 | −19.3 ± 5.4 | .0004* | −30.1 to −8.5** |
| Secondary Outcomes | |||||
| Body weight (kg) | 5 | 640 | −1.7 ± 0.5 | .18 | −1.7 to 0.3 |
| BMI (kg/m2) | 3 | 284 | −0.5 ± 0.6 | .40 | −1.6 to 0.7 |
| Vo2max (mL·kg−1·min−1) | 4 | 507 | 4.7 ± 0.4 | <.0001* | 3.1 to 6.3** |
Studies indicate total number of studies included in each analysis; Subjects, total number of subjects included in each analysis; mean ± SEM, mean ± standard error of the mean; CI, confidence interval; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; BMI, body mass index; Vo2max, maximum oxygen consumption.
Statistically significant at P < .05.
Significantly different from zero (0).
Figure 1.
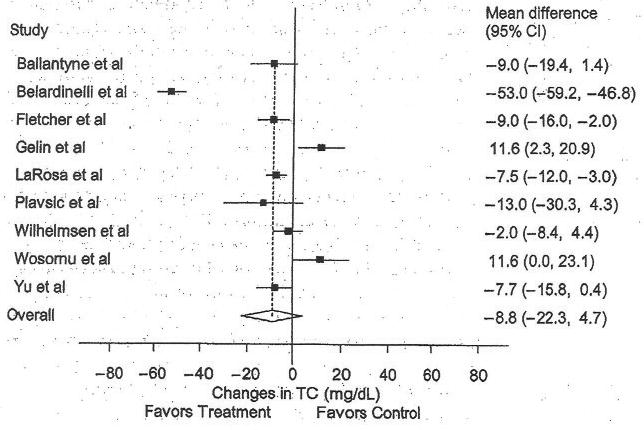
Forest plot for changes in total cholesterol (TC). The black boxes represent the mean change in TC for each study and the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in TC across all listed studies, whereas the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
High-density Lipoprotein Cholesterol
The results for HDL-C are shown in Table 4 and Figure 2. Across all studies, a statistically significant increase of approximately 9% was found. With each study deleted from the model once, results remained statistically significant. No statistically significant publication bias was observed (t = 1.93, P = .13). Increases in HDL-C were associated with decreases in BMI (r = 0.90, P = .002), but not body weight (r = 0.61, P = .29). In addition, increased compliance to the exercise protocol was associated with increases in HDL-C (r = 0.86, P = .02). Furthermore, a trend was found for increases in HDL-C and increases in Vo2max (r = 0.74, P = .09). No other statistically significant differences or relationships were observed (P > .05 for all).
Figure 2.
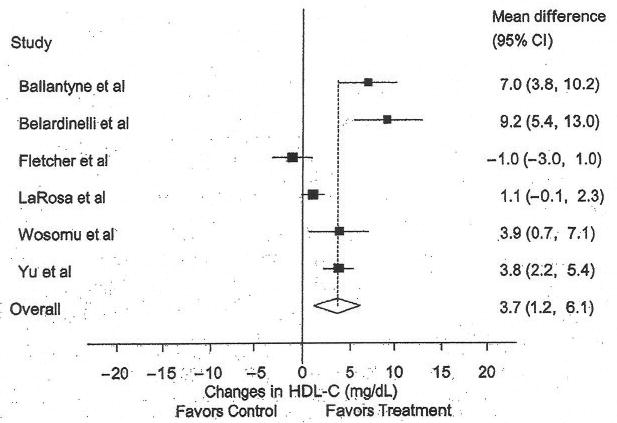
Forest plot for changes in high density lipoprotein cholesterol (HDL-C). The black boxes represent the mean change in HDL-C for each study, and the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in HDL-C across all listed studies, whereas the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Low-density Lipoprotein Cholesterol
The results for LDL-C are shown in Table 4 and Figure 3. Across all studies, a nonsignificant reduction of approximately 5% was found. However, with each study deleted from the model once, results were statistically significant when the Wosomu et al11 study was deleted from the model (mean ± SEM, −12.4 ± 3.5 mg/dL, 95% CI, −23.6 to −1.1 mg/dL). Decreases in LDL-C were associated with increased compliance to the exercise protocol (r = 0.97, P = .0001). In addition, there was a trend for greater decreases in LDL-C with greater decreases in BMI (r = 0.79, P = .06). No other statistically significant differences or relationships were observed (P > .05 for all).
Figure 3.
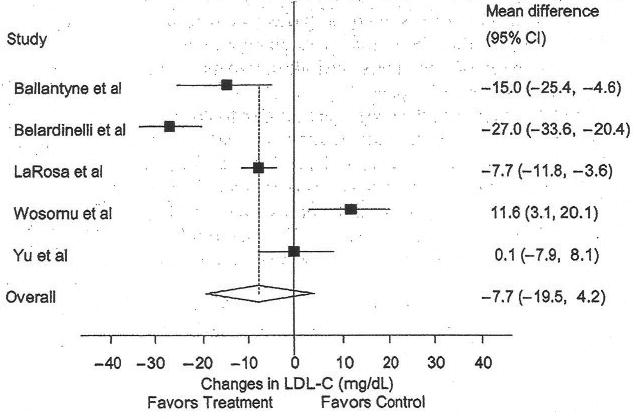
Forest plot for changes in low density lipoprotein cholesterol (LDL-C). The black boxes represent the mean change in LDL-C for each study, and the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in LDL-C across all listed studies, whereas the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Triglycerides
The results for TG can be found in Table 4 and Figure 4. Across all studies, a statistically significant decrease of approximately 11% was found. No statistically significant publication bias was found (t = −0.86, P = .42). With each study deleted from the model once, results remained statistically significant. Decreases in TG were associated with greater compliance to the exercise protocol (r = 0.82, P = .003). In addition, there was a trend for greater decreases in TG for those with higher initial TG levels (r = 0.58, P = .07) and studies of lower quality (r = 0.59, P = .09). No other statistically significant differences or relationships were observed (P > .05 for all).
Figure 4.
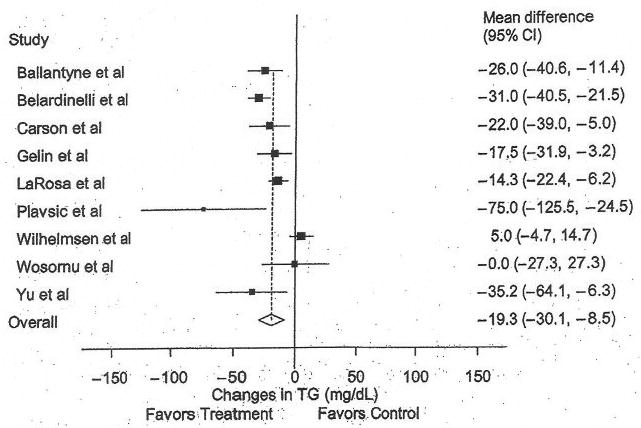
Forest plot for changes in triglycerides (TG). The black boxes represent the mean change in TG for each study, and the lines represent the 95% confidence intervals. The diamond and dashed lines represent the overall mean change in TG across all listed studies, whereas the left and right ends of the diamond represent the 95% confidence interval for all studies combined.
Discussion
Our overall results suggest that aerobic exercise increases HDL-C and decreases TG in this cohort. From a clinical perspective, these improvements (+9% for HDL-C and −11% for TG) are probably important. For example, although LDL-C is currently the primary target of lipid-lowering therapy,2 increases in HDL-C (+6%) and decreases in TG (−31%) in patients with coronary heart disease have been shown to reduce nonfatal myocardial infarction and coronary heart disease death by 22% in the absence of changes in LDL-C.30 Thus, it appears that even without lowering LDL-C, improvements in HDL-C and TG can benefit patients with CVD.
Although we did not find a statistically significant reduction in TC or LDL-C, important cardioprotective improvements in LDL-C subfractions may have occurred. For example, it has recently been shown that aerobic exercise in previously sedentary, overweight men and women with mild to moderate dyslipidemia decreases the concentrations of the more atherogenic small LDL particles while increasing the average size of LDL particles despite a lack of a statistically significant reduction in LDL-C.31 Thus, the beneficial effects of aerobic exercise on LDL-C may not be apparent when limited to the assessment of total LDL-C alone. However, Varady et al32 reported that aerobic exercise decreased LDL peak particle size in previously sedentary, hypercholesterolemic adults. The authors concluded that aerobic exercise may potentially increase the risk for coronary heart disease by decreasing LDL peak particle size.32 Alternatively, Elosua et al33 concluded that aerobic exercise had no effect on LDL particle diameter. Given these discrepant results, additional research dealing with the effects of aerobic exercise on LDL subfractions in adults appears warranted. Because we are not aware of any research to date that has examined this issue in patients with CVD, future studies should include a representative sample of this population.
Because 5 studies reported providing dietary advice to both the exercise and control groups,6,8–10,12 improvements in lipids and lipoproteins in the exercise groups may have been blunted by any possible and equivalent changes in diet that may have occurred in both groups. This is especially true because we calculated differences in lipids and lipoproteins as the exercise minus control group difference. One exception to this is the study by Fletcher et al6 in which it was reported that dietary changes were limited to subjects in the exercise group only.
Our LDL-C and TG results are in agreement with a recent meta-analysis of the effects of exercise on lipids and lipoproteins.34 However, although we found a statistically significant increase in HDL-C and a nonsignificant decrease in TC, the previously conducted meta-analysis found no statistically significant changes in HDL-C, but a statistically significant reduction in TC.34 One of the possible reasons for the conflicting findings may be our stricter inclusion criteria. For example, we limited our inclusion criteria to only those studies in which aerobic exercise was an intervention, whereas the previously conducted meta-analysis included studies employing other types of exercise (eg, yoga). In addition, we only included studies in which the only difference between the exercise and usual care groups was the aerobic exercise intervention.
We found several interesting relationships between changes in lipids and lipoproteins and selected variables, most notably, a statistically significant relationship between greater compliance to the exercise protocol and improvements in all of the lipids and lipoproteins reported in this study (TC, HDL-C, LDL-C, TG). Intuitively, this makes sense if aerobic exercise is indeed the primary or sole factor associated with improvements in lipids and lipoproteins. We also found a statistically significant relationship between decreases in BMI and greater improvements in TC and HDL-C, along with a trend for greater reductions in LDL-C. This raises the possibility that exercise-mediated decreases in BMI may play a role in improving TC, HDL-C, and LDL-C in adults with CVD. However, these results need to be interpreted with caution as we did not find any relationship between changes in body weight and lipids and lipoproteins. One of the reasons for these conflicting findings probably has to do with the larger sample size for body weight. Consequently, our findings for body weight may be more appropriate than our BMI findings.
The trend for greater increases in HDL-C with greater increases in Vo2max suggests that those that are the least fit may have the most to gain from an aerobic exercise program in relation to increasing HDL-C levels. Alternatively, it could suggest that training at higher intensities leads to greater increases in HDL-C. For example, a recent review found that the preponderance of evidence favored greater increases in Vo2max from higher intensities of training.35 Similarly, Wenger and Bell36 concluded that higher exercise intensities elicit greater improvements in Vo2max. Unfortunately, only 20% of the studies in our meta-analysis provided intensity data.
The trend for greater decreases in TG with higher baseline TG levels suggests that those with higher initial levels of TG may have more to gain than those with lower initial TG levels. Finally, the trend for greater decreases in TG with studies of lower quality may warrant caution with respect to the interpretation of our TG findings.
Clinicians today may rely too heavily on lipid-lowering drugs (ie, statins). In our opinion, we should not abandon aggressive lifestyle alterations (diet and/or exercise) which have been shown to significantly improve the lipid and lipoprotein profile equivalent to drug monotherapy and beyond improvements with statins alone. Several investigations have examined the interaction between dietary changes, exercise training, drug therapy, or combinations thereof, on both lipid and lipoprotein levels in patients with hypercholesterolemia.37–39 Although pharmacotherapies (ie, statins) are widely considered as a first-line strategy to stabilize overt CVD, aggressive diet therapy (less than 10% of calories from fat) can result in improvements in the lipid and lipoprotein profile that are comparable to those reported in trials of drug monotherapy,38,39 and when combined with daily aerobic exercise, can result in additional substantial reductions in TC, LDL-C, and TG, beyond those achieved with cholesterol-lowering drugs.37
Meta-analysis, like any type of review, is limited by the accuracy and availability of data in the included studies. Thus, our results should not be generalized beyond the characteristics of the studies included in this meta-analysis. For example, most subjects were men and none of the studies reported lipid and/or lipoprotein outcome date for women only. Given that 37.6 million women (33.9%) have CVD1 and that suboptimal lipid and lipoprotein levels are major risk factors for CVD in both men and women, it would seem appropriate to suggest that future trials examining the effects of aerobic exercise on lipids and lipoproteins enroll increasing numbers of women.
Because of missing data, we were unable to conduct any type of multiple regression analyses. Consequently, this increased our risk for a Type I error. Moreover, we made no adjustment for multiple comparisons because we did not want to assume that chance was the first-order explanation for our findings.40 Thus, although the associations between lipids and lipoproteins and our selected variables are interesting, these should be tested in well-designed, randomized, controlled trials prior to reaching any clinical decisions regarding these relationships. Finally, we were unable to estimate exercise energy expenditure from each study because of missing data for different variables from different studies. Because this information may have yielded valuable insight regarding the dose of exercise necessary for bringing about changes in lipids and lipoproteins, it is suggested that future studies either estimate energy expenditure or provide complete data for the calculation of such.
In conclusion, the results of our study suggest that aerobic exercise increases HDL-C and decreases TG in adults, especially men, with CVD.
Acknowledgments
This study was supported by a grant from the National Institutes of Health—National Heart, Lung and Blood Institute, Award #R01-HL069802 (G. A. Kelley, Principal Investigator).
Footnotes
The authors have no conflict of interest.
Contributor Information
George A. Kelley, Department of Community Medicine, School of Medicine, West Virginia University, Morgantown.
Kristi S. Kelley, Department of Community Medicine, School of Medicine, West Virginia University, Morgantown.
Barry Franklin, Cardiac Rehabilitation and Exercise Laboratories, William Beaumont Hospital, Royal Oak, Mich.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas, Tex: American Heart Association; 2005. [Google Scholar]
- 2.National Cholesterol Education Program, National Heart Lung and Blood Institute, National Institutes of Health. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3.Ballantyne FC, Clark RS, Simpson HS, Ballantyne D. High density and low density lipoprotein subfractions in survivors of myocardial infarction and in control subjects. Metabolism. 1982;31:433–437. doi: 10.1016/0026-0495(82)90230-x. [DOI] [PubMed] [Google Scholar]
- 4.Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol. 2001;37:1891–1900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 5.Carson P, Phillips R, Lloyd M, et al. Exercise after myocardial infarction: a controlled trial. J R Coll Physicians Lond. 1982;16:147–151. [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher BJ, Dunbar SB, Felner JM, et al. Exercise testing and training in physically disabled men with clinical evidence of coronary artery disease. Am J Cardiol. 1994;73:170–174. doi: 10.1016/0002-9149(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 7.Gelin J, Jivegard L, Karlsson J, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients: I. One year results of functional and physiological improvements. Eur J Vasc Endovasc Surg. 2001;22:107–113. doi: 10.1053/ejvs.2001.1413. [DOI] [PubMed] [Google Scholar]
- 8.LaRosa JC, Cleary P, Muesing RA, Gorman P, Hellerstein HK, Naughton J. Effect of long-term moderate physical exercise on plasma lipoproteins: the national exercise and heart disease project. Arch Intern Med. 1982;142:2269–2274. doi: 10.1001/archinte.142.13.2269. [DOI] [PubMed] [Google Scholar]
- 9.Plavsic C, Turkulin K, Perman Z, et al. The results of “exercise therapy in coronary prone individuals and coronary patients”. G Ital Cardiol. 1976;6:422–432. [PubMed] [Google Scholar]
- 10.Wilhelmsen L, Sanne H, Elmfeldt D, Grimby G, Tibblin G, Wedel H. A controlled trial of physical training after myocardial infarction. Effects on risk factors, nonfatal reinfarction, and death. Prev Med. 1975;4:491–508. doi: 10.1016/0091-7435(75)90035-3. [DOI] [PubMed] [Google Scholar]
- 11.Wosomu D, Bedford D, Ballantyne D. A comparison of the effects of strength and aerobic exercise training on exercise capacity and lipids after coronary artery bypass surgery. Eur Heart J. 1996;17:854–863. doi: 10.1093/oxfordjournals.eurheartj.a014966. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Li LS, Ho HH, Lau CP. Long-term changes in exercise capacity, quality of life, body anthropometry, and lipid profiles after a cardiac rehabilitation program in obese patients with coronary heart disease. Am J Cardiol. 2003;91:321–325. doi: 10.1016/s0002-9149(02)03159-4. [DOI] [PubMed] [Google Scholar]
- 13.Hedges LV, Olkin I. Vote-counting methods in research synthesis. Psychol Bull. 1980;88:359–369. [Google Scholar]
- 14.Petitti DB. Meta-analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 15.Mann GV, Teel K, Hayes O, McNally A, Bruno D. Exercise in the disposition of dietary calories: regulation of serum lipoprotein and cholesterol levels in human subjects. N Engl J Med. 1955;253:349–355. doi: 10.1056/NEJM195509012530901. [DOI] [PubMed] [Google Scholar]
- 16.ProCite for Windows. 5.0.3. ISI ResearchSoft. Carlsbad, Calif: ProCite; 2003. [Google Scholar]
- 17.Microsoft Excel 2002 (10.6501.6626) SP3. Redmond, Wash: Microsoft Corporation; [Google Scholar]
- 18.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 19.Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psycbol Methods. 2001;6:203–217. [PubMed] [Google Scholar]
- 20.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 22.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Hunter JE, Schmidt FL. Fixed effects vs. random effects meta-analysis models: implications for cumulative research knowledge. Int J Sel Assess. 2000;8:275–292. [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Hedges LV, Olkin I. Statistical Methods for Meta-analysis. San Diego, Calif: Academic Press; 1985. [Google Scholar]
- 27.StataCorp. Stata/SE 8.2 for Windows. College Station, Tex: Stata Corporation; 2005. [Google Scholar]
- 28.SPSS 13.0 for Windows. Chicago, Ill: SPSS, Inc; 2004. [Google Scholar]
- 29.Agren B, Olin C, Castenfors J, Nilsson-Ehle P. Improvements of the lipoprotein profile after coronary bypass surgery: additional effects of an exercise training program. Eur Heart J. 1989;10:451–458. doi: 10.1093/oxfordjournals.eurheartj.a059509. [DOI] [PubMed] [Google Scholar]
- 30.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 31.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 32.Varady KA, St-Pierre AC, Lamarche B, Jones PJ. Effect of plant sterols and endurance training on LDL particle size and distribution in previously sedentary hypercholesterolemic adults. Eur J Clin Nutr. 2005;59:518–525. doi: 10.1038/sj.ejcn.1602106. [DOI] [PubMed] [Google Scholar]
- 33.Elosua R, Molina L, Fito M, et al. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–334. doi: 10.1016/s0021-9150(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 34.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Swain DP, Franklin BA. VO(2) reserve and the minimal intensity for improving cardiorespiratory fitness. Med Sci Sports Exerc. 2002;34:152–157. doi: 10.1097/00005768-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 36.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3:346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 37.Barnard RJ, DiLauro SC, Inkeles SB. Effects of intensive diet and exercise intervention in patients taking cholesterol-lowering drugs. Am J Cardiol. 1997;79:1112–1114. doi: 10.1016/s0002-9149(97)00058-1. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins DJA, Kendall CWC, Marchie A, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs. lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 39.Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129–133. doi: 10.1016/0140-6736(90)91656-u. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]


