Abstract
Prophet of Pit-1 (Prop1) is an early transcription factor that delays the appearance of gonadotropin in the developing pituitaries. Prop1 transgenic (Tg) mice have been shown to generate pituitary tumors that either produce TSH or are non-hormone producing. In our series of Prop1 Tg mice, only 5 out of 9 female mice produced pituitary adenomas, and the adenomas were only GH, PRL, GH and PRL, PRL and gonadotropin or TSH producing. The pituitary cells that surrounded these adenomas showed hyperplasia of the corresponding hormone producing cells; i.e. the GH cells were increased in the pituitary that contained GH producing adenoma. In addition, although the adenomas lacked the expression of Prop1, the non-neoplastic pituitary cells showed expression of Prop1.
The Prop1 Tg mice also showed vacuolated cells with eccentric nuclei, which are characteristic of “signet-ring hypertrophic cells”. Using immunohistochemistry, these signet ring hypertrophic cells were found to be positive for gonadotropin.
Taken together, our results suggest a (1) tumorigenic effect of Prop1 in the pituitaries, and (2) causative effects of signet ring-type gonadotropes.
Keywords: Prop1, pituitary, adenoma, pituitary signet-ring cell
I. Introduction
The pituitary gland develops from Rathke’s pouch and its primordium appears on embryonic day (e) 8.5 in mice. The hormone producing cells of the pituitary gland initially appear as α-glycoprotein hormone subunit (αGSU) positive cells on e11 and, subsequently, differentiate into anterior pituitary hormone producing cells [41]. Pituitary cell types can be classified into three lineages: the growth hormone (GH)-prolactin (PRL)-thyroid stimulating hormone (TSH) (GH-PRL-TSH) cell lineage, the proopiomelanocortin (POMC; precursor of adrenocorticotropic hormone, ACTH) lineage, and the gonadotropin (luteinizing hormone/follicle stimulating hormone; LH/FSH) lineage. Various transcription factors have been reported to play roles in the differentiation of these lineages. Differentiation into the POMC lineage depends on the expression of NeuroD1 and Tpit [21, 28]. Gata2 [7, 36] and SF1 [16] expression indicate differentiation into the gonadotropin lineage. The GH-PRL-TSH lineage, which is regulated by Pit1 [3, 15], is also dependent on the function of the ‘paired’-like homeodomain transcription factor, Prop1, as indicated by studies in Prop1 mutants (Ames dwarf mutant mice (Prop1df/df) and combined pituitary hormone deficiency (CPHD) in humans) [1, 8, 31, 43]. Prop1 is an early regulator of Pit1 in the developing mouse pituitary gland [10]. With maximum expression at e12.5, Prop1 mRNA expression rapidly decreases after e14.5, but may persist at detectable levels in some species [34]. The temporal regulation of Prop1 gene expression is critical to its function.
In human pituitary adenomas, transcription factors and synergistic interactions are involved in the adenomatous differentiation of the pituitary gland, as well as normal cell differentiation [27, 32, 37, 39]. Persistent Prop1 expressing mice have delayed gonadotrope development and a propensity for tumorigenesis [6]. It has been reported that non-functioning tumors or focal thyrotrope hyperplasia appear in the pituitaries of aged Prop1 transgenic mice.
In order to explore the effects of Prop1 overexpression on pituitary function, the tumorigenesis and differentiation rates of pituitary cells from Prop1 transgenic mice were examined. We identified tumors of the Pit1-dependent cell lineage. In addition to tumor formation, the appearance of signet-ring type gonadotropes was observed. This study was designed to elucidate the roles of Prop1 in tumorigenesis and its effect on the differentiation of pituitary cells.
II. Materials and Methods
The generation of Prop1 transgenic mice
Mice carrying the Prop1 alleles were supplied by the University of Michigan Medical School and bred at Tokai University. Mice were housed in ventilated cages under 12-h light and 12-h dark cycles. All mice were maintained under specific pathogen-free conditions at Tokai University School of Medicine (Isehara, Japan), and the experiments proceeded according to the Guidelines for Animal Experimentation published by the Japanese Association for Laboratory Animal Science (1987). Prop1 transgenic mice were generated with mouse Prop1 genomic sequences under the control of the αGSU (Cga) promoter and with splice sites and polyadenylation sequences from mouse protamine 1 [6]. Six lines of Prop1 transgenic mice were generated (D1–D6). In the present study, transgenic mice from lines D4 and D6 were bred to C57BL/6J mice. The D4 line of Prop1 transgenics was analyzed in detail. The D4 line of Prop1 transgenic mice was officially named TgN(Cga-Prop1)D4Sac. Genomic DNA was prepared from tail biopsies of the newborn progeny, and PCR was performed to identify mice that carried the transgene using a Tissue Direct PCR kit (GenScript Corp., Piscataway, NJ). A forward primer located in the Cga promoter (5'-ATG GCT CCT TCT TTG AGC TTC-3') and a reverse primer located in the coding sequence of Prop1 (5'-TCA ACT TTC AGG ATG TTT TGT ATA A-3') were used for PCR.
Immunohistochemistry of hormones Pit1 and ERa in Prop1 transgenic mouse pituitaries
The pituitary glands of the Prop1 transgenic mice at 1.5 years of age were fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4 at 4°C. The fixed tissues were washed in PBS and dehydrated through successively more concentrated ethanol solutions and finally embedded in paraffin. Tissue sections of 4 µm thickness were prepared for hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC). For IHC, the slides were dewaxed and rehydrated before staining. For transcription factor immunostaining, epitopes were exposed by autoclaving for 5 min in Antigen Retrieval Citra Plus Solution (BioGenex, San Ramon, CA). Anti-Pit1 rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used at a 1:100 dilution. Anti-ERα rabbit polyclonal antibody (Santa Cruz Biotechnology) was used at 1:2000. Anti-Gata2 rabbit polyclonal antibody (Santa Cruz Biotechnology) was used at 1:200. Anti-Sf1 rabbit polyclonal antibody (Affinity BioReagents, Golden, CO) was used at 1:1000. Anti-PRL (NHPP, NIDDK, Bethesda, MD), anti-human GH (DakoCytomation, Denmark) and anti-αGSU (NHPP) rabbit antibodies were used at 1:400, 1:400 and 1:200, respectively. Anti-human LHβ (Beckman-Coulter, Fullerton, CA), anti-human TSHβ (Advanced Immunochemical Inc., Long Beach, CA) and anti-human ACTH (DakoCytomation) monoclonal antibodies were used at 1:200, 1:100 and 1:200, respectively. Sections were incubated with these primary antibodies for 1 hr at room temperature and then with biotin-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Signals were amplified using the horseradish peroxidase (HRP) conjugated ENVISION plus kit (DakoCytomation) according to the manufacturer’s instructions. HRP activity was visualized with 3,3'-diaminobenzodine. Sections were lightly counterstained with methyl green or hematoxylin. Selected slides were stained with hemotoxylin and eosin to show morphology.
Quantification of immunopositive areas for pituitary hormones on Prop1 transgenic mouse pituitaries
Using immunohistochemical slides, individual pituitary hormone (PRL, GH, ACTH, αGSU, TSHβ, LHβ and FSHβ) positive cell areas and whole anterior pituitary areas (as background) were counted by two independent observers. Five fields at 25× magnification were randomly selected and counted using digital-image analyzing software, ImageJ 1.37v, developed at the National Institutes of Health, Bethesda, MD, USA.
Laser microdissection and RT-PCR
Tissue sections of 8 µm thickness were prepared from the same formalin fixed paraffin embedded tissue blocks and counterstained with toluidine blue. For the separation of the adenomas or hyperplastic cells in the Prop1 Tg pituitary sections, a laser capture assay was performed using a Laser Capture Microdissection system (LCM) (MMI Molecular Machines & Industries Inc, Rockledge, FL). Total RNA extraction was performed using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA), and RNA was reverse transcribed using the SuperScript First-Strand Synthesis System RT-PCR kit (Invitrogen Life Technologies) after incubation with proteinase K. RNase inhibitor (RNasin), SuperScript III reverse transcriptase, RNase-free DNase I and oligo (dT)12–18 primers were from Invitrogen Life Technologies. PCR was performed with AmpliTaq Gold PCR kits according to the manufacturer’s instructions, and each specific primer used was as follows: mouse PRL primers, 5'-AGC CCC CGA ATA CAT CCT AT-3' and 5'-ATC CCA TTT CCT TTG GCT TC-3'; mouse GH primers, 5'-TCC TCA GCA GGA TTT TCA CC-3' and 5'-CAT GTT GGC GTC AAA CTT GT-3' and mouse GAPDH primers, 5'-TGC GAC TTC AAC AGC AAC TC-3' and 5'-ATG TAG GCC ATG AGG TCC AC-3'. These primer sets were designed to span one intron to allow distinction of genomic contamination. cDNA samples for PCR were incubated for 50 cycles of PCR amplification on a Mastercycler thermal cycler (Eppendorf AG, Hamburg, Germany). The Prl, Gh and Gapdh PCR products were detected as bands of 117 bp, 173 bp and 143 bp, respectively. Moreover, quantitative PCR was performed using TaqMan Gene Expression Assays according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). The TaqMan probes for Mouse Prop1 (Mm00839471_m1) and b-actin (Mm00607939_s1) were obtained from Applied Biosystems. Quantitative real-time PCR was run for 50 cycles on an ABI Prism 7700 thermal cycler (Applied Biosystems).
III. Results
Changes in body weight and pituitary weight in Prop1 transgenic mice
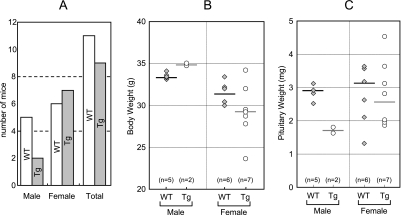
Prop1 transgenic mice were generated with mouse Prop1 genomic sequences under the control of the Cga promoter, which is active in the progenitor cells of Rathke’s pouch from e9.5 to e12.5 and, subsequently, activated in the gonadotrope and TSH producing cell (thyrotrope) [19]. Certain types of adenomas, hyperplastic and hypertrophic change in the anterior pituitary gland, arose in Prop1 transgenic mice at 1.5 years of age (Fig. 1A). Two of seven males and seven of thirteen female mice were the founders of the Prop1 transgene population. The body weight of transgenic males were similar or slightly greater than that of wild-type (WT) males; however, the weight of the pituitary was decreased (Fig. 1B, C). In contrast, no correlation between body weight and pituitary weight in transgenic or control female mice was observed.
Fig. 1.
Body and pituitary weights of aged transgenic mice. Two of seven male and seven of thirteen female mice were the founders of the Prop1 transgene population (A). In the male transgenic mice, body weights are significantly increased (B-left), while pituitary weights are decreased (C-left) when compared to that of WT mice. On the other hand, body and pituitary weights show no significant change in female transgenic mice (Tg) (B, C-right). WT, wild-type mice; Tg, Cga-Prop1 Tg; Gray diamond, data from WT; white circle, data from Prop1 Tg.
Prop1 transgene expression increases the incidence of pituitary adenomas
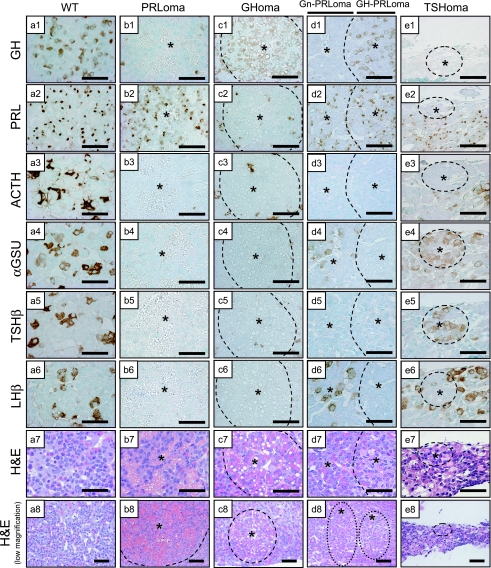
Analysis of all sections from Prop1 transgenic pituitaries confirmed the presence of pituitary adenomas and morphological changing in the anterior lobe of each gland. To determine the characteristics of these diseased pituitaries, sections were stained by immunohistochemistry using antibodies against each of the pituitary hormones. The adenomas were present in the background of only female pituitaries and in 5 out of 9 Prop1 transgenic mice (Table 1). Two cases of PRL producing adenomas (PRLomas) and one GH producing adenoma (GHoma) were observed (Fig. 2 b, c). Two cases exhibited focal acidophilic PRLomas undergoing angiogenesis. These tumors that produced PRL without other hormones in the cytoplasm demonstrated high vascularity (Fig. 2 b1–7). One GHoma with microvesicular fat did not produce other hormones, and this tumor was not vascularized (Fig. 2 c1–7). One case of a multihormonal tumor that including both a gonadotropin (Gn)-PRL double positive region (Gn-PRLoma) and a somatomammotroph (GH-PRL double positive) cell region (GH-PRLoma) was induced in a Prop1 transgenic pituitary (Fig. 2 d, Table 1 Tg No. 4). Moreover, one case of a small TSHβ and αGSU-positive adenoma (TSHoma) was induced without other pituitary hormones (Fig. 2 e). We designated these lesions as “adenomas” because the production of single hormones and/or nodules were featured in their pituitary pathology.
Table 1.
Diagnosis for Prop1 transgenic pituitary diseases
| Tg No. | sex | diagnosis | Hormones and Transcription factors in Prop1 transgenic pituitaries |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GH | PRL | αGSU | TSHβ | LHβ | FSHβ | ACTH | Pit1 | ER | GATA2 | SF1 | |||
| 1 | f | PRLoma | − | +++ | − | − | − | − | − | +++ | +++ | − | − |
| 2 | f | PRLoma | − | ++ | − | − | − | − | − | +++ | ++ | + | − |
| 3 | f | GHoma | ++ | − | − | − | − | − | − | +++ | + | − | − |
| 4 | f | Gn-PRLoma* | − | + | ++ | − | + | + | − | + | + | − | − |
| GH-PRLoma** | ++ | ++ | − | − | − | − | − | + | + | + | − | ||
| 5 | f | TSHoma | − | − | + | +++ | − | − | − | + | − | ++ | − |
| 6 | m | signet-ring*** | − | − | ++ | − | + | + | − | − | + | − | − |
| signet-ring | − | − | ++ | − | + | − | − | − | + | − | − | ||
| 7 | m | signet-ring | − | − | ++ | − | + | + | − | − | + | − | − |
| signet-ring | − | − | − | − | + | + | − | − | + | − | − | ||
| 8 | f | signet-ring | − | − | ++ | − | + | + | − | − | + | − | − |
| 9 | f | signet-ring | − | − | − | − | + | + | − | − | + | − | − |
Gn-PRLoma; Gonadotropin and PRL producing adenoma.
GH-PRLoma; Somatomammotroph adenoma.
signet-ring; Pituitary signet-ring cells.
Immunoreactivity: −; negative, +; less intense, ++; moderate, +++; intense.
Fig. 2.
Immunocharacterization of pituitary hormones in Prop1 transgenic adenomas. Light microscopy of coronal sections of a wild-type mouse pituitary (WT; a) and Prop1 Tg mice including PRLoma (b), Prop1 Tg including GHoma (c), Gn-PRLoma/GH-PRLoma (d) and small TSHoma (e). Immunostaining of GH, PRL, ACTH, αGSU, TSHβ, LHβ and H&E stain. All sections were stained by methyl green nuclear stain. Asterisk: adenoma region. Bars=50 µm.
Each adenoma expresses specific transcription factors
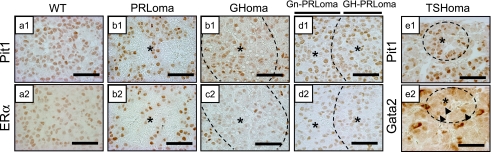
In order to further characterize these adenomas, sections were immunostained with specific antibodies against Pit1 (Fig. 3 a–e1) and ERα (Fig. 3 a–e2). Pit1 and ERα were both detected in the PRLoma and the multihormonal tumor (Gn-PRLoma/GH-PRLoma) (Fig. 3 b1, b2, d1, d2). In the GHoma, ERα expression was weakly positive compared with the surrounding region of the nodule (Fig. 3 c2). Gata2 was expressed in the nucleus of small TSHoma cells (Fig. 3 e2).
Fig. 3.
Immunocharacterization of transcription factors in Prop1 transgenic adenomas. Light microscopy of coronal sections of a wild-type mouse pituitary (WT; a) and Prop1 Tg mice including PRLoma (b), Prop1 Tg including GHoma (c), Gn-PRLoma/GH-PRLoma (d) and small TSHoma (e). Immunostaining of Pit1, ERα, Gata2 and H&E stain. Arrowhead: Gata2 expression in the nucleus of small TSHoma region, Asterisk: adenoma region. Bars=50 µm.
The Prl and Gh mRNA expression levels are different in Prop1 transgenic adenomas
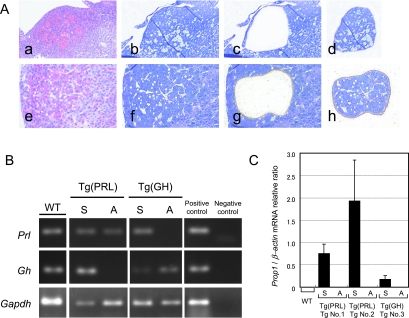
To confirm the results of the immunohistochemistry and image analyses, Prl and Gh mRNA accumulation in the adenomatous regions (Fig. 4A-d and h) was compared with the surrounding region (Fig. 4A-c and g) by laser microdissection (Fig. 4B). RT-PCR products of Prl and Gh mRNA were identified as 117 bp and 173 bp bands on 2% agarose gels, respectively. Prl expression was detected in all cases, except the GHoma (Fig. 4B, top). We observed Gh expression in WT pituitaries, GHoma and the surrounding pituitary regions of PRLoma, but Gh mRNA was not detected in the PRLoma (Fig. 4B, middle). These RT-PCR results are consistent with the patterns obtained by immunohistochemistry.
Fig. 4.
RT-PCR analysis of Prl, Gh and Prop1 expression in PRLomas and GHoma sampled by laser microdissection (LCM). Dividing between the adenoma and non-diseased pituitary by LCM (A). PRLoma (a–d) and GHoma (e–h) were identified in different Prop1 Tg. All sections were stained by H&E (a, e) and toluidine blue (b, f). Tissues were divided into adenomatous nodules (d, h) or these surrounding pituitary regions (c, g) by LCM. RT-PCR analysis of Gh and Prl (B). mRNA from LCM samples was reverse transcribed. RT-PCR analysis reveals Prl (117 bp), Gh (173 bp) and Gapdh (143 bp) fragments. The Cga-Prop1 Tg does not express Gh mRNA in PRLoma, nor Prl mRNA in GHoma, in agreement with the results of immunohistochemistry (Fig. 2). Quantitative RT-PCR analysis of Prop1 expression (C). Prop1 mRNA expressions were analyzed by quantitative RT-PCR. Prop1 is expressed in the hyperplastic surrounding regions of the adenomas. Prop1 expression is not observed in PRLoma and GHoma. M, 50 bp ladder marker; WT, wild-type; Tg (PRL), Prop1 Tg with PRLoma; Tg (GH), Prop1 Tg with GHoma; S, surrounding anterior pituitary region of adenoma; A, adenoma; Positive control, cDNA from normal fresh mouse pituitary; Negative control, non-template control.
Prop1 expression in adenomas and these surrounding pituitaries
To quantify the expression of Prop1 relative to the house-keeping gene β-actin, real-time RT-PCR was performed using TaqMan probes for Prop1. No Prop1 expression was detected in matched WT animals. Prop1 expression was elevated in the surrounding pituitaries of adenomas compared with the WT pituitaries. Prop1 expression, however, was not observed in any adenoma nodules of Prop1 transgenic mice (Fig. 4C).
Signet-ring like hypertrophic cells are gonadotropes
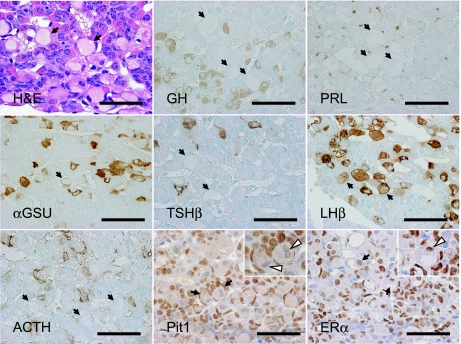
Four of nine transgenic pituitaries had regions of widespread hypertrophic signet-ring cells that were not present in non-transgenic controls (Fig. 5 H&E, Table 1, Tg No. 6–9). The pituitary signet-ring cells included one or two nuclei. αGSU, LHβ and FSHβ were diffusely immunopositive in the cytoplasm of pituitary signet-ring cells (data not shown in FSHβ). However, these immunoreactivities were weaker than those in non-disease pituitary gonadotroph cells. ERα was expressed, but Pit1 was not expressed in the pituitary signet-ring cells (Fig. 5, arrow).
Fig. 5.
Characterization of ‘pituitary signet-ring cells’ in Prop1 transgenic pituitary. H&E stain, immunostaining of GH, PRL, αGSU, TSHβ, LHβ, ACTH, Pit1 and ERα. All sections were counter-stained by methyl green or hematoxylin nuclear stain. Four cases of Prop1 Tg pituitaries reveal signet-ring hypertrophic gonadotropes. Arrow: pituitary signet-ring cells, white arrowhead: nuclei of signet-ring cells. Bars=50 µm.
Prop1 transgene expression induces hyperplastic changes in the surrounding anterior pituitaries of adenomas and of pituitary signet-ring cells
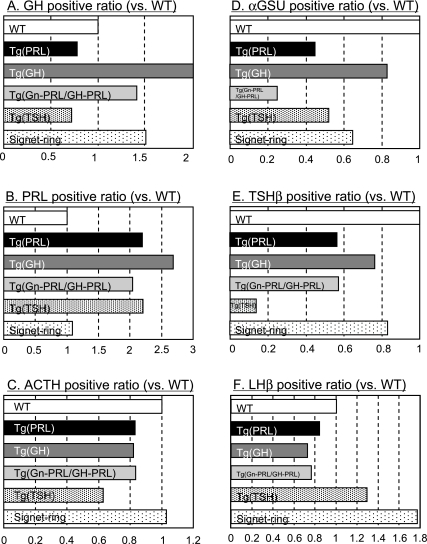
We also compared the hormone-positive areas of WT pituitaries with the surrounding pituitary cells of the adenomas or the pituitary signet-ring cells (as detected by immunostaining) in the Prop1 transgenic pituitaries. This was performed using digital-image analyzing software. In the surrounding region of the Tg pituitary gland, which contained GHoma, the GH-positive areas were approximately 1.8-fold larger than those of WT pituitaries or those of the Tg pituitary containing PRLoma (Fig. 6A). PRL positive areas in Prop1 Tg with GHoma or PRLoma were 2-fold larger than WT pituitary (Fig. 6B). LHβ positive areas were about 1.8-fold larger than WT pituitaries in the pituitaries which contained signet-ring cells (Fig. 6F). In contrast, ACTH positive areas in both Prop1 transgenic adenomas were similar to those in the WT pituitary. In the surrounding region of small TSHoma, TSH positive areas were smaller than those observed in WT pituitaries (Fig. 6E). αGSU-, TSHβ- and LHβ-positive areas in neoplastic pituitaries were less than that of WT pituitary (Fig. 6D, E). We described these pituitary changes as transgenic pituitary adenomas as “hyperplasia”.
Fig. 6.
Quantification of hormone positive regions in the surrounding anterior pituitaries of adenomas and of pituitary signet-ring cells. Quantification of immunopositive areas for individual pituitary hormones (A: GH, B: PRL, C: ACTH, D: αGSU, E: TSHβ and F: LHβ) were measured using ImageJ analyzer. Data were normalized to areas of the surrounding pituitaries of the adenomas or the signet-ring gonadotropes in the same field (relative values vs. WT) from Prop1 Tg pituitaries. WT, wild-type mouse pituitary; Tg(PRL), surrounding region of Prop1 Tg PRLoma; Tg(GH), surrounding region of Prop1 Tg GHoma; Tg(Gn/GH-PRL), surrounding region of Prop1 Tg Gn-PRLoma/GH-PRLoma case; Tg(TSH), surrounding region of Prop1 Tg TSHoma; signet-ring, surrounding region of signet-ring gonadotroph cells.
IV. Discussion
In the present study, mice overexpressing Prop1 under the control of the Cga promoter tended to develop pituitary adenomas. Persistent Prop1 expression has been shown to induce tumors with non-hormonal nodules or a TSH-producing adenoma in aged Tg mice [6]. Moreover, Prop1 is also expressed in the dorsal area of Rathke’s pouch, which was shown to be a proliferating region in mouse pituitary development [30]. Here, we report that all Prop1 transgenes clearly induced pituitary adenomas or the pituitary signet-ring cells. Therefore, these results suggest that persistent Prop1 overexpression may lead to dysregulated pituitary cell proliferation and function.
Prop1 binds to early enhancer sites of the Pit1 gene [10]. Pit1 is a critical regulator of GH production and somatotroph cell differentiation [11, 20, 23]. In our study, the PRLoma in the Prop1 Tg pituitary was vascularized in a manner similar to the estrogen-inducible PRL-producing tumors in rodents [12]. Estrogen may act directly through ERα and β, and regulate expression of the pituitary tumor-derived transforming gene (Pttg), which is known as an angiogenic mechanism in pituitary tumors. Pttg expression coincides with the early lactotrophic hyperplastic response, angiogenesis and PRLoma development [13]. Together, these results suggest that PRLomas form synchronously with angiogenesis in the development of tumorigenesis in Prop1 Tg pituitary.
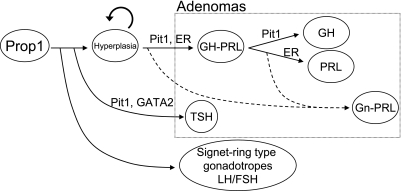
Transcription factors and synergistic co-factors, including Prop1, Pit1, Gata2 [4, 7], Sf1 [45], Tpit [40] and several hypothalamic releasing hormone receptors [18, 22], are required for the determination of cell phenotypes and lineage-specific cell proliferation. PRL expression and lactotroph cell differentiation are regulated by the synergistic effects of Pit1 and ERα [33, 44]. According to our immunohistochemical data, PRLomas of aged Prop1 Tg were positive for both Pit1 and ERα. GHoma in a Prop1 Tg was Pit1-positive, but only very weakly ERα immunoreactive in our study. Activation of the Prop1-Pit1-ERα or Prop1-Pit1 sequence may correlate to the differentiation of PRL- or GH-producing adenoma, respectively [5]. Pit1 and Gata2 were expressed in the nucleus of a small TSHoma (Fig. 3 e1, 2). Synergic function of Pit1 and Gata2 leads the expression of TSHβ [7, 24]. In human growth hormone-releasing hormone (hGHRH) Tg, Pit1 overexpression has been suggested to result in adenomas through a “hyperplasia-adenoma” sequence [26, 38]. The regions of both GH-producing cells in the surrounding pituitary regions of GHomas and PRL-producing cells in the surrounding pituitary regions of PRLomas from Prop1 Tg pituitary was larger than that of WT pituitaries (Fig. 6A, B). Therefore, the surrounding pituitary cells of these adenomas were thought to be at an early stage in the “hyperplasia-adenoma” sequence in Prop1 Tg. In the pituitary signet-ring cells, the LHβ positive region was larger than that of WT pituitaries (Fig. 6F), hence we designated this as “hyperplasia” based on pituitary pathology. We suggest that the tumorigenesis occurred during the transition from normal to hyperplasia to adenoma in the Prop1 Tg pituitaries (Fig. 7).
Fig. 7.
A scheme of the hyperplasia-adenoma sequence and the formation of signet-ring cells in Prop1 transgenic mouse pituitary. Tumorigenesis occurs through the sequence of normal to hyperplasia to adenoma in the Prop1 Tg pituitaries. The pituitary signet-ring cells are derived from a type of gonadotroph.
Transcription factors are divided into two groups: transcription factors involved in early development and transcription factors involved in later functional differentiation. Prop1 is included in both of these categories. Its expression leads to the ontogenesis of pituitary gonadotropes, as well as somatotropes, lactotropes, and caudomedial thyrotropes in mouse studies [25, 42]. Additionally, as we report here, Prop1 has a role not only in tumorigenesis, but also in pituitary cell differentiation in the Prop1 Tg model.
αGSU is one of the earliest markers of anterior pituitary development. The Prop1 positive ventral pituitary region is known to differentiate as other hormone-producing quiescent cells arise with pituitary development [35]. In PRLoma or GHoma of the Prop1 Tg mice, there was no expression of Prop1 mRNA. Prop1 transgene expression is dependent on Cga promoter activation in these mice, but this may be attenuated with progression of monohormonal (PRL or GH-producing) adenomatous differentiation. However, αGSU was expressed in small TSHoma and Gn-PRLoma. Prop1 is thought to be a critical initiation factor for Pit1 lineage differentiation. It is known that GH- and PRL-cell differentiations are induced after TSH-cell differentiation in the Pit1 lineage [29]. The absence of Prop1 may be induced after differentiation of the TSH lineage and Gn cell lineage in the Prop1 Tg pituitary.
In half of all Prop1 Tg mice, pituitary signet-ring cells were observed and expressed gonadotropins consisting of αGSU, LHβ and FSHβ. The signet-ring cells also expressed the transcription factor, ERα, without Gata2 and Sf1. It is indicated that the pituitary signet-ring cells occur in the gonadotropin producing cells, but may not be typical because Gata2 and Sf1 are known to activate the expression of gonadotropic hormones [4, 45]. These signet-ring cells are morphologically similar to those of typical castration cells [2, 14]. The possibilities include that these changes may be interpreted to be due to the feedback response to the physiologically gonadectomized condition of the aged Prop1 Tg mice. It has been also reported that the signet-ring changes may also be associated with tumor behavior such as invasion [9]. Interestingly, the signet-ring cells were prominent in two human pituitary adenoma cases, GHoma and clinically non-functioning adenoma [9, 17]. Further ultrastructural studies are necessary to clarify the character of the pituitary signet-ring cells in Prop1 Tg mice.
In summary, based on these experimental studies, we propose that constitutively expressed Prop1 contributes to the development of Pit1-lineage adenomas (PRLoma, GHoma and TSHoma) in Prop1 Tg female mice through the “normal-hyperplasia-adenoma” sequence only. PRL and GH differentiation may be dependent on activation by a Pit1-ERα combination or Pit1 only, respectively, while TSH differentiation may be dependent on the synergistic function between Pit1 and Gata2. Prop1 may be related to the differentiated Pit1-lineage adenoma and the levels of expression may act as a critical regulator of Pit1-lineage hormones since PRL expression depends on ERα and TSHβ expression depends on Gata2 [41]. In addition, persistent Prop1 expression led to the development of a multihormonal adenoma (Gn-PRLoma/GH-PRLoma). Thus, we conclude that Prop1 acts as an important proliferation and differentiation factor in the hyperplasia-adenoma and the hyperplasia-pituitary signet-ring cell sequence in pituitary cells of Prop1 Tg. The absence of Prop1 in adenomas and the formation of pituitary signet-ring cells remain for further investigation.
V. Acknowledgements
We thank Dr. Johbu Itoh (Tokai University School of Medicine Teaching and Research Support Center) for technical assistance, and Dr. Hanako Kajiya (Tokai University) and Dr. Lori T. Raetzman (University of Illinois, Urbana-Champaign, IL, USA) for their expert advice. We are grateful to Dr. Albert F. Parlow for antibodies, the US National Hormone and Pituitary Program (NIDDK), and NTC/NIPPN TechnoCluster, Inc. for laser microdissection assays. This work was supported by a Grant-in-Aid for Scientific Research Projects (#16390110) of the Japanese Ministry of Education, Culture, Sports, Science and Technology, by the Research on Measures for Intractable Diseases Project of the Hypothalamo-Pituitary Dysfunction Research Group of the Japanese Ministry of Health, Labor and Welfare, and by a Grant from the Tokai University School of Medicine Research Aid (2005–2007).
VI. References
- 1.Agarwal G., Bhatia V., Cook S., Thomas P. Q. Adrenocorticotropin deficiency in combined pituitary hormone deficiency patients homozygous for a novel PROP1 deletion. J. Clin. Endocrinol. Metab. 2000;85:4556–4561. doi: 10.1210/jcem.85.12.7013. [DOI] [PubMed] [Google Scholar]
- 2.Akazawa N., Taniguchi K., Mikami S. Effects of vitamin A deficiency on the function of pituitary-gonadal system in male rats. Jpn. J. Vet. Sci. 1989;51:1209–1217. doi: 10.1292/jvms1939.51.1209. [DOI] [PubMed] [Google Scholar]
- 3.Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988;55:505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- 4.Charles M. A., Saunders T. L., Wood W. M., Owens K., Parlow A. F., Camper S. A., Ridgway E. C., Gordon D. F. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol. Endocrinol. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- 5.Chuang F. M., West B. L., Baxter J. D., Schaufele F. Activities in Pit-1 determine whether receptor interacting protein 140 activates or inhibits Pit-1/nuclear receptor transcriptional synergy. Mol. Endocrinol. 1997;11:1332–1341. doi: 10.1210/mend.11.9.9978. [DOI] [PubMed] [Google Scholar]
- 6.Cushman L. J., Watkins-Chow D. E., Brinkmeier M. L., Raetzman L. T., Radak A. L., Lloyd R. V., Camper S. A. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum. Mol. Genet. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- 7.Dasen J. S., O’Connell S. M., Flynn S. E., Treier M., Gleiberman A. S., Szeto D. P., Hooshmand F., Aggarwal A. K., Rosenfeld M. G. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97:587–598. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- 8.Deladoey J., Fluck C., Buyukgebiz A., Kuhlmann B. V., Eble A., Hindmarsh P. C., Wu W., Mullis P. E. “Hot spot” in the PROP1 gene responsible for combined pituitary hormone deficiency. J. Clin. Endocrinol. Metab. 1999;84:1645–1650. doi: 10.1210/jcem.84.5.5681. [DOI] [PubMed] [Google Scholar]
- 9.Deniz K., Tanriverdi F., Selcuklu A., Kontas O., Kelestimur F. Signet ring-like cells in pituitary adenoma. Pathol. Res. Pract. 2008;204:209–212. doi: 10.1016/j.prp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.DiMattia G. E., Rhodes S. J., Krones A., Carriere C., O’Connell S., Kalla K., Arias C., Sawchenko P., Rosenfeld M. G. The Pit-1 gene is regulated by distinct early and late pituitary-specific enhancers. Dev. Biol. 1997;182:180–190. doi: 10.1006/dbio.1996.8472. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Puig C., Seoane S., Blanco M., Macia M., Garcia-Caballero T., Segura C., Perez-Fernandez R. Pit-1 is expressed in normal and tumorous human breast and regulates GH secretion and cell proliferation. Eur. J. Endocrinol. 2005;153:335–344. doi: 10.1530/eje.1.01962. [DOI] [PubMed] [Google Scholar]
- 12.Gomez O., Balsa J. A. Autocrine/paracrine action of pituitary vasoactive intestinal peptide on lactotroph hyperplasia induced by estrogen. Endocrinology. 2003;144:4403–4409. doi: 10.1210/en.2003-0261. [DOI] [PubMed] [Google Scholar]
- 13.Heaney A. P., Horwitz G. A., Wang Z., Singson R., Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat. Med. 1999;5:1317–1321. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim S. N., Moussa S. M., Childs G. V. Morphometric studies of rat anterior pituitary cells after gonadectomy: correlation of changes in gonadotropes with the serum levels of gonadotropins. Endocrinology. 1986;119:629–637. doi: 10.1210/endo-119-2-629. [DOI] [PubMed] [Google Scholar]
- 15.Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55:519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- 16.Ingraham H. A., Lala D. S., Ikeda Y., Luo X., Shen W. H., Nachtigal M. W., Abbud R., Nilson J. H., Parker K. L. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes. Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 17.Ironside J. W., Jefferson A. A., Timperley W. R. Growth hormone-secreting pituitary adenoma of mixed cell type: a histological, ultrastructural and immunocytochemical study. Clin. Neuropathol. 1986;5:28–33. [PubMed] [Google Scholar]
- 18.Kaiser U. B., Sabbagh E., Chen M. T., Chin W. W., Saunders B. D. Sp1 binds to the rat luteinizing hormone beta (LHbeta) gene promoter and mediates gonadotropin-releasing hormone-stimulated expression of the LHbeta subunit gene. J. Biol. Chem. 1998;273:12943–12951. doi: 10.1074/jbc.273.21.12943. [DOI] [PubMed] [Google Scholar]
- 19.Kendall S. K., Gordon D. F., Birkmeier T. S., Petrey D., Sarapura V. D., O’Shea K. S., Wood W. M., Lloyd R. V., Ridgway E. C., Camper S. A. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone alpha-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Mol. Endocrinol. 1994;8:1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- 20.Kurotani R., Yoshimura S., Iwasaki Y., Inoue K., Teramoto A., Osamura R. Y. Exogenous expression of Pit-1 in AtT-20 corticotropic cells induces endogenous growth hormone gene transcription. J. Endocrinol. 2002;172:477–487. doi: 10.1677/joe.0.1720477. [DOI] [PubMed] [Google Scholar]
- 21.Lamolet B., Pulichino A. M., Lamonerie T., Gauthier Y., Brue T., Enjalbert A., Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 22.Lin C., Lin S. C., Chang C. P., Rosenfeld M. G. Pit-1-dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature. 1992;360:765–768. doi: 10.1038/360765a0. [DOI] [PubMed] [Google Scholar]
- 23.Miyai S., Itoh J., Kajiya H., Takekoshi S., Osamura R. Y. Pit-1 gene inhibition using small interfering RNAs in rat pituitary GH secreting cell line. Acta Histochem. Cytochem. 2005;38:107–114. [Google Scholar]
- 24.Nakano K., Matsushita A., Sasaki S., Misawa H., Nishiyama K., Kashiwabara Y., Nakamura H. Thyroid-hormone-dependent negative regulation of thyrotropin beta gene by thyroid hormone receptors: study with a new experimental system using CV1 cells. Biochem. J. 2004;378:549–557. doi: 10.1042/BJ20031592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasonkin I. O., Ward R. D., Raetzman L. T., Seasholtz A. F., Saunders T. L., Gillespie P. J., Camper S. A. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum. Mol. Genet. 2004;13:2727–2735. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- 26.Osamura R. Y., Oda K., Utsunomiya H., Inada K., Umemura S., Shibuya M., Katakami H., Voss J. W., Mayo K. E., Rosenfeld M. G. Immunohistochemical expression of PIT-1 protein in pituitary glands of human GRF transgenic mice: its relationship with hormonal expressions. Endocr. J. 1993;40:133–139. doi: 10.1507/endocrj.40.133. [DOI] [PubMed] [Google Scholar]
- 27.Oyama K., Sanno N., Teramoto A., Osamura R. Y. Expression of neuro D1 in human normal pituitaries and pituitary adenomas. Mod. Pathol. 2001;14:892–899. doi: 10.1038/modpathol.3880408. [DOI] [PubMed] [Google Scholar]
- 28.Poulin G., Turgeon B., Drouin J. NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol. Cell. Biol. 1997;17:6673–6682. doi: 10.1128/mcb.17.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radian S., Coculescu M., Morris J. F. Somatotroph to thyrotroph cell transdifferentiation during experimental hypothyroidism—a light and electron-microscopy study. J. Cell. Mol. Med. 2003;7:297–306. doi: 10.1111/j.1582-4934.2003.tb00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raetzman L. T., Ward R., Camper S. A. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- 31.Riepe F. G., Partsch C. J., Blankenstein O., Monig H., Pfaffle R. W., Sippell W. G. Longitudinal imaging reveals pituitary enlargement preceding hypoplasia in two brothers with combined pituitary hormone deficiency attributable to PROP1 mutation. J. Clin. Endocrinol. Metab. 2001;86:4353–4357. doi: 10.1210/jcem.86.9.7828. [DOI] [PubMed] [Google Scholar]
- 32.Sanno N., Teramoto A., Matsuno A., Takekoshi S., Itoh J., Osamura R. Y. Expression of Pit-1 and estrogen receptor messenger RNA in prolactin-producing pituitary adenomas. Mod. Pathol. 1996;9:526–533. [PubMed] [Google Scholar]
- 33.Schaufele F. Regulation of estrogen receptor activation of the prolactin enhancer/promoter by antagonistic activation function-2-interacting proteins. Mol. Endocrinol. 1999;13:935–945. doi: 10.1210/mend.13.6.0298. [DOI] [PubMed] [Google Scholar]
- 34.Sloop K. W., McCutchan Schiller A., Smith T. P., Blanton J. R., Jr, Rohrer G. A., Meier B. C., Rhodes S. J. Biochemical and genetic characterization of the porcine Prophet of Pit-1 pituitary transcription factor. Mol. Cell. Endocrinol. 2000;168:77–87. doi: 10.1016/s0303-7207(00)00318-x. [DOI] [PubMed] [Google Scholar]
- 35.Sornson M. W., Wu W., Dasen J. S., Flynn S. E., Norman D. J., O’Connell S. M., Gukovsky I., Carriere C., Ryan A. K., Miller A. P., Zuo L., Gleiberman A. S., Andersen B., Beamer W. G., Rosenfeld M. G. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 36.Steger D. J., Hecht J. H., Mellon P. L. GATA-binding proteins regulate the human gonadotropin alpha-subunit gene in the placenta and pituitary gland. Mol. Cell. Biol. 1994;14:5592–5602. doi: 10.1128/mcb.14.8.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahara S., Kurotani R., Sanno N., Takumi I., Yoshimura S., Osamura R. Y., Teramoto A. Expression of pituitary homeo box 1 (Ptx1) in human non-neoplastic pituitaries and pituitary adenomas. Mod. Pathol. 2000;13:1097–1108. doi: 10.1038/modpathol.3880204. [DOI] [PubMed] [Google Scholar]
- 38.Umemura S., Oda K., Utsunomiya H., Sanno N., Itoh J., Katakami H., Osamura R. Y. Immunohistochemical characterization of “hyperplasia-adenoma sequence” in the pituitaries of transgenic mice expressing a human growth hormone-releasing factor gene. Tokai. J. Exp. Clin. Med. 1995;20:71–79. [PubMed] [Google Scholar]
- 39.Umeoka K., Sanno N., Osamura R. Y., Teramoto A. Expression of GATA-2 in human pituitary adenomas. Mod. Pathol. 2002;15:11–17. doi: 10.1038/modpathol.3880484. [DOI] [PubMed] [Google Scholar]
- 40.Vallette-Kasic S., Brue T., Pulichino A. M., Gueydan M., Barlier A., David M., Nicolino M., Malpuech G., Dechelotte P., Deal C., Van Vliet G., De Vroede M., Riepe F. G., Partsch C. J., Sippell W. G., Berberoglu M., Atasay B., de Zegher F., Beckers D., Kyllo J., Donohoue P., Fassnacht M., Hahner S., Allolio B., Noordam C., Dunkel L., Hero M., Pigeon B., Weill J., Yigit S., Brauner R., Heinrich J. J., Cummings E., Riddell C., Enjalbert A., Drouin J. Congenital isolated adrenocorticotropin deficiency: an underestimated cause of neonatal death, explained by TPIT gene mutations. J. Clin. Endocrinol. Metab. 2005;90:1323–1331. doi: 10.1210/jc.2004-1300. [DOI] [PubMed] [Google Scholar]
- 41.Voss J. W., Rosenfeld M. G. Anterior pituitary development: short tales from dwarf mice. Cell. 1992;70:527–530. doi: 10.1016/0092-8674(92)90422-9. [DOI] [PubMed] [Google Scholar]
- 42.Ward R. D., Raetzman L. T., Suh H., Stone B. M., Nasonkin I. O., Camper S. A. Role of PROP1 in pituitary gland growth. Mol. Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- 43.Wu W., Cogan J. D., Pfaffle R. W., Dasen J. S., Frisch H., O’Connell S. M., Flynn S. E., Brown M. R., Mullis P. E., Parks J. S., Phillips J. A., 3rd, Rosenfeld M. G. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat. Genet. 1998;18:147–149. doi: 10.1038/ng0298-147. [DOI] [PubMed] [Google Scholar]
- 44.Ying C., Lin D. H. Estrogen-modulated estrogen receptor x Pit-1 protein complex formation and prolactin gene activation require novel protein synthesis. J. Biol. Chem. 2000;275:15407–15412. doi: 10.1074/jbc.275.20.15407. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L., Bakke M., Krimkevich Y., Cushman L. J., Parlow A. F., Camper S. A., Parker K. L. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–154. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]