Abstract
Successful adaptation to changes in an animal's emotional and motivational environment depends on behavioral flexibility accompanied by changes in bodily responses, e.g., autonomic and endocrine, which support the change in behavior. Here, we identify the orbitofrontal cortex (OFC) as pivotal in the flexible regulation and coordination of behavioral and autonomic responses during adaptation. Using an appetitive Pavlovian task, we demonstrate that OFC lesions in the marmoset (i) impair an animal's ability to rapidly suppress its appetitive cardiovascular arousal upon termination of a conditioned stimulus and (ii) cause an uncoupling of the behavioral and autonomic components of the adaptive response after reversal of the reward contingencies. These findings highlight the role of the OFC in emotional regulation and are highly relevant to our understanding of disorders such as schizophrenia and autism in which uncoupling of emotional responses may contribute to the experiential distress and disadvantageous behavior associated with these disorders.
Keywords: behavioral inhibition, emotion, reversal learning
The orbitofrontal cortex (OFC) has long been implicated in behavioral flexibility as measured by tests of discrimination reversal learning and extinction (1, 2). In reversal learning, an animal is first taught to respond to one of two visual stimuli to receive food reward, a response to the other being unrewarded. Lesions of the OFC do not affect initial acquisition of the visual discrimination, but the ability to alter responding when the association between the stimuli and reward is reversed is markedly impaired across a range of species (3–9). Similarly, animals with OFC lesions display prolonged responding during extinction when the response no longer results in the receipt of food reward (1, 10, 11). However, an alteration in behavioral output is just one component of the overall adaptive response of an animal to changes in its environment. It is important to recognize that behavioral adaptation is accompanied by alterations in the bodily state, including autonomic and endocrine activity, appropriate to the motivational and emotional context. Thus, Pavlov showed that dogs stopped salivating to a buzzer when it no longer predicted reward (12), and, had the behavioral response also been measured, it would have presumably shown that they stopped approaching the buzzer too. Indeed, if, despite inhibiting their salivation during extinction, Pavlov's dogs found themselves still approaching the buzzer, or vice versa, such incongruency between the somatic and autonomic feedback might be expected to produce emotional ambiguity, an issue that will be considered in more detail in Discussion.
Currently, we have very little understanding of the neural circuitry underlying the coordination of behavioral and bodily responses in adaptive responding. Although the OFC is critical for behavioral adaptation, its role in the overall coordination of the adaptive response is unknown. Indeed, few studies have investigated the role of the OFC in the regulation of autonomic and other bodily responses (13–16), despite evidence from anatomical studies that this region has the appropriate connections both at the level of the hypothalamus and brainstem (17–21). Thus, the present study investigated the effects of excitotoxic lesions of the OFC in the marmoset monkey on the adaptation of behavioral and cardiovascular responses [blood pressure (BP) and heart rate (HR)] in two distinct contexts: first, after the unexpected termination of an appetitive conditioned stimulus (CS) and the subsequent failure to receive food reward (single trial extinction) and second, after reversal of the reward contingencies (Pavlovian discrimination reversal).
Results
Conditioned Appetitive Cardiovascular and Behavioral Responses Do Not Depend on the OFC for Their Expression.
We first determined whether lesions of the OFC would disrupt the expression of conditioned cardiovascular and behavioral responses in marmosets. After habituation to the test apparatus and implantation of a biotelemetric probe [see supporting information (SI) Text], all subjects were trained on an appetitive Pavlovian conditioning task (Fig. 1). Each session consisted of one to three presentations of an auditory stimulus (tone or white noise). Twenty seconds after the onset of each stimulus presentation (CS period), the monkeys gained access to a box (US period, 120 s) that was either full of high-incentive food (+) or was empty (−). The auditory stimulus continued to sound throughout the US period, and each CS/US pairing was separated by a variable intertrial interval (70–110 s). A session could include both CS/US− and CS/US+ trials, but the latter occurred only as the last trial in a session and in only 5 of every 10 sessions. Behavioral measurements included head jerking, an orienting response shown specifically to auditory appetitive CSs (refs. 21 and 55; see Methods for details).
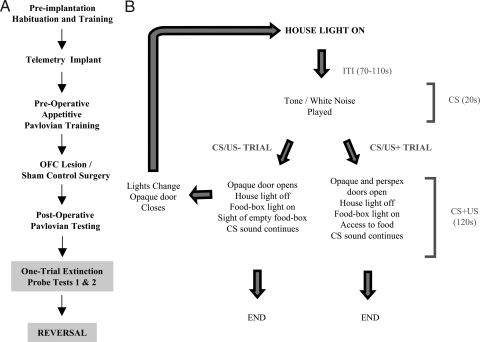
Fig. 1.
Task design. (A) A flow diagram depicting the various stages of training. Habituation to the testing apparatus lasted for 2–3 weeks. In between the two, one-trial extinction tests and the subsequent contingency reversal, animals received reminder conditioning sessions. (B) A schematized illustration of the events in a typical Pavlovian session in which animals learned to associate a 20-s tone or white noise (CS) with a subsequent 120-s period in which they had access to highly palatable food (appetitive US; US+) or an empty food box (US−). Marmosets were trained 5 days per week, one session per day. Sessions consisted of a single CS/US− or CS/US+ trial, two or three CS/US− trials or one or two CS/US− and one CS/US+ trial. See SI Text for details.
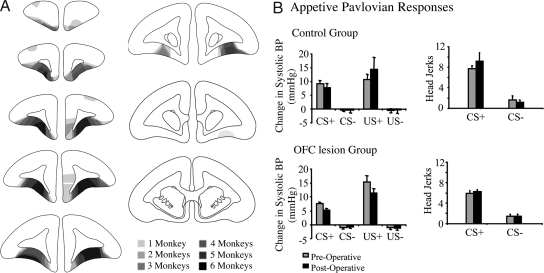
The mean number of CS+ and CS− trials, respectively, before reaching stable, conditioned BP (systolic) and behavioral (head jerking) responses to the appetitive CS was 13 ± 2 and 21.4 ± 3 for the to-be sham-lesioned control group and 17.5 ± 3 and 22.7 ± 3 for the to-be OFC-lesioned group. All animals then received either a bilateral, excitotoxic lesion of the OFC (n = 6) or sham-control surgery (n = 5; see Methods and Fig. 2A for lesion analysis). Postoperatively, animals with OFC lesions exhibited a similar expression of conditioned autonomic and behavioral responses as they did preoperatively and a similar level to that of controls postoperatively (Fig. 2B). Three-way ANOVAs [condition(CS+/CS−) × group(lesion/control) × surgery (pre/post)] revealed that all groups showed a significant main effect of condition during both the CS period [F(1, 9) = 6.3, P = 0.03] and US period [F(1, 9) = 160.2, P < 0.001] for systolic BP, but there were no effects of group after surgery (Fs <1). The same pattern of effects was seen for diastolic BP and HR (Table S1). CS-directed head-jerking behavior also showed a main effect of condition [F(1, 9) = 165.3, P < 0.001] but no other significant effects. There were no differences between the groups in the latency to eat the food or in the amount eaten (Table S2). However, marked differences between the control and OFC- lesioned groups emerged during subsequent tests of emotion regulation, i.e., extinction and reversal.
Fig. 2.
Lesion analysis and effects of OFC lesions on the expression of conditioned autonomic and behavioral responses. (A) Schematic diagram of a series of coronal sections through the frontal lobe of the marmoset illustrating the extent of damage in all six of the OFC lesioned monkeys. The different levels of shading, ranging from solid black to pale gray, represent the areas of cortex that were damaged in one (palest shading), two, three, four, five, and six (darkest shading) marmosets, respectively. In general, OFC damage extended from just caudal to the frontal pole to just rostral to the genu of the corpus callosum. In most animals, the majority of the dysgranular portion of the OFC was damaged, sparing the lateral granular regions (see ref. 21 for detailed cytoarchitectonic description of this region). In two of the animals, there was damage to the ventromedial convexity, primarily on one side only, and two of the six animals also had variable damage to the medial wall, unilaterally. (B) Histograms of the mean changes in systolic BP for control and OFC-lesioned animals during the 20-s CS+/CS− period (compared with the immediately preceding 20-s baseline period) and during the first 60 s of the US+/US− period (compared with the 20-s CS period) are shown on the Left. Histograms of mean conditioned behavior (head jerks) during the CS+/CS− (compared with baseline) are shown on the Right.
OFC Lesions Prolong Autonomic Arousal After CS Termination and Reward Omission.
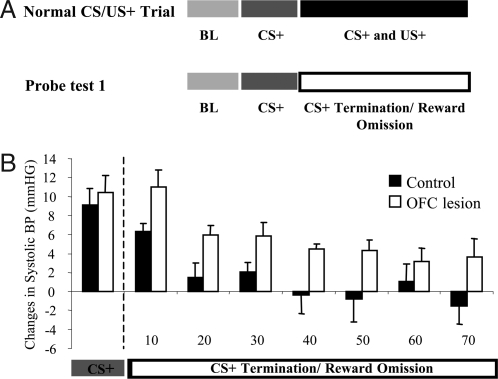
To determine the role of OFC in the regulation of conditioned autonomic arousal, BP and HR levels were monitored during a one-trial extinction session in which a single CS+ unexpectedly terminated after 20 s, and reward was omitted (probe test 1; Fig. 3A). Control animals exhibited a rapid decline in cardiovascular arousal (systolic BP) with levels returning to baseline after approximately 30 s (Fig. 3B). In contrast, the arousal levels of animals with OFC lesions remained significantly elevated over the 70-s period of recording. Two-way ANOVA [group × time bin (7 × 10 s)] of the systolic BP across the US omission period revealed a main effect of Group [F(1, 9) = 6.8, P = 0.03]. There was also a main effect of time bin [F(6, 54) = 11, P < 0.001), whereby appetitive systolic BP responses in both groups tended to decline over the seven 10-s time-bins after CS termination. No other significant effects were observed (Fs <1). The same pattern of prolonged arousal was also seen in the measures of diastolic BP and HR, although the HR did not reach significance (Table S3). Very little behavior was observed during this period in either group (see SI Text). In particular, neither group displayed head jerking upon termination of the CS+, because head jerking is seen only in the presence of a CS. In a second, one-trial extinction session, after the first 20 s of the CS+, the auditory stimulus was not terminated as in probe test 1 but was maintained for an additional 120 s (the normal length of the US period), still in the absence of reward (total playing time, 140 s; probe test 2). Performance in this session did not differ between the groups (Fs <1, ANOVA, Table S4). Instead, the presence of the CS+ throughout the US omission period resulted in maintained systolic BP arousal in both groups. Thus, these findings demonstrate that marmosets with excitotoxic lesions of the OFC show markedly slower cardiovascular recovery in response to the termination of a conditioned appetitive stimulus. It is unlikely that this slowed recovery reflects an enhanced frustration to the loss of expected reward compared with controls because there were no differences between the groups when the CS remained on for the entire US period, despite omission of the reward (probe test 2). Moreover, there is little evidence in the literature to link selective damage to the OFC with enhanced frustration, monkeys with OFC lesions, if anything, exhibit less frustrative behavior (11).
Fig. 3.
Effects of OFC lesions on one-trial extinction. (A) A schematic illustration of a typical rewarded (CS+) trial in which animals, after the first 20 s of an auditory CS, received access to high-incentive food for 2 min. After surgery, animals were exposed to a single, one-trial extinction probe session in which the auditory CS+ was terminated after 20 s, and there was no access to the food box (probe test 1). (B) Histogram illustrating the prolonged arousal in OFC-lesioned animals (open bars, n = 6) compared with controls (solid bars, n = 5) after the termination of the CS+ and omission of the reward in probe test 1. Systolic BP (change from BL) was measured during the CS period and for a subsequent 70-s, “No-US” period.
OFC Is Critical for the Coordination of Behavioral and Autonomic Output During Reversal Learning.
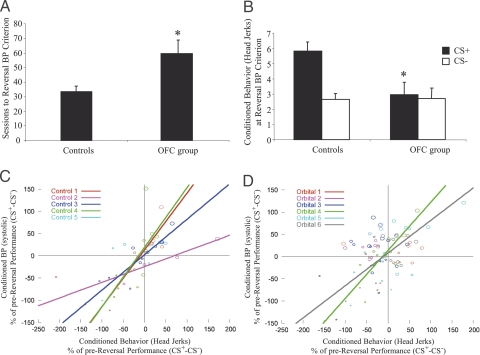
To determine the role of OFC in the coordination of adaptive responses, control and OFC-lesioned groups were subjected to a reversal of the reward contingencies, such that the previously rewarded stimulus was no longer rewarded, and the previously unrewarded stimulus became rewarded. All animals were tested on this reversal task until their systolic BP responses to the new CS+ were significantly greater than their systolic BP responses to the new CS− (BP reversal criterion; see Fig. 4 legend and SI Text for details). Compared with controls, animals with OFC lesions were far slower to reverse their conditioned BP, requiring significantly more sessions to reach criterion than controls [Group, F(1, 9) = 6.2, P = 0.03; Fig. 4A], although they took the same number of sessions to reach “chance” performance (SI Text and results in Table S5). However, having reached criterion, they showed comparable levels of conditioned BP to controls (controls: CS+, 3.3 ± 0.4; CS−, −0.2 ± 0.5; OFC lesions: CS+, 4.0 ± 0.4; CS−, 1.0 ± 0.1). They also showed comparable latency and consumption measures to controls (Table S6).
Fig. 4.
Effects of OFC lesions on Pavlovian reversal. (A) After reversal of the reward contingencies, OFC-lesioned animals took a greater number of sessions to reach reversal BP criterion (mean systolic BP response across six consecutive CS+ trials was significantly greater than that for the intervening (6–14) CS− trials at P < 0.02) compared with controls. (B) At reversal BP criterion, OFC lesioned animals displayed significantly impaired behavioral responses to the new CS+ compared with controls. (C and D) Correlation graphs depicting behavioral (head jerk) and systolic BP responses across reversal for control and OFC-lesioned animals, respectively. All data points are difference measures between CS+ and CS− responses during the reversal calculated as a percentage of prereversal performance. Each point (circle) reflects the mean of five sessions (final data point: mean of 3–7 sessions) with small and large circles reflecting performance earlier and later in reversal, respectively. Lines represent significant correlations between behavior and BP in individual animals. Asterisks in A and B indicate that the lesioned group is significantly different from controls.
Reversal of the conditioned BP in the control group was accompanied by reversal of their conditioned behavioral response. In contrast, when the conditioned BP of the animals with OFC lesions reflected the new contingencies, their conditioned behavior did not (Fig. 4B). Two-way ANOVA of the behavior (at BP reversal criterion) showed an overall condition effect [F(1, 9) = 10.9, P < 0.01] and a group × condition interaction [F(1, 9) = 7.9, P = 0.02]. Further analysis verified that OFC lesions significantly impaired behavioral responses to the new CS+ [F(1, 9) = 7.3, P = 0.02] but not to the new CS− (F <1). These findings suggested that the conditioned behavioral and autonomic responses in the OFC-lesioned group had become uncoupled. Thus, correlational analyses were performed on the behavioral and BP responses of each animal across the reversal (see Methods). ANOVA of the correlation coefficients (r′; r transformed) showed significantly reduced correlation coefficients in the lesioned group compared with controls [mean values ± SEM, Control: 1.06 ± 0.17; OFC lesion: 0.45 ± 0.14; Group, F(1, 9) = 7.97, P = 0.02; individual r values in Table S7], confirming that the OFC lesion had significantly weakened the coupling between these different response outputs. This can be clearly seen when comparing the scatter plots of the two response outputs, in control and lesioned animals, in Fig. 4 C and D, respectively. Points in the lower left quadrant indicate that both the conditioned behavioral and BP responses reflect the former contingencies, and points in the upper right quadrant indicate successful reversal of both conditioned responses. Points restricted to these two quadrants, as seen in the scatter plot for controls (Fig. 4C), reflect strong coupling between the two responses during learning of the reversal. In contrast, points more broadly scattered across all four quadrants, as seen in the scatter plot for lesions (Fig. 4D), reflects uncoupling of the response outputs as learning progresses.
Discussion
The present results demonstrate the critical involvement of the OFC in the flexible regulation and coordination of appetitive conditioned responses. Excitotoxic lesions of the primate OFC left intact the expression of conditioned, appetitive behavioral and autonomic responses that had been acquired before surgery. They did prolong, however, the otherwise rapid decline in cardiovascular arousal that accompanied the termination of the appetitive CS and omission of the reward in the one-trial extinction test. In addition, they not only delayed the relearning of a Pavlovian discrimination after reversal of the reward contingencies but caused the uncoupling of the conditioned behavioral and cardiovascular responses during reversal learning.
OFC and Extinction.
That OFC lesions were without effect on the expression of conditioned cardiovascular and behavioral responses is consistent with our (22) and others previous findings (7, 23). However, adaptation of the cardiovascular response in a one-trial extinction test was disrupted by the lesion. Whereas the cardiovascular arousal of control animals showed a rapid return to baseline after termination of the eliciting stimulus (CS+) and reward omission, cardiovascular recovery in animals with OFC lesions was markedly slower. This result highlights the contribution of the OFC to the on-line regulation of cardiovascular arousal, ensuring the rapid adjustment of that arousal to ongoing changes in the environment. It stands in contrast with the effects of lesions of ventromedial PFC in rats on the spontaneous recovery of both appetitive and aversive conditioning the day after extinction (24–26), an effect most likely due to the involvement of the ventromedial PFC in the use of contextual information to disambiguate cues that have formed multiple associations (27).
Slowed recovery from appetitive arousal after OFC lesions in monkeys parallels the findings of slowed recovery from negative emotional responses reported in a number of human studies (28, 29). For example, slowed recovery is seen in individuals with low trait resilience (29) and in individuals with greater relative left-sided prefrontal activation in scalp-recorded electrical signals (28), although the specific neural underpinnings of such effects are unknown. The experimental preparation developed in this study therefore provides a unique opportunity for future investigations to identify the brain structures and neurochemical systems that contribute to the recovery from positive and negative emotional responses and to determine the extent of their overlap.
OFC and Reversal Learning.
By measuring simultaneously both cardiovascular and behavioral responses to an appetitive CS, this study extends the results of previous studies of reversal learning that implicated the OFC in behavioral flexibility and reveals the additional importance of the OFC in the flexibility of autonomic responses. Indeed, it identifies the OFC as being critical for a coordinated response output as an animal adapts to changes in its environment. In the absence of the OFC, not only were animals slower to adapt their behavioral and autonomic responses to the reversed reward contingencies, but these responses became uncoupled. Thus, despite showing cardiovascular arousal to the currently rewarded CS, their behavioral arousal was often still directed toward the previously rewarded CS.
Whether the OFC acts to coordinate the different response outputs directly remains to be determined. Alternatively, response coordination may be an emergent property of emotional circuits that are regulated by the OFC. In this latter scenario, response uncoupling after OFC lesions may be a byproduct of the loss of this regulation. Such emotional circuits are likely to include the amygdala, striatum, hypothalamus, and periaqueductal gray, all of which receive direct projections from the OFC. Thus, it has been shown in rats performing a reversal of an odorant go/no-go discrimination task, that far fewer amygdala neurons reverse their odor preference after loss of OFC input (30), highlighting the dependence on the OFC of flexible neuronal firing in the amygdala. Although lesions of the amygdala tend not to affect overall performance on the reversal of appetitive, instrumental discrimination tasks (31, 32), their effect on performance of appetitive, Pavlovian reversal tasks is unknown. However, it has been shown that, unlike instrumental responses on discrimination tasks, the expression of conditioned cardiovascular responses in Pavlovian tasks depends on an intact amygdala (33). Moreover, the acquisition of various conditioned behaviors including CS-elicited approach (34) and orienting responses also depend on an intact amygdala (35, 36), despite their expression being amygdala-independent (M.S.M., K.B., Y. Mikheenko, and A.C.R., unpublished results and refs. 35–37). Other structures within the emotional circuit that may be involved in the expression of these conditioned behavioral responses, include the ventral (37) and dorsal striatum (38). In addition, the hypothalamus and periaqueductal gray are likely involved in regulation of the autonomic response (18, 20, 39).
OFC and Emotional Regulation.
The finding that lesions of the OFC cause an uncoupling of the elements of an adaptive response provides insight into the role of the OFC in emotional regulation. Emotions are highly adaptive and complex states, simultaneously engaging psychological, physiological, and behavioral responses that are triggered by the cognitive appraisal of external events. However, our immediate reactions to emotive stimuli are not always beneficial, and, therefore, an important element of emotion is the ability to appropriately adapt and rapidly modify emotional responses on a moment-by-moment basis. The contribution of the OFC to the regulation of positive emotional states is clearly demonstrated in the present study. Indeed, as animals without an OFC attempted to adapt to changes in the emotional significance of stimuli in the environment, there was fractionation of the emotional response. Such fractionation may have deleterious consequences on overall emotionality. Thus, interoceptive feedback, including that from the autonomic and somatic systems, is a core feature of many theories of emotion and is linked, in particular, to subjective emotional experience or “feelings” (40, 41). In addition, there is accumulating evidence that peripheral feedback can modulate emotional processing (42, 43), although the extent to which it does may depend on an individual's interoceptive awareness (44). Consequently, disjunctions between the autonomic and somatic components of peripheral feedback (e.g., increased cardiovascular arousal but no concomitant behavioral arousal) may have a marked impact on emotional experience.
There have been few, if any, investigations in humans comparing both behavioral and physiological arousal with respect to emotion regulation. However, an uncoupling of autonomic arousal from emotion perception (45) and from activations in emotion-specific brain regions (46) has been reported in schizophrenic patients, and a hyper- or hyporesponsive autonomic system has been reported in autistic children (47). These findings have led to the proposal that such disjunctions may contribute to the paranoid symptoms of patients with schizophrenia (46) and the development of certain stereotyped behaviors in autistic children (47). Since disrupted processing within the OFC is associated with both schizophrenia (48) and autism (49), the present results thus identify OFC dysfunction as a possible cause of the uncoupling of emotional responses that occurs in these disorders.
Methods
All procedures were conducted in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act under project license PPL 80/1770.
Cardiovascular Measurements.
Telemetry details have been described (33) and are explained in SI Text.
Behavioral Measurements.
Pavlovian conditioning procedures have the potential to generate a number of associations between a CS and a US in the brain, and responses elicited as a result of these representations have often been classified as “preparatory” (US-nonspecific) and “consummatory” (US-specific) (50) or “CS-directed” and “US-directed” (21, 51). Initially described in rats (21, 51), head jerks are defined as short, very rapid movements (a flick or snap) of the head mainly from side to side in response to auditory appetitive CS presentations. Never before assessed in primates, the marmosets in the present task were found to show selective head jerking to conditioned auditory stimuli that had been paired with food reward. See SI Text for details of additional measures.
Surgeries.
When fully anesthetized, marmosets were implanted with telemetry devices (33) and received excitotoxic lesions of the OFC or sham surgery (52) in two separate operations separated by at least 1 month (Table S8 and SI Text).
Statistical Analysis.
All cardiovascular and behavioral data were analyzed using SPSS software version 12.0.01.
Lesion effects were analyzed by using one-, two-, and three-way ANOVAs as described in Results. Any significant interactions were analyzed by using simple interaction and simple main effects, by using the mean square error term (SEM) from the original interaction where appropriate. Data that were skewed and that violated the distribution requirement of ANOVA, were transformed appropriately (53, 54). In addition, systolic BP and head-jerk behaviors during the reversal were statistically correlated by using a two-tailed bivariate correlation. The correlations provided a correlation coefficient (r) for each animal. To compare the correlation coefficients across control and lesioned groups Fisher's transformation of r, r′ was used in a one-way ANOVA.
Supplementary Material
Acknowledgments.
We thank Profs. T. W. Robbins and B. J. Everitt for comments on an earlier draft. We thank the James F McDonnell Foundation for supporting Y.L.R. for 4 months. The work was carried out within the Behavioural and Clinical Neurosciences Institute, supported by a consortium award from the Wellcome Trust and the Medical Research Council (MRC) of the U.K. This work was supported by MRC Program Grant G0401411 (to A.C.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800417105/DCSupplemental.
References
- 1.Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- 2.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Experimental Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 3.Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiology and Behavior. 1969;4:163–171. [Google Scholar]
- 4.Jones B, Mishkin M. Limbic lesions and the problem of stimulus–reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- 5.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 7.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 9.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butter CM, McDonald JA, Snyder DR. Orality, preference behavior, and reinforcement value of nonfood object in monkeys with orbital frontal lesions. Science. 1969;164:1306–1307. doi: 10.1126/science.164.3885.1306. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- 12.Pavlov IP. Conditioned Reflexes. Oxford: Oxford Univ Press; 1927. Extinction of conditioned reflexes; pp. 48–67. [Google Scholar]
- 13.Brutkowski S. Effect of excision of the frontal lobes on salivary conditioned reflexes in dogs. Acta Physiol Pol. 1954;5:505–507. [PubMed] [Google Scholar]
- 14.Brutkowski S. Effects of prefrontal ablations on salivation during the alimentary unconditioned reflex and after its cessation. Acta Biol Exp. 1959;19:281–290. [Google Scholar]
- 15.Auleytner B, Brutkowski S. Effects of bilateral prefrontal lobectomy on the classical (type i) defensive conditioned reflexes and some other responses related to defensive behaviour in dogs. Acta Biol Exp. 1960;20:243–262. [Google Scholar]
- 16.Hall RE, Cornish K. Role of the orbital cortex in cardiac dysfunction in unanesthetized rhesus monkey. Exp Neurol. 1977;56:289–297. doi: 10.1016/0014-4886(77)90348-x. [DOI] [PubMed] [Google Scholar]
- 17.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 18.Öngür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 19.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Process. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Roberts AC. A componential analysis of the functions of primate orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The Oribitofrontal Cortex. Oxford: Oxford Univ Press; 2006. pp. 237–264. [Google Scholar]
- 23.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan MA, LeDoux JE. Differential Contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 25.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;11:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci. 2007;26:2654–2660. doi: 10.1111/j.1460-9568.2007.05855.x. [DOI] [PubMed] [Google Scholar]
- 28.Jackson DC, et al. Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 29.Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. J Pers Soc Psychol. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Braesicke K, et al. Autonomic arousal in an appetitive context in primates: A behavioural and neural analysis. Eur J Neurosci. 2005;21:1733–1740. doi: 10.1111/j.1460-9568.2005.03987.x. [DOI] [PubMed] [Google Scholar]
- 34.Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- 35.McDannald M, Kerfoot E, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. Eur J Neurosci. 2004;20:240–248. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- 36.Groshek F, et al. Amygdala central nucleus function is necessary for learning, but not expression, of conditioned auditory orienting. Behav Neurosci. 2005;119:202–212. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardinal RN, et al. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- 38.Han JS, McMahn RW, Holland P, Gallagher M. The role of an amygdala-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 40.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 41.Damasio A. Feelings of emotion and the self. Ann NY Acad Sci. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- 42.Nicotra A, Critchley HD, Mathias CJ, Dolan RJ. Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain. 2006;129:718–728. doi: 10.1093/brain/awh699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Critchley HD, et al. Vagus nerve stimulation for treatment-resistant depression: behavioral and neural effects on encoding negative material. Psychosom Med. 2007;69:17–22. doi: 10.1097/PSY.0b013e31802e106d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 45.Aleman A, Kahn RS. Strange feelings: Do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Williams LM, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155:29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proc Biol Sci. 2001;268:1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baare WF, et al. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- 49.Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Konorski J. Integrative Activity of the Brain. Chicago: Chicago Univ Press; 1967. [Google Scholar]
- 51.Holland PC. The Psychology of Learning and Motivation. Englewood Cliffs, NJ: Prentice–Hall; 1984. Origins of behaviour in Pavlovian conditioning. [Google Scholar]
- 52.Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howell DC. Graduate-Level Statistics for Psychology and Neuroscience: ANOVA in Practice, and Complex ANOVA Designs. Belmont, CA: Duxbury; 2002. [Google Scholar]
- 54.Cardinal R, Aitken MRF. ANOVA for the Behavioural Sciences Researcher. Mahwah, NJ: Lawrence Erlbaum; 2006. [Google Scholar]
- 55.Roberts AC, et al. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): An anterograde and retrograde tract-tracing study. J Comp Neurol. 2007;502:86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.